- 1Department of Pathology, Obstetrics and Gynecology Hospital of Fudan University, Shanghai, China
- 2Department of Gynecological Oncology, Obstetrics and Gynecology Hospital of Fudan University, Shanghai, China
- 3Department of Radiology, Obstetrics and Gynecology Hospital of Fudan University, Shanghai, China
COL1A1–PDGFB gene fusion uterine sarcoma is an especially rare malignant mesenchymal tumor that was previously classified as an undifferentiated uterine sarcoma due to the lack of specific features of differentiation. Till now, only five cases have been reported, and here we presented another case recently diagnosed in a Chinese woman who had vaginal bleeding. She presented with a cervical mass at the anterior lip of the cervix invading the vagina and was treated with laparoscopic total hysterectomy plus bilateral salpingo-oophorectomy (TH+BSO) and partial vaginal wall resection with the final pathology of COL1A1–PDGFB fusion uterine sarcoma. Our aim is to emphasize the importance of differential diagnosis of this rare tumor, as early precise diagnosis may allow patients to benefit from the targeted therapy imatinib. This article also serves as further clinical evidence of this disease, serving to increase clinical awareness of this rare sarcoma to avoid misdiagnosis.
Introduction
Uterine mesenchymal tumors consist of a group of heterogeneous tumors with various morphological features, immunohistochemical (IHC) presentations, and genetic mutations. Undifferentiated uterine sarcomas are malignant mesenchymal tumors that lack specific features of differentiation and are diagnosed with the exclusion of others. With the rapid development of molecular technology including fluorescence in situ hybridization (FISH) and next-generation sequencing (NGS), the pathological classification and prognosis assessment of these tumors achieved remarkable progression (1, 2).
COL1A1 gene is located at chromosomes 17q21.3 to q22.1 with 52 exons. The gene is highly variable, as it contains multiple fragile breakpoints spanning a wide range. PDGFB gene is located at chromosomes 22q12.3 to q13.1 containing 7 exons. Its breakpoint is consistently present in intron 1. When these two genes merged, the expression of PDGFB would be muted from the regulation of upstream inhibitory factors, and COL1A1–PDGFB chimeric mRNAs would be generated, resulting in PDGFB and its receptor (PDGFBRB) stimulating cell proliferation in an autocrine or paracrine manner, which was reported to be oncogenic (3–5).
According to the previous literature, COL1A1–PDGFB gene fusion occurred mainly in soft tissue dermatofibrosarcoma protuberans (6–8) and pediatric giant cell fibroblastoma (9), while it was rarely reported in the female genital tract. Croce (10) first reported three cases with COL1A1–PDGFB fusion in uterine sarcomas in 2019, and subsequently, Samuel and Adriana respectively reported one case each in 2020 and 2022 (11, 12).
The relevant literature had been reviewed, and no reports in China had been found on this tumor so far. Here, we presented the first uterine sarcoma located at the cervix with COL1A1–PDGFB gene fusion in China.
Case report
Clinical presentation
A 57-year-old woman presented with uninduced post-menopausal vaginal bleeding for 2 weeks. Gynecological examination revealed a 4-cm mass on the anterior lip of the cervix protruding to the vagina. Pelvic ultrasound showed a 51 × 45 × 35 mm3 hypoechoic mass in the lower segment of the uterus extending to the anterior lip of the cervix with a rich blood supply. MRI displayed an irregular exophytic mass on the cervix that presented slightly high signal intensity on T2-weighted imaging (T2WI) with significant enhancement (Supplementary Figure 1), and cervical myoma was suspected for which malignancy could not be excluded. As for medical history, she had undergone surgery for papillary thyroid cancer in another hospital with iodine-131 radiation after an operation in 2011, and regular follow-ups showed no abnormality currently. She denied any family history. For obstetric history, she was G2P2 with two children born through vaginal delivery in her 30s. She denied any unprotected sex, and no bleeding was noticed during intercourse. The pre-op tumor markers were all within normal range. A comprehensive pre-op evaluation was performed with the human papillomavirus (HPV) test as negative, liquid based cytology test (LCT) as negative for intraepithelial lesion or malignancy (NILM), and abdominal+chest CT with contrast showing no suspicious lymph node or any other abnormality.
As cervical myoma was considered and malignancy could not be excluded, total hysterectomy plus bilateral salpingo-oophorectomy (TH+BSO) plus partial vaginal wall resection was suggested for this patient who has had menopause for 11 years already. The patient received laparoscopic surgery with frozen pathology reported as cervical myoma, and the whole specimen was extracted through the vagina. As the tumor size was slightly too big to pass through the atrophic vagina, the uterus was dissected under the protection of a specimen bag, and there was no dissemination of the tumor during the operation. The tumor was completely resected, and no extrauterine lesions were detected during the operation. Postoperative RNA sequencing of tumor tissue was performed, which supported the diagnosis of COL1A1–PDGFB fusion uterine sarcoma. Considering the rarity of this tumor and limited data available as to the treatment and prognosis, thorough communication with the patient was conducted, and the decision was reached as no further adjuvant therapy was given post-operation and close follow-up was required. The patient was suggested to undergo follow-ups every 3–6 months in the first 2 years post-operation, and the frequency could be extended to 6–12 months since the third year after surgery. The patient was recommended to undergo lifelong follow-ups, and the latest follow-up at 6 months after surgery showed no abnormality. The patient had been compliant with regular follow-ups, and no adverse events have been reported so far.
