- 1Medical Research and Biometrics Center, National Center for Cardiovascular Diseases, Fuwai Hospital, Peking Union Medical College & Chinese Academy of Medical Sciences, Beijing, China
- 2Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 3Department of Oncology, China-Japan Friendship Hospital, Beijing, China
- 4Jockey Club School of Public Health and Primary Care, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
Objective: Patients with breast cancer carrying BRCA1 and BRCA2 genetic alterations show poor prognoses. However, the efficacy of pharmacotherapies for patients with advanced breast cancer carrying BRCA1/2 pathogenic variants remains unclear. This study aimed to conduct a network meta-analysis to assess the efficacy and safety of various pharmacotherapies for patients with metastatic, locally advanced, or recurrent breast cancer carrying BRCA1/BRCA2 pathogenic variants.
Methods: A literature search was conducted using Embase, PubMed, and Cochrane Library (CENTRAL), from inception to 11th May 2022. The references of included articles were screened to identify relevant literature. This network meta-analysis included patients with metastatic locally advanced or recurrent breast cancer who received pharmacotherapy and carried deleterious variants of BRCA1/2. The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were followed for conducting and reporting this systematic meta-analysis. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method was employed to evaluate evidential certainty. Frequentist random-effect model was applied. Results of objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and rates of any-grade adverse events were presented.
Results: Nine randomized controlled trials were obtained comprising six treatment regimens, including 1912 patients with pathogenic variants of BRCA1 and BRCA2. The orchestration of PARP inhibitors with platinum-based chemotherapy was found to be the most effective with a pooled odds ratio (OR) of 3.52 (95% CI 2.14, 5.78) for ORR; 1.53 (1.34,1.76), 3.05 (1.79, 5.19), and 5.80 (1.42, 23.77) for 3-, 12-, and 24-month PFS, respectively, and 1.04 (1.00, 1.07), 1.76 (1.25, 2.49) and 2.31 (1.41, 3.77) for 3-, 12-, and 36-month OS, respectively compared to those receiving non-platinum-based chemotherapy. However, it posed an elevated risk of some adverse events. Platinum-based chemotherapy alone or PARP inhibitors markedly improved ORR, PFS, and OS compared to non-platinum-based chemotherapy. Interestingly, platinum-based chemotherapy surpassed PARP inhibitors in terms of efficacy. Evidence on programmed death-ligand 1(PD-L1) inhibitors and sacituzumab govitecan (SG) suggested low quality and insignificant results.
Conclusions: Among all treatment regimens, PARP inhibitors with platinum exhibited the best efficacy, although with a trade-off of elevated risk of some types of adverse events. Future research on direct comparisons between different treatment regimens specifically targeting patients with breast cancer carrying BRCA1/2 pathogenic variants with a pre-specified adequate sample size is warranted.
Introduction
Breast cancer is the most diagnosed cancer in women and the fifth leading cause of cancer mortality worldwide, with an estimated 685,000 deaths in 2020 (1). Breast cancer is also the leading cause of cancer-related disability-adjusted life years (DALYs) for females globally, as reported in 2019 (2). It has a rapidly rising incidence rate in transitioning countries in South America, Africa, and Asia, as well as high-income Asian countries (1).
Pathogenic variants of breast cancer susceptibility genes 1 or 2 (BRCA1/BRCA2) reportedly occur in nearly 5% of patients with breast cancer (3, 4). These patients are more likely to have a family history, receive an early diagnosis, or show a worse prognosis, especially at an advanced cancer stage (5, 6). Genetic alterations in BRCA1/BRCA2 cause the weakening of DNA double-strand break (DSB) repair ability, making the tumor cells highly dependent on the pathways involved in single-strand break repair (7, 8). The enzyme, poly(adenosine diphosphate-ribose) polymerase (PARP) crucially controls this pathway, making PARP inhibitors a promising treatment strategy for patients with breast cancer carrying BRCA1/2 pathogenic variants (9). The U.S. Food and Drug Administration approved two PARP inhibitors, olaparib and talazoparib, as treatment options for patients with metastatic or advanced breast cancer carrying germline BRCA1/2 pathogenic variants. Other PARP inhibitors have also been tested for breast cancer therapy, including veliparib and niraparib. As a class, PARP inhibitors share some similarities (10). Platinum agents are reportedly more effective for patients with breast cancer carrying germline BRCA1/2 pathogenic variants (11). These treatments are recommended as preferred treatment options for recurrent or stage IV TNBC in the updated guidelines (12).
Platinum-based chemotherapy and PARP inhibitors are common regimens for patients with breast cancer carrying BRCA pathogenic variants. Randomized controlled trials (RCTs) using PARP inhibitors or platinum for treating patients with metastatic, locally advanced, or recurrent breast cancer carrying BRCA1/2 pathogenic variants have shown efficacy, as evidenced by improved survival duration (13–15). However, the comparative performances of these regimens remain unknown.
A previous network meta-analysis compared the efficacy and safety of various drug regimens for patients with BRCA-pathogenic variant-associated breast cancer. However, the primary analysis mixed studies on patients at different disease stages, and no comparative results were provided for patients with advanced breast cancer and BRCA1/2 pathogenic variants (16).
We undertook this network meta-analysis to assess the efficacy and safety of pharmacotherapies for patients with metastatic, locally advanced, or recurrent breast cancer carrying BRCA1/2 pathogenic variants.
Methods
The network meta-analysis was performed following the guidelines of the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (17).
Data sources and search strategies
From inception until May 11th, 2022, a systematic literature search was conducted in Embase, PubMed, and Cochrane Library (CENTRAL). To identify relevant studies, we screened the references cited in the included publications. Terms related to breast cancer and its synonyms, BRCA1/2 pathogenic variants, and RCTs were used (please refer to the detailed search string in Appendix 1).
Study selection and data extraction
Eligibility criteria included the following: (1) studies of patients with advanced or metastatic breast cancer. (2) Studies targeting patients carrying BRCA1/2 pathogenic variants or those reporting relevant subgroup results. (3) Studies with chemotherapy or targeted therapies as the treatment strategy. (4) Studies reporting at least one of the following outcomes: objective response rate (ORR), progression-free survival (PFS), or overall survival (OS). (5) Studies with an RCT design.
Exclusion criteria were as follows: (1) studies including patients with BRCA methylation. (2) Studies including patients treated with non-platinum-based chemotherapy both in the intervention and control arms. (3) Trials published in languages other than English. Only reports with the most updated results were used to retrieve information for studies derived from the same trial.
The screening was conducted by meticulously reading the titles and abstracts of each potential article, and full texts were scrupulously scrutinized when necessary. The following data were collected: author’s names, publication year, study’s abbreviation, registration number, sample size, BRCA pathogenic variant type, the proportion of TNBC patients, patients’ indication, treatment regimens, patients’ median age, and efficacy and safety outcomes. Two investigators (ZY and LY) independently conducted study selection and data extraction. Any disparity was adjudicated by a senior reviewer (LW).
