- 1Markey Cancer Center, University of Kentucky, Lexington, KY, United States
- 2Division of Cancer Biostatistics, University of Kentucky, Lexington, KY, United States
- 3Department of Radiology, University of Kentucky, Lexington, KY, United States
- 4Department of Internal Medicine, University of Kentucky, Lexington, KY, United States
- 5Department of Obstetrics and Gynecology, University of Kentucky, Lexington, KY, United States
- 6Department of Pharmacy Practice and Science, University of Kentucky, Lexington, KY, United States
Background: Appalachia is a region with significant cancer disparities in incidence and mortality compared to Kentucky and the United States. However, the contribution of these cancer health disparities to subsequent primary cancers (SPCs) among survivors of adult-onset cancers is limited. This study aimed to quantify the overall and cancer type-specific risks of SPCs among adult-onset cancer survivors by first primary cancer (FPC) types, residence and sex.
Methods: This retrospective cohort study from the Kentucky Cancer Registry included 148,509 individuals aged 20-84 years diagnosed with FPCs from 2000-2014 (followed until December 31, 2019) and survived at least 5 years. Expected numbers of SPC were derived from incidence rates in the Kentucky population; standardized incidence ratio (SIR) compared with those expected in the general Kentucky population.
Results: Among 148,509 survivors (50.2% women, 27.9% Appalachian), 17,970 SPC cases occurred during 829,530 person-years of follow-up (mean, 5.6 years). Among men, the overall risk of developing any SPCs was statistically significantly higher for 20 of the 30 FPC types, as compared with risks in the general population. Among women, the overall risk of developing any SPCs was statistically significantly higher for 20 of the 31 FPC types, as compared to the general population. The highest overall SIR were estimated among oral cancer survivors (SIR, 2.14 [95% CI, 1.97-2.33] among men, and among laryngeal cancer survivors (SIR, 3.62 [95% CI, 2.93-4.42], among women. Appalachian survivors had significantly increased risk of overall SPC and different site specific SPC when compared to non-Appalachian survivors. The highest overall SIR were estimated among laryngeal cancer survivors for both Appalachian and non-Appalachian residents (SIR, 2.50: 95%CI, 2.10-2.95; SIR, 2.02: 95% CI, 1.77-2.03, respectively).
Conclusion: Among adult-onset cancer survivors in Kentucky, several FPC types were significantly associated with greater risk of developing an SPC, compared with the general population. Risk for Appalachian survivors was even higher when compared to non-Appalachian residents, but was not explained by higher risk of smoking related cancers. Cancers associated with smoking comprised substantial proportions of overall SPC incidence among all survivors and highlight the importance of ongoing surveillance and efforts to prevent new cancers among survivors.
1 Introduction
The United States (US) 5-year survival rate for all cancers increased from 49% to 68% between the mid-1970s to 2011 and 2017 (1). In 2022, there were an estimated 18 million cancer survivors in the US (2) and the number is projected to continue to increase to 22.1 million by the year 2030 (3). Cancer survivors are at risk for the development of new malignancies, or subsequent primary cancers (SPCs) diagnosed after their first primary cancer (FPC) (4). Nationally, SPCs account for approximately 25% of newly diagnosed cancers in patients 65 years or older and 11% for adults younger than 65 (5). Older age at diagnosis and a modifiable risk factor [e.g.; smoking, obesity, alcohol or infection related (6–9)] associated FPC both increase the risk of developing SPCs (10–12). Sung and colleagues recently reported an analysis of twelve Surveillance, Epidemiology, and End Results (SEER) registries in the United States assessing the risk of SPCs in individuals diagnosed with a FPC and surviving 5 years, demonstrating that risk of death due to SPCs was higher among cancer survivors than the general population and that cancers associated with modifiable risk factors, like smoking and obesity, comprise a significant proportion of SPCs (11).