Methods
The IHC staining was performed on formalin-fixed and paraffin-embedded (FFPE) tissue blocks automatically (Leica Bond Max, Wetzlar, Germany), and then antibodies (Table 1) were applied according to the manufacturer’s instructions. RNA extraction, RNA-seq library preparation, sequencing, and analysis were carried out as previously described (13–15). Total RNA was extracted from FFPE tissue blocks, and rRNA was removed to obtain mRNA, which was then processed into short fragments. The interrupted mRNA segments were reverse-transcribed with random primers. After the synthesis of the first strand of cDNA by reverse transcription, the second strand of cDNA was synthesized, which became double-stranded cDNA. cDNA was purified by Beckman AMPPure XP magnetic beads and repaired at the end, and a sequencing joint was added. The target fragments were recovered by purifying magnetic beads and then amplified by PCR. The library constructed was sequenced by Illumina HiSeq2000.
Pathologic analyses
Histopathologic findings
A gross examination of the specimen revealed a 5.5-cm mass at the anterior lip of the cervix protruding toward the vagina. The tumor cross-section was firm, white, and whorled with relatively clear boundaries (Supplementary Figure 2). Microscopically, the tumor boundary was generally clear, while infiltration into the cervical mucosa and fibromyometrium was noticed locally (Figure 1A). The tumor consisted of relatively uniform spindle cells densely arranged in more prominent storiform or herringbone patterns. The nuclei were oval- to spindle-shaped, and the cytoplasm was eosinophilic and scarce, with blurred cell boundaries (Figure 1B). However, some minor regions with sparse cell distribution and dilated small blood vessels were also seen (Figure 1C). Mitoses were relatively active, up to 30 per 10 high-power fields (HPF), with mild-to-moderate nuclear heteromorphism (Figure 1D).
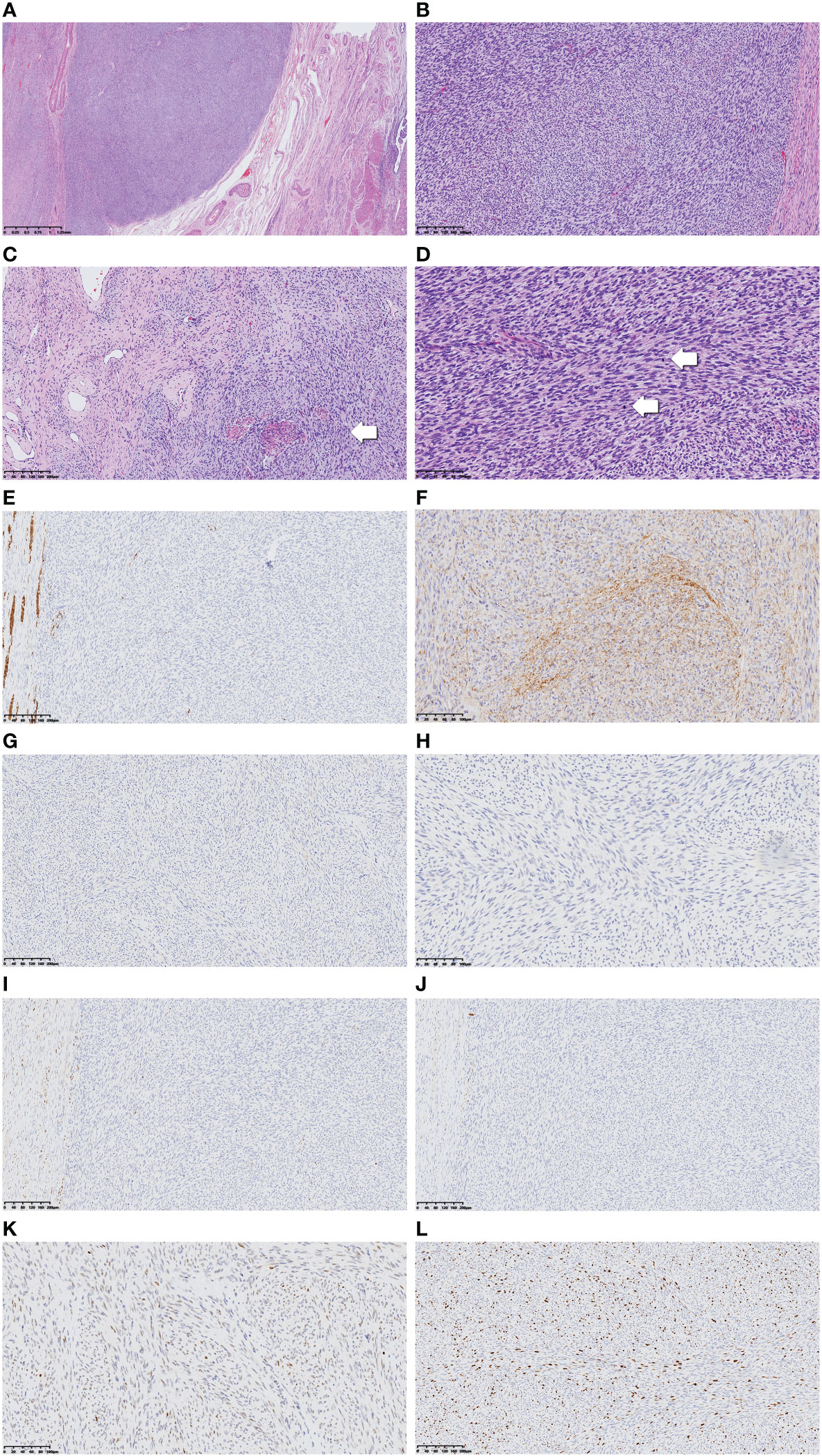
Figure 1 Low-power view of the tumor revealed relatively clear boundary (A, ×40). Tumor cells were arranged in a more prominent storiform or herringbone pattern in the cellular view (B, ×100), whereas in the cell-sparse area, more dilated small blood vessels can be seen. Normal cervical fibromuscular tissue not invaded by the tumor as pointed by the arrow (C, ×100). Tumor nuclei in oval to spindle shape are shown in high-power view, and the cytoplasm appears sparse and eosinophilic. The nuclear heteromorphism appears mild to moderate, while mitoses are numerous and obvious (arrow) (D, ×200). Immunohistochemical results including desmin, CD34, TRK, S100, ER, PR, and P53 are displayed (E–K, ×200). The Ki-67 proliferation index was high (L, ×200).
