- Department of Radiation Oncology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
Background: Currently, the optimal adjuvant regional nodal irradiation (RNI) volume for breast cancer (BC) remained controversial. We aimed to define the optimal RNI treatment volume for BC by using a comprehensive network meta-analysis (NMA) of published studies.
Materials and methods: PubMed, Embase, Medline, and Cochrane Central Register of Controlled Trials were searched from database inception to 30 May 2022. Studies assessing different adjuvant RNI volumes for BC were eligible for inclusion. The primary outcome was overall survival (OS), and secondary outcome was disease-free survival (DFS) and distant-metastasis-free survival (DMFS).
Results: A total of 29,640 BC patients from twenty studies were included. The pooled hazard ratio demonstrated that internal mammary node irradiation (IMNI) in BC patients significantly improved OS giving HR (hazard ratio) of 0.87 (95%CI: 0.83–0.91, p<0.001), DFS with HR of 0.78 (95%CI: 0.68–0.90, p<0.01), and DMFS with HR of 0.87 (95%CI: 0.79–0.97, p<0.01) when compared to controls. Sub-group analysis indicated that RNI with IMNI significantly improved OS (HR 0.87, 95%CI: 0.81–0.93, p<0.01), DFS (HR 0.65, 95%CI: 0.56–0.77, p<0.01), and DMFS (HR 0.90, 95%CI: 0.82–0.98, p=0.02) when compared to RNI without IMNI. NMA showed that CW/WB (chest wall/whole breast) + RNI with IMNI significantly improved DFS (HR 0.93, 95%CI: 0.86–1.00) and DMFS (HR 0.90, 95%CI: 0.81–0.99), but not for OS (HR 0.93, 95%CI: 0.84–1.03) when compared to CW/WB alone. Based on the analysis of the treatment ranking, CW/WB+RNI with IMNI appeared as the best treatment approach for BC patients.
Conclusions: Our pooled results demonstrated that RNI with IMNI yielded a significant survival advantage for BC patients. NMA showed that CW/WB+RNI with IMNI was the optimal radiation volume for BC patients.
Introduction
According to the global cancer statistics 2020, female breast cancer (BC) ranked the most commonly diagnosed cancer, accounting for 11.7% of total tumors with an estimated 2.3 million new cases (1). Radiation therapy (RT) played a key role in the management of BC after breast-conserving surgery or mastectomy, and regional nodal irradiation (RNI) was widely used for the treatment of node-positive BC due to its potential survival benefit (2). Since the publication of two large phase III randomized controlled trials MA-20 (3) and EORTC-22922/10925 (4), comprehensive RNI including supraclavicular lymph nodes (SVCs) and internal mammary lymph nodes (IMNs) had become the standardized adjuvant RT therapy for high-risk node-negative or involving one to three node-positive BC in international guidelines. However, despite an abundance of data supporting the benefit of comprehensive RNI for BC, uncertainty existed regarding the appropriate RNI volume for BC. Indeed, significant inter-physician variability existed in real-world practice especially in IMN irradiation (IMNI) because the inclusion of the IMN would lead to cardiopulmonary extra doses, which increase the risks for developing late adverse events such as cardiac events and pulmonary fibrosis. In a more recent randomized clinical trial (KROG 0806), the authors failed to demonstrate superiority of the IMNI group over those treated without IMNI for node-positive BC patients because of sample size calculation issue, poor protocol compliance, and RT quality assurance issue (5). Thus, an unanswered question about the optimal regional nodal irradiation volume for BC patients remains, and the optimal RNI strategy gained great interest for radiation oncologists with the aim of archiving maximal clinical benefit of RNI, with the minimal possible radiotherapy toxicity to maintain the quality of life. As a result, we performed the present comprehensive meta-analysis to investigate the efficacy of radiotherapy with IMNI vs. RNI without IMNI in BC patients. In addition, we also compared the efficacy difference of RNI regimens head-to-head via network meta-analysis in terms of combined clinically meaningful overall survival (OS), disease-free survival (DFS), and distant-metastasis-free survival (DMFS) benefits.
Materials and methods
Data source
Several databases including PubMed, Embase, Medline, and Cochrane Central Register of Controlled Trials were searched for relevant studies. Studies comparing different regional nodal irradiation volumes were included. The search keywords were breast cancer, breast carcinoma, radiotherapy, regional node radiation, and clinical studies. Clinical studies should meet the following criteria (1): clinical studies involving BC patients (2), clinical studies comparing efficacy of different adjuvant RNI volumes, and (3) available survival data regarding RNI in BC patients. BC patients treated with neoadjuvant therapy were excluded for analysis in the present study.
Data extraction
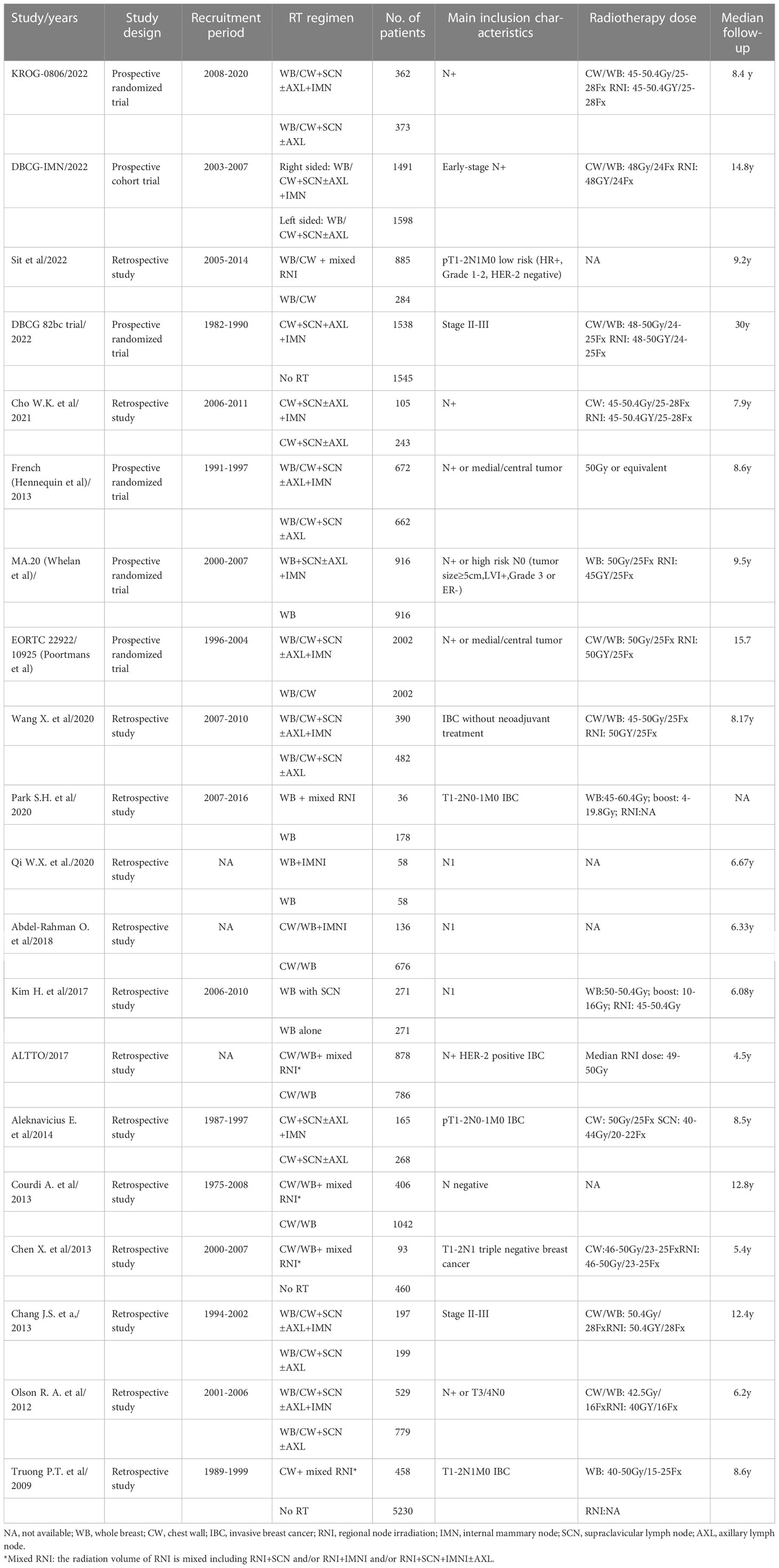
Four independent investigators conducted the data extraction, and any discrepancy between the reviewers was resolved by consensus. The following information was extracted for each study: first author’s name, year of publication, number of enrolled patients, study design, radiation regimen, main inclusion characteristics, radiotherapy dose, and median follow-up time. If the radiation volume of RNI was not specifically defined including RNI+SCN (supraclavicular lymph node) and/or RNI+IMNI and/or RNI+SCN+IMNI, we defined it as mixed RNI group. The primary outcome of interest was OS, and the secondary outcomes were DFS and DMFS.