While cancer incidence and mortality rates have steadily declined in the U.S., rural Appalachia Kentucky is an exception, where incidence continues to increase, mortality rates are 36% higher than urban non-Appalachians and 5 year survival is only 50% (13). In addition, Kentucky leads the nation in both lung cancer incidence and mortality. Excess cancer incidence and mortality is typically attributed to high rates of cancer associated risk factors, however a recent cross-sectional study demonstrated that the impact of risk factors on cancer mortality varies by region. In the South, smoking, receipt of Supplemental Nutrition Assistance Program (SNAP) (a surrogate indicator of low income) benefits and physical inactivity were over 60% relative importance. In Appalachia receiving SNAP benefits was of high importance and in Kentucky, smoking was of high importance (14). Interestingly, risk factor importance did not correspond completely with risk factor prevalence, which suggests the potential of unobserved and location specific risk factors for further exploration.
Given Kentucky’s high incidence and poor survival related to cancers, significant disparities and the presence of region-specific cancer risk factors, the objective of this study was to determine the frequency of SPCs in patients diagnosed with cancer in Kentucky and Appalachian Kentucky.
2 Materials and methods
2.1 Study population and first primary cancer
Cancer cases diagnosed at ages 20 to 84 during 2000-2014 in the Kentucky cancer registry were selected from SEER 17 research plus 2021 November submission data (15). FPC sites were categorized based on the International Classification of Disease for Oncology Third Edition (ICD-O-3)-World Health Organization 2008 definition (16). Patients with at least five years of survival were included to estimate the long-term survivorships and permit comparison of findings with published results (11).
2.2 Subsequent primary cancer cases ascertainment
All new SPCs were included based on the SEER’s rule for multiple primaries, except non-melanoma skin cancer (17). The follow-up of SPC began after five years of their FPC diagnosis, a 60-months latency exclusion, and continued until death or loss to follow-up, attained age 90 or older, or at the study cut-off time (December 31, 2019), whichever came first. Multiple events of SPC were allowed so the survivor could continue to contribute person-time at risk for their entire time in the study (18).
2.3 Analytical variables
Variables included in this study include cancer survivors’ age categorized into four groups: 20-49, 50-64, 65-74, and 75 years older. The race was coded as white, black, other, and unknown based on the SEER race recode group (19). Only patients with male or female sex were included. The years of FPC diagnosis were grouped in five year increments. SEER historic stages were selected for consistency of stage comparison due to the change of stage definitions over the years (20). According to the Appalachia Regional Commission, Appalachian regions, especially Kentucky Appalachia, suffer more significant economic and cancer burdens than non-Appalachia regions. The geographical variable indicated whether patients’ residences were in Appalachia or not at the time the cancer diagnosis were created (21).
2.4 Statistical analysis
The estimates of SPC were compared with the expected incidence in the general Kentucky population by calculating standardized incidence ratios (SIRs) and 95% confidence intervals (CI). SIR was calculated as the ratio of the observed to the expected number of SPCs (22). The expected numbers of SPC were derived from Kentucky incidence rates in the SEER incidence file 2021 submission, stratified by age (5-year groups), race (white/unknown, black, other), sex, and calendar year (5-year groups), multiplied by the appropriate person-years at risk. Statistics of patients’ SPC on any site, same site, and sites other than the FPC were estimated using the SIRs and 95% CI. Results were further stratified by sex and Appalachian region to compare site-specific risks of developing SPC. Cancer sites with 200 or more survivors in the full cohort were presented for site-specific risk assessment in the Tables and Figures.
To further understand the impacts of risk factors on SPC, the standard definitions for risk factor-associated cancers were adopted from the Centers for Disease Control and Prevention (CDC) (23). The SIRs were estimated to assess the risk of developing any risk factor associated with SPC within the same category of FPC for 12 tobacco-associated cancers [oropharynx, esophagus, stomach, colorectal, liver, pancreas, larynx, lung, cervix, kidney, urinary bladder, acute myeloid leukemia], 13 obesity-associated cancers [esophageal adenocarcinoma, gastric cardia, colorectal, liver, gallbladder, pancreas, multiple myeloma, postmenopausal female breast, corpus and uterus, ovary, kidney, meningioma, thyroid], six alcohol-associated cancers [oropharynx, esophagus, colorectal, liver, larynx, female breast], three physical inactivity-associated cancers [colon, postmenopausal female breast, corpus and uterus, NOS], and six Human Papillomavirus (HPV)-associated cancers [oropharyngeal squamous cell carcinoma, anal and rectal squamous cell carcinoma, vulvar squamous cell carcinoma, vaginal squamous cell carcinoma, cervical carcinoma, penile squamous cell carcinoma]. All statistical tests were two-sided with a significance level of p < 0.05. Analysis was conducted utilizing SAS 9.4(Cary, NC) and SEER*Stat 8.4.0.
