- 1Oncology Department, Oncovida Cancer Center, Santiago, Chile
- 2Brazilian National Cancer Institute, Grupo Oncoclínicas, Rio de Janeiro, Brazil
- 3Public Health Department, Americas Health Foundation, Bogota, Colombia
- 4Centro Oncológico, Hospital Médica Sur, Mexico City, Mexico
- 5Departamento de Oncología, Hospital Universitario San Ignacio, Centro Javeriano de Oncología, Bogotá, Colombia
- 6Hospital do Coracao (HCor), Oncology Department, São Paulo, SP, Brazil
- 7Departamento de Oncología, Fundación Valle del Lili, Cali, Colombia
- 8Instituto de Oncología Ángel Roffo, Universidad de Buenos Aires, Fundación CIDEA, Buenos Aires, Argentina
Melanoma represents an increasing public health burden with extensive unmet needs in Latin America (LA). A mutation in the BRAF gene is present in approximately 50% of all melanomas in White populations and is a target of precision medicine, with the potential to dramatically improve patient outcomes. Thus, increased access to BRAF testing and therapy is LA must be explored. At a multi-day conference, a panel of Latin American experts in oncology and dermatology were provided with questions to address the barriers limiting access to testing for BRAF mutation in patients with melanoma in LA, who may be eligible for targeted therapy to improve their prognosis. During the conference, responses were discussed and edited until a consensus on addressing the barriers was achieved. Identified challenges included ignorance of BRAF-status implications, limited human and infrastructural resources, affordability and reimbursement, fragmented care delivery, pitfalls in the sample journey, and lack of local data. Despite the clear benefits of targeted therapies for BRAF-mutated melanoma in other regions, there is no clear path to prepare LA for a sustainable personalized medicine approach to this disease. Due to melanoma’s time-sensitive nature, LA must aim to provide early access to BRAF testing and consider mutational status within treatment decision making. To this end, recommendations are provided and include establishing multidisciplinary teams and melanoma referral centers and improving access to diagnosis and treatment.
Introduction
Melanoma represents an increasing public health burden with extensive unmet needs in Latin America (LA). Globally, melanoma incidence has risen and accounts for most skin cancer-related mortality (1), with 324,635 new cases in 2020 and 57,000 deaths worldwide (2) Moreover, studies report that patients with skin melanoma in low-and-middle-income countries, such as those in LA, are more likely to present with advanced disease and have poorer survival when compared to high-income countries (3–5).
Nevertheless, the prognosis of patients with advanced melanoma has dramatically improved in recent years. Before immunotherapy and targeted therapy emerged, the average five-year survival for patients with stage IV melanoma was 2·3%, and the median survival was eight to ten months (6). More recently, the advent of precision medicine has leveraged the increased understanding of tumor biology and the immune system’s role in developing personalized cancer therapies. One target of precision medicine is the BRAF protein, encoded by BRAF.
BRAF is a potent oncogene, present in approximately 50% of all melanomas in the White population, that plays a critical role in the Ras-Raf-mitogen-activated protein kinase/extracellular signal-related kinase (MEK) cell-signaling pathway (7, 8). People with melanoma who possess BRAF gene variants exhibit distinctive clinical features, with particularly aggressive biological behavior; patients are often younger and have tumors in areas without chronic sun exposure, with superficial spreading or nodular histology, and have an increased nevus count (9, 10). Additionally, BRAF-mutated tumors are more likely to metastasize to the brain (11, 12).
Data regarding melanoma mutations primarily comes from high-income countries (HIC), likely due to greater access to testing. Unlike in Europe and the US (13), data on melanoma incidence in LA is scarce. The available reports are mainly based on hospital records or private institutions that do not represent the general population, likely leading to underestimating the burden of this disease in the region (3, 14, 15). Additionally, the available data regarding mutational status has not been collected prospectively, thus having limited accuracy. Therefore, increased epidemiologic and data collection efforts are necessary to characterize the different populations and perform improved clinicopathologic correlation studies.
Melanoma epidemiology and BRAF-mutation frequency are heterogeneous and affected by ethnicity. For instance, a meta-analysis comparing the incidence rates of Asians and Whites found 19.5% and 40.3%, respectively (9). BRAF-mutation prevalence in LA has been found to be lower than in predominantly White populations. This is potentially a result of higher proportions of indigenous heritage and higher rates of acral-lentiginous melanoma in LA. However, ethnic variations among and within countries in LA are wide (9), and prior genomic knowledge of country-specific populations key to mutation screenings is largely lacking.7
Still, a few studies have documented BRAF-related melanoma in LA. In a single-institution cohort of 459 patients with melanoma in Barretos, Brazil, 34% carried a BRAF mutation (16). Another cohort showed V600 BRAF mutations in around 40% of cases (17, 18). However, this data is from a private institution and may not represent the general population (17–19). In Argentina, BRAF V600 mutation was reported in 50·2% of 354 patients (20).. Most of this sample is from patients from either the city or province of Buenos Aires, where most residents are White. In Mexico, studies identified BRAF V600E variant frequencies in primary melanoma studies ranging from 6·4% (3/47 patients in Mexico City) (21) to 73·0% (24/33 in Northeast Mexico) (22). In Chile, there is a complete lack of epidemiologic data.
Patients with melanoma with BRAF V600E and V600K mutations respond to clinically available BRAF inhibitors. Targeted treatment for patients with BRAF melanoma has reversed the poor prognosis associated with this molecular alteration (10). The success of targeted therapies in BRAF-mutated melanoma has led to the recommendation that patients with advanced disease and at high relapse risk be screened for V600 mutations to help guide therapeutic decision making (23).
Despite these advances, unmet needs remain, especially in regions such as LA, where determining mutational status and access to timely diagnosis and treatment are challenging. This review discusses the unmet needs of patients with BRAF-mutated melanoma in LA, including molecular testing strategies for detecting the mutation and their appropriate use within the regional context. The content is from the literature and panelists’ experience and opinion. The challenges to providing adequate and effective diagnosis and treatment for patients with BRAF-mutated melanoma are discussed, and recommendations on overcoming these barriers will be provided.
Methods
Study design and panelists
Americas Health Foundation (AHF) identified seven experts in oncology and dermatology from Argentina, Brazil, Chile, Colombia, and Mexico who have published in BRAF-mutated, melanoma, or health economics since 2016. As it was not practical to gather panelists from all the countries in Latin America together for a conference, the panel was chosen to provide a perspective of oncologists and dermatologists from countries across Latin America. The panel convened for a three-day virtual meeting on October 26-29, 2021, to discuss the need for region-specific recommendations. To identify the panel, AHF conducted a literature review to identify scientists and clinicians from the above countries who have publications relating to BRAF-mutated melanoma since 2016. Augmenting this search, AHF contacted opinion leaders from LA’s medical field to corroborate the list of individuals who adequately represented the necessary fields of study. All the experts who attended the meeting are named authors of this paper. An AHF staff member moderated the discussion. The authors retain complete control over the content of the paper.