Outcome measures
The outcome data were pooled and reported as hazard ratio (HR). Between-study heterogeneity was estimated using the χ2-based Q statistics (6). Heterogeneity was considered statistically significant when pheterogeneity< 0.1. The presence of publication bias was evaluated by using the Begg and Egger tests (7, 8). A statistical test with a p-value <0.05 was considered significant. Sensitivity analysis was performed to assess the bias risk of one single study on the pooled result by a leave-one-out approach. Study quality of prospective randomized studies was assessed by using the Jadad scale based on the reporting of the studies’ methods and results (9). The quality of the retrospective or non-randomized studies was evaluated according to the Newcastle–Ottawa Scale (NOS) (10). For studies that did not report HR of OS, DFS, or DMFS, we used Engauge Digitizer 4.1 software to calculate the HR and 95% CI from the survival curve described by Tierney et al. (11).
An NMA offered methods to visualize and interpret a broader picture of current evidence and assessed the comparative effectiveness among various RNI volumes. Therefore, a network meta-analysis was performed using a frequentist framework (12). A network plot was generated for each disease setting to show all interventions included in the NMA. Comparative effectiveness results of all possible RNI comparisons were summarized with an HR and 95% CI (13). We investigated which RNI treatment volume most effectively reduced the hazards of BC progression, distant metastasis, and death by allowing multiple comparison treatment effects. Rank probabilities of treatments for efficacy were estimated by p-score. When the treatment chosen was the best option, the p-score approached 1 (100%), while p-score for the worst treatment option approached zero. Statistical analysis was performed using R software (Version 4.1.1, “meta,” “netmeta” package; R Foundation for Statistical Computing, Vienna, Austria) and Review Manager (RevMan) Version 5.0 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008).
Results
Search results
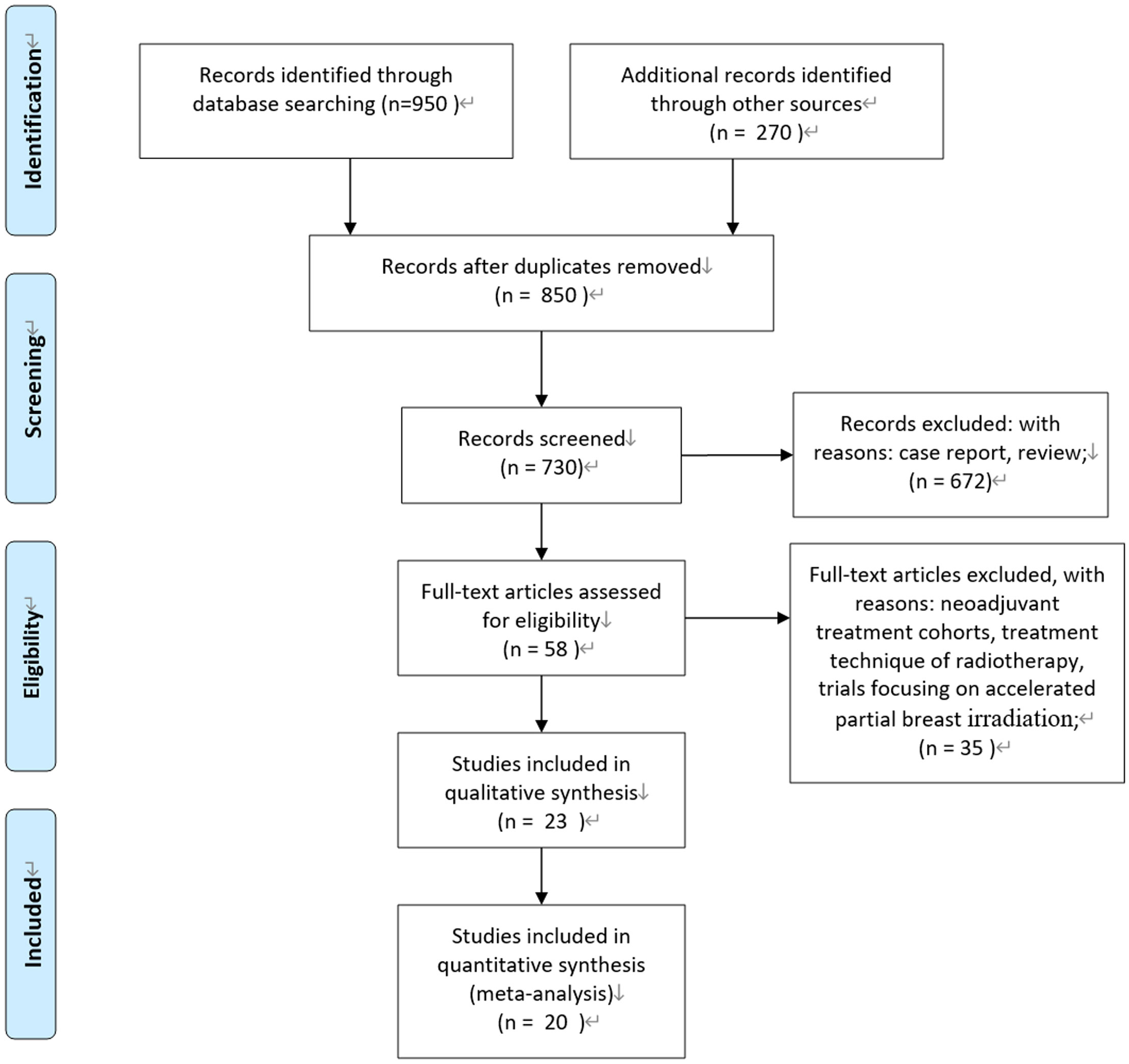
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement, we conducted the present meta-analysis (14). Our initial search yielded 850 potentially relevant reports. After excluding review articles, neoadjuvant treatment cohorts, treatment technique of radiotherapy, studies focusing on accelerated partial breast irradiation, case reports, meta-analyses, and observation studies, a total of 23 clinical studies were included. After reviewing the included studies, three studies were undated analysis of previously published studies (15–17). Finally, 20 publications of 23 clinical studies were included (3–5, 15–34). Of them, five studies were prospective randomized controlled trials. One study was a nationwide, prospective cohort study, and patients with right-sided disease were allocated to IMNI, whereas patients with left-sided disease were allocated to no IMNI because of the risk of radiation-induced heart disease (25); two studies were individual meta-analyses of two prospective cohorts (33, 34); and the remaining 12 studies were retrospective publications. Figure 1 show the process of selection.
