- 1Immunodeficiency Centre for Wales, University Hospital of Wales, Cardiff, United Kingdom
- 2Division of Hematologic Malignancies, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 3Faculty of Medicine and Health Sciences, Ghent University Hospital, Ghent, Belgium
- 4Department of Medicine, Hematology-Oncology, Case Western Reserve University, Cleveland, OH, United States
- 5Rochester Regional Health, Rochester, NY, United States
- 6Department of Medicine, Allergy/Immunology and Rheumatology, University of Rochester, Rochester, NY, United States
- 7Department of Biomedical Sciences and Human Oncology, University of Bari Aldo Moro Medical School, Bari, Italy
- 8Department of Medicine, McGill University Health Centre, Montreal, QC, Canada
Introduction: Patients with hematological malignancies (HMs), like chronic lymphocytic leukemia (CLL), multiple myeloma (MM), and non-Hodgkin lymphoma (NHL), have a high risk of secondary immunodeficiency (SID), SID-related infections, and mortality. Here, we report the results of a systematic literature review on the potential association of various cancer regimens with infection rates, neutropenia, lymphocytopenia, or hypogammaglobulinemia, indicative of SID.
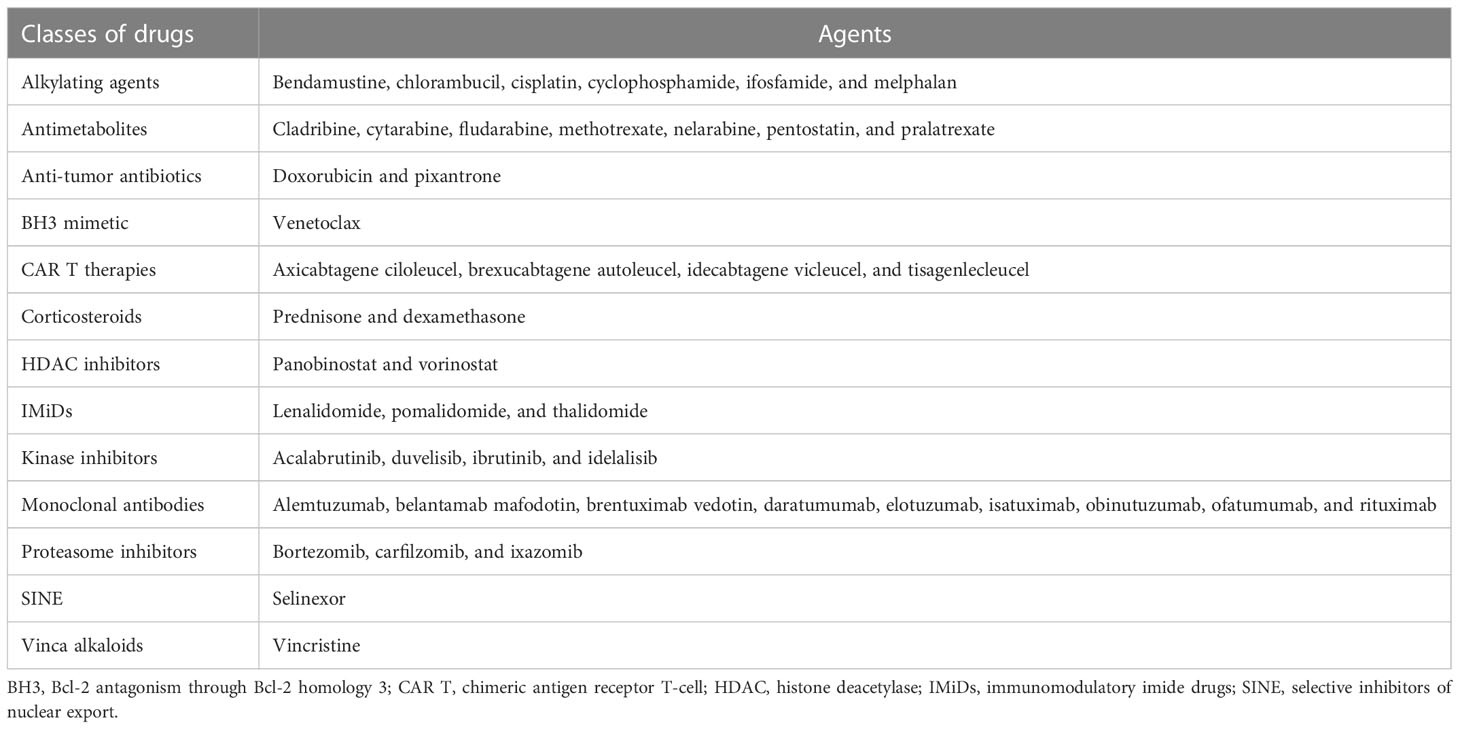
Methods: A systematic literature search was performed in 03/2022 using PubMed to search for clinical trials that mentioned in the title and/or abstract selected cancer (CLL, MM, or NHL) treatments covering 12 classes of drugs, including B-lineage monoclonal antibodies, CAR T therapies, proteasome inhibitors, kinase inhibitors, immunomodulators, antimetabolites, anti-tumor antibiotics, alkylating agents, Bcl-2 antagonists, histone deacetylase inhibitors, vinca alkaloids, and selective inhibitors of nuclear export. To be included, a publication had to report at least one of the following: percentages of patients with any grade and/or grade ≥3 infections, any grade and/or grade ≥3 neutropenia, or hypogammaglobulinemia. From the relevant publications, the percentages of patients with lymphocytopenia and specific types of infection (fungal, viral, bacterial, respiratory [upper or lower respiratory tract], bronchitis, pneumonia, urinary tract infection, skin, gastrointestinal, and sepsis) were collected.
Results: Of 89 relevant studies, 17, 38, and 34 included patients with CLL, MM, and NHL, respectively. In CLL, MM, and NHL, any grade infections were seen in 51.3%, 35.9% and 31.1% of patients, and any grade neutropenia in 36.3%, 36.4%, and 35.4% of patients, respectively. The highest proportion of patients with grade ≥3 infections across classes of drugs were: 41.0% in patients with MM treated with a B-lineage monoclonal antibody combination; and 29.9% and 38.0% of patients with CLL and NHL treated with a kinase inhibitor combination, respectively. In the limited studies, the mean percentage of patients with lymphocytopenia was 1.9%, 11.9%, and 38.6% in CLL, MM, and NHL, respectively. Two studies reported the proportion of patients with hypogammaglobulinemia: 0–15.3% in CLL and 5.9% in NHL (no studies reported hypogammaglobulinemia in MM).
Conclusion: This review highlights cancer treatments contributing to infections and neutropenia, potentially related to SID, and shows underreporting of hypogammaglobulinemia and lymphocytopenia before and during HM therapies.
1 Introduction
1.1 Secondary immunodeficiency (SID) in patients with hematological malignancies (HMs)
SID is a group of disorders in which cell-mediated immunity and/or humoral immune responses are compromised by non-inherited factors, increasing the risk of infections (1, 2). SID can be caused by several factors, including non-genetic metabolic diseases (e.g., protein-losing enteropathy, diabetes mellitus, chronic kidney disease, etc.), malnutrition, medications and malignancies, among others (2–4). Patients with HMs, including chronic lymphocytic leukemia (CLL), multiple myeloma (MM), and non-Hodgkin lymphoma (NHL), have a higher risk of SID, SID-related infections, and mortality compared with immunocompetent individuals (3, 5). Their risk of developing SID and SID-related infections is influenced by the distinct intrinsic pathophysiology of the disease, the use of and exposure time to different cancer treatments, and the presence of certain comorbidities, such as chronic lung or heart disease, kidney failure, diabetes, chronic obstructive pulmonary disease, and hypertension, of which some are caused or aggravated by cancer treatments (1, 3, 5–8). Interestingly, differences exist in both the sites and pathogen spectrum associated with certain HMs and their treatments (9–13), which might also be different from those observed in primary immunodeficiency (PID) (14). Additionally, there is a growing body of evidence that suggests that PID-related genes might influence the development of certain HMs and the likelihood of SID development in cohorts of patients with HMs (15–19).
1.2 Agents contributing to SID development in patients with HMs
Various agents used to treat HMs have been reported to increase the risk of infection due to their mode of action or as associated adverse effects on the immune system that are not clearly related to the pharmacologic activity of the molecule (2, 5, 8). These agents can affect the innate and/or adaptive immune systems in different ways, depending on which component they target (e.g., neutrophils, dendritic cells [DC], granulocytes, monocytes, and macrophages, which regulate the innate immune response; antibodies, B and T cells, which regulate the adaptive immune response; natural killer cells [NK], which are involved in both the innate and adaptive immune responses) (20, 21).
Anti-cancer monoclonal antibodies can be detrimental to both the innate and the adaptive immune systems based on the antigens they target. For instance, anti-CD20 antibodies primarily induce B-cell depletion, since CD20 is expressed by B cells only. However, since CD52 is expressed by T cells, B cells, granulocytes, monocytes, macrophages, NK cells, and DC, monoclonal antibodies directed against CD52 will impact both the innate and adaptive immune systems (22, 23). In addition, monoclonal antibodies can lead to infections, neutropenia, and sometimes cause a prolonged delay of functional recovery of the targeted cell population (24–28). In a similar way, the effects of chimeric antigen receptor T-cell (CAR T) therapies on the immune systems are influenced by which antigens the T cells are engineered to target; but can also lead to other adverse events related directly to its mode of action (e.g., cytokine release syndrome and hypogammaglobulinemia) and other adverse effects considered ‘on-target off-tumor’, like infections, neutropenia, and fatigue (22, 24, 29).
Proteasome inhibitors can induce neutropenia, reduce the number of T cells, NK cells and DC, alter NK-cell and CD8+ T-cell function, and cytokine production, therefore affecting both innate and adaptive processes (1, 30). Several kinases are involved in both the proliferation, activation, and survival of malignant cells, as well as the regulation of signaling pathways of immune cells (e.g., granulocytes, monocytes, DC, and NK cells for the regulation of the innate immune response; antibody production, T and B cells for the regulation of the adaptive immune response) (31).
Kinase inhibitors have drastically helped manage HMs; however, they can compromise the correct functioning of different immune cells, leading to infections and neutropenia (24, 31). For instance, ibrutinib inhibits the Bruton’s tyrosine kinase (BTK), which regulates granulocyte and monocyte function, DC maturation and activation, and B-cell development (31–33).
The precise mode of action of immunomodulatory imide drugs (IMiDs) remains unclear and current hypotheses are mainly based on in vitro studies. The immune modulation of IMiDs has been linked to both the innate and adaptive immune responses, including CD4+ and CD8+ T-cell co-stimulation, NK-cell activation, regulatory T-cell (Treg) suppression, cytokine production, neutropenia, and increased antibody-dependent cellular cytotoxicity (1, 34). Finally, several drugs with a systemic mode of action not directly linked with the immune system have been used to treat patients with HMs and have adverse events associated with immune system dysfunction. For instance, cytotoxic conventional chemotherapeutics have been shown to affect both the innate and adaptive immune systems by targeting DC, Treg, NK cells, cytokine production, and neutrophil and macrophage activity (35). Therefore, physicians need to be aware of the likely immunodeficiency resulting from the combined use of these agents, which affect the correct functioning of multiple immune cell types.
1.3 Secondary antibody deficiency (SAD), neutropenia, lymphocytopenia, and hypogammaglobulinemia
Various sub-types of SID have been described based on the components of both the innate and adaptive immune systems that are missing and/or are impaired/malfunctioning (4). For instance, neutropenia, loss of skin and mucosal barrier function, as well as reduced phagocytosis and cytotoxicity are examples of SID related to the innate immune response (4). On the other hand, compromised antibody function and production, and impaired T cells are examples of SID related to the adaptive immune response (4). In an increasing number of cases, defects in T, B, and NK cells may be present at the same time resulting in a combined immunodeficiency (CID) (15).
In this systematic literature review, we will focus on SAD, neutropenia, and lymphocytopenia or diminished lymphocyte function. SAD is defined as a reduction in serum immunoglobulin (Ig) concentration and/or diminished Ig function/quality (3), with hypogammaglobulinemia specifically referring to the aspect of reduction in serum Ig concentration rather than loss of functionality (36). Several cut-offs for hypogammaglobulinemia are used in the literature, suggesting a potential lack of consistency across studies (37–40). The authors agree with the recent expert consensus review published in Blood Reviews, where mild (4–6 g/L) and severe (<4 g/L) definitions of hypogammaglobulinaemia are suggested (41).
Neutropenia is a reduction in the absolute number of neutrophils circulating in the blood, graded per the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 as grade 1, less than the lower limit of normal–1,500 per mm3; grade 2, 1,499–1,000 per mm3; grade 3, 999–500 per mm3; grade 4, <500 per mm3 (42, 43). Lymphocytopenia is a reduction in the total lymphocyte count (i.e., T cells, B cells, and NK cells) graded per NCI-CTAE, version 5.0 as grade 1, less than the lower limit of normal–800 per mm3; grade 2, 799–500 per mm3; grade 3, 499–200 per mm3; grade 4, <200 per mm3 or a decreased function of these cells (42–44). However, different institutions may use slightly different reference ranges to determine grading. Lymphocytopenia might not be an ideal marker of a dysfunctional immune system in patients with CLL due to lymphocytosis; however, it could be useful in patients with NHL and MM to define the risk of infections (44–46).