Search strategy AHF conducted a literature review using PubMed, MEDLINE, and EMBASE. The following search terms were used: “BRAF,” “melanoma treatment,” and “cancer,” in combination with “Latin America,” “Mexico,” “Colombia,” “Argentina,” “Brazil,” and “Chile,” “molecular testing,” from 01/01/2016 until 04/10/2021. The articles identified were in English, Portuguese, and Spanish. Particular attention was paid to identifying literature and research in LA.
AHF developed specific questions to address barriers limiting access to testing BRAF variants in LA and assigned one to each panel member (Supplementary Table 1). A written response to each question was drafted by individual panel members based on the literature review and personal expertise. Each narrative was reviewed and edited by the entire panel during the three-day conference through numerous rounds of discussion until a complete agreement was reached. For issues where there was disagreement among the panel, additional dialogues took place until all panel members agreed to the content included in this paper. The recommendations developed were based on the evidence gathered, expert opinion, and personal experience and were approved by the entire panel. After the conference, the final manuscript was distributed by email to the panel for review and approval.
Role of the funding source
This manuscript was supported by an unrestricted grant given to AHF, a 501(c)3 nonprofit organization dedicated to improving health care throughout LA, by Novartis. The funder had no role in the study design, data collection, data analysis, interpretation, and writing of the report.
BRAF mutation testing
Patient selection and timing
International clinical practice guidelines (CPG) suggest that BRAF mutation testing be mandatory in patients with stage III or stage IV melanoma and high-risk resected disease (24). When metastases occur, it is recommended to use the metastatic sample. If it is unavailable, the analyses may be performed on lymph node metastases or the primary tumor, as there is a high degree of concordance between the BRAF status of primary melanomas and their metastatic lesions (25).
In the appropriate clinical context, initiating reflex testing at an earlier stage (IIB, IIC) for patients with limited access to frequent visits and specialist care can prevent unnecessary delays in targeted BRAF/MEK inhibitor therapy (26). They may prove to have a similar benefit for BRAF-mutated melanoma, as earlier treatment initiation may improve clinical outcomes for patients.
Testing methods
Different methods for BRAF testing exist and can be considered based on their utility in screening, confirmatory, and reference testing (27). Testing decisions often depend upon available methods and infrastructure, specificity and sensitivity, and variable cost and access throughout the region (Table 1) (28). Primary cutaneous melanomas, metastatic lymph nodes, or radiologically detected lesions are fixed with formaldehyde and paraffin-embedded in blocks that can be used for testing (29). Essential to accurate molecular testing, the tissue sample journey is complicated and involves prefixation, fixation, and post-fixation processes. The specific procedures of each step must be optimized to achieve preservation and ensure a high-quality sample.
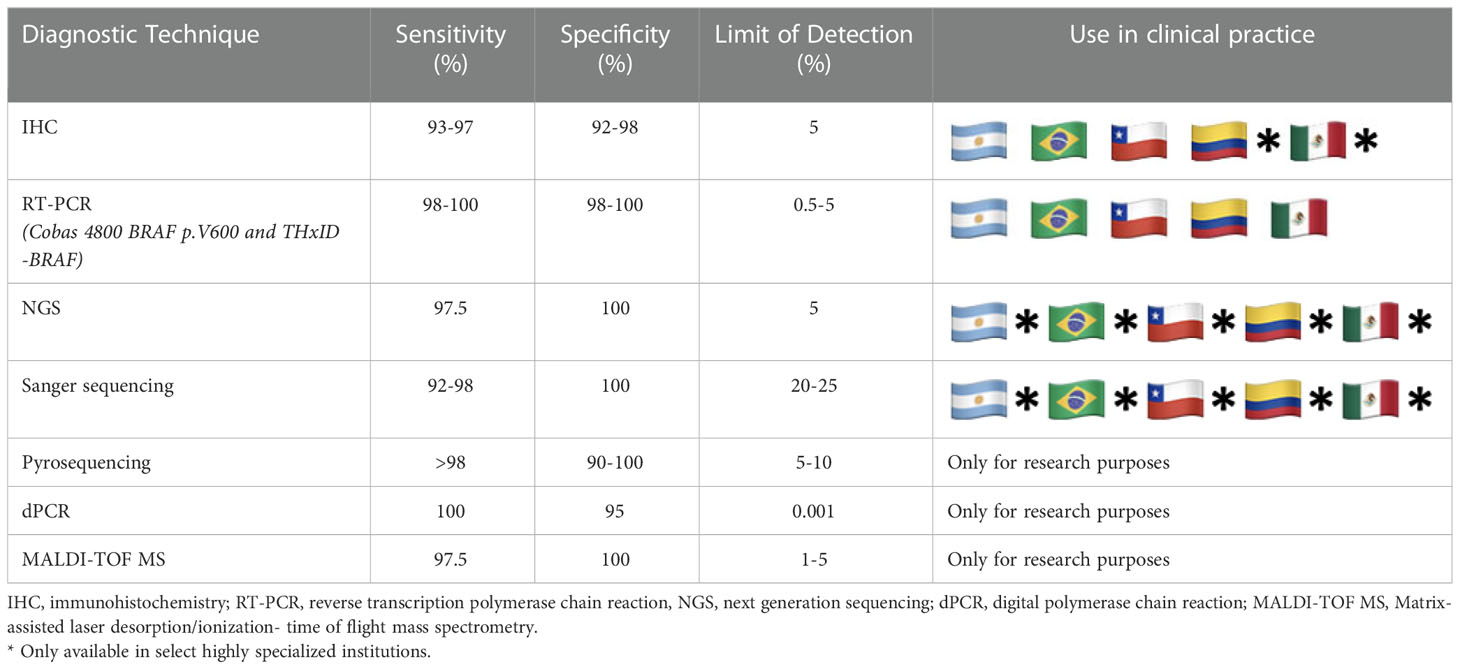
Table 1 Sensitivity, specificity, and use in LA clinical practice for common BRAF diagnostic techniques.