Characteristics of the included studies
The main characteristics of included studies are summarized in Table 1. A total of twenty studies comprising 29,640 patients met the eligibility criteria. Both early-stage BC with pT1 or pT2 tumors with pN0 or pN1 disease or locally advanced BC patients were included. Patients treated with mastectomy or breast-conservation surgery (BCS) and planned axillary lymph node dissection were included. The median follow-up time ranges from 4.5 to 30 years. The common prescribed radiation dose for CW/WB and RNI was 45–50 Gy in conventional fraction size. The detailed information of the included studies is listed in Table 1.
Quality of included studies
For the five prospective randomized studies (5, 15–17, 27), all of them were open-label randomized controlled trial, thus had a Jadad score of 3. For the remaining 14 retrospective trials and 1 non-randomized trial, the quality was evaluated according to the NOS table. All 15 studies had good quality, with a ≥6 (Supplementary Table 1).
Effect of different RNI volumes on outcomes of BC
A total of 17 studies (3–5, 15–21, 26–28, 30, 31, 33, 34) reported OS data were included to analyze the efficacy of CW/WB+RNI with IMNI vs. without IMNI in BC patients. The pooled hazard ratio for OS demonstrated that CW/WB+RNI with IMNI in BC patients significantly improved OS, giving HR of 0.87 (95%CI: 0.83–0.91, p<0.01, Figure 2A). There was no significant heterogeneity between studies (I2=26%, p=0.16), and the pooled HR for OS was performed by using fixed-effects model. As for DFS, 16 studies were included for analysis (5, 15–20, 23, 24, 26, 28–30, 32, 34). The pooled result showed that CW/WB+RNI with IMNI in BC patients significantly improved DFS with an HR of 0.78 (95%CI: 0.68–0.90, p<0.01, Figure 2B). There was significant heterogeneity between studies (I2=77%, p<0.01), and the pooled HR for DFS was performed by using random-effects model. A total of 10 studies included DMFS data for analysis (5, 15–18, 21, 23, 25, 26, 31), and the pooled results showed that CW/WB+RNI with IMNI in BC patients significantly improved DMFS giving HR of 0.87 (95%CI: 0.79–0.97, p<0.01, Figure 2C). There was significant heterogeneity between studies (I2=48%, p=0.04), and the pooled HR for DMFS was performed by using random-effects model.
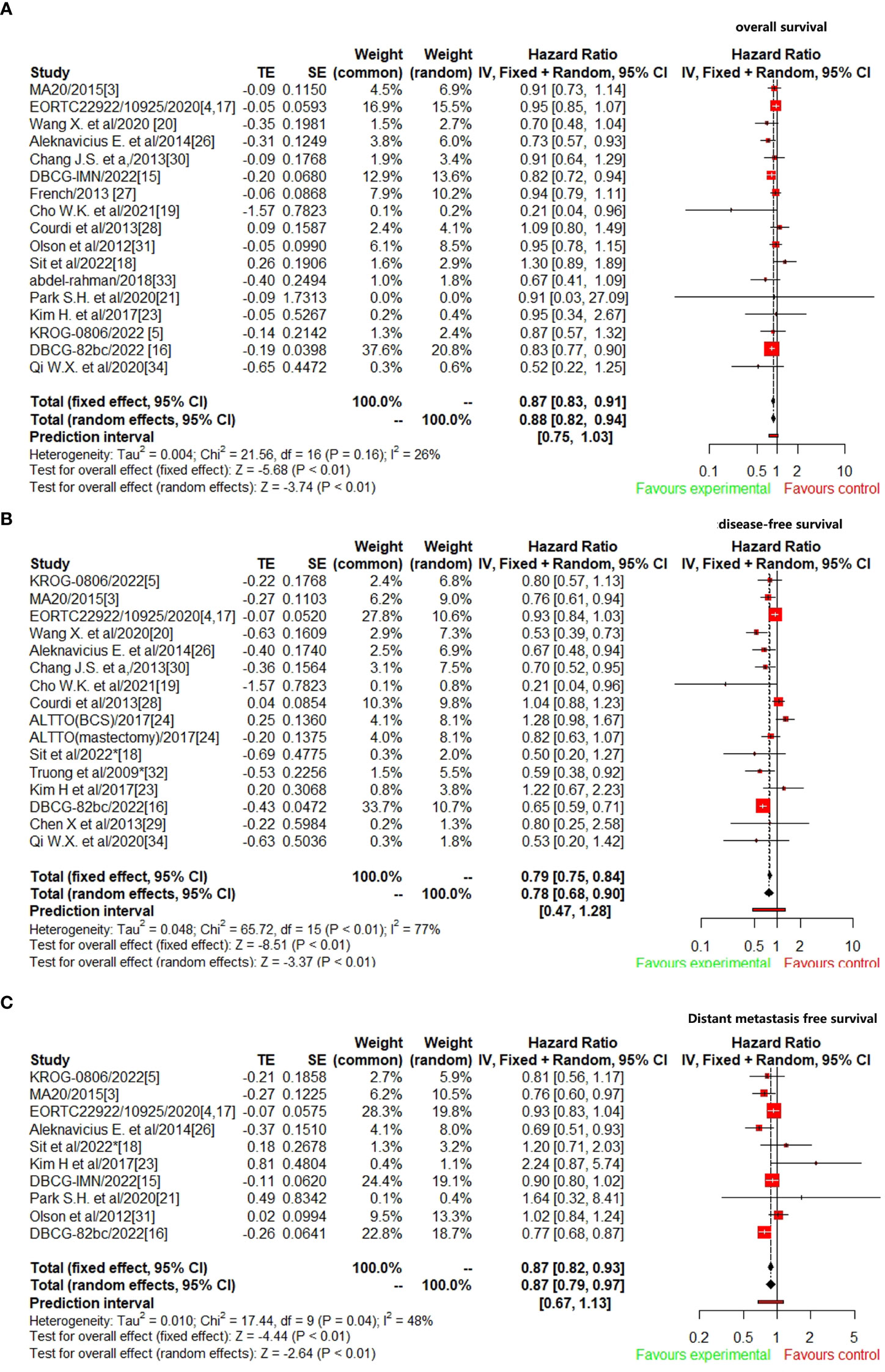
Figure 2 Comparison survival outcomes of RNI with IMNI vs. RNI without IMNI in early-stage BC. (A) OS; (B) DFS; (C) DMFS.
