Abstract
Purpose:
This study aims to perform a pooled analysis to compare the outcomes of robot-assisted partial nephrectomy (RAPN) between complex tumors (hilar, endophytic, or cystic) and non-complex tumors (nonhilar, exophytic, or solid) and evaluate the effects of renal tumor complexity on outcomes in patients undergoing RAPN.
Methods:
Four databases were systematically searched, including Science, PubMed, Web of Science, and Cochrane Library, to identify relevant studies published in English up to December 2022. Review Manager 5.4 was used for statistical analyses and calculations. The study was registered with PROSPERO (Registration number: CRD42023394792).
Results:
In total, 14 comparative trials, including 3758 patients were enrolled. Compared to non-complex tumors, complex tumors were associated with a significantly longer warm ischemia time (WMD 3.67 min, 95% CI 1.78, 5.57; p = 0.0001), more blood loss (WMD 22.84 mL, 95% CI 2.31, 43.37; p = 0.03), and a higher rate of major complications (OR 2.35, 95% CI 1.50, 3.67; p = 0.0002). However, no statistically significant differences were found between the two groups in operative time, length of stay, transfusion rates, conversion to open nephrectomy and radical nephrectomy rates, estimated glomerular filtration rate (eGFR) decline, intraoperative complication, overall complication, positive surgical margins (PSM), local recurrence, and trifecta achievement.
Conclusions:
RAPN can be a safe and effective procedure for complex tumors (hilar, endophytic, or cystic) and provides comparable functional and oncologic outcomes to non-complex tumors.
Systematic review registration:
https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=394792, identifier CRD42023394792.
1 Introduction
Partial nephrectomy (PN) is currently considered the optimal treatment for small renal tumors, as recommended by the AUA and EAU guidelines (1, 2). In addition to achieving comparable surgical outcomes and cancer control to radical nephrectomy, PN allows for the preservation of the nephrons (3). The field of PN has seen innovative advances with the introduction of robotic technology, and open PN has gradually given way to robot-assisted partial nephrectomy (RAPN) (4). Furthermore, Choi et al. (5) have demonstrated that RAPN was associated with a shorter warm ischemic time, lower rate of conversion to open nephrectomy, and less estimated glomerular filtration rate (eGFR) decline compared to laparoscopic PN. Recently, RAPN has increasingly been applied to technically challenging complex tumors.
The renal tumor complexity mainly depends on some tumor-associated factors, such as tumor size and type (endophytic, hilar, or cystic) (6). In renal hilar tumors, the mass is in close proximity to the urinary collecting system and the major renal vessels, which adds to the difficulty of the PN (7). Due to the surgeon being unable to identify the tumor location and size, PN for endophytic renal tumors is very challenging (8). In addition, performing PN for cystic renal tumors is also more difficult than for solid renal tumors because of the risk of cyst wall damage and tumor cell spillage (9). Garisto et al. (10) reported that RAPN provided acceptable results in terms of perioperative, functional and oncological outcomes for complex tumors (RENAL score > 9). The RENAL score (11) is a scoring tool to predict the difficulty of nephrectomy, but it has some shortcomings. It does not sufficiently evaluate some factors closely related to the complexity of renal tumors, such as renal hilar tumors and cystic renal tumors. Therefore, the effectiveness and safety of RAPN for these types of tumors remain controversial.
This systematic review and meta-analysis aim to integrate the data from comparative studies to evaluate the efficacy and safety of RAPN for complex tumors (hilar, endophytic, or cystic), providing guidance for clinical decision-making.
2 Methods
The present study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement 2020 (12, 13), and was registered in PROSPERO (Registration number: CRD42023394792).
2.1 Literature search strategy, study selection and data collection
Four databases, including Science, PubMed, Web of Science, and Cochrane Library, were systematically searched to identify fully published studies till December 2022. The search terms were as follows: ((Renal hilar tumors OR hilar tumors OR renal hilar masses) AND (Endophytic renal tumors OR endophytic renal masses) AND (Cystic renal cell carcinoma OR cystic renal tumors OR cystic renal masses) AND (Robotic partial nephrectomy OR Robot-assisted partial nephrectomy OR Robot-assisted nephron-sparing surgery)). Furthermore, the relevant references were manually searched to avoid omissions and expand the search scope.
The PICOS approach was used to define the inclusion criteria (1): all the patients were diagnosed with localized renal tumors; (2): in the experimental group, the patients were diagnosed with renal hilar tumors, endophytic renal tumors, or cystic renal tumors, and underwent RAPN; (3): in the control group, patients were diagnosed with renal nonhilar tumors, exophytic renal tumors, or solid renal tumors, and underwent RAPN; (4): the studies measured the perioperative, complication, renal functional and oncologic outcomes; (5): randomized controlled trials (RCTs), prospective or retrospective comparative studies. The exclusion criteria included: (1) non-comparative studies and duplicate studies, (2) letters, comments, case reports and unpublished studies, and (3) studies that did not contain the required data for meta‐analysis.
Two reviewers independently extracted the data from each qualified literature. The following data were extracted: (1) first author, year of publication, center, and country. (2) Age, body mass index (BMI), sample size, preoperative eGFR, PADUA score, and follow-up period. (3) Perioperative outcomes, including operative time, blood loss, transfusion rates, hospital stay, warm ischemia time, conversion to open nephrectomy and radical nephrectomy rates, intraoperative complications, major complications (defined as Clavien grade ≥ 3), and overall complications (defined as Clavien grade ≥ 1) (14) (4). Renal functional and oncologic outcomes, including eGFR decline, positive surgical margins (PSM), local recurrence, trifecta achievement: margin status (negative), warm ischemia time (< 25 min), and complications (Clavien grade ≤ 2), tumor size, tumor stage and pathology. Any discrepancies and disagreements were resolved by reaching a consensus with a third reviewer.
In these studies, the risk of bias in non-randomized studies of interventions (ROBINS-I) was used to assess the quality of the literature (15). Two independent reviewers estimated the quality of the included studies, and any discrepancies were solved through discussion.
2.2 Statistical analysis
The statistical analysis of this study was processed using Cochrane Collaborative RevMan5.4 software. The weighted mean difference (WMD) was calculated for continuous variables, whereas the odds ratio (OR) was calculated for dichotomous variables, and the results were presented with 95% confidence intervals (CI). Furthermore, the I2 test was used to measure the heterogeneity of each indicator among the studies (16), and statistical significance was defined as p < 0.05. For outcomes with significant heterogeneity (I2 > 75%), sensitivity analysis was performed by excluding one study from the pooled effect at a time to identify the source of heterogeneity and to assess the robustness.
2.3 Subgroup analysis
A subgroup analysis was performed to compare the different tumor types: renal hilar vs. renal nonhilar tumors, endophytic vs. exophytic renal tumors, and cystic vs. solid renal tumors.
2.4 Publication bias
Publication bias was examined using the Begg’s method funnel plot.
3 Results
3.1 Baseline characteristics
Initially, a comprehensive electronic search yielded 209 studies, which were subsequently reduced to 32 after the removal of duplicate entries. A preliminary evaluation of the titles and abstracts of these studies led to the selection of 14 studies, which involved a total of 3758 patients, for inclusion in our meta-analysis (Figure 1) (17–30). All 14 non-RCTs were retrospective comparisons, with three studies being multi-institutional (21, 23, 29), while the others were single-center. The studies were conducted in different countries, including the USA, Korea, China, and Japan, with a follow-up period of between 3.3 to 48 months. The key characteristics of all patients included in each study are summarized in Table 1 and Table 2 (including sample size, age, BMI, gender, tumor diameter, tumor site, preoperative eGFR, RENAL score and tumor types). Table 3 displays the oncologic outcomes.
Figure 1
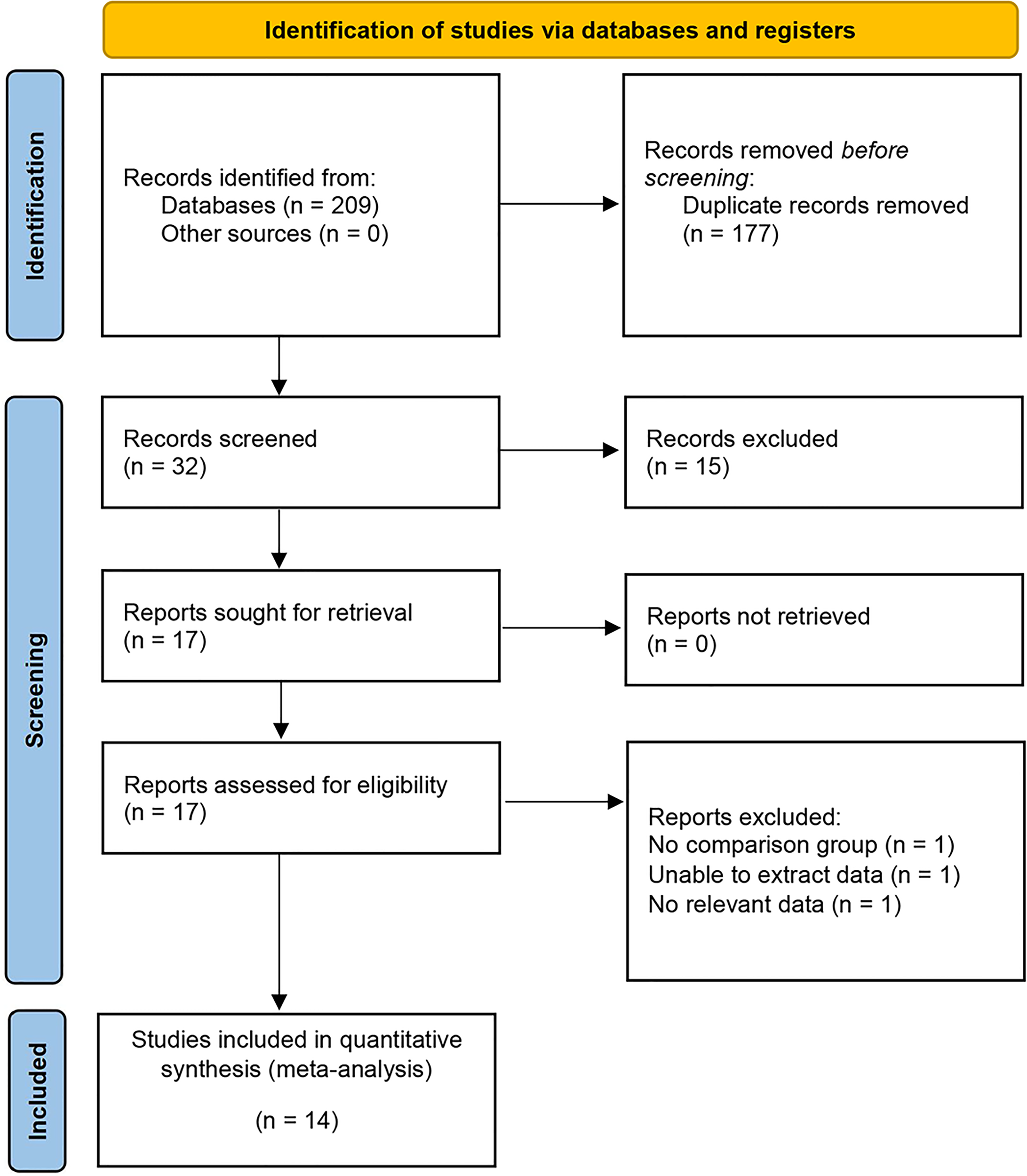
PRISMA flow diagram for the systematic review.