Outcomes and measures
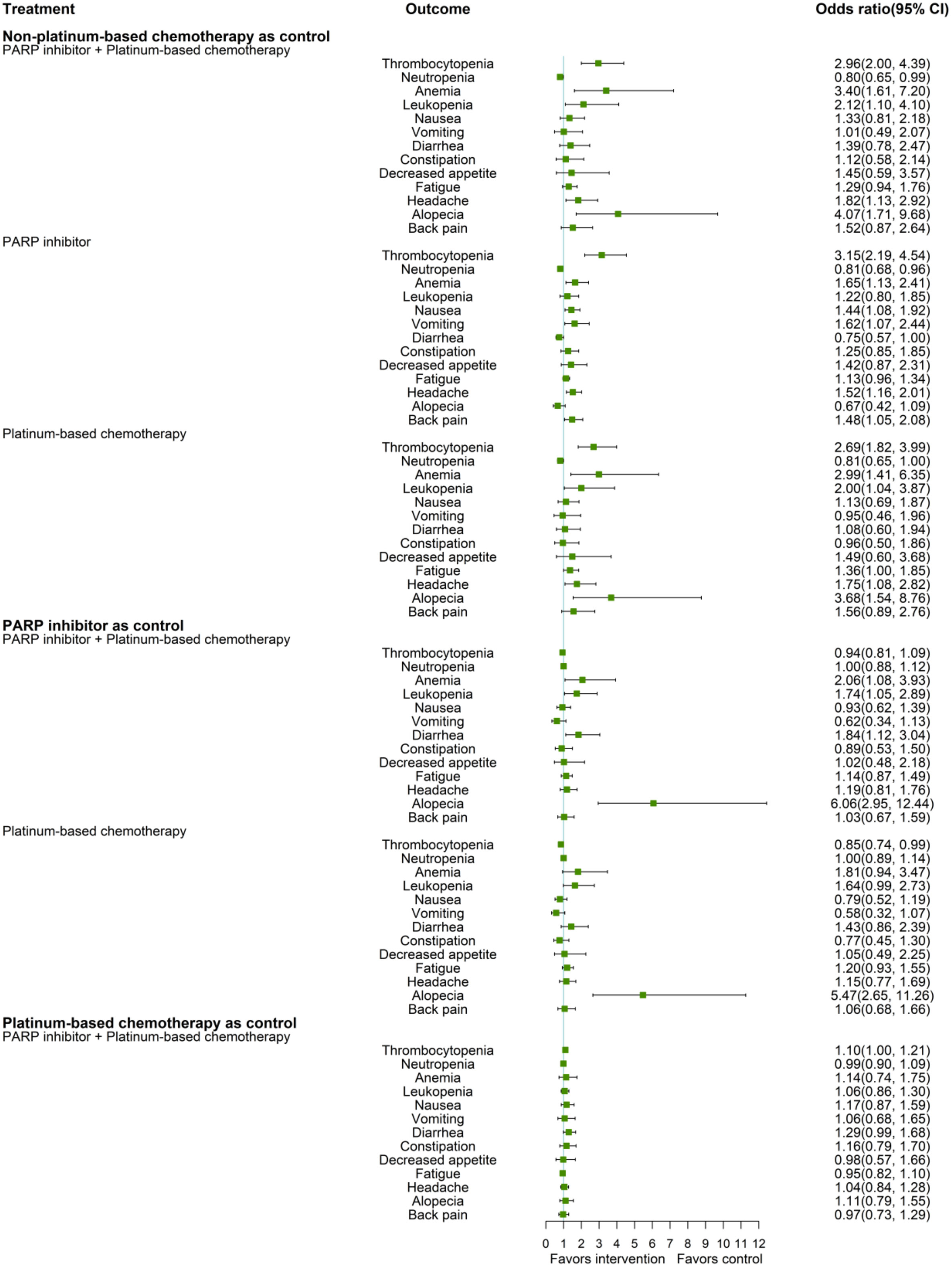
Efficacy outcomes included ORR and PFS rates at 3-, 12-, and 24 months, and OS rates at 3-, 12-, 24, and 36 months. Raw data on the number of patients experiencing/not experiencing the outcome were obtained from Kaplan-Meier survival curves. The toxicological effects were measured as rates of any-grade adverse events (thrombocytopenia, neutropenia, anemia, leukopenia, nausea, vomiting, diarrhea, constipation, decreased appetite, fatigue, headache, alopecia, and back pain).
Data analysis and evidential quality assessment
All eligible studies were included in the network meta-analysis utilizing the frequentist method and the random-effects model (18). The network estimates were visualized using net-league tables and forest plots. Odds ratios (ORs) with 95% confidence intervals (CIs) were created to quantify outcomes. The P-score, measuring the degree to which one therapy was guaranteed to be superior compared to its counterparts, was used to rank various treatment regimens (19).
Two independent reviewers (ZY and LY) evaluated the risk of bias in each study using the Cochrane risk of bias tool 2.0 for RCTs. All efficacy outcomes were assessed, and the effect of assignment to intervention was regarded as the effect of interest. The study’s overall risk of bias was divided into three categories as follows: low risk of bias if all domains showed low risk; some concerns if there was at least one domain showing some concerns but not at high risk, and high if there was at least one domain at high risk or multiple domains showing some concerns (20). We applied the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to assess the certainty of the evidence and rated it as high, moderate, low, or very low (21).
Cochran’s Q statistic was decomposed into within-design and between-design values to test the heterogeneity (22). Local inconsistency was tested by splitting and comparing indirect and direct effects, and the former estimates were calculated by back-calculation method (23). We also assessed the transitivity by comparing the distributions of potential effect modifiers across treatment regimens. Comparison-adjusted funnel plots were applied to detect publication biases for direct comparisons with treatment ranked by their P-scores (24). Egger’s regression and Begg’s rank tests were also performed to test for asymmetry in any potential publication biases. To assess the robustness of these results, sensitivity analyses were conducted using the surface under the cumulative ranking curve (SUCRA) values to rank the treatments and excluding studies reporting somatic BRCA deleterious variants, as the corresponding patients may not share the same advantage as those carrying germline mutations (12). The R package, netmeta, in R version 4.2.0, was used for data analyses.