Tumor heterogeneity in advanced-stage melanoma must be considered as it may have implications for molecular testing and, thus, treatment (30). To mitigate the risk of misinterpreting BRAF mutational status due to intratumor heterogeneity, testing should always be conducted on metastatic lesions when available (31, 32). Sequential analysis using confirmatory methods for detecting BRAF mutations is usually performed in high income countries (HIC). Nevertheless, this approach is not always feasible or cost-effective in limited-resource contexts. Ideally, confirmatory testing with real-time polymerase chain reaction (PCR), Sanger sequencing, or Next-Generation Sequencing (NGS) should be conducted in all patients with a negative test result through immunohistochemistry (IHC). Alternatively, a blood sample may be assayed for circulating tumor DNA, but its lower sensitivity compared with a tissue-based biopsy must be considered (33). Figure 1 proposes a suggested pathway for BRAF testing in limited-resource settings.
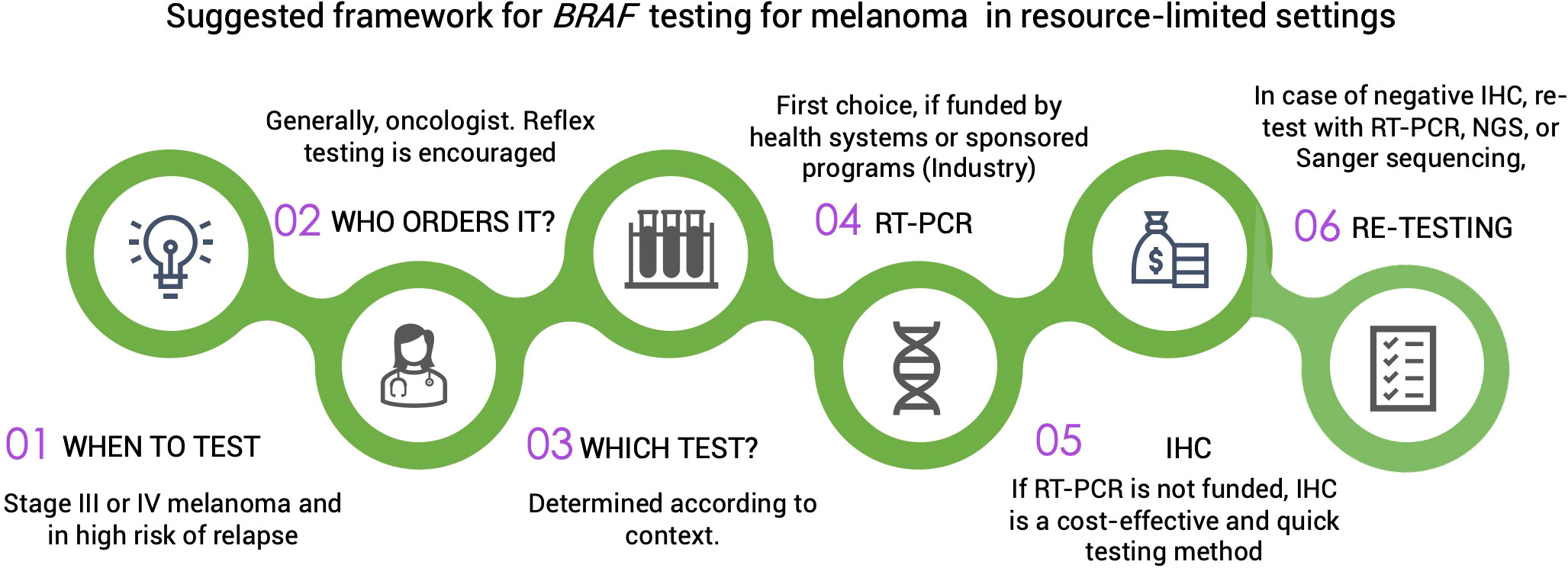
Figure 1 IHC, immunohistochemistry; RT-PCR, reverse transcription polymerase chain reaction; NGS, next generation sequencing.
Immunohistochemistry (IHC)
IHC is relatively simple and inexpensive, providing rapid results. BRAF V600E is the only variant that can be detected through this method (26, 34). Of note, IHC is not the best method regarding sensitivity: mutations may not be detected in some patients (31). However, employing IHC can be a cost-effective tool and a valuable supplement to conventional mutation testing to allow patients with V600E-variant metastatic melanoma to be triaged rapidly into appropriate treatment pathways (31, 32).
Real-time PCR-based techniques
Various real-time PCR-based methods are available, including Cobas 4800 and THxID-BRAF. Although these methods offer the advantages of a relatively quick turnaround time, they do not allow direct identification of the specific nucleotide sequence. These are commercially available companion diagnostic kits, each targeted to identify mainly V600E and V600K mutations (8).
Next-generation sequencing (NGS)
Despite NGS time, cost, and data-analysis demands, it can provide robust data on all BRAF and other actionable variants and quantify allele frequency. High specificity and sensitivity can be achieved with a small proportion of tumor DNA from the tissue sample. Its role is especially prominent in research, large mutation analysis, clinical trials, therapeutics, and confirmatory testing.
Sanger sequencing
Sanger sequencing, long known as the gold standard for reference testing in somatic variants, is 100% specific, providing quality sample preparation (8). It primarily serves as a confirmatory test in the case of inconclusive PCR methods, given its relatively higher costs and turnaround time (35).
Other methods
Several other methods for BRAF testing that offer high sensitivity and specificity exist but are generally not available in LA outside of the research context, primarily due to high costs and technological requirements. These methods include pyrosequencing, matrix-assisted laser desorption ionization time of flight mass spectrometry, allele-specific PCR, and droplet digital PCR.
Systemic treatment for BRAF-mutated melanoma
Targeted therapy defied the conventional thinking in the United States, Europe, and Australia that patients in poor clinical condition due to advancing disease should not be treated. The uptake of these therapies throughout LA has been slower than in other regions due to access limitations, infrastructure issues, and cost constraints. Nevertheless, healthcare systems in the region could benefit from increasing the use of a precision medicine approach regarding patient outcomes, cost-effectiveness, and resource allocation.
Despite the fact that targeted therapy and immunotherapy have been highly effective in treating advanced BRAF-mutated melanoma (36–38), data comparing the two treatments revealed that targeted therapy has a greater response relative to immunotherapy. In contrast, immunotherapy confers longer-lasting results (39). Therefore, disease features, safety profiles, medical history, patient preferences, and access must be considered when making treatment decisions.
The first BRAF inhibitor developed, vemurafenib, surprised the oncological community with its Phase-I trial results showing rapid and profound responses that had never been seen in melanoma, though short-lived (40). The combination of MEK and BRAF inhibitors improved response rates, progression-free survival, and overall survival (OS) compared to monotherapy with BRAF inhibitors, as demonstrated with dabrafenib + trametinib (38); vemurafenib + cobimetinib (36); and encorafenib + binimetinib (37). For these three therapies, five-year progression-free and OS results were almost 20% and 35%, respectively. Some advanced melanoma characteristics associated with long-term responses to these drugs are normal lactate dehydrogenase (LDH) levels, less than three metastatic sites, and a good Eastern Cooperative Oncology Group performance status (38). These clinical features may help select patients with BRAF-mutated melanoma that would benefit most from targeted therapy in the long term. Patients with a high tumor burden, rapid progression, or poor performance status represent an unmet need for currently available drugs; reasonable access to target therapy for these patients is vital because of the rapidly progressing disease.