Table 1
| Reference | Year | Country | Center | Patients | Age(y) | Male/Female | BMI (kg/m2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Complex group | Non-complex group | Complex group | Non-complex group | Complex group | Non-complex group | Complex group | Non-complex group | ||||
| Tyagi | 2021 | India | single-center | 41 | 41 | 50.4(12.7) | 51.5(11.7) | 24/17 | 25/16 | 24.9(3.3) | 24.4(3.0) |
| Liu | 2021 | China | single-center | 75 | 98 | 55(16) | 55.5(13.25) | 43/32 | 71/27 | NA | |
| Lu | 2018 | China | single-center | 30 | 170 | 52.4(15.3) | 58(13.5) | 14/16 | 99/71 | 23.7(3.3) | 25.4(3.9) |
| Eyraud | 2013 | USA | single-center | 70 | 294 | 58(14.07) | 59(11.11) | 45/25 | 170/124 | 29.39(5.19) | 29.29(6.01) |
| Dulabon | 2011 | USA | multi-institutional | 41 | 405 | 59.3(12.8) | 60.0(11.3) | 29/12 | 233/172 | 28.6(6.3) | 30.2(6.4) |
| Motoyama | 2022 | Japan | single-center | 26 | 127 | 64.5(12.5) | 68(15.75) | 18/8 | 83/44 | 24.8(5.43) | 24.2(8.05) |
| Carbonara | 2020 | USA and Europe | multi-institutional | 147 | 510 | 57.7(11.8) | 60.9(12.7) | 93/54 | 296/214 | 27.4(5.7) | 27.7(5.2) |
| Komninos | 2014 | Korea | single-center | 45 | 64 | 50(9.63) | 51(10.37) | 31/14 | 30/34 | 26.1(3.33) | 25.5(3.7) |
| Autorino | 2014 | USA | single-center | 65 | 179 | 56(1.4) | 61.2(0.9) | 31/34 | 111/68 | 29.4(6.3) | 31.2(7.4) |
| Yagisawa | 2022 | Japan | single-center | 83 | 83 | 55(14) | 54(13) | 65/18 | 60/23 | 24(4) | 23(4) |
| Zennami | 2021 | Japan | single-center | 46 | 271 | 58(14.07) | 62(12.59) | 33/13 | 202/69 | 23.5(2.67) | 23.8(3.04) |
| Raheem | 2016 | Korea | single-center | 32 | 263 | 51.3(11.3) | 52.1(12.5) | 16/16 | 168/95 | 24.4(2.6) | 24.7(3.5) |
| Novara | 2016 | Europe | multi-institutional | 54 | 411 | 62(12.22) | 58(12.59) | 36/18 | 281/130 | NA | |
| Akca | 2014 | USA | single-center | 55 | 55 | 55.82(14.9) | 58.67(12.1) | 26/29 | 28/27 | 29.11(54) | 30.22(5.6) |
The trials included in the systemic review.
BMI, Body mass index; Mean (SD).
Table 2
| Reference | Tumor site (Lt/Rt) | Tumor diameter (cm) | Preoperative eGFR (ml/min/1.73 m) | RENAL score | Type | Follow-up duration (month) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Complex group | Non-complex group |
Complex group | Non-complex group |
Complex group | Non-complex group |
Complex group | Non-complex group |
Complex group | Non-complex group |
||
| Tyagi | 22/19 | 18/23 | 4.4(1.6) | 3.5(1.5) | 100.8(41.8) | 92.3(28.0) | 7.9(1.7) | 7.8(1.7) | Renal hilar tumors |
Range: 3-12 | |
| Liu | NA | 4.8(1.98) | 4.5(1.65) | 113.9(33.08) | 121.5(11.6) | 9(1.5) | 6(1.75) | Renal hilar tumors |
Mean: 30 | ||
| Lu | 14/16 | 74/96 | 4.8(2) | 3.7(1.8) | 94.1(13.3) | 86.5(25.3) | 9(1.2) | 7.4(1.7) | Renal hilar tumors |
Mean: 28 (range: 3-12) |
Mean: 32.3 (range: 3-12) |
| Eyraud | NA | 3.9(1.63) | 2.6(1.26) | 84.8(19.26) | 85.6(21.04) | Low: 0; Intermediate: 29; High: 41 |
Low: 146; Intermediate: 132; High: 16 |
Renal hilar tumors |
Mean: 7.4 (range: 1.3-18.5) |
||
| Dulabon | NA | 3.46(1.35) | 2.88(1.53) | NA | NA | Renal hilar tumors |
Range: 3-45 | ||||
| Motoyama | 8/18 | 63/64 | 1.9(1.0) | 2.9(1.75) | NA | 9(1.25) | 6(1.5) | Endophytic renal tumors |
NA | ||
| Carbonara | NA | 4.2(2.5) | 3.2(4.1) | 84.2(22.7) | 83.6(21.4) | 10(1.48) | 4(1.48) | Endophytic renal tumors |
21.6(20) | 32.3(25.4) | |
| Komninos | 26/19 | 29/35 | 2.6(1.56) | 2.5(2.96) | 84.4(10.37) | 90(14.07) | 9(1.48) | 5.5(2.22) | Endophytic renal tumors |
Mean: 48 (range: 20-59) |
Mean: 38 (range: 16-63) |
| Autorino | 32/33 | 90/89 | 2.6(1.0) | 3.7(2.1) | 89.6(22.9) | 80.1(23.2) | 8.7(1.4) | 6.4(2.2) | Endophytic renal tumors |
12.6(11.0) | 14.5(13.8) |
| Yagisawa | NA | 2.8(1.3) | 3.0(1.3) | 67(17) | 69(17) | Low: 17; Intermediate: 59; High: 8 |
Low: 32; Intermediate: 36; High: 15 |
Cystic renal tumors |
23(22) | 21(18) | |
| Zennami | NA | 3.2(1.48) | 2.9(1.11) | 68.6(16.67) | 69.5(15.41) | Low: 19; Intermediate: 23; High: 4 |
Low: 120; Intermediate: 137; High: 14 |
Cystic renal tumors |
Mean: 41 | Mean: 37 | |
| Raheem | NA | 3.7(1.9) | 3.3(1.8) | 88(14.82) | 90(8.89) | Low: 5; Intermediate: 11; High: 16 |
Low: 67; Intermediate: 91; High: 104 |
Cystic renal tumors |
Mean: 58 (range: 24–63) |
Mean: 46 (range: 24–60) |
|
| Novara | NA | 3.45(2.16) | 3.2(1.30) | 79(20.74) | 81(19.26) | Low: 13; Intermediate: 28; High: 13 |
Low: 201; Intermediate: 118; High: 92 |
Cystic renal tumors |
Mean: 10 (IQR: 3–24) |
Mean: 6 (IQR: 3–19) |
|
| Akca | 25/30 | 29/26 | 2.7(2.6) | 3.1(2.0) | 89.6(38.1) | 80.1(27.1) | 9(2.96) | 9(2.96) | Cystic renal tumors |
Mean: 10(IQR: 22) |
Mean: 7(IQR: 9) |
The trials included in the systemic review.
eGFR, estimated glomerular filtration rate; Mean (SD); IQR, interquartile range.
