- Peking University People’s Hospital, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Beijing, China
Introduction: Allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains a major strategy to cure patients with acute lymphoblastic leukemia (ALL). The aim of this study was to evaluate whether isolated flow cytometry (FCM)-positive central nervous system (CNS) involvement before allo-HSCT is clinically significant.
Methods: The effects of isolated FCM-positive CNS involvement prior to transplantation on the outcomes of 1406 ALL patients with complete remission (CR) were retrospectively investigated.
Results: Patients were classified into isolated FCM-positive CNS involvement (n=31), cytology-positive CNS involvement (n = 43), and negative CNS involvement (n = 1332) groups. Among the three groups, the 5-year cumulative incidence of relapse (CIR) values were 42.3%, 48.8%, and 23.4%, respectively (P<0.001). The 5-year leukemia-free survival (LFS) values were 44.7%, 34.9%, and 60.8%, respectively (P<0.001). Compared with the negative CNS group (n=1332), the 5-year CIR of the pre-HSCT CNS involvement group (n=74) was higher (46.3% vs. 23.4%, P<0.001], and the 5-year LFS was inferior (39.1% vs. 60.8%, P<0.001). Multivariate analysis indicated that four variables, T-cell ALL, in second complete remission or beyond (CR2+) at HSCT, pre-HSCT measurable residual disease positivity, and pre-HSCT CNS involvement, were independently associated with a higher CIR and inferior LFS. A new scoring system was developed using the following four variables: low-risk, intermediate-risk, high-risk, and extremely high-risk groups. The 5-year CIR values were 16.9%, 27.8%, 50.9%, and 66.7%, respectively (P<0.001), while the 5-year LFS values were 67.6%, 56.9%, 31.0%, and 13.3%, respectively (P<0.001).
Conclusion: Our results suggest that ALL patients with isolated FCM-positive CNS involvement are at a higher risk of recurrence after transplantation. Patients with pre-HSCT CNS involvement had higher CIR and inferior survival outcomes.
1 Introduction
Presently, allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains one of the main ways to cure patients with acute lymphoblastic leukemia (ALL) (1–6). However, recurrence after transplantation remains one of the main factors affecting the survival of ALL patients (4, 7). Several risk factors before HSCT, such as complete remission (CR) status (8), positive measurable residual disease (MRD) (9–17), and central nervous system (CNS) involvement (18–20), were associated with relapse in patients with ALL who underwent allo-HSCT. Shigematsu et al. (21) confirmed that ALL patients with CNS involvement who received allografting experienced a higher cumulative incidence of relapse (CIR) and inferior survival compared to those without. Aldoss et al. (22) showed that compared to allografts without CNS involvement pre-HSCT, patients with CNS involvement had a higher risk of CNS relapse (2-year CNS relapse: 9.6% vs. 1.4%, P<0.0001), inferior event-free survival (EFS) (hazard ratio [HR]: 1.52; P=0.003), and worse overall survival (OS, HR: 1.55; P=0.003) after transplantation. In allo-HSCT settings, Kharfan-Dabaja et al. (23) demonstrated that CNS involvement at diagnosis was also associated with a significantly higher incidence of relapse (HR: 1.58, P=0.03) and a trend towards worse leukemia-free survival (LFS, HR: 1.38, P=0.057). However, these studies failed to answer the question of whether isolated flow cytometry (FCM)-positive CNS involvement before transplantation is clinically significant, although available studies (24–28) suggest that FCM is superior to conventional cytology (CC) in identifying leukemia cells in cerebrospinal fluid (CSF).
In our previous study (29), we found that transplant patients with ALL could be classified into subgroups with high and low risk of relapse according to pre-HSCT disease status, immunophenotype of ALL, and MRD before transplantation. However, the effects of isolated FCM-positive CNS involvement before transplantation on outcomes in these subgroup patients is unclear because of the low number of participants enrolled in the studies reported by other researchers (18, 22). In addition, available studies performed by others (9, 30) and our previous studies (17, 29, 31) suggest that the risk score system is superior to single variables in predicting transplant outcomes. However, data are lacking on whether incorporating positive CNS involvement with other variables, including disease status, immunophenotype of ALL, and MRD status, before transplantation could further improve the risk stratification of patients with ALL. Therefore, we performed a retrospective study to investigate the association of isolated FCM positivity in CNS before transplantation with outcomes in all patients with ALL and subgroup cases who underwent allo-HSCT. We also established a risk score system based on positive CNS involvement and other variables to improve the stratification of transplant outcomes.
2 Patients and methods
2.1 Study design
This retrospective analysis included 1406 patients with ALL who underwent allo-stem cell transplantation at Peking University People’s Hospital between January 2009 and December 2018. All participants signed an informed consent document and had relatively complete medical records. The study protocol was in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Peking University. All patients were treated according to the transplantation protocol, as previously described (32).
2.2 Transplantation protocols
Granulocyte colony-stimulating factor (G-CSF, 5 μg/kg/d for 5 days) was used to mobilize the bone marrow (G-BM) or peripheral blood (G-PB). The target mononuclear cell count in the total allografts was greater than 6×108 cells/kg of recipient weight.
For patients who received haploidentical transplantation, the Bu-based conditioning regimen was as follows: cytarabine (4 g/m2/d) on days –10 to –9, busulfan (3.2 mg/kg/d) on days –8 to –6, cyclophosphamide (CTX, 1.8 g/m2/d) on days –5 to –4, oral Me-CCNU (250 mg/m2, once) on day –3, and anti-thymocyte globulin (ATG, 2.5 mg/kg/d) on days –5 to –2. The total body irradiation (TBI)-based conditioning regimen consisted of TBI (770 cGy) on day –6, CTX (1.8 g/m2/d) on days –5 to –4, oral Me-CCNU (250 mg/m2, once) on day –3, and ATG (2.5 mg/kg/d) on days –5 to –2. Patients who underwent human leukocyte antigen-matched sibling donor transplantation received the same regimen mentioned above, but without ATG. The graft-versus-host disease (GVHD) prophylaxis regimen comprised immunosuppressive agents, including cyclosporine A, mycophenolate mofetil, and short-term methotrexate. The detailed protocol is described in our previous publication (32).
2.3 Investigation of CNS involvement
Fresh CSF samples were collected, centrifuged, and stained for morphological examination. An expert cytopathologist interpreted each case. Positive cytology was defined as unequivocal morphological evidence of leukemic blasts in the CSF.
Simultaneously, CSF samples were examined by multiparameter FCM, which was performed using a combination of six-to-eight color antibodies, according to the immunophenotype of blasts identified in the bone marrow at the initial diagnosis. The antibody combination panel consisted of mCD3, CD2, CD4, CD5, CD7, CD8, CD10, CD19, CD20, CD34, CD38, CD45, CD58, and CD123. FCM positivity was considered when a cluster of more than 10 cells was characterized by a leukemia-associated immunophenotype (33).
2.4 Definitions
Hyperleukocytosis was defined as leukocyte count greater than 30×109/L in B cell ALL (B-ALL) or 100×109/L in T cell ALL (T-ALL). Patients were classified as high-risk if they met the following criteria: age >35 years, hyperleukocytosis, adverse cytogenetics (t[4;11], complex karyotype, low hypodiploidy-near triploidy), or delayed CR1 (remission required more than 4 weeks after induction of therapy) (34). CNS involvement was defined as infiltration of leukemic blasts in the CSF, as determined by either FCM, CC, or both methods. Isolated FCM-positive CNS involvement was defined as infiltration in the CNS detected only by multiparameter FCM (33). Hematological relapse was diagnosed when blasts reappeared in the PB or >5% in the BM aspirate. Extramedullary recurrence was diagnosed through physical examination, imaging, pathology, or cytology. The cumulative incidence rate of hematological recurrence (CIHR) involves only hematological recurrence, whereas the CIR involves hematological recurrence and extramedullary recurrence. OS was defined from the day of transplantation to the day of death for any reason or to the last day of follow-up. Leukemia-free survival (LFS) was defined from the date of transplantation as the starting point to the date of the first event or last follow-up as the endpoint. The events for LFS included morphological or extramedullary relapse and death from any cause. Non-relapse mortality (NRM) was defined as death from any cause within 28 days after HSCT or death without evidence of disease recurrence after 28 days. Neutrophil recovery was defined as the first day of an absolute neutrophil count that exceeded 0.5×109/L for 3 consecutive days. Platelet engraftment was defined as the first of 7 consecutive days with a platelet count exceeding 20×109/L without platelet transfusion support. Acute and chronic GVHD was defined as previously described (35).
