- 1Cardiothoracic Surgery Departments, Weill Cornell Medicine, New York, NY, United States
- 2Surgical Oncology Department, National Cancer Institute, Cairo University, Cairo, Egypt
- 3Cardiac Surgery Department, Spedali Civili di Brescia, University of Brescia, Brescia, Italy
- 4Cardiothoracic Surgery Department, Mayo Clinic, Jacksonville, FL, United States
- 5Department of Surgery, Zagazig University Faculty of Medicine, Zagazig, Egypt
- 6Department of Cardio-Thoracic Surgery, Maastricht University Medical Centre, Maastricht University, Maastricht, Netherlands
- 7Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, Netherlands
Introduction: Primary malignant cardiac tumors (PMCTs) are rare. Geographical distribution has been demonstrated to affect cancer outcomes, making the reduction of geographical inequalities a major priority for cancer control agencies. Geographic survival disparities have not been reported previously for PMCT and the aim of this study is to compare the prevalence and the long-term survival rate with respect to the geographic location of PMCTs using the Surveillance, Epidemiology, and End Results (SEER) research plus data 17 registries between 2000 and 2019.
Methods: The SEER database was queried to identify geographic variation among PMCTs. We classified the included states into 4 geographical regions (Midwest, Northeast, South and West regions) based on the U.S. Census Bureau-designated regions and divisions. Different demographic and clinical variables were analyzed and compared between the four groups. Kaplan Meier curves and Cox regression were used for survival assessment.
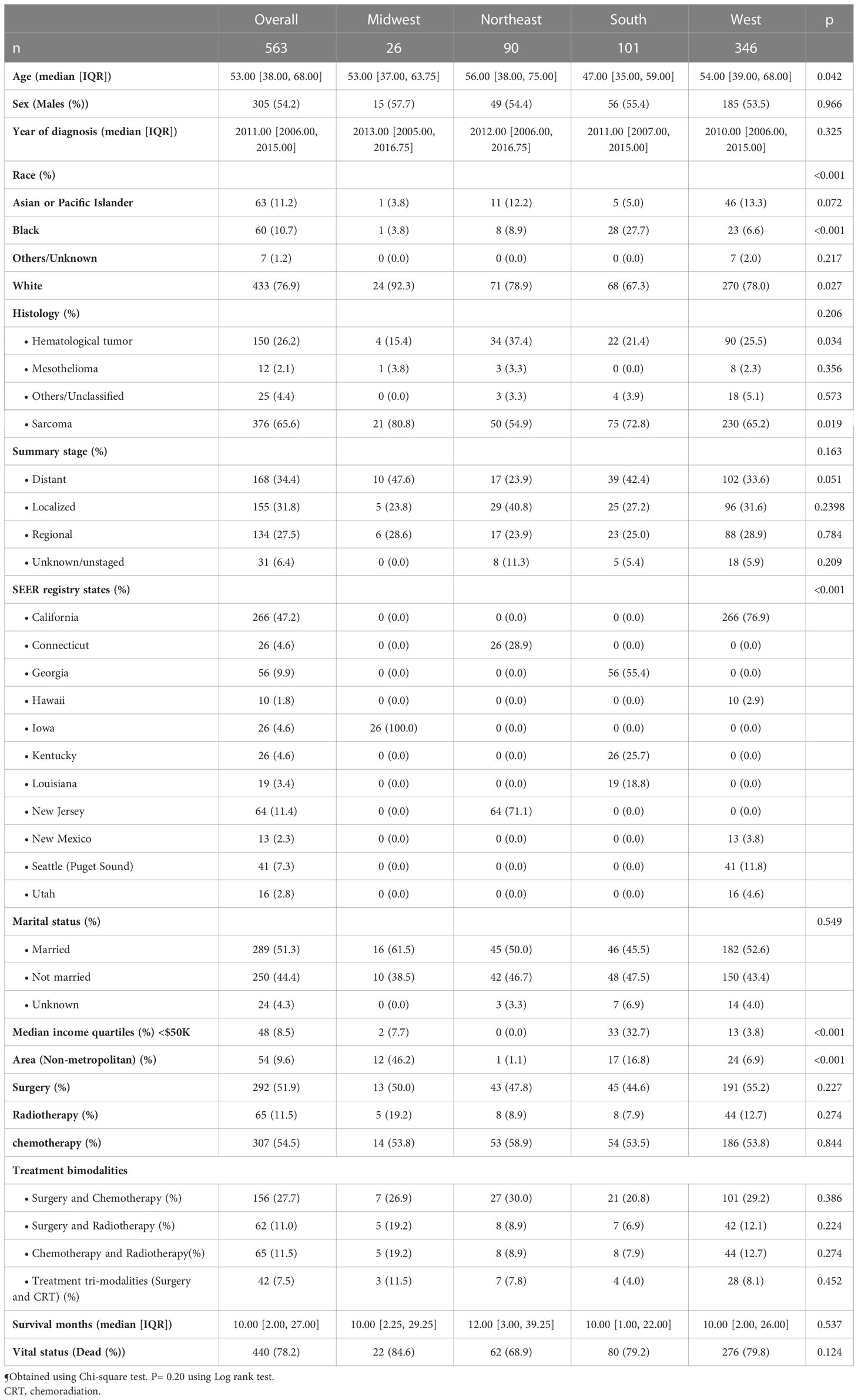
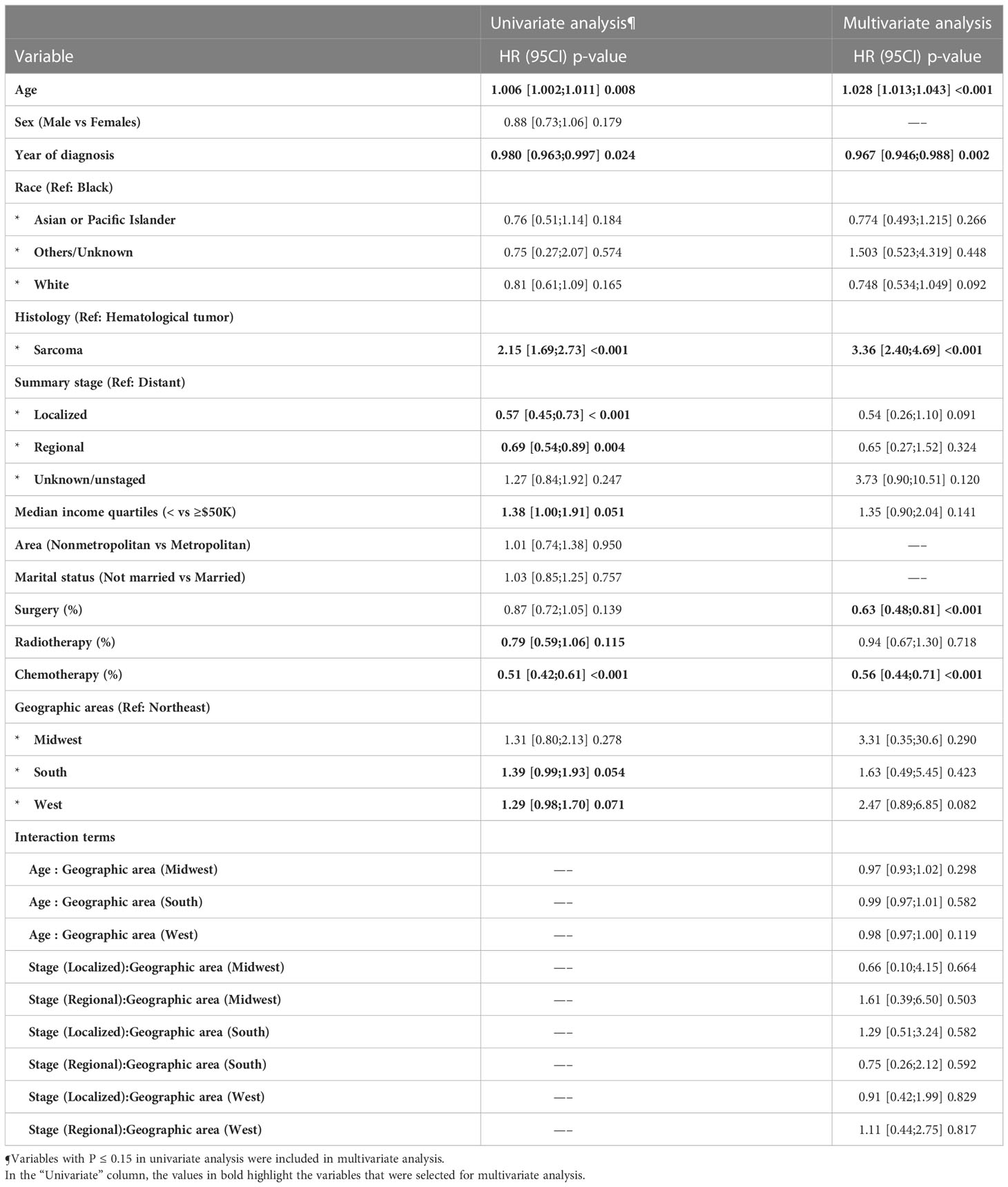
Results: A total of 563 patients were included in our analysis. The median age was 53 years (inter-quartile range (IQR): 38 - 68 years) and included 26, 90, 101, and 346 patients from the Midwest, Northeast, South, and West regions respectively. Sarcoma represented 65.6% of the cases, followed by hematological tumors (26.2%), while mesothelioma accounted for 2.1%. Treatment analysis showed no significant differences between different regions. Median overall survival was 11, 21, 13, and 11 months for Midwest, Northeast, South and West regions respectively and 5-year overall survival was 22.2%, 25.4%, 14.9%, and 17.6% respectively. On multivariate Cox regression, significant independent predictors of late overall mortality among the entire cohort included age (Hazard Ratio [HR] 1.028), year of diagnosis (HR 0.967), sarcoma (HR 3.36), surgery (HR 0.63) and chemotherapy (HR 0.56).