Table 3
| Reference | Tumor stage | Tumor pathology | ||
|---|---|---|---|---|
| Complex tumor group | Non-complex tumor group | Complex tumor group | Non-complex tumor group | |
| Tyagi | pT1a:12; pT1b:21; pT2a:1; pT2b:2; pT3a:1 | pT1a:22; pT1b:14; pT2a:2; pT2b:2; pT3a:0 | Benign: 3; Malignant: 38 | Benign: 4; Malignant: 37 |
| Liu | pTa:24; pT1b:39; pT2a:8 | pTa:59; pT1b:31; pT2a:6 | Clear cell: 64; Papillary: 3; Chromophobe: 4; Others: 4 | Clear cell: 90; Papillary: 1; Chromophobe: 6; Others: 1 |
| Lu | pTa:9; pT1b:5; pT2:0; pT3a:2 | pTa:93; pT1b:20; pT2:1; pT3a:10 | Clear cell: 16;lymphovascular invasion:2 | Clear cell: 117;lymphovascular invasion:5 |
| Eyraud | pT1a:26; pT1b:19; pT2:1; pT3a:7 | pT1a:160; pT1b:30; pT2:4; pT3a:11 | Clear cell: 53 | Clear cell: 205 |
| Dulabon | pT1a:23; pT1b:6; pT2:1; pT3a:5; pT3b:2 | pT1a:248; pT1b:36; pT2:3; pT3a:8; pT3b:1 | Clear cell: 29; Papillary: 6; Chromophobe: 1; Others: 0 | Clear cell: 193; Papillary: 67; Chromophobe: 26; Others: 5 |
| Motoyama | NA | Clear cell: 18; Others: 2; Benign: 6 | Clear cell: 75; Others: 24; Benign: 28 | |
| Carbonara | pT1a:68; pT1b:33; pT2a:13; pT2b:2; pT3a:10 | pT1a:307; pT1b:70; pT2a:9; pT2b:4; pT3a:18 | Benign: 31; Malignant: 116 | Benign: 121; Malignant: 389 |
| Komninos | pT1a:30; pT1b:9; pT2:1; pT3a:0 | pT1a:30; pT1b:10; pT2:4; pT3a:2 | Benign: 5; Malignant: 40 | Benign: 18; Malignant: 46 |
| Autorino | pT1a:47; pT1b:3; pT2:0; pT3a:2 | pT1a:84; pT1b:41; pT2:4; pT3a:11 | Benign: 17; Malignant: 48 | Benign: 40; Malignant: 139 |
| Yagisawa | NA | NA | ||
| Zennami | pT1a:34; pT1b:3; pT2:0; pT3a:0 | pT1a:226; pT1b:20; pT2:1; pT3a:5 | Clear cell: 33; Papillary: 2; Chromophobe: 1; Others: 1; Benign: 9 | Clear cell: 205; Papillary: 24; Chromophobe: 18; Others: 5; Benign: 19 |
| Raheem | cT1a:18; cT1b:13; cT2a:1; cT2b:0 | cT1a:195; cT1b:58; cT2:8; cT3a:2 | Benign: 3; Malignant: 29 | Benign: 46; Malignant: 217 |
| Novara | pT1a:33; pT1b:6; pT2:0; pT3a:4 | pT1a:241; pT1b:54; pT2:3; pT3a:23 | Clear cell: 31; Papillary: 10; Chromophobe: 1; Others: 1; Benign: 11 | Clear cell: 251; Papillary: 46; Chromophobe: 25; Others: 5; Benign: 84 |
| Akca | pT1a:14; pT1b:12; pT2a:1; pT3a:3 | pT1a:28; pT1b:12; pT2a:0; pT3a:7 | Clear cell: 18; Papillary: 6; Chromophobe: 2; Others: 4; | Clear cell: 21; Papillary: 16; Chromophobe: 7; Others: 3; |
Oncologic outcomes.
No significant difference was found in age (p = 0.06), tumor diameter (p = 0.26), BMI (p = 0.14) and preoperative eGFR (p = 0.86). However, the tumor diameter was significantly larger in the renal hilar tumor subgroup compared to the renal nonhilar tumors subgroup (p < 0.0001) (Table S1).
3.2 Assessment of quality
A comparative analysis was performed on all the included studies, revealing a moderate risk of bias (Table S2).
3.3 Outcome analysis
3.3.1 Perioperative effectiveness
The pooled results demonstrated no difference in operative time (14 studies; p = 0.40) between the complex and non-complex tumor groups (17–30). However, the subgroup analysis indicated that renal hilar tumors had a longer operative time than renal nonhilar tumors (WMD 19.17 min, 95% CI 4.30, 34.04; p = 0.01) (Figure 2). The meta‐analysis included 14 studies that reported the warm ischemia time (17–30). The combined results revealed that the complex tumor group was associated with a longer warm ischemia time than the non-complex tumor group (WMD 3.67 min, 95% CI 1.78, 5.57; p = 0.0001). Similar results were found in renal hilar and endophytic renal tumors groups (renal hilar tumors: WMD 6.85 min, 95% CI 2.20, 11.49; p = 0.004; endophytic renal tumors: WMD 5.41 min, 95% CI 4.14, 6.67; p < 0.00001) (Figure 3). The cumulative analysis revealed no significant difference in length of hospital stay between the two groups (14 studies; p = 0.69) (17–30). Furthermore, the subgroup analysis demonstrated no statistically significant differences in the length of hospital stay among the three subgroups (Figure S1).
Figure 2
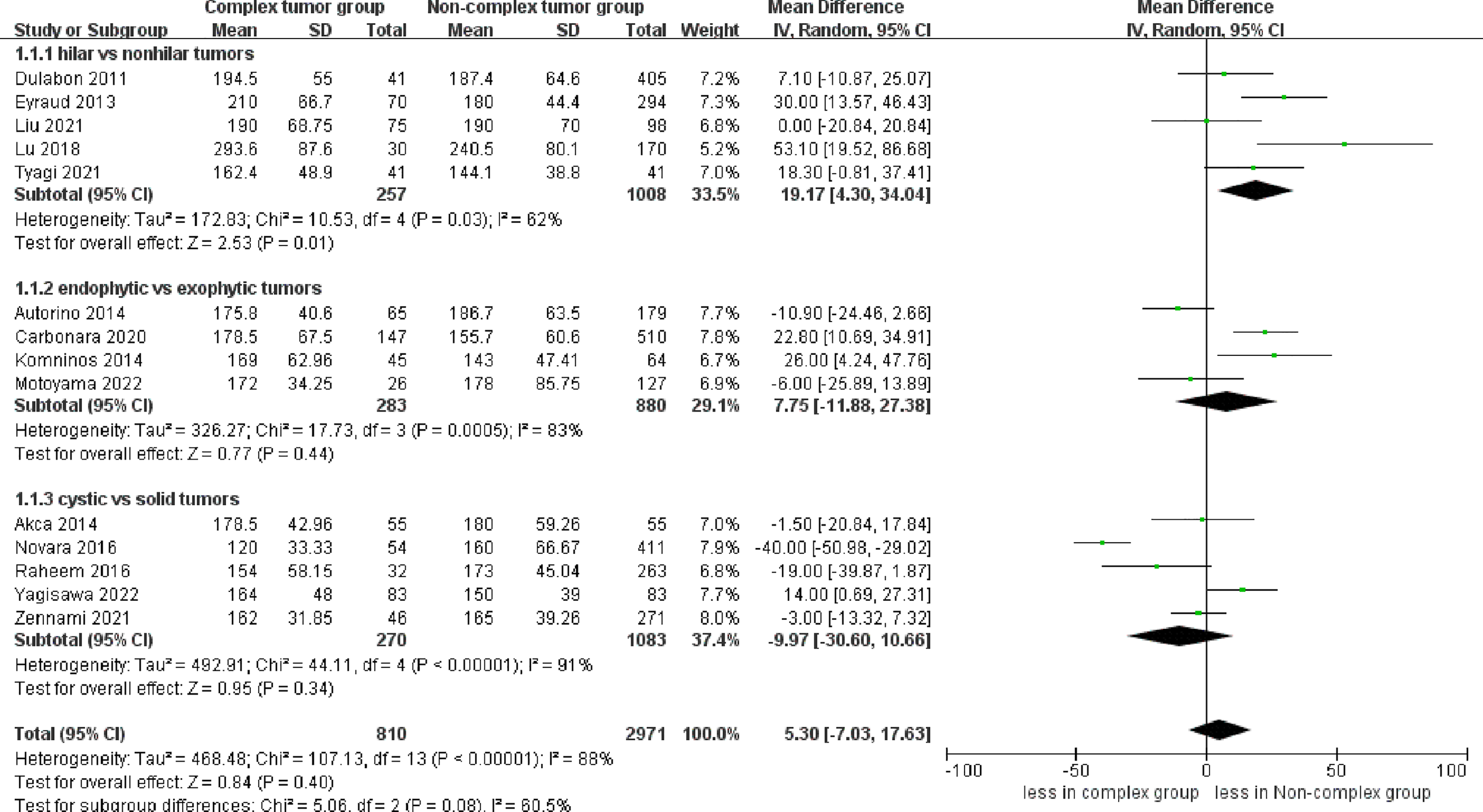
Forest plots of perioperative outcome- operative time.
Figure 3
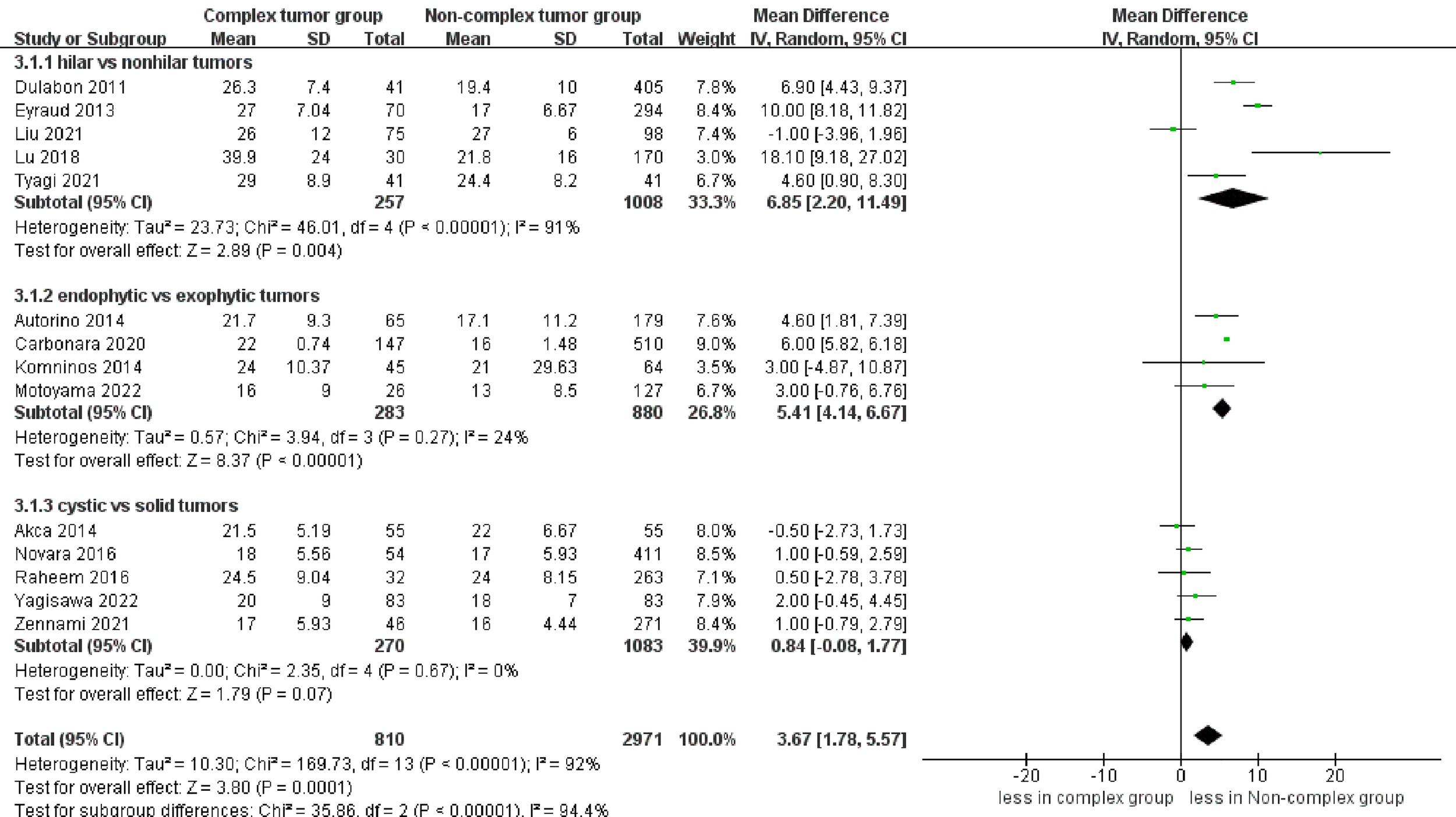
Forest plots of perioperative outcome- warm ischemia time.