The endpoint of the last follow-up for all surviving patients was June 18, 2022.
2.5 Statistical analysis
Patient characteristics were compared among the positive blasts in CSF detected by CC or FCM and negative subgroups using the Chi-square test for categorical variables and Mann–Whitney test for continuous variables. Cumulative incidence curves were used with a competing risk setting, and relapse was treated as a competing event to calculate the NRM probability. Death from any cause was a competing risk for relapse. The probabilities of LFS, OS, incidence of hematological relapse, and NRM were estimated using the Kaplan–Meier method and log–rank test. Age, sex, immunophenotype of ALL, hyperleukocytosis at diagnosis, risk stratification, BCR/ABL positive or negative at diagnosis, and concomitant extramedullary (except CNS involvement) or not at diagnosis were included in the univariate analysis for CNS involvement. Pre-HSCT isolated FCM-positive CNS involvement, cytology-positive or negative CNS involvement, and all variables in Table 1, except for donor-recipient sex-matched graft, were included in the univariate analysis for the outcomes. Parameters with P ≤ 0.1 were selected to enter the multivariable analysis using the Cox proportional hazards model. In the multivariate model, the proportional hazard assumption and linear relation between covariates were verified. P ≤ 0.05 indicated a significant difference. SPSS version 26.0 software (IBM Corporation, Armonk, NY, USA) and RStudio were used to perform the statistical analysis.
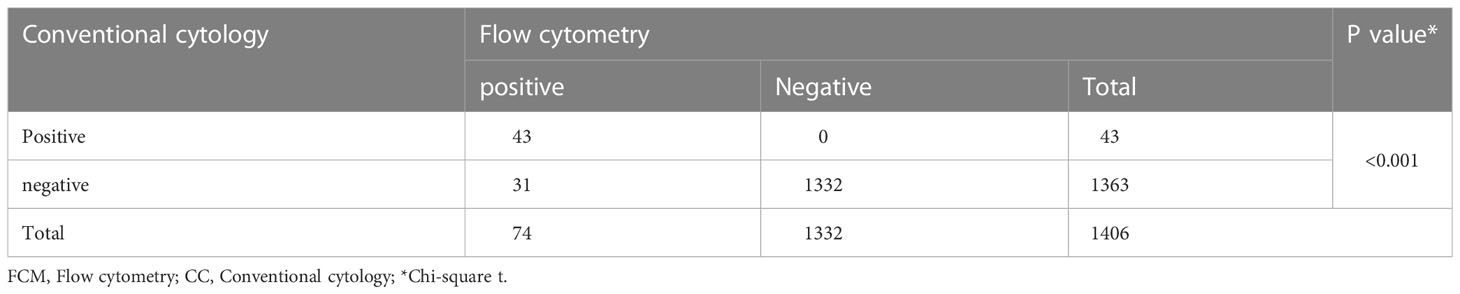
Table 1 FCM and CC analysis of CSF for the detection of CNS involvement in patients with ALL (N=1406).
3 Results
3.1 Patient characteristics and transplant outcomes
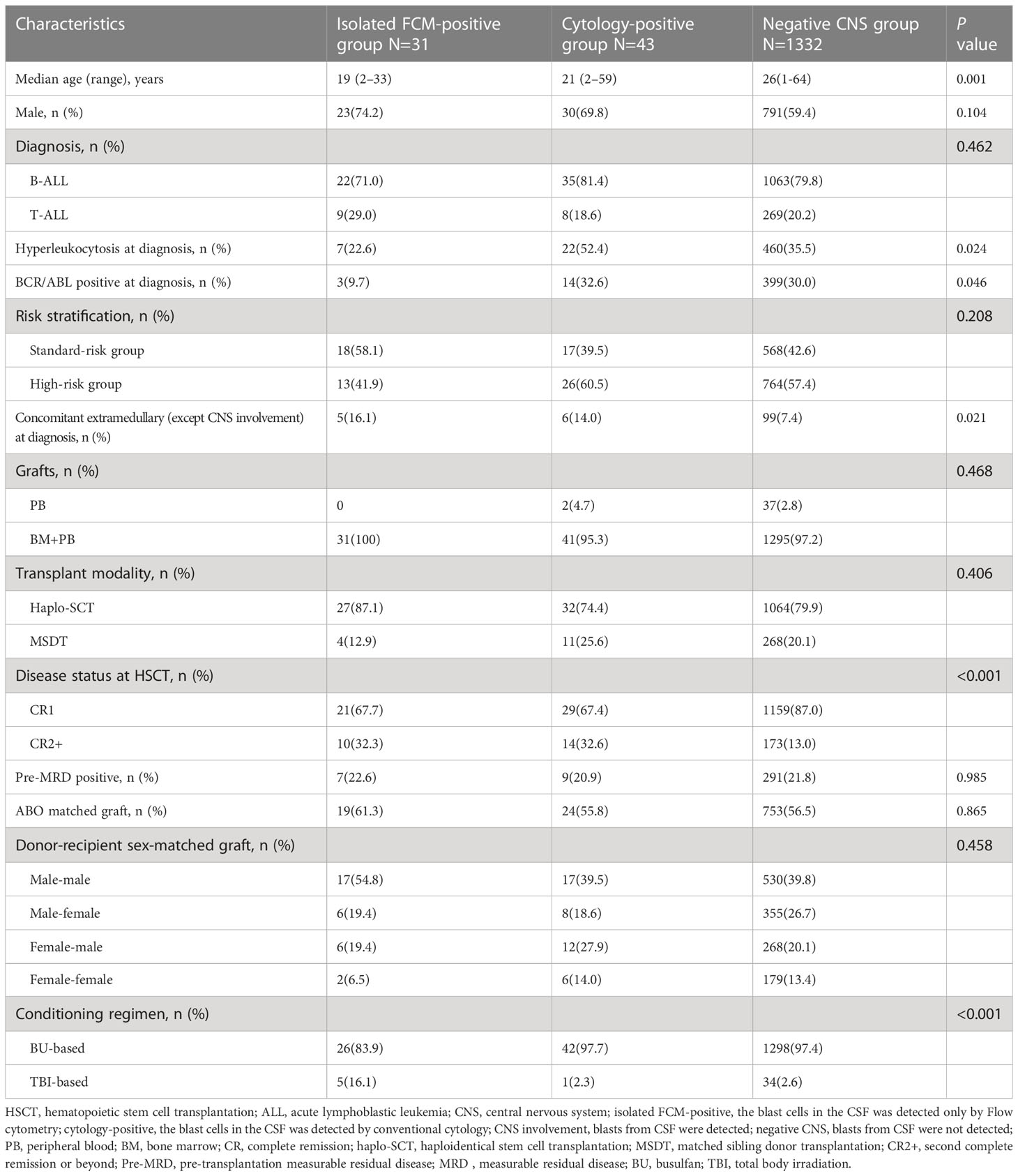
From January 2009 to December 2018, 1406 ALL patients with CR underwent allo-HSCT at our center, and before transplantation, blasts were detected in the CSF of 74 patients (5.4%). Forty-three patients (3.1%) had cytology-positive CNS involvement, which was detected by both FCM and CC, and 31 patients (2.2%) had isolated FCM-positive CNS involvement (Table 1). Twenty-two patients (51.2%) and 14 patients (45.2%) in the cytology-positive and isolated FCM-positive groups, respectively, were detected at diagnosis. The remaining patients were detected during treatment, and none of them was detected at transplantation after treatment with intrathecal injection. The baseline characteristics of the isolated FCM-positive, cytology-positive, and negative CNS involvement groups are presented in Table 2. The age of the patients with CNS involvement was lower (P=0.001). The incidence rates of hyperleukocytosis at diagnosis (P=0.024) and BCR/ABL positive (P=0.046) were higher in the cytology-positive group. Patients with extramedullary involvement (except CNS involvement) at diagnosis (P<0.001) and in second complete remission or beyond (CR2+) at transplantation were more common in the isolated FCM-positive or cytology-positive group (P<0.001).
