- 1INSERM Unité Mixte de Recherche (UMR)_S_1310, Université Paris Saclay, Villejuif, France
- 2INGESTEM National IPSC Infrastructure, Villejuif, France
- 3CITHERA, Center for iPSC Therapies, Evry, France
- 4APHP Paris Saclay, Division of Hematology, Paris Saclay University Hospitals, Le Kremlin Bicêtre, and Villejuif, France
- 5Inserm Unité Mixte de Recherche (UMR) 1193 Centre-Hepato Biliaire, Paul Brousse, Villejuif, France
The classical natural history of chronic myeloid leukemia (CML) has been drastically modified by the introduction of tyrosine kinase inhibitor (TKI) therapies. TKI discontinuation is currently possible in patients in deep molecular responses, using strict recommendations of molecular follow-up due to risk of molecular relapse, especially during the first 6 months. We report here the case of a patient who voluntarily interrupted her TKI therapy. She remained in deep molecular remission (MR4) for 18 months followed by detection of a molecular relapse at +20 months. Despite this relapse, she declined therapy until the occurrence of the hematological relapse (+ 4 years and 10 months). Retrospective sequential transcriptome experiments and a single-cell transcriptome RNA-seq analysis were performed. They revealed a molecular network focusing on several genes involved in both activation and inhibition of NK-T cell activity. Interestingly, the single-cell transcriptome analysis showed the presence of cells expressing NKG7, a gene involved in granule exocytosis and highly involved in anti-tumor immunity. Single cells expressing as granzyme H, cathepsin-W, and granulysin were also identified. The study of this case suggests that CML was controlled for a long period of time, potentially via an immune surveillance phenomenon. The role of NKG7 expression in the occurrence of treatment-free remissions (TFR) should be evaluated in future studies.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative neoplasm initiated by the occurrence of the Philadelphia chromosome (Ph1) in a primitive hematopoietic stem cell. The natural history of disease from a chronic phase towards accelerated and blast phases has now been drastically modified by the introduction of the targeted therapies. BCR::ABL1 tyrosine kinase inhibitors (TKIs) improved overall survival in patients with chronic phase CML (1), and recent data suggest that the life expectancy of CML patients responding to TKI could currently be similar to that of the general population of the same age and sex (2).
Up to 80% of newly diagnosed CML patients in chronic phase (CP) achieve a molecular response under TKI therapies especially by the use of second-generation TKI such as Nilotinib (3). A major issue remaining today in the CML field is the persistence of stem cells in deep molecular response due to the resistance of leukemic stem cells (LSC) to TKI (4–6). Indeed, TKI treatment is unable to eliminate quiescent stem cell fraction (7), and the survival of LSC is independent on BCR::ABL TK activity (8). In half of the CML patients in deep molecular responses, TKI discontinuation can allow a treatment-free remission (TFR) (9, 10). In several TKI-discontinuation trials, it has been shown that an immunological mechanism contributes to the absence of relapse, in particular with increased levels of NK cells (11).
We report here the case of a patient who voluntarily stopped her TKI therapy and remained in deep molecular response without therapy for 18 months. At the end of this period of 18 months, which was operationally a TFR, a molecular relapse was diagnosed, but the patient declined therapy for an additional 3 years until hematological relapse. A transcriptome analysis was performed at several time points of this TKI-discontinuation condition with low or high BCR::ABLIS levels. In addition, a single-cell transcriptome analysis of the circulating mononuclear cells was performed.
Case description
A CML was diagnosed in 2014 in a 30-year-old female patient with low Sokal score. She was initially treated with Imatinib, but this drug was rapidly stopped because of intolerance, and she was switched to Dasatinib (Figure 1). A deep molecular response (RM4) was rapidly obtained with a clinical and molecular follow-up every 3 months. At January 2016, she stopped her therapy voluntarily. In August 2016, molecular analysis identified a relapse with BCR::ABL/ABLIS levels, which rose to 3%. She disclosed at that time that she was not taking Dasatinib. Despite this molecular recurrence, she declined therapy while accepting regular hematological and molecular analyses. BCR::ABLIS levels rose to 25% in October 2017 and to 38% in September 2019 (Figure 1). During this 3-year period of molecular progression, blood counts and clinical examinations remained normal. In October 2019, BCR::ABLIS was 51%, and she was in hematological relapse. She refused bone marrow aspirate but accepted to start TKI therapy by Dasatinib 100 mg/day. After an initial response, she reduced the dose of Dasatinib to 50 mg/day because of GI intolerance and stopped it again in September 2020. In March 2021, she was again in hematological relapse with BCR::ABLIS level at 59%. She accepted Dasatinib at a very low dose (20 mg/day), which induced a major molecular response (MMR) that is currently ongoing.