Sub-group analysis according to different RNI volumes
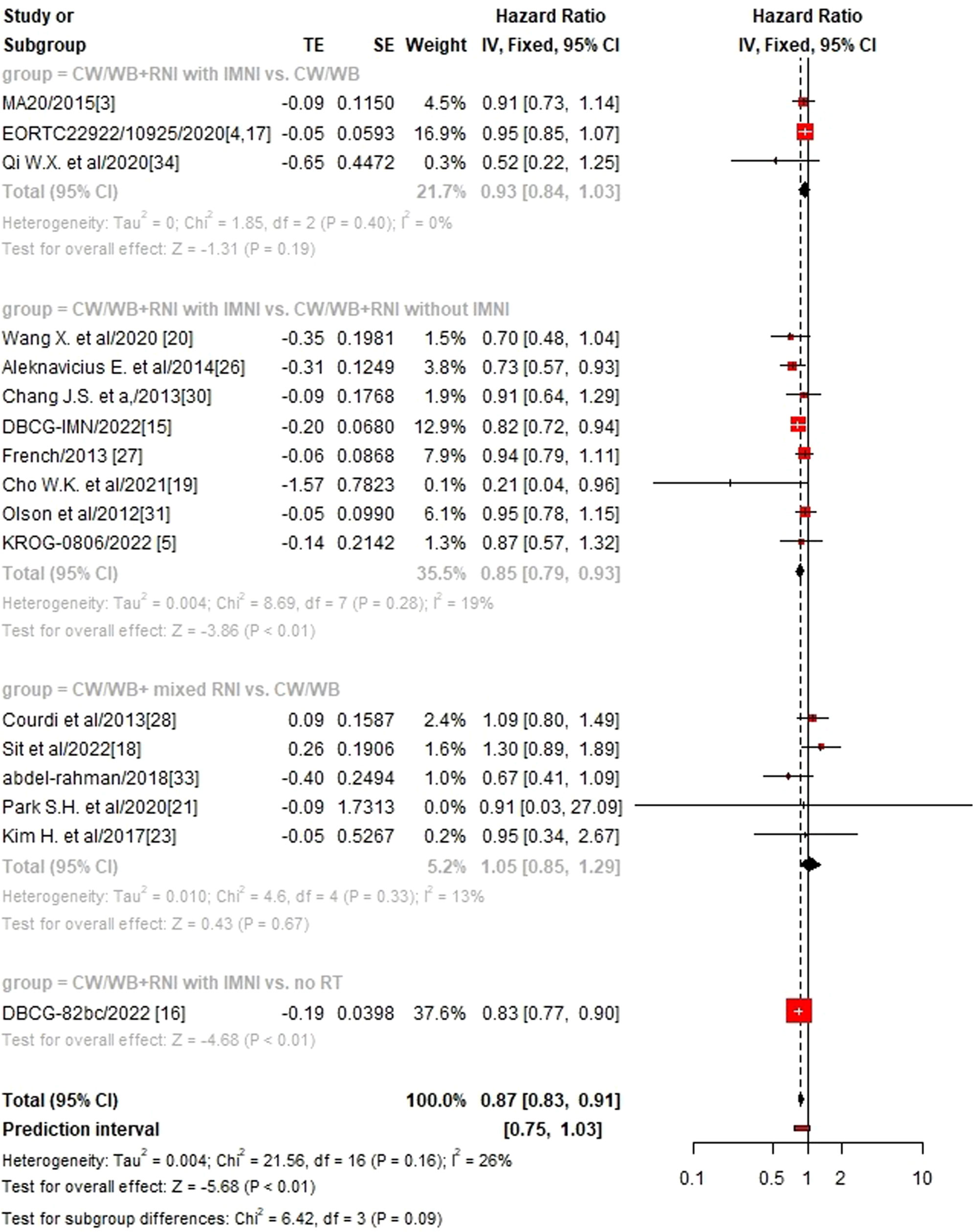
We then performed sub-group analysis according to different RNI volumes and found that CW/WB+RNI with IMNI significantly improved OS (HR 0.85, 95%CI: 0.79–0.93, p<0.01, Figure 3), DFS (HR 0.65, 95%CI: 0.56–0.77, p<0.01, Supplementary Figure 1), and DMFS (HR 0.90, 95%CI: 0.82–0.98, p=0.02, Supplementary Figure 2) when compared to CW/WB+RNI without IMNI, while no significant difference could be found between CW/WB+RNI with IMNI vs. CW/WB in terms of OS (HR 0.94, 95%CI: 0.85–1.03, p=0.19) and DFS (HR 0.93, 95%CI: 0.86–1.00, p=0.06), but not for DMFS (HR 0.90, 95%CI: 0.81–0.99, p=0.04). Similarly, CW/WB+ mixed RNI did not significantly improve the OS (HR1.05, 95%CI: 0.85–1.29, p=0.67), DFS (HR 0.94, 95%CI: 0.80–1.11, p=0.48), and DMFS (HR 1.41, 95%CI: 0.91–2.19, p=0.13) of BC patients treated with CW/WB alone.
Network meta-analysis
For quantitative synthesis within NMA, treatment approaches from eight studies were categorized into groups as follows: 1) CW/WB+RNI with IMNI-R (right-side breast cancer), 2) CW/WB+RNI with IMNI, 3) CW/WB+ RNI without IMNI, 4) CW/WB +mixed RNI, 5) SW/WB+SVC, 6) CW/WB alone, and 7) no radiotherapy (RT).
An NMA of seven different RNI radiotherapy regimens was conducted with regards to OS in BC patients (Supplementary Figure 3A). Compared to CW/WB, CW/WB+RNI with IMNI had a tendency to improve OS (HR 0.93, 95%CI: 0.84–1.03, Figure 4A), while no significant survival difference could be observed between CW/WB+RNI with IMNI-R and CW/WB (HR 0.88, 95%CI: 0.72–1.07, Figure 4A). Similarly, CW/WB+RNI without IMNI did not improve the OS when compared to CW/WB alone (HR 1.07, 95%CI: 0.93–1.23, Figure 4A). However, RT did not significantly decrease the OS when compared to CW/WB in BC patients (HR 1.13, 95%CI: 0.99–1.28). p-score for each treatment is shown in Supplementary Table 2. In the BC patients, CW/WB+RNI with IMNI-R had the highest p-score for OS followed by CW/WB+RNI with IMNI, indicating that it was a better treatment option for preventing breast cancer death based on treatment ranking according to p-score (Supplementary Table 2).