However, significantly more blood loss was observed in the complex tumor group compared with the non-complex tumor group (14 studies; WMD 22.84 mL, 95% CI 2.31, 43.37; p = 0.03) (17–30). The subgroup analysis demonstrated no statistically significant differences in blood loss between the endophytic renal tumors and the cystic renal tumors compared to the non-complex group (p = 0.67; p = 0.54) (Figure 4). Transfusion rates were reported in 12 studies (17–26, 28, 30). No statistically significant difference in transfusion rates was observed between the two groups (p = 0.05) (Figure S2). Similarly, the cumulative analysis revealed no significant difference in the prevalence of conversion to open nephrectomy and radical nephrectomy rates between the two groups (five studies; p = 0.23 and six studies; p = 0.23) (Figure S3) (19–22, 30) (19–22, 27, 28);.
Figure 4
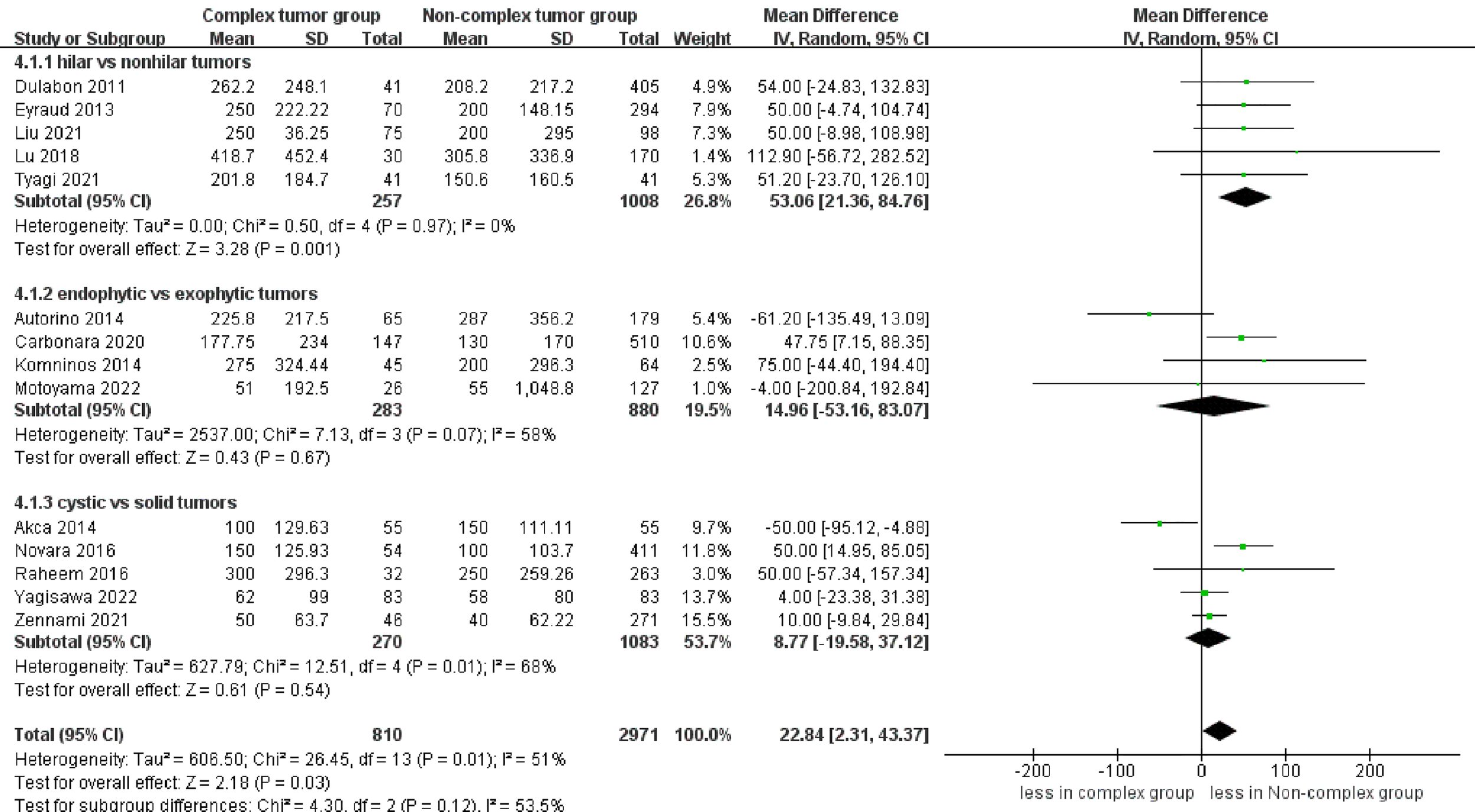
Forest plots of perioperative outcome-blood loss.
3.3.2 Complications
No statistically significant difference in intraoperative complications was observed between the two groups (seven studies; p = 0.49) (Figure S4) (23–25, 27–30). However, the complex group had more major complications compared to the non-complex group (12 studies; OR 2.35, 95% CI 1.50 to 3.67, p = 0.0002) (18–20, 22–30), while the subgroup analysis revealed that no significant difference in major complications between renal hilar and nonhilar tumors (p = 0.18) (Figure 5). Overall complications occurred in 27.9% (207 of 743 cases) of patients in the complex group and 20.0% (480 of 2439 cases) of patients in the non-complex group. The cumulative analysis revealed no significant difference in overall complications between the two groups (13 studies; p = 0.08) (Figure 6) (17–20, 22–30).
Figure 5
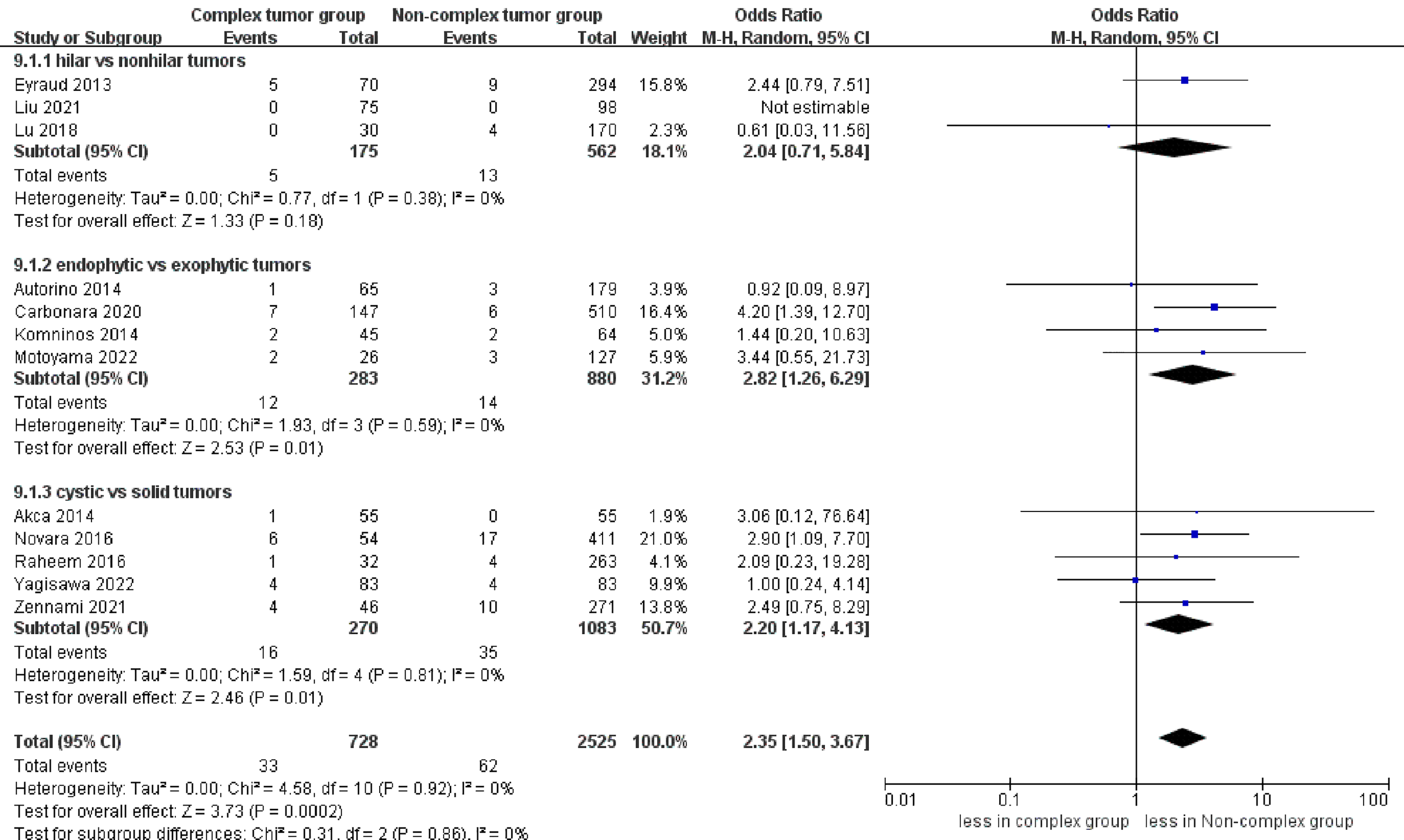
Forest plots of complication- major complications.