Combination therapy with immunotherapy (nivolumab/ipilimumab) followed by target therapy (dabrafenib/trametinib) is emerging as an option that may yield greater overall survival in patients with BRAFV600-mutated advanced melanoma (38, 39, 41, 42). The CheckMate 067 is a phase III trial which randomized previously untreated unresectable stage III or stage IV melanoma patients to receive nivolumab plus ipilimumab (four doses) followed by nivolumab; or nivolumab alone; or ipilimumab alone. The 6.5-year overall survival rates were respectively 57%, 43%, and 25% in patients with BRAF-mutant tumors and 46%, 42%, and 22% in those with BRAF-wild-type tumors, and the median overall survival is the longest in a phase III melanoma trial reported to date (43).
Few prospective data are available on sequential immunotherapy and BRAF/MEK inhibition for BRAF-mutant metastatic melanoma. The SECOMBIT is a noncomparative phase II trial which randomized patients with untreated, metastatic BRAF-mutant melanoma to receive A) encorafenib plus binimetinib until progressive disease followed by ipilimumab plus nivolumab; or B) ipilimumab plus nivolumab until progressive disease followed by encorafenib plus binimetinib; or C) encorafenib plus binimetinib for 8 weeks followed by ipilimumab plus nivolumab until progressive disease followed by encorafenib plus binimetinib. At a median follow-up of 32.2 months, the median overall survival was not reached in any arm. However, the 2 and 3-year OS rates showed that sequential immunotherapy and targeted therapy provide clinically meaningful survival benefits (44).
Access to BRAF testing and treatment in LA
Although the proportion of patients with a melanoma diagnosis that undergoes BRAF-mutation testing in LA is unknown, it is likely lower than that of HIC due to restricted access. Access to diagnostic methods and treatments varies widely among and within countries in LA. Moreover, vast inequities exist in access between the regional private and public healthcare systems. Molecular testing demands an infrastructure comprising technological, financial, and human resources, which few institutions in LA possess (45). The complex technologies and processes are mostly only available in highly specialized centers, usually concentrated in major cities, leaving large populations underserved. In general, public hospitals do not offer this testing.
In countries in LA where targeted therapies are approved, BRAF testing is sometimes provided cost-free by the pharmaceutical industry, making it available to a large portion of the population for as long as the sponsored programs exist (45). Although this offers a short-term solution to access, it is not without problems. Logistical issues arise in the sample-handling journey, often resulting in diagnostic and treatment initiation delays. Further, these programs displace the government’s responsibility to provide reimbursement for testing, which is an undesirable situation.
Ideally, access to BRAF testing must be accompanied by access to targeted therapies. This is often not the case in LA, mainly due to economic constraints. Although at least one targeted therapy for BRAF-mutated melanoma is approved by regulatory agencies and available in many countries in LA, including Argentina, Brazil, Chile, Colombia, Mexico, and Uruguay, a lack of reimbursement, particularly within the public healthcare systems, continues to limit drug access (46). Of note, since advanced melanoma is a time-sensitive malignancy, access to both testing and therapy must be prompt.
Discussion
Challenges to BRAF-mutated melanoma management in LA
A precision medicine approach to BRAF-mutated melanoma has demonstrated potential to improve health outcomes; however, factors inherent to precision medicine and LA’s healthcare systems create significant implementation obstacles. Given molecular pathology’s technical considerations and complexities, achieving precision medicine’s potential requires overcoming these hurdles. The barriers to quality management of BRAF-mutated melanoma in LA throughout the patient journey are depicted in Figure 2.
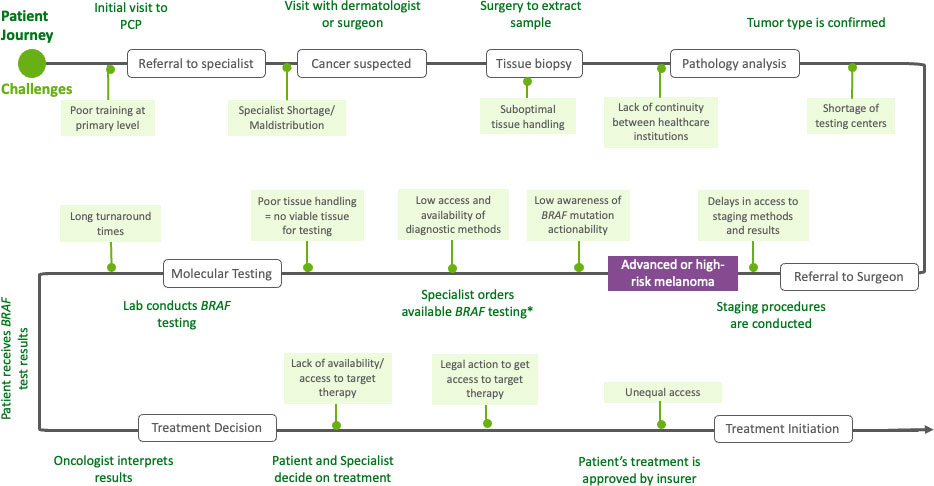
Figure 2 Patient journey of people with BRAF melanoma in Latin America. PCP, primary care physician.
Affordability/Reimbursement
Precision medicine approaches demand high up-front investments in infrastructure, personnel training, and funding for molecular testing and targeted therapies. Although access to testing methods is not uncommon in the region, the corresponding targeted therapies are not always reimbursed or available. Additionally, anti-cancer drugs are proportionally much more expensive in LA when compared with higher income regions (47) because drugs are acquired with weaker currencies, and procurement strategies are not optimized. In the authors’ experience, the extent to which financing for testing limits access varies from not being an issue to affecting 75% of the time. The majority believe that financing for therapy is a limiting factor 25% of the time, although some report it is a factor 50-75% of the time.
Limited infrastructure
Healthcare centers with the capacity to provide melanoma care in LA are disproportionately located in major cities, leaving large areas underserved with unequal access levels. Even in major cities, pathology laboratories that meet the infrastructural demands of molecular tests used to diagnose BRAF mutations are scarce, resulting in long turnaround times for testing or unreliable test results.