2.5 Ethical considerations
This study was approved by the University of Kentucky Institutional Review Board (IRB # 78447). All data were treated as confidential and only accessible in password-protected files for authorized study staff.
3 Results
Between 2000-2014, 148,509 patients in KY diagnosed with an invasive FPC who survived at least 5 years were included in the study (Table 1). Women (51%, 74,508) and men (49%, 74,001) were approximately equally represented. The majority of patients were white (93%, 138,178) and resided in non-Appalachian regions (72%, 107,121). Participants lived a mean of 5.59 years after SPC and longer than 10 years after FPC diagnosis, accruing 829,530 person-years of follow-up.
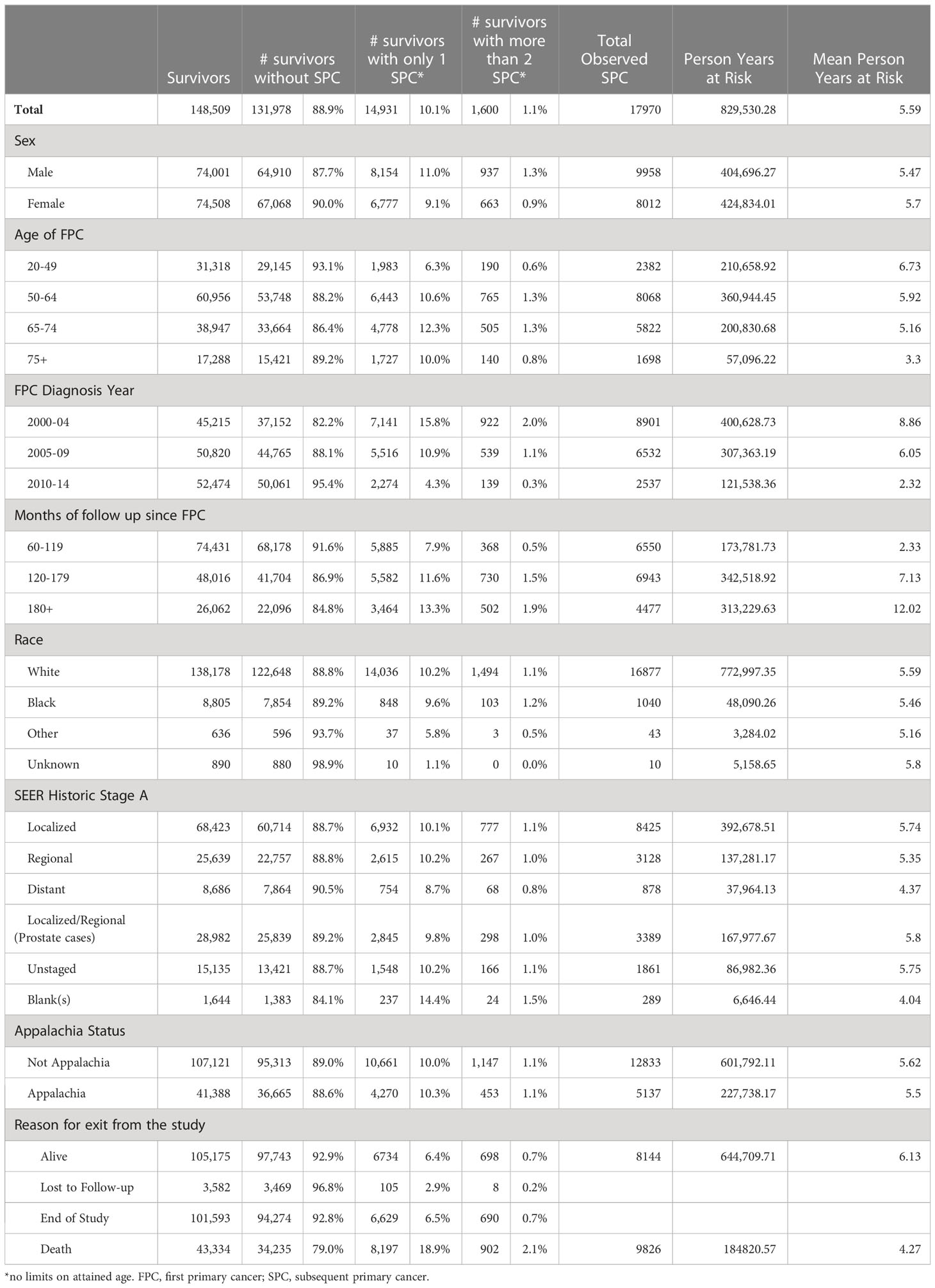
Table 1 Characteristics of patients diagnosed with Invasive FPC in 2000-2014, KY, 20-84, with >= 5 years of survival.