Results
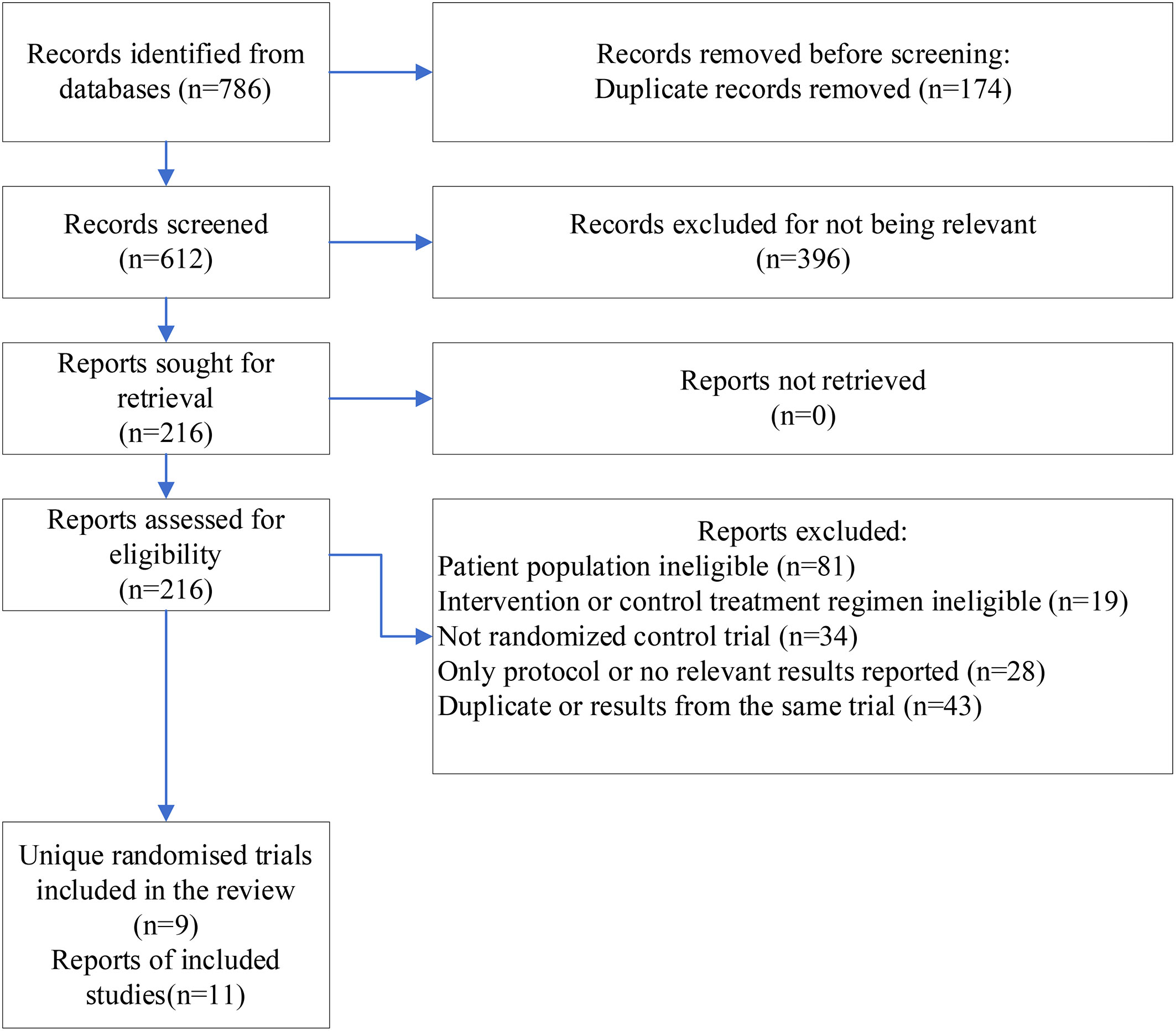
We identified 786 records, and after screening the titles and abstracts, 216 reports were retrieved for screening their full-text (Figure 1). Nine RCTs involving 1912 participants with six treatment regimens, including non-platinum-based chemotherapy, platinum-based chemotherapy, PARP inhibitor-containing regimen, PARP inhibitor plus platinum-based chemotherapy, programmed death-ligand 1 (PD-L1) inhibitor, and sacituzumab govitecan (SG), with one multi-arm study, were included. For EMBRACA and OlympiAD studies, additional final analysis reports from updated survival data were included. Finally, 11 reports were included (25–35).
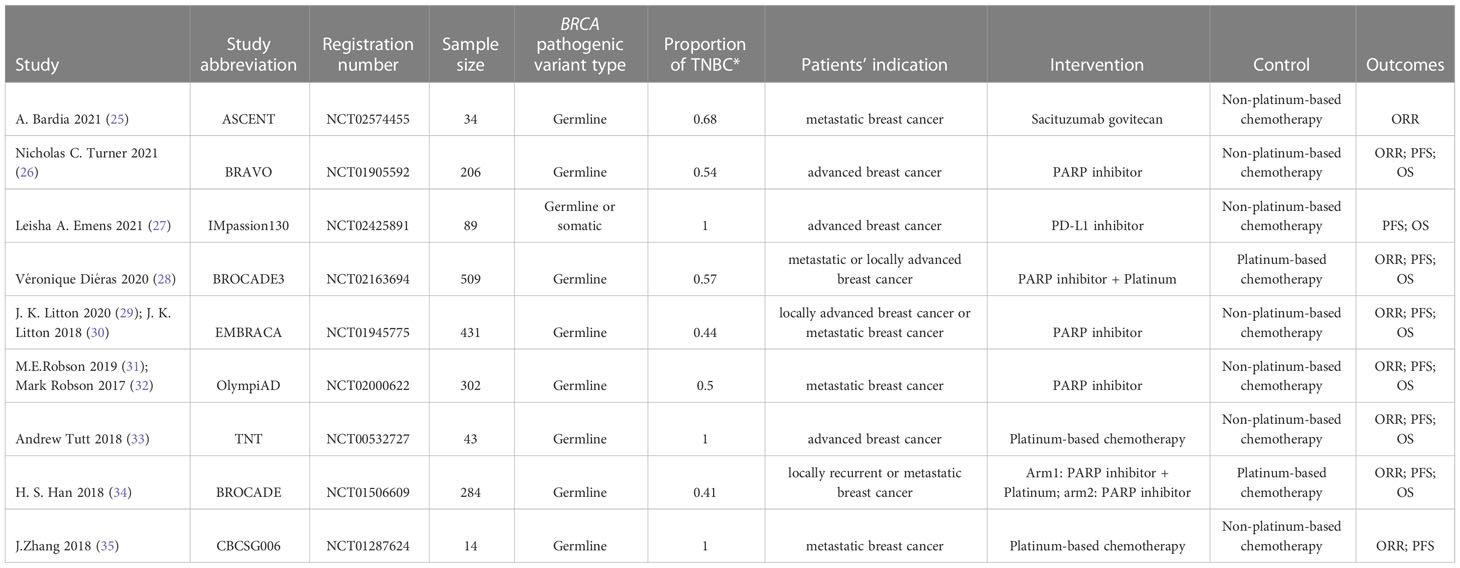
The features of the included studies are summarized in Table 1. The range of publications dated from 2018 to 2021, suggesting recent research attention has been drawn toward BRCA1/2 deleterious variants. Eight studies explicitly reported the results of patients carrying germline BRCA deleterious variants, and one reported a mix of patients carrying germline or somatic mutations. Three of the nine studies targeted only TNBC patients. Eight studies provided the outcome as ORR, while another set of eight studies provided the outcome for survival rate as PFS; seven stated the outcome as the OS rate, and five offered comprehensive information on adverse events included in the analysis.