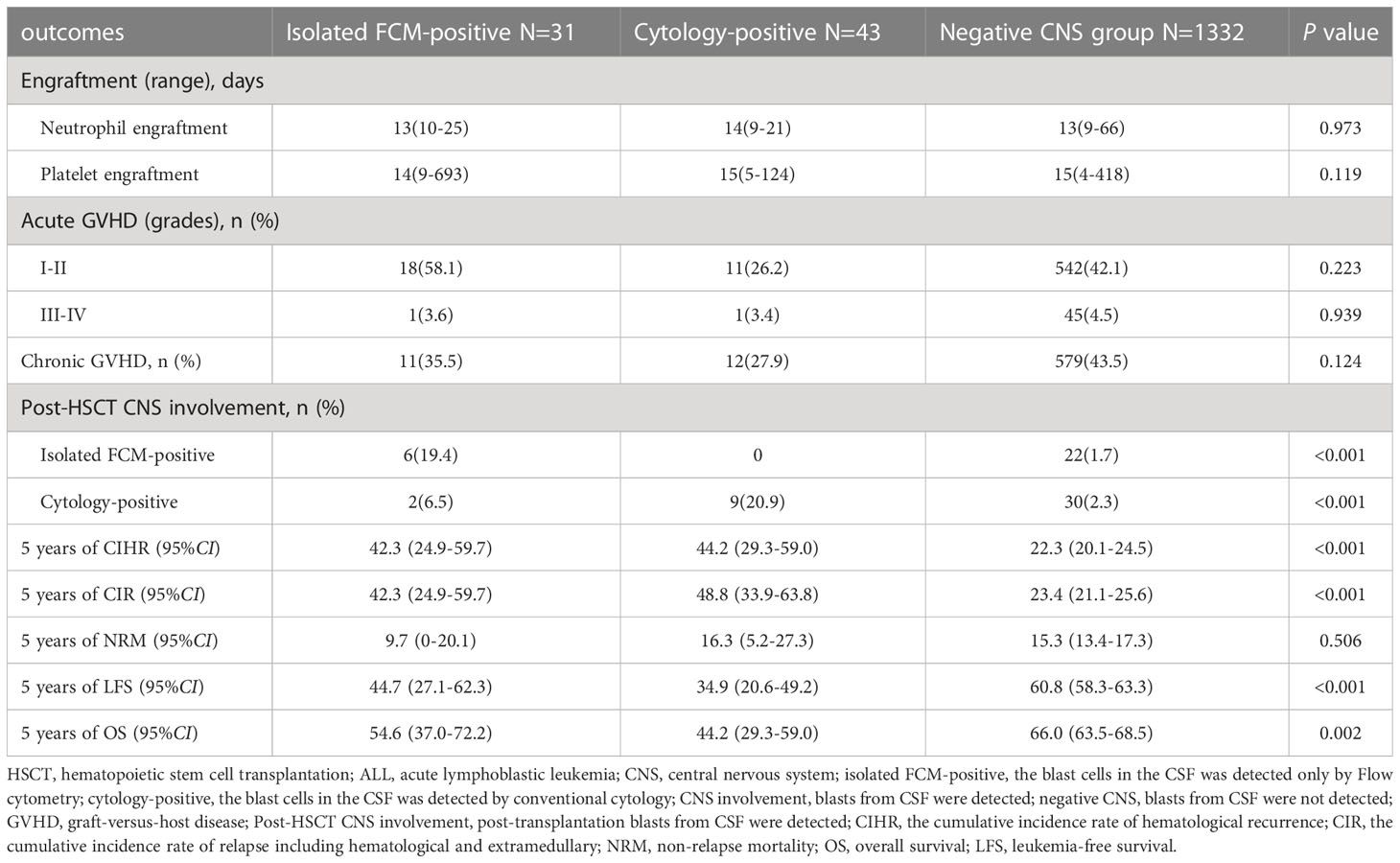
The results are presented in Table 3. Of the 74 patients with pre-HSCT CNS involvement, 18 (24.3%) had CNS involvement recurrence after transplantation (post-HSCT CNS involvement). Among the 31 patients in the isolated FCM-positive CNS involvement group before transplantation, six patients were confirmed to have isolated FCM-positive CNS involvement and two patients had cytology-positive CNS involvement after transplantation. Of the 43 patients in the cytology-positive CNS involvement group before transplantation, nine patients had cytology-positive CNS involvement after transplantation. Of the remaining 1332 patients with negative CNS involvement before transplantation, 52 (3.9%) had post-HSCT CNS involvement, including 22 with isolated FCM-positive CNS involvement and 30 with cytology-positive CNS involvement. Among the 1406 patients with allograft ALL with a median follow-up of 56.5 months, the estimated 5-year OS rate was 65.0% (95% confidence interval [CI]: 62.5–67.6%), the 5-year LFS rate was 59.8% (95% CI: 57.3–62.3%), the 5-year CIHR was 23.5% (95% CI: 21.3–25.7%), the 5-year CIR was 25.1% (95% CI: 22.8–27.4%), and the 5-year NRM rate was 15.3% (95% CI: 13.4–17.2%).
3.2 Risk factors for pre-HSCT CNS involvement
Univariate analysis showed that age above 25 years (median) (P<0.001), male patients (P=0.039), and concomitant extramedullary at diagnosis (P=0.024) were associated with CNS involvement before transplantation. Further multivariate analysis showed that only age above 25 years (median) (odds ratio: 0.395; 95% CI: 0.228–0.632; P<0.001) was an independent prognostic factor for pre-HSCT CNS involvement (Table S1).
3.3 Association of isolated FCM-positive CNS involvement pre-HSCT with transplant outcomes
Among the isolated FCM-positive CNS involvement group, the cytology-positive CNS involvement group, and negative CNS group, the 5-year CIHR was 42.3% (95% CI: 24.9–59.7%), 44.2% (95% CI: 29.3–59.0%), and 22.3% (95% CI: 20.1–24.5%), respectively (P<0.001). The 5-year CIR was 42.3% (95% CI: 24.9–59.7%), 48.8% (95% CI: 33.9–63.8%), and 23.4% (95% CI: 21.1–25.6%), respectively (P<0.001). Compared to the negative CNS group, the isolated FCM-positive CNS involvement group had a higher CIHR (P=0.007) and CIR (P=0.003), and the cytology-positive CNS involvement group had a higher CIHR (P<0.001) and CIR (P<0.001). There were no significant differences in the CIHR (P=0.761) or CIR (P=0.662) between the two pre-HSCT CNS involvement groups (Figure 1). We assessed the risk factors that might influence outcomes in ALL patients undergoing allo-HSCT. The univariate analysis results are shown in Table S2. On multivariate analysis, T-ALL, CR2+ at HSCT, pre-HSCT MRD positivity, and pre-HSCT cytology-positive CNS involvement were independent risk factors associated with higher CIHR, CIR, and inferior OS and LFS. Pre-HSCT isolated FCM-positive CNS involvement was the only independent risk factor associated with higher CIHR and CIR. Details of the multivariate analyses of the outcomes are shown in Table 3. Univariate and multivariate analyses showed that acute GVHD grades III–IV were the only independent risk factors for NRM (data not shown).
