- 1Department of Gastrointestinal Surgery, Chengdu Second People’s Hospital, Chengdu, Sichuan, China
- 2Lung Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Department of General Surgery, Affiliated Hospital of Zunyi Medical University, Affiliated Digestive Hospital of Zunyi Medical University, Zunyi, China
Introduction: The number of overweight patients with gastric cancer (GC) is increasing, and no previous study has compared laparoscopic gastrectomy (LG) and robotic gastrectomy (RG) in obese patients with GC. To investigate the perioperative and oncologic outcomes of RG and LG in obese GC patients, we performed a meta-analysis of propensity matched scores and retrospective studies to compare the perioperative parameters, oncologic findings, and short-term postoperative outcomes between the two groups.
Methods: This study was performed according to the PRISMA guidelines. A search was performed on PubMed, Web of Science, EMBASE, and Cochrane Central Register to identify eligible propensity matched scores and retrospective studies conducted and published before December 2022. Data on perioperative and oncological outcomes were included in the meta-analysis.
Results: Overall, we identified 1 propensity score match study and 5 randomized control trials of RG and LG, enrolling a total of 718 patients (197 and 521 patients received RG and LG, respectively). No significant differences were observed between the two groups in terms of complications, bleeding, or lymph node dissection. Of note, RG had a longer procedure time (P = 0.03), earlier oral intake (P = 0.0010), shorter hospital stay (P = 0.0002), and shorter time to defecation (P < 0.00001).
Conclusions: This meta-analysis concluded that patients in the RG group had shorter hospital stays, earlier postoperative feeding, and earlier postoperative ventilation; however, no differences were found in blood loss, number of lymph nodes removed, or overall complications. RG is an effective, safe, and promising treatment for obese patients with GC, compensating for the shortcomings of laparoscopy and allowing for less trauma and faster recovery.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022298967.
1 Introduction
Gastric cancer (GC) is a disease of global concern. With more than 1 million new cases of GC annually, GC is the fifth most commonly diagnosed malignancy and third most common cause of cancer-related deaths worldwide (1). GC is a complexand heterogeneous disease, resulting from the interaction among genetic, environmental, and host factors (2). Recent summaries and epidemiological studies conducted by the International Agency for Research on Cancer have reiterated obesity as a risk factor for GC (3), with the strength of the positive association between excess weight and GC risk increasing as a function of increasing body mass index (BMI) (4). Since 1980, the number of obese patients has tripled in 70 countries (5), with obesity having become a global epidemic, affecting more than 600 million adults worldwide (6). The Japanese Association for the Study of Obesity defines obesity as a BMI ≥25 kg/m2 (2, 7), with the criteria of the World Health Organization (WHO) differentiating between overweight (BMI between 25-30 kg/m2) and obesity (BMI >30 kg/m2) (8).
For patients with GC, radical gastrectomy with D2 lymph node dissection remains the standard treatment (9). However, minimally invasive surgery (MIS), including laparoscopic and robotic approaches, has become an effective option for the treatment of GC, particularly in patients with early-stage tumors (10, 11). Compared to conventional open gastrectomy, laparoscopic gastrectomy (LG) offers the advantages of minimal invasiveness, which include good visualization and magnification of the anatomy; less surgical trauma and pain; less intraoperative blood loss; and earlier postoperative recovery (12, 13). Since the first successful LG was reported by Kitano et al. (14) in 1994, LG has routinely been used worldwide to treat GC (15). However, obesity is considered as a major technical limitation of laparoscopic surgery, with a large amount of visceral fat narrowing the surgical field (16). Moreover, the volume and fragility of abdominal fat can also hinder pancreatic tissue and fat deposition and cause error in the intraoperative anatomical plane, resulting in inadequate lymph node dissection, which can be further exacerbated by lymph node clearance tissue and blood exudate (17).
In order to address the limitations of conventional LG in obese patients, the feasibility of robot-assisted gastrectomy (RG) has inferred from the results of earlier publications (17). Robotic surgery systems were created to overcome the effect of surgeons’ physiological hand tremor and provide a larger and more accurate range of motion, as well as to provide surgeons with a 3-dimensional (3D) magnified view of the surgical field and an ergonomic surgical environment (18). Since first reported by Hashizume et al. (19) in 2002, studies on RG have been increasingly reported. However, differences in performance parameters and surgical outcomes between robotic and laparoscopic surgery for GC have not been evaluated in obese patients. The use of MIS in this high-risk group could be improved by quality evidence regarding the risk and benefits of RG in obese patients with GC, an important clinical issue considering the rapid increase in incidence of both obesity and GC. Therefore, our aim was to perform a meta-analysis to compare perioperative parameters, oncologic findings, and short-term postoperative outcomes between RG and LG performed in obese patients with GC.
2 Methods
2.1 Search strategy and study selection
The protocol for this study was prospectively registered with PROSPERO (registration number CRD42022298967) (20) and the methods adhered to the PRISMA guidelines. The PICO model was used to ensure completeness and accuracy of the search strategy. The population of interest was obese patients with GC. The interventions evaluated were RG and LG, with outcomes compared between the two procedures or a control group. The following outcomes were evaluated: operative time, volume of blood loss, extent of lymph node dissection, overall rate of complications, time to first flatus, time to oral intake, and length of hospital stay.
A systematic literature search for published propensity score match (PSM) studies and randomized control trials (RCTs) was performed in PubMed, EMBASE, Web of Science, and Cochrane Central Register, from January 2003 to December 2022. The following keywords were used for the search: gastric cancer, obesity, laparoscopic gastrectomy, robotic gastrectomy, propensity score matching, retrospective studies, and minimally invasive surgery. A manual search of the reference lists of included studies was performed to identify additional relevant references.
2.2 Selection criteria and exclusion criteria
Two reviewers (XY and LZ) performed an independent screening of identified studies. In the first stage, the abstracts and titles were screened to include potentially relevant studies. Full texts of selected studies were subsequently retrieved and reviewed entirely to confirm inclusion. Disagreements were resolved by consensus with a third author (QF). The selection criteria for studies were as follows: (1) participants – mean age >18 years, confirmed diagnosis of GC, and obesity, defined by a BMI ≥25 kg/m2; (2) types of interventions – RG and LG; (3) types of studies – PSM and retrospective studies; and (4) inclusion of necessary data for statistical analysis or at least one of the following clinical outcomes – estimated blood loss, time to flatus, time to oral intake, number of harvested lymph nodes, operative time, length of hospital stay, and overall rate of complications. The exclusion criteria were as follows: (1) absence of differentiation between patients with GC and other indications for surgery; (2) reported on open gastrectomy only; (3) combined reporting of outcomes for LG and RG; and (4) non-comparative study designs, such as letters, reviews, comments, posters, and agreements. Observational studies in which patients were sampled based on exposure were included as cohort studies, whereas those in which patients were sampled based partially or entirely on outcomes were included as case series, irrespective of the sample size.