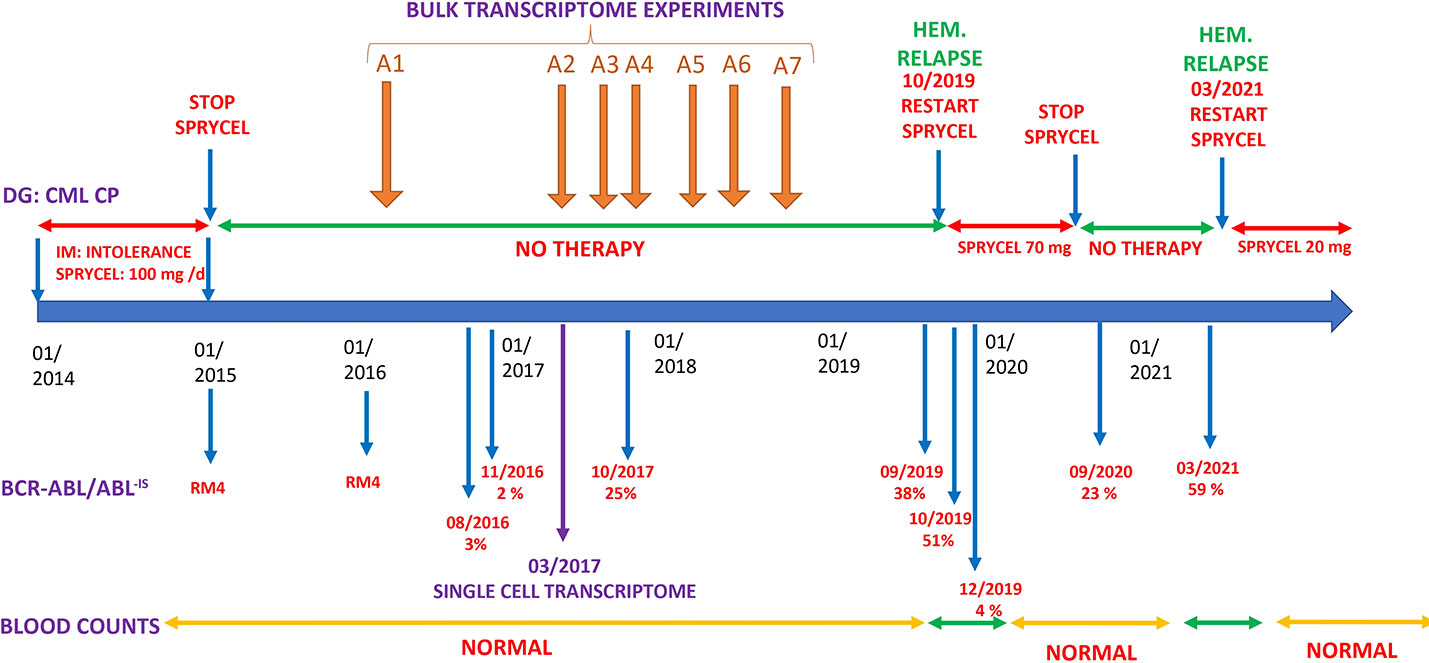
Figure 1 Clinical history. DG, diagnosis; IM, imatinib; Hem. relapse, hematological relapse (see text).
To gain insights into the molecular events occurring during this “self-induced” drug interruption, we have performed with the informed consent of the patient, serial transcriptome analyses in frozen peripheral blood cells (PBMCs). The first three were performed during periods of low BCR::ABL expression, whereas the last four were performed in samples with high BCR::ABL expression (Figure 1). All these analyses were performed without TKI administration. In one sample, a single-cell RNA-seq analysis was performed. For all retrospective samples for which BCR::ABLIS quantification was performed, peripheral blood mononuclear cells have been processed to perform total RNA extraction and whole transcriptome experiment on microarray chip ClariomS human (Thermo Fisher Scientific).
Discussion
Although this patient declined therapy for a long period of time (5 years), the progression of the disease was very slow. This prompted us to perform bulk transcriptome analyses from serially collected blood samples to study the expression of the genes involved during this natural history (Figure 1). For each timepoint of these samples, the results of BCR::ABLIS quantifications were available. BCR::ABL-IS quantification was taken as predictor for Pavlidis Template Matching algorithm (12) to elucidate gene expression profile, which followed the amount of BCR::ABL transcript during the time course of the disease. After false discovery rate adjustment of the gene signature, 188 genes were found to be connected to BCR::ABL-IS (Supplemental Table S1). One of the top associated molecule was the BCR transcript (rank = 30), suggesting a good correlation between BCR::ABL-IS quantification and transcriptome time-course experiments.