Fragmented care
From diagnosis to treatment, melanoma care in LA is generally fragmented, a stark contrast to the current standard of care that involves a multidisciplinary team approach. Delays occur at virtually every step of the patient journey due to miscoordinations in care efforts at the primary level, during the diagnostic phase, and in treatment decision making. Tumor boards for melanoma, which can aid in overcoming diagnostic and management barriers, are not widely implemented.
Lack of human resources
There is a generalized shortage of medical personnel involved in melanoma care, including dermatologists, clinical oncologists, and pathologists, leading to high workloads and delays in diagnosis and treatment. This shortage may be related to the relatively small number of training opportunities for residencies and fellowships in these specialties within the region. Furthermore, a lack of resources in smaller cities or rural areas disincentivizes specialists from practicing in these areas, creating severe access gaps due to geographic resource maldistribution (48). At the primary care level, medical personnel lack training in identifying suspicious lesions and appropriate referral, which may also result in more advanced stages at diagnosis.
Lack of awareness of BRAF-status implications
Despite BRAF testing for advanced melanoma being compulsory based on unanimous international CPG, a generalized lack of awareness among stakeholders involved in melanoma decision making exists. Treating physicians do not always adhere to CPG, and similar treatment approaches are often taken for all or most patients with melanoma in LA, without regard to mutational status. Likewise, government, regulatory agencies, and payers do not always make evidence-based decisions to approve and reimburse targeted therapies and their corresponding diagnostic methods.
Pitfalls in the sample journey/quality assurance
Adequate institutional protocols and quality-control standards to regulate sample preparation are not standard across laboratories (49). Suboptimal practices within the tissue sample journey, including insufficient sample quantities, create technical challenges to BRAF testing. Because of limited infrastructural resources, there is a lack of continuity across the different healthcare institutions which the sample must pass in its journey, causing further delays.
Lack of local data
Region- and country-specific data to characterize the BRAF-variation prevalence in each population, enable accurate treatment decisions, and guide public policy are severely deficient. Global data corroborate the positive impact of introducing precision medicine strategies for melanoma treatment; however, the lack of local data hinders advancing this approach in the region. It is also necessary to define adequate metrics and indicators to track patient outcomes and determine the cost-effectiveness of targeted therapies for melanoma. Most decisions on healthcare resource allocation in LA are primarily based on cost. Yet coordinated (international) efforts to negotiate more accessible prices are not initiated by the governing bodies.
Recommendations
This panel has addressed the lack of access to diagnosis and treatment for BRAF-mutated melanoma. With increasing health care costs and limited resources, a critical need exists to understand the root causes of these technologies’ underuse in the population for which they were developed. Additionally, efforts to increase access should be a collaborative, multi-stakeholder endeavor. The recommendations below address the challenges to widespread access to these diagnostic tools and, as a result, adequate treatments. Because these access issues are not exclusive to this region or this cancer, these recommendations may be tailored on a country-by-country and cancer-by-cancer basis.
1. Improve affordability and reimbursement
Governments must work toward achieving a sustainable approach to sourcing high-cost cancer diagnostic methods and therapies as a region by:
- Implementing procurement and contracting strategies such as managed entry agreements or risk-sharing strategies (50).
- Leveraging negotiation power using pooled procurement, several countries can unite as a single buying bloc by combining their resources and requesting tests and doses.
- Improving the coherence of approval and reimbursement for both pieces of the companion diagnostic (i.e., testing and therapy). Ideally, these regulatory pathways should provide an aligned channel for co-developed products to ensure innovation is not stifled.
2. Improve testing infrastructure
Stakeholders must develop high-quality laboratories that can perform the molecular testing required for BRAF detection by:
- Increasing investment in pathology departments and laboratories to meet the technological and training demands of high-quality molecular testing.
- Establishing adequate quality-control standards, accreditation programs, and institutional protocols that regulate sample preparation and the quality of BRAF testing (51).
- Creating centralized laboratories to perform the tests throughout the countries may help optimize turnaround times and save costs.
3. Establish multidisciplinary teams and melanoma referral centers
Healthcare institutions should provide a multidisciplinary approach to melanoma management by:
- Establishing multidisciplinary teams that include primary care physicians, dermatologists, clinical oncologists, surgeons, pathologists, geneticists, radiation oncologists, palliative care doctors, oncology nurses, and social workers.
- Providing oncology navigators to guide and support patients through the medical and administrative process complexities related to cancer care.
- Increasing referral centers for skin cancer care to promote care continuity and reduce delays (52–55).
4. Raise awareness of melanoma and the actionability of BRAF variants
Medical societies and healthcare professionals must engage in continuous medical education at every level of care on the importance of determining BRAF mutational status.
- Primary care physicians must be trained to recognize suspicious lesions and understand appropriate referral situations.
- Specialists must understand the importance of determining BRAF mutations and facilitate diagnostic testing. Leveraging the concept of reflex testing may help reduce diagnostic delays.
- Pathologists and molecular biologists must be adequately trained to conduct and interpret tests reliably and accurately.
5. Increase BRAF testing
When indicated, healthcare professionals, medical societies, government, and patient organizations should promote testing for BRAF mutations.
- All patients with metastatic or unresectable melanoma or those at high risk of relapse should be screened for BRAF V600 mutations, preferably in a metastatic lesion, and for adjuvant therapy in the primary tumor to guide therapeutic decision making (56).
6. Address shortage and maldistribution of specialists
Governments, medical societies, and academic institutions must address the shortage and maldistribution of specialists by:
- Increasing training opportunities in residency and fellowship programs in dermatology, surgical and clinical oncology, and pathology to address the lack of specialists in these fields.
- Implementing virtual tumor boards or second opinion networks to bridge the gaps created by geographical disparities.
7. Increase data collection and outcomes tracking
All stakeholders must prioritize funding for melanoma research to:
- Establish national disease-specific registries to generate local data on which to base health policies tailored to the national context (50).
- Define and quantify quality metrics, including indicators for access to diagnostic tools and therapies, to monitor patient outcomes and the impact of precision medicine strategies.
Conclusion
There are wide opportunities in LA to improve the landscape of melanoma prevention, early detection, characterization, and management. Strategies to bolster primary and secondary prevention of skin melanomas must be prioritized as essential steps to improving the OS of patients with skin melanoma and also considering the economic implications this may have for health systems by avoiding disease evolution to more complex stages (57–60). That said, healthcare systems in LA must be better equipped to adapt to the complexities of the advanced stages of melanoma. Delaying diagnosis and treatment initiation negatively impacts progression-free survival and OS. In HIC, broad access to effective therapy for advanced disease has led to reductions in mortality. Similar results can be expected in LA if access is improved (55, 61). Despite the clear outcome benefits from targeted therapies for BRAF-mutated melanoma in other regions, there is no clear path to prepare LA for a sustainable personalized medicine approach to this disease. Due to melanoma’s time-sensitive nature, LA countries must provide early access to BRAF testing in appropriate situations and for mutational status to be considered within treatment decision making.