2.3 Data extraction and quality assessment
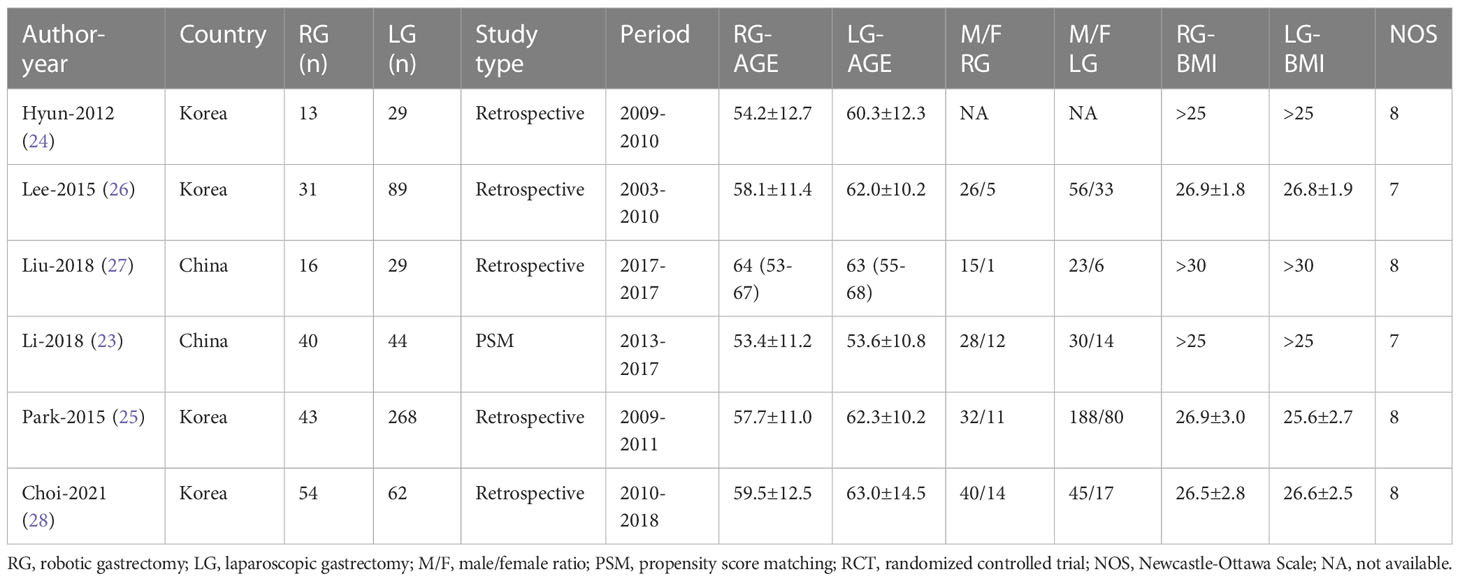
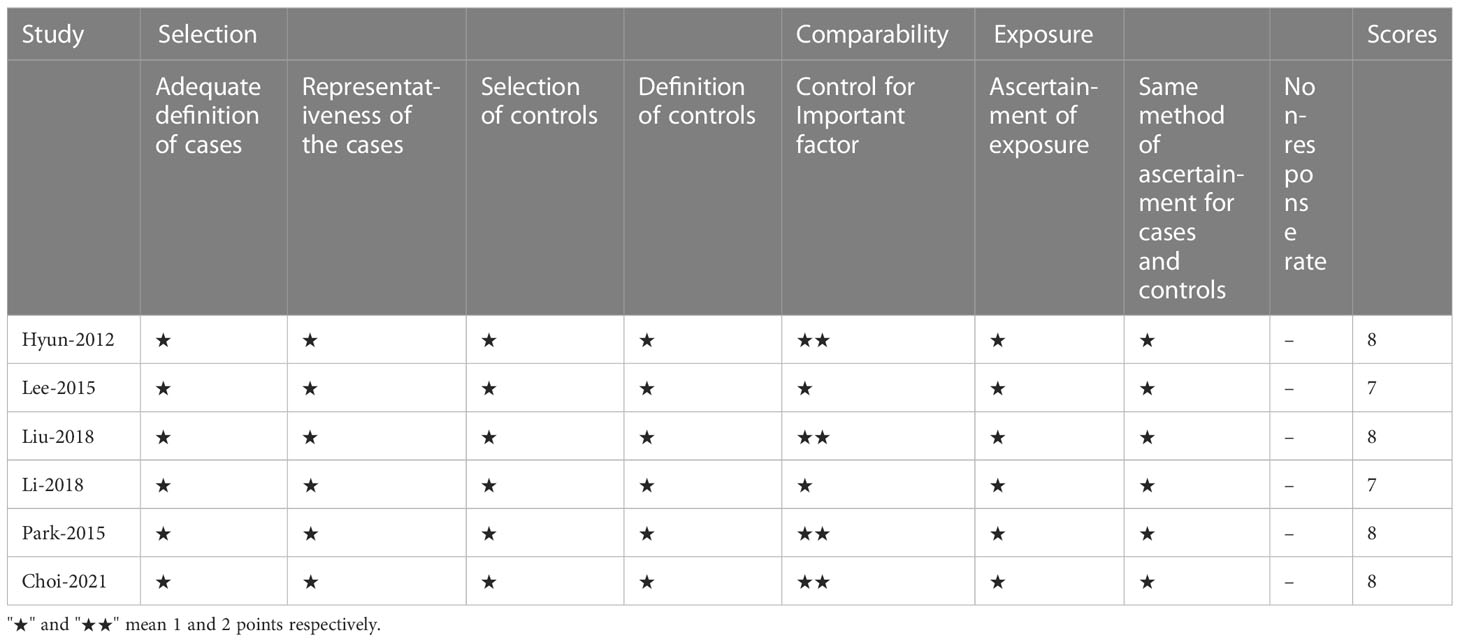
XY imported the search results into the document manager EndNoteX9. After eliminating duplicate documents, XY and LZ screened the documents by browsing titles, abstracts, and full-text reading, and XY and LZ independently extracted data from the literature that met the inclusion and exclusion criteria. The major data extraction included the following: name of first or corresponding author, study design, publication year, country, sample size, mean age, sex, BMI, operative time, bleeding, overall complications, number of retrieved lymph nodes, time to first flatus, time to oral intake, and hospital length of stay. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of studies (21). All included studies were independently evaluated by two authors (XY and LZ), and RCTs and PSM studies with an NOS score >6 were considered to be high-quality studies.