Evidence of the enrichment of an immunological signature during the time out of therapy
Functional enrichment performed with time course transcriptome expression profile (Supplemental Table S1) on ToppCell Atlas database revealed that this signature mainly characterized natural killer-T (NK-T) lymphocyte cell type (Figure 2A), with specific upregulation of IL21R, ADGR1, S1PR5, AUTS2, BCR, BPGM, ANKRD16, EPHA4, OCLN, CHIC1, and CEP41 (Figure 2B). These results suggested that during the natural evolution of the disease, the molecular signature of an NK/T lymphocyte cell population could be detected in the PBMC.
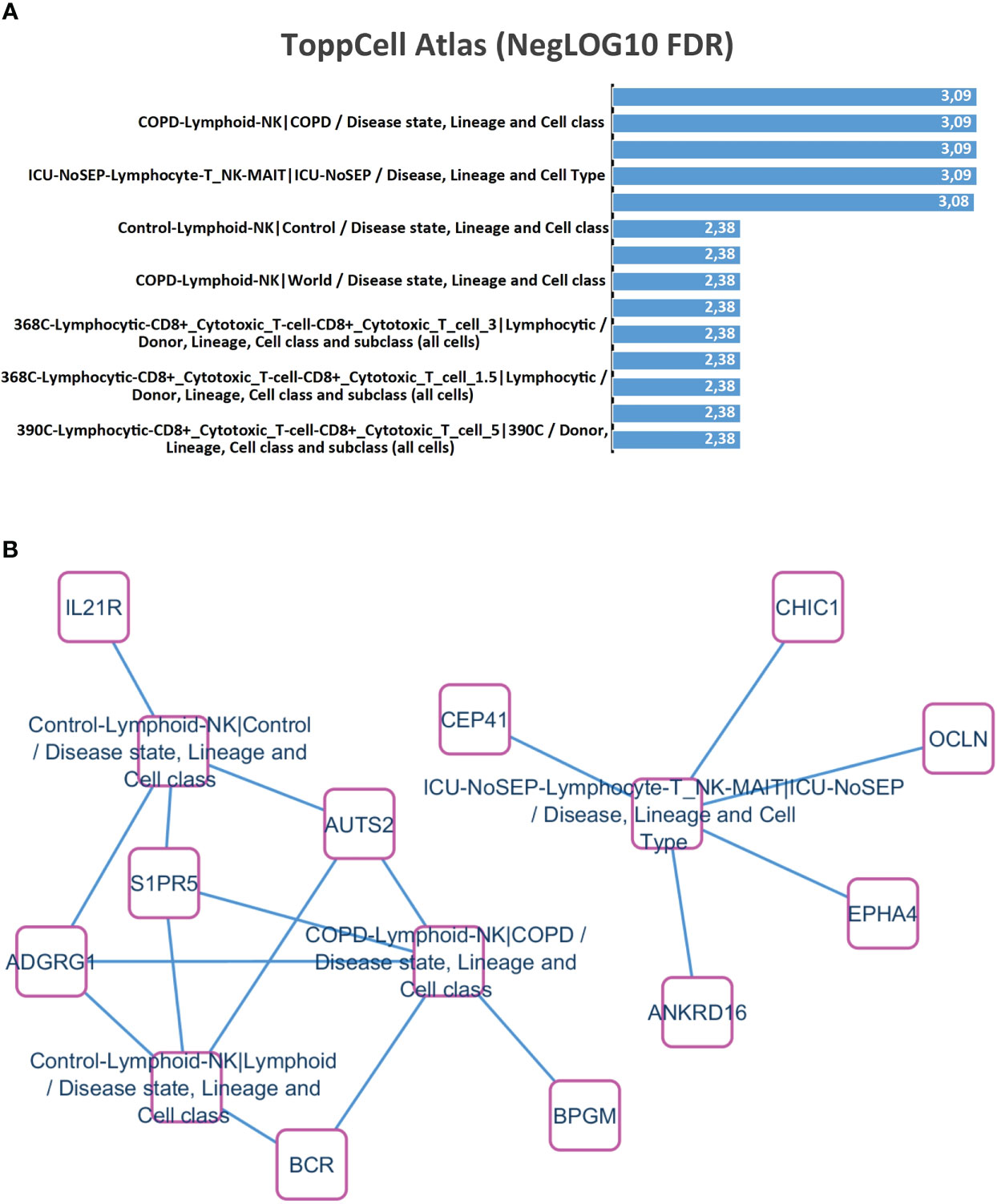
Figure 2 NK/T lymphocyte cell-type enrichment during time course transcriptome experiments correlated with BCR::ABL-IS quantification. (A) Barplot of functional enrichment performed on ToppCell Atlas database (negative log10 of false discovery rate p-values); (B) NK-T lymphocyte cell enrichment network.