1.4 Unmet needs
Epidemiological data on the prevalence of infections and infection-related mortality in patients with HMs suggest infections may account for up to 50% of deaths in CLL, and up to 22% and 33% of deaths in MM and NHL, respectively (7, 47–49). However, it is difficult to confirm whether these infections are linked to hypogammaglobulinemia, impacts on other immune components, comorbidities, or a combination thereof. Furthermore, data are lacking regarding differences in rates of hypogammaglobulinemia and hypogammaglobulinemia-related infections across HMs and across classes of drugs, rates of lymphocytopenia and related infections, and types of infections across HMs. The lack of data may result in a lesser awareness of the issue of hypogammaglobulinemia and infections within this population and therefore a lower uptake in assessment and management strategies for SID in HMs (Table 1).
1.5 Scope of the systematic literature review
In this systematic literature review, we aim to provide insights into the cancer agents used to treat HMs that are associated with SID, including differences in incidence of SID and infections among patients undergoing systemic treatment for CLL, MM, and NHL.
2 Methods
This review is reported in accordance with PRISMA guidelines on reporting reviews of the literature. On March 16th, 2022, a systematic literature search was performed from the PubMed database, searching for studies that mentioned in the title and/or abstract the following categories of drugs (licensed to treat CLL, MM, or NHL in the EU and US) divided per class of drug (Table 2): monoclonal antibodies, CAR T therapies, proteasome inhibitors, kinase inhibitors, IMiDs, corticosteroids, antimetabolites, anti-tumor antibiotics, alkylating agents, Bcl-2 antagonism through Bcl-2 homology 3 (BH3) mimetic, histone deacetylase (HDAC) inhibitors, vinca alkaloids, or selective inhibitors of nuclear export (SINE). In addition, the search strings included the MeSH terms for three types of HMs that are more indolent than others, in which SID is known to be a current unresolved challenge, and for which sufficient studies were expected to be found in order to carry out the analysis: CLL, or MM, or NHL. Finally, the following studies were included: interventional, or observational, or retrospective, or cohort, or meta-analysis, or prospective, or database, or multicenter, or case-control. Further inclusion criteria were applied to identify articles written in English, including humans, labeled as clinical trials in PubMed, and published between 2011 and 2022. Based on agreement among the authors, this period reflects the rapid evolution of the treatment landscape over the last decade.
This initial search resulted in 738 publications, which were then further refined to include phase III, phase IV and observational studies only, excluding phase I and phase II studies to avoid considering doses or settings that might not reflect the approved labels and are more likely to have fewer patients enrolled compared with phase III and phase IV studies. We obtained 243 publications in total (Supplementary Figure 1) that were then screened for relevance by type of HM, drug regimen, number of patients, year of publication, and class of drug. The screening was performed in parallel to minimize the risk of bias. Double counting was avoided by using the numerical identifier unique to each article and the Excel functionality called ‘distinct count’. In order to be included in this systematic literature review, a publication had to report at least one of the following details related to adverse events (defined per the CTCAE): percentages of patients with any grade or grade ≥3 infections; percentages of patients with any grade or grade ≥3 neutropenia; and percentages of patients with hypogammaglobulinemia. These types of infections were selected as they were the most frequently reported and comparable across all studies. In addition, studies that reported grade 1 and/or grade 2 adverse events only were excluded because of incompatibility with the any grade or grade ≥3 events criteria used in our paper.
Of the 243 studies evaluated, 89 were considered relevant. From the relevant publications, the percentages of patients with lymphocytopenia (composition of lymphocytopenia was not specified) and specific types of infection (fungal, viral, bacterial, lower respiratory tract infection [LRTI], upper respiratory tract infection [URTI], sinusitis, nasopharyngitis, respiratory, bronchitis, pneumonia, urinary tract infection [UTI], skin, gastrointestinal [GI], Candida, and sepsis) were collected if available. Of note, not all studies reported values for both any grade and grade ≥3 events; this has led to the situation where in some categories, individual studies only reporting grade ≥3 results reported higher levels of grade ≥3 events than other studies did for any grade events, leading to the average of grade ≥3 events being higher than the average for any grade events. These instances are highlighted in the analysis for clarity.
2.1 Types of infection analyses
Further analyses were performed on sinopulmonary bacterial infections and the types of infections that were most reported in the studies evaluated as part of the systematic literature review. The mean percentage of patients with the following types of infections were collected for these analyses: fungal, viral, bacterial, bacteremia, staphylococcal bacteremia, varicella-zoster virus (VZV) reactivation, LRTI, URTI, sinusitis, nasopharyngitis, respiratory, bronchitis, pneumonia, lung, UTI, skin, GI, herpes simplex virus, Candida only, and sepsis. Due to some of these descriptors overlapping (e.g., respiratory, lung, LRTI, and URTI), we categorized herpes simplex virus and VZV reactivation within the herpes group viral subtype; sinusitis and nasopharyngitis within the URTI subtype; bacteremia and staphylococcal bacteremia within the bacterial subtype; and lung with the respiratory subtype. Sinopulmonary bacterial infections were calculated by including LRTI, URTI, sinusitis, nasopharyngitis, bronchitis, and/or pneumonia.
3 Results
3.1 Infection and neutropenia rates in patients with CLL, MM, and NHL
Of the 89 relevant publications, 17 included patients with CLL (50–66), 38 with MM (67–99), and 34 with NHL (100–134) (Table 3). The mean proportion of patients who had any grade or grade ≥3 infections was 51.3% and 19.8% in CLL, 35.9% and 16.3% in MM, and 31.1% and 11.3% in NHL, respectively (Table 3). The mean percentage of patients who had any grade neutropenia was 36.3% in CLL, 36.4% in MM, and 35.4% in NHL. The mean percentage of patients with grade ≥3 neutropenia was 29.8% in patients with CLL, 23.2% in patients with MM, and 38.7% in patients with NHL.
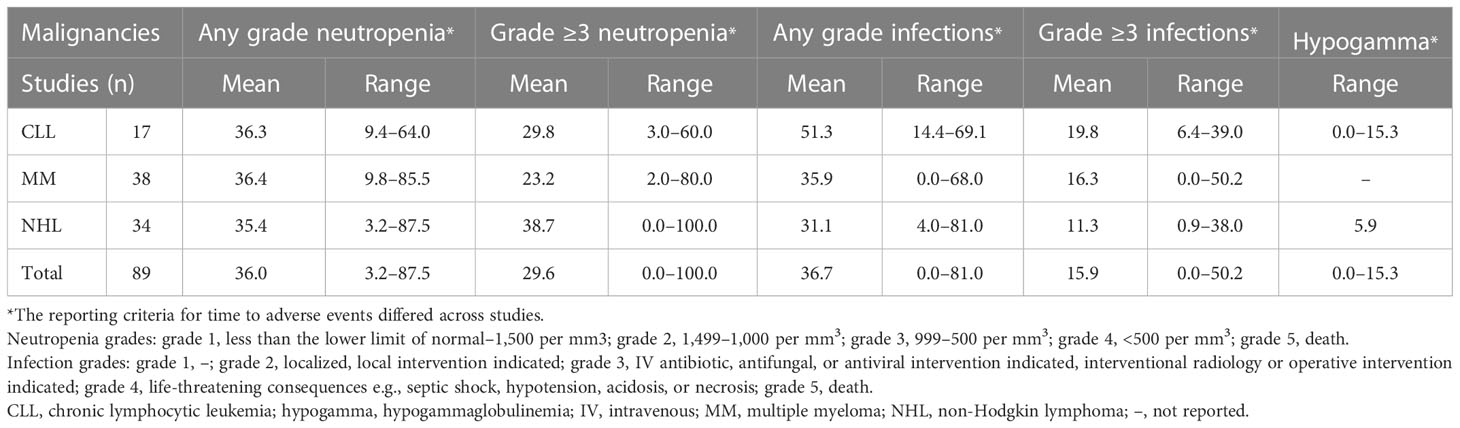
Table 3 Percentages of patients with CLL, MM, and NHL who had infections (any grade and grade ≥3), neutropenia (any grade or grade ≥3), or hypogammaglobulinemia.
In addition, rates of any grade and grade ≥3 infections, neutropenia, and hypogammaglobulinemia were divided into two timeframe groups to reflect changes in the treatment landscape, 2011–2016 and 2017–2022 (Supplementary Table 1). The rates of grade ≥3 infections were higher in the 2017–2022 group versus the 2011–2016 group across CLL, MM, and NHL.
The high variability across studies resulted in extremely wide ranges of neutropenia and infection rates. For this reason, box and whisker plots (Figure 1) were created to locate each percentage of patients within the ranges. As shown in the box and whisker plots, patients with CLL seem to be more susceptible to any grade and grade ≥3 infections than patients with MM and NHL.
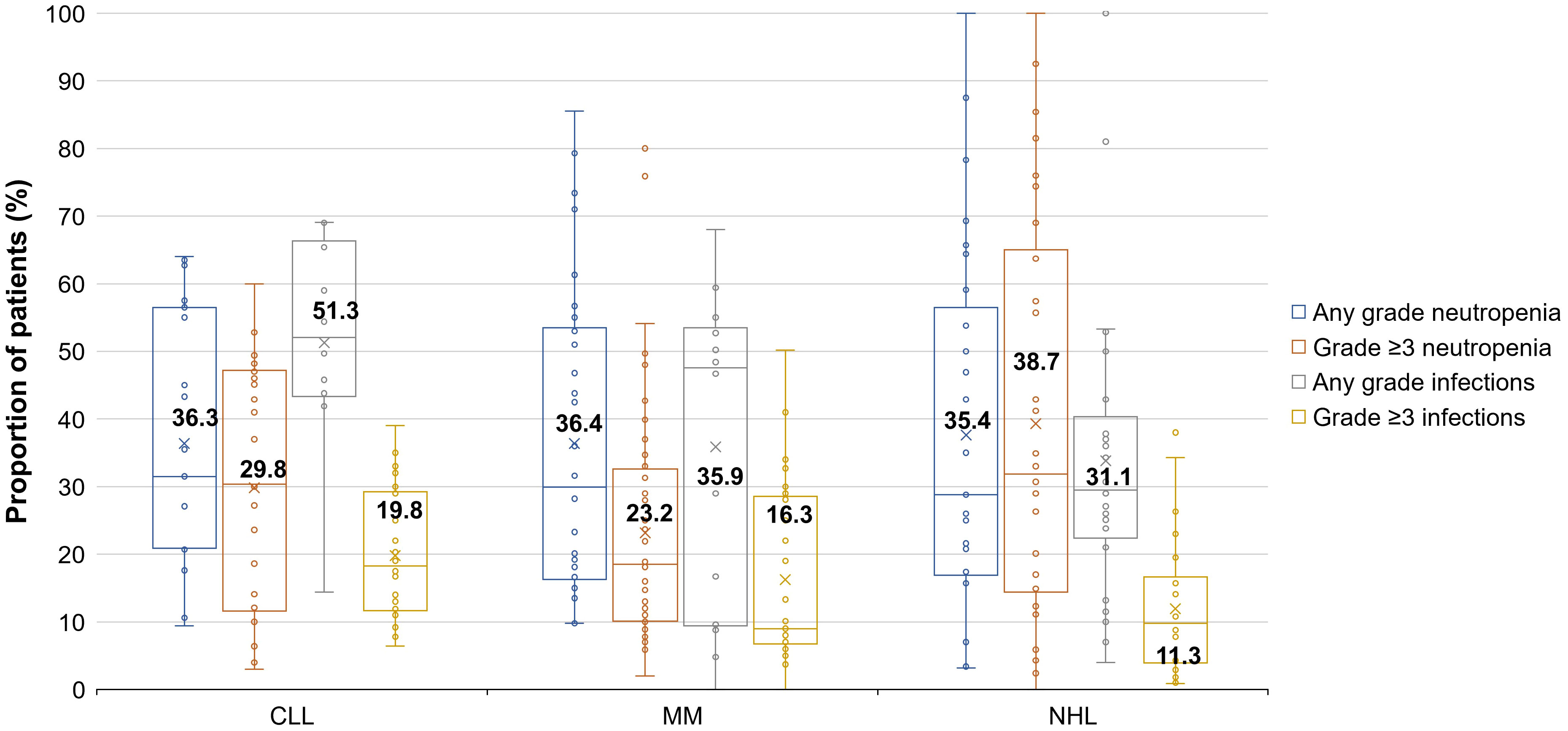
Figure 1 Proportions of patients with CLL, MM, and NHL who had infections (any grade and grade ≥3) or neutropenia (any grade or grade ≥3). Each box displays data distribution through their quartile (i.e., upper quartile, median, and lower quartile), with the bars representing the variability outside the upper and lower quartile (i.e., upper extreme and lower extreme). A dot outside the bars represents an outlier. The x symbols and corresponding data label represent the mean values for each data set. Not all studies reported values for both any grade and grade ≥3 events; this has led to the situation where in some categories, individual studies reported higher levels of grade ≥3 events than other studies did for any grade events, leading to the average of grade ≥3 events being higher than the average for any grade events Neutropenia grades: grade 1, less than the lower limit of normal–1,500 per mm3; grade 2, 1,499–1,000 per mm3; grade 3, 999–500 per mm3; grade 4, <500 per mm3; grade 5, death. Infection grades: grade 1, –; grade 2, localized, local intervention indicated; grade 3, IV antibiotic, antifungal, or antiviral intervention indicated, interventional radiology or operative intervention indicated; grade 4, life-threatening consequences e.g., septic shock, hypotension, acidosis, or necrosis; grade 5, death. CLL, chronic lymphocytic leukemia; IV, intravenous; MM, multiple myeloma; NHL, non-Hodgkin lymphoma.