Overall, out of 16,531 FPC survivors with SPCs, 10.1% (14,931) of survivors developed one SPC and 1.1% (1,600) had two or more SPCs. Development of an SPC was more common among individuals initially diagnosed with an FPC between the ages of 50 and 64 (44%, 6443 + 765/16,531) and between 65 and 74 (32%, (4778 + 505)/16,531), and least common among those initially diagnosed between 20 and 40, (13%), despite having the longest average exposure of 6.73 years. Among all FPC survivors, 29% (43,334/148,509) were deceased, in those without a SPC, 26% were deceased (32,235/131,978), while the majority (55%, 8197 + 902/16,531) of survivors with a SPC were deceased.
3.1 Any site SPC
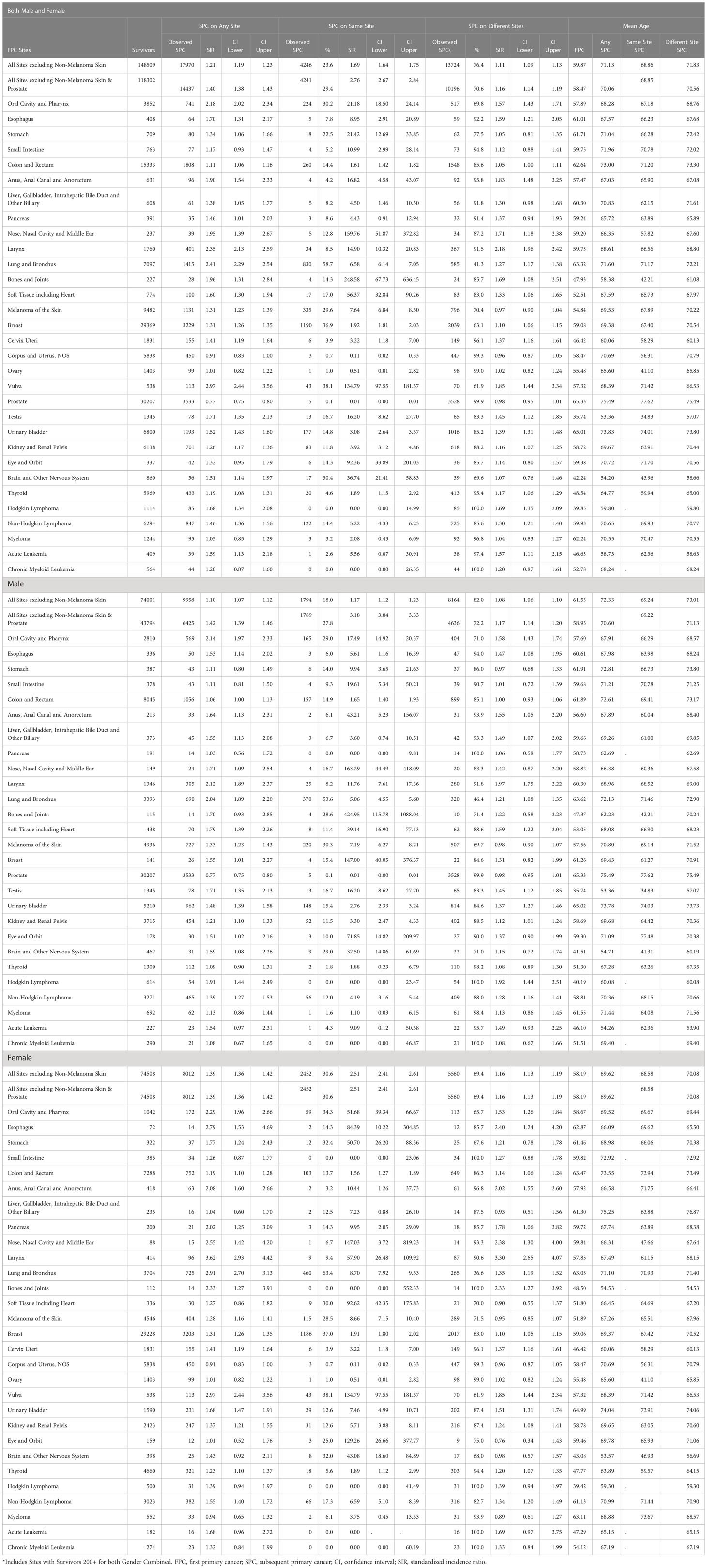
Among all survivors, 12.1% (17,970/148,509) were diagnosed with one or more SPC at any site (Table 2). In comparison to the general Kentucky population, Kentucky cancer survivors had a 21% higher risk of developing a new cancer with a standardized incidence ratio (SIR) of 1.21 (95%CI: 1.19-1.23), and a mean age of 60 at diagnosis of FPC and 71 at SPC. Of the 31 evaluated FPC cancer types, survivors were at increased risk of 26 of them. This effect was consistently observed for men and women with SIR 1.1 (95%CI: 1.04-1.12) and SIR 1.39 (95%CI: 1.36-1.42), respectively, although overall, women were more likely to develop a SPC than men, with an increased risk of almost 40%. Consistent with a prior report,11 Kentucky men with a FPC of prostate cancer were the only group less likely to develop a SPC, SIR 0.80 (95% CI: 0.77-0.75). However, when