Conclusion: Primary malignant cardiac tumors are rare and associated with poor prognosis. Sarcoma is the most common pathological type. Younger age, recent era diagnosis, surgical resection, and chemotherapy were the independent predictors of better survival. While univariate analysis revealed that patients in the South areas had a worse survival trend compared to other areas, geographic disparity in survival was nullified in multivariate analysis.
Introduction
Primary malignant cardiac tumors (PMCTs) much less common than primary benign or secondary/metastatic cardiac tumors (1), although their existence dates back to the 15th century (2). Most early reported cases have been postmortem diagnoses based on autopsy findings (3) due to a lack of advanced cardiac imaging and echocardiography in previous eras. The first antemortem diagnosis occurred in 1934 by Barnes et al (4) with the diagnosis made from electrocardiogram findings and biopsy of tumor embolic foci (4). In the recent era of advanced diagnostic imaging, an increase in the known incidence of cardiac tumors has been documented (5, 6). Sarcoma has been documented as the most common pathological type, followed by lymphoma and mesothelioma (5, 7, 8), with an increase in the incidence of cardiac lymphoma, especially in 1980s, and 1990s (Autoimmune deficiency Syndrome (AIDS) era) (1, 9), but this has been stable since 2000 due to marked improvement in the Human Immunodeficiency Virus (HIV) management (9). The incidence of cardiac mesothelioma has decreased due to less asbestos exposure (1, 10). The prognosis is generally poor, and complete surgical resection is the mainstay of the treatment, with better survival associated with complete resection in comparison to other treatment modalities (3). For instance, Simpson et al. reported a median survival of 17 months with resection versus 6 months with other modalities (3). The first successful surgery for PMCTs was reported by Maurer et al. in the year 1952 (11).
Area-level and geographic characteristics have been demonstrated to affect the cancer survival, independent of patient-level characteristics, making alleviation of the geographical inequalities in cancer outcomes a major priority for cancer control agencies (12). Understanding of the factors underlying geographical disparities is important for targeting policies. Geographic survival disparities have been reported previously in the setting of other malignant tumors (13–17), but not reported for PMCTs, therefore the aim of this study is to compare the geographic locations regarding the prevalence, and the long-term survival rate of PMCTs using the Surveillance, Epidemiology, and End Results (SEER) research plus registry 17.