3.2 Drug class-related analyses
Drug class-related analyses were performed and included all studies where a B-lineage monoclonal antibody, a proteasome inhibitor, a kinase inhibitor, or immunomodulatory drugs were used either as monotherapy or in combination with different classes of drugs as a doublet or triplet regimen (Table 4). The sum of the number of studies for monotherapy and doublet/triplet regimens in Table 4 may be higher than the total number of studies reported in Table 3 as some studies may have both monotherapy and doublet/triplet arms, therefore may have been counted twice. Only one study on the use of CAR T therapies in patients with NHL resulted from the systematic literature review; the proportion of patients with any grade infection was 29.5% (104).
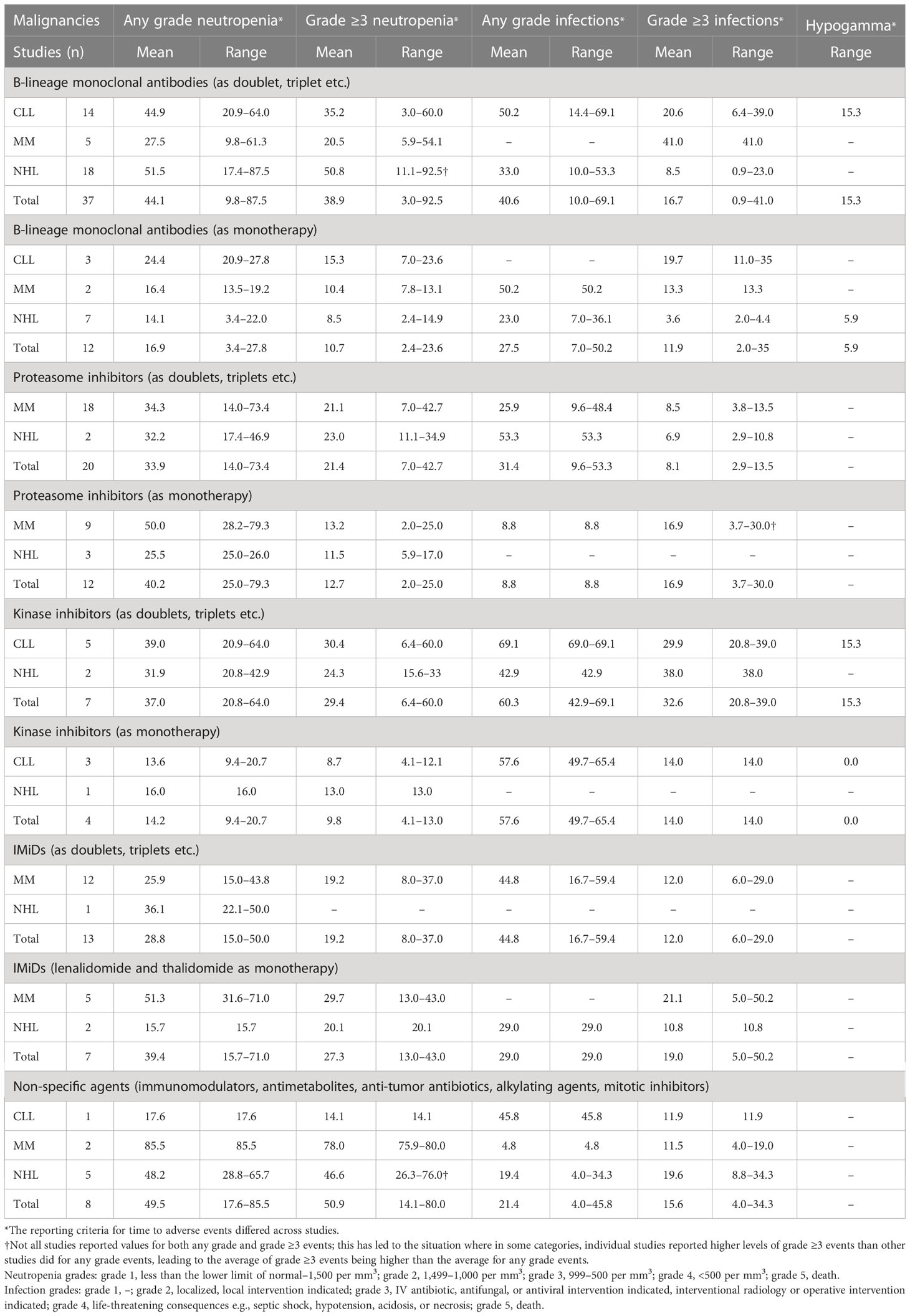
Table 4 Percentages of patients with CLL, MM, and NHL treated with different classes of drugs as monotherapy or in combination with other therapies who had infections (any grade and grade ≥3), neutropenia (any grade or grade ≥3), or hypogammaglobulinemia.
3.2.1 B-lineage monoclonal antibodies
B-lineage monoclonal antibodies (anti-CD20: rituximab, ofatumumab, and obinutuzumab; anti-CD38: isatuximab and daratumumab; anti-CD30: brentuximab vedotin; anti-CD52: alemtuzumab; anti-CD269: belantamab mafodotin; or anti-CD319: elotuzumab) were used in three studies in CLL (54, 59, 66), two in MM (68, 91), and seven in NHL (110, 113, 122, 124, 127, 128, 134) as monotherapy. In addition, 14 studies in CLL (50–58, 61–65), five studies in MM (87, 90, 92, 97, 99), and 18 studies in NHL (105–107, 111, 112, 114, 115, 117–121, 124, 127, 130–133) reported use of B-lineage monoclonal antibodies in combination with other agents (Table 4).
The mean proportion of patients treated with a B-lineage monoclonal antibody as monotherapy who had any grade infections was 50.2% in MM and 23.0% in NHL. In patients with CLL, no publications reported the rate of any grade infections when a B-lineage monoclonal antibody was used as monotherapy. Grade ≥3 infections were similar in patients with CLL and NHL regardless of using a B-lineage monoclonal antibody as monotherapy (19.7% in CLL and 3.6% in NHL) or in combination with other agents (20.6% in CLL and 8.5% in NHL); however, grade ≥3 infections were numerically lower in patients with MM treated with a B-lineage monoclonal antibody as monotherapy (13.3%) compared with patients treated with doublet/triplet regimen that included a B-lineage monoclonal antibody (41.0%; Table 4). The mean proportion of patients who reported any grade and grade ≥3 neutropenia was often numerically lower in patients treated with a B-lineage monoclonal antibody as monotherapy compared with patients treated with doublet/triplet regimen in patients with CLL, MM, and NHL (Table 4).
3.2.2 Proteasome inhibitors
Proteasome inhibitors used as monotherapy were reported in nine studies in MM (69, 70, 80, 81, 83, 94, 95, 98, 135) and three in NHL (109, 123, 126). In addition, 18 studies in MM (71, 76–78, 82, 84–87, 89, 90, 93, 96–99, 136) and two studies in NHL (107, 124) reported the use in combination with other agents (Table 4). No data were reported on the use of proteasome in patients with CLL. Data on infections were not reported in patients treated with proteasome inhibitor monotherapy in patients with NHL. In patients with MM, any grade infections were numerically lower in patients treated with a proteasome inhibitor as monotherapy compared with doublet/triplet regimen; however, grade ≥3 infections were numerically higher in patients who received mono versus combination therapy (Table 4). The mean proportion of patients with grade ≥3 neutropenia was lower in patients treated with proteasome inhibitor monotherapy compared with patients treated with doublet/triplet regimen (Table 4). However, fewer studies reported the use of a proteasome inhibitor as monotherapy compared with combination therapy, which might have skewed the results.
3.2.3 Kinase inhibitors
Kinase inhibitors were reported in three studies in CLL (51, 60, 62) and one in NHL (123) when used as monotherapy, and in five studies in CLL (50, 51, 56, 61, 62) and two studies in NHL (100, 127) in combination with other therapies. The mean proportion of patients treated with a kinase inhibitor as monotherapy who had any grade and grade ≥3 infections was 57.6% and 14.0% in CLL, respectively. Data on any grade and grade ≥3 infections were not reported in patients with MM or NHL treated with a kinase inhibitor. The mean proportion of patients treated with a kinase inhibitor in combination with other therapies who had any grade and grade ≥3 infections was 69.1% and 29.9% in CLL, and 42.9% and 38.0% in NHL, respectively. The mean proportion of patients with any grade/grade ≥3 infections and neutropenia was numerically lower in patients with CLL treated with a kinase inhibitor as monotherapy versus combination therapy (any grade and grade ≥3 infections: 57.6% and 14.0% versus 69.1% and 29.9%, respectively; any grade and grade ≥3 neutropenia: 13.6% and 8.7% versus 39.0% and 30.4%, respectively, Table 4).
3.2.4 IMiDs
IMiDs were used as monotherapy in five studies in MM (69, 73, 75, 79, 135) and two in NHL (108, 125). In addition, IMiDs were used in combination with other therapies in 12 studies in MM (67, 71–74, 77, 79, 84, 89, 92, 136, 137) and one study in NHL (111). No studies reported data on IMiDs in CLL. In patients with MM treated with monotherapy, only grade ≥3 infections were reported, and the mean rate was 21.1%. The mean proportion of patients treated with monotherapy who had any grade and grade ≥3 neutropenia was 51.3% and 29.7% in MM, respectively. In patients with NHL, the mean proportion of patients with any grade and grade ≥3 infections was 29.0% and 10.8%, respectively, and the mean proportion of patients who had any grade and grade ≥3 neutropenia was 15.7% and 20.1%, respectively. When used as monotherapy in patients with MM, IMiDs led to a numerically higher rate of grade ≥3 infections compared with IMiDs used in combination with other therapies (Table 4).
3.2.5 Non-specific agents
Additional analyses for non-specific agents were performed and included only corticosteroids, antimetabolites, anti-tumor antibiotics, alkylating agents, and mitotic inhibitors regardless of whether these were monotherapy or combination regimen (Table 4). These analyses did not include B-lineage monoclonal antibodies, tyrosine kinase inhibitors, proteasome inhibitors, or IMiDs. Non-specific agents were used in eight studies, one in CLL (65), two in MM (79, 82), and five in NHL (102, 115, 116, 119, 129). In CLL, the proportion of patients with any grade and grade ≥3 infections was 45.8% and 11.9%, respectively. The mean percentage for any grade and grade ≥3 infections was 4.8% and 11.5% in patients with MM, respectively (this is due to individual studies reporting only grade ≥3 results that were higher than other studies reported for any grade events), and 19.4% and 8.8% in patients with NHL, respectively. The mean proportion of patients who had any grade and grade ≥3 neutropenia are shown in Table 4.
3.3 Specific drug analyses
Analyses were performed to estimate the ranges of patients with infections, neutropenia or hypogammaglobulinemia associated with specific drug use (Table 5). These analyses included the use of drugs as monotherapy or in combination with other agents. Only the drugs with the highest number of studies in each class of drug were selected for these analyses.
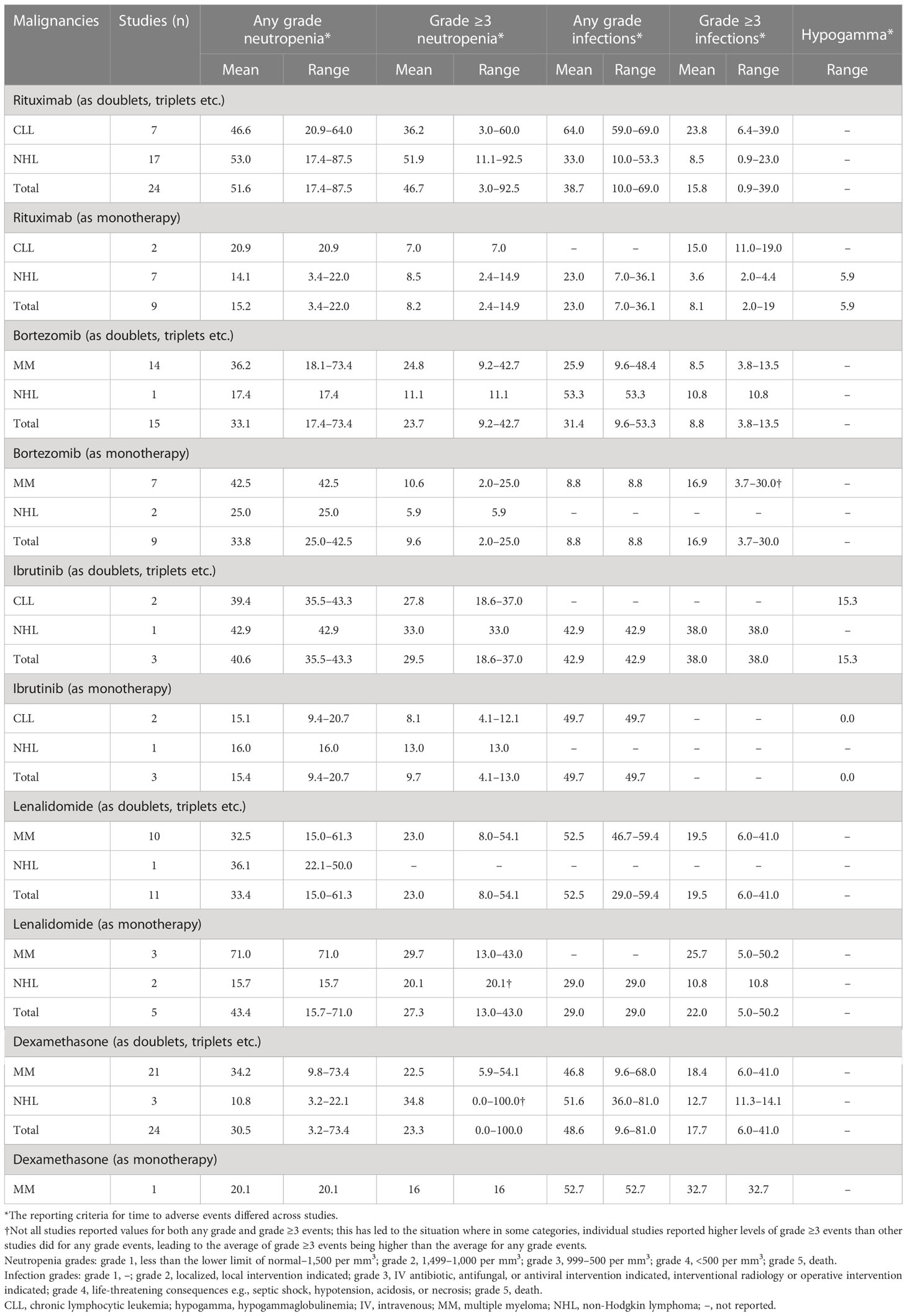
Table 5 Percentages of patients with CLL, MM, and NHL treated with rituximab, bortezomib, ibrutinib, lenalidomide, or dexamethasone as monotherapy or in combination with other therapies who had infections (any grade and grade ≥3), neutropenia (any grade or grade ≥3), or hypogammaglobulinemia.