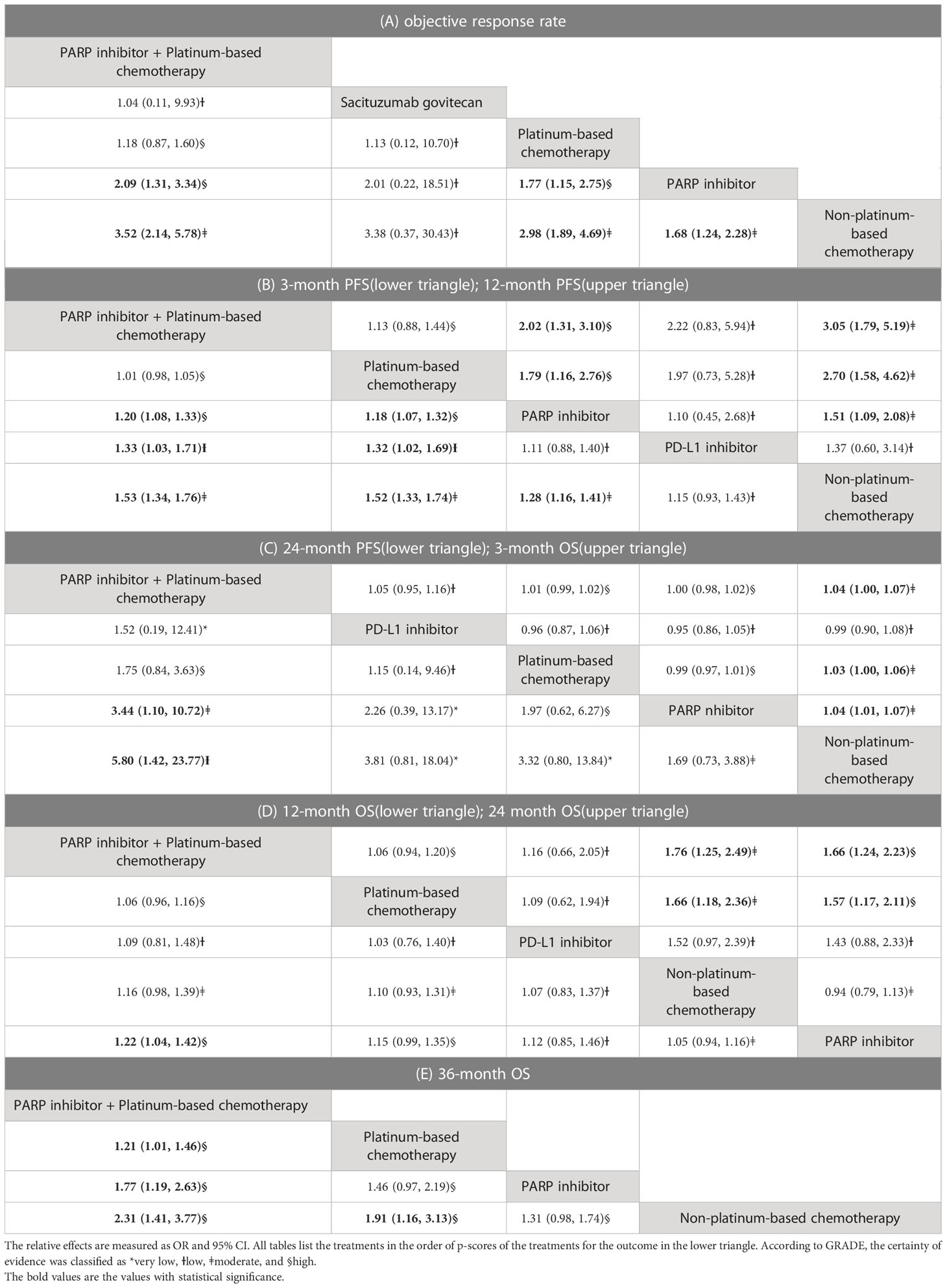
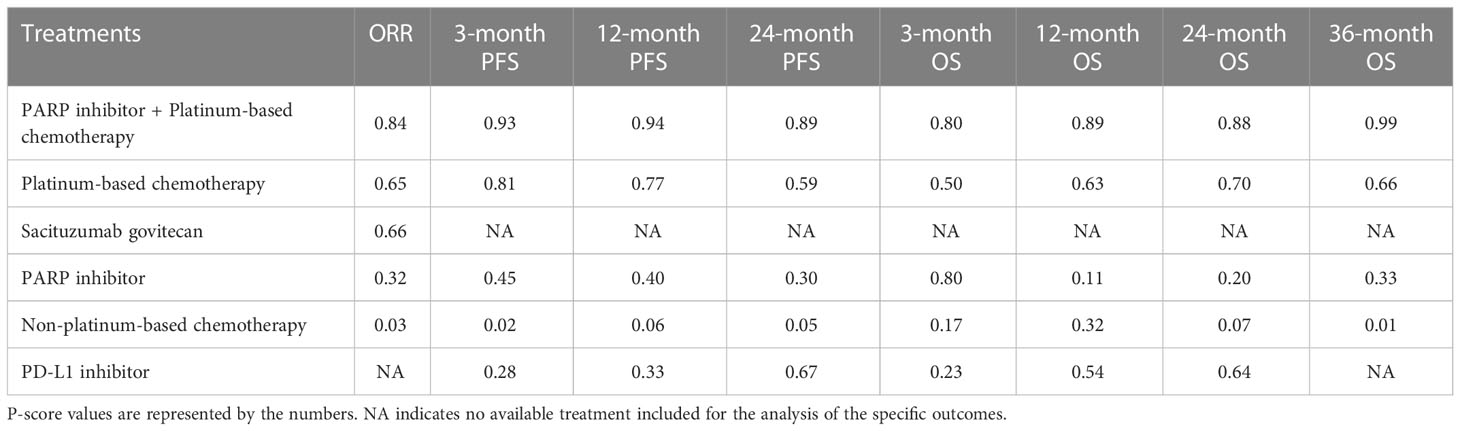
Figure 2 illustrates the network of available direct comparisons for efficacy outcomes. Network plots for safety outcomes are provided in Appendix 2. Table 2 shows the network meta-analysis results for the efficacy outcomes of eligible trials. Rankings of efficacy outcomes are shown in Table 3.