Author contributions
PS and AM Writing-original draft, investigation, formal analysis, validation. MR-R Writing-review and editing, visualization, conceptualization, methodology, project administration. JR, AR, RS, AZ, and GC Writing-original draft, investigation, formal analysis, validation. All authors contributed to the article and approved the submitted version.
Funding
This manuscript was supported by an unrestricted grant given to AHF, a 501(c)3 nonprofit organization dedicated to improving health care throughout LA, by Novartis. The funder had no role in the study design, data collection, analysis, interpretation, and report writing.
Conflict of interest
GC received: grants or had contracts from Novartis, Merck Sharp & Dohme, Pfizer, Roche/Genentech, Bristol Myers Squibb, Array, Springer, Grupo Español de Melanoma; consulting fees from Merck Sharp & Dohme, Novartis, Bristol Myers Squibb, Roche/Genentech; honoraria for presentations from Novartis, Merck Sharp & Dohme, Pfizer, Roche, Bristol Myers Squibb, Array, Springer, and Grupo Español de Melanoma; support for attending meetings from Novartis, Merck Sharp & Dohme, Bristol Myers Squibb, Roche; payment for board participation from Novartis, Merck Sharp & Dohme, Bristol Myers Squibb, Roche/Genentech. AM’s institution received grants from Clovis Oncology, Regeneron, Merck Sharp & Dohme, Bristol Myers Squibb, GlaxoSmithKline, AstraZeneca, Novartis, Amgen, and Roche for clinical trials. AM received payment for lectures from Merck Sharp & Dohme, Bristol Myers Squibb, GlaxoSmithKline, AstraZeneca, Novartis, Roche, Sanofi. Advisory Board participation from Merck Sharp & Dohme, Bristol Myers Squibb, GlaxoSmithKline, AstraZeneca, Novartis, Roche. RS received: consulting fees from Merck Sharp & Dohme and L’Oreal; payment for presentations from Merck Sharp & Dohme, Bristol Myers Squibb, Sanofi Genzyme, Pfizer, Merck Serono, Novartis; support from Bristol Myers Squibb for expert testimony; support for attending meetings from Sanofi Genzyme and Merck Sharp & Dohme.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1032300/full#supplementary-material
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin (2017) 67(1):7–30. doi: 10.3322/caac.21387
2. Melanoma of skin. International Agency for Research on Cancer (2121). Available at: https://gco.iarc.fr/today/data/factsheets/cancers/16-Melanoma-of-skin-fact-sheet.pdf.
3. de Vries E, Sierra M, Piñeros M, Loria D, Forman D. The burden of cutaneous melanoma and status of preventive measures in central and south America. Cancer Epidemiol (2016) 44:S100–S9. doi: 10.1016/j.canep.2016.02.005
4. Schmerling RA, Loria D, Cinat G, Ramos WE, Cardona AF, Sánchez JL, et al. Cutaneous melanoma in Latin America: The need for more data. Rev Panam Salud Publica (2011) 30(5):431–8. doi: 10.1590/S1020-49892011001100005
5. Sortino-Rachou AM, Curado MP, de Camargo Cancela M. Cutaneous melanoma in Latin America: A population-based descriptive study. Cad Saúde Pública (2011) 27(3). doi: 10.1590/S0102-311X2011000300016
6. Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol (2008) 26(4):527–34. doi: 10.1200/JCO.2007.12.7837
7. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature (2002) 417(6892):949–54. doi: 10.1038/nature00766
8. Vanni I, Tanda ET, Spagnolo F, Andreotti V, Bruno W, Ghiorzo P. The current state of molecular testing in the BRAF-mutated melanoma landscape. Front Mol Biosci (2020) 7. doi: 10.3389/fmolb.2020.00113
9. Kim S, Kim S, Hahn H, Lee Y, Choe Y, Ahn K. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol (2015) 72(6):1036–46.e2. doi: 10.1016/j.jaad.2015.02.1113
10. Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol (2011) 29(10):1239–46. doi: 10.1200/JCO.2010.32.4327
11. O'Shea PJ, Tatineni V, Rauf Y, Jia X, Murphy ES, Chao ST, et al. Outcomes of BRAF mutated vs. wild type tumors in melanoma brain metastasis. Int J Radiat Oncol Biol Phys (2021) 111(3, Supplement):e576. doi: 10.1016/j.ijrobp.2021.07.1551
12. Venur VA, Kotecha R, Chen Z, Chao ST, Elson P, Suh JH, et al. Impact of BRAF mutation in patients with brain metastasis from melanoma. J Clin Oncol (2015) 33(15_suppl):e13016-e. doi: 10.1200/jco.2015.33.15_suppl.e13016
13. Bauer J, Büttner P, Murali R, Okamoto I, Kolaitis NA, Landi MT, et al. BRAF mutations in cutaneous melanoma are independently associated with age, anatomic site of the primary tumor, and the degree of solar elastosis at the primary tumor site. Pigment Cell Melanoma Res (2011) 24(2):345–51. doi: 10.1111/j.1755-148X.2011.00837.x
14. Reyes E, Uribe C, de Vries E. Population-based incidence and melanoma-specific survival of cutaneous malignant melanoma in a Colombian population 2000–2009. Int J Dermatol (2018) 57(1):21–7. doi: 10.1111/ijd.13839
15. dos Santos CA, Souza DLB. Melanoma mortality in Brazil: Trends and projections (1998-2032). Ciênc saúde Colet (2019) 24(4). doi: 10.1590/1413-81232018244.13932017
16. da Costa LMM, Crovador CDS, de Carvalho CEB, Vazquez VDL. Characteristics of Brazilian melanomas: Real-world results before and after the introduction of new therapies. BMC Res Notes (2019) 12(1):296. doi: 10.1186/s13104-019-4336-7
17. Fouto Matias JE, Jung JE, Böer A, Bresch M, Falk TM. BRAF mutations in cutaneous melanoma: No correlation with histological prognostic factors or overall survival. Jornal Brasileiro Patologia e Medicina Laboratorial (2010) 46(6):487–93. doi: 10.1590/S1676-24442010000600009
18. Carranza H, Cardona AF, Vargas CA, Archila P, Otero JM, Bernal L, et al. Genotyping melanoma in Colombia. J Clin Oncol (2014) 32(15_suppl):e20054-e. doi: 10.1200/jco.2014.32.15_suppl.e20054
19. Vicente A, Crovador CS, Macedo G, Scapulatempo-Neto C, Reis RM, Vazquez VL. Mutational profile of driver genes in Brazilian melanomas. J Glob Oncol (2019) 5:1–14. doi: 10.1200/JGO.19.00169
20. Prost D, llanos A, Bence P, Boixadera L, Krasnapolski M, Bilbao E, et al. Frequency of BRAF V600 mutation in 354 locallyadvanced or metastatic melanoma patients froman academic center in Argentina: The apple does not fall very far from the tree. Pigment Cell Melanoma Res (2017) 31:5. doi: 10.1016/j.jmoldx.2012.10.002
21. Zepeda-Lopez PD, Salas-Alanis JC, Toussaint-Caire S, Gutierrez-Mendoza D, Vega-Memije E, Silva SL, et al. BRAF mutation (V600E) prevalence in Mexican patients diagnosed with melanoma. Case Rep Oncol (2016) 9(1):241–5. doi: 10.1159/000445939
22. Fajardo Ramírez OR, Salas Alanis JC, Guzmán Huerta E, Martínez U, Barbosa A, Scott SP, et al. BRAF mutations among patients from the northeast of Mexico with malignant melanoma. Clin Res J (2014) 66(3):2.