3.3.1 Rituximab
In the nine studies that evaluated the anti-CD20 agent rituximab as monotherapy [two in CLL (54, 66) and seven in NHL (110, 113, 115, 122, 124, 127, 128)], the mean percentage for any grade and grade ≥3 infections was 23% and 3.6% in patients with NHL, respectively (Table 5). Only grade ≥3 infections and neutropenia were reported in patients with CLL, and the rates were 15.0% and 7.0%, respectively. The mean percentage for any grade and grade ≥3 neutropenia was 14.1% and 8.5% in patients with NHL, respectively.
Twenty-four studies evaluated rituximab [seven in CLL (50, 53, 54, 56–58, 63) and 17 in NHL (105–107, 111, 112, 114, 115, 117, 118, 120, 121, 124, 127, 130–133)] in combination with other therapies (Table 5).When rituximab was used as monotherapy, the rates of any grade and ≥3 infections and neutropenia were numerically lower across CLL, MM, and NHL compared with rituximab used in combination with other therapies (Table 5).
3.3.2 Bortezomib
Bortezomib was evaluated in nine studies as monotherapy [seven in MM (69, 81, 83, 94, 95, 98, 135) and two in NHL (109, 126)] and in 15 studies (14 in MM (71, 74, 76–78, 82, 84–86, 89, 90, 96, 98, 136) and one in NHL) in combination with other therapies. No studies reported the use of bortezomib in patients with CLL. When used as monotherapy in patients with MM, bortezomib led to a higher rate of grade ≥3 infections compared with its use in combination with other therapies (Table 5).
3.3.3 Ibrutinib
Three studies evaluated the use of ibrutinib as monotherapy, two in CLL (60, 62) and one in NHL (123), and three studies in combination with other therapies [two in CLL (61, 62) and one in NHL (100)]. In patients treated with ibrutinib monotherapy, only the mean percentage for any grade infections was reported and only in patients with CLL (49.7%). When used in combination with other therapies, only the mean percentage for any grade and grade ≥3 infections was reported in patients with NHL, and the rates were 42.9% and 38.0%, respectively (Table 5).
Both any grade and grade ≥3 neutropenia were numerically lower in both patients with CLL and NHL when treated with ibrutinib monotherapy compared with ibrutinib included in doublet/triplet regimen (Table 5).
3.3.4 Lenalidomide
Lenalidomide was used as monotherapy in five studies [three in MM (69, 75, 79) and two in NHL (108, 125)], and in combination with other therapies in 11 studies [10 in MM (67, 71–74, 77, 79, 92, 93, 137) and one in NHL (111)]. When used as monotherapy, the mean percentage for grade ≥3 infections was 25.7% in patients with MM, and the mean percentage for any grade and grade ≥3 infections was 29% and 10.8% in patients with NHL, respectively. Data on any grade infections were not reported in patients with MM treated with lenalidomide monotherapy (Table 5). The mean percentage for any grade and grade ≥3 neutropenia was 71% and 29.7% in patients with MM, respectively, and 15.7% and 20.1% in patients with NHL, respectively. Data for combination with other therapies, are shown in Table 5.
3.3.5 Dexamethasone
The use of dexamethasone as monotherapy was reported in only one MM study (88). Combination with other therapies was reported in 24 studies, 21 in MM (67, 71–74, 77–79, 82, 84, 86–88, 92, 93, 96, 97, 99, 136–138) and three in NHL (101, 103, 111).
For combination regimens, in which dexamethasone was used with a diverse range of agents, the mean percentage for any grade and grade ≥3 infections was 46.8% and 18.4% in patients with MM, and 51.6% and 12.7% in patients with NHL, respectively. The mean percentage for any grade and grade ≥3 neutropenia was 34.2% and 22.5% in patients with MM, and 10.8% and 34.8% in patients with NHL, respectively.
3.4 Infection and neutropenia rates in patients receiving regimen combinations commonly used in clinical practice
The drugs with the highest number of studies in each class of drug were selected for the drug specific analyses. However, in clinical practice, certain specific drug combinations are more commonly used than others, such as those recommended by the European Society for Medical Oncology (ESMO) (139–141).
When assessing these more commonly used combinations, seven studies reported the use of rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in patients with NHL (106, 107, 114, 120, 130, 131, 133). In this population, the mean percentage for any grade and grade ≥3 infections was 34.9% and 11.3%, respectively; and the mean percentage for any grade and grade ≥3 neutropenia was 67.1% and 64.5%, respectively.
In patients with CLL, the use of chlorambucil in combination with obinutuzumab was reported in five studies (G-Clb); fludarabine, cyclophosphamide, and rituximab (FCR) in three studies; and bendamustine plus rituximab in four studies. None of the selected studies reported data on the use of venetoclax in combination with obinutuzumab. In the G-Clb group, the mean percentage for any grade and grade ≥3 infections was 29.1% and 11.1%, respectively; and the mean percentage for any grade and grade ≥3 neutropenia was 46.5% and 38.4%, respectively (51, 52, 61, 63, 64). In the FCR group, only the mean percentage for grade ≥3 infections and neutropenia was reported: 24.1% and 26.0%, respectively (53, 54, 57). In patients who received bendamustine plus rituximab, the mean percentage for any grade and grade ≥3 infections was 42.2% and 18.0%, respectively; and the mean percentage for any grade and grade ≥3 neutropenia was 59.7% and 43.4%, respectively (54, 56, 58, 63).
In one study that investigated the use of daratumumab in combination with lenalidomide and dexamethasone in patients with MM, the rate for grade ≥3 infections was 41.0% and the rates for any grade and grade ≥3 neutropenia were 61.3% and 54.1%, respectively (92). Only the rate for grade ≥3 neutropenia (39.9%) was reported in patients with MM who received daratumumab in combination with bortezomib, melphalan, and prednisone (90). None of the selected studies reported data on both the use of bortezomib in combination with lenalidomide and dexamethasone (VRd) and the use of daratumumab in combination with bortezomib, thalidomide, and dexamethasone (daraVTD) in patients with MM.
None of these studies reported data on the rates of hypogammaglobulinemia, highlighting the need for further reporting on immunoglobulin G (IgG) levels, especially in regimen combinations including drugs known to have a mode of action likely to impact IgG levels directly, such as B-lineage monoclonal antibodies like daratumumab.
3.5 Lymphocytopenia in patients with CLL, MM, and NHL
The rates of lymphocytopenia were reported in a limited number of studies only: one in patients with CLL (64), seven in MM (68, 70, 78, 91, 92, 97, 138), and three in NHL (116, 120, 127) (data not shown). The mean percentage of patients with lymphocytopenia was 1.9% in patients with CLL, 11.9% in MM, and 38.6% in NHL.
3.6 Hypogammaglobulinemia and sinopulmonary bacterial infection analyses
Only two of the evaluated studies reported data on hypogammaglobulinemia (Table 3). In patients with CLL, one study reported hypogammaglobulinemia in 15.3% of patients who received combination therapy with ublituximab (anti-CD20) and ibrutinib, and in 0.0% of patients who received ibrutinib monotherapy (62). In patients with NHL, one study reported hypogammaglobulinemia in 5.9% of patients who received rituximab maintenance therapy for up to 2 years (122). Neither study had a confirmed definition of what was classed as hypogammaglobulinemia nor was testing reported prior to the initiation of treatment.
Patients with hypogammaglobulinemia commonly present with recurrent bacterial sinopulmonary infections (e.g., otitis, sinusitis, pneumonia, nasopharyngitis), which often are due to encapsulated bacteria such as S. pneumoniae (3–5, 26, 142). In this systematic literature review, we classed sinopulmonary bacterial infections to include LRTI, URTI, sinusitis, nasopharyngitis, bronchitis, and/or pneumonia, which were collected from 17 studies in CLL, 38 in MM, and 34 in NHL. However, not all relevant studies reported all types of infections used to calculate the rate of sinopulmonary bacterial infections (e.g., no relevant studies reported data on the percentages of patients with LRTI and sinusitis in MM; as a result, the data for sinopulmonary bacterial infections in patients with MM did not include LRTI and sinusitis values). The mean proportion of patients with sinopulmonary bacterial infections was 7.6%, 14.4%, and 6.3% in patients with CLL, MM, and NHL, respectively. Sinopulmonary bacterial infections were reported in 15.7% (54, 59, 66), 8.5% (68, 91), and 7.8% (110, 113, 115, 122, 124, 127, 128, 134) of patients with CLL, MM, and NHL, respectively, when treated with B-lineage monoclonal antibodies as monotherapy (Table 6). In patients who received proteasome inhibitor as monotherapy, sinopulmonary bacterial infections were reported in 7.7% (69, 70, 80, 81, 83, 94, 95, 98, 135) of patients with MM. In patients treated with kinase monotherapy, sinopulmonary bacterial infections were reported in 8.0% of patients with CLL (51, 60, 62). In those patients who received non-specific agents as monotherapy and/or double/triplet regimen, sinopulmonary bacterial infections were reported in 9.9% (102, 115, 116, 119, 129) of patients with NHL.
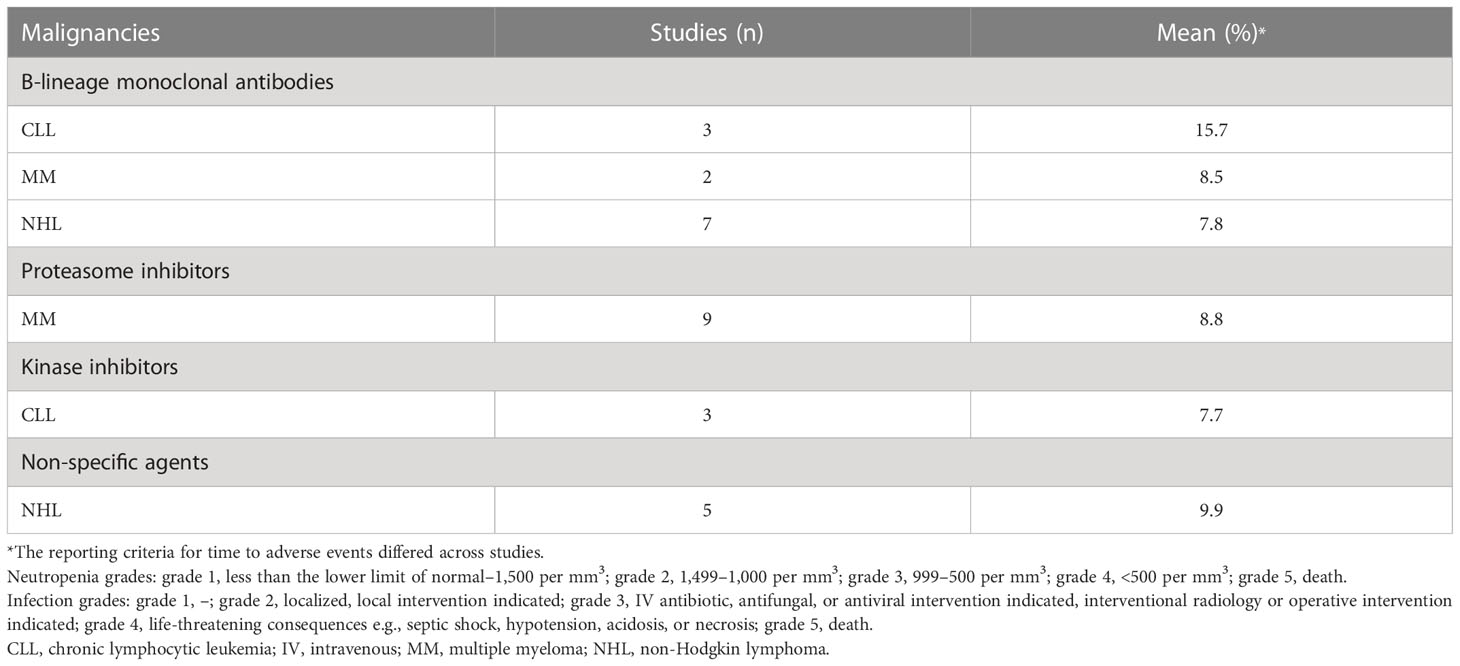
Table 6 Proportions of patients with CLL, MM, and NHL treated with different classes of drugs as monotherapy who had sinopulmonary bacterial infections.