Material and methods
Data sources
The SEER database of the National Cancer Institute (NCI) was queried to identify geographic variation among PMCTs patients based on inpatient status.
The public use version (https://www.cdc.gov/cancer/uscs/technical_notes/contributors/seer.htm) of data collected from the SEER research plus data 17 registries NOV-2021 (2000–2019) was used for this study. This registry is an extended form of the SEER data containing survival and treatment data and was released November of 2021 and includes patients from years 2000-2019. We used U.S. Census Bureau-designated regions and divisions as shown in Supplementary Table 1 for the geographic classification of the included states in SEER database (18). The Census Bureau region definition is widely used for data collection and analysis and is the most used classification system. We included only patients with single primary cardiac tumor (sequence number = 0 or 1), as survival in patients with multiple primary tumors could not be ascribed to a single anatomical cancer site.
Study population and inclusion criteria
Among our cohort, we classified the included states into 4 geographical regions based on the U.S. Census Bureau-designated regions and divisions as shown in Supplementary Table 1 (18). The Census Bureau region definition is widely used for data collection and analysis, and is the most commonly used classification system (18). There were 26 (4.6%) patients from the Midwest, 90 (16%) from Northeast, 101 (18%) from the South, and 346 (61.4%) from the West regions.
Patients were excluded if follow up data was missing or in case of presence of sequence of malignant neoplasms of more than 1 over the lifetime of the patient.
Study variables
General baseline demographics and clinical variables included in the analysis are year of diagnosis, age, sex, race, histological subtype, stage, and treatment modalities (surgery, chemotherapy, or radiotherapy). Relevant socioeconomic variables were included in the analysis which included median income, area (nonmetropolitan or metropolitan), marital status, SEER registry state, and geographical region. Survival months and vital status, whether the patient was alive or dead at the last follow-up were retrieved.
Survival and follow-up data
Overall survival (OS) was defined as the time from the date of diagnosis until the date of death from any cause or the date of the last follow-up. Overall median follow-up was 82 months (95%CI: 68-107). Median follow-up was 127 months in Midwest vs 64, 72 and 96 months in Northeast, South, and West regions respectively.
Statistical analysis
Baseline demographics and clinical characteristics were compared between the four groups. Continuous variables are presented as median and interquartile range (IQR) and are compared between groups utilizing Kruskal-Wallis test. Categorical variables were presented as a frequency count and percentage and compared between groups using Chi-Squared test. Survival was estimated and presented using a Kaplan-Meier curve and compared across groups using the Log-Rank Test. Univariate and multivariate predictors of late mortality were estimated using the Cox proportional hazards method and are reported as a hazard ratio (HR) and 95% confidence interval (95%CI). Univariate predictors were selected for inclusion within the multivariate model if p<0.15.
All p values are two-sided and considered statistically significant if <0.05. The statistical analysis was performed using R version 4.2.1 within RStudio.
Results
Demographics
Among the 714 PMCTs cases in the SEER database, 27 cases were excluded due to missing survival time and 124 cases due to the presence of sequence of malignant neoplasms of more than 1 over the lifetime of the patient. Therefore, a total of 563 patients were included in the study analysis (Supplementary Figure 1).
Median age was 53 years (interquartile range (IQR): 38 to 68 years) and included 26, 90, 101 and 346 patients from Midwest, Northeast, South, and West regions respectively. Overall, there were 305 males (54.2%). More than three-quarters of the study population was White, followed by Asian race (11.2%), Black race (10.7%) and other (1.2%). Each stage (localized, regional, and distant) represented about one third of the included cohort (Table 1).
The four regions showed some significant differences: among the different regions, South region had younger patients with a median age of 47 vs 53 in Midwest (P=0.042). White race was the most prevalent and represented 92.3% vs 67.3% in Midwest vs South regions (P=0.027), while black race represented 27.7% vs 3.8% in South vs Midwest regions (P<0.001). One-third of South region has a median income of less than $50K vs zero percent in Northeast region (P<0.001). The South area had higher stages when compared to the Northeast area (P=0.051) (Table 1).
A subgroup analysis based on the number of treatment modalities showed that more treatments options were used in younger patients (p<0.001). Hematological tumors were more prevalent in the no treatment subgroup (p<0.001), while sarcoma was less prevalent in the no treatment subgroup (p<0.001). Patients receiving no treatment had a dismal prognosis with a median survival of 1 month (IQR: 0.0-5.0) (Supplementary Table 2).
The annual trend of PMCTs among the entire cohort, and the 4 regions is shown in Supplementary Figure 2.
Histology and treatment
Among all patients, sarcoma was the most common histological category (65.6%), followed by hematological tumors (26.2%), while mesothelioma accounted for 2.1%. Sarcomas represented almost three-quarters of included cases in each of Midwest and South regions, while almost 60% in the other 2 regions (P=0.019). Hematological tumors were most common in Northeast region (37.4%, P=0.034) (Table 1).
A total of 292 patients (51.9%) underwent surgery, 307 (54.5%) received chemotherapy, and 65 (11.5%) received radiotherapy. Treatment analysis showed absence of significant differences between different regions either by studying each single modality, as bimodalities, or as tri-modalities (Table 1).
Survival analysis
Median overall survival was 11 months (95%CI: 7-47), 21 (95%CI: 12-41), 13 (95%CI:8-17), and 11 (95%CI: 9-16) for Midwest, Northeast, South, and West regions respectively. Two-year overall survival was 36.4%, 44.7%,27.6%, and 31.7% for Midwest, Northeast, South, and West regions respectively. 5-year overall survival was 22.2%, 25.4%, 14.9%, and 17.6% for Midwest, Northeast, South, and West regions respectively (Overall log rank P=0.24, Figure 1A).