A single-cell RNA-seq analysis was performed on the PBMC sample collected on March 2017 where the patient had evident molecular progression with a blood BCR::ABL-IS level at 2%. She was out of therapy at that time (Figure 1), and her blood counts were normal.
Single-cell transcriptome of 9,036 PBMC could be demultiplexed after Cell Ranger pipeline. A mean number of 9,054 transcripts has been found to be expressed by cell. After preprocessing of single-cell transcriptome of PBMCs, dimension reduction by PCA (Figure 3A) and UMAP identified three main topological cell clusters including T-lymphocytes (expressing CD3E), B-lymphocytes (expressing MS4A1), and monocytes expressing CD68 (Figure 3B). UMAP dimension reduction conjugated to unsupervised clustering identified 11 distinct cell communities (Figure 3C), which expressed distinct patterns of markers (Figure 3D). Clusters 7–10 were found to be less represented quantitatively in the blood of the patient (Figure 3D).
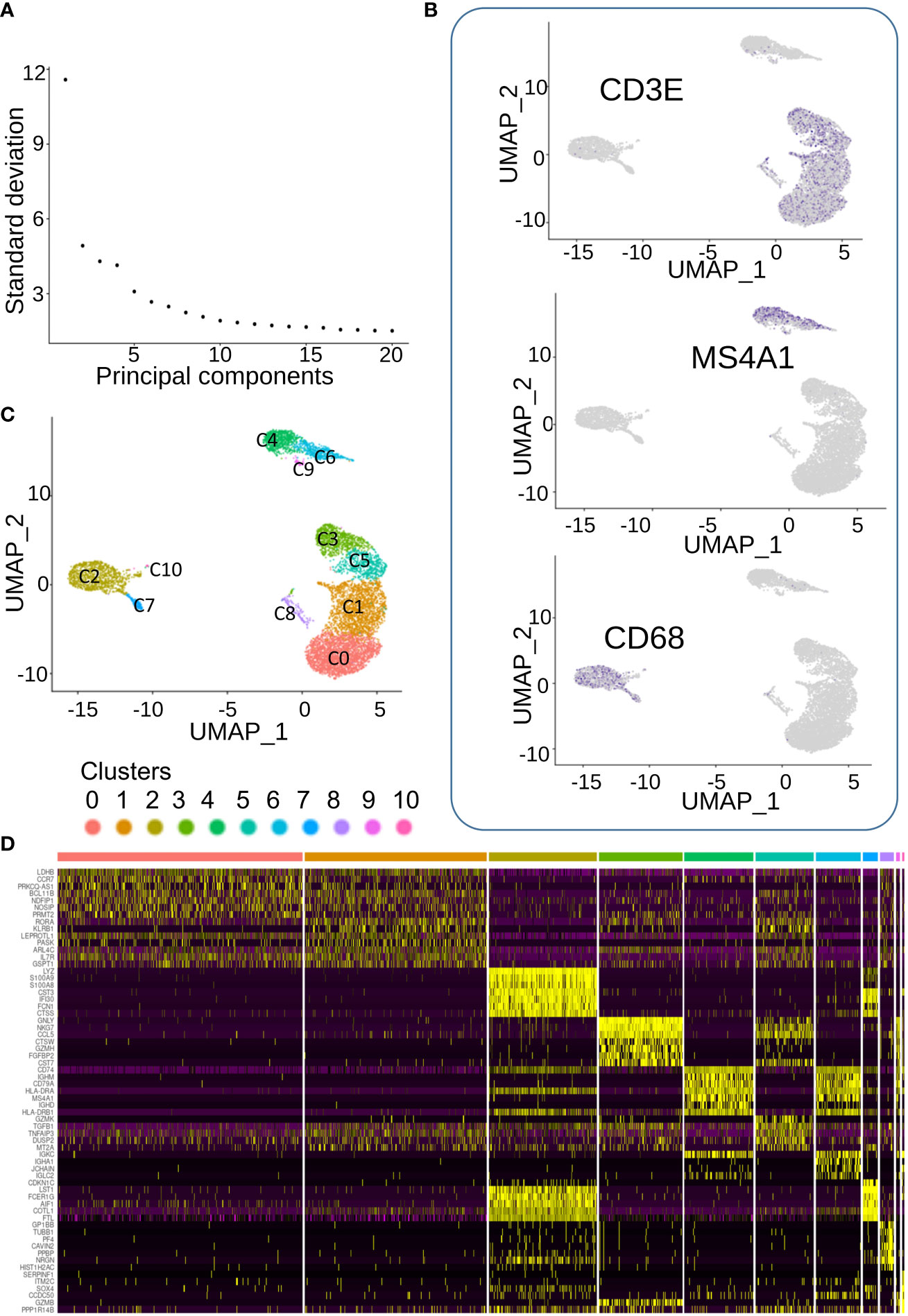
Figure 3 Single cell transcriptome analysis of CML blood cells. (A) Elbow plot of dimension reduction. (B) Feature plot of markers characterizing three main cell populations: CD3E for T lymphocyte, CD68 for monocytes, and MS4A1 for B lymphocytes. (C) Unsupervised clustering on UMAP dimension reduction. (D) Expression heatmap of best molecular markers of the eleven cell communities.