Immunohistochemical findings
The tumor cells were stained positive for CD34, P16, SMARCA4, and INI1 and scattered weak-positive for CCND1. S-100, estrogen receptor (ER), progesterone receptor (PR), desmin, caldesmon, SMA, CD10, BCOR, ALK, TRK, Melan-A, HMB-45, NSE, and CD99 were stained negative. P53 was stained as wild type, and Ki-67 was expressed in 30% of tumor cells (Figures 1E–L).
Molecular findings
Illumina NextSeq RNA sequencing was adopted, which covered all exons including 632 genes, and special attention was focused on 148 genes (listed in the Supplementary Material), and COL1A1 (NM_000088.3: Exon45)–PDGFB (NM_002608.2: Exon2) gene fusion was detected in this case (Figure 2).
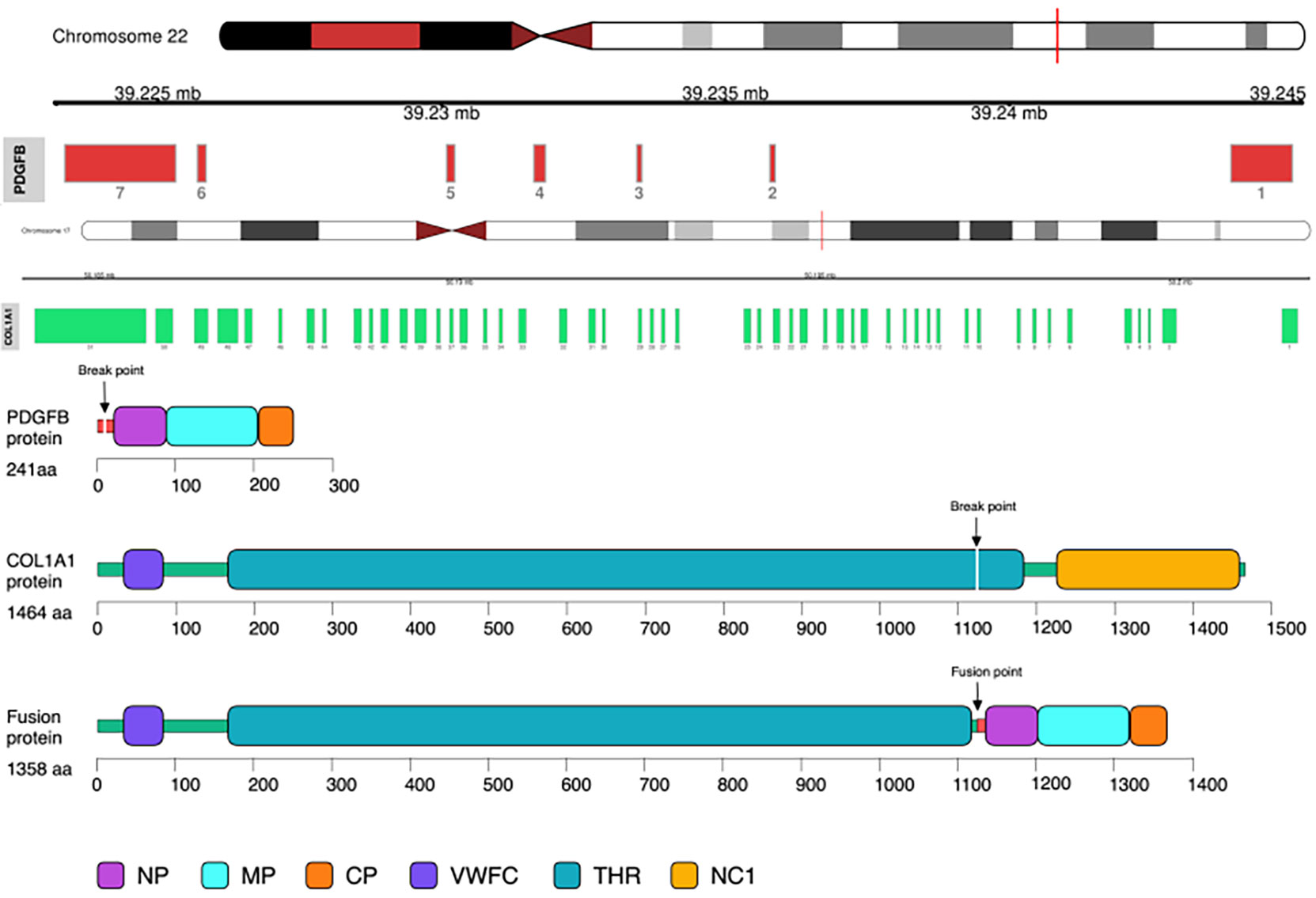
Figure 2 COL1A1–PDGFB gene fusion was detected by RNA sequencing. Note the unbalanced breakpoints (arrows) on CH17q (COL1A1) and 22q (PDGFB).