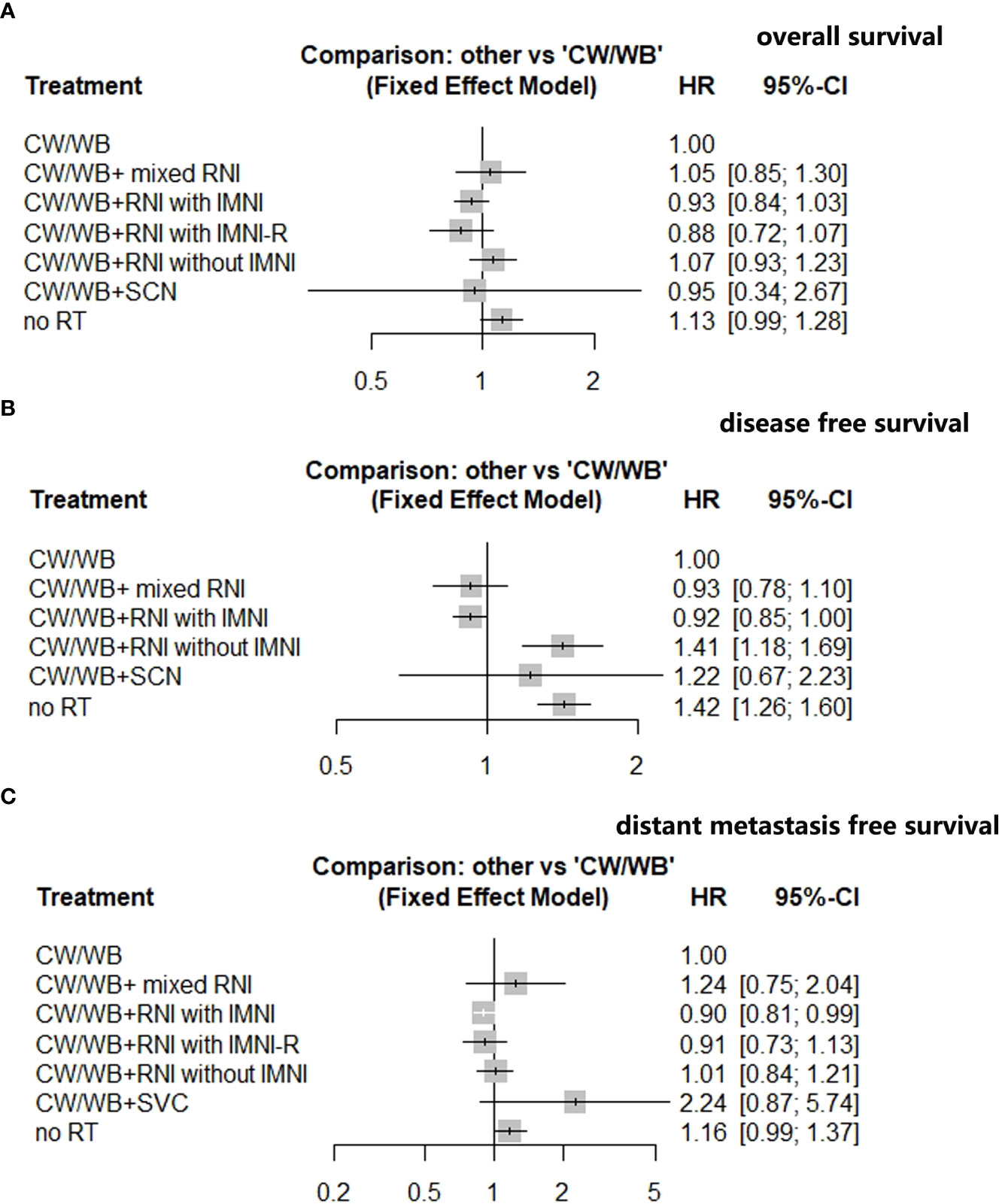
Figure 4 Network analysis of different RNI volume versus CW/WB alone early-stage BC. (A) OS; (B) DFS; (C) DMFS.
An NMA of six different maintenance therapy regimens was conducted with regards to DFS in BC patients (Supplementary Figure 3B). Compared to CW/WB, CW/WB+RNI with IMNI significantly improved DFS (HR 0.92, 95%CI: 0.85–1.00, Figure 4B), while CW/WB+RNI without IMNI (HR 1.41, 95%CI: 1.18–1.69, Figure 4B), CW/WB+ mixed RNI (0.93, 95%CI: 0.78–1.10, Figure 4B) or CW/WB+SVC (HR 1.22, 95%CI: 0.67–2.23, Figure 4B) was not significantly associated with a lower likelihood of disease progression. According to the p-score for each treatment, CW/WB+RNI with IMNI had the highest p-score (Supplementary Table 3).
An NMA of seven different RNI radiotherapy regimens was conducted with regards to DMFS in BC patients (Supplementary Figure 3C). Compared to CW/WB, CW/WB+RNI with IMNI significantly improved DMFS (HR 0.90, 95%CI: 0.81–0.99, Figure 4C), while CW/WB+RNI without IMNI-R (HR 0.91, 95%CI: 0.73–1.13, Figure 4C), CW/WB+RNI without IMNI (HR 1.01, 95%CI: 0.84–1.21), CW/WB+ mixed RNI (1.24, 95%CI: 0.75–2.04, Figure 4C), or CW/WB+SVC (HR 2.24, 95%CI: 0.87–5.74, Figure 4C) was not significantly associated with a lower likelihood of disease progression. According to the p-score for each treatment, CW/WB+RNI with IMNI had the highest p-score (p=0.8883, Supplementary Table 4). Dummy Supplementary Table 5, 6
Publication bias and sensitivity analysis
As shown in Supplementary Figure 4, there was no obvious asymmetry in modified funnel plots for OS, DFS, and DMFS, indicating the absence of significant publication bias. We also performed Egger’s tests to detect the publication bias, and no statistically significant publication bias was detected for OS (Egger’s test, p=0.74), DFS (Egger’s test, p=0.71), DMFS (Egger’s test, p=0.43). The sensitivity analysis (Supplementary Figure 5) was performed to test the stability of our findings. The results suggested that the effects of CW/WB+RNI with IMNI or mixed RNI therapy on OS, DFS, and DMFS were reliable.
Discussion
Over the past decades, primary breast tumor resection followed by adjuvant radiation to the lymphatic drainage for both early-stage and locally advanced BC had become the standard treatment strategy. However, the treatment volume of RNI significantly varied among those published studies. Thus, there remained a matter of debate on which regional nodal volume should be irradiated for BC patients in the era of effective systematic therapies (35). Prior to the present study, Budach et al. performed two meta-analyses to define the optimal RNI volume based on four prospective randomized trials, and the pooled results indicated a statistically significant improvement in overall survival for CW/WB+ comprehensive RNI when compared to CW/WB alone in stage I–III breast cancer (36, 37). In 2019, the Early Breast Cancer Trialists’ Collaborative Group conducted an updated meta-analysis to investigate the efficacy of regional nodal irradiation in early-stage BC patients according to treatment periods and found that RT to regional lymph nodes in older (1961–1978) studies increased the overall risk of death, while nodal RT in more recent (1989–2003) studies reduced breast cancer recurrence, breast cancer mortality, and overall mortality (38). Since the publication of these meta-analyses, more clinical trials had been performed in recent years, but the optimal treatment RNI strategy remained undetermined. We therefore performed this meta-analysis to assess the benefit of different RNI treatments in BC patients and compare the efficacy of different RNI regimens via network meta-analysis.
A total of 29,640 BC patients from 20 studies were included for analysis. In consistent with previous result (39), our pooled HR demonstrated that radiotherapy with IMNI in BC patients significantly improved OS, DFS, and DMFS when compared to RNI without IMNI (p<0.05). We then performed a sub-group analysis according to RNI volume and found that CW/WB+RNI with IMNI significantly improved OS (HR 0.85, p<0.01), DFS (HR 0.65, p<0.01), and DMFS (HR 0.90, p=0.02) when compared to CW/WB+RNI without IMNI. In contrast to the report of Budach et al. (36), no significant difference could be found between CW/WB+RNI with IMNI vs. CW/WB alone in terms of OS (p=0.19) and DFS (p=0.48), while a significantly decreased risk of developing distant metastasis could be observed in CW/WB+RNI with IMNI group when compared to CW/WB alone (p=0.04). Additionally, CW/WB+ mixed RNI did not improve OS, DFS, and DMFS when compared to CW/WB alone (p>0.05). We further explored the optimal RNI treatment strategy for BC patients by using network meta-analysis. Based on those published studies, CW/WB+RNI with IMNI remained a better treatment option for preventing BC progression and death, and CW/WB combined with comprehensive RNI could be recommended for BC patients after curative surgery.