2.4 Statistical analysis
Meta-analyses were performed using Review Manager (version 5.3). The 95% confidence interval (CI) and mean difference (MD) were used for continuous data, whereas the odds ratio (OR) with 95% CI were used for dichotomous data. When outcomes were reported as a median and range, the methods described by Hozo et al. (22) were used to convert the values to the mean and standard deviation. Statistical heterogeneity was quantified using Higgin’s I2 index. Larger values of I2 indicate increasing heterogeneity, with values of 25%, 50%, and 75% reflecting low, moderate, and high degrees of heterogeneity, respectively. If the analysis still showed significant heterogeneity, the random effects model was used for meta-analysis. A sensitivity analysis was performed by omitting one study at a time and recalculating the combined OR. Finally, Egger’s test and Begg’s method were used to evaluate bias.
3 Results
3.1 Characteristics of the included studies
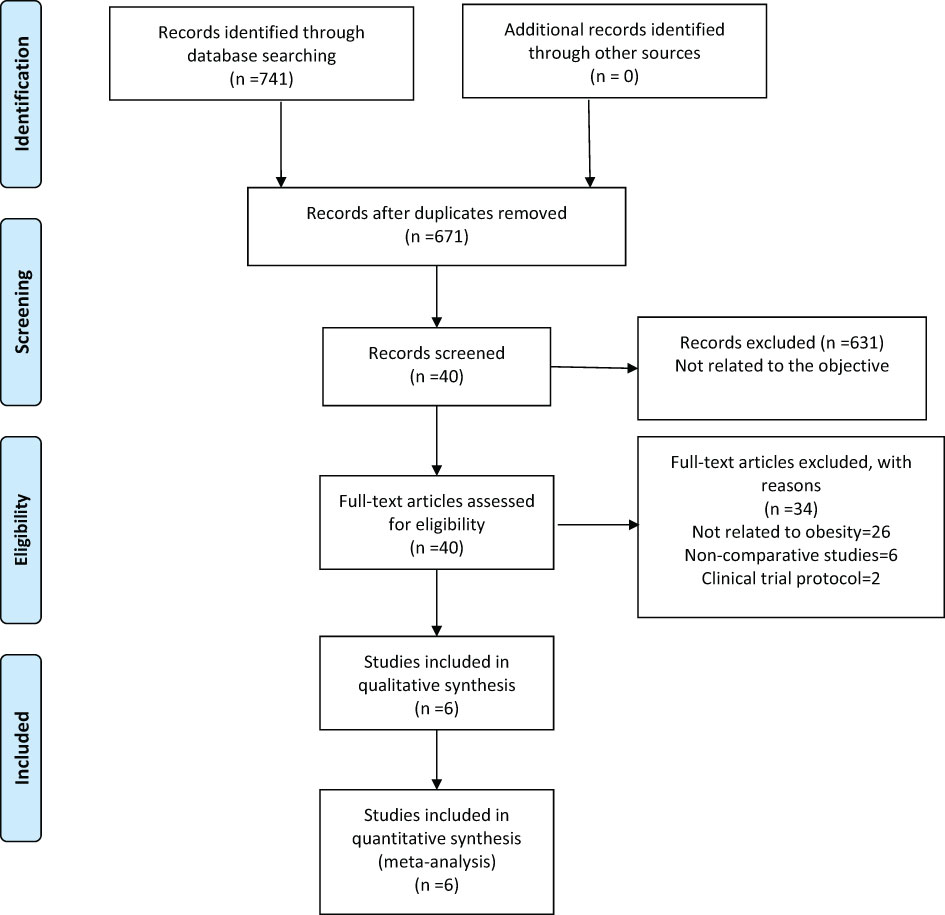
Our search retrieved 741 publications, of which six were included in the meta-analysis, one PSM (23) and five retrospective (24–28) studies, published between 2012 and 2018, reporting on a total of 718 patients. Of these, 197 patients were treated with RG for GC and 521 with LG. The detailed flow chart of the identification, screening, and selection of studies is shown in Figure 1. The characteristics and demographic data of patients included, as well as the summary of NOS of all included studies, are summarized in Table 1. The six studies included were of relatively high quality, according to the NOS (7-8) (Table 2).
3.2 Short-term outcomes
3.2.1 Operative time
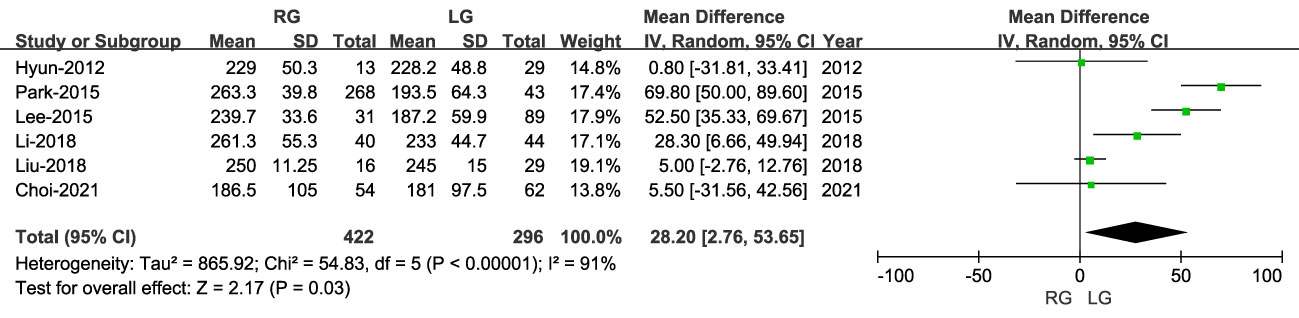
The operative time was reported in six studies, with a longer operative time for RG than LG (MD: 28.20 min; 95% CI: 2.76 to 53.65; P < 0.00001), with high heterogeneity (I2 = 91%) (Figure 2).