Figure 6
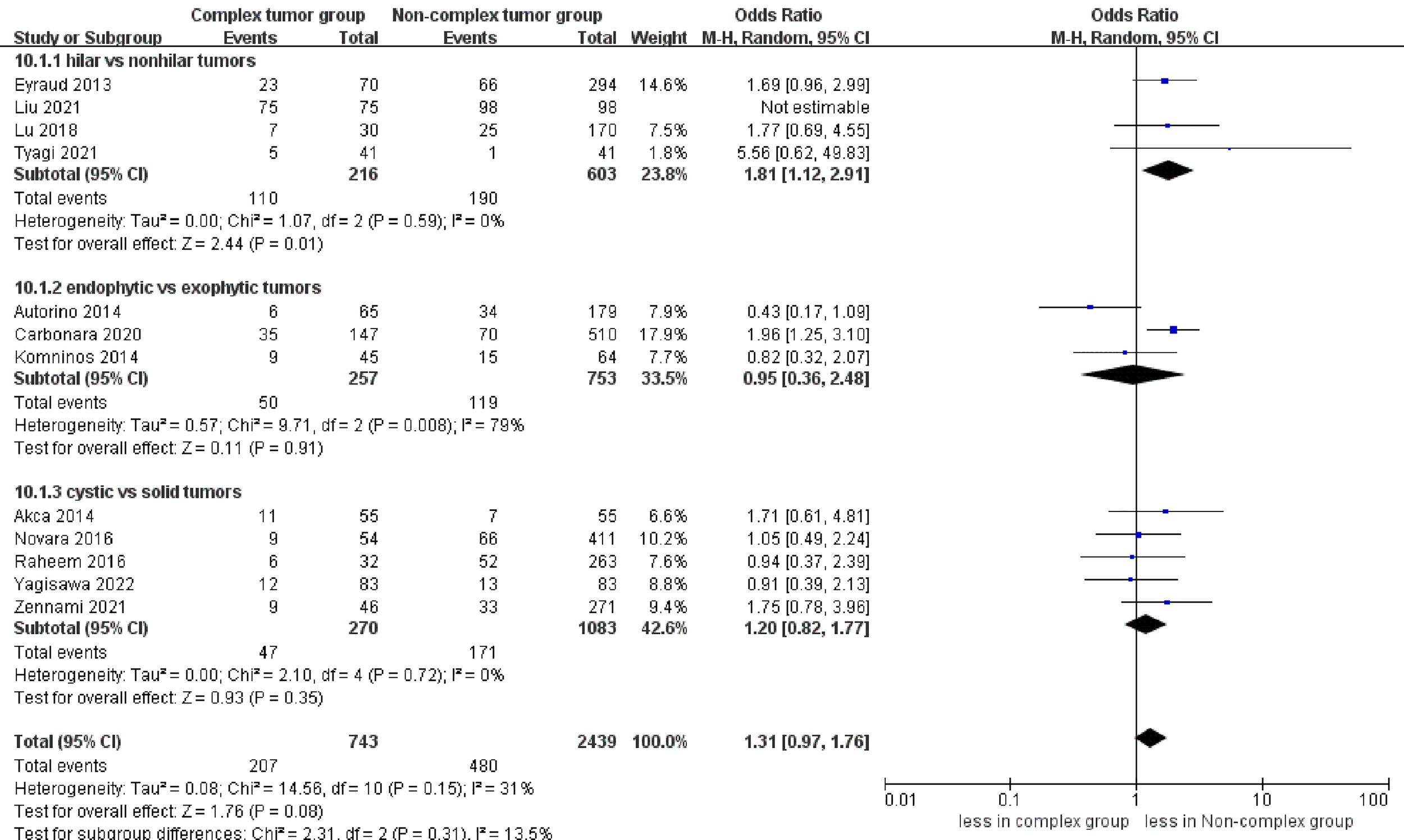
Forest plots of complication- overall complications.
3.3.3 Renal functional and oncologic outcomes
eGFR decline was reported in 12 studies (17–20, 23–30), demonstrating no significant differences between the two groups (p = 0.72). However, the subgroup analysis revealed that endophytic renal tumors were associated with a larger eGFR decline compared to exophytic renal tumors (WMD 3.71 mL/min/1.73 m, 95% CI 1.08, 6.34; p = 0.006) (Figure 7).
Figure 7
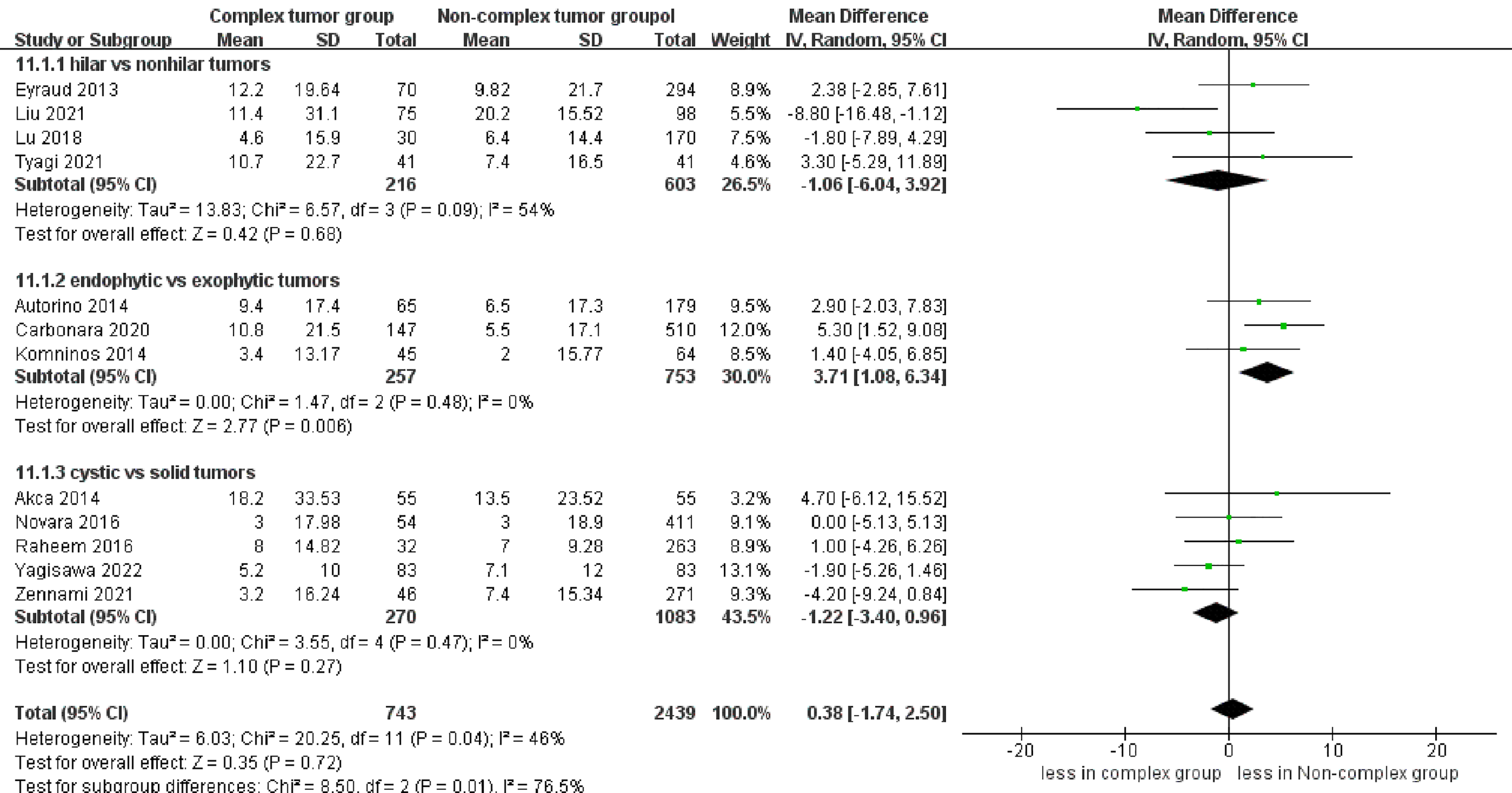
Forest plots of renal functional outcome- eGFR decline.
No significant differences were found regarding PSM between the complex tumor and non-complex tumor groups (14 studies; p = 0.19) (17–30). Furthermore, our subgroup analysis also demonstrated that the three subgroups had no statistically significant differences in PSM compared to the non-complex tumor group (p = 0.35; p = 0.18; p = 0.76) (Figure 8). Similarly, no statistically significant difference in recurrence was found between the two groups (11 studies; p = 0.43) (Figure 9) (17, 18, 20, 21, 23–30), and the subgroup analysis also illustrated no significant difference between the three subgroups and the non-complex group (p = 0.28; p = 0.16; p = 0.23). In terms of trifecta achievement, the pooled results revealed no difference between the two groups (seven studies; p = 0.05) (Figure 9) (17, 22–25, 27, 28).