In this systematic literature review, the most common types of infections reported in patients with CLL and MM were related to the respiratory system, whereas in patients with NHL they were bacterial infections, pneumonia, and viral infections (data not shown). Other less common types of infections included viral and UTI infections in CLL, viral and skin infections in MM, and UTI and Candida infections in NHL (data not shown).
4 Discussion
Despite infections related to SID accounting for 22–50% of deaths in patients with HMs (7, 47–49), we still observed a lack of data reported on hypogammaglobulinemia, lymphocytopenia, and consistent infection reporting in phase III, phase IV, and observational studies, suggesting a likely underestimate of hypogammaglobulinemia and cellular immunodeficiency in the development of recurrent and fatal infections in patients with HMs. In addition, there is still a lack of data in the literature regarding differences in rates of hypogammaglobulinemia-related infections, rates of hypogammaglobulinemia and infections across classes of drugs, and types of infections across HMs (Table 1).
This level of granularity in reporting rates of specific subtypes of SID and related infections might not be a priority for hematologists and hemato-oncologists with the main focus on treating the malignancy. However, we believe that collecting these data might help highlight trends and possible correlations that could inform changes in the management of HMs and related infections in everyday clinical practice, such as improving supportive care and serving as a stimulus for development of approaches that include the early testing and detection of immunodeficiency alongside prevention and treatment of infection as part of the routine management of these HMs (41). Therefore, we undertook this systematic literature review to provide insight into the cancer treatments associated with SID, including the incidence of infections, neutropenia, and hypogammaglobulinemia among patients undergoing systemic treatment for CLL, MM, and NHL.
In this systematic literature review, the highest proportion of patients with grade ≥3 infections across classes of drugs was 41.0% in patients with MM treated with a B-lineage monoclonal antibody combination; and 29.9% and 38.0% of patients with CLL and NHL treated with a kinase inhibitor combination, respectively. As expected, the incidence of neutropenia did not always correlate with the incidence of infections. Interestingly, the higher rates of grade ≥3 infections in the 2017–2022 group versus the 2011–2016 group across all the selected HMs might be due to numerous factors such as the concomitant use of old and novel therapeutic agents (e.g., B-lineage monoclonal antibodies, tyrosine kinase inhibitors, and proteasome inhibitors) and HM therapies becoming increasingly more potent and correspondingly more immunosuppressive, as well as longer survival and more comorbidities.
As many CAR T therapies are still in phase I or II clinical development (143–145) (and were therefore excluded from this systematic literature review) SID data associated with CAR T therapies is still emerging and not fully represented in this systematic literature review. For example, lisocabtagene maraleucel and ciltacabtagene autoleucel were not included in our analyses because these drugs were not approved in both the US and EU markets by March 16, 2022, and therefore there is a relatively small number of patients treated with these agents. Further work is needed in the rapidly evolving field of CAR T to report data on SID-related and hypogammaglobulinemia-related infections (144, 146).
Notably, the use of monotherapy was mostly associated with a numerically lower risk of infection or neutropenia. For instance, the mean proportion of patients with any grade infections was numerically lower when rituximab was used as monotherapy across patients with CLL, MM, and NHL compared with its use in combination with other agents. The use of ibrutinib as monotherapy led to a numerically lower mean percentage of patients with any grade and grade ≥3 neutropenia versus combination therapies. On the contrary, bortezomib used as monotherapy was associated with a numerically higher mean percentage of patients with grade ≥3 infections and a numerically lower mean percentage of patients with any grade infections; as already mentioned, this is due to individual studies reporting only grade ≥3 results that were higher than other studies did for any grade events. Unfortunately, further analyses to compare anti-CD20 versus anti-CD38 agents could not be undertaken due to sample sizes and mismatched disease cohorts.
The infection spectrum observed in this patient population has some similarities with those observed in primary antibody deficiency (PAD) but also some differences. While sinopulmonary infections are common in HMs and PAD, infection sites that are less common in PAD were also observed in this systematic literature review, such as the urinary tract and skin (with herpes group viral reactivation/infection in particular). The occurrence of viral and fungal, as well as bacterial pathogen groups, speaks to a potential CID phenotype in many patients with HM. The variability in the types of infection across patients with CLL, MM, and NHL might be due to both the disease and different related treatments that influence the infection profile of patients with HMs. Future data highlighting the differences between bacterial, fungal, and viral infection distribution with higher statistical power might be useful to predict patients’ infection risk and inform clinical decision making. While coronavirus disease 2019 (COVID-19) infection data were not collected in the studies analyzed, it is recognized that patients with HMs are at risk for severe COVID-19. In addition, the information gained from the use of vaccines against COVID-19 in these patients has been extremely informative in terms of providing functional vaccine response data to refine risk stratification.
Interestingly, despite both B-lineage monoclonal antibodies against CD20 and tyrosine kinase inhibitors being detrimental to B-cell development, hypogammaglobulinemia was detected only in patients with CLL who received ublituximab and ibrutinib (BTK) combination therapy compared with patients treated with ibrutinib monotherapy who did not show a decrease in their IgG serum concentration. Notably, neutropenia, pneumonia, bronchitis, and Herpes zoster infections were also higher in patients treated with ublituximab and ibrutinib combination therapy compared with ibrutinib monotherapy (62). It is possible that monotherapy has been used in less severe disease settings and that a balance exists between immunosuppression from the therapy on normal immune cells and reduction in tumor-related immunosuppression due to the therapy.
This systematic literature review has several limitations: i) as not all the studies analyzed specified precise definitions for hypogammaglobulinemia, infections and SAD, this might have influenced the data as slightly different outcomes may have been captured; ii) systematic literature reviews are not powered to have statistical significance; therefore, data should be considered as exploratory. However, they can help highlight trends and possible correlations that lay the foundation for further studies; iii) most of the data came from phase III clinical trials, which do not necessarily reflect real-life clinical practice (147). Some investigators recognize the pivotal role of real-world data and evidence that can be optimized (148, 149). Meta-analysis of data from hematological databases is one avenue that could provide insightful follow-up to extrapolate information on the rate of patients with HMs and hypogammaglobulinemia due to various cancer treatments in real-life settings; iv) finally, these drugs may be used at various times throughout a disease course and as induction or maintenance therapy. Therefore, as the risk of infection can vary depending on both the timing from diagnosis and severity of disease, direct comparison of infections rates between drugs must be undertaken with caution since data were not normalized for time exposure to agents and infection reporting. Future analyses will be crucial in evaluating the rates of hypogammaglobulinemia and infections in early versus late disease course. Moreover, distinction between BTK and phosphoinositide 3-kinase (PI3K) inhibitors were not performed due to the low number of studies that tested PI3K inhibitors, therefore an overall kinase inhibitor category was used, which may limit practical application of data from this category.
With this systematic literature review, the authors wish to shed light on which treatments might contribute to the development of SID in this rapidly evolving therapeutic area and to highlight the importance of reporting data on hypogammaglobulinemia, both before and during therapy in patients with HMs. The authors believe that, while treatment of the malignancy is clearly of primary importance, there are still several knowledge gaps on the management of SID (Table 1); therefore, efforts need to be undertaken to improve awareness of how to diagnose and treat patients with hypogammaglobulinemia, CID, and infections in HMs, as well as optimize treatments to prevent recurrent and severe infections. Without increased recording and reporting of Ig levels in this patient population, the benefits of a range management strategies such as infection exposure mitigation strategies, vaccination, antibiotics, antiviral drugs, and immunoglobulin replacement therapy (IgRT) cannot be fully evaluated (41).
Data availability statement
The datasets presented in this study are the results of analyses conducted with data taken from online publications. The name of the publications can be found in the reference list.
Author contributions
All authors contributed equally to the review approach/design, consensus meetings, recommendations, interpretation of literature, writing, and critical review of this article. All authors reviewed the final manuscript and agreed on the decision to submit it for publication.
Acknowledgments
Thanks to Daniele Guido and Alexandra Philiastides for categorizing the studies.
Conflict of interest
Literature searches, article screening, data abstraction, and medical writing support was provided by Meridian HealthComms Ltd (Manchester, UK) in accordance with Good Publication Practice guidelines and funded by CSL Behring. SJ has received support for conferences, speaker, advisory boards, trials, data and safety monitoring boards, and projects with CSL Behring, Takeda, Swedish Orphan Biovitrum, Biotest, Binding Site, Grifols, BPL, Octapharma, LFB, Pharming, GSK, Weatherden, Zarodex, Sanofi, and UCB Pharma. SG has received research funding from Miltenyi Biotec, Takeda, Celgene, Amgen, Sanofi, Johnson and Johnson, and Actinium and is on the advisory boards for: Kite Pharmaceuticals, Celgene, Sanofi, Novartis, Johnson and Johnson, Amgen, Takeda, Jazz Pharmaceuticals, Actinium Pharmaceuticals, and CSL Behring. TK has received support, never on a personal account, for conferences, speaker bureaus, advisory boards, trials, data and safety monitoring boards, and projects with Sanofi, Takeda, BMS, Johnson & Johnson, MSD, Novartis, Gilead, Octapharma, and CSL Behring. HML has received consultancy fees and support for advisory boards from CSL Behring and Actinium Pharmaceuticals; is a consultant, promotional speaker, and a member of the advisory boards for Jazz Pharmaceuticals and Seattle Genetics; is a member of the data safety and monitoring boards for BMS, BioSight, and Celgene; is a promotional speaker for AstraZeneca; has received consultancy fees and has stock options with Partner Therapeutics; and has received consultancy fees from Pluristem. SSM has received support for an advisory board and speaker bureaus with CSL Behring. RR has received support for advisory boards, data and safety monitoring boards, and speaker bureaus with Janssen Cilag, BMS, Celgene, Takeda, CSL Behring, and Octapharma. DCV has received salary support from Fonds de Recherche du Québec - Santé Senior Clinician-Scientist scholar program; has received clinical trial support from CSL Behring, Cidara Therapeutics, and Moderna; has received honoraria for advisory board consultations or speaker presentations from Astra Zeneca, CSL Behring, Merck Canada, Moderna, Novartis Canada, Qu biologics, and UCB Biosciences GmbH; and has a patent application pending Electronic Filing System ID: 40101099 and a report of invention submitted to McGill University Track code: D2021-0043.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1098326/full#supplementary-material
References
1. Allegra A, Tonacci A, Musolino C, Pioggia G, Gangemi S. Secondary immunodeficiency in hematological malignancies: Focus on multiple myeloma and chronic lymphocytic leukemia. Front Immunol (2021) 12:738915. doi: 10.3389/fimmu.2021.738915
2. Chinen J, Shearer WT. Advances in basic and clinical immunology in 2009. J Allergy Clin Immunol (2010) 125(3):563–8. doi: 10.1016/j.jaci.2010.01.022
3. Patel SY, Carbone J, Jolles S. The expanding field of secondary antibody deficiency: Causes, diagnosis, and management. Front Immunol (2019) 10:33. doi: 10.3389/fimmu.2019.00033
4. Tuano KS, Seth N, Chinen J. Secondary immunodeficiencies: An overview. Ann Allergy Asthma Immunol (2021) 127(6):617–26. doi: 10.1016/j.anai.2021.08.413
5. Friman V, Winqvist O, Blimark C, Langerbeins P, Chapel H, Dhalla F. Secondary immunodeficiency in lymphoproliferative malignancies. Hematol Oncol (2016) 34(3):121–32. doi: 10.1002/hon.2323
6. Morrison VA. Infectious complications of chronic lymphocytic leukaemia: Pathogenesis, spectrum of infection, preventive approaches. Best Pract Res Clin Haematol (2010) 23(1):145–53. doi: 10.1016/j.beha.2009.12.004
7. Blimark C, Holmberg E, Mellqvist UH, Landgren O, Björkholm M, Hultcrantz M, et al. Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients. Haematologica (2015) 100(1):107–13. doi: 10.3324/haematol.2014.107714
8. Jolles S, Smith BD, Vinh DC, Mallick R, Espinoza G, DeKoven M, et al. Risk factors for severe infections in secondary immunodeficiency: A retrospective US administrative claims study in patients with hematological malignancies. Leuk Lymphoma (2022) 63(1):64–73. doi: 10.1080/10428194.2021.1992761
9. Hamblin AD, Hamblin TJ. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull (2008) 87:49–62. doi: 10.1093/bmb/ldn034
10. Andersen MA, Moser CE, Lundgren J, Niemann CU. Epidemiology of bloodstream infections in patients with chronic lymphocytic leukemia: A longitudinal nation-wide cohort study. Leukemia (2019) 33(3):662–70. doi: 10.1038/s41375-018-0316-5
11. Hamblin TJ. Chronic lymphocytic leukaemia. Baillieres Clin Haematol (1987) 1(2):449–91. doi: 10.1016/S0950-3536(87)80009-4
12. Vacca A, Melaccio A, Sportelli A, Solimando AG, Dammacco F, Ria R. Subcutaneous immunoglobulins in patients with multiple myeloma and secondary hypogammaglobulinemia: A randomized trial. Clin Immunol (2018) 191:110–5. doi: 10.1016/j.clim.2017.11.014
13. Anderson LA, Atman AA, McShane CM, Titmarsh GJ, Engels EA, Koshiol J. Common infection-related conditions and risk of lymphoid malignancies in older individuals. Br J Cancer (2014) 110(11):2796–803. doi: 10.1038/bjc.2014.173
14. McCusker C, Upton J, Warrington R. Primary immunodeficiency. Allergy Asthma Clin Immunol (2018) 14(Suppl 2):141–52. doi: 10.1186/s13223-018-0290-5
15. Ballow M, Sánchez-Ramón S, Walter JE. Secondary immune deficiency and primary immune deficiency crossovers: Hematological malignancies and autoimmune diseases. Front Immunol (2022) 13:928062. doi: 10.3389/fimmu.2022.928062
16. Ochoa-Grullón J, Guevara-Hoyer K, Pérez López C, Pérez de Diego R, Peña Cortijo A, Polo M, et al. Combined immune defect in b-cell lymphoproliferative disorders is associated with severe infection and cancer progression. Biomedicines (2022) 10(8):2020. doi: 10.3390/biomedicines10082020
17. Leeksma OC, de Miranda NF, Veelken H. Germline mutations predisposing to diffuse large b-cell lymphoma. Blood Cancer J (2017) 7(3):e541. doi: 10.1038/bcj.2017.15
18. Kiaee F, Azizi G, Rafiemanesh H, Zainaldain H, Sadaat Rizvi F, Alizadeh M, et al. Malignancy in common variable immunodeficiency: A systematic review and meta-analysis. Expert Rev Clin Immunol (2019) 15(10):1105–13. doi: 10.1080/1744666X.2019.1658523
19. Guevara-Hoyer K, Fuentes-Antrás J, de la Fuente-Muñoz E, Fernández-Arquero M, Solano F, Pérez-Segura P, et al. Genomic crossroads between non-hodgkin’s lymphoma and common variable immunodeficiency. Front Immunol (2022) 13:937872. doi: 10.3389/fimmu.2022.937872
20. Marshall JS, Warrington R, Watson W, Kim HL. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol (2018) 14(Suppl 2):49. doi: 10.1186/s13223-018-0278-1
21. Pierce S, Geanes ES, Bradley T. Targeting natural killer cells for improved immunity and control of the adaptive immune response. Front Cell Infect Microbiol (2020) 10:231. doi: 10.3389/fcimb.2020.00231
22. Freeman CL, Gribben JG. Immunotherapy in chronic lymphocytic leukaemia (CLL). Curr Hematol Malig Rep (2016) 11(1):29–36. doi: 10.1007/s11899-015-0295-9
23. Rashidi M, Bandala-Sanchez E, Lawlor KE, Zhang Y, Neale AM, Vijayaraj SL, et al. CD52 inhibits toll-like receptor activation of NF-κB and triggers apoptosis to suppress inflammation. Cell Death Differ (2018) 25(2):392–405. doi: 10.1038/cdd.2017.173
24. Sochacka-Ćwikła A, Mączyński M, Regiec A. FDA-Approved drugs for hematological malignancies - the last decade review. Cancers (2021) 14(1):87. doi: 10.3390/cancers14010087
25. Shortt J, Spencer A. Adjuvant rituximab causes prolonged hypogammaglobulinaemia following autologous stem cell transplant for non-hodgkin's lymphoma. Bone Marrow Transplant (2006) 38(6):433–6. doi: 10.1038/sj.bmt.1705463
26. Srivastava S, Wood P. Secondary antibody deficiency - causes and approach to diagnosis. Clin Med (Lond) (2016) 16(6):571–6. doi: 10.7861/clinmedicine.16-6-571
27. Shimony S, Bar-Sever E, Berger T, Itchaki G, Gurion R, Yeshurun M, et al. Late onset neutropenia after rituximab and obinutuzumab treatment - characteristics of a class-effect toxicity. Leuk Lymphoma (2021) 62(12):2921–7. doi: 10.1080/10428194.2021.1948037