IMNI would inevitably increase RT dose to the heart and lungs regardless of using modern radiation technology, which might be associated with an increased risk of developing RT-related toxicities (40). But in a large cohort study based on SEER database, Giordano et al. (41). found that the risk of death from ischemic heart disease associated with radiation for breast cancer had been substantially decreased over time. In a more recent meta-analysis of three randomized trials, Budach et al. found that IMNI was not associated with an excess of cardiac death or cardiac toxicity rate (36). Although there was a lack of formal guidelines on whether or not to treat IMN for BC patients, radiation oncologists in our institute had achieved a consensus that CW/WB+RNI with IMNI could be recommended for BC patients due to the low incidence of cardiac toxicities. Between June 2017 and August 2019, we performed a prospective randomized controlled trial to investigate the early cardiac event in pre-specified dose constraints for the heart vs. choice of radiation oncologists for breast cancer treated with postoperative intensity-modulated radiotherapy, which had been registered in ClinicalTrials.gov (NCT02942615). By 31 December 2019, 143 patients completed 1-year follow-up (77 in study group and 66 in control group). The Dmean of the heart was 374.9 ± 205.3cGy and 376.7 ± 204.7cGy in the study and control group, respectively (p=0.96). No clinical cardiac toxicity was observed. Subclinical cardiac events occurred in 29 patients in the study group and 29 in the control group (p=0.45) (42). Therefore, mean heart dose in BC treated with IMNI was low and did not increase the risk of developing early-stage cardiac toxicities. As for late cardiac toxicities, EORTC Trial 22922/10925 reported 15-year side effects after IMNI and showed that IMNI did not significantly increase the risk of developing cardiac fibrosis when compared to without IMNI group (1.1% vs. 1.9%, p=0.07) but not for any cardiac disease (9.4% vs. 11.1%, p=0.04). RT technique improvement would also impact the cardiac toxicity of RT. The Danish Breast Cancer Group performed a population-based study and found a higher risk of cardiac toxicity in left- vs. right-sided patients irradiated during the non-CT-based period, while no increased risk of coronary artery disease in left-sided versus right-sided patients was observed in the CT-based period (43). As a result, with the improvement of RT techniques, IMNI could be safely applied for BC patients, even in the left-side BC.
Another major concern was that BC was a heterogeneous disease, and the risk of developing recurrence significantly varied. For BC patients with multiple risk factors, including young age, large tumor size, lymph vascular invasion, medial/central tumor location, or high nuclear grade (44), the incidence of local regional recurrence could increase to 20%. Therefore, one size did not fit all, and not all BCs could benefit from comprehensive RNI. Based on our established clinical risk model for N1 breast cancer (45, 46), our group initiated a prospective randomized clinical trial to investigate whether a part of pN1 breast cancer patients could be safely omitted from IMNI by using a clinical-genomic model, and the trial was under recruiting(NCT04517266). The preliminary results of enrolled 75 patients showed that among clinically high-risk pN1 BC, which is defined as having at least two of the five clinical risk factors (age≤40, three positive LN(lymph node), T2 stage, grade 3, and Ki-67 index≥14%), 70% of them present with genomic high risk, and 30% present with genomic low risk, and whether those clinical high-risk but genomic low-risk BC patients could be omitted from IMNI still needs long-term follow-up of our research (47). Another potential method was performing lymphoscintigraphy by injecting radiotracer for axillary sentinel node biopsy and peritumor to identify high risk for IMN metastases. It had been reported that lymphoscintigraphy not only identifies axillary sentinel node biopsy but also depicts drainage to the internal mammary (IM) basin, present in approximately 20% of patients (48, 49). Internal mammary sentinel lymph node biopsy (IM-SLNB) was another way to clearly determine the status of IMN metastases, although routine performance of IM-SLNB in clinical axillary lymph node (ALN)-negative patients remained debated. Qiu et al. (50) conducted a prospective cohort study and found that IM-SLNB visualization rate was 71.9% among BC patients who received initial surgery and 33.1% among BC patients treated with neoadjuvant systemic therapy.
In the real-world practice, there is a severe heterogeneity issue of RNI administration. In the ALTTO trial, only a third of the pN1 patients (36.8%) received RNI, while 82.2% of patients with four or more positive lymph nodes were treated with RNI. Among patients treated with RNI, 60.9% of the patients RNI targeting only one regional node area, while only 3.9% received RNI targeting the three regional nodal areas (24). One possible explanation for this finding was that the ALTTO trial was conducted between 2007 and 2011, before the first publication of the MA.20 and EORTC 22922/10925 trials. The quality of the regional nodal plan was another issue that should caution radiation oncologists. Ling et al. performed a network to determine the compliance with regional nodal coverage, contouring quality, target coverage, and organ-at-risk dosimetric parameters and found that 18% of plans presented with unacceptable nodal contour quality and 15% of them had inadequate coverage (51). In the KROG-0806 trial, the individual case review demonstrated that overall protocol compliance, including IMNI, significantly varied, and only 59.0% of the prescribed dose was delivered to the IMNI group (52, 53).
Nevertheless, there were some limitations that needed to be concerned of. First, both prospective and retrospective studies were included in this meta-analysis. Although the quality of included studies was high, selection bias between groups could not be avoided despite the fact that we performed subgroup analysis according to RNI volumes that successfully reduced the heterogeneity to a low grade. Second, this study was conducted at the base trial level but not at the individual level. Therefore, we could not perform pooled analysis according to patient characteristics, such as nodal stage or tumor location, although it had been established that medial-located tumor is a well-known predictor for IMNI benefit. In addition, both early-stage and locally advanced BC were included in the present meta-analysis, which might be another source of heterogeneity. Finally, the present study included both old or modern systematic therapy, and advances in systematic treatment in the modern era might affect the benefit of RNI, which might be another source of heterogeneity.
Conclusion
In conclusion, the present study demonstrated that RNI with IMNI yielded a significant survival advantage for BC patients. Subgroup analysis according to RNI volumes showed that CW/WB+RNI+IMNI significantly improved DFS and DMFS when compared to CW/WB+RNI without IMNI, but not for OS. NMA found that CW/WB+RNI+IMNI was the optimum RNI treatment strategy for BC patients that reduced mortality and disease progression. Additionally, further studies evaluating the impact of RNI volume on late cardiac and lung toxicities are strongly needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
Conceptualization: JC and W-XQ. Project administration: CX and LC. Methodology: W-XQ, GC, and JC. Data curation: JC and CX. Formal analysis: W-XQ. Manuscript preparation: W-XQ, CX, GC, JC, and LC. Final approval of manuscript: all authors.