3.2.2 Blood loss
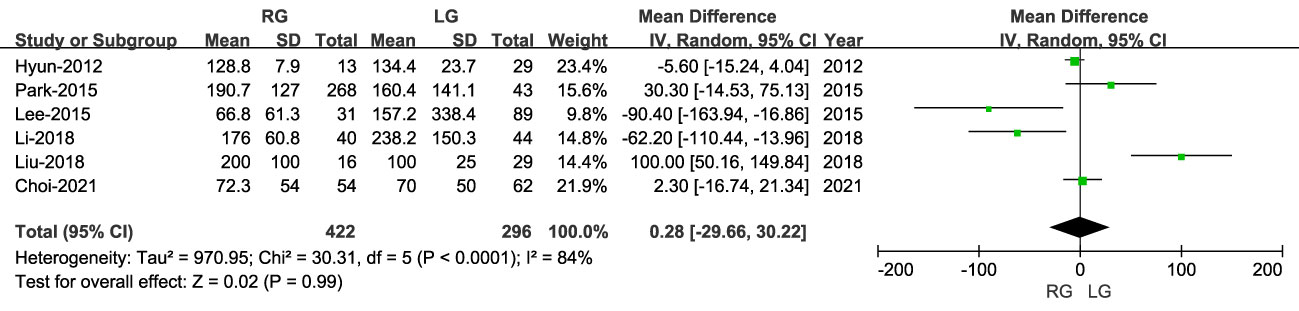
The volume of blood loss was reported in six studies, including 718 patients, with lower blood loss for RG than LG (MD: 0.28 ml; 95% CI: -29.66 to 30.22; P = 0.99), with high heterogeneity (I2 = 84%) (Figure 3).
3.2.3 Number of retrieved lymph nodes
The number of harvested lymph nodes was reported in six studies, with the extent of lymph node dissection not being different between RG and LG (MD: 1.50; 95% CI: -3.25 to 6.26; P = 0.54), with high heterogeneity (I2 = 76%) (Figure 4).
3.3 Postoperative outcomes
3.3.1 Overall complications
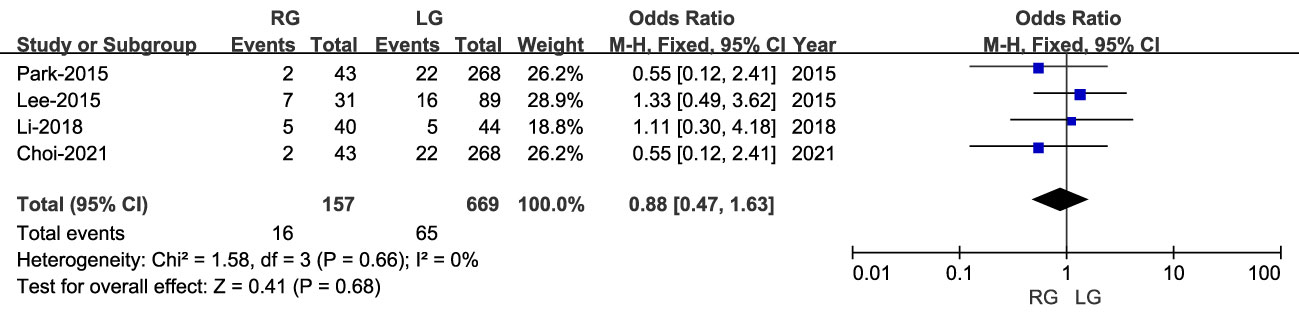
Overall surgical complications were reported in four studies, including 631 patients (168 RG and 463 LG). Although the rate of overall complications was lower for RG than LG, this difference was not statistically significant (OR: 0.99; 95% CI: 0.55 to 1.79; P = 0.98), with low heterogeneity (I2 = 0%) (Figure 5).
3.3.2 Time to first flatus
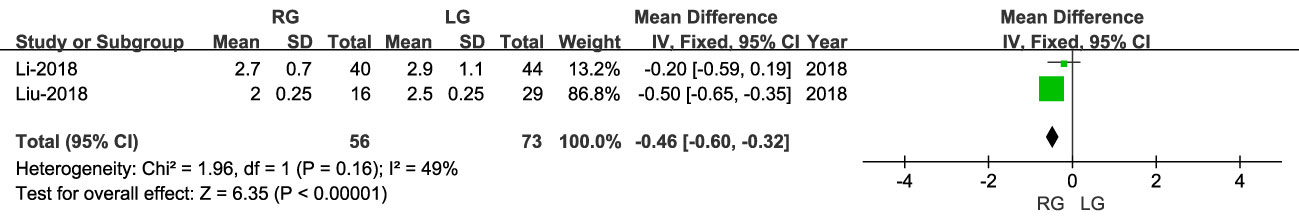
Time to first flatus was reported in two studies, including 129 patients (56 RG and 73 LG), and was lower for RG than LG (OR: -0. 46; 95% CI: -0.60 to -0.32; P < 0.00001), with moderate heterogeneity (I2 = 49%) (Figure 6).
3.3.3 Time to oral intake
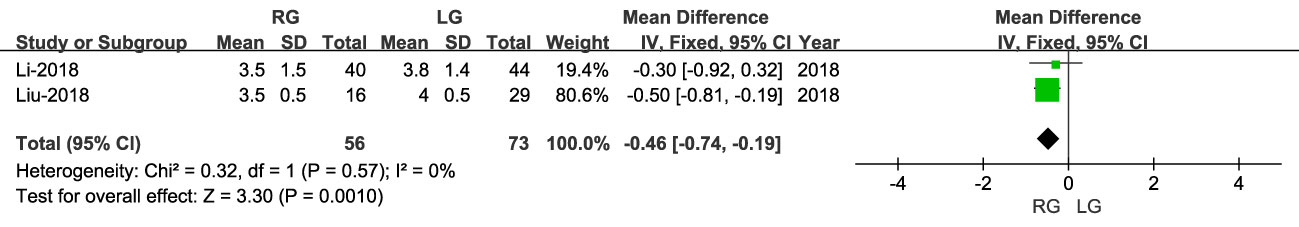
Time to oral intake was reported in two studies, including 129 patients (56 RG and 73 LG). The time to oral intake was earlier for RG than LG (OR: -0.46; 95% CI: -0.74, -0.19; P = 0.0010), with low heterogeneity (I2 = 0%) (Figure 7).