28. Moore DC. Drug-induced neutropenia: A focus on rituximab-induced late-onset neutropenia. P T (2016) 41(12):765–8.
29. O'Leary MC, Lu X, Huang Y, Lin X, Mahmood I, Przepiorka D, et al. FDA Approval summary: Tisagenlecleucel for treatment of patients with relapsed or refractory b-cell precursor acute lymphoblastic leukemia. Clin Cancer Res (2019) 25(4):1142–6. doi: 10.1158/1078-0432.CCR-18-2035
30. Merin NM, Kelly KR. Clinical use of proteasome inhibitors in the treatment of multiple myeloma. Pharm (Basel) (2014) 8(1):1–20. doi: 10.3390/ph8010001
31. Jacobs CF, Eldering E, Kater AP. Kinase inhibitors developed for treatment of hematologic malignancies: Implications for immune modulation in COVID-19. Blood Adv (2021) 5(3):913–25. doi: 10.1182/bloodadvances.2020003768
32. Zarrin AA, Bao K, Lupardus P, Vucic D. Kinase inhibition in autoimmunity and inflammation. Nat Rev Drug Discovery (2021) 20(1):39–63. doi: 10.1038/s41573-020-0082-8
33. Vargas L, Hamasy A, Nore BF, Smith CI. Inhibitors of BTK and ITK: state of the new drugs for cancer, autoimmunity and inflammatory diseases. Scand J Immunol (2013) 78(2):130–9. doi: 10.1111/sji.12069
34. Quach H, Ritchie D, Stewart AK, Neeson P, Harrison S, Smyth MJ, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia (2010) 24(1):22–32. doi: 10.1038/leu.2009.236
35. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell (2015) 28(6):690–714. doi: 10.1016/j.ccell.2015.10.012
36. Otani IM, Lehman HK, Jongco AM, Tsao LR, Azar AE, Tarrant TK, et al. Practical guidance for the diagnosis and management of secondary hypogammaglobulinemia: a work group report of the AAAAI primary immunodeficiency and altered immune response committees. J Allergy Clin Immunol (2022) 149(5):1525–60. doi: 10.1016/j.jaci.2022.01.025
37. Jablonka A, Etemadi H, Adriawan IR, Ernst D, Jacobs R, Buyny S, et al. Peripheral blood lymphocyte phenotype differentiates secondary antibody deficiency in rheumatic disease from primary antibody deficiency. J Clin Med (2020) 9(4):1049. doi: 10.3390/jcm9041049
38. Perriguey M, Maarouf A, Stellmann JP, Rico A, Boutiere C, Demortiere S, et al. Hypogammaglobulinemia and infections in patients with multiple sclerosis treated with rituximab. Neurol Neuroimmunol Neuroinflamm (2022) 9(1):e1115. doi: 10.1212/NXI.0000000000001115
39. Samson M, Audia S, Lakomy D, Bonnotte B, Tavernier C, Ornetti P. Diagnostic strategy for patients with hypogammaglobulinemia in rheumatology. Joint Bone Spine (2011) 78(3):241–5. doi: 10.1016/j.jbspin.2010.09.016
40. Tieu J, Smith RM, Gopaluni S, Kumararatne DS, McClure M, Manson A, et al. Rituximab associated hypogammaglobulinemia in autoimmune disease. Front Immunol (2021) 12:671503. doi: 10.3389/fimmu.2021.671503
41. Jolles S, Giralt S, Kerre T, Lazarus HM, Mustafa SS, Papanicolaou GA, et al. Secondary antibody deficiency in chronic lymphocytic leukemia and non-Hodgkin lymphoma: Recommendations from an international expert panel. Blood Rev In press. (2022). doi: 10.1016/j.blre.2022.101020
42. Warny M, Helby J, Nordestgaard BG, Birgens H, Bojesen SE. Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PloS Med (2018) 15(11):e1002685. doi: 10.1371/journal.pmed.1002685
43. National Cancer Institute: Division of Cancer Treatment & Diagnosis. Common terminology criteria for adverse events (CTCAE) v5.0 (2017). Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (Accessed August 23, 2022).
44. Mims MP. Lymphocytosis, lymphocytopenia, hypergammaglobulinemia, and hypogammaglobulinemia. Hematology (2018) 682–690. doi: 10.1016/B978-0-323-35762-3.00049-4
45. Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood (2015) 126(5):573–81. doi: 10.1182/blood-2015-03-567388
46. Andersen MA, Grand MK, Brieghel C, Siersma V, Andersen CL, Niemann CU. Pre-diagnostic trajectories of lymphocytosis predict time to treatment and death in patients with chronic lymphocytic leukemia. Commun Med (Lond) (2022) 2:50. doi: 10.1038/s43856-022-00117-4
47. Oscier D, Dearden C, Eren E, Fegan C, Follows G, Hillmen P, et al. Guidelines on the diagnosis, investigation and management of chronic lymphocytic leukaemia. Br J Haematol (2012) 159(5):541–64. doi: 10.1111/bjh.12067
48. Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al. Early mortality after diagnosis of multiple myeloma: Analysis of patients entered onto the united kingdom medical research council trials between 1980 and 2002. J Clin Oncol (2005) 23(36):9219–26. doi: 10.1200/JCO.2005.03.2086
49. Ostrow S, Diggs CH, Sutherland J, Wiernik PH. Causes of death in patients with non-hodgkin's lymphoma. Cancer (1981) 48(3):779–82. doi: 10.1002/1097-0142(19810801)48:3<779::AID-CNCR2820480320>3.0.CO;2-3
50. Eyre TA, Preston G, Kagdi H, Islam A, Nicholson T, Smith HW, et al. A retrospective observational study to evaluate the clinical outcomes and routine management of patients with chronic lymphocytic leukaemia treated with idelalisib and rituximab in the UK and Ireland (RETRO-idel). Br J Haematol (2021) 194(1):69–77. doi: 10.1111/bjh.17475
51. Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): A randomised, controlled, phase 3 trial. Lancet (2020) 395(10232):1278–91. doi: 10.1016/S0140-6736(20)30262-2
52. Herishanu Y, Shaulov A, Fineman R, Bašić-Kinda S, Aviv A, Wasik-Szczepanek E, et al. Frontline treatment with the combination obinutuzumab ± chlorambucil for chronic lymphocytic leukemia outside clinical trials: Results of a multinational, multicenter study by ERIC and the Israeli CLL study group. Am J Hematol (2020) 95(6):604–11. doi: 10.1002/ajh.25766
53. Herling CD, Cymbalista F, Groß-Ophoff-Müller C, Bahlo J, Robrecht S, Langerbeins P, et al. Early treatment with FCR versus watch and wait in patients with stage binet a high-risk chronic lymphocytic leukemia (CLL): A randomized phase 3 trial. Leukemia (2020) 34(8):2038–50. doi: 10.1038/s41375-020-0747-7
54. Sylvan SE, Asklid A, Johansson H, Klintman J, Bjellvi J, Tolvgård S, et al. First-line therapy in chronic lymphocytic leukemia: A Swedish nation-wide real-world study on 1053 consecutive patients treated between 2007 and 2013. Haematologica (2019) 104(4):797–804. doi: 10.3324/haematol.2018.200204
55. Stilgenbauer S, Leblond V, Foà R, Böttcher S, Ilhan O, Knauf W, et al. Obinutuzumab plus bendamustine in previously untreated patients with CLL: A subgroup analysis of the GREEN study. Leukemia (2018) 32(8):1778–86. doi: 10.1038/s41375-018-0146-5
56. Zelenetz AD, Barrientos JC, Brown JR, Coiffier B, Delgado J, Egyed M, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: Interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol (2017) 18(3):297–311. doi: 10.1016/S1470-2045(16)30671-4
57. Awan FT, Hillmen P, Hellmann A, Robak T, Hughes SG, Trone D, et al. A randomized, open-label, multicentre, phase 2/3 study to evaluate the safety and efficacy of lumiliximab in combination with fludarabine, cyclophosphamide and rituximab versus fludarabine, cyclophosphamide and rituximab alone in subjects with relapsed chronic lymphocytic leukaemia. Br J Haematol (2014) 167(4):466–77. doi: 10.1111/bjh.13061
58. Špaček M, Obrtlíková P, Hrobková S, Cmunt E, Karban J, Molinský J, et al. Prospective observational study in comorbid patients with chronic lymphocytic leukemia receiving first-line bendamustine with rituximab. Leuk Res (2019) 79:17–21. doi: 10.1016/j.leukres.2019.02.002
59. van Oers MH, Kuliczkowski K, Smolej L, Petrini M, Offner F, Grosicki S, et al. Ofatumumab maintenance versus observation in relapsed chronic lymphocytic leukaemia (PROLONG): An open-label, multicentre, randomised phase 3 study. Lancet Oncol (2015) 16(13):1370–9. doi: 10.1016/S1470-2045(15)00143-6
60. Pula B, Iskierka-Jazdzewska E, Dlugosz-Danecka M, Szymczyk A, Hus M, Szeremet A, et al. Long-term efficacy of ibrutinib in relapsed or refractory chronic lymphocytic leukemia: Results of the polish adult leukemia study group observational study. Anticancer Res (2020) 40(7):4059–66. doi: 10.21873/anticanres.14403
61. Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(1):43–56. doi: 10.1016/S1470-2045(18)30788-5
62. Sharman JP, Brander DM, Mato AR, Ghosh N, Schuster SJ, Kambhampati S, et al. Ublituximab plus ibrutinib versus ibrutinib alone for patients with relapsed or refractory high-risk chronic lymphocytic leukaemia (GENUINE): A phase 3, multicentre, open-label, randomised trial. Lancet Haematol (2021) 8(4):e254–66. doi: 10.1016/S2352-3026(20)30433-6
63. Panovská A, Němcová L, Nekvindová L, Špaček M, Šimkovič M, Papajík T, et al. Real-world data on efficacy and safety of obinutuzumab plus chlorambucil, rituximab plus chlorambucil, and rituximab plus bendamustine in the frontline treatment of chronic lymphocytic leukemia: The GO-CLLEAR study by the Czech CLL study group. Hematol Oncol (2020) 38(4):509–16. doi: 10.1002/hon.2744
64. Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink AM, Robrecht S, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): Follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2020) 21(9):1188–200. doi: 10.1016/S1470-2045(20)30443-5
65. Hillmen P, Robak T, Janssens A, Babu KG, Kloczko J, Grosicki S, et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): A randomised, multicentre, open-label phase 3 trial. Lancet (9980) 2015:1873–83:385. doi: 10.1016/S0140-6736(15)60027-7
66. Greil R, Obrtlíková P, Smolej L, Kozák T, Steurer M, Andel J, et al. Rituximab maintenance versus observation alone in patients with chronic lymphocytic leukaemia who respond to first-line or second-line rituximab-containing chemoimmunotherapy: Final results of the AGMT CLL-8a mabtenance randomised trial. Lancet Haematol (2016) 3(7):e317–29. doi: 10.1016/S2352-3026(16)30045-X
67. Puig N, Hernández MT, Rosiñol L, González E, de Arriba F, Oriol A, et al. Lenalidomide and dexamethasone with or without clarithromycin in patients with multiple myeloma ineligible for autologous transplant: A randomized trial. Blood Cancer J (2021) 11(5):101. doi: 10.1038/s41408-021-00490-8
68. Iida S, Ishikawa T, Min CK, Kim K, Yeh SP, Usmani SZ, et al. Subcutaneous daratumumab in Asian patients with heavily pretreated multiple myeloma: Subgroup analyses of the noninferiority, phase 3 COLUMBA study. Ann Hematol (2021) 100(4):1065–77. doi: 10.1007/s00277-021-04405-2
69. Baertsch MA, Mai EK, Hielscher T, Bertsch U, Salwender HJ, Munder M, et al. Lenalidomide versus bortezomib maintenance after frontline autologous stem cell transplantation for multiple myeloma. Blood Cancer J (2021) 11(1):1. doi: 10.1038/s41408-020-00390-3
70. Li J, Bao L, Xia Z, Wang S, Zhou X, Ding K, et al. Ixazomib-based frontline therapy in patients with newly diagnosed multiple myeloma in real-life practice showed comparable efficacy and safety profile with those reported in clinical trial: A multi-center study. Ann Hematol (2020) 99(11):2589–98. doi: 10.1007/s00277-020-04234-9