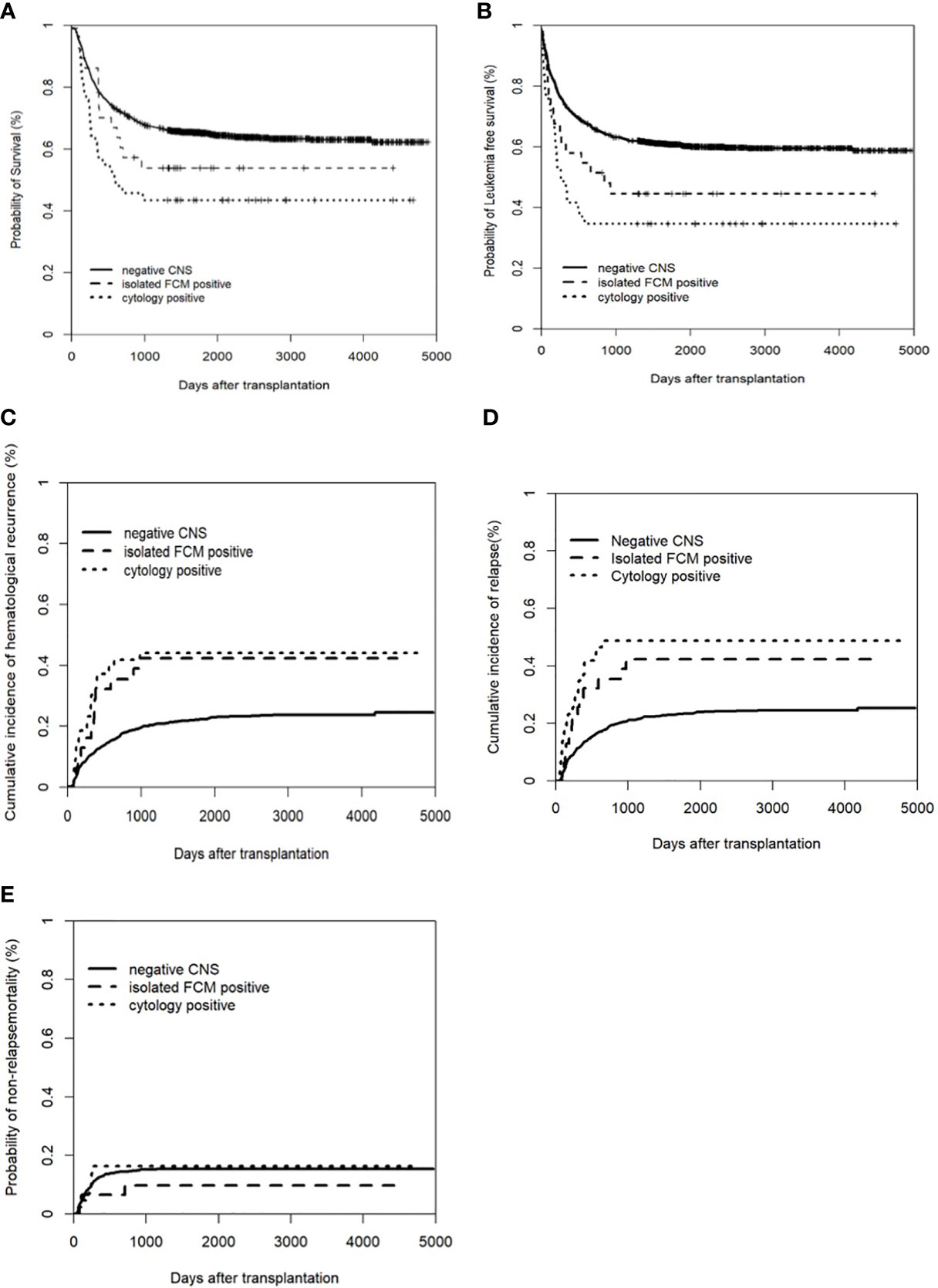
Figure 1 Clinical outcomes of patients with and without CNS involvement. (A) Overall survival (OS); (B) Leukemia free of survival (LFS): * Leukemia free survival was defined as survival without hematologic relapse or extramedullary relapse; (C) cumulative incidence of hematological recurrence; (D) cumulative incidence of relapse; (E) non-relapse mortality (NRM).
3.4 Association of pre-HSCT CNS involvement with transplant outcomes
Considering the higher CIR of patients either in the isolated FCM-positive CNS involvement group or in the cytology-positive CNS involvement group compared to those in the negative CNS group, we investigated the association of pre-HSCT CNS involvement determined either by FCM or cytology. The 5-year CIHR of patients with pre-HSCT CNS involvement was 43.4% (95% CI: 32.1–54.7%), which was significantly higher than that of the negative CNS (22.3%, 95% CI: 20.1–24.5%, P<0.001), and the 5-year CIR was 46.3% (95% CI: 34.7–57.4%) vs. 23.4% (95% CI: 21.1–25.6%) (P<0.001). The 5-year LFS rate of patients with pre-HSCT CNS involvement was lower (39.1% [95% CI: 27.9–50.3%] vs. 60.8% [95% CI: 58.3–63.3%], P<0.001), and the 5-year OS rate of pre-HSCT CNS involvement was also inferior (48.6% [95% CI: 37.2–60.0%] vs. 66.0% [95% CI: 63.5–68.5%], P=0.001). There was no significant difference in NRM between the two groups (13.5% [95% CI: 5.7–21.3%] vs. 15.3% [95% CI: 13.4–17.3%], P=0.679). We then assessed the risk factors that might influence the outcomes; the univariate analysis results are shown in Table S2. On multivariate analysis, T-ALL, CR2+ at HSCT, pre-HSCT MRD positivity, and pre-HSCT CNS involvement were independent risk factors associated with higher CIHR and CIR and inferior OS and LFS. Details of the multivariate analyses of the outcomes are shown in Table 4. Univariate and multivariate analyses showed that acute GVHD grades III–IV were the only independent risk factors for NRM.