3.3.4 Length of hospital stay
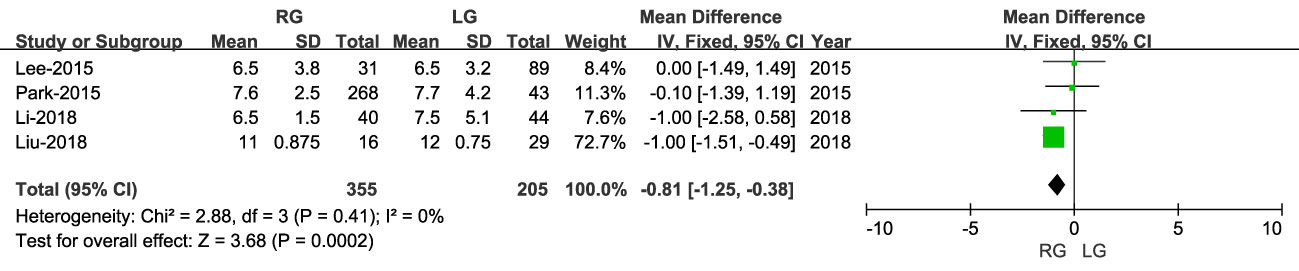
Length of hospital stay was reported in four studies, including 560 patients (355 RG and 205 LG), with a shorter stay for RG than LG (MD: - 0.81; 95% CI: -1.25 to 0.38; P = 0.0002), with low heterogeneity (I2 = 0%) (Figures 8, 9).
4 Discussion
MIS has revolutionized gastrectomy surgery, with comparable oncological outcomes to those for open surgery having been reported, making it an ideal alternative to open surgery to facilitate an earlier return to daily activities postoperatively (29). MIS offers many advantages over conventional open surgery, including reduced post-operative pain, rapid recovery of gastrointestinal function, and shorter hospital stays (30). In their meta-analysis study, Milone et al. (31) showed that for distal gastrectomy, a total laparoscopic approach, with intraperitoneal anastomosis, was safe and feasible, compared to laparoscopic-assisted surgery with extraperitoneal anastomosis, with a lower volume of blood loss (P=0.003), lower number of lymph nodes harvested (P=0.022), and shorter length of hospital stay (P=0.037). However, differences between RG an LG have not previously beenclarified for obese patients with GC with regards to perioperativeparameters, oncological findings, and short-term postoperative outcomes.
The increasing prevalence of obesity and overweight is now considered to be a global epidemic, with 1.9 billion and 650 million of adults being obese and overweight, respectively, in 2016 (32). The International Obesity Task Force recommended that a BMI ≥25 kg/m2 be used to define obesity (33). Obesity poses a specific risk for radical laparoscopy for GC treatment. While radical laparoscopic treatment for GC offers significant advantages over conventional open surgery in terms of minimizing invasive operative time and shortening the postoperative recovery period (34), the approach is limited by two-dimensional images, decreased tactile sensation, magnification of physiological hand tremor, decreased dexterity due to instruments used, and limited range of instrument motion (35). The large amount of fatty tissue in the abdominal wall and cavity in obese patients increases the difficulty of adequately exposing the surgical field, further increasing the technical difficulty of radical laparoscopic treatment (36). This increased technical difficulty has been associated with longer operative times, increased intra-operative blood loss, decreased extent of lymph node dissection, and a higher risk of postoperative complications (37, 38). Major complications of distal gastrectomy for GC treatment in obese patients include pancreatic fistula and significant increase in anastomotic leakage (39). Robotic surgery has been shown to overcome the limitations associated with a thick abdominal wall and excess intra-abdominal fat for colectomy.
Therefore, new technology is required to overcome these limitations. For patients who have undergone colectomy (40), robotic surgery has been shown to overcome the difficulties associated with thick abdominal walls and excess intra-abdominal fat, improving the visual field, instrumentation, and ergonomics. Therefore, the benefits of robotic surgery might be more pronounced for patients with a high BMI compared to those with BMI within normal range. RG improves complex reconstruction after gastrectomy and lymph node dissection to ensure oncological safety in patients with advanced GC (15), providing an enlarged 3D view and steadily motion of tweezers, allowing for precise identification of anatomical layers and avoiding injury to the adjacent organs (41). In particular, wrist mounted surgical instruments combined with the shock absorption function of RG can provide accurate anatomical rendering near the pancreas for precise in vivo anastomosis, which can reduce postoperative intraperitoneal complications (42). These features minimize surgical trauma and facilitate precise control of intra-abdominal bleeding in technically challenging tasks, such as lymph node dissection, intracorporeal anastomosis, and application of ligatures within closed cavities (43). As surgical instruments continue to advance and surgical performance gradually improve, more procedures have been attempted by experienced surgeons using Da Vinci robots, with the safety and feasibility of these procedures having been confirmed (44). Moreover, the learning curve for RG is less steep than that for LG (45), with better outcomes obtained in initial cases for RG than LG, indicative of an easier adaptation to robot-assisted than laparoscopic surgery. However, no previous studies have reported on the advantages of RG over LG with regards to reducing surgical stress and improving short-term outcomes in patients with obesity.
Longer operative time for RG than LG might be attributed to the longer docking time associated with older robotic systems, which often involve complex docking procedures and port-placement configurations (25). Longer operative time for RG in obese patients include unsuitable length of ports for obese patients, inefficient port placement, camera movements that interrupt the procedure, and unsuitable optical systems that do not allow for a large surgical field of view, all of which prevent safe continuous dissection and require careful manipulation (46). However, with the newer Da Vinci Xi system, docking can be accomplished more quickly, using an arm-mounted robotic arm and a laser targeting system. In addition, the multi-quadrant capability of Xi system reduces the need for redocking or mixing procedures, thereby reducing the procedure time (41). Song et al. (47) reported a decrease in time to dock and set up of the robotic arm after the initial 25 learning cases, with further shortening of docking time with accumulating experience. The effect of learning curve may have contributed to the longer operative time in the RG group as some cases were performed as a part of the surgeon’s learning. This is a limitation of the evidence we present in this study and, therefore, the prolonged operation time should not limit the research on new applications of RG.
Radical surgery for GC requires extensive lymph node dissection for accurate assessment of GC stage and prognosis, reducing the risk for metastasis and recurrence (48, 49). In our analysis, there was no difference in the extent of lymph node dissection between RG and LG (P = 0.82). Moreover, surgical blood loss (P = 0.99) and rate of complications (P = 0.98) were also comparable between the two approaches. However, RG offered surgeons the benefit of a 3D surgical field of view, with a magnification of 10-15x, improving direct viewing of the relationship between blood vessels and surrounding tissues and ability to clearly recognize different tissue structures. In addition, the manipulator arm (e.g., “hand” of the robotic surgical system) removes physiological tremor, improving surgical stability and accuracy, thus ensuring safety during gastric vascular dissection and ligation (50). In this regard, the rate of postoperative complication is an important short-term outcome, being lower for RG than LG group, although this difference was not statistically significant. These results demonstrate that RG is as safe and as viable as LG for the treatment of GC in obese patients.