Discussion
In 2019, Croce recommended that uterine spindle cell sarcomas could be divided into three categories: NTRK fusion group, COL1A1–PDGFB fusion group, and a group that was tentatively classified as malignant peripheral nerve sheath tumor as positively stained with S100 and contained neither of the molecular abnormalities above (10), a category that excluded leiomyosarcoma (LMS) and high-grade endometrial stromal sarcoma (HGESS). Identification of gene fusion-associated sarcomas is extremely important, as patients can potentially benefit from specific targeted treatments. The first drug that targeted NTRK gene fusion-positive tumors and received approval from the Food and Drug Administration (FDA) was larotrectinib in 2018, which had an overall response rate (ORR) of 75% (95% CI: 61–85%, independent review) in a pooled analysis of three phase I and II single-arm trials of 55 combined pediatric and adult patients (16). An updated pooled analysis from three phase I and II clinical trials of larotrectinib (NCT02122913, TNCT02637687, and NCT02576431) resulted in an ORR of 75% (95% CI: 68–81%) based on an investigator review of 206 patients evaluable for response (17). In 2019, entrectinib was also approved, and a pooled analysis of three phase I and II studies showed an ORR of 57% (95% CI: 43–71%, independent review) in 54 adult patients (18). As ORRs for larotrectinib and entrectinib were averaged across different tumor types, the underlying assumption was that the efficacy or effectiveness was the same regardless of histology. As for uterine cancer, the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Uterine Neoplasms (NCCN Guidelines®) explicitly recommended trying either larotrectinib or entrectinib for NTRK 1/2/3 fusion-positive uterine sarcoma (19). Imatinib was first approved by the FDA for the treatment of patients with soft tissue tumors bearing COL1A1–PDGFB fusion in 2006 and, according to a recent systematic review, was associated with objective responses in more than 60% of advanced cases (20).
COL1A1–PDGFB fusion uterine sarcomas were often reported to be asymptomatic. Patient 4 was noticed to have a palpable mass on physical examination, and the tumor grew rapidly during follow-up. The patient in the fifth case came with vaginal bleeding and lower abdominal pain. A cervical mass was found on vaginal examination. The fourth case described that the tumor had a pink–tan–white whorled appearance with areas of necrosis. Our case shared a similar gross appearance, with no obvious necrotic area. Interestingly, our case had a relatively clear boundary and mainly invaded in an expansive manner, compressing on surrounding normal tissue, while local infiltration was seen. Further comparisons between our case and patients reported previously are discussed in detail in Table 2 (10–12).
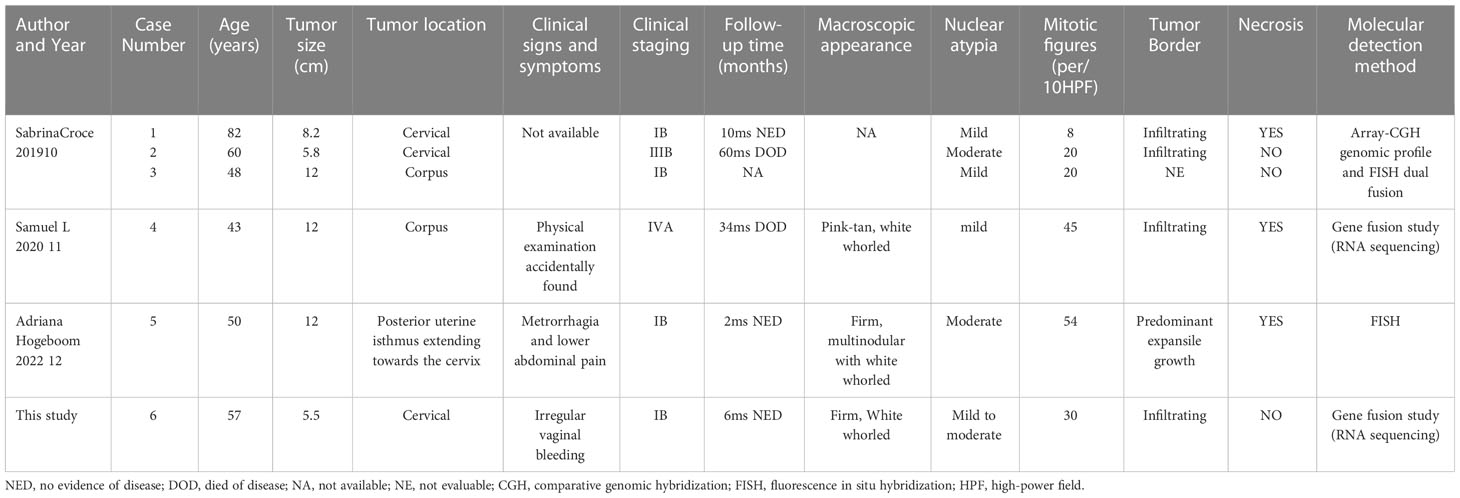
Table 2 Clinicopathological findings of COL1A1–PDGFB fusion uterine sarcoma in this case and comparison with previous cases in the literature.