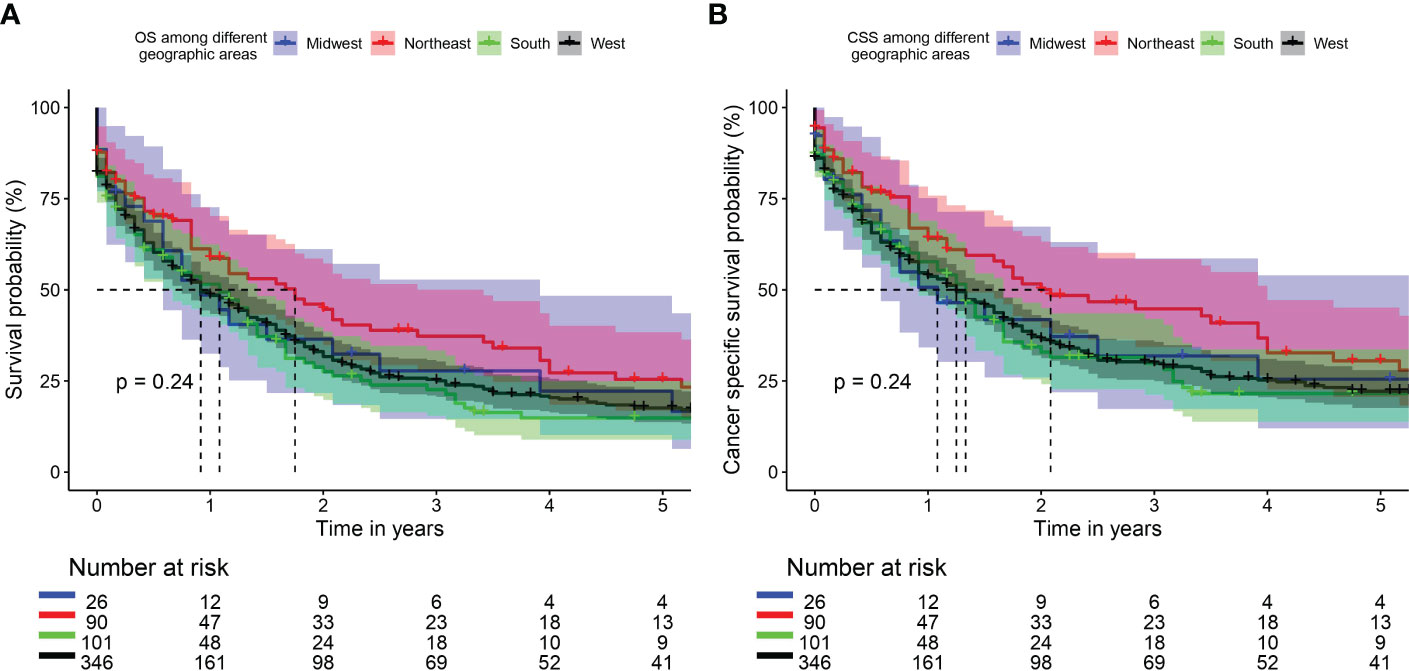
Figure 1 Kaplan Meier curve of overall survival (OS) (A) and cancer specific survival (CSS) (B) among different geographic areas.
Median cancer-specific survival was 13 months (95%CI: 7-47), 25 (95%CI: 16-48), 16 (95%CI:11-20), and 15 (95%CI: 11-19) for Midwest, Northeast, South, and West regions respectively. Two-year cancer-specific survival was 41.8%, 50.1%, 32.9%, and 36.4% for Midwest, Northeast, South, and West regions respectively. 5-year cancer-specific survival was 25.5%, 30.5%, 21.6%, and 22.2% for Midwest, Northeast, South, and West regions respectively (Overall log rank P=0.24, Figure 1B).
The median survival of surgery, chemotherapy and radiotherapy groups were 18 months (95%CI: 14-20), 20 months (95%CI: 16-22), and 23 months (95%CI: 16-38), respectively. Survival subgroup analysis on the number of treatments presented the following outcomes: “none” 1 month (95%CI: 0-2), “single modality” 13 months (95%CI: 9-16), “bimodality” 21 months (95%CI: 20-25), and “trimodality” 23 months (95%CI: 15-45).
On univariate Cox regression, South region was associated with a trend toward higher late mortality versus Northeast region (HR 1.39, 95%CI 0.99-1.93, P=0.054). On multivariable Cox regression, independent predictors of late overall mortality among the entire cohort included age (HR 1.028, 95%CI: 1.013-1.043, p<0.001), year of diagnosis (HR 0.967, 95%CI: 0.946-0.988, p=0.002), sarcoma (HR 3.36, 95%CI: 2.40;4.69, P<0.001, hematological tumor set as reference), surgery (HR 0.63, 95%CI: 0.48;0.81, p<0.001), and chemotherapy (HR 0.56, 95%CI 0.44-0.71, p<0.001) (Table 2).