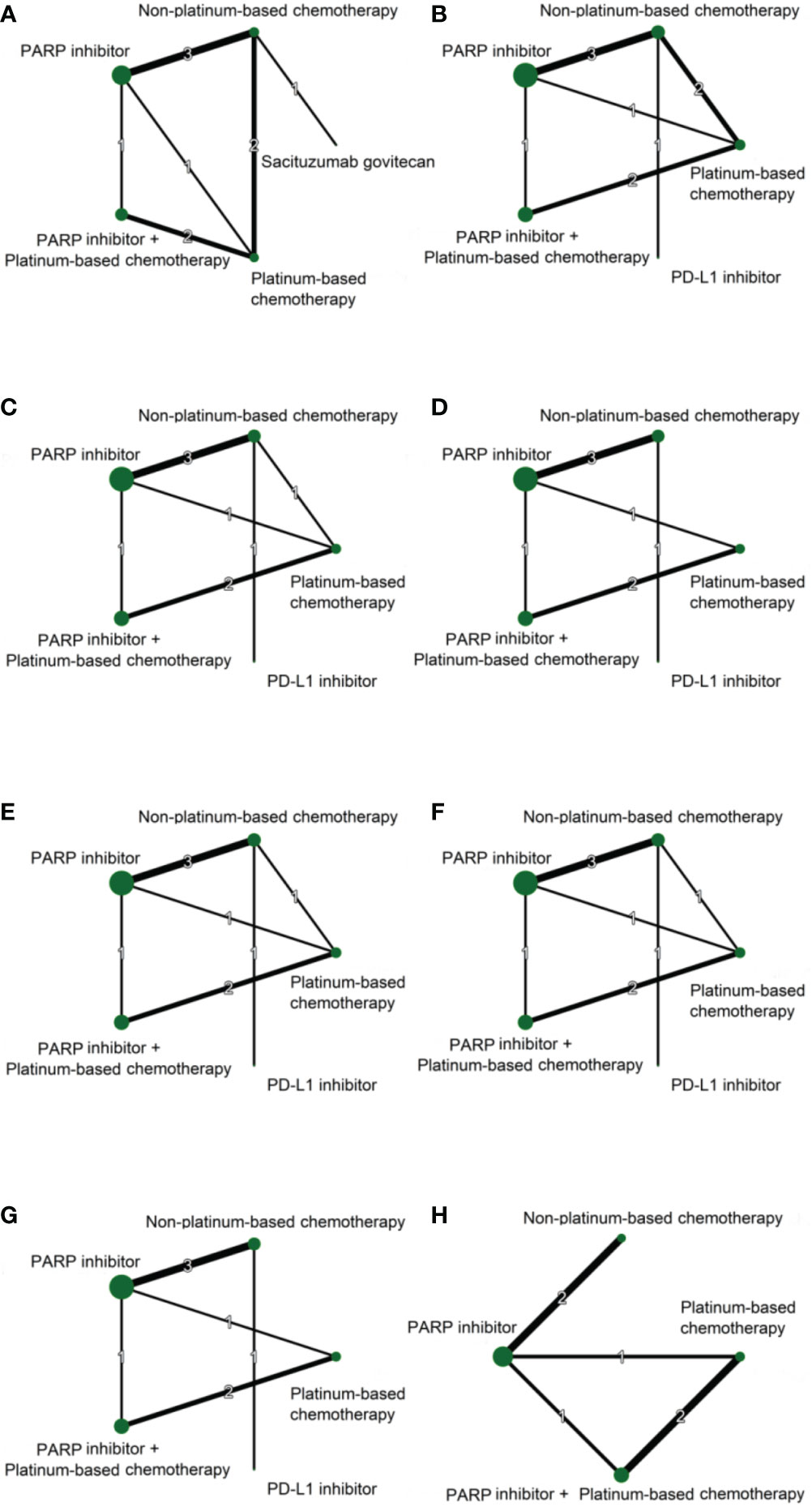
Figure 2 Network plots of direct comparisons for ORR (A), 3-month PFS (B), 12-month PFS (C), 24-month PFS (D), 3-month OS (E), 12-month OS (F), 24-month OS (G), and 36-month OS (H) Each node represents a treatment regimen. The thickness of the lines is related to the number of randomized trials that included relevant direct comparisons, and the size of the nodes is proportional to the number of individuals allocated to the corresponding intervention group.
ORR comparison
In terms of ORR, the treatment regimens containing both PARP inhibitors and platinum-based chemotherapies yielded the best benefit versus SG (OR 1.04, 95% CI 0.11 to 9.93), platinum-based chemotherapy (OR 1.18, 95% CI 0.87 to 1.60), PARP inhibitors (OR 2.09, 1.31 to 3.34), and non-platinum-based chemotherapy (OR 3.52, 95% CI 2.14, 5.78). Additionally, platinum-based chemotherapy markedly improved ORR compared to PARP inhibitors (OR 1.77, 95% CI 1.15 to 2.75) and non-platinum-based chemotherapy (OR 1.68, 95% CI 1.24 to 2.28). PARP inhibitors showed a significantly higher ORR compared to non-platinum-based chemotherapy (OR 1.68, 95% CI 1.24 to 2.28).
PFS comparison
For the outcome of PFS, the treatment regimens containing both PARP inhibitor and platinum-based chemotherapy were most likely to be ranked the best among all treatments. The PFS improved significantly at months 3 (OR 1.20, 95% CI 1.08, 1.33), 12 (OR 2.02, 95% CI 1.31, 3.10), and 24 (OR 3.44, 95% CI 1.10, 10.72) with PARP inhibitor plus platinum compared to other regimens comprising PARP inhibitors alone. The orchestration of PARP inhibitors with platinum-based chemotherapy also showed a significantly better PFS than those of the non-platinum-based chemotherapy at months 3 (OR 1.53, 95% CI 1.34,1.76), 12 (OR 3.05, 95% CI 1.79, 5.19), and 24 (OR 5.80, 95% CI 1.42, 23.77). A significant advantage of PARP inhibitor plus platinum over PD-L1 inhibitor was found for 3-month PFS (OR 1.33 95% CI 1.03, 1.71) but not 12-month (OR 2.22, 95% CI 0.83, 5.94) or 24-month (OR 1.52 95% CI 0.19, 12.41) PFS rates. Furthermore, platinum-based chemotherapy showed significantly higher 3-month and 12-month PFS rates than PARP inhibitor alone (OR 1.18, 95% CI 1.07, 1.32; OR 1.79, 95% CI 1.16, 2.76); however, the relative effect was statistically insignificant for 24-month PFS with a wider confidence interval (OR 1.97, 95% CI 0.62, 6.27). Platinum-based chemotherapy also had a higher 3-month PFS than PD-L1 inhibitor (OR 1.32, 95% CI1.02, 1.69), as well as significantly higher 3-month and 12-month PFS rates than non-platinum-based chemotherapy (OR 1.52, 95% CI 1.33, 1.74; OR 2.70, 95% CI 1.58, 4.62). The treatment regimen containing PARP inhibitors alone showed better 3-month PFS (OR 1.28, 95% CI 1.16, 1.41) and 12-month PFS (OR 1.51, 95% CI 1.09, 2.08) rates than non-platinum-based chemotherapy.