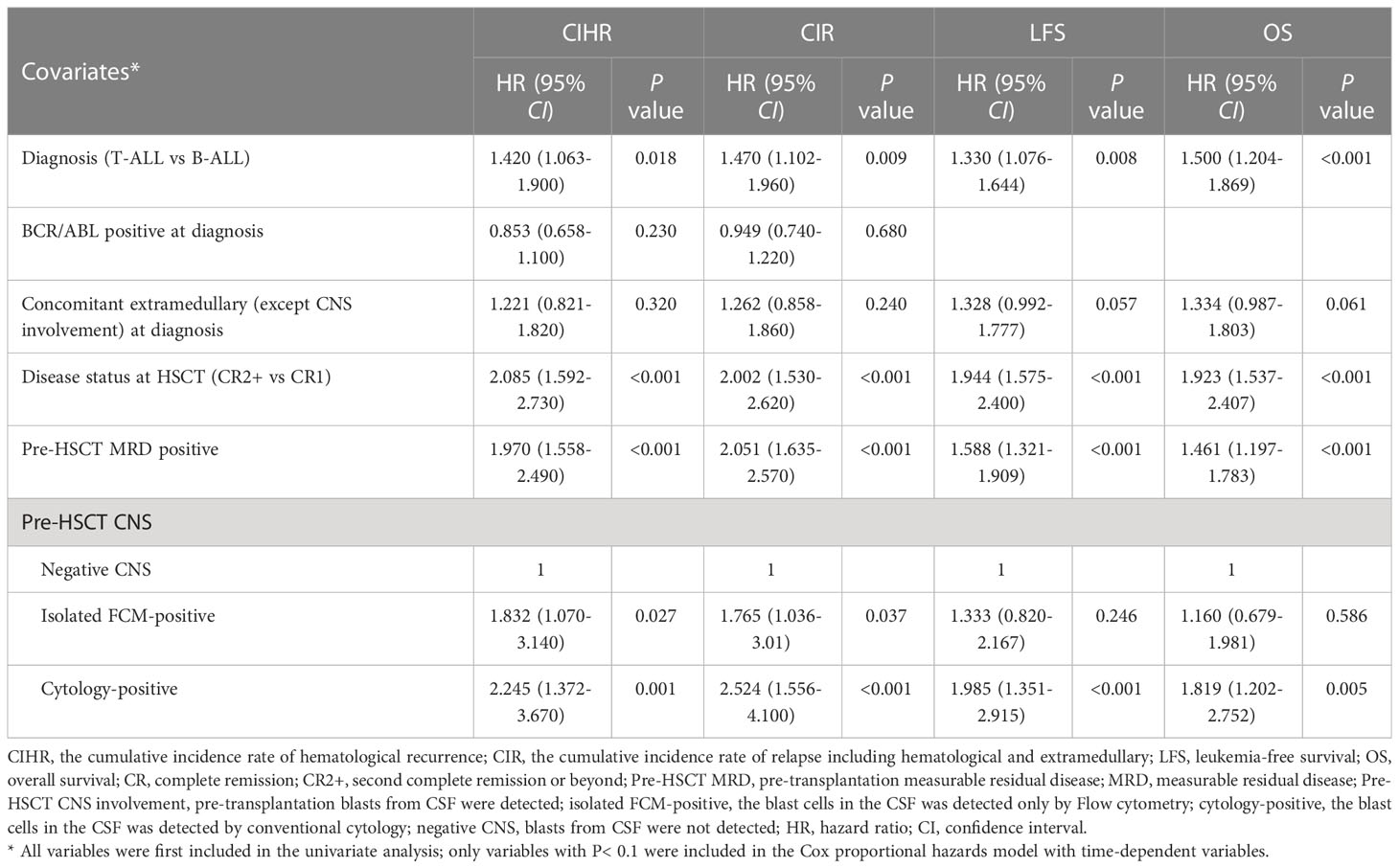
Table 4 Multivariate analysis of the factors associated with CIHR, CIR LFS and OS about isolated FCM-positive CNS involvement group.
3.5 A new scoring system with transplant outcomes in the entire cohort of patients
Based on the multivariate analysis (Table S1), we developed a risk score for transplant outcome prediction. The score was the number of risk factors, including T-ALL, CR2+ at HSCT, pre-HSCT MRD positivity, and pre-HSCT CNS involvement. By combining the risk scores for these four major variables, patients were stratified into four distinctive risk groups: low-risk, intermediate-risk, high-risk, and extremely high-risk, which had scores of 0, 1, 2, and 3–4, respectively. The 5-year CIR of the four groups was 16.9% (95% CI: 14.1–19.7%), 27.8% (95% CI: 24.0–31.6%), 50.9% (95% CI: 42.0–59.8%), and 66.7% (95% CI: 40.3–93.1%), respectively; the 5-year LFS was 67.6% (95% CI: 64.3–70.9%), 56.9% (95% CI: 52.3–61.0%), 31.0% (95% CI: 22.8–39.2%), and 13.3% (95% CI: 0–30.5%), respectively; and the 5-year OS was 71.8% (95% CI: 68.5–75.1%), 63.4% (95% CI: 59.3–67.5%), 38.3% (95% CI: 29.7–46.9%), and 13.3% (95% CI: 0–30.5%), respectively (all P<0.001) (Figure 2). The multivariate analysis indicated that the new scoring system was the only independent risk factor simultaneously associated with higher CIHR and CIR and inferior OS and LFS. In addition, concomitant extramedullary involvement at diagnosis was another factor that affected OS (Table S2).
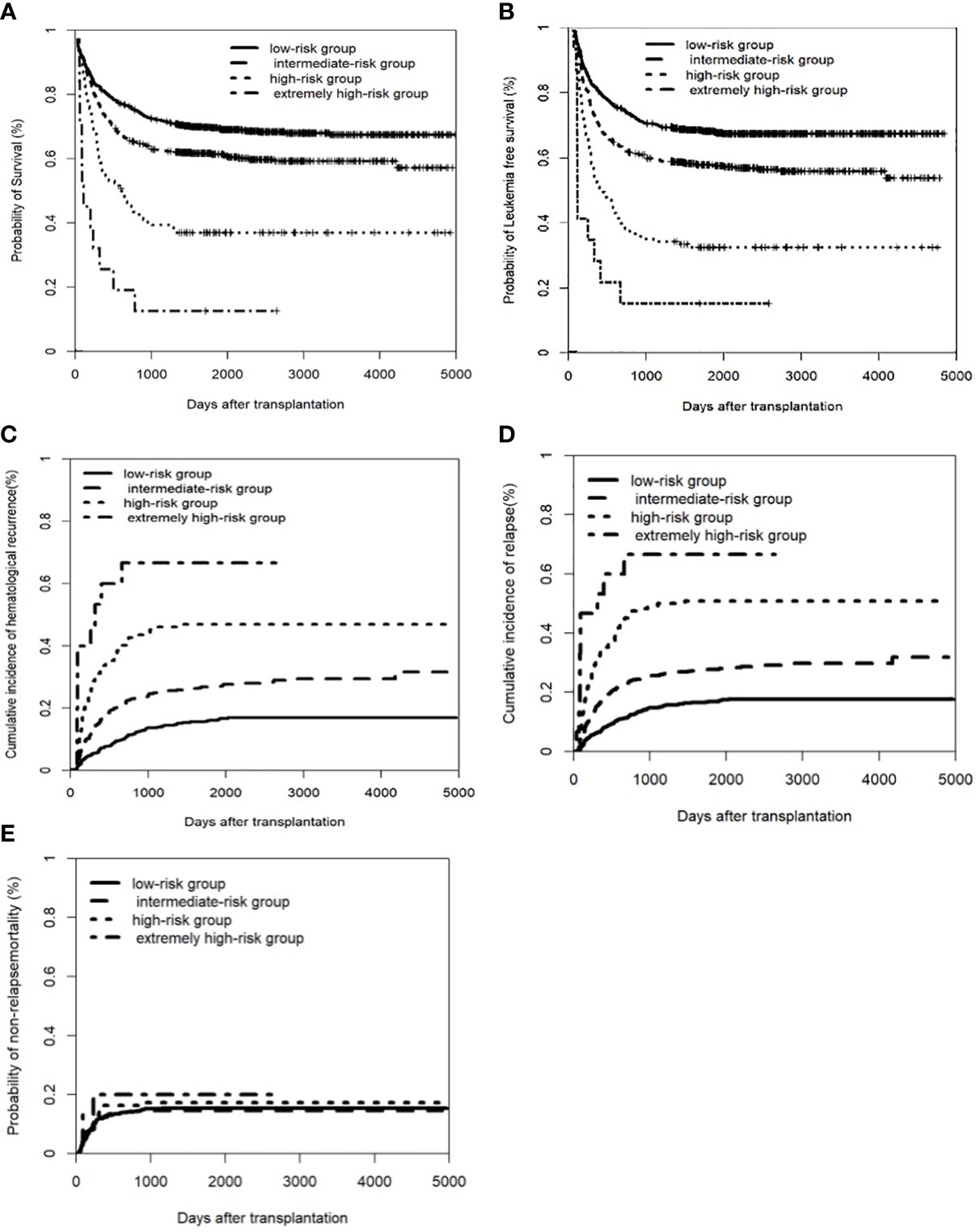
Figure 2 Clinical outcomes of a new scoring system in the entire cohort of patients. The factors including pre-CNS involvement, pre-HSCT MRD postive, disease status of CR2+ before transplant, and T-ALL. (A) Overall survival (OS); (B) Leukemia free of survival (LFS): * Leukemia free survival was defined as survival without hematologic relapse or extramedullary relapse; (C) cumulative incidence of hematological recurrence; (D) cumulative incidence of relapse; (E) non-relapse mortality (NRM).