Figure 8
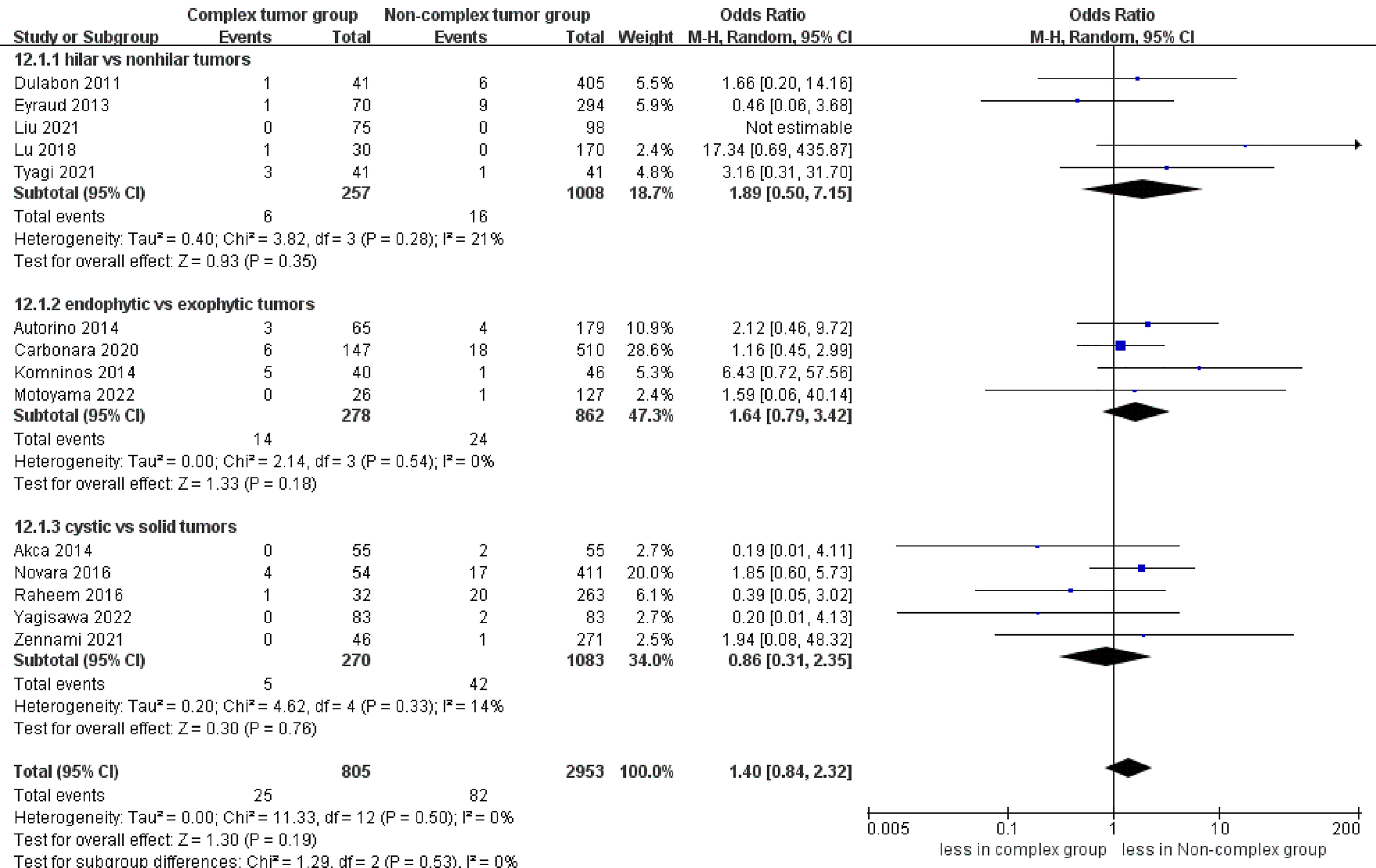
Forest plots of oncologic outcome-PSM.
Figure 9
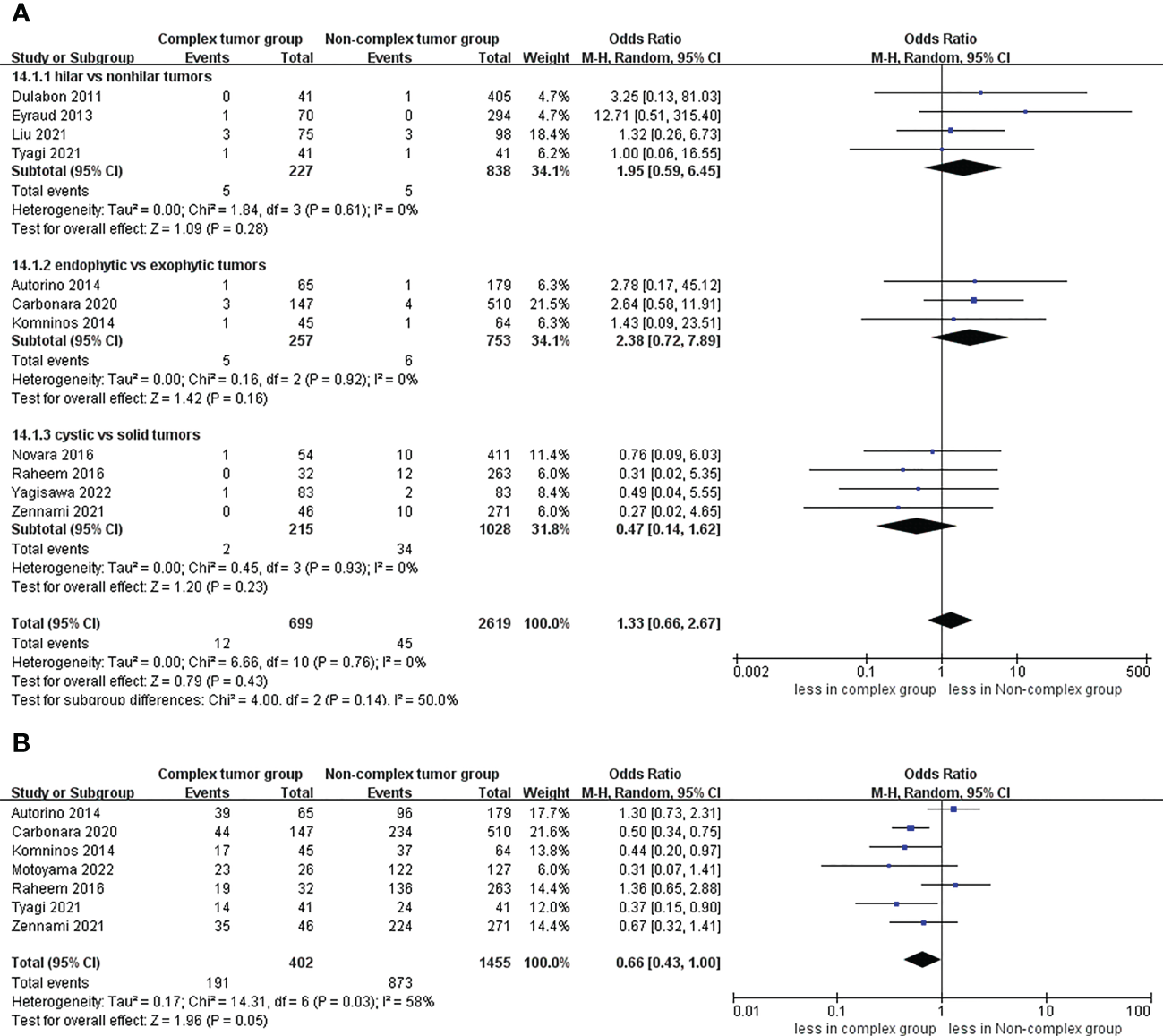
Funnel plot of oncologic outcomes (A) recurrence, (B) trifecta achievement.
3.4 Heterogeneity
Most outcomes showed low to moderate heterogeneity between the included studies. Nevertheless, high heterogeneity was found in warm ischemia time and operative time (I2 = 92%; I2 = 88%).
3.5 Sensitivity analysis
Leave-one-out tests were performed to identify changes in heterogeneity in outcomes with high heterogeneity (operative time and warm ischemia time). Finally, no substantial change in heterogeneity was observed among the two perioperative outcomes, indicating that the source of heterogeneity in operative time and warm ischemia time was stable.
3.6 Publication bias
A funnel plot was used to assess publication bias, including operative time, warm ischemia time, blood loss, and major complications. The findings showed a roughly tapered distribution of included studies, while there is still some publication bias (Figure S5).
4 Discussion
This is the first study to assess the perioperative, functional, and oncologic outcomes of RAPN for renal hilar tumors, endophytic renal tumors, and cystic renal tumors. Moreover, some important findings from this analysis need further discussion.
No statistically significant difference in operative time was found between the two groups. Nevertheless, the subgroup analysis reported that renal hilar tumors had a longer operative time than renal nonhilar tumors. The renal hilar tumors had a larger tumor diameter than renal nonhilar tumors, increasing the difficulty of surgery, which might explain the increase in the operative time for renal hilar tumors. In addition to tumor characteristics, many factors influence the operative time, such as the experience of the surgeon and the assistant, BMI, and intraoperative complications (28). Therefore, further research is required to investigate this aspect. In terms of length of hospital stay, no statistical significance was found between the two groups. However, the length of stay for robotic surgery is mostly affected by surgeon expertise and institutional volume rather than surgical methods (31). The combined results demonstrated that the complex tumor group was associated with a longer warm ischemia time than the non-complex tumor group. This finding may be attributed to multiple reasons. First, the increase in warm ischemia time is related to the more challenging dissection, resection and anastomosis of renal hilar tumors and endophytic renal tumors. Second, careful dissection is required for cystic renal tumors to avoid tumor cell spillage, which would increase the warm ischemia time. However, there are certain aspects that warrant our attention, particularly with regard to the optimal duration of warm ischemia during PN, which continues to be a topic of debate within the urological community. Several studies have suggested that warm ischemia time should be limited to 25 or 30 minutes to minimize the risk of renal function impairment (32–34). It is noteworthy that all studies reported an ischemia time of fewer than 30 minutes in the complex tumor group. Considering the above, the ischemia time of the complex tumors is acceptable.