OS comparison
In terms of the 3-month OS, treatment regimens containing both PARP inhibitors and platinum-based chemotherapy (OR 1.04, 95% CI 1.00, 1.07), platinum-based chemotherapy (OR 1.03, 95% CI 1.00, 1.06), and the treatment using PARP inhibitors (OR 1.04, 95% CI 1.01, 1.07) were significantly superior to non-platinum-based chemotherapy. For the 12-month OS, the treatment regimens containing both PARP inhibitors and platinum demonstrated a significant advantage over treatment with PARP inhibitors alone (OR 1.22, 95% CI 1.04, 1.42). At month 24, the treatment regimen containing both PARP inhibitors and platinum showed a higher OS rate than treatment with PARP inhibitors alone (OR 1.66, 95% CI 1.24, 2.23) and treatment using non-platinum-based chemotherapy (OR 1.76, 1.25, 2.49). Platinum-based chemotherapy also showed a better 24-month OS compared to PARP inhibitors alone (OR 1.57, 95% CI 1.17, 2.11) and non-platinum-based chemotherapy (OR 1.66, 95% CI 1.18, 2.36). For 36-month OS, only four treatments were included in the analysis. The treatment regimen containing both PARP inhibitors and platinum showed a significantly higher 36-month OS rate versus platinum-based chemotherapy (OR 1.21, 95% CI 1.01, 1.46), PARP inhibitors (OR 1.77, 95% CI 1.19, 2.63), and non-platinum-based chemotherapy (OR 2.31, 95% CI 1.41, 3.77). Platinum-based chemotherapy also showed a better 36-month OS compared to non-platinum-based chemotherapy (OR 1.91, 95% CI 1.16, 3.13).
Results from the IMpassion130 trial, whereby some of the patients carried somatic BRCA variants, were excluded from the sensitivity analysis. The principal findings were supported by the results of our sensitivity analysis (Appendix 9).
Safety analysis
Figure 3 shows the network estimates of ORs for adverse events of any grade. Compared to non-platinum-based chemotherapy, the treatment regimen of PARP inhibitor plus platinum had a significantly higher OR for thrombocytopenia (OR 2.96, 95% CI 2.00, 4.39), anemia (OR 3.40, 95% CI 1.61, 7.20), leukopenia (OR 2.12, 95% CI 1.10, 4.10), headache (OR 1.82, 95% CI 1.13, 2.92), and alopecia (OR 4.07, 95% CI 1.71, 9.68), while treatment with PARP inhibitors showed a significantly increased risk of thrombocytopenia (OR 3.15, 95% CI 2.19, 4.54), anemia (OR 1.65, 95% CI 1.13, 2.41), nausea (OR 1.44 95% CI 1.08, 1.92), vomiting (OR 1.62, 95% CI 1.07, 2.44), headache (OR 1.52 95% CI 1.16, 2.01), and back pain (OR 1.48 95% CI 1.05, 2.08), compared to non-platinum-based chemotherapy. Platinum-based chemotherapy had higher ORs for thrombocytopenia (OR 2.69 95% CI 1.82, 3.99), anemia (OR 2.99, 95% CI 1.41, 6.35), leukopenia (OR 2.00, 95% CI 1.04, 3.87), fatigue (OR 1.36, 95% CI 1.00, 1.85), headache (OR 1.75, 95% CI 1.08, 2.82), and alopecia (OR 3.68, 95% CI 1.54, 8.76), compared to non-platinum-based chemotherapy. All three treatment regimens, PARP inhibitor plus platinum (OR 0.8, 95% CI 0.65, 0.99), PARP inhibitor (OR 0.81 95% CI 0.68, 0.96), and platinum-based chemotherapy (OR 0.81 95% CI 0.65, 1.00), had a significantly lower risk of neutropenia than non-platinum-based chemotherapy.
Compared to a treatment regimen containing PARP inhibitor, significantly higher ORs of anemia (OR, 2.06, 95% CI 1.08, 3.93), leukopenia (OR 1.74, 95% CI 1.05, 2.89), diarrhea (OR 1.84, 95% CI 1.12, 3.04), and alopecia (OR 6.06, 95% CI 2.95, 12.44) were observed for the treatment regimens including both PARP inhibitors and platinum.
Platinum-based chemotherapy had a significantly higher OR for alopecia (OR 5.47, 95% CI 2.65, 11.26) than PARP inhibitor but a significantly lower OR for thrombocytopenia (OR 0.85, 95% CI 0.74, 0.99). Compared to platinum-based chemotherapy, the treatment with PARP inhibitors plus platinum showed a substantially higher OR for thrombocytopenia (OR 1.10, 95% CI 1.00, 1.21) but no significant differences were noted for most adverse events.
Supplementary Appendix 3 summarizes the results of the risk of bias assessment. The network meta-analysis’ heterogeneity, intransitivity, inconsistency, and publication bias were also assessed (Supplementary Appendices 4–7). No evidence of significant inconsistency was detected.
Discussion
This network meta-analysis revealed that incorporating a PARP inhibitor in platinum-based chemotherapy was the most efficient treatment plan for all specified efficacy outcomes, that is ORR, PFS, and OS. Additionally, platinum-based chemotherapy was superior to PARP inhibitor alone in terms of ORR, 3-month PFS, 12-month PFS, and 24-month OS. Among safety outcomes, the treatment regimens comprising both PARP inhibitors and platinum, PARP inhibitor alone, or platinum-based chemotherapy were all associated with a significantly elevated risk for hematological and non-hematological side effects compared to non-platinum-based chemotherapy. The treatment regimen comprising both a PARP inhibitor and platinum showed a higher risk of anemia, leukopenia, diarrhea, and alopecia, compared to PARP inhibitors without platinum; the safety profile to platinum-based chemotherapy was comparable to PARP inhibitor plus platinum. Thus, adding PARP inhibitors to platinum-based chemotherapy would hardly cause more safety burdens.
A previous network meta-analysis of hazard ratios for PFS and ORR found that for patients with advanced breast cancer carrying germline BRCA variants, treatment with PARP inhibitor plus platinum were ideal regimens (16). Our study included updated articles and additional treatment regimens. We further evaluated more outcomes of OS and PFS rates at different times and various types of adverse events in detail with a specialized focus on patients with metastatic, locally advanced, or recurrent breast cancer. We found similar results, whereby treatment with PARP inhibitor plus platinum was the most effective. Furthermore, we also identified that platinum-based chemotherapy had a better prognosis in terms of most efficacy outcomes than the treatment with PARP inhibitors alone. Nevertheless, there was only one study that included a direct comparison between platinum-based chemotherapy and PARP inhibitors, which had a major contribution to the pooled results. Further verification in the future is needed.