Since the first description of RG in 2003, its use has increased rapidly, especially in East Asia (51). Our analysis identified two important advantages of RG over LG, namely: a faster return to bowel function and shorter length of hospital stay. Earlier time to first flatus and time to first oral intake are two crucial factors of postoperative recovery. The superiority of RG over LG on functional recovery of bowel function may reflect the stability and flexibility of the movement of the robotic arm, avoiding excessive traction forces on the tissues and accidental damage to the blood vessels, and, thus, causing less trauma to the patient (52). Therefore, RG causes minimal disturbance to the gastrointestinal tract, facilitating an earlier recovery of bowel function, resulting in earlier return to oral intake, evacuation, and early discharge from hospital. However, few studies have evaluated the long-term oncological efficacy of RG, with most of these having limitations, such as imbalanced covariates, a small number of cases, and the inability to compare outcomes with the same surgical team (53). Moreover, owing to the short history of clinical use of RG, the long-term survival outcomes between RG compared to other surgical methods for GC in obese patients remains to be evaluated.
Owing to insufficient data, the cost-effectiveness of RG and LG in obese patients with GC was not compared in this meta-analysis. Robotic surgical systems have well-known capital and maintenance costs, as well as additional costs depending on the robot-assisted procedures (54). However, there is growing evidence that robotic surgery is more cost effective in the long term, compared to traditional open surgery, reducing the length of hospital stay and lowering the risk of complications (55). Robotic surgery might have specific cost benefits for high-risk clinical populations, such as the elderly, morbidly obese patients, and patients with comorbidities (56). Moreover, the cost of robotic gastrectomy surgery will gradually decrease with continued improvement in robotic technology and increased use.
In summary, our meta-analysis provides evidence of specific benefits of RG over LG for GC treatment in obese patients, namely shorter hospital stay, and earlier oral intake and bowel function. Therefore, RG might be considered as an effective, safe, and promising treatment for obese patients with GC, resulting in less trauma than LG and facilitating a faster postoperative recovery. Long-term oncological outcomes and survival of RG will need to be evaluated. It is anticipated that future prospective trials with long-term outcomes will provide a better understanding of the role of RG in the treatment of GC in obese patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
Study concept and design (XY and LZ). Acquisition of data (QF). Analysis and interpretation of data (QF). Drafting of the manuscript (XY and LZ). Critical revision of the manuscript for important intellectual content (YZ and LZ). Administrative, technical, or material support; and study supervision (YZ and QF). All authors have contributed to the manuscript and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMI, body mass index; CI, confidence interval; GC, gastric cancer; LG, laparoscopic gastrectomy; MD, mean difference; NOS, MIS, minimally invasive surgery; Newcastle-Ottawa scale; OR, odds ratio; PSM, propensity score match; RCT, randomized controlled study; RG, robotic gastrectomy; WHO, World Health Organization.
References
1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (London England) (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
2. Patel TH, Cecchini M. Targeted therapies in advanced gastric cancer. Curr Treat Opt Oncol (2020) 21:70. doi: 10.1007/s11864-020-00774-4
3. Li S, Wu T, Lu YX, Wang JX, Yu FH, Yang MZ, et al. Obesity promotes gastric cancer metastasis via diacylglycerol acyltransferase 2-dependent lipid droplets accumulation and redox homeostasis. Redox Biol (2020) 36:101596. doi: 10.1016/j.redox.2020.101596
4. Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer (Oxford Engl (2009) 1990) 45:2867–73. doi: 10.1016/j.ejca.2009.04.019
5. Tsekrekos A, Lovece A, Chrysikos D, Ndegwa N, Schizas D, Kumagai K, et al. Impact of obesity on the outcomes after gastrectomy for gastric cancer: a meta-analysis. Asian J Surg (2022) 45:15–26. doi: 10.1016/j.asjsur.2021.04.033
6. Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity's impact. Fertility sterility (2017) 107:840–7. doi: 10.1016/j.fertnstert.2017.01.017
7. Shimada S, Sawada N, Ishiyama Y, Nakahara K, Maeda C, Mukai S, et al. Impact of obesity on short- and long-term outcomes of laparoscopy assisted distal gastrectomy for gastric cancer. Surg endoscopy (2018) 32:358–66. doi: 10.1007/s00464-017-5684-9
8. Wang L, Zhou B, Zhao Z, Yang L, Zhang M, Jiang Y, et al. Body-mass index and obesity in urban and rural China: findings from consecutive nationally representative surveys during 2004-18. Lancet (London England) (2021) 398:53–63. doi: 10.1016/S0140-6736(21)00798-4
9. Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, et al. A randomized phase III trial comparing adjuvant single-agent S1, s-1 with oxaliplatin, and postoperative chemoradiation with s-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial(✩). Ann Oncol Off J Eur Soc Med Oncol (2021) 32:368–74. doi: 10.1016/j.annonc.2020.11.017
10. Kong Y, Cao S, Liu X, Li Z, Wang L, Lu C, et al. Short-term clinical outcomes after laparoscopic and robotic gastrectomy for gastric cancer: a propensity score matching analysis. J gastrointestinal Surg Off J Soc Surg Alimentary Tract (2020) 24:531–9. doi: 10.1007/s11605-019-04158-4