3.6 Subgroup analysis of pre-HSCT CNS involvement compared to negative CNS
As shown in Table S3, in the B-ALL or T-ALL subgroup, disease status of the CR1 stage subgroup or CR2+ subgroup, and pre-HSCT MRD negative subgroup or pre-HSCT MRD positive subgroup, patients with CNS involvement pre-HSCT had significantly higher CIHR or CIR than those with negative CNS pre-HSCT. Regarding survival, the cases of CNS involvement pre-HSCT from all the other subgroups, except those from the CR2+ subgroup, had worse LFS and OS. In addition, cases with CNS involvement pre-HSCT in the T-ALL subgroup, disease status of CR1 stage subgroup, pre-HSCT MRD negative subgroup, and pre-HSCT MRD positive subgroup had an inferior OS compared to those with negative CNS. The multivariate analysis indicated that pre-HSCT CNS involvement was an independent risk factor associated with higher CIHR and CIR and inferior OS and LFS in each subgroup (data not shown).
The participants (n=1406) were divided into three groups according to age at diagnosis: 199 cases (14.2%), pediatric (1~14 years); 944 cases (67.2%), adolescent and young adult (AYA) (15~39 years); and 263 cases (18.7%), adults (age above 39 years), respectively. Among the three groups, no statistical differences were observed in OS (P= 0.154), LFS (P=0.135), and relapse (P=0.660). Subgroup analysis showed that CNS involvement in pediatric or adult patients was not independently associated with OS, LFS, relapse, and NRM. In the AYA group, isolated FCM-positive CNS involvement before transplantation had a higher risk of recurrence after transplantation and worse LFS and OS compared to negative CNS; these results were similar to those of patients with cytology-positive CNS involvement (data not shown).
Among the above subgroups, there was no difference in NRM.
4 Discussion
To our knowledge, this is the first study to show that isolated FCM-positive CNS involvement pre-HSCT was associated with higher CIR after transplantation (Tables 2–4). The negative effects of pre-HSCT CNS involvement by either cytology or FCM on transplant outcomes of ALL patients were demonstrated in both the total patient group and subgroups of total patients (Tables S1, S3). More importantly, a new scoring system based on four risk factors, including pre-HSCT CNS involvement, could better predict transplant outcomes (Figure 2; Table S2). Our study suggests that pre-HSCT CNS involvement by either cytology or FCM is an independent variable for predicting transplant outcomes in patients with ALL.
Regarding the clinical significance of CNS involvement detected by FCM, previous studies have demonstrated that CNS involvement by FCM at diagnosis is associated with a higher risk of relapse in childhood ALL (36, 37). In adult ALL/lymphoblastic lymphoma patients, Del Principe et al. (38) analyzed the association of CNS involvement in 38 newly diagnosed patients; 53% of the patients received allo-HSCT. The results showed that 2-year OS rates were 0%, 22%, and 53% (P=0.008) for the FCM+/CC+, FCM+/CC–, and FCM–/CC– subgroup, respectively. Gong et al. (33) found that the OS of patients with FCM+/CC– was similar to that of patients with FCM+/CC+, both of which were significantly shorter than those of patients with FCM–/CC–; however, only 142 patients in the study received allo-HSCT. In another retrospective study, Del Principe et al. (39) analyzed the data from 13 Italian hematological centers and found that patients with CNS involvement detected by FCM or CC had similar hematological recurrence rates, disease-free survival, and OS; however, compared to patients without CNS involvement, they had higher relapse rates and worse survival. In this study, only 55.1% of the patients underwent allo-HSCT. Consistent with studies in childhood ALL (36, 37) and the study by Del Principe et al. (39), we found that isolated FCM-positive CNS involvement before transplantation had a higher risk of recurrence after transplantation, including higher CIHR or CIR, which was similar to those with cytology-positive CNS involvement. In contrast to the studies by Del Principe et al. (38), our study showed that the isolated FCM-positive CNS involvement was not independently associated with a worse OS on multivariate analysis. Several factors may account for the differences in the effects of isolated FCM-positive CNS involvement on survival between the results of other studies (33, 38, 39). 1) The heterogeneity of treatment in the studies by others (33, 38, 39), but only including allo-HSCT in our study; 2) the differences in leukemia burden between isolated FCM-positive and cytology-positive CNS involvement, and 3) the graft-versus-leukemia effects of allo-HSCT might overcome the negative effect of isolated FCM-positive CNS involvement on survival.
Since the CIR of patients with isolated FCM-positive CNS involvement or cytology-positive CNS involvement was higher than that of patients with negative CNS pre-HSCT in our study, we combined the two groups into one group, namely, pre-HSCT CNS involvement. Our data showed that patients with pre-HSCT CNS involvement had a higher CIHR and CIR, as well as inferior survival, compared to those with negative CNS involvement. Previous studies (21, 22, 40, 41) have shown inconsistent results regarding whether CNS involvement has an adverse effect on the survival of ALL patients. For adult patients with ALL who received either chemotherapy alone or allo-HSCT, Lazarus et al. (40) showed that CNS involvement at diagnosis was an independent risk factor for OS (P=0.03), but not for EFS (P=0.07). Data from 69 patients with CNS involvement showed that allo-HSCT improved the survival of patients with CNS involvement compared with chemotherapy (43% vs. 26%). Hamdi et al. (41) showed that pre-transplant CNS involvement was associated with post-transplant CNS recurrence but did not affect survival. Shigematsu et al. (21) studied 2582 patients with ALL who underwent adult allogeneic stem cell therapy and showed that patients with CNS involvement pre-HSCT had a higher CNS recurrence rate (P =0.02) or overall recurrence rate (P<0.01), and a worse 3-year OS rate (P<0.01). Aldoss et al. (22) demonstrated that pre-HSCT CNS involvement was an independent risk factor for EFS and OS. Although controversy remains (21, 22, 41), most of these studies, including our study, have shown the negative effects of CNS involvement pre-HSCT on transplant outcomes, suggesting that CNS involvement pre-HSCT is a risk factor for poor prognosis in patients with ALL.
A previous study indicated that disease status, pre-HSCT MRD, and immunophenotype of leukemia cells were independent risk factors for CIR and survival in ALL patients who received allografting (29). Therefore, we investigated the effects of pre-HSCT CNS involvement on transplant outcomes of patients in each subgroup. In contrast to other studies, we found that pre-HSCT CNS involvement was associated with a higher CIR in the total and subgroups of patients (Table S3). Except for patients with CR2+, cases in the total group and other subgroups with pre-HSCT CNS involvement were associated with poor survival (Table S3). We speculate that the lack of negative effects of CNS involvement before HSCT on survival may be explained by the small number of patients with CR2+. Therefore, a large sample of ALL patients with pre-HSCT CNS involvement is needed to confirm our hypothesis.
Based on the results of the multivariate analysis (Table S1), we established a new prognostic scoring system combining this with risk factors of disease status, immunophenotype of ALL, and pre-HSCT MRD that affected the transplant outcomes of ALL patients in our previous study (29). Thus, patients were stratified into four distinct risk groups. The risk categories were significantly different for relapse, LFS, and OS, thus providing evidence for more individualized and precise clinical treatment.
This study has some limitations. First, it was a retrospective study; therefore, more multicenter prospective studies are required to confirm our findings. Second, the patients included in our study underwent haploidentical allograft transplants and matched sibling donor transplantation; thus, more studies need to be conducted to confirm this in other transplant modalities, such as matched unrelated donor transplants and umbilical cord blood transplant. Third, our study was conducted only in the allograft model based on G-CSF and ATG-induced immune tolerance; whether the same conclusion can be reached in the allograft of the PT/CY mode (42) requires additional studies. In addition, the number of cases of TBI-based conditioning regimen in our study was small. The role of TBI in ALL patients with CNS involvement should be elucidated in further studies.