The combined results also revealed that the complex tumor group was associated with more blood loss than the non-complex tumor group, with no statistically significant difference in blood loss among endophytic and cystic renal tumors compared to the non-complex tumor group. In renal hilar tumors, the hilar vessels are responsible for the blood supply to the tumors, supporting their growth (19). Expectedly, these hilar tumors exhibited a larger diameter compared to nonhilar tumors across all the included studies. Furthermore, renal hilar tumors are located close to hilar vessels, which increases the risk of bleeding during the operation. The above reasons might explain the higher blood loss in the hilar tumor group compared to the non-hilar tumor group (19). Although no significant difference was found, larger blood loss was found in endophytic and cystic renal tumors than in the non-complex group in most included studies. The difference might not have been statistically significant due to the small number of included studies in the subgroup analysis. However, the increased blood loss was not likely clinically significant because no significant difference was found in transfusion rates between the two groups. The cumulative analysis revealed no significant difference in the prevalence of conversion to open nephrectomy and radical nephrectomy rates between the two groups. Nevertheless, all the operations in the included studies were performed by experienced surgeons; thus, this outcome should be interpreted with caution.
The meta-analysis revealed that the complex tumor group was associated with more major complications than the non-complex tumor group. The results may be attributed to the complexity of RAPN in tumor resection and reconstruction. However, no patients died due to major complications, suggesting no statistically significant difference in trifecta achievement between the two groups. Moreover, the cumulative analysis revealed no significant difference in intraoperative and overall complications between the two groups. Therefore, higher-level evidence is required to verify our outcomes.
Although the results demonstrated that the complex tumor group was associated with longer warm ischemia time than the non-complex tumor group, no statistically significant difference in eGFR decline was observed between the two groups. It may be due to the following reasons. Recent studies have demonstrated that preoperative renal function and the number of preserved kidneys are the primary factors that are significantly associated with long-term renal function outcomes. In contrast, warm ischemia time has been found to play a relatively minor role in influencing long-term renal function outcomes (35, 36). Furthermore, Fergany et al. (37) showed that age played an important role in the recovery of postoperative long-term renal function. For cystic renal tumors, the extent of renal parenchymal resection may be larger than expected due to the risk of cyst wall damage, which might lead to kidney function loss. Interestingly, the subgroup analysis revealed no significant difference in eGFR decline between cystic renal tumors and solid renal tumors.
The oncologic outcomes are important indicators of surgical quality. Our analysis demonstrated that the complex tumor group had no statistically significant differences in PSM compared to the non-complex tumor group. The PSM rate was 3.10% in the complex tumor group, which is consistent with a high-volume institution that reported PSM rates varying from 0 to 3.7% for RAPN (38). Furthermore, some important aspects of this result need further discussion. First, Marszalek et al. (39) showed that PSM might not be a deciding factor of recurrence. Second, many factors could affect PSM, such as tumor diameter, surgical approach, and tumor stage (40). Therefore, additional studies are required to validate our outcomes. In the included studies, no significant difference in PSM was found between the endophytic tumors and exophytic tumors. In contrast, the endophytic tumors demonstrated slightly higher PSM rates, which might be caused by the higher surgical complexity of endophytic tumors. No statistically significant difference in recurrence was found between the two groups. In cystic renal tumors, cyst rupture might increase the risk of recurrence. Pradere et al. (41) performed a retrospective study evaluating the occurrence of cyst rupture and its effects on recurrence and PSM (50 cyst ruptures out of 268).
Interestingly, there were no recurrence and metastasis in the 50 patients. However, further studies are required to verify this outcome, and particular caution should still be exercised in the manipulation of cystic tumors during the operation. On the other hand, due to insufficient literature, the metastatic recurrence, overall survival, and recurrence-free survival between the two groups cannot be confirmed. Therefore, more studies with a larger sample are required to verify the oncologic results. The difference in trifecta achievement rates between the two groups showed marginal significance (p = 0.05). The trifecta rates were 47.5% for the complex tumor group, which were lower than in cases from the RAPN series for small renal tumors (42). However, many factors could affect the trifecta rates, including tumor diameter and tumor complexity. Our result is consistent with a previous study which reported that trifecta rates of RAPN for highly complex renal tumors (43). On the other hand, the longer warm ischemia time in the complex tumor group seems to be a primary cause affecting trifecta achievement. Nevertheless, trifecta achievement cannot evaluate long-term renal functional and oncologic outcomes, and additional long-term follow-up studies are required to assess the outcomes.
Sagalovich et al. (44) reported the effectiveness and safety between RAPN and open PN for renal hilar tumors. The results of this study demonstrated that RAPN provided similar effectiveness and safety to open PN while it was less invasive. Kara et al. (45) conducted a study to compare the outcomes between RAPN and open PN for completely endophytic renal tumors. The results indicated similar outcomes when performed by experienced surgeons, whereas RAPN exhibited less blood loss and shorter length of stay compared to open PN. Moreover, Pinheiro et al. (46) demonstrated that laparoscopic PN was safe and effective for cystic renal tumors. However, these results remain controversial. Owing to the insufficient literature included, RAPN cannot be compared to other surgical methods. In the included studies, all procedures were performed in large high-volume institutions by experienced laparoscopic and robotic surgeons. Therefore, the outcomes might not be generalized to other institutions, and the results should be interpreted with caution.
However, the limitations of this study should be acknowledged. First, all the studies included in the analysis were non-RCTs, which undoubtedly had potential misclassification bias. Second, no subgroup analysis was performed based on the surgical method (transperitoneal and retroperitoneal), which may lead to subtle differences in outcomes. Third, the tumor complexity (hilar, endophytic, or cystic) might occasionally overlap. Due to insufficient literature, this aspect could not be further analyzed. Last, the follow-up period of some studies is relatively short (3.3-7 months), limiting the comparison between the two groups in terms of renal functional and oncological outcomes.
5 Conclusions
The outcomes of the present study demonstrated that RAPN could be a safe and effective procedure for complex tumors (hilar, endophytic, or cystic) with similar perioperative, functional and oncologic outcomes compared to non-complex tumors (nonhilar, exophytic, or solid). Nevertheless, a larger sample size, more long-term follow-up, and data from multicenter studies are required to verify the conclusions.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Protocol development: X-BC, Y-GL and TW; literature search and database creation: X-BC, Y-GL, TW, Z-BD, C-LT and X-DY; formal analysis: X-BC, Y-GL, TW, Z-BD, and X-DY; methodology: X-BC, QZ and X-DY; supervision: X-BC, Y-GL, and X-DY; writing manuscript: X-BC, Y-GL, TW, Z-BD, QZ, C-LT and X-DY. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1178592/full#supplementary-material
Supplementary Figure 1Forest plots of perioperative outcome- length of hospital stay.
Supplementary Figure 2Forest plots of perioperative outcome- transfusion rates.
Supplementary Figure 3Forest plots of perioperative outcome (A) conversion to open nephrectomy rates, (B) conversion to radical nephrectomy rates.
Supplementary Figure 4Forest plots of complication- intraoperative complications.
Supplementary Figure 5Funnel plot of the studies represented in the meta-analysis (A) operative time, (B) warm ischemia time, (C) blood loss, (D) major complications.
References
1
Campbell S Uzzo RG Allaf ME Bass EB Cadeddu JA Chang A et al . Renal mass and localized renal cancer: AUA guideline. J Urol (2017) 198(3):520–9. doi: 10.1016/j.juro.2017.04.100
2
Ljungberg B Cowan NC Hanbury DC Hora M Kuczyk MA Merseburger AS et al . EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol (2010) 58(3):398–406. doi: 10.1016/j.eururo.2010.06.032
3
Wang J Lu Y Wu G Wang T Wang Y Zhao H et al . The role of three-dimensional reconstruction in laparoscopic partial nephrectomy for complex renal tumors. World J Surg Oncol (2019) 17(1):159. doi: 10.1186/s12957-019-1701-x
4
Chang KD Abdel Raheem A Kim KH Oh CK Park SY Kim YS et al . Functional and oncological outcomes of open, laparoscopic and robot-assisted partial nephrectomy: a multicentre comparative matched-pair analyses with a median of 5 years' follow-up. BJU Int (2018) 122(4):618–26. doi: 10.1111/bju.14250
5
Choi JE You JH Kim DK Rha KH Lee SH . Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol (2015) 67(5):891–901. doi: 10.1016/j.eururo.2014.12.028
6
Motoyama D Sato R Watanabe K Matsushita Y Watanabe H Matsumoto R et al . Perioperative outcomes in patients undergoing robot-assisted partial nephrectomy: comparative assessments between complex and non-complex renal tumors. Asian J Endosc Surg (2021) 14(3):379–85. doi: 10.1111/ases.12872
7
George AK Herati AS Rais-Bahrami S Waingankar N Kavoussi LR . Laparoscopic partial nephrectomy for hilar tumors: oncologic and renal functional outcomes. Urology (2014) 83(1):111–5. doi: 10.1016/j.urology.2013.08.059
8
Larcher A Capitanio U De Naeyer G Fossati N D'Hondt F Muttin F et al . Is robot-assisted surgery contraindicated in the case of partial nephrectomy for complex tumours or relevant comorbidities? a comparative analysis of morbidity, renal function, and oncologic outcomes. Eur Urol Oncol (2018) 1(1):61–8. doi: 10.1016/j.euo.2018.01.001
9
Papadimitriou VG Takos D Adamopoulos V Vegertaki U Heretis JM Stamatiou KN et al . Unusual case of multilocular cystic renal cell carcinoma treated with nephron-sparing technique. G Chir (2009) 30(8-9):345–8.