In our case, the uterine sarcoma displayed relatively uniform spindle cells with elongated nuclei, uniform chromatin, sparse cytoplasm, and poorly defined cell boundaries. The nuclei were mildly anomalous, and mitoses were relatively active with up to 30 per 10 HPF, which looked like dermatofibrosarcoma protuberans (DFSP). Previous studies (3, 21, 22) suggested DFSP is a relatively inert, low-to-moderate-malignant soft tissue tumor of the dermis. The morphological feature was poorly defined nodular masses infiltrating subcutaneous or skeletal muscle. The morphology is usually presented with a uniform arrangement of spindle-shaped cell bundles in a typical storiform pattern. Particular attention should be paid to the presence of fibrosarcomatous change or other high-risk features, and CD34 is often highly and diffusely expressed in the cytoplasm. Both histomorphologic manifestations and IHC staining of CD34 overlap with COL1A1–PDGFB fusion uterine sarcomas. DFSP has a characteristic molecular feature of t(17;22) (q22; q13), and therefore, COL1A1–PDGFB fusion could be detected in more than 90% of DFSP cases. For DFSP without COL1A1–PDGFB fusion, the molecular assay showed multiple gene translocations, P53 mutation or overexpression, or murine double minute 2 (MDM2) overexpression. The most common sites of DFSP were the torso of the body and extremities, and in rare cases, it could also occur at the head and neck, while DFSP in the female genital tract was only reported in the vulvar region. Therefore, although some scholars prefer to refer to uterine tumors bearing COL1A1–PDGFB fusion as dermatofibrosarcoma in the uterus (considering the morphologic and IHC similarity), the nomenclature of uterine sarcoma with COL1A1–PDGFB fusion seems more appropriate.
In a pathological setting, leiomyoma, LMS, HGESS, and undifferentiated uterine sarcoma should be considered as the main differential diagnosis of COL1A1–PDGFB fusion uterine sarcomas, and NTRK fusion uterine sarcoma should be the most challenging one to be differentiated (23–25). NTRK fusion uterine sarcoma was first reported in 2011 (24, 26) and mainly occurred in young women, with an age range of 23–60 years (average 35 years). The lesions were mostly located at the cervix instead of the corpus, which is the same for COL1A1–PDGFB fusion uterine sarcomas. The morphology usually presented with fibrosarcoma-like spindle cells arranged in a storiform or fishbone pattern. Other features such as vascular hyalinosis and hemangiopericytoma-like changes, vascular infiltration, and significant inflammatory cell infiltration are rarely seen in COL1A1–PDGFB fusion uterine sarcomas. IHC markers could be critical clues for diagnosis; usually, TRK, S100, and CD34 were stained positive, with markers of smooth muscle (desmin and caldesmon) and hormone receptors (ER and PR) stained negative for NTRK fusion uterine sarcoma. However, the absence or weak expression of TRK cannot rule out the NTRK fusion uterine sarcoma due to the poor sensitivity and specificity of IHC staining of TRK, which should be verified by molecular testing if necessary. Of cases of NTRK fusion uterine sarcomas, 90% were found to be confined to the uterus at the time of initial clinical evaluation and were potentially responsive to anticancer therapy (27). Boyle and Rabban reported four cases of uterine sarcoma with NTRK fusion presented as rare cervical polypoid masses, which could be easily confused with adenosarcoma with stromal overgrowth. However, adenosarcoma usually presented with negative S100 and TRK on IHC, and molecular detection without NTRK rearrangement should be the gold standard to facilitate differentiation (28, 29). Similar to COL1A1–PDGFB fusion uterine sarcomas, both were more common in the cervix and shared similar morphological manifestations. However, the age onset of COL1A1–PDGFB fusion uterine sarcomas was older, ranging from 43 to 82 years (average at 56.7 years, median at 53.5 years). IHC features usually provided more clues for differential diagnosis, as CD34 was usually stained remarkably positive, while TRK, S100, myogenic markers, and hormone receptors were often stained negative. Although in most cases IHC staining is a simple and cost-effective method to assist diagnosis, confirmatory FISH or gene sequencing is mandatory in cases that are hard to identify. Due to the rarity of this tumor, limited experience with clinical treatment and prognosis, and lack of knowledge about the effectiveness of targeted therapy, it was particularly critical to correctly identify the tumor as the first step.
Under a microscope, a relatively sparse area of tumor cells with rare mitoses inspected could also be confused with leiomyoma. However, IHC markers for smooth muscle differentiation (desmin, caldesmon, and SMA) stained negative could exclude benign leiomyoma. LMS usually displayed moderate-to-severe nuclear heteromorphism, active mitoses, and remarkable necrosis and stained positive for the markers of smooth muscle differentiation (23), which were inconsistent with COL1A1–PDGFB fusion uterine sarcomas. The tumor cells of HGESS were often smaller with irregular or tongue-like invasion into the myometrium, and CCND1 and BCOR were often positive on IHC. They usually presented with specific gene fusion of YWHAE–NUTM2 A/B fusion and ZC3H7B–BCOR fusion. Other mutations such as EPC1, SUZ12, BRD8, PHF1, TPR, LMNA, TPM3, RBPMS, EML4, and STRN were also reported (30–32).
The prognosis for COL1A1–PDGFB fusion uterine sarcomas does not seem optimistic so far, as two patients of the five cases reported before have died. Both of them were at advanced clinical stages IIIB and IVA. Our patient (case 6 in Table 2) did not show any recurrence or progression for the past 6 months. The rarity of COL1A1–PDGFB fusion uterine sarcomas occurring in the female genital tract and unspecific morphology, especially without molecular tests, resulted in frequent misdiagnosis. Misdiagnosis could be one of the main reasons for poor prognosis, as adequate adjuvant therapy was delayed or missed for these patients. Therefore, awareness is encouraged when morphology and IHC markers do not match, and assistance from molecular tests (especially RNA sequencing for gene fusion in sarcoma) is critical for precise diagnosis.