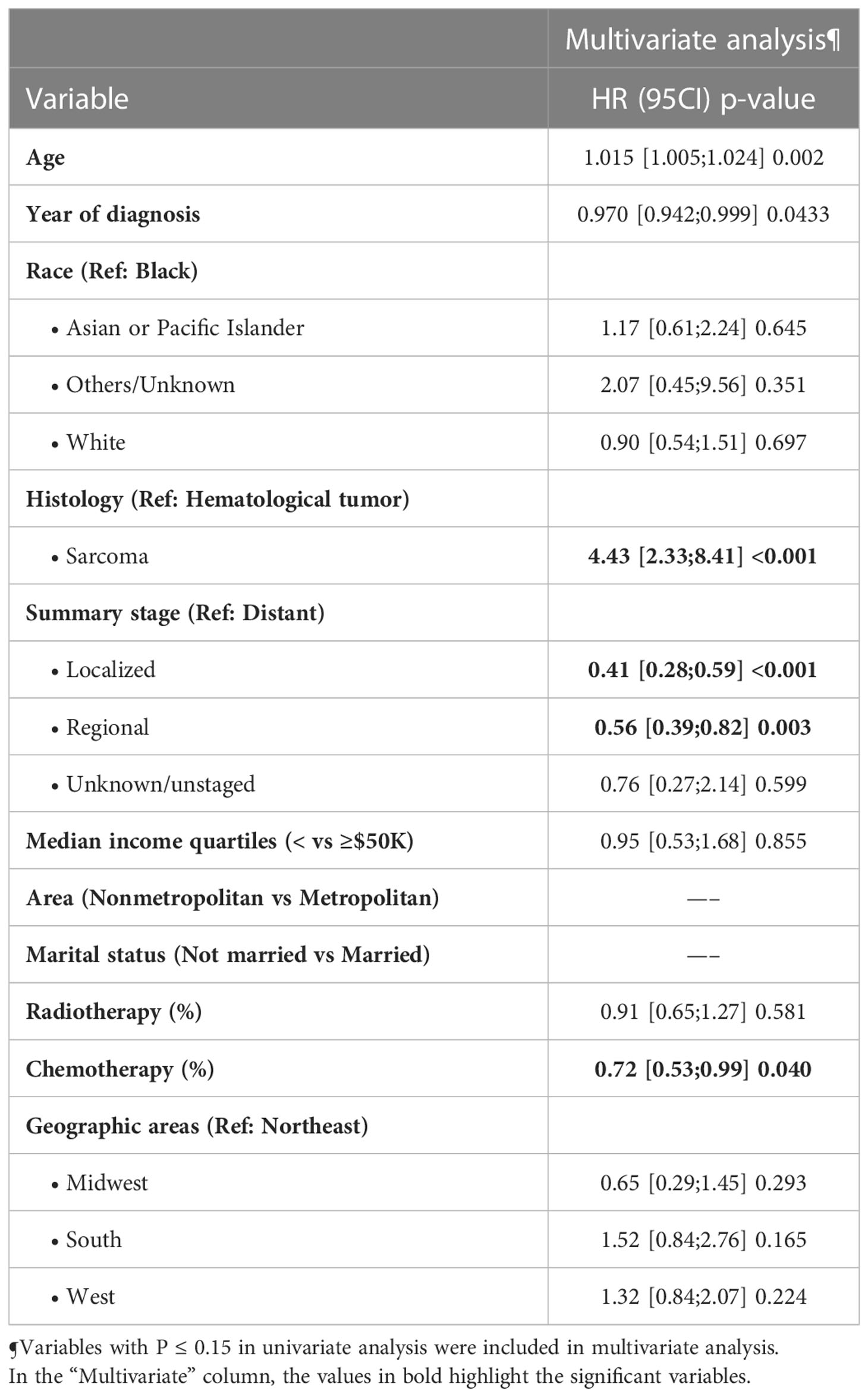
On multivariable Cox regression among surgical subgroup (n=292), independent predictors of late overall mortality included advanced age, earlier year of diagnosis, sarcoma vs hematological tumor set as reference, localized and regional stages vs distant stage, and chemotherapy (Table 3).
A graphical summary of the findings of our study is represented in Supplementary Figure 3.
Discussion
Primary malignant cardiac tumors are rare and only account for 0.008% of reported cancer, and 9.4% of all primary cardiac tumors in the SEER database (1, 19). While prior series reported that secondary cardiac tumors (SCTs) are 20 to 30 times more common than primary cardiac tumors, with the most common tumor source being hematological malignancies (leukemia and lymphoma), and other solid tumors especially melanoma, lung, and breast cancer (6), recent meta-analysis reported almost equal prevalence of both primary and SCTs (19). A low incidence and prevalence of PMCTs has been demonstrated over different decades (5, 20–27). In the recent era, despite their rarity, the incidence of these tumors appeared to have increased due to recent advance in the cardiac imaging (MRI and CT) (1). Moreover, cardiac imaging can evaluate the characteristics of cardiac tumors by visualizing the relationship between the tumor and the surrounding tissues, and is essential for the surgical plan, the assessment of tumor progression, and the monitoring of postoperative tumor recurrence and metastasis (28). Angiosarcoma was and still the most common, followed by lymphoma (especially in immunocompromised patients; AIDS, and following organ transplant) (5, 7, 8).
Due to the relative scarcity of reported cases of these tumors, there is no conclusive study showing any survival disparities between geographical locations in these patients. Therefore, the objective of this study was to assess survival outcomes for different geographical locations in United States based on the SEER data.
In univariate analysis, a worse survival trend for patients in the South area compared to patients in the Northeast area (HR: 1.39, 95%CI 0.99-1.93], p = 0.054) was observed. However, at multivariable analysis, none of the geographic areas showed a significant disparity in long term survival. With respect to the demographic and the clinicopathological characteristics between these 4 geographic locations, we found that patients in South region (worst survival in univariate analysis) were relatively younger in age, with relatively lower income (p<0.001) and higher tumor stages (p=0.051) when compared to the Northeast region (the best survival). There were more patients from non-metropolitan areas (p<0.001) compared to Northeast area. There was no difference in sex distribution or treatment options provided between the groups. In the whole cohort, the most common racial designation was Caucasian. The South region had a higher proportion of black patients as compared to Northeast. There is no evidence that racial difference affects the long-term survival among patients with cardiac tumors (29, 30). In previous reports about impact of geographic location on cancer survival, the authors surmised that the survival advantages of one geographic region area over others was mainly attributed to socioeconomic status, income and education levels, healthcare facilities inequalities, and less access to medical care (in rural vs. urban areas) rather than racial differences (12–17). While prior series showed better survival for patients with private insurance/managed care insurance vs Medicare (HR= 0.67 (95%CI 0.52-0.87), P=0.002) (31), we were not able to investigated these factors as the SEER version used did not contain data on insurance, education levels, or healthcare facility type. In private insurance countries, the economic disparity may impact the outcomes of the patients as non-insured patients are less likely to receive costly treatments. However, also countries with national healthcare system may not always guarantee a full equity of care (32). The health insurance status is also related to the socioeconomic status. In fact, in clinical trials where treatments are free, low socioeconomic status is associated with worse outcomes (33). Besides, nationwide cardiovascular studies have reported that patients with a low socioeconomic status may be under- or late diagnosed and have limited access to several treatments (34, 35).