5 Conclusions
In this large sample study, we found that isolated FCM-positive CNS involvement pre-HSCT was associated with a higher relapse rate but had no effect on survival after transplantation. Pre-HSCT CNS involvement affects both relapse and survival rates. Our results not only provide further evidence suggesting that pre-HSCT CNS involvement, determined by cytology or FCM, is a risk factor for poor outcomes, but also provide a new scoring system based on pre-HSCT CNS involvement and other risk factors for a more precise evaluation of clinical prognosis. These two factors might be helpful for individualized therapy for ALL patients receiving allografting.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
XJH and YJC designed the study and revised the paper. LM and YJC collected the data, analyzed the data, and drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the eighth Central Health Research Project of National Health Commission of the People’s Republic of China (grant number: 2022YB54).
Acknowledgments
We thank all the faculty members who participated in this study. The authors would also like to thank Editage (www.editage.cn) for assistance in editing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1166990/full#supplementary-material
Abbreviations
ALL, acute lymphoblastic leukemia; Allo-HSCT, Allogeneic hematopoietic stem cell transplantation; ATG, anti-thymocyte globulin; B-ALL, B-cell acute lymphoblastic leukemia; BM, bone marrow; CC, conventional cytology; CI, confidence interval; CIHR, cumulative incidence rate of hematological recurrence; CIR, cumulative incidence of relapse; CNS, central nervous system; CR, complete remission; CR2+, second complete remission or beyond; CSF, cerebrospinal fluid; CTX, cyclophosphamide; EFS, event-free survival; FCM, flow cytometry; G-CSF, granulocyte colony-stimulating factor; GVHD, graft-versus-host disease; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; LFS, leukemia-free survival; MRD, measurable residual disease; NRM, non-relapse mortality; OS, overall survival; PB, peripheral blood; T-ALL, T-cell acute lymphoblastic leukemia; TBI, total body irradiation.
References
1. Dhedin N, Huynh A, Maury S, Tabrizi R, Beldjord K, Asnafi V, et al. Role of allogeneic stem cell transplantation in adult patients with ph-negative acute lymphoblastic leukemia. Blood. (2015) 125(16):2486–96. doi: 10.1182/blood-2014-09-599894
2. Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant. (2019) 54(10):1525–52. doi: 10.1038/s41409-019-0516-2
3. Khazal S, Kebriaei P. Hematopoietic cell transplantation for acute lymphoblastic leukemia: review of current indications and outcomes. Leuk Lymphoma. (2021) 62(12):2831–44. doi: 10.1080/10428194.2021.1933475
4. Shem-Tov N, Peczynski C, Labopin M, Itala-Remes M, Blaise D, Labussiere-Wallet H, et al. Haploidentical vs. unrelated allogeneic stem cell transplantation for acute lymphoblastic leukemia in first complete remission: on behalf of the ALWP of the EBMT. Leukemia. (2020) 34(1):283–92. doi: 10.1038/s41375-019-0544-3
5. Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical versus matched-sibling transplant in adults with Philadelphia-negative high-risk acute lymphoblastic leukemia: a biologically phase III randomized study. Clin Cancer Res (2016) 22(14):3467–76. doi: 10.1158/1078-0432.CCR-15-2335
6. Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. The consensus from the Chinese society of hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol (2021) 14(1):145. doi: 10.1186/s13045-021-01159-2
7. Pulsipher MA, Carlson C, Langholz B, Wall DA, Schultz KR, Bunin N, et al. IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood. (2015) 125(22):3501–8. doi: 10.1182/blood-2014-12-615757
8. Sanchez-Garcia J, Serrano J, Serrano-Lopez J, Gomez-Garcia P, Martinez F, Garcia-Castellano JM, et al. Quantification of minimal residual disease levels by flow cytometry at time of transplant predicts outcome after myeloablative allogeneic transplantation in ALL. Bone Marrow Transplant. (2013) 48(3):396–402. doi: 10.1038/bmt.2012.147
9. Bader P, Salzmann-Manrique E, Balduzzi A, Dalle JH, Woolfrey AE, Bar M, et al. More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling. Blood Adv (2019) 3(21):3393–405. doi: 10.1182/bloodadvances.2019000449
10. Ciudad J, San Miguel JF, López-Berges MC, Vidriales B, Valverde B, Ocqueteau M, et al. Prognostic value of immunophenotypic detection of minimal residual disease in acute lymphoblastic leukemia. J Clin Oncol (1998) 16(12):3774–81. doi: 10.1200/JCO.1998.16.12.3774
11. Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM study group. J Clin Oncol (2009) 27(3):377–84. doi: 10.1200/JCO.2008.17.6065
12. Balduzzi A, Di Maio L, Silvestri D, Songia S, Bonanomi S, Rovelli A, et al. Minimal residual disease before and after transplantation for childhood acute lymphoblastic leukaemia: is there any room for intervention? Br J Haematol (2014) 164(3):396–408. doi: 10.1111/bjh.12639
13. Bar M, Wood BL, Radich JP, Doney KC, Woolfrey AE, Delaney C, et al. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leuk Res Treat (2014) 2014:421723. doi: 10.1155/2014/421723
14. Dworzak MN, Froschl G, Printz D, Mann G, Potschger U, Muhlegger N, et al. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood. (2002) 99(6):1952–8. doi: 10.1182/blood.V99.6.1952
15. Gandemer V, Pochon C, Oger E, Dalle JH, Michel G, Schmitt C, et al. Clinical value of pre-transplant minimal residual disease in childhood lymphoblastic leukaemia: the results of the French minimal residual disease-guided protocol. Br J Haematol (2014) 165(3):392–401. doi: 10.1111/bjh.12749
16. Shen Z, Gu X, Mao W, Yin L, Yang L, Zhang Z, et al. Influence of pre-transplant minimal residual disease on prognosis after allo-SCT for patients with acute lymphoblastic leukemia: systematic review and meta-analysis. BMC Cancer. (2018) 18(1):755. doi: 10.1186/s12885-018-4670-5
17. Wang XY, Fan QZ, Xu LP, Wang Y, Zhang XH, Chen H, et al. The quantification of minimal residual disease pre- and post-unmanipulated haploidentical allograft by multiparameter flow cytometry in pediatric acute lymphoblastic leukemia. Cytometry B Clin Cytom. (2020) 98(1):75–87. doi: 10.1002/cyto.b.21840
18. Ganem G, Kuentz M, Bernaudin F, Gharbi A, Cordonnier C, Lemerle S, et al. Central nervous system relapses after bone marrow transplantation for acute lymphoblastic leukemia in remission. Cancer. (1989) 64(9):1796–804. doi: 10.1002/1097-0142(19891101)64:9<1796::AID-CNCR2820640907>3.0.CO;2-7
19. Larson RA. Managing CNS disease in adults with acute lymphoblastic leukemia. Leuk Lymphoma. (2018) 59(1):3–13. doi: 10.1080/10428194.2017.1326597
20. Oshima K, Kanda Y, Yamashita T, Takahashi S, Mori T, Nakaseko C, et al. Central nervous system relapse of leukemia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2008) 14(10):1100–7. doi: 10.1016/j.bbmt.2008.07.002