As the clinical signs and symptoms in patients with COL1A1–PDGFB fusion uterine sarcomas in the female genital tract were usually silent in early stages, two out of six patients were initially diagnosed at late stages (IIIB and IVA in cases 2 and 4) as shown in Table 2. These two patients died of the disease at 60 and 34 months on follow-up. In case 4, the lack of response to chemotherapy prompted genomic testing for potential targeted therapies. It was at that time the COL1A1–PDGFB fusion was identified. Treatment with imatinib was initiated and continued for 6 months. The effects lasted and achieved the peak at the 11-month follow-up, as the intrabdominal mass reduced in size from 22.4 to 6.5 cm. CT progression was noticed at the 14-month follow-up after initiation of imatinib, as multiple abdominal masses that previously decreased in size grew back rapidly. Further investigations of more targeted therapy at COL1A1–PDGFB fusion are urgently needed to improve prognosis. Routine physical examination and clinical identification with the coordination of gynecologists and radiologists are crucial to guarantee early diagnosis and prompt treatment.
As for the surgical approach, the risk of inadvertent dissemination of occult malignancies of presumed benign tissue must be considered, as COL1A1–PDGFB fusion uterine sarcomas could be judged as benign leiomyoma on imaging (33). Morcellation should be avoided or carefully performed under the protection of a specimen bag. Laparotomy, colpotomy, or laparoscopic hysterectomy with contained specimen extraction through the vagina is appropriate.
This study expands the clinicopathological features of COL1A1–PDGFB fusion uterine sarcomas of the cervix, adding the first Chinese case to the five reported cases and highlighting a potential pitfall in the morphological differential diagnosis with NTRK fusion uterine sarcoma, leiomyoma, LMS, HGESS, and undifferentiated uterine sarcoma. A lack of knowledge has been seldom discussed previously. The prognosis for COL1A1–PDGFB fusion uterine sarcomas does not seem optimistic so far, as the current clinical evidence, long-term follow-up of these patients, and more clinical analyses with bigger sample size are urgently needed to better study the prognosis of this particularly rare type of uterine sarcoma. More investigations are warranted to clarify the pathogenesis and development of this disease and help improve the prognosis.
Conclusion
The new categorization of uterine spindle cell sarcomas in 2009 started a new era of pathological diagnosis depending on molecular features from classical morphology. With only five cases previously reported, we presented the sixth case of COL1A1–PDGFB fusion uterine sarcoma in the female genital tract. Its pathological morphology is easily confused with benign uterine leiomyoma. Moreover, other mesenchymal malignancies such as NTRK fusion uterine sarcoma, LMS, and HGESS also need to be differentiated. The assistance by immunohistochemistry and molecular detection is critical for precise pathological diagnosis of different categories of uterine sarcoma. Precise identification could allow patients to benefit from further treatment, especially targeted therapy such as imatinib.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained for the publication of this case report.
Author contributions
Author Contributions: Conceptualization, YN and YS. Methodology, YN and LL. Software, LL. Clinical resources, YN and LL. Lab work, SW, FZ and LL. Writing—original draft preparation, YS and LL. Writing—review and editing, YN and YS. Visualization, HS and FM. Supervision, YN and YS. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1108586/full#supplementary-material
Supplementary information of genes covered by the sarcoma gene panel | AKT2, AKT3, ALK, AR, AXL, BCL2, BCOR, BCR, BRAF, BRCA1, BRCA2, BRD4, CD74, CDK12, CDKN2A, CIC, CTNNB1, DDR2, EGFR, ERBB2, ERBB4, ERG, ESR1, ETV1, ETV6, FGFR1, FGFR2, FGFR3, FGR, FLT3, FOXO1, GLI1, JAK2, JAK3, KIT, KMT2A, KRAS, MDM4, MET, MSH2, MYC, NF1, NOTCH1, NOTCH2, NRG1, NTRK1, NTRK2, NTRK3, PDGFB, PDGFRA, PDGFRB, PIK3CA, PPARG, PTEN, RAD51B, RAF1, RARA, RB1, RET, ROS1, SMARCB1, STK11, TERT, TFE3, TMPRSS2, WT1, ETV4, ETV5, EWSR1, MYB, NUTM1, EZR, SLC34A2, SDC4, ACTB, ATF1, CAMTA1, COL1A1, COL1A2, CREB3L1, CREB3L2, CSF1, DNAJB1, FEV, FLI1, FUS, JAZF1, LPP, MGEA5, NAB2, NCOA2, NOTCH4, NR4A3, PHF1, PLAG1, PRKACA, RANBP2, RELA, RIPK4, RPS6KB2, SS18, SSX1, USP6, YWHAE, HMGA2, TAF15, TFG, CCNB3, EPC1, MEAF6, MKL2, STAT6, TCF12, NUP214, PRKACB, PRKCA, TACC3, DDIT3, SLC45A3, NCOA1, EIF3E, RSP02, PAX8, MAML2, PAX3, PAX7, PTPRK, RSPO3, NUP107, PBX1, C110RF95, COL6A3, MAML3, MYBL1, PLK2, PRDM10, PRKCB, TFEB, TRIO, VGLL2, GRB7, MAST1, MAST2, BRD3, CDH11, ESRP1, MYH9, YAP1.