In PMCT, age has proven to negatively impact survival outcomes of a prior analysis of the National Cancer Database (36).With increasing age, patients presented a more significant comorbidity burden compared to younger ones and were treated more conservatively. This was confirmed also in the current analysis were patients that did not receive any treatment were significantly older than the other treatment subgroups (p<0.001).
Sarcoma was the most common pathological type (65.6%), followed by hematological malignancies (26.2%) and mesothelioma (2.1%) in the whole cohort. There was a higher percent of sarcoma in the Southern region. Our results from the SEER database are similar to reports from non-American databases regarding the incidence of tumor types of PMCT (5, 20–22, 26). Blondeau et al. in a French study of 533 cases reported that sarcoma represented the majority of PMCT (90%) (20). Approximately 50% of our cohort underwent surgery, 53% received chemotherapy, and 11.3% of patients underwent radiation therapy. The reasons for non-cancer directed surgery was mentioned in Supplementary Table 3. Surgery has been established to be the most effective treatment option for PMCTs (3), which can also be performed through minimally invasive approaches in selected patients. Sultan and colleagues in their multi-institutional study from the National Cancer Database confirm that the surgery group as compared to the no surgery groups had significantly better long-term survival (p<0.0001) (37). Simpson et al. reported a median survival of 17 months for those who underwent surgery compared with only 6 months in patients received other treatment options (3). Chemotherapy was used for advanced non operable cases, or as a neoadjuvant before surgery, and the reasons why radiotherapy was not widely used might be related to radio-insensitivity of some cardiac sarcomas, as well as its cardiac-related-toxicity which furthermore deteriorate the cardiac functions (38). However, previous studies have shown that radiotherapy was associated with improved progression-free survival (PFS) on multivariate analysis (39).
Randhawa et al. reported their 25 years’ experience that patients who received multimodality treatment (any combination of surgery, radiation therapy, and chemotherapy) had an improved median survival compared with patients treated with surgery, radiation therapy, or chemotherapy only (P=0.05) (40). This was also reported in other studies (41, 42). Saleh and colleagues showed that neoadjuvant chemotherapy followed by radical surgery is safe and effective strategy also in patients with right-sided heart sarcomas (43). Besides, due to the propensity for brain metastases in cardiac tumors, brain MRI at the time of diagnosis should be considered (44).
Primary malignant cardiac tumors are generally associated with poor outcomes, and 78.2% of patients in our analysis were dead at the end of follow-up, with median survival of 10 months. In multivariate analysis, older age, earlier era diagnosis, sarcoma, and non-surgical treatment options were associated with poor survival. Our results are similar to those reported by Bui et al (29) and Yin et al (30). Bui et al (29) demonstrated survival improvement in patients diagnosed in the recent era compared to old era (The 1-year survival rate: 13.3% (1975–1998), 40.9% (1999–2004), 50% (2005–2010), and 59.7% (2011–2016), p-value = 0.0064), however, Yin et al (30) study did not show this survival advantages although there was an obvious trend (P = 0.13).
Limitations
This study utilized the SEER database, which is considered a highly reliable source of epidemiologic information (incidence and prevalence), and survival assessment. However, inaccurate estimation of the prevalence of cardiac tumors in the SEER data might be present as these tumors can present by sudden cardiac death (45), hence not documented, as the SEER data reported only living cases not the postmortem diagnosis. Studying the impact of the geographic location on survival from the SEER data might be changed over time given the fact of patients’ migration to different areas. Assessment of comorbidity information, genetic details or individual risk factors were not reported in the SEER data. We used a SEER version that had both states and survival data but no insurance, education levels, or healthcare facilities type data. Furthermore, the details of the received treatment such as the doses, local and systemic side effects of the chemotherapy or radiotherapy were not reported, so its impact on survival cannot be assessed.
Conclusion
Primary malignant cardiac tumors are rare and associated with poor prognosis. Sarcoma is the most common pathological type. Younger age, recent era diagnosis, surgical resection, and chemotherapy are identified as the independent predictors of better survival. While univariate analysis suggested that patients in the Southern region had worse survival trend compared to other geographic areas, the geographic disparity in survival was nullified in multivariate analysis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceptualization: MR, SK, MB. Methodology: MR. Software, Validation, Formal analysis: MR. Data curation: MR, SK, AD, MB, MA, IG, CL, YME. Investigation: MR, SK, MB. Writing-review and editing: All authors. Visualization: MR, SK, AD, MB, MA, IG, CL, YME. Resources: MR, MA, SM. Supervision: MR, SK, MA, CL, RL, SM. Project administration: MR, SK, MA, SM.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1071770/full#supplementary-material
Supplementary Table 1 | U.S. Census Bureau-designated regions and divisions.
Supplementary Table 2 | Criteria of included studies based on the number of treatments received.
Supplementary Table 3 | Reason for no cancer directed surgery.
Supplementary Figure 1 | Flowchart of our included cases.
Supplementary Figure 2 | Annual trend of PMCTs among (A) the entire cohort, (B) Midwest, (C) Northeast, (D) South, and (E) West regions.
Supplementary Figure 3 | Graphical summary of the geographical findings.
References
1. Oliveira GH, Al-Kindi SG, Hoimes C, Park SJ. Characteristics and survival of malignant cardiac tumors: a 40-year analysis of> 500 patients. Circulation (2015) 132(25):2395–402. doi: 10.1161/CIRCULATIONAHA.115.016418