BRCA1/2 are crucial for homologous recombination (HR) during DSB repair, and pathogenic variants are linked to genome instability and the progression of cancer (36). It was reported that HR deficiency assays, such as detecting nuclear RAD51 foci in tumor cells, could identify patients with BRCA pathogenic variants that are more likely to respond to platinum-containing therapy and PARP inhibitors (37–40). Platinum drugs, like cisplatin and carboplatin, act as DNA cross-linking agents forming intra-strand crosslinks, and in turn inhibiting DNA synthesis, function, and transcription (41). BRCA pathogenic variant carriers without sufficient DNA repair ability are, therefore, more sensitive to platinum (42). PARP1 and PARP2 enzymes are critical to the DNA damage response (DDR), and HR deficiency and PARP inhibitors result in synthetic lethality through mechanisms related to catalytic inhibition of the PARP enzyme and trapping of PARP-DNA complexes (9). Our findings showed that the treatment combining both PARP inhibitors and platinum had better efficacy than either regimen alone; however, there may be an increased risk of some adverse events in the former. Since both PARP inhibitors and platinum target and impede DNA synthesis, identifying which of the two treatments is more effective for patients carrying BRCA pathogenic variants would be an interesting topic. Although treating patients with advanced breast cancer carrying pathogenic variants of BRCA with platinum-based chemotherapy is more advantageous than PARP inhibitors according to our analyses, the results need further verification in a sizable RCT that includes direct comparisons.
Sacituzumab govitecan is a Trop-2-directed antibody-drug conjugate that can increase double-stranded DNA breaks (43). It benefits metastatic TNBC patients regardless of germline BRCA1/2 variants, as evidenced in an original trial (25). SG was included in the network analysis for ORR comparison, yet the related results were all statistically insignificant, and the evidence was of low certainty. As for PD-L1 inhibitors, the original study also found that BRCA1/2 status was not a prognostic factor for PFS or OS outcomes (27). Network meta-analysis results for PD-L1 revealed that the quality of evidence was relatively poor. Thus, further investigation is warranted and should be more pertinent to the specific corresponding biomarker (Trop-2 and PD-L1) expression than the BRCA1/2 variants.
There are also some limitations of our study. First, the sample sizes of several trials were small, which may have led to a broader estimate of the CIs of effects and impaired the evidence quality. This happens mainly because the targeted group with BRCA1/2 pathogenic variants is only a subgroup from the original trial. Second, not all studies were included for comparing each outcome, as some data were unavailable from the initial studies.
Conclusion
In conclusion, PARP inhibitor combined with platinum-based chemotherapy was proved as the optimal treatment for patients with metastatic, locally advanced, or recurrent breast cancer carrying BRCA1/2 pathogenic variants in terms of efficacy outcomes, namely ORR, PFS, and OS. Although the combination of both PARP inhibitor and platinum resulted in more adverse events compared to PARP inhibitor alone and non-platinum-based chemotherapy regimens, which should raise caution in clinical settings, adding PARP inhibitor to platinum barely caused an extra risk of unfavorable events compared to platinum-based chemotherapy alone. Thus, this combined regimen should be considered for patients with advanced breast cancer carrying BRCA pathogenic variants for better prognostic outcomes. Confirmatory RCTs of sufficient, pre-specified sample sizes that directly compare currently available treatment regimens and are explicitly aimed at patients carrying BRCA1/2 pathogenic variants should be conducted in the future.
Author contributions
WL had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. YZ: Conceptualization, Methodology, Software, Investigation, Formal analysis, Writing - Original Draft, Visualization. YL: Conceptualization, Investigation, Data Curation Writing - Review & Editing. WDL: Software, Validation, Visualization, Writing - Review & Editing. RZ: Writing - Review & Editing, Conceptualization. LT: Writing - Review & Editing Supervision. YW: Methodology Supervision. WL: Supervision, Writing - Review & Editing, Acquisition of the financial support for the project leading to this publication, Project administration All authors contributed to the article and approved the submitted version.
Funding
Clinical trial biobank and statistical methodology service platform construction (2022-FWTS08).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1080297/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209−49. doi: 10.3322/caac.21660
2. Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019. JAMA Oncol (2022) 8(3):420. doi: 10.1001/jamaoncol.2021.6987
3. Malone KE, Daling JR, Doody DR, Hsu L, Bernstein L, Coates RJ, et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res (2006) 66(16):8297−308. doi: 10.1158/0008-5472.CAN-06-0503
4. Kurian AW, Gong GD, John EM, Miron A, Felberg A, Phipps AI, et al. Performance of prediction models for BRCA mutation carriage in three Racial/Ethnic groups: Findings from the northern California breast cancer family registry. Cancer Epidemiol Biomark Ampmathsemicolon Prev (2009) 18(4):1084−91. doi: 10.1158/1055-9965.epi-08-1090
5. Kwong A, Shin VY, Ho JCW, Kang E, Nakamura S, Teo S-H, et al. Comprehensive spectrum of BRCA1 and BRCA2 deleterious mutations in breast cancer in Asian countries. J Med Genet (2016) 53(1):15−23. doi: 10.1136/jmedgenet-2015-103132
6. Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, Miron P, et al. Inherited mutations in 17 breast cancer susceptibility genes among a Large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol (2015) 33(4):304−11. doi: 10.1200/jco.2014.57.1414
7. Zhao W, Wiese C, Kwon Y, Hromas R, Sung P. The BRCA tumor suppressor network in chromosome damage repair by homologous recombination. Annu Rev Biochem (2019) 88(1):221−45. doi: 10.1146/annurev-biochem-013118-111058
8. Tarsounas M, Sung P. The antitumorigenic roles of BRCA1–BARD1 in DNA repair and replication. Nat Rev Mol Cell Biol (2020) 21(5):284−99. doi: 10.1038/s41580-020-0218-z
9. Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science (2017) 355(6330):1152−8. doi: 10.1126/science.aam7344
10. Palleschi M, Tedaldi G, Sirico M, Virga A, Ulivi P, De Giorgi U. Moving beyond PARP inhibition: Current state and future perspectives in breast cancer. Int J Mol Sci (2021) 22(15):7884. doi: 10.3390/ijms22157884
11. Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, et al. TBCRC009: A multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol (2015) 33:1902–9. doi: 10.1200/jco.2014.57.6660
12. Network NCC. Breast cancer (Version 2.2022), in: NCCN website (2022). Available at: https://www.nccn.org/guidelines/category_1 (Accessed July 11, 2022).