Clusters 0 and 1, which were the largest ones, seemed to share expression of markers (Figure 3D) corresponding to T lymphocytes (Figures 3B, C). In terms of expression specificity, cluster 5 shared some markers with clusters 0 and 1(Figure 3D). Interestingly, among the cluster of T-lymphocytes (Figures 3B, C), we could observe another cluster (cluster C3) that expressed a distinct signature from the three other T-lymphocyte clusters (0, 1, and 5, Figure 3D). Among the best markers characterized during single-cell transcriptome analysis, a high expression of NKG7 was revealed in individual cluster C3 (Figure 4A), which correspond to a subpopulation of CD3E+ T lymphocytes (Figure 4B). Differential expression analysis between the distinct cell clusters showed that a high expression of natural killer effective markers characterized cluster C3 (Figure 4C), such as GNLY (granulysin), GZMH (granzyme H), and CTSW (cathepsin W). In addition, a high expression of CCL5 chemokine and FGFBP2 secreted protein (cytotoxic marker) was found in cluster C3 of natural killer cells from this patient (Figure 4C). These molecular results on single-cell transcriptome performed on PBMC suggested therefore the presence of an immunologically competent cells in the blood sample analyzed.
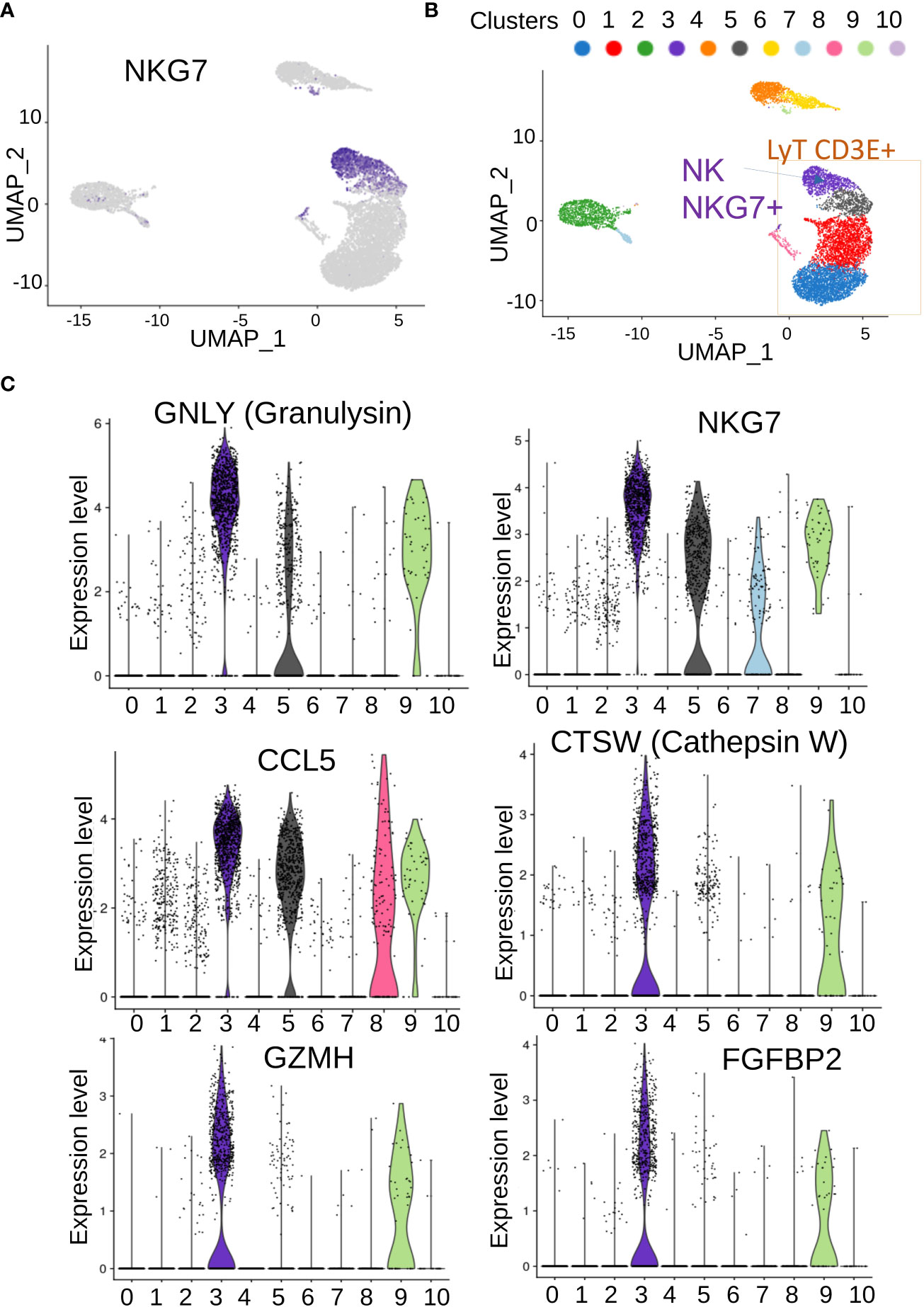
Figure 4 Characterization of genes associated with NK/T cells in PBMC. (A) UMAP of NKG7-positive cells in single-cell transcriptome; (B) inference of NKG7-positive cell on cells communities identified; (C) best over expressed markers identified in cluster 3 of NKG7-positive cells.
Interestingly, functional enrichment of the gene signature on ToppCell atlas signature highlighted that activation of genes involved in natural killer/T lymphocytes following BCR::ABL quantification. NK cells play important roles in innate immunity against virus or tumors by secreting cytotoxic granules (13). In the case reported here, the transcriptomic profile was compatible with both NK and a NK/T-cell population, the latter known to exhibit anti-leukemia activity (14, 15). The deficient function of the invariant NK/T cells in CML and their restoration on TKI therapy has previously been reported (16, 17). The role of NK cells in CML immune surveillance has also been suggested in patients remaining in deep molecular response after TKI discontinuation (11).