Funding
This study was supported in part by Clinical Research Plan of SHDC (grant SHDC2020CR4070 and SHDC2020CR2052B).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1081201/full#supplementary-material
Abbreviations
BC, breast cancer; RNI, regional nodal irradiation; NMA, network meta-analysis; OS, overall survival; DFS, disease-free survival; DMFS, distant-metastasis-free survival; IMNI, internal mammary node irradiation; HR, hazard ratio; CW/WB, (chest wall/whole breast); SVCs, supraclavicular lymph nodes; IMNs, internal mammary lymph nodes; SCN, supraclavicular lymph node; NOS, Newcastle–Ottawa Scale; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; BCS, breast-conservation surgery; RT, radiotherapy; MHD, mean heart dose; LN, lymph node.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Ebctcg, McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet (2014) 383:2127–35. doi: 10.1016/S0140-6736(14)60488-8
3. Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. Regional nodal irradiation in early-stage breast cancer. New Engl J Med (2015) 373:307–16. doi: 10.1056/NEJMoa1415340
4. Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. New Engl J Med (2015) 373:317–27. doi: 10.1056/NEJMoa1415369
5. Kim YB, Byun HK, Kim DY, Ahn SJ, Lee HS, Park W, et al. Effect of elective internal mammary node irradiation on disease-free survival in women with node-positive breast cancer: A randomized phase 3 clinical trial. JAMA Oncol (2022) 8:96–105. doi: 10.1001/jamaoncol.2021.6036
6. Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol (2005) 28:123–37. doi: 10.1002/gepi.20048
7. Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis (1985) 27:335–71. doi: 10.1016/S0033-0620(85)80003-7
8. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50:1088–101. doi: 10.2307/2533446
9. Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet (1998) 352:609–13. doi: 10.1016/S0140-6736(98)01085-X
10. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
11. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
12. Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using r: a review of currently available automated packages. PloS One (2014) 9:e115065. doi: 10.1371/journal.pone.0115065
13. Helali AE, Wong CHL, Choi HCW, Chan WWL, Dickson N, Siu SWK, et al. A comprehensive systematic review and network meta-analysis: the role of anti-angiogenic agents in advanced epithelial ovarian cancer. Sci Rep (2022) 12:3803. doi: 10.1038/s41598-022-07731-1
14. Moher D LA, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
15. Thorsen LBJ, Overgaard J, Matthiessen LW, Berg M, Stenbygaard L, Pedersen AN, et al. Internal mammary node irradiation in patients with node-positive early breast cancer: Fifteen-year results from the Danish breast cancer group internal mammary node study. J Clin Oncol (2022) 40:4198–206. doi: 10.1200/JCO.22.00044
16. Overgaard M, Nielsen HM, Tramm T, Hojris I, Grantzau TL, Alsner J, et al. Postmastectomy radiotherapy in high-risk breast cancer patients given adjuvant systemic therapy. a 30-year long-term report from the Danish breast cancer cooperative group DBCG 82bc trial. Radiother Oncol (2022) 170:4–13. doi: 10.1016/j.radonc.2022.03.008
17. Poortmans PM, Weltens C, Fortpied C, Kirkove C, Peignaux-Casasnovas K, Budach V, et al. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I-III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial. Lancet Oncol (2020) 21:1602–10. doi: 10.1016/S1470-2045(20)30472-1
18. Sit D, Lalani N, Chan E, Tran E, Speers C, Gondara L, et al. Association between regional nodal irradiation and breast cancer recurrence-free interval for patients with low-risk, node-positive breast cancer. Int J Radiat oncology biology physics (2022) 112:861–9. doi: 10.1016/j.ijrobp.2021.10.149
19. Cho WK, Chang JS, Park SG, Kim N, Choi DH, Kim H, et al. Internal mammary node irradiation in node-positive breast cancer treated with mastectomy and taxane-based chemotherapy. Breast (2021) 59:37–43. doi: 10.1016/j.breast.2021.05.012
20. Wang X, Luo J, Jin K, Chen X, Zhang L, Meng J, et al. Internal mammary node irradiation improves 8-year survival in breast cancer patients: results from a retrospective cohort study in real-world setting. Breast Cancer (Tokyo Japan) (2020) 27:252–60. doi: 10.1007/s12282-019-01015-9
21. Park SH, Kim JC. Regional nodal irradiation in pT1-2N1 breast cancer patients treated with breast-conserving surgery and whole breast irradiation. Radiat Oncol J (2020) 38:44–51. doi: 10.3857/roj.2019.00647
22. Kim K, Jeong Y, Shin KH, Kim JH, Ahn SD, Kim SS, et al. Impact of regional nodal irradiation for breast cancer patients with supraclavicular and/or internal mammary lymph node involvement: A multicenter, retrospective study (KROG 16-14). Cancer Res Treat Off J Korean Cancer Assoc (2019) 51:1500–8. doi: 10.4143/crt.2018.575
23. Kim H, Park W, Yu JI, Choi DH, Huh SJ, Kim YJ, et al. Prognostic impact of elective supraclavicular nodal irradiation for patients with N1 breast cancer after lumpectomy and anthracycline plus taxane-based chemotherapy (KROG 1418): A multicenter case-controlled study. Cancer Res Treat Off J Korean Cancer Assoc (2017) 49:970–80. doi: 10.4143/crt.2016.382
24. Gingras I, Holmes E, De Azambuja E, Nguyen DH, Izquierdo M, Anne Zujewski J, et al. Regional nodal irradiation after breast conserving surgery for early HER2-positive breast cancer: Results of a subanalysis from the ALTTO trial. J Natl Cancer Inst (2017) 109:djw331. doi: 10.1093/jnci/djw331
25. Thorsen LB, Offersen BV, Dano H, Berg M, Jensen I, Pedersen AN, et al. DBCG-IMN: A population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol Off J Am Soc Clin Oncol (2016) 34:314–20. doi: 10.1200/JCO.2015.63.6456
26. Aleknavicius E, Atkocius V, Kuzmickiene I, Steponaviciene R. Postmastectomy internal mammary nodal irradiation: a long-term outcome. Medicina (2014) 50:230–6. doi: 10.1016/j.medici.2014.09.010
27. Hennequin C, Bossard N, Servagi-Vernat S, Maingon P, Dubois JB, Datchary J, et al. Ten-year survival results of a randomized trial of irradiation of internal mammary nodes after mastectomy. Int J Radiat oncology biology physics (2013) 86:860–6. doi: 10.1016/j.ijrobp.2013.03.021
28. Courdi A, Chamorey E, Ferrero JM, Hannoun-Levi JM. Influence of internal mammary node irradiation on long-term outcome and contralateral breast cancer incidence in node-negative breast cancer patients. Radiother Oncol (2013) 108:259–65. doi: 10.1016/j.radonc.2013.06.028
29. Chen X, Yu X, Chen J, Yang Z, Shao Z, Zhang Z, et al. Radiotherapy can improve the disease-free survival rate in triple-negative breast cancer patients with T1-T2 disease and one to three positive lymph nodes after mastectomy. Oncologist (2013) 18:141–7. doi: 10.1634/theoncologist.2012-0233
30. Chang JS, Park W, Kim YB, Lee IJ, Keum KC, Lee CG, et al. Long-term survival outcomes following internal mammary node irradiation in stage II-III breast cancer: results of a large retrospective study with 12-year follow-up. Int J Radiat oncology biology physics (2013) 86:867–72. doi: 10.1016/j.ijrobp.2013.02.037