13. Wang C-J, Xu Y, Lin Y, Zhu H-J, Zhou Y-D, Mao F, et al. Platinum-based neoadjuvant chemotherapy for breast cancer with BRCA mutations: A meta-analysis. Front Oncol (2020) 10:592998. doi: 10.3389/fonc.2020.592998
14. Caramelo O, Silva C, Caramelo F, Frutuoso C, Almeida-Santos T. The effect of neoadjuvant platinum-based chemotherapy in BRCA mutated triple negative breast cancers -systematic review and meta-analysis. Hered Cancer Clin Pract (2019) 17(1):11–21. doi: 10.1186/s13053-019-0111-y
15. Wang J, Zhang Y, Yuan L, Ren L, Zhang Y, Qi X. Comparative efficacy, safety, and acceptability of single-agent poly (ADP-ribose) polymerase (PARP) inhibitors in BRCA-mutated HER2-negative metastatic or advanced breast cancer: A network meta-analysis. Aging (2020) 13(1):450−9. doi: 10.18632/aging.202152
16. Jiang Y, Meng X-Y, Deng N-N, Meng C, Li L-H, He Z-K, et al. Effect and safety of therapeutic regimens for patients with germline BRCA mutation-associated breast cancer: A network meta-analysis. Front Oncol (2021) 11:718761. doi: 10.3389/fonc.2021.718761
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev (2021) 10(1):89–100. doi: 10.1186/s13643-021-01626-4
18. Rücker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods (2012) 3(4):312−24. doi: 10.1002/jrsm.1058
19. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol (2015) 15(1):58–67. doi: 10.1186/s12874-015-0060-8
20. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
21. Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE working group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ (2014) 349(sep24 5):g5630−g5630. doi: 10.1136/bmj.g5630
22. Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med (2012) 31(29):3805−20. doi: 10.1002/sim.5453
23. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med (2010) 29(7−8):932−44. doi: 10.1002/sim.3767
24. Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods (2012) 3(2):161−76. doi: 10.1002/jrsm.57
25. Bardia A, Tolaney SM, Punie K, Loirat D, Oliveira M, Kalinsky K, et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol (2021) 32(9):1148−56. doi: 10.1016/j.annonc.2021.06.002
26. Turner NC, Balmaña J, Poncet C, Goulioti T, Tryfonidis K, Honkoop AH, et al. Niraparib for advanced breast cancer with germline BRCA1 and BRCA2 mutations: the EORTC 1307-BCG/BIG5–13/TESARO PR-30–50–10-C BRAVO study. Clin Cancer Res (2021) 27(20):5482−91. doi: 10.1158/1078-0432.CCR-21-0310
27. Emens LA, Molinero L, Loi S, Rugo HS, Schneeweiss A, Diéras V, et al. Atezolizumab and nab -paclitaxel in advanced triple-negative breast cancer: Biomarker evaluation of the IMpassion130 study. JNCI J Natl Cancer Inst (2021) 113(8):1005−16. doi: 10.1093/jnci/djab004
28. Diéras V, Han HS, Kaufman B, Wildiers H, Friedlander M, Ayoub J-P, et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2020) 21(10):1269−82. doi: 10.1016/s1470-2045(20)30447-2
29. Litton JK, Hurvitz SA, Mina LA, Rugo HS, Lee K-H, Gonçalves A, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann Oncol (2020) 31(11):1526−35. doi: 10.1016/j.annonc.2020.08.2098
30. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee K-H, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med (2018) 379(8):753−63. doi: 10.1056/NEJMoa1802905
31. Robson ME, Tung N, Conte P, Im S-A, Senkus E, Xu B, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol (2019) 30(4):558−66. doi: 10.1093/annonc/mdz012
32. Robson M, Im S-A, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med (2017) 377(6):523−33. doi: 10.1056/NEJMoa1706450
33. Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT trial. Nat Med (2018) 24(5):628−37. doi: 10.1038/s41591-018-0009-7
34. Han HS, Diéras V, Robson M, Palácová M, Marcom PK, Jager A, et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Ann Oncol (2018) 29(1):154−61. doi: 10.1093/annonc/mdx505
35. Zhang J, Lin Y, Sun XJ, Wang BY, Wang ZH, Luo JF, et al. Biomarker assessment of the CBCSG006 trial: A randomized phase III trial of cisplatin plus gemcitabine compared with paclitaxel plus gemcitabine as first-line therapy for patients with metastatic triple-negative breast cancer. Ann Oncol (2018) 29(8):1741−7. doi: 10.1093/annonc/mdy209
36. Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer (2011) 12(1):68−78. doi: 10.1038/nrc3181
37. Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC, et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer HRD predicts response to platinum therapy in TNBC. Clin Cancer Res (2016) 22(15):3764–73. doi: 10.1158/1078-0432.CCR-15-2477
38. Chopra N, Tovey H, Pearson A, Cutts R, Toms C, Proszek P, et al. Homologous recombination DNA repair deficiency and PARP inhibition activity in primary triple negative breast cancer. Nat Commun (2020) 11(1):1–12. doi: 10.1038/s41467-020-16142-7
39. Pellegrino B, Herencia-Ropero A, Llop-Guevara A, Pedretti F, Moles-Fernández A, Viaplana C, et al. Preclinical in vivo validation of the RAD51 test for identification of homologous recombination-deficient tumors and patient stratification. Cancer Res (2022) 82(8):1646–57. doi: 10.1158/0008-5472.CAN-21-2409
40. Llop-Guevara A, Loibl S, Villacampa G, Vladimirova V, Schneeweiss A, Karn T, et al. Association of RAD51 with homologous recombination deficiency (HRD) and clinical outcomes in untreated triple-negative breast cancer (TNBC): Analysis of the GeparSixto randomized clinical trial. Ann Oncol Off J Eur Soc Med Oncol (2021) 32:1590–6. doi: 10.1016/j.annonc.2021.09.003
41. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol (2014) 740:364−78. doi: 10.1016/j.ejphar.2014.07.025
42. Torrisi R, Zuradelli M, Agostinetto E, Masci G, Losurdo A, Sanctis RD, et al. Platinum salts in the treatment of BRCA-associated breast cancer: A true targeted chemotherapy? Crit Rev Oncol Hematol (2019) 135:66−75. doi: 10.1016/j.critrevonc.2019.01.016
43. Cardillo TM, Sharkey RM, Rossi DL, Arrojo R, Mostafa AA, Goldenberg DM. Synthetic lethality exploitation by an anti–Trop-2-SN-38 antibody–drug conjugate, IMMU-132, plus PARP inhibitors in BRCA1/2 –wild-type triple-negative breast cancer. Clin Cancer Res (2017) 23(13):3405−15. doi: 10.1158/1078-0432.CCR-16-2401
Keywords: breast neoplasms, genes, BRCA1, BRCA2, network meta-analysis
Citation: Zhu Y, Li Y, Liu W, Zhou R, Tse LA, Wang Y and Li W (2023) Efficacy and safety of treatment regimens for patients with metastatic, locally advanced, or recurrent breast cancer carrying BRCA1/BRCA2 pathogenic variants: A network meta-analysis. Front. Oncol. 13:1080297. doi: 10.3389/fonc.2023.1080297
Received: 26 October 2022; Accepted: 24 January 2023;
Published: 14 February 2023.
Edited by:
Benedetta Pellegrino, University of Parma, ItalyReviewed by:
Martina Pagliuca, University of Naples Federico II, ItalyGianluca Tedaldi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), Italy
Copyright © 2023 Zhu, Li, Liu, Zhou, Tse, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Li, TGl3ZWlAbXJiYy1uY2NkLmNvbQ==
 Yingxuan Zhu
Yingxuan Zhu Yang Li1
Yang Li1 Lap Ah Tse
Lap Ah Tse Yang Wang
Yang Wang Wei Li
Wei Li