We then analyzed genes expressed in NK cell clusters among NK-T lymphocyte population in single-cell transcriptome, while the patient was in molecular recurrence and without TKI therapy but without hematological relapse. This NK cell cluster was positive for NKG7 expression and cells highly expressed effectors of cytotoxicity genes. Granulysin is an antimicrobial peptide (AMP) highly active against cancer cells such as melanoma (18). Cathepsin W (lymphopain) is a papain-like cysteine protease of unknown function that is specifically expressed in NK cells and to a lesser extent in cytotoxic T cells (CTL), and its expression in NK-92 (NK cell model) is linked to the cytotoxicity observed against K562 (19). Granzyme H is well-known cytotoxic effector of NK cells (20). NKG7+ positive NK cell cluster also highly expressed FGFBP2-secreted protein that is known to be a marker of the cytotoxicity of lymphocytes (21).
These molecular findings could also be linked to the immuno-molecular data obtained from the whole transcriptome analyses. Indeed, transcriptome experiments showed that the IL21R expression (receptor to interleukin 21) followed the BCR::ABL-IS quantification analyses during TKI interruption period. IL-21 has been shown to participate to the NK cell expansion of K562 cell line engineered to express membrane-bound IL-21 feeder in combination with IL-2 (22, 23).
S1P (5) receptor found in NK cell network is also known to be essential for human NK cell trafficking (24, 25). Another interesting receptor expression was that of GPR56, which is an inhibitory receptor of NK cells, negatively regulating effector functions such as production of inflammatory cytokines and cytolytic proteins, degranulation, and target cell killing through association with the tetraspanin CD81 (26).
Overall, the molecular analyses reported during the long-term TKI discontinuation in this patient allowed to detect major modulation of genes involved with NK cell activity. Interestingly, several genes that we identified to be part of the immunological pathway seemed to be activated with a successful TFR after TKI discontinuation (10, 27–29). Importantly we have identified for the first time NKG7 expression in single cell transcriptome from CML PBMC, this gene being a major regulator of granule exocytosis in cancer (30). Its expression deserves further evaluation in patients with CML.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving human participants were reviewed and approved by INSERM ethical committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the patients/participants for the publication of this case report.