2. Realdo C. De re anatomica. Venice: Nicolae Beuil-acquae [Facsimile Culture et Civilisation, Bruxelles (1969).
3. Simpson L, Kumar SK, Okuno SH, Schaff HV, Porrata LF, Buckner JC, et al. Malignant primary cardiac tumors: review of a single institution experience. Canc: Interdiscip Int J Am Cancer Soc (2008) 112(11):2440–6. doi: 10.1002/cncr.23459
4. Barnes AR, Beaver DC, Snell AM. Primary sarcoma of the heart: report of a case with electrocardiographic and pathological studies. Am Heart J (1934) 9(4):480–91. doi: 10.1016/S0002-8703(34)90096-X
5. Cresti A, Chiavarelli M, Glauber M, Tanganelli P, Scalese M, Cesareo F, et al. Incidence rate of primary cardiac tumors: a 14-year population study. J Cardiovasc Med (2016) 17(1):37–43. doi: 10.2459/JCM.0000000000000059
6. Poterucha TJ, Kochav J, O’Connor DS, Rosner GF. Cardiac tumors: clinical presentation, diagnosis, and management. Curr Treat opt Oncol (2019) 20(8):1–15. doi: 10.1007/s11864-019-0662-1
7. Yu L, Gu T, Shi E, Xiu Z, Fang Q, Wang C, et al. Primary malignant cardiac tumors. J Cancer Res Clin Oncol (2014) 140(6):1047–55. doi: 10.1007/s00432-014-1651-1
8. Petrich A, Cho SI, Billett H. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer (2011) 117(3):581–9. doi: 10.1002/cncr.25444
9. Shiels MS, Engels EA, Linet MS, Clarke CA, Li J, Hall HI, et al. The epidemic of non–Hodgkin lymphoma in the united states: Disentangling the effect of HIV, 1992–2009U. s. NHL trends excluding HIV-infected cases, 1992–2009. Cancer Epidemiol Biomarkers Prev (2013) 22(6):1069–78. doi: 10.1158/1055-9965.EPI-13-0040
10. Weill H, Hughes J, Churg A. Changing trends in US mesothelioma incidence. Occup Environ Med (2004) 61(5):438–41. doi: 10.1136/oem.2003.010165
11. Maurer ER. Successful removal of tumor of the heart. J Thorac Surg (1952) 23(5):479–85. doi: 10.1016/S0096-5588(20)31121-1
12. Dasgupta P, Baade PD, Aitken JF, Turrell G. Multilevel determinants of breast cancer survival: association with geographic remoteness and area-level socioeconomic disadvantage. Breast Cancer Res Treat (2012) 132(2):701–10. doi: 10.1007/s10549-011-1899-y
13. Zhang H, Dziegielewski PT, Nguyen TJ, Jeffery CC, O’Connell DA, Harris JR, et al. The effects of geography on survival in patients with oral cavity squamous cell carcinoma. Oral Oncol (2015) 51(6):578–85. doi: 10.1016/j.oraloncology.2015.03.012
14. Yu XQ, Luo Q, Smith DP, O’Connell DL, Baade PD. Geographic variation in prostate cancer survival in new south Wales. Med J Australia. (2014) 200(10):586–90. doi: 10.5694/mja13.11134
15. Johnson AM, Hines RB, Johnson JA III, Bayakly AR. Treatment and survival disparities in lung cancer: the effect of social environment and place of residence. Lung Cancer. (2014) 83(3):401–7. doi: 10.1016/j.lungcan.2014.01.008
16. Huang L, Pickle LW, Stinchcomb D, Feuer EJ. Detection of spatial clusters: application to cancer survival as a continuous outcome. Epidemiology (2007) 18(1):73–87. doi: 10.1097/01.ede.0000249994.30736.24
17. Henry KA, Niu X, Boscoe FP. Geographic disparities in colorectal cancer survival. Int J Health geograph (2009) 8(1):1–13. doi: 10.1186/1476-072X-8-48
18. Commerce UDo. Census regions and divisions of the united states. Economic Statistics Administration, US Census Bureau, Geography Division (1995).
19. Rahouma M, Arisha MJ, Elmously A, El-Sayed Ahmed MM, Spadaccio C, Mehta K, et al. Cardiac tumors prevalence and mortality: A systematic review and meta-analysis. Int J Surg (2020) 76:178–89. doi: 10.1016/j.ijsu.2020.02.039
20. Blondeau P. Primary cardiac tumors-French studies of 533 cases. Thorac Cardiovasc surg (1990) 38(S 2):192–5. doi: 10.1055/s-2007-1014065
21. Burke A. Primary malignant cardiac tumors. Semin Diagn Pathol (2008) 25(1):39–46. doi: 10.1053/j.semdp.2007.10.006
22. Alfaro-Gómez F, Careaga-Reyna G, Valero-Elizondo G, Argüero-Sánchez R. Tumors of the heart 16 years experience in hospital de cardiología, del centro médico nacional siglo XXI in Mexico city. Cirugia y cirujanos. (2003) 71(3):179–85.
23. Odim J, Reehal V, Laks H, Mehta U, Fishbein MC. Surgical pathology of cardiac tumors: two decades at an urban institution. Cardiovasc Pathol (2003) 12(5):267–70. doi: 10.1016/S1054-8807(03)00087-5
24. Piazza N, Chughtai T, Toledano K, Sampalis J, Liao C, Morin J-F. Primary cardiac tumours: eighteen years of surgical experience on 21 patients. Can J Cardiol (2004) 20(14):1443–8.