Supplementary Figure 1 | Pelvic MRI showed that an oval mass protruding from the cervix to the vagina and compressed on the anterior bladder with a slightly higher signal (A). The mass was significantly enhanced, remarkably higher than that of the uterus post-enhancement (B).
Supplementary Figure 2 | On gross examination of the specimen, the tumor was mainly located on anterior lip of the cervix, growing in a pushing manner toward the vagina. The cross-section of the mass was firm and white-whorled with relatively clear boundary.
References
1. Momeni-Boroujeni A, Chiang S. Uterine mesenchymal tumours: recent advances. Histopathology (2020) 76(1):64–75. doi: 10.1111/his.14008
2. Chiang S, Oliva E. Recent developments in uterine mesenchymal neoplasms. Histopathology (2013) 62(1):124–37. doi: 10.1111/his.12048
3. Thway K, Noujaim J, Jones RL, Fisher C. Dermatofibrosarcoma protuberans: pathology, genetics, and potential therapeutic strategies. Ann Diagn Pathol (2016) 25:64–71. doi: 10.1016/j.anndiagpath.2016.09.013
4. Simon MP, Navarro M, Roux D, Pouysségur J. Structural and functional analysis of a chimeric protein COL1A1-PDGFB generated by the translocation t(17;22) (q22;q13.1) in dermatofibrosarcoma protuberans (DP). Oncogene (2001) 20(23):2965–75. doi: 10.1038/sj.onc.1204426
5. Takahira T, Oda Y, Tamiya S, Higaki K, Yamamoto H, Kobayashi C, et al. Detection of COL1A1-PDGFB fusion transcripts and PDGFB/PDGFRB mRNA expression in dermatofibrosarcoma protuberans. Mod Pathol (2007) 20(6):668–75. doi: 10.1038/modpathol.3800783
6. Pedeutour F, Simon MP, Minoletti F, Barcelo G, Terrier-Lacombe MJ, Combemale P, et al. Translocation, t(17;22) (q22;q13), in dermatofibrosarcoma protuberans: A new tumor-associated chromosome rearrangement. Cytogenet Cell Genet (1996) 72(2-3):171–4. doi: 10.1159/000134178
7. Simon MP, Pedeutour F, Sirvent N, Grosgeorge J, Minoletti F, Coindre JM, et al. Deregulation of the platelet-derived growth factor b-chain gene via fusion with collagen gene COL1A1 in dermatofibrosarcoma protuberans and giant-cell fibroblastoma. Nat Genet (1997) 15(1):95–8. doi: 10.1038/ng0197-95
8. Karanian M, Pérot G, Coindre JM, Chibon F, Pedeutour F, Neuville A. Fluorescence in situ hybridization analysis is a helpful test for the diagnosis of dermatofibrosarcoma protuberans. Mod Pathol (2015) 28(2):230–7. doi: 10.1038/modpathol.2014.97
9. Maire G, Martin L, Michalak-Provost S, Gattas GJ, Turc-Carel C, Lorette G, et al. Fusion of COL1A1 exon 29 with PDGFB exon 2 in a der(22)t(17;22) in a pediatric giant cell fibroblastoma with a pigmented bednar tumor component. evidence for age-related chromosomal pattern in dermatofibrosarcoma protuberans and related tumors. Cancer Genet Cytogenet (2002) 134(2):156–61.
10. Croce S, Hostein I, Longacre TA, Mills AM, Pérot G, Devouassoux-Shisheboran M, et al. Uterine and vaginal sarcomas resembling fibrosarcoma: a clinicopathological and molecular analysis of 13 cases showing common NTRK-rearrangements and the description of a COL1A1-PDGFB fusion novel to uterine neoplasms. Mod Pathol (2019) 32(7):1008–22. doi: 10.1038/s41379-018-0184-6
11. Grindstaff SL, DiSilvestro J, Hansen K, DiSilvestro P, Sung CJ, Quddus MR. COL1A1-PDGFB fusion uterine fibrosarcoma: A case report with treatment implication. Gynecol Oncol Rep (2020) 31:100523. doi: 10.1016/j.gore.2019.100523
12. Hogeboom A, Bárcena C, Parrilla-Rubio L, Revilla E, Ruano Y, Gallego-Gutiérrez I, et al. A case of COL1A1-PDGFB fusion uterine sarcoma. Int J Gynecol Pathol (2022) 42(2):147–50. doi: 10.1097/PGP.0000000000000875
13. Ren H, Li Y, Yao Q, Lv H, Tang S, Zhou X, et al. Epithelioid leiomyosarcoma of broad ligament harboring PGR-NR4A3 and UBR5-PGR gene fusions: a unique case report. Virchows Arch (2022) 480(4):933–8. doi: 10.1007/s00428-021-03169-4
14. Cloutier JM, Allard G, Bean GR, Hornick JL, Charville GW. PDGFB RNA in situ hybridization for the diagnosis of dermatofibrosarcoma protuberans. Mod Pathol (2021) 34(8):1521–9. doi: 10.1038/s41379-021-00800-2
15. Zhu R, Yan J, Li B, Tan F, Yan W, Shen J, et al. Determination of COL1A1-PDGFB breakpoints by next-generation sequencing in the molecular diagnosis of dermatofibrosarcoma protuberans. Exp Mol Pathol (2021) 122:104672. doi: 10.1016/j.yexmp.2021.104672
16. Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol (2020) 21(4):531–40. doi: 10.1016/S1470-2045(19)30856-3
17. Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol (2020) 21(2):271–82. doi: 10.1016/S1470-2045(19)30691-6
18. Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol (2018) 15(12):731–47. doi: 10.1038/s41571-018-0113-0
19. Abu-Rustum NR, Yashar CM, Bradley K, Campos SM, Chino J, Chon HS, et al. NCCN guidelines® insights: Uterine neoplasms, version 3.2021. J Natl Compr Canc Netw (2021) 19(8):888–95. doi: 10.6004/jnccn.2021.0038
20. Navarrete-Dechent C, Mori S, Barker CA, Dickson MA, Nehal KS. Imatinib treatment for locally advanced or metastatic dermatofibrosarcoma protuberans: a systematic review. JAMA Dermatol (2019) 155(3):361–9. doi: 10.1001/jamadermatol.2018.4940
21. Allen A, Ahn C, Sangueza OP. Dermatofibrosarcoma protuberans. Dermatol Clin (2019) 37(4):483–8. doi: 10.1016/j.det.2019.05.006
22. Hao X, Billings SD, Wu F, Stultz TW, Procop GW, Mirkin G, et al. Dermatofibrosarcoma protuberans: Update on the diagnosis and treatment. J Clin Med (2020) 9(6):1752. doi: 10.3390/jcm9061752
23. Chiang S, Cotzia P, Hyman DM, Drilon A, Tap WD, Zhang L, et al. NTRK fusions define a novel uterine sarcoma subtype with features of fibrosarcoma. Am J Surg Pathol (2018) 42(6):791–8. doi: 10.1097/PAS.0000000000001055
24. Croce S, Hostein I, McCluggage WG. NTRK and other recently described kinase fusion positive uterine sarcomas: A review of a group of rare neoplasms. Genes Chromosomes Cancer (2021) 60(3):147–59. doi: 10.1002/gcc.22910
25. Markl B, Hirschbuhl K, Dhillon C. NTRK-fusions - a new kid on the block. Pathol Res Pract (2019) 215(10):152572. doi: 10.1016/j.prp.2019.152572
26. Mills AM, Karamchandani JR, Vogel H, Longacre TA. Endocervical fibroblastic malignant peripheral nerve sheath tumor (neurofibrosarcoma): report of a novel entity possibly related to endocervical CD34 fibrocytes. Am J Surg Pathol (2011) 35(3):404–12. doi: 10.1097/PAS.0b013e318208f72e
27. Chiang S. S100 and pan-trk staining to report NTRK fusion-positive uterine sarcoma: Proceedings of the ISGyP companion society session at the 2020 USCAP annual meeting. Int J Gynecol Pathol (2021) 40(1):24–7. doi: 10.1097/PGP.0000000000000702
28. Boyle W, Williams A, Sundar S, Yap J, Taniere P, Rehal P, et al. TMP3-NTRK1 rearranged uterine sarcoma: A case report. Case Rep Womens Health (2020) 28:e00246. doi: 10.1016/j.crwh.2020.e00246
29. Rabban JT, Devine WP, Sangoi AR, Poder L, Alvarez E, Davis JL, et al. NTRK fusion cervical sarcoma: a report of three cases, emphasising morphological and immunohistochemical distinction from other uterine sarcomas, including adenosarcoma. Histopathology (2020) 77(1):100–11. doi: 10.1111/his.14069
30. Kim Y, Kim D, Jung Sung W, Hong J. High-grade endometrial stromal sarcoma: Molecular alterations and potential immunotherapeutic strategies. Front Immunol (2022) 13:837004. doi: 10.3389/fimmu.2022.837004
31. Hoang L, Chiang S, Lee CH. Endometrial stromal sarcomas and related neoplasms: new developments and diagnostic considerations. Pathology (2018) 50(2):162–77. doi: 10.1016/j.pathol.2017.11.086
32. Chiang S, Lee CH, Stewart CJR, Oliva E, Hoang LN, Ali RH, et al. BCOR is a robust diagnostic immunohistochemical marker of genetically diverse high-grade endometrial stromal sarcoma, including tumors exhibiting variant morphology. Mod Pathol (2017) 30(9):1251–61. doi: 10.1038/modpathol.2017.42
Keywords: COL1A1-PDGFB fusion, uterine sarcoma, RNA sequencing, dermatofibrosarcoma protuberans (DFSP), NTRK fusion, target therapy
Citation: Lu L, Wang S, Shen H, Zhang F, Ma F, Shi Y and Ning Y (2023) Case Report: A case of COL1A1–PDGFB fusion uterine sarcoma at cervix and insights into the clinical management of rare uterine sarcoma. Front. Oncol. 13:1108586. doi: 10.3389/fonc.2023.1108586
Received: 26 November 2022; Accepted: 13 February 2023;
Published: 13 March 2023.
Edited by:
Pierluigi Giampaolino, University of Naples Federico II, ItalyReviewed by:
Luigi Della Corte, University of Naples Federico II, ItalyKomsun Suwannarurk, Thammasat University, Thailand
Copyright © 2023 Lu, Wang, Shen, Zhang, Ma, Shi and Ning. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Shi, c2hpeXVlNzQ2N0BmY2t5eS5vcmcuY24=; Yan Ning, bmluZ3lhbjEzNzBAZmNreXkub3JnLmNu
†These authors have contributed equally to this work and share first authorship
 Linghui Lu
Linghui Lu Shunni Wang1†
Shunni Wang1† Yue Shi
Yue Shi