Author contributions
JI, PM, NO and DC: Performed molecular Single cell experiments; CD: Bioinformatics analyses; XF performed BCR-ABL quantification experiments, JF, AB-G: validated BCR-ABL data, AT, AB-G: Clinical follow-up, plan experiments, JI, PM, CD, AB-G, AT: wrote the paper. All authors contributed to the article and approved the submitted version
Funding
This study was performed with the internal funds from Inserm.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1117781/full#supplementary-material
References
1. Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. New Engl J Med (2017) 376(10):917–27. doi: 10.1056/NEJMoa1609324
2. Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM-L. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol (2016) 34(24):285157. doi: 10.1200/JCO.2015.66.2866
3. Kantarjian HM, Hughes TP, Larson RA, Kim D-W, Issaragrisil S, le Coutre P, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia (2021) 35(2):44053. doi: 10.1038/s41375-020-01111-2
4. Chomel J-C, Bonnet M-L, Sorel N, Bertrand A, Meunier M-C, Fichelson S, et al. Leukemic stem cell persistence in chronic myeloid leukemia patients with sustained undetectable molecular residual disease. Blood (2011) 118(13):365760. doi: 10.1182/blood-2011-02-335497
5. Chomel JC, Bonnet M-L, Sorel N, Sloma I, Bennaceur-Griscelli A, Rea D, et al. Leukemic stem cell persistence in chronic myeloid leukemia patients in deep molecular response induced by tyrosine kinase inhibitors and the impact of therapy discontinuation. Oncotarget (2016) 7(23):35293301. doi: 10.18632/oncotarget.9182
6. Chu S, McDonald T, Lin A, Chakraborty S, Huang Q, Snyder DS, et al. Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood (2011) 118(20):556572. doi: 10.1182/blood-2010-12-327437
7. Graham SM, Jørgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood (2002) 99(1):319–25. doi: 10.1182/blood.v99.1.319
8. Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest (2011) 121(1):396409. doi: 10.1172/JCI35721
9. Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Intergroupe français des leucémies myéloïdes chroniques. discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre stop imatinib (STIM) trial. Lancet Oncol (2010) 11(11):1029–35. doi: 10.1016/S1470-2045(10)70233-3
10. Saussele S, Richter J, Guilhot J, Gruber FX, Hjorth-Hansen H, Almeida A, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol (2018) 19(6):747–57. doi: 10.1016/S1470-2045(18)30192-X
11. Hughes A, Clarson J, Tang C, Vidovic L, White DL, Hughes TP, et al. CML patients with deep molecular responses to TKI have restored immune effectors and decreased PD-1 and immune suppressors. Blood (2017) 129(9):116676. doi: 10.1182/blood-2016-10-745992
12. Pavlidis P, Noble WS. Analysis of strain and regional variation in gene expression in mouse brain. Genome Biol (2001) 2(10):2859–68. doi: 10.1186/gb-2001-2-10-research0042
13. Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? the example of natural killer cells. Sci (New York N.Y.) (2011) 331(6013):4449. doi: 10.1126/science.1198687
14. Yan Y, Steinherz P, Klingemann HG, Dennig D, Childs BH, McGuirk J, et al. Antileukemia activity of a natural killer cell line against human leukemias. Clin Cancer Res (1998) 4(11):285968. doi: 10.1021/pr200210w
15. Yotnda P, Firat H, Garcia-Pons F, Garcia Z, Gourru G, Vernant JP, et al. Cytotoxic T cell response against the chimeric P210 BCR-ABL protein in patients with chronic myelogenous leukemia. J Clin Invest (1998) 101(10):229096. doi: 10.1172/JCI488
16. Shimizu K, Hidaka M, Kadowaki N, Makita N, Konishi N, Fujimoto K, et al. Evaluation of the function of human invariant NKT cells from cancer patients using alpha-galactosylceramide-loaded murine dendritic cells. J Immunol (2006) 177(5):3484–92. doi: 10.4049/jimmunol.177.5.3484