71. Kumar SK, Jacobus SJ, Cohen AD, Weiss M, Callander N, Singh AK, et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol (2020) 21(10):1317–30. doi: 10.1016/S1470-2045(20)30452-6
72. Jackson GH, Pawlyn C, Cairns DA, Striha A, Collett C, Waterhouse A, et al. Optimising the value of immunomodulatory drugs during induction and maintenance in transplant ineligible patients with newly diagnosed multiple myeloma: Results from myeloma XI, a multicentre, open-label, randomised, phase III trial. Br J Haematol (2021) 192(5):853–68. doi: 10.1111/bjh.16945
73. Usmani SZ, Schjesvold F, Oriol A, Karlin L, Cavo M, Rifkin RM, et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): A randomised, open-label, phase 3 trial. Lancet Haematol (2019) 6(9):e448–58. doi: 10.1016/S2352-3026(19)30109-7
74. Stadtmauer EA, Pasquini MC, Blackwell B, Hari P, Bashey A, Devine S, et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: Results of the BMT CTN 0702 trial. J Clin Oncol (2019) 37(7):589–97. doi: 10.1200/JCO.18.00685
75. Jackson GH, Davies FE, Pawlyn C, Cairns DA, Striha A, Collett C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2019) 20(1):57–73. doi: 10.1016/S1470-2045(18)30687-9
76. Kim MK, Kim K, Min CK, Kwak JY, Bae SB, Yoon SS, et al. A prospective, open-label, multicenter, observational study to evaluate the efficacy and safety of bortezomib-melphalan-prednisone as initial treatment for autologous stem cell transplantation-ineligible patients with multiple myeloma. Oncotarget (2017) 8(23):37605–18. doi: 10.18632/oncotarget.16790
77. Gentile M, Magarotto V, Offidani M, Musto P, Bringhen S, Teresa Petrucci M, et al. Lenalidomide and low-dose dexamethasone (Rd) versus bortezomib, melphalan, prednisone (VMP) in elderly newly diagnosed multiple myeloma patients: A comparison of two prospective trials. Am J Hematol (2017) 92(3):244–50. doi: 10.1002/ajh.24621
78. Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol (2016) 17(1):27–38. doi: 10.1016/S1470-2045(15)00464-7
79. Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: A randomised, multicentre, phase 3 trial. Lancet Oncol (2015) 16(16):1617–29. doi: 10.1016/S1470-2045(15)00389-7
80. Muchtar E, Gatt ME, Rouvio O, Ganzel C, Chubar E, Suriu C, et al. Efficacy and safety of salvage therapy using carfilzomib for relapsed or refractory multiple myeloma patients: A multicentre retrospective observational study. Br J Haematol (2016) 172(1):89–96. doi: 10.1111/bjh.13799
81. Lin M, Hou J, Chen W, Huang X, Liu Z, Zhou Y, et al. Improved response rates with bortezomib in relapsed or refractory multiple myeloma: An observational study in Chinese patients. Adv Ther (2014) 31(10):1082–94. doi: 10.1007/s12325-014-0159-z
82. Cook G, Williams C, Brown JM, Cairns DA, Cavenagh J, Snowden JA, et al. High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI myeloma X relapse [Intensive trial]): A randomised, open-label, phase 3 trial. Lancet Oncol (2014) 15(8):874–85. doi: 10.1016/S1470-2045(14)70245-1
83. Nooka AK, Kaufman JL, Behera M, Langston A, Waller EK, Flowers CR, et al. Bortezomib-containing induction regimens in transplant-eligible myeloma patients: A meta-analysis of phase 3 randomized clinical trials. Cancer (2013) 119(23):4119–28. doi: 10.1002/cncr.28325
84. Garderet L, Iacobelli S, Moreau P, Dib M, Lafon I, Niederwieser D, et al. Superiority of the triple combination of bortezomib-thalidomide-dexamethasone over the dual combination of thalidomide-dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: The MMVAR/IFM 2005-04 randomized phase III trial from the chronic leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol (2012) 30(20):2475–82. doi: 10.1200/JCO.2011.37.4918
85. Cavo M, Gay F, Beksac M, Pantani L, Petrucci MT, Dimopoulos MA, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): A multicentre, randomised, open-label, phase 3 study. Lancet Haematol (2020) 7(6):e456–68. doi: 10.1016/S2352-3026(20)30099-5
86. Kumar SK, Harrison SJ, Cavo M, de la Rubia J, Popat R, Gasparetto C, et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol (2020) 21(12):1630–42. doi: 10.1016/S1470-2045(20)30525-8
87. Moreau P, Dimopoulos MA, Mikhael J, Yong K, Capra M, Facon T, et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): A multicentre, open-label, randomised phase 3 trial. Lancet (2021) 397(10292):2361–71. doi: 10.1016/S0140-6736(21)00592-4
88. Miguel JS, Weisel K, Moreau P, Lacy M, Song K, Delforge M, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol (2013) 14(11):1055–66. doi: 10.1016/S1470-2045(13)70380-2
89. Walter-Croneck A, Grzasko N, Soroka-Wojtaszko M, Jurczyszyn A, Torosian T, Rymko M, et al. Case-adjusted bortezomib-based strategy in routine therapy of relapsed/refractory multiple myeloma shown to be highly effective–a report by polish myeloma study group. Leuk Res (2014) 38(7):788–94. doi: 10.1016/j.leukres.2014.04.011
90. Mateos MV, Cavo M, Blade J, Dimopoulos MA, Suzuki K, Jakubowiak A, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): A randomised, open-label, phase 3 trial. Lancet (2020) 395(10218):132–41. doi: 10.1016/S0140-6736(19)32956-3
91. Mateos MV, Nahi H, Legiec W, Grosicki S, Vorobyev V, Spicka I, et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): A multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol (2020) 7(5):e370–80. doi: 10.1016/S2352-3026(20)30070-3
92. Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): Overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(11):1582–96. doi: 10.1016/S1470-2045(21)00466-6
93. Terpos E, Ramasamy K, Maouche N, Minarik J, Ntanasis-Stathopoulos I, Katodritou E, et al. Real-world effectiveness and safety of ixazomib-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma. Ann Hematol (2020) 99(5):1049–61. doi: 10.1007/s00277-020-03981-z
94. Musto P, Simeon V, Cascavilla N, Falcone A, Petrucci MT, Cesini L, et al. Is re-challenge still an option as salvage therapy in multiple myeloma? the case of REal-life BOrtezomib re-use as secoND treatment for relapsed patients exposed frontline to bortezomib-based therapies (the REBOUND study). Ann Hematol (2019) 98(2):361–7. doi: 10.1007/s00277-018-3524-1
95. Knauf W, Tapprich C, Schlag R, Schütz S, Alkemper B, Gaede B, et al. Bortezomib-containing regimens are effective in multiple myeloma-results of a non-interventional phase IV study. Oncol Res Treat (2015) 38(4):167–73. doi: 10.1159/000381297
96. San-Miguel JF, Hungria VT, Yoon SS, Beksac M, Dimopoulos MA, Elghandour A, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: A multicentre, randomised, double-blind phase 3 trial. Lancet Oncol (2014) 15(11):1195–206. doi: 10.1016/S1470-2045(14)70440-1
97. Dimopoulos M, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Results from a randomised, multicentre, open-label, phase 3 study. Lancet (2020) 396(10245):186–97. doi: 10.1016/S0140-6736(20)30734-0
98. Dimopoulos M, Siegel DS, Lonial S, Qi J, Hajek R, Facon T, et al. Vorinostat or placebo in combination with bortezomib in patients with multiple myeloma (VANTAGE 088): A multicentre, randomised, double-blind study. Lancet Oncol (2013) 14(11):1129–40. doi: 10.1016/S1470-2045(13)70398-X
99. Usmani SZ, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Updated outcomes from a randomised, multicentre, open-label, phase 3 study. Lancet Oncol (2022) 23(1):65–76. doi: 10.1016/S1470-2045(21)00579-9
100. Wang M, Ramchandren R, Chen R, Karlin L, Chong G, Jurczak W, et al. Concurrent ibrutinib plus venetoclax in relapsed/refractory mantle cell lymphoma: The safety run-in of the phase 3 SYMPATICO study. J Hematol Oncol (2021) 14(1):179. doi: 10.1186/s13045-021-01188-x
101. Marín-Sánchez A, Martínez-Fernández G, Gómez-Catalán I, Montoya-Morcillo MC, Algarra-Algarra JL, Ibañez-García Á, et al. Efficacy of chemotherapy protocols for hematological malignancies: H-CVAD versus GELA/BURKIMAB/PETHEMA LAL. Exp Hematol (2021), 101:49–57. doi: 10.1016/j.exphem.2021.08.003
102. Shi YK, Hong XN, Yang JL, Xu W, Huang HQ, Xiao XB, et al. Bendamustine treatment of Chinese patients with relapsed indolent non-Hodgkin lymphoma: A multicenter, open-label, single-arm, phase 3 study. Chin Med J (Engl) (2021) 134(11):1299–309. doi: 10.1097/CM9.0000000000001463
103. Bishton MJ, Rule S, Wilson W, Turner D, Patmore R, Clifton-Hadley L, et al. The UK NCRI study of chlorambucil, mitoxantrone and dexamethasone (CMD) versus fludarabine, mitoxantrone and dexamethasone (FMD) for untreated advanced stage follicular lymphoma: Molecular response strongly predicts prolonged overall survival. Br J Haematol (2020) 190(4):545–54. doi: 10.1111/bjh.16555
104. Gupta S, Seethapathy H, Strohbehn IA, Frigault MJ, O'Donnell EK, Jacobson CA, et al. Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large b-cell lymphoma. Am J Kidney Dis (2020) 76(1):63–71. doi: 10.1053/j.ajkd.2019.10.011
105. Pettengell R, Długosz-Danecka M, Andorsky D, Belada D, Georgiev P, Quick D, et al. Pixantrone plus rituximab versus gemcitabine plus rituximab in patients with relapsed aggressive b-cell non-Hodgkin lymphoma not eligible for stem cell transplantation: A phase 3, randomized, multicentre trial (PIX306). Br J Haematol (2020) 188(2):240–8. doi: 10.1111/bjh.16255
106. Poeschel V, Held G, Ziepert M, Witzens-Harig M, Holte H, Thurner L, et al. Four versus six cycles of CHOP chemotherapy in combination with six applications of rituximab in patients with aggressive b-cell lymphoma with favourable prognosis (FLYER): A randomised, phase 3, non-inferiority trial. Lancet (2019) 394(10216):2271–81. doi: 10.1016/S0140-6736(19)33008-9
107. Davies A, Cummin TE, Barrans S, Maishman T, Mamot C, Novak U, et al. Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large b-cell lymphoma (REMoDL-b): An open-label, randomised, phase 3 trial. Lancet Oncol (2019) 20(5):649–62. doi: 10.1016/S1470-2045(18)30935-5
108. Broccoli A, Casadei B, Chiappella A, Visco C, Tani M, Cascavilla N, et al. Lenalidomide in pretreated patients with diffuse large b-cell lymphoma: An Italian observational multicenter retrospective study in daily clinical practice. Oncologist (2019) 24(9):1246–52. doi: 10.1634/theoncologist.2018-0603
109. Skarbnik AP, Ma E, Lafeuille MH, Fortier J, Feldman T, Duh MS, et al. Treatment patterns and outcomes with subcutaneous bortezomib in patients with relapsed mantle cell lymphoma: A retrospective, observational study of patient medical records from US community oncology practices. Leuk Lymphoma (2017) 58(8):1968–72. doi: 10.1080/10428194.2016.1272688
110. Rummel M, Kim TM, Aversa F, Brugger W, Capochiani E, Plenteda C, et al. Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20+ diffuse large b-cell lymphoma or follicular lymphoma: Results from a prospective, randomized, open-label, crossover study (PrefMab). Ann Oncol (2017) 28(4):836–42. doi: 10.1093/annonc/mdw685