21. Shigematsu A, Kako S, Mitsuhashi K, Iwato K, Uchida N, Kanda Y, et al. Allogeneic stem cell transplantation for adult patients with acute lymphoblastic leukemia who had central nervous system involvement: a study from the adult ALL working group of the Japan society for hematopoietic cell transplantation. Int J Hematol (2017) 105(6):805–11. doi: 10.1007/s12185-017-2197-1
22. Aldoss I, Al Malki MM, Stiller T, Cao T, Sanchez JF, Palmer J, et al. Implications and management of central nervous system involvement before allogeneic hematopoietic cell transplantation in acute lymphoblastic leukemia. Biol Blood Marrow Transplant. (2016) 22(3):575–8. doi: 10.1016/j.bbmt.2015.10.016
23. Kharfan-Dabaja MA, Labopin M, Bazarbachi A, Salmenniemi U, Mielke S, Chevallier P, et al. CNS involvement at initial diagnosis and risk of relapse after allogeneic HCT for acute lymphoblastic leukemia in first complete remission. Hemasphere. (2022) 6(11):e788. doi: 10.1097/HS9.0000000000000788
24. Cesana C, Klersy C, Scarpati B, Brando B, Faleri M, Bertani G, et al. Flow cytometry and cytomorphology evaluation of hematologic malignancy in cerebrospinal fluids: comparison with retrospective clinical outcome. Ann Hematol (2011) 90(7):827–35. doi: 10.1007/s00277-010-1145-4
25. Del Principe MI, Maurillo L, Buccisano F, Sconocchia G, Cefalo M, De Santis G, et al. Central nervous system involvement in adult acute lymphoblastic leukemia: diagnostic tools, prophylaxis, and therapy. Mediterr J Hematol Infect Dis (2014) 6(1):e2014075. doi: 10.4084/mjhid.2014.075
26. Garcia KA, Cherian S, Stevenson PA, Martino CH, Shustov AR, Becker PS, et al. Cerebrospinal fluid flow cytometry and risk of central nervous system relapse after hyperCVAD in adults with acute lymphoblastic leukemia. Cancer. (2022) 128(7):1411–7. doi: 10.1002/cncr.34073
27. Ranta S, Nilsson F, Harila-Saari A, Saft L, Tani E, Soderhall S, et al. Detection of central nervous system involvement in childhood acute lymphoblastic leukemia by cytomorphology and flow cytometry of the cerebrospinal fluid. Pediatr Blood Cancer. (2015) 62(6):951–6. doi: 10.1002/pbc.25363
28. Sayed D, Badrawy H, Ali AM, Shaker S. Immunophenotyping and immunoglobulin heavy chain gene rearrangement analysis in cerebrospinal fluid of pediatric patients with acute lymphoblastic leukemia. Leuk Res (2009) 33(5):655–61. doi: 10.1016/j.leukres.2008.09.033
29. Zhao XS, Liu YR, Xu LP, Wang Y, Zhang XH, Chen H, et al. Minimal residual disease status determined by multiparametric flow cytometry pretransplantation predicts the outcome of patients with ALL receiving unmanipulated haploidentical allografts. Am J Hematol (2019) 94(5):512–21. doi: 10.1002/ajh.25417
30. Pui CH, Pei D, Coustan-Smith E, Jeha S, Cheng C, Bowman WP, et al. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. Lancet Oncol (2015) 16(4):465–74. doi: 10.1016/S1470-2045(15)70082-3
31. Zhao X, Zhao X, Chen H, Qin Y, Xu L, Zhang X, et al. Comparative analysis of flow cytometry and RQ-PCR for the detection of minimal residual disease in Philadelphia chromosome-positive acute lymphoblastic leukemia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2018) 24(9):1936–43. doi: 10.1016/j.bbmt.2018.03.015
32. Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. (2006) 107(8):3065–73. doi: 10.1182/blood-2005-05-2146
33. Gong X, Lin D, Wang H, Wang Y, Liu B, Wei H, et al. Flow cytometric analysis of cerebrospinal fluid in adult patients with acute lymphoblastic leukemia during follow-up. Eur J Haematol (2018) 100(3):279–85. doi: 10.1111/ejh.13011
34. Piemontese S, Ciceri F, Labopin M, Bacigalupo A, Huang H, Santarone S, et al. A survey on unmanipulated haploidentical hematopoietic stem cell transplantation in adults with acute leukemia. Leukemia. (2015) 29(5):1069–75. doi: 10.1038/leu.2014.336
35. Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. (2015) 125(25):3956–62. doi: 10.1182/blood-2015-02-627786
36. Haas Vd, Pieters R, Sluijs-Gelling AJVD, Zwaan CM, Velden VHJVD. Flowcytometric evaluation of cerebrospinal fluid in childhood ALL identifies CNS involvement better then conventional cytomorphology. Leukemia (2021) 35(6):1773–6. doi: 10.1038/s41375-020-01029-9
37. Thastrup M, Marquart HV, Levinsen M, Grell K, Abrahamsson J, Albertsen BK, et al. Flow cytometric detection of leukemic blasts in cerebrospinal fluid predicts risk of relapse in childhood acute lymphoblastic leukemia: a Nordic society of pediatric hematology and oncology study. Leukemia. (2020) 34(2):336–46. doi: 10.1038/s41375-019-0570-1
38. Del Principe MI, Buccisano F, Cefalo M, Maurillo L, Di Caprio L, Di Piazza F, et al. High sensitivity of flow cytometry improves detection of occult leptomeningeal disease in acute lymphoblastic leukemia and lymphoblastic lymphoma. Ann Hematol (2014) 93(9):1509–13. doi: 10.1007/s00277-014-2080-6
39. Del Principe MI, Buzzatti E, Piciocchi A, Forghieri F, Bonifacio M, Lessi F, et al. Clinical significance of occult central nervous system disease in adult acute lymphoblastic leukemia. a multicenter report from the campus ALL network. Haematologica. (2021) 106(1):39–45. doi: 10.3324/haematol.2019.231704
40. Lazarus HM, Richards SM, Chopra R, Litzow MR, Burnett AK, Wiernik PH, et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: results from the international ALL trial MRC UKALL XII/ECOG E2993. Blood. (2006) 108(2):465–72. doi: 10.1182/blood-2005-11-4666
41. Hamdi A, Mawad R, Bassett R, di Stasi A, Ferro R, Afrough A, et al. Central nervous system relapse in adults with acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2014) 20(11):1767–71. doi: 10.1016/j.bbmt.2014.07.005
42. Bacigalupo A, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplant. (2015) 50 Suppl 2:S37. doi: 10.1038/bmt.2015.93
Keywords: acute lymphoblastic leukemia, central nervous system involvement, isolated flow cytometry positive, allogeneic hematopoietic stem cell transplantation, transplant outcomes
Citation: Ma L, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, Wang FR, Han W, Sun YQ, Yan CH, Lv M, Tang FF, Mo XD, Wang ZD, Jiang Q, Lu J, Jiang H, Liu YR, Liu KY, Chang YJ and Huang XJ (2023) Effects of isolated central nervous system involvement evaluated by multiparameter flow cytometry prior to allografting on outcomes of patients with acute lymphoblastic leukemia. Front. Oncol. 13:1166990. doi: 10.3389/fonc.2023.1166990
Received: 15 February 2023; Accepted: 24 April 2023;
Published: 10 May 2023.
Edited by:
Martin Maiers, National Marrow Donor Program, United StatesReviewed by:
Jiaqian Qi, The First Affiliated Hospital of Soochow University, ChinaMarco Cerrano, University Hospital of the City of Health and Science of Turin, Italy
Copyright © 2023 Ma, Xu, Wang, Zhang, Chen, Chen, Wang, Han, Sun, Yan, Lv, Tang, Mo, Wang, Jiang, Lu, Jiang, Liu, Liu, Chang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojun Huang, aHVhbmd4aWFvanVuQGJqbXUuZWR1LmNu
 Ling Ma
Ling Ma Lan-Ping Xu
Lan-Ping Xu Yu Wang
Yu Wang Xiao-Hui Zhang
Xiao-Hui Zhang Huan Chen
Huan Chen Meng Lv
Meng Lv Fei-Fei Tang
Fei-Fei Tang Xiao-Dong Mo
Xiao-Dong Mo Qian Jiang
Qian Jiang Jin Lu
Jin Lu Ying-Jun Chang
Ying-Jun Chang Xiao-Jun Huang
Xiao-Jun Huang