23. Basurto-Lozada P, Molina-Aguilar C, Castaneda-Garcia C, Vázquez-Cruz ME, Garcia-Salinas OI, Álvarez-Cano A, et al. Acral lentiginous melanoma: Basic facts, biological characteristics and research perspectives of an understudied disease. Pigment Cell Melanoma Res (2021) 34(1):59–71. doi: 10.1111/pcmr.12885
24. Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol (2019) 30(12):1884–901. doi: 10.1093/annonc/mdz411
25. Godoy-Gijón E, Yuste-Chaves M, Santos-Briz Á. BRAF mutation status concordance between primary cutaneous melanomas and corresponding metastases: A review of the latest evidence. Actas Dermosifiliogr (2017) 108(10):894–901. doi: 10.1016/j.ad.2016.12.025
26. Colomba E, Hélias-Rodzewicz Z, Von Deimling A, Marin C, Terrones N, Pechaud D, et al. Detection of BRAF p.V600E mutations in melanomas: Comparison of four methods argues for sequential use of immunohistochemistry and pyrosequencing. J Mol Diagn (2013) 15(1):94–100. doi: 10.1016/j.jmoldx.2012.09.001
27. List of cleared or approved companion diagnostic devices. US Food and Drug Administration (2021) Washington, D.C., USA. Available at: https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools.
28. Ihle MA, Fassunke J, König K, Grünewald I, Schlaak M, Kreuzberg N, et al. Comparison of high resolution melting analysis, pyrosequencing, next generation sequencing and immunohistochemistry to conventional Sanger sequencing for the detection of p.V600E and non-p.V600E BRAF mutations. BMC Cancer (2014) 14:13. doi: 10.1186/1471-2407-14-13
29. Cheng L, Lopez-Beltran A, Massari F, MacLennan GT, Montironi R. Molecular testing for BRAF mutations to inform melanoma treatment decisions: A move toward precision medicine. Mod Pathol (2018) 31(1):24–38. doi: 10.1038/modpathol.2017.104
30. Grzywa TM, Paskal W, Włodarski PK. Intratumor and intertumor heterogeneity in melanoma. Transl Oncol (2017) 10(6):956–75. doi: 10.1016/j.tranon.2017.09.007
31. Manfredi L, Meyer N, Tournier E, Grand D, Uro-Coste E, Rochaix P, et al. Highly concordant results between immunohistochemistry and molecular testing of mutated V600E BRAF in primary and metastatic melanoma. Acta Derm Venereol (2016) 96(5):630–4. doi: 10.2340/00015555-2326
32. Long GV, Wilmott JS, Capper D, Preusser M, Zhang YE, Thompson JF, et al. Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. Am J Surg Pathol (2013) 37(1):61–5. doi: 10.1097/PAS.0b013e31826485c0
33. Santiago-Walker A, Gagnon R, Mazumdar J, Casey M, Long GV, Schadendorf D, et al. Correlation of BRAF mutation status in circulating-free DNA and tumor and association with clinical outcome across four BRAFi and MEKi clinical trials. Clin Cancer Res (2016) 22(3):567–74. doi: 10.1158/1078-0432.CCR-15-0321
34. Pearlstein MV, Zedek DC, Ollila DW, Treece A, Gulley ML, Groben PA, et al. Validation of the VE1 immunostain for the BRAF V600E mutation in melanoma. J Cutan Pathol (2014) 41(9):724–32. doi: 10.1111/cup.12364
35. McEvoy AC, Wood BA, Ardakani NM, Pereira MR, Pearce R, Cowell L, et al. Droplet digital PCR for mutation detection in formalin-fixed, paraffin-embedded melanoma tissues: A comparison with Sanger sequencing and pyrosequencing. J Mol Diagn (2018) 20(2):240–52. doi: 10.1016/j.jmoldx.2017.11.009
36. Ascierto PA, Dreno B, Larkin J, Ribas A, Liszkay G, Maio M, et al. 5-year outcomes with cobimetinib plus vemurafenib in BRAFV600 mutation–positive advanced melanoma: Extended follow-up of the coBRIM study. Clin Cancer Res (2021) 27:10. doi: 10.1158/1078-0432.CCR-21-0809
37. Dummer R, Flaherty K, Robert C, Arance AM, de Groot JW, Garbe C, et al. Five-year overall survival (OS) in COLUMBUS: A randomized phase 3 trial of encorafenib plus binimetinib versus vemurafenib or encorafenib in patients (pts) with BRAF V600-mutant melanoma. J Clin Oncol (2021) 39(15_suppl):9507. doi: 10.1200/JCO.2021.39.15_suppl.9507
38. Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med (2019) 381(7):626–36. doi: 10.1056/NEJMoa1904059
39. Lelliott EJ, McArthur GA, Oliaro J, Sheppard KE. Immunomodulatory effects of BRAF, MEK, and CDK4/6 inhibitors: Implications for combining targeted therapy and immune checkpoint blockade for the treatment of melanoma. Front Immunol (2021) 12. doi: 10.3389/fimmu.2021.661737
40. Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med (2010) 363(9):809–19. doi: 10.1056/NEJMoa1002011
41. National Library of Medicine (US). Dabrafenib and trametinib followed by ipilimumab and nivolumab or ipilimumab and nivolumab followed by dabrafenib and trametinib in treating patients with stage III-IV BRAFV600 melanoma (2022). Available at: https://www.clinicaltrials.gov/ct2/show/NCT02224781.