25. Fernandes F, Soufen HN, Ianni BM, Arteaga E, Ramires FJ, Mady C. Primary neoplasms of the heart. clinical and histological presentation of 50 cases. Arquivos brasileiros cardiologia. (2001) 76:235–7. doi: 10.1590/S0066-782X2001000300006
26. Kamiya H, Yasuda T, Nagamine H, Sakakibara N, Nishida S, Kawasuji M, et al. Surgical treatment of primary cardiac tumors 28 years’ experience in kanazawa university hospital. Japanese Circ J (2001) 65(4):315–9. doi: 10.1253/jcj.65.315
27. Hoffmeier A, Schmid C, Deiters S, Drees G, Rothenburger M, Tjan T, et al. Neoplastic heart disease-the Muenster experience with 108 patients. Thorac Cardiovasc surg (2005) 53(01):1–8. doi: 10.1055/s-2004-830389
28. Li X, Chen Y, Liu J, Xu L, Li Y, Liu D, et al. Cardiac magnetic resonance imaging of primary cardiac tumors. Quantitat Imaging Med Surg (2020) 10(1):294. doi: 10.21037/qims.2019.11.13
29. Bui Q, Ngo TN, Mazur J, Pham V, Palmer C, Truong BQ, et al. Long-term outcomes of primary cardiac malignant tumors: Difference between African American and Caucasian population. Cancer Med (2021) 10(24):8838–45. doi: 10.1002/cam4.4385
30. Yin K, Luo R, Wei Y, Wang F, Zhang Y, Karlson KJ, et al. Survival outcomes in patients with primary cardiac sarcoma in the united states. J Thorac Cardiovasc Surg (2021) 162(1):107–15.e2. doi: 10.1016/j.jtcvs.2019.12.109
31. Rahouma M, Baudo M, Shmushkevich S, Chadow D, Mohamed A, Gaudino M, et al. Association between insurance status and survival among patients with malignant cardiac tumours. Br J Surg (2022) 109(2):e24–e25. doi: 10.1093/bjs/znab423
32. Lestuzzi C. Impact of health insurance and socioeconomic status in the outcome of cancer and cardiac diseases. Int J Cardiol (2021) 344:184–5. doi: 10.1016/j.ijcard.2021.09.032
33. Unger JM, Moseley AB, Cheung CK, Osarogiagbon RU, Symington B, Ramsey SD, et al. Persistent disparity: socioeconomic deprivation and cancer outcomes in patients treated in clinical trials. J Clin Oncol (2021) 39(12):1339. doi: 10.1200/JCO.20.02602
34. Andell P, Li X, Martinsson A, Nilsson PM, Zöller B, Smith JG, et al. Neighborhood socioeconomic status and aortic stenosis: a Swedish study based on nationwide registries and an echocardiographic screening cohort. Int J Cardiol (2020) 318:153–9. doi: 10.1016/j.ijcard.2020.06.034
35. von Kappelgaard L, Gislason G, Davidsen M, Zwisler A-D, Juel K. Temporal trends and socioeconomic differences in treatment and mortality following a diagnosis of aortic stenosis. Int J Cardiol (2021) 336:87–92. doi: 10.1016/j.ijcard.2021.05.039
36. Rahouma M, Baudo M, Dabsha A, Dimagli A, Mohamed A, Mick SL, et al. Outcomes of octogenarians with primary malignant cardiac tumors: National cancer database analysis. J Clin Med (2022) 11(16):4899. doi: 10.3390/jcm11164899
37. Sultan I, Bianco V, Habertheuer A, Kilic A, Gleason TG, Aranda-Michel E, et al. Long-term outcomes of primary cardiac malignancies: multi-institutional results from the national cancer database. J Am Coll Cardiol (2020) 75(18):2338–47. doi: 10.1016/j.jacc.2020.03.041
38. Truong PT, Jones SO, Martens B, Alexander C, Paquette M, Joe H, et al. Treatment and outcomes in adult patients with primary cardiac sarcoma: the British Columbia cancer agency experience. Ann Surg Oncol (2009) 16(12):3358–65. doi: 10.1245/s10434-009-0734-8
39. Isambert N, Ray-Coquard I, Italiano A, Rios M, Kerbrat P, Gauthier M, et al. Primary cardiac sarcomas: a retrospective study of the French sarcoma group. Eur J Cancer. (2014) 50(1):128–36. doi: 10.1016/j.ejca.2013.09.012
40. Randhawa JS, Budd GT, Randhawa M, Ahluwalia M, Jia X, Daw H, et al. Primary cardiac sarcoma. Am J Clin Oncol (2016) 39(6):593–9. doi: 10.1097/COC.0000000000000106
41. Chan EY, Ali A, Zubair MM, Nguyen DT, Ibarra-Cortez SH, Graviss EA, et al. Primary cardiac sarcomas: Treatment strategies. J Thorac Cardiovasc Surg (2022) S0022-5223(22)00100–3. doi: 10.1016/j.jtcvs.2021.10.070
42. Hasan SM, Witten J, Collier P, Tong MZ, Pettersson GB, Smedira NG, et al. Outcomes after resection of primary cardiac sarcoma. JTCVS Open (2021) 8:384–90. doi: 10.1016/j.xjon.2021.08.038
43. Abu Saleh WK, Ramlawi B, Shapira OM, Al Jabbari O, Ravi V, Benjamin R, et al. Improved outcomes with the evolution of a neoadjuvant chemotherapy approach to right heart sarcoma. Ann Thorac Surg (2017) 104(1):90–6. doi: 10.1016/j.athoracsur.2016.10.054
44. Siontis BL, Leja M, Chugh R. Current clinical management of primary cardiac sarcoma. Expert Rev Anticancer Ther (2020) 20(1):45–51. doi: 10.1080/14737140.2020.1711738
Keywords: primary malignant cardiac tumors, database analysis, geographic variation, cardiac surgery, oncology
Citation: Rahouma M, Khairallah S, Dabsha A, Baudo M, El-Sayed Ahmed MM, Gambardella I, Lau C, Esmail YM, Mohamed A, Girardi L, Gaudino M, Lorusso R and Mick SL (2023) Geographic variation in malignant cardiac tumors and their outcomes: SEER database analysis. Front. Oncol. 13:1071770. doi: 10.3389/fonc.2023.1071770
Received: 16 October 2022; Accepted: 04 January 2023;
Published: 24 January 2023.
Edited by:
Rohit Moudgil, Cleveland Clinic, United StatesReviewed by:
Guoqing Zhang, First Affiliated Hospital of Zhengzhou University, ChinaChiara Lestuzzi, Santa Maria degli Angeli Hospital Pordenone, Italy
Copyright © 2023 Rahouma, Khairallah, Dabsha, Baudo, El-Sayed Ahmed, Gambardella, Lau, Esmail, Mohamed, Girardi, Gaudino, Lorusso and Mick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed Rahouma, bW1yMjAxMUBtZWQuY29ybmVsbC5lZHU=; bWhtZHJhaG91bWFAZ21haWwuY29t
†These authors have contributed equally to this work
 Mohamed Rahouma
Mohamed Rahouma Sherif Khairallah1,2†
Sherif Khairallah1,2† Massimo Baudo
Massimo Baudo Roberto Lorusso
Roberto Lorusso