11. Cianchi F, Indennitate G, Paoli B, Ortolani M, Lami G, Manetti N, et al. The clinical value of fluorescent lymphography with indocyanine green during robotic surgery for gastric cancer: a matched cohort study. J gastrointestinal Surg Off J Soc Surg Alimentary Tract (2020) 24:2197–203. doi: 10.1007/s11605-019-04382-y
12. Sugimoto M, Kinoshita T, Shibasaki H, Kato Y, Gotohda N, Takahashi S, et al. Short-term outcome of total laparoscopic distal gastrectomy for overweight and obese patients with gastric cancer. Surg endoscopy (2013) 27:4291–6. doi: 10.1007/s00464-013-3045-x
13. Sun L, Zhao B, Huang Y, Lu H, Luo R, Huang B. Feasibility of laparoscopy gastrectomy for gastric cancer in the patients with high body mass index: a systematic review and meta-analysis. Asian J Surg (2020) 43:69–77. doi: 10.1016/j.asjsur.2019.03.017
14. Terashima M. The 140 years' journey of gastric cancer surgery: from the two hands of billroth to the multiple hands of the robot. Ann Gastroenterol Surg (2021) 5:270–7. doi: 10.1002/ags3.12442
15. Marano L, Fusario D, Savelli V, Verre L, Neri A, Marrelli D, et al. Robotic versus laparoscopic gastrectomy for gastric cancer: protocol for umbrella review of systematic reviews and meta-analyses. BMJ Open (2020) 10:e033634. doi: 10.1136/bmjopen-2019-033634
16. Jung JH, Ryu SY, Jung MR, Park YK, Jeong O. Laparoscopic distal gastrectomy for gastric cancer in morbidly obese patients in south Korea. J Gastric Cancer (2014) 14:187–95. doi: 10.5230/jgc.2014.14.3.187
17. Duchalais E, Machairas N, Kelley SR, Landmann RG, Merchea A, Colibaseanu DT, et al. Does obesity impact postoperative outcomes following robotic-assisted surgery for rectal cancer? Surg endoscopy (2018) 32:4886–92. doi: 10.1007/s00464-018-6247-4
18. Li J, Xi H, Guo X, Gao Y, Xie T, Qiao Z, et al. Surgical outcomes and learning curve analysis of robotic gastrectomy for gastric cancer: multidimensional analysis compared with three−dimensional high−definition laparoscopic gastrectomy. Int J Oncol (2019) 55:733–44. doi: 10.3892/ijo.2019.4851
19. Zheng-Yan L, Feng Q, Yan S, Ji-Peng L, Qing-Chuan Z, Bo T, et al. Learning curve of robotic distal and total gastrectomy. Br J Surg (2021) 108:1126–32. doi: 10.1093/bjs/znab152
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed.) (2021) 372:n71. doi: 10.1136/bmj.n71
21. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Method (2014) 14:45. doi: 10.1186/1471-2288-14-45
22. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Method (2005) 5:13. doi: 10.1186/1471-2288-5-13
23. Li Z, Li J, Li B, Bai B, Liu Y, Lian B, et al. Robotic versus laparoscopic gastrectomy with D2 lymph node dissection for advanced gastric cancer: a propensity score-matched analysis. Cancer Manag Res (2018) 10:705–14. doi: 10.2147/CMAR.S161007
24. Hyun MH, Lee CH, Kwon YJ, Cho SI, Jang YJ, Kim DH, et al. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol (2013) 20:1258–65. doi: 10.1245/s10434-012-2679-6
25. Park JY, Ryu KW, Reim D, Eom BW, Yoon HM, Rho JY, et al. Robot-assisted gastrectomy for early gastric cancer: is it beneficial in viscerally obese patients compared to laparoscopic gastrectomy? World J Surg (2015) 39:1789–97. doi: 10.1007/s00268-015-2998-4
26. Lee J, Kim YM, Woo Y, Obama K, Noh SH, Hyung WJ. Robotic distal subtotal gastrectomy with D2 lymphadenectomy for gastric cancer patients with high body mass index: comparison with conventional laparoscopic distal subtotal gastrectomy with D2 lymphadenectomy. Surg endoscopy (2015) 29:3251–60. doi: 10.1007/s00464-015-4069-1
27. Liu HB, Wang WJ, Li HT, Han XP, Su L, Wei DW, et al. Robotic versus conventional laparoscopic gastrectomy for gastric cancer: a retrospective cohort study. Int J Surg (London England) (2018) 55:15–23. doi: 10.1016/j.ijsu.2018.05.015
28. Choi S, Song JH, Lee S, Cho M, Kim YM, Hyung WJ, et al. Surgical merits of open, laparoscopic, and robotic gastrectomy techniques with D2 lymphadenectomy in obese patients with gastric cancer. Ann Surg Oncol (2021) 28:7051–60. doi: 10.1245/s10434-021-09952-6
29. Herrera-Almario G, Strong VE. Minimally invasive gastric surgery. Ann Surg Oncol (2016) 23:3792–7. doi: 10.1245/s10434-016-5429-3
30. Milone M, Manigrasso M, Burati M, Elmore U, Gennarelli N, Cesare Giglio M, et al. Intracorporeal versus extracorporeal anastomosis after laparoscopic gastrectomy for gastric cancer. A systematic Rev meta-analysis. J visceral Surg (2019) 156:305–18. doi: 10.1016/j.jviscsurg.2019.01.004
31. Parisi A, Nguyen NT, Reim D, Zhang S, Jiang ZW, Brower ST, et al. Current status of minimally invasive surgery for gastric cancer: a literature review to highlight studies limits. Int J Surg (London England) (2015) 17:34–40. doi: 10.1016/j.ijsu.2015.02.021
32. Castagneto-Gissey L, Casella-Mariolo J, Casella G, Mingrone G. Obesity surgery and cancer: what are the unanswered questions? Front Endocrinol (2020) 11:213. doi: 10.3389/fendo.2020.00213
33. Liu M, Xing J, Arslan A, Tan F, Fan Y, Xu K, et al. Safety and efficacy of laparoscopic gastrectomy in obese patients with gastric cancer. Med (Baltimore) (2019) 98:e17991. doi: 10.1097/MD.0000000000017991
34. Mao C, Chen X, Sun X, Wang X, Zhu C, Chen W, et al. Laparoscopic gastrectomy reduces adverse postoperative outcomes and decreases morbidity for gastric cancer patients with visceral obesity: a propensity score-matched analysis. J Cancer (2021) 12:2113–21. doi: 10.7150/jca.47552
35. Ma J, Li X, Zhao S, Zhang R, Yang D. Robotic versus laparoscopic gastrectomy for gastric cancer: a systematic review and meta-analysis. World J Surg Oncol (2020) 18:306. doi: 10.1186/s12957-020-02080-7