31. Olson RA, Woods R, Speers C, Lau J, Lo A, Truong PT, et al. Does the intent to irradiate the internal mammary nodes impact survival in women with breast cancer? a population-based analysis in British Columbia. Int J Radiat oncology biology physics (2012) 83:e35–41. doi: 10.1016/j.ijrobp.2011.11.066
32. Truong PT, Jones SO, Kader HA, Wai ES, Speers CH, Alexander AS, et al. Patients with t1 to t2 breast cancer with one to three positive nodes have higher local and regional recurrence risks compared with node-negative patients after breast-conserving surgery and whole-breast radiotherapy. Int J Radiat oncology biology physics (2009) 73:357–64. doi: 10.1016/j.ijrobp.2008.04.034
33. Abdel-Rahman O. Impact of regional nodal irradiation on the outcomes of N1 breast cancer patients referred for adjuvant treatment: A patient-level pooled analysis of 2 clinical trials. Clin Breast cancer (2018) 18:504–10. doi: 10.1016/j.clbc.2018.07.016
34. Qi WX, Cao L, Xu C, Zhao S, Chen J. Adjuvant regional nodal irradiation did not improve outcomes in T1-2N1 breast cancer after breast-conserving surgery: A propensity score matching analysis of BIG02/98 and BCIRG005 trials. Breast (2020) 49:165–70. doi: 10.1016/j.breast.2019.11.001
35. Duma MN. An update on regional nodal irradiation: Indication, target volume delineation, and radiotherapy techniques. Breast Care (2020) 15:128–35. doi: 10.1159/000507040
36. Budach W, Kammers K, Boelke E, Matuschek C. Adjuvant radiotherapy of regional lymph nodes in breast cancer - a meta-analysis of randomized trials. Radiat Oncol (2013) 8:267. doi: 10.1186/1748-717X-8-267
37. Haussmann J, Budach W, Tamaskovics B, Bolke E, Corradini S, Djiepmo-Njanang FJ, et al. Which target volume should be considered when irradiating the regional nodes in breast cancer? results of a network-meta-analysis. Radiat Oncol (2019) 14:102. doi: 10.1186/s13014-019-1280-6
38. Dodwell D, Taylor C, McGale P, Coles C, Duane F, Gray R, et al. Regional lymph node irradiation in early stage breast cancer: An EBCTCG meta-analysis of 13,000 women in 14 trials. Cancer Res (2019) 79:GS4–02. doi: 10.1158/1538-7445.SABCS18-GS4-02
39. Jia S, Liu Z, Zhang J, Zhao C, Zhu L, Kong J, et al. Can internal mammary lymph nodes irradiation bring survival benefits for breast cancer patients? a systematic review and meta-analysis of 12,705 patients in 12 studies. Radiat Oncol (2021) 16:42. doi: 10.1186/s13014-021-01772-y
40. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. New Engl J Med (2013) 368:987–98. doi: 10.1056/NEJMoa1209825
41. Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Institute (2005) 97:419–24. doi: 10.1093/jnci/dji067
42. Ou DCL, Xu C, Fang Y, Chen J. Interim analysis of a phase III study of management of cardiac toxicity in breast cancer patients treated with multidisciplinary therapy. Int J Radiat Oncol Biol Phys (2020) 108:e37. doi: 10.1016/j.ijrobp.2020.07.1069
43. Milo MLH, Thorsen LBJ, Johnsen SP, Nielsen KM, Valentin JB, Alsner J, et al. Risk of coronary artery disease after adjuvant radiotherapy in 29,662 early breast cancer patients: A population-based Danish breast cancer group study. Radiother Oncol (2021) 157:106–13. doi: 10.1016/j.radonc.2021.01.010
44. Mamtani A, Patil S, Stempel MM, Morrow M. Are there patients with T1 to T2, lymph node-negative breast cancer who are "high-risk" for locoregional disease recurrence? Cancer (2017) 123:2626–33. doi: 10.1002/cncr.30658
45. Qi WX, Cao L, Xu C, Zhao S, Chen J. Established and validated novel nomogram for predicting prognosis of post-mastectomy pN0-1 breast cancer without adjuvant radiotherapy. Cancer Manag Res (2021) 13:3517–27. doi: 10.2147/CMAR.S292233
46. Xu FF, Cao L, Xu C, Cai G, Wang SB, Qi WX, et al. Practical model to optimize the strategy of adjuvant postmastectomy radiotherapy in T1-2N1 breast cancer with modern systemic therapy. Front Oncol (2022) 12:789198. doi: 10.3389/fonc.2022.789198
47. Qi WX, Cao L, Zheng S, Xu C, Cai R, Xu H, et al. IMNI PRECISION trial protocol: a phase II, open-label, non-inferior randomized controlled trial of tailoring omission of internal mammary node irradiation for early-stage breast cancer. BMC cancer (2022) 22:1356. doi: 10.1186/s12885-022-10454-1
48. Byrd DR, Dunnwald LK, Mankoff DA, Anderson BO, Moe RE, Yeung RS, et al. Internal mammary lymph node drainage patterns in patients with breast cancer documented by breast lymphoscintigraphy. Ann Surg Oncol (2001) 8:234–40. doi: 10.1007/s10434-001-0234-y
49. Hindie E, Groheux D. Patient selection for internal mammary node irradiation: Lymphoscintigraphy can help. J Clin Oncol Off J Am Soc Clin Oncol (2022) 40:3669–70. doi: 10.1200/JCO.22.01178
50. Qiu PF, Zhao RR, Wang W, Sun X, Chen P, Liu YB, et al. Internal mammary sentinel lymph node biopsy in clinically axillary lymph node-positive breast cancer: Diagnosis and implications for patient management. Ann Surg Oncol (2020) 27:375–83. doi: 10.1245/s10434-019-07705-0
51. Ling DC, Moppins BL, Champ CE, Gorantla VC, Beriwal S. Quality of regional nodal irradiation plans in breast cancer patients across a Large network-can we translate results from randomized trials into the clinic? Pract Radiat Oncol (2021) 11:e30–e5. doi: 10.1016/j.prro.2020.06.007
52. Yoon HI, Yoon J, Chung Y, Nam CM, Cha H, Choi J, et al. Individual case review in a phase 3 randomized trial to investigate the role of internal mammary lymph node irradiation for breast cancer: Korean radiation oncology group 08-06 study. Radiother Oncol (2017) 123:15–21. doi: 10.1016/j.radonc.2017.01.017
53. Chung Y, Kim JW, Shin KH, Kim SS, Ahn SJ, Park W, et al. Dummy run of quality assurance program in a phase 3 randomized trial investigating the role of internal mammary lymph node irradiation in breast cancer patients: Korean radiation oncology group 08-06 study. Int J Radiat oncology biology physics (2015) 91:419–26. doi: 10.1016/j.ijrobp.2014.10.022
Keywords: regional irradiation volume, breast cancer, network meta-analysis, survival, systematic review
Citation: Qi W-X, Cao L, Xu C, Cai G and Chen J (2023) The optimal regional irradiation volume for breast cancer patients: A comprehensive systematic review and network meta-analysis of published studies. Front. Oncol. 13:1081201. doi: 10.3389/fonc.2023.1081201
Received: 27 October 2022; Accepted: 10 January 2023;
Published: 31 January 2023.
Edited by:
Vittorio Gebbia, University of Palermo, ItalyReviewed by:
Roberta Castriconi, San Raffaele Hospital (IRCCS), ItalyJee Suk Chang, Yonsei University, Republic of Korea
Copyright © 2023 Qi, Cao, Xu, Cai and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Xiang Qi, cWl3ZWl4aWFuZzExMTNAMTYzLmNvbQ==; Jiayi Chen, Y2hlbmppYXlpMDE4OEBhbGl5dW4uY29t
 Wei-Xiang Qi
Wei-Xiang Qi Lu Cao
Lu Cao Jiayi Chen
Jiayi Chen