10
Garisto J Bertolo R Dagenais J Sagalovich D Fareed K Fergany A et al . Robotic versus open partial nephrectomy for highly complex renal masses: comparison of perioperative, functional, and oncological outcomes. Urol Oncol (2018) 36(10):471.e1–9. doi: 10.1016/j.urolonc.2018.06.012
11
Kutikov A Uzzo RG The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol (2009) 182(3):844–53. doi: 10.1016/j.juro.2009.05.035
12
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj (2021) 372:n71. doi: 10.1136/bmj.n71
13
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
14
Dindo D Demartines N Clavien PA . Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
15
Sterne JA Hernán MA Reeves BC Savović J Berkman ND Viswanathan M et al . ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj (2016) 355:i4919. doi: 10.1136/bmj.i4919
16
Higgins JP Thompson SG Deeks JJ Altman DG . Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
17
Tyagi S Sharma G Bora GS Mavuduru RS Sharma AP Devana SK et al . Trifecta and pentafecta outcomes following robot-assisted partial nephrectomy for hilar versus nonhilar tumors: a propensity-matched analysis. Indian J Urol (2021) 37(4):318–24. doi: 10.4103/iju.iju_136_21
18
Liu T Zhao Z Feng B Wang X Li T Xie S et al . Robotic-assisted laparoscopic tumor enucleation is a feasible technique for renal hilar tumors: a retrospective study. J Surg Oncol (2021) 124(1):135–42. doi: 10.1002/jso.26479
19
Lu SY Chung HJ Huang EY Lin TP Lin ATL . The perioperative outcomes between renal hilar and non-hilar tumors following robotic-assisted partial nephrectomy (RAPN). J Chin Med Assoc (2018) 81(8):676–81. doi: 10.1016/j.jcma.2017.11.014
20
Eyraud R Long JA Snow-Lisy D Autorino R Hillyer S Klink J et al . Robot-assisted partial nephrectomy for hilar tumors: perioperative outcomes. Urology (2013) 81(6):1246–51. doi: 10.1016/j.urology.2012.10.072
21
Dulabon LM Kaouk JH Haber GP Berkman DS Rogers CG Petros F et al . Multi-institutional analysis of robotic partial nephrectomy for hilar versus nonhilar lesions in 446 consecutive cases. Eur Urol (2011) 59(3):325–30. doi: 10.1016/j.eururo.2010.11.017
22
Motoyama D Ito T Sugiyama T Otsuka A Miyake H . Comparison of perioperative outcomes among patients with exophytic, mesophytic, and endophytic renal tumors undergoing robot-assisted partial nephrectomy. Int J Urol (2022) 29(9):1026–30. doi: 10.1111/iju.14946
23
Carbonara U Simone G Minervini A Sundaram CP Larcher A Lee J et al . Outcomes of robot-assisted partial nephrectomy for completely endophytic renal tumors: a multicenter analysis. Eur J Surg Oncol (2021) 47(5):1179–86. doi: 10.1016/j.ejso.2020.08.012
24
Komninos C Shin TY Tuliao P Kim DK Han WK Chung BH et al . Robotic partial nephrectomy for completely endophytic renal tumors: complications and functional and oncologic outcomes during a 4-year median period of follow-up. Urology (2014) 84(6):1367–73. doi: 10.1016/j.urology.2014.08.012
25
Autorino R Khalifeh A Laydner H Samarasekera D Rizkala E Eyraud R et al . Robot-assisted partial nephrectomy (RAPN) for completely endophytic renal masses: a single institution experience. BJU Int (2014) 113(5):762–8. doi: 10.1111/bju.12455
26
Yagisawa T Takagi T Yoshida K Hata K Iizuka J Muromiya Y et al . Surgical outcomes of robot-assisted laparoscopic partial nephrectomy for cystic renal cell carcinoma. J Robot Surg (2022) 16(3):649–54. doi: 10.1007/s11701-021-01292-7
27
Zennami K Takahara K Matsukiyo R Nukaya T Takenaka M Fukaya K et al . Long-term functional and oncologic outcomes of robot-assisted partial nephrectomy for cystic renal tumors: a single-center retrospective study. J Endourol (2021) 35(7):1006–12. doi: 10.1089/end.2020.0994
28
Schwen ZR Pierorazio PM . Editorial comment from Dr schwen and Dr pierorazio to robot-assisted partial nephrectomy confers excellent long-term outcomes for the treatment of complex cystic renal tumors: median follow up of 58 months. Int J Urol (2016) 23(12):983. doi: 10.1111/iju.13244
29
Novara G La Falce S Abaza R Adshead J Ahlawat R Buffi NM et al . Robot-assisted partial nephrectomy in cystic tumours: analysis of the vattikuti global quality initiative in robotic urologic surgery (GQI-RUS) database. BJU Int (2016) 117(4):642–7. doi: 10.1111/bju.13256
30
Akca O Zargar H Autorino R Brandao LF Laydner H Krishnan J et al . Robotic partial nephrectomy for cystic renal masses: a comparative analysis of a matched-paired cohort. Urology (2014) 84(1):93–8. doi: 10.1016/j.urology.2014.03.017
31
Leow JJ Heah NH Chang SL Chong YL Png KS . Outcomes of robotic versus laparoscopic partial nephrectomy: an updated meta-analysis of 4,919 patients. J Urol (2016) 196(5):1371–7. doi: 10.1016/j.juro.2016.06.011
32
Becker F Van Poppel H Hakenberg OW Stief C Gill I Guazzoni G et al . Assessing the impact of ischaemia time during partial nephrectomy. Eur Urol (2009) 56(4):625–34. doi: 10.1016/j.eururo.2009.07.016
33
Thompson RH Lane BR Lohse CM Leibovich BC Fergany A Frank I et al . Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol (2010) 58(3):340–5. doi: 10.1016/j.eururo.2010.05.047
34
Zargar H Akca O Autorino R Brandao LF Laydner H Krishnan J et al . Ipsilateral renal function preservation after robot-assisted partial nephrectomy (RAPN): an objective analysis using mercapto-acetyltriglycine (MAG3) renal scan data and volumetric assessment. BJU Int (2015) 115(5):787–95. doi: 10.1111/bju.12825
35
Lane BR Russo P Uzzo RG Hernandez AV Boorjian SA Thompson RH et al . Comparison of cold and warm ischemia during partial nephrectomy in 660 solitary kidneys reveals predominant role of nonmodifiable factors in determining ultimate renal function. J Urol (2011) 185(2):421–7. doi: 10.1016/j.juro.2010.09.131
36
Mir MC Campbell RA Sharma N Remer EM Simmons MN Li J et al . Parenchymal volume preservation and ischemia during partial nephrectomy: functional and volumetric analysis. Urology (2013) 82(2):263–8. doi: 10.1016/j.urology.2013.03.068
37
Fergany AF Saad IR Woo L Novick AC . Open partial nephrectomy for tumor in a solitary kidney: experience with 400 cases. J Urol (2006) 175(5):1630–3. doi: 10.1016/s0022-5347(05)00991-2
38
Patton MW Salevitz DA Tyson MD 2nd Andrews PE Ferrigni EN et al . Robot-assisted partial nephrectomy for complex renal masses. J Robot Surg (2016) 10(1):27–31. doi: 10.1007/s11701-015-0554-8
39
Marszalek M Carini M Chlosta P Jeschke K Kirkali Z Knüchel R et al . Positive surgical margins after nephron-sparing surgery. Eur Urol (2012) 61(4):757–63. doi: 10.1016/j.eururo.2011.11.028
40
Malkoç E Maurice MJ Kara Ö. Ramirez D Nelson RJ Dagenais J et al . Predictors of positive surgical margins in patients undergoing partial nephrectomy: a large single-center experience. Turk J Urol (2019) 45(1):17–21. doi: 10.5152/tud.2018.57767
41
Pradere B Peyronnet B Delporte G Manach Q Khene ZE Moulin M et al . Intraoperative cyst rupture during partial nephrectomy for cystic renal masses-does it increase the risk of recurrence? J Urol (2018) 200(6):1200–6. doi: 10.1016/j.juro.2018.06.025
42
Bianchi L Schiavina R Borghesi M Chessa F Casablanca C Angiolini A et al . Which patients with clinical localized renal mass would achieve the trifecta after partial nephrectomy? the impact of surgical technique. Minerva Urol Nefrol (2020) 72(3):339–49. doi: 10.23736/s0393-2249.19.03485-4
43
Bertolo R Autorino R Fiori C Amparore D Checcucci E Mottrie A et al . Expanding the indications of robotic partial nephrectomy for highly complex renal tumors: urologists' perception of the impact of hyperaccuracy three-dimensional reconstruction. J Laparoendosc Adv Surg Tech A (2019) 29(2):233–9. doi: 10.1089/lap.2018.0486
44
Sagalovich D Dagenais J Bertolo R Garisto JD Kaouk JH . Trifecta outcomes in renal hilar tumors: a comparison between robotic and open partial nephrectomy. J Endourol (2018) 32(9):831–6. doi: 10.1089/end.2018.0445
45
Kara O Maurice MJ Malkoc E Ramirez D Nelson RJ Caputo PA et al . Comparison of robot-assisted and open partial nephrectomy for completely endophytic renal tumours: a single centre experience. BJU Int (2016) 118(6):946–51. doi: 10.1111/bju.13572
46
Pinheiro T Sepulveda F Natalin RH Metrebian E Medina R Goldman SM et al . Is it safe and effective to treat complex renal cysts by the laparoscopic approach? J Endourol (2011) 25(3):471–6. doi: 10.1089/end.2010.0254
Summary
Keywords
renal hilar tumors, endophytic renal tumors, cystic renal tumors, robot-assisted partial nephrectomy, meta-analysis
Citation
Chen X-b, Li Y-g, Wu T, Du Z-b, Tan C-l, Zhang Q and Yu X-d (2023) Perioperative, oncologic, and functional outcomes of robot-assisted partial nephrectomy for special types of renal tumors (hilar, endophytic, or cystic): an evidence-based analysis of comparative outcomes. Front. Oncol. 13:1178592. doi: 10.3389/fonc.2023.1178592
Received
03 March 2023
Accepted
05 April 2023
Published
20 April 2023
Volume
13 - 2023
Edited by
Francesco Chierigo, San Martino Hospital, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), Italy
Reviewed by
Matteo Droghetti, University of Bologna, Italy; Simone Sforza, University of Florence, Italy
Updates
Copyright
© 2023 Chen, Li, Wu, Du, Tan, Zhang and Yu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-dong Yu, 21434379@qq.com
†These authors have contributed equally to this work
This article was submitted to Genitourinary Oncology, a section of the journal Frontiers in Oncology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.