36. Pata G, Solaini L, Roncali S, Pasini M, Ragni F. Impact of obesity on early surgical and oncologic outcomes after total gastrectomy with "over-D1" lymphadenectomy for gastric cancer. World J Surg (2013) 37:1072–81. doi: 10.1007/s00268-013-1942-8
37. Chen K, Pan Y, Zhang B, Maher H, Wang XF, Cai XJ. Robotic versus laparoscopic gastrectomy for gastric cancer: a systematic review and updated meta-analysis. BMC Surg (2017) 17:93. doi: 10.1186/s12893-017-0290-2
38. Lee JH, Park B, Joo J, Kook MC, Kim YI, Lee JY, et al. Body mass index and mortality in patients with gastric cancer: a large cohort study. Gastric Cancer (2018) 21:913–24. doi: 10.1007/s10120-018-0818-x
39. Shibasaki S, Suda K, Obama K, Yoshida M, Uyama I. Should robotic gastrectomy become a standard surgical treatment option for gastric cancer? Surg Today (2020) 50:955–65. doi: 10.1007/s00595-019-01875-w
40. Harr JN, Luka S, Kankaria A, Juo YY, Agarwal S, Obias V. Robotic-assisted colorectal surgery in obese patients: a case-matched series. Surg endoscopy (2017) 31:2813–9. doi: 10.1007/s00464-016-5291-1
41. Ojima T, Nakamura M, Hayata K, Kitadani J, Takeuchi A, Yamaue H, et al. Comparison of short-term surgical outcomes using da Vinci S, Si And xi surgical system for robotic gastric cancer surgery. Sci Rep (2021) 11:11063. doi: 10.1038/s41598-021-90741-2
42. Kinoshita T, Sato R, Akimoto E, Tanaka Y, Okayama T, Habu T. Reduction in postoperative complications by robotic surgery: a case-control study of robotic versus conventional laparoscopic surgery for gastric cancer. Surg endoscopy (2022) 36:1989–98. doi: 10.1007/s00464-021-08483-1
43. Xiong J, Nunes QM, Tan C, Ke N, Chen Y, Hu W, et al. Comparison of short-term clinical outcomes between robotic and laparoscopic gastrectomy for gastric cancer: a meta-analysis of 2495 patients. J laparoendoscopic Adv Surg techniques. Part A (2013) 23:965–76. doi: 10.1089/lap.2013.0279
44. Kuang Y, Lei S, Zhao H, Cui B, Liu K, Yao H. Totally robotic distal gastrectomy: a safe and feasible minimally invasive technique for gastric cancer patients who undergo distal gastrectomy. Digest Surg (2020) 37:360–7. doi: 10.1159/000507809
45. Bobo Z, Xin W, Jiang L, Quan W, Liang B, Xiangbing D, et al. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: meta-analysis and trial sequential analysis of prospective observational studies. Surg endoscopy (2019) 33:1033–48. doi: 10.1007/s00464-018-06648-z
46. Xiong B, Ma L, Zhang C. Robotic versus laparoscopic gastrectomy for gastric cancer: a meta-analysis of short outcomes. Surg Oncol (2012) 21:274–80. doi: 10.1016/j.suronc.2012.05.004
47. Song J, Kang WH, Oh SJ, Hyung WJ, Choi SH, Noh SH. Role of robotic gastrectomy using da Vinci system compared with laparoscopic gastrectomy: initial experience of 20 consecutive cases. Surg endoscopy (2009) 23:1204–11. doi: 10.1007/s00464-009-0351-4
48. Mogal H, Fields R, Maithel SK, Votanopoulos K. In patients with localized and resectable gastric cancer, what is the optimal extent of lymph node dissection-D1 versus D2 versus D3? Ann Surg Oncol (2019) 26:2912–32. doi: 10.1245/s10434-019-07417-5
49. Aiolfi A, Lombardo F, Matsushima K, Sozzi A, Cavalli M, Panizzo V, et al. Systematic review and updated network meta-analysis of randomized controlled trials comparing open, laparoscopic-assisted, and robotic distal gastrectomy for early and locally advanced gastric cancer. Surgery (2021) 170:942–51. doi: 10.1016/j.surg.2021.04.014
50. Zhang Z, Zhang X, Liu Y, Li Y, Zhao Q, Fan L, et al. Meta-analysis of the efficacy of da Vinci robotic or laparoscopic distal subtotal gastrectomy in patients with gastric cancer. Med (Baltimore) (2021) 100:e27012. doi: 10.1097/MD.0000000000027012
51. Cui H, Cao B, Liu G, Xi H, Chen Z, Liang W, et al. Comparison of short-term outcomes and quality of life in totally laparoscopic distal gastrectomy and totally robotic distal gastrectomy for clinical stage I-III gastric cancer: study protocol for a multi-institutional randomised clinical trial. BMJ Open (2021) 11:e043535. doi: 10.1136/bmjopen-2020-043535
52. Guerrini GP, Esposito G, Magistri P, Serra V, Guidetti C, Olivieri T, et al. Robotic versus laparoscopic gastrectomy for gastric cancer: the largest meta-analysis. Int J Surg (London England) (2020) 82:210–28. doi: 10.1016/j.ijsu.2020.07.053
53. Tian Y, Cao S, Kong Y, Shen S, Niu Z, Zhang J, et al. Short- and long-term comparison of robotic and laparoscopic gastrectomy for gastric cancer by the same surgical team: a propensity score matching analysis. Surg endoscopy (2022) 36:185–95. doi: 10.1007/s00464-020-08253-5
54. O'Kelly F, Farhat WA, Koyle MA. Cost, training and simulation models for robotic-assisted surgery in pediatric urology. World J Urol (2020) 38:1875–82. doi: 10.1007/s00345-019-02822-7
55. Ashrafian H, Clancy O, Grover V, Darzi A. The evolution of robotic surgery: surgical and anaesthetic aspects. Br J anaesthesia 119 (2017) 119:i72–84. doi: 10.1093/bja/aex383
Keywords: gastric cancer, robotic gastrectomy, laparoscopic gastrectomy, obesity, short-term outcomes, meta-analysis
Citation: Yu X, Zhu L, Zhang Y and Feng Q (2023) Robotic versus laparoscopic gastrectomy for gastric cancer in patients with obesity: systematic review and meta-analysis. Front. Oncol. 13:1158804. doi: 10.3389/fonc.2023.1158804
Received: 13 March 2023; Accepted: 04 May 2023;
Published: 19 May 2023.
Edited by:
Ye Zhou, Fudan University, ChinaReviewed by:
Michele Manigrasso, University of Naples Federico II, ItalyKai Xu, Peking University, China
Taha Anbara, Arman International Hospital, Iran
Copyright © 2023 Yu, Zhu, Zhang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingbo Feng, ZnFiOTE3NWRvY0AxNjMuY29t
†These authors contributed equally to this work
 Xianzhe Yu
Xianzhe Yu Lingling Zhu
Lingling Zhu Yan Zhang2†
Yan Zhang2† Qingbo Feng
Qingbo Feng