17. Rossignol A, Levescot A, Jacomet F, Robin Aurélie, Basbous S, Giraud C, et al. Evidence for BCR-ABL-Dependent dysfunctions of INKT cells from chronic myeloid leukemia patients. Eur J Immunol (2012) 42(7):187075. doi: 10.1002/eji.201142043
18. Al-Wasaby S, Guerrero-Ochoa P, Ibáñez-Pérez R, Soler R, Conde B, Martínez-Lostao L, et al. In vivo potential of recombinant granulysin against human melanoma. Cancer Treat Res Commun (2021) 27:100355. doi: 10.1016/j.ctarc.2021.100355
19. Wex T, Wex H, Hartig R, Wilhelmsen S, Malfertheiner P. Functional involvement of cathepsin W in the cytotoxic activity of NK-92 cells. FEBS Lett (2003) 552(23):11519. doi: 10.1016/s0014-5793(03)00895-0
20. Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol (2015) 15(6):388–400. doi: 10.1038/nri3839
21. Ogawa K, Tanaka K, Ishii A, Nakamura Y, Kondo S, Sugamura K, et al. A novel serum protein that is selectively produced by cytotoxic lymphocytes. J Immunol (2001) 166(10):6404–12. doi: 10.4049/jimmunol.166.10.6404
22. Streltsova MA, Barsov E, Erokhina SA, Kovalenko EI. Retroviral gene transfer into primary human NK cells activated by IL-2 and K562 feeder cells expressing membrane-bound IL-21. J Immunol Methods (2017) 450(novembre):9094. doi: 10.1016/j.jim.2017.08.003
23. Kweon S, Phan M-TT, Chun S, Yu H, Kim J, Kim S, et al. Expansion of human NK cells using K562 cells expressing OX40 ligand and short exposure to IL-21. Front Immunol (2019) 10:879. doi: 10.3389/fimmu.2019.00879
24. Drouillard A, Mathieu A-L, Marçais A, Belot A, Viel S, Mingueneau M, et al. S1PR5 is essential for human natural killer cell migration toward sphingosine-1 phosphate. J Allergy Clin Immunol (2018) 141(6):2265–2268.e1. doi: 10.1016/j.jaci.2017.11.022
25. Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, et al. T-Bet-Dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med (2009) 206(11):246981. doi: 10.1084/jem.20090525
26. Chang G-W, Hsiao C-C, Peng Y-M, Braga FAV, Kragten NAM, Remmerswaal EBM, et al. The adhesion G protein-coupled receptor GPR56/ADGRG1 is an inhibitory receptor on human NK cells. Cell Rep (2016) 15(8):175770. doi: 10.1016/j.celrep.2016.04.053
27. Ilander M, Olsson-Strömberg U, Schlums H, Guilhot J, Brück O, Lähteenmäki H, et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia (2017) 31(5):110816. doi: 10.1038/leu.2016.360
28. Rea D, Henry G, Khaznadar Z, Etienne G, Guilhot F, Nicolini F, et al. Natural killer-cell counts are associated with molecular relapse-free survival after imatinib discontinuation in chronic myeloid leukemia: The IMMUNOSTIM study. Haematologica (2017) 102(8):136877. doi: 10.3324/haematol.2017.165001
29. Kumagai T, Nakaseko C, Nishiwaki K, Yoshida C, Ohashi K, Takezako N, et al. Dasatinib cessation after deep molecular response exceeding 2 years and natural killer cell transition during dasatinib consolidation. Cancer Sci (2018) 109(1):18292. doi: 10.1111/cas.13430
Keywords: chronic myelogenous leukemia, natural killer cells, NKG7, TFR, CML
Citation: Imeri J, Desterke C, Marcoux P, Chaker D, Oudrhiri N, Fund X, Faivre J, Bennaceur-Griscelli A and Turhan AG (2023) Case report: Long-term voluntary Tyrosine Kinase Inhibitor (TKI) discontinuation in chronic myeloid leukemia (CML): Molecular evidence of an immune surveillance. Front. Oncol. 13:1117781. doi: 10.3389/fonc.2023.1117781
Received: 06 December 2022; Accepted: 16 February 2023;
Published: 16 March 2023.
Edited by:
Simona Soverini, University of Bologna, ItalyReviewed by:
Michele Bianchini, National Scientific and Technical Research Council (CONICET), ArgentinaDaniela Damiani, Hematology and Stem Cell Transplant, Italy
Fabio Guolo, San Martino Hospital (IRCCS), Italy
Copyright © 2023 Imeri, Desterke, Marcoux, Chaker, Oudrhiri, Fund, Faivre, Bennaceur-Griscelli and Turhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali G. Turhan, dHVydml2MzNAZ21haWwuY29t
 Jusuf Imeri
Jusuf Imeri Christophe Desterke1,2
Christophe Desterke1,2 Paul Marcoux
Paul Marcoux Ali G. Turhan
Ali G. Turhan