42. Schummer P, Schilling B, Gesierich A. Long-term outcomes in BRAF-mutated melanoma treated with combined targeted therapy or immune checkpoint blockade: Are we approaching a true cure? Am J Clin Dermatol (2020) 21(4):493–504. doi: 10.1007/s40257-020-00509-z
43. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol (2022) 40(2):127–37. doi: 10.1200/JCO.21.02229
44. Ascierto PA, Mandalà M, Ferrucci PF, Guidoboni M, Rutkowski P, Ferraresi V, et al. Sequencing of ipilimumab plus nivolumab and encorafenib plus binimetinib for untreated BRAF-mutated metastatic melanoma (SECOMBIT): A randomized, three-arm, open-label phase II trial. J Clin Oncol. 2022, 41(2):212–21. doi: 10.1200/JCO.21.02961
45. da Cunha IW, de Almeida Coudry R, de Macedo MP, de Assis EACP, Stefani S, Soares FA. A call to action: Molecular pathology in Brazil. Surg Exp Pathol (2021) 4(1):15. doi: 10.1186/s42047-021-00096-1
46. Valdes A. Access to high-cost medicines in the americas. Pan American Health Organization / World Health Organization (2021) Washington, D.C., USA. Available at: https://www.paho.org/hq/index.php?option=com_content&view=article&id=2149%3A2008-el-acceso-medicamentos-alto-costo-americas&catid=1266%3Amedicines-access-innovation&Itemid=1178&lang=en. PAHO/WHO.
47. Morel CM, McGuire A, Mossialos E. The level of income appears to have no consistent bearing on pharmaceutical prices across countries. Health Aff (Millwood) (2011) 30(8):1545–52. doi: 10.1377/hlthaff.2010.0317
48. Maceira D, Paraje G, Aramayo F, Masi SD, Sánchez YD. Financiamiento público de la investigación en salud en cinco países de américa latina. Rev Panam Salud Publica (2010) 27(6):10.
49. da Cunha IW, de Almeida Coudry R, de Macedo MP, de Assis EACP, Stefani SD, Soares FA. A call to action: Molecular pathology in Brazil. Surg Exp Pathol (2021) 4:1–27. doi: 10.1186/s42047-021-00096-1
50. Wouters MW, Michielin O, Bastiaannet E, Beishon M, Catalano O, Del Marmol V, et al. ECCO essential requirements for quality cancer care: Melanoma. Crit Rev Oncol Hematol (2018) 122:164–78. doi: 10.1016/j.critrevonc.2017.12.020
51. Volger S, Paris V, Panteli D. Ensuring access to medicines: how to redesign pricing, reimbursement and procurement. Copenhagen: WHO Regional Office for Europe (2018). Available at: https://www.euro.who.int/:data/assets/pdf_file/0009/379710/PolicyBrief_AUSTRIA_PB30_web_13082018.pdf.
52. Cornelius LA, Fields RC, Tarhini A. Multidisciplinary care of BRAF-mutant stage III melanoma: A physicians perspective review. Oncologist (2021) 26(9):e1644–e51. doi: 10.1002/onco.13852
53. Denton E, Conron M. Improving outcomes in lung cancer: The value of the multidisciplinary health care team. J Multidiscip Healthc (2016) 9:137–44. doi: 10.2147/JMDH.S76762
54. Kesson EM, Allardice GM, George WD, Burns HJ, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: Retrospective, comparative, interventional cohort study of 13 722 women. Bmj (2012) 344:e2718. doi: 10.1136/bmj.e2718
55. Macdermid E, Hooton G, Macdonald MR, McKay G, Grose D, Mohammed N, et al. Improving patient survival with the colorectal cancer multi-disciplinary team. Colorectal Dis (2008) 11(3):4. doi: 10.1111/j.1463-1318.2008.01580.x
56. Dummer R, Hauschild A, Guggenheim M, Keilholz U, Pentheroudakis G. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2012) 23(Suppl 7):vii86–91. doi: 10.1093/annonc/mds229
57. Perez M, Abisaad JA, Rojas KD, Marchetti MA, Jaimes N. Skin cancer: Primary, secondary, and tertiary prevention. part I. J Am Acad Dermatol (2022) 87(2):255–68. doi: 10.1016/j.jaad.2021.12.066
58. Rojas KD, Perez ME, Marchetti MA, Nichols AJ, Penedo FJ, Jaimes N. Skin cancer: Primary, secondary, and tertiary prevention. part II. J Am Acad Dermatol (2022) 87(2):271–88. doi: 10.1016/j.jaad.2022.01.053
59. Trager MH, Queen D, Samie FH, Carvajal RD, Bickers DR, Geskin LJ. Advances in prevention and surveillance of cutaneous malignancies. Am J Med (2020) 133(4):417–23. doi: 10.1016/j.amjmed.2019.10.008
60. Gordon L, Shih S, Watts C, Goldsbury D, Green A. The economics of skin cancer prevention with implications for Australia and new Zealand: Where are we now? Public Health Res Pract. 2022, 32(1):e31502119. doi: 10.17061/phrp31502119
61. Cornelissen JJ, van der Holt B, Verhoef GEG, van 't Veer MB, van Oers MHJ, Schouten HC, et al. Myeloablative allogeneic versus autologous stem cell transplantation in adult patients with acute lymphoblastic leukemia in first remission: A prospective sibling donor versus no-donor comparison. Blood (2009) 113(6):1375–82. doi: 10.1182/blood-2008-07-168625
Keywords: BRAF-mutated melanoma, Latin America, melanoma, V600E, access, health policy
Citation: Salman P, de Melo AC, Rico-Restrepo M, Rodriguez J, Russi A, Schmerling RA, Zambrano A and Cinat G (2023) Addressing the unmet needs of patients with BRAF-mutated melanoma in Latin America: Expert perspective. Front. Oncol. 13:1032300. doi: 10.3389/fonc.2023.1032300
Received: 12 October 2022; Accepted: 27 February 2023;
Published: 14 March 2023.
Edited by:
Sapna Patel, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Enrica Teresa Tanda, San Martino Hospital (IRCCS), ItalyJuan Esteban Garcia-Robledo, Mayo Clinic Arizona, United States
José Manuel Lopes, University of Porto, Portugal
Copyright © 2023 Salman, de Melo, Rico-Restrepo, Rodriguez, Russi, Schmerling, Zambrano and Cinat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pamela Salman, cHZzYWxtYW5AZ21haWwuY29t
 Pamela Salman1*
Pamela Salman1* Andreia Cristina de Melo
Andreia Cristina de Melo Mariana Rico-Restrepo
Mariana Rico-Restrepo Jeronimo Rodriguez
Jeronimo Rodriguez Andrea Russi
Andrea Russi Gabriela Cinat
Gabriela Cinat