111. Zinzani PL, Rigacci L, Cox MC, Devizzi L, Fabbri A, Zaja F, et al. The efficacy of lenalidomide combination therapy in heavily pretreated non-Hodgkin lymphoma patients: An Italian observational, multicenter, retrospective study. Leuk Lymphoma (2017) 58(1):226–9. doi: 10.1080/10428194.2016.1184755
112. Xue K, Gu JJ, Zhang Q, Liu X, Wang J, Li XQ, et al. Cardiotoxicity as indicated by LVEF and troponin T sensitivity following two anthracycline-based regimens in lymphoma: Results from a randomized prospective clinical trial. Oncotarget (2016) 7(22):32519–31. doi: 10.18632/oncotarget.8685
113. Witzens-Harig M, Benner A, McClanahan F, Klemmer J, Brandt J, Brants E, et al. Rituximab maintenance improves survival in male patients with diffuse large b-cell lymphoma. results of the HD2002 prospective multicentre randomized phase III trial. Br J Haematol (2015) 171(5):710–9. doi: 10.1111/bjh.13652
114. Wieringa A, Boslooper K, Hoogendoorn M, Joosten P, Beerden T, Storm H, et al. Comorbidity is an independent prognostic factor in patients with advanced-stage diffuse large b-cell lymphoma treated with r-CHOP: A population-based cohort study. Br J Haematol (2014) 165(4):489–96. doi: 10.1111/bjh.12765
115. Amiot A, Lévy M, Copie-Bergman C, Dupuis J, Szablewski V, Le Baleur Y, et al. Rituximab, alkylating agents or combination therapy for gastric mucosa-associated lymphoid tissue lymphoma: A monocentric non-randomised observational study. Aliment Pharmacol Ther (2014) 39(6):619–28. doi: 10.1111/apt.12635
116. Pettengell R, Coiffier B, Narayanan G, de Mendoza FH, Digumarti R, Gomez H, et al. Pixantrone dimaleate versus other chemotherapeutic agents as a single-agent salvage treatment in patients with relapsed or refractory aggressive non-Hodgkin lymphoma: A phase 3, multicentre, open-label, randomised trial. Lancet Oncol (2012) 13(7):696–706. doi: 10.1016/S1470-2045(12)70212-7
117. Rummel M, Kaiser U, Balser C, Stauch M, Brugger W, Welslau M, et al. Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: A multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol (2016) 17(1):57–66. doi: 10.1016/S1470-2045(15)00447-7
118. Schwartzberg LS, Saleh M, Whittaker S, Abella E. Severe neutropenia and relative dose intensity among patients <65 and ≥65 years with non-hodgkin's lymphoma receiving CHOP-based chemotherapy. Support Care Cancer (2014) 22(7):1833–41. doi: 10.1007/s00520-014-2157-8
119. Sehn LH, Chua N, Mayer J, Dueck G, Trněný M, Bouabdallah K, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): A randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol (2016) 17(8):1081–93. doi: 10.1016/S1470-2045(16)30097-3
120. Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet (2013) 381(9873):1203–10. doi: 10.1016/S0140-6736(12)61763-2
121. Morigi A, Argnani L, Lolli G, Broccoli A, Pellegrini C, Nanni L, et al. Bendamustine-rituximab regimen in untreated indolent marginal zone lymphoma: Experience on 65 patients. Hematol Oncol (2020) 38(4):487–92. doi: 10.1002/hon.2773
122. Witzens-Harig M, Foá R, Di Rocco A, van Hazel G, Chamone DF, Rowe JM, et al. Maintenance with rituximab is safe and not associated with severe or uncommon infections in patients with follicular lymphoma: Results from the phase IIIb MAXIMA study. Ann Hematol (2014) 93(10):1717–24. doi: 10.1007/s00277-014-2103-3
123. Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: An international, randomised, open-label, phase 3 study. Lancet (2016) 387(10020):770–8. doi: 10.1016/S0140-6736(15)00667-4
124. Coiffier B, Osmanov EA, Hong X, Scheliga A, Mayer J, Offner F, et al. Bortezomib plus rituximab versus rituximab alone in patients with relapsed, rituximab-naive or rituximab-sensitive, follicular lymphoma: A randomised phase 3 trial. Lancet Oncol (2011) 12(8):773–84. doi: 10.1016/S1470-2045(11)70150-4
125. Ladetto M, Cortelazzo S, Ferrero S, Evangelista A, Mian M, Tavarozzi R, et al. Lenalidomide maintenance after autologous haematopoietic stem-cell transplantation in mantle cell lymphoma: Results of a fondazione italiana linfomi (FIL) multicentre, randomised, phase 3 trial. Lancet Haematol (2021) 8(1):e34–44. doi: 10.1016/S2352-3026(20)30358-6
126. Zinzani PL, Pellegrini C, Merla E, Ballerini F, Fabbri A, Guarini A, et al. Bortezomib as salvage treatment for heavily pretreated relapsed lymphoma patients: A multicenter retrospective study. Hematol Oncol (2013) 31(4):179–82. doi: 10.1002/hon.2036
127. Matasar MJ, Capra M, Özcan M, Lv F, Li W, Yañez E, et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol (2021) 22(5):678–89. doi: 10.1016/S1470-2045(21)00145-5
128. Ogura M, Sancho JM, Cho SG, Nakazawa H, Suzumiya J, Tumyan G, et al. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 in comparison with rituximab in patients with previously untreated low-tumour-burden follicular lymphoma: A randomised, double-blind, parallel-group, phase 3 trial. Lancet Haematol (2018) 5(11):e543–53. doi: 10.1016/S2352-3026(18)30157-1
129. Farhi J, Laribi K, Orvain C, Hamel JF, Mercier M, Sutra Del Galy A, et al. Impact of front line relative dose intensity for methotrexate and comorbidities in immunocompetent elderly patients with primary central nervous system lymphoma. Ann Hematol (2018) 97(12):2391–401. doi: 10.1007/s00277-018-3468-5
130. Hara T, Yoshikawa T, Goto H, Sawada M, Yamada T, Fukuno K, et al. R-THP-COP versus r-CHOP in patients younger than 70 years with untreated diffuse large b cell lymphoma: A randomized, open-label, noninferiority phase 3 trial. Hematol Oncol (2018) 36(4):638–44. doi: 10.1002/hon.2524
131. Chiappella A, Martelli M, Angelucci E, Brusamolino E, Evangelista A, Carella AM, et al. Rituximab-dose-dense chemotherapy with or without high-dose chemotherapy plus autologous stem-cell transplantation in high-risk diffuse large b-cell lymphoma (DLCL04): Final results of a multicentre, open-label, randomised, controlled, phase 3 study. Lancet Oncol (2017) 18(8):1076–88. doi: 10.1016/S1470-2045(17)30444-8
132. Zinzani PL, Pellegrini C, Broccoli A, Gandolfi L, Stefoni V, Casadei B, et al. Fludarabine-Mitoxantrone-Rituximab regimen in untreated indolent non-follicular non-hodgkin's lymphoma: Experience on 143 patients. Hematol Oncol (2015) 33(3):141–6. doi: 10.1002/hon.2151
133. Xu PP, Fu D, Li JY, Hu JD, Wang X, Zhou JF, et al. Anthracycline dose optimisation in patients with diffuse large b-cell lymphoma: A multicentre, phase 3, randomised, controlled trial. Lancet Haematol (2019) 6(6):e328–37. doi: 10.1016/S2352-3026(19)30051-1
134. Feng X, Guo W, Wang Y, Li J, Zhao Y, Qu L, et al. The short-term efficacy and safety of brentuximab vedotin plus cyclophosphamide, epirubicin and prednisone in untreated PTCL: A real-world, retrospective study. Adv Ther (2022) 39(1):532–43. doi: 10.1007/s12325-021-01943-z
135. Castro TB, Hallack Neto AE, Atalla A, Ribeiro LC. Pharmacovigilance of patients with multiple myeloma being treated with bortezomib and/or thalidomide. Braz J Med Biol Res (2016) 49(6):e5128. doi: 10.1590/1414-431x20165128
136. Moreau P, Hulin C, Macro M, Caillot D, Chaleteix C, Roussel M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood (2016) 127(21):2569–74. doi: 10.1182/blood-2016-01-693580
137. Leleu X, Terpos E, Sanz RG, Cooney J, O'Gorman P, Minarik J, et al. An international, multicenter, prospective, observational study of neutropenia in patients being treated with lenalidomide + dexamethasone for relapsed or relapsed/refractory multiple myeloma (RR-MM). Am J Hematol (2016) 91(8):806–11. doi: 10.1002/ajh.24416
138. Dimopoulos MA, Palumbo A, Corradini P, Cavo M, Delforge M, Di Raimondo F, et al. Safety and efficacy of pomalidomide plus low-dose dexamethasone in STRATUS (MM-010): A phase 3b study in refractory multiple myeloma. Blood (2016) 128(4):497–503. doi: 10.1182/blood-2016-02-700872
139. Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large b-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2015) 26 Suppl 5:v116–25. doi: 10.1093/annonc/mdv304
140. Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2021) 32(1):23–33. doi: 10.1016/j.annonc.2020.09.019
141. Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up(†). Ann Oncol (2021) 32(3):309–22. doi: 10.1016/j.annonc.2020.11.014
142. Ogi M, Yokoyama H, Tomosugi N, Hisada Y, Ohta S, Takaeda M, et al. Risk factors for infection and immunoglobulin replacement therapy in adult nephrotic syndrome. Am J Kidney Dis (1994) 24(3):427–36. doi: 10.1016/S0272-6386(12)80899-7
143. Gill S, Brudno JN. CAR T-cell therapy in hematologic malignancies: Clinical role, toxicity, and unanswered questions. Am Soc Clin Oncol Educ Book (2021) 41:1–20. doi: 10.1200/EDBK_320085
144. Zhang X, Zhu L, Zhang H, Chen S, Xiao Y. CAR-T cell therapy in hematological malignancies: Current opportunities and challenges. Front Immunol (2022) 13:927153. doi: 10.3389/fimmu.2022.927153
145. Elsallab M, Levine BL, Wayne AS, Abou-El-Enein M. CAR T-cell product performance in haematological malignancies before and after marketing authorisation. Lancet Oncol (2020) 21(2):e104–16. doi: 10.1016/S1470-2045(19)30729-6
146. Lu J, Jiang G. The journey of CAR-T therapy in hematological malignancies. Mol Cancer (2022) 21(1):194. doi: 10.1186/s12943-022-01663-0
147. Heneghan C, Goldacre B, Mahtani KR. Why clinical trial outcomes fail to translate into benefits for patients. Trials (2017) 18(1):122. doi: 10.1186/s13063-017-1870-2
148. Passamonti F, Corrao G, Castellani G, Mora B, Maggioni G, Gale RP, et al. The future of research in hematology: Integration of conventional studies with real-world data and artificial intelligence. Blood Rev (2022) 54:100914. doi: 10.1016/j.blre.2021.100914
Keywords: secondary immunodeficiency, hematological malignances, neutropenia, hypogammaglobulinemia, secondary antibody deficiency, B-lineage monoclonal antibodies, Bruton kinase inhibitors
Citation: Jolles S, Giralt S, Kerre T, Lazarus HM, Mustafa SS, Ria R and Vinh DC (2023) Agents contributing to secondary immunodeficiency development in patients with multiple myeloma, chronic lymphocytic leukemia and non-Hodgkin lymphoma: A systematic literature review. Front. Oncol. 13:1098326. doi: 10.3389/fonc.2023.1098326
Received: 14 November 2022; Accepted: 04 January 2023;
Published: 07 February 2023.
Edited by:
Robert Ohgami, The University of Utah, United StatesReviewed by:
Joshua Richter, Icahn School of Medicine at Mount Sinai, United StatesRahul Banerjee, University of Washington, United States
Copyright © 2023 Jolles, Giralt, Kerre, Lazarus, Mustafa, Ria and Vinh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen Jolles, Sm9sbGVzU1JAY2FyZGlmZi5hYy51aw==
†ORCID: Stephen Jolles, orcid.org/0000-0002-7394-6804
 Stephen Jolles
Stephen Jolles Sergio Giralt
Sergio Giralt Tessa Kerre
Tessa Kerre Hillard M. Lazarus
Hillard M. Lazarus S. Shahzad Mustafa
S. Shahzad Mustafa Roberto Ria
Roberto Ria Donald C. Vinh8
Donald C. Vinh8