prostate cancer as a FPC was excluded the risk of a SPC in men was SIR 1.42 (95%CI 1.39-1.46), which was similar to risk in women. Of the 30 evaluated FPC cancer types in men, survivors with 20 different FPC were at a risk of SPC. Of the 31 evaluated FPC cancer types in women, survivors with 20 different FPC were at a risk of SPC. Men were diagnosed on average at age 62 for FPC and 72 for SPC, while women had a mean age of diagnosis of FPC at age 58 and 70 for SPC. Among men, the most common FPC and SPC sites numerically were prostate, colon and rectum, and urinary bladder, Supplementary Figure 1. In women, breast, colon and rectum, and corpus and uterus were the most frequent FPC sites, while breast, colon and rectum, and lung and bronchus had the highest number of SPCs.
Interestingly, while numerically the most SPCs (3533) occurred in men with an FPC of prostate cancer, their risk of a SPC was lower than the Kentucky general population with an SIR 0.77 (95% CI:0.75-0.80), Table 2. Men with a FPC of the oral cavity, SIR 2.14 (95% CI: 1.97-2.33), larynx, SIR 2.12 (95% CI:1.89-2.37), and lung, SIR 2.04 (95% CI:1.89-2.20) had the highest risk of a SPC, while women with a FPC of the larynx, SIR 3.62 (95% CI:2.93-4.42), vulva, SIR 2.97 (95% CI:2.44-3.56), and lung and bronchus cancers, SIR 2.91 (95% CI:2.70-3.13) were at the highest risk of SPC. Unlike men with an FPC of prostate cancer, women surviving an FPC were not at a decreased risk of any SPC.
3.2 Same site SPC
Among all survivors with an FPC, 2.9% (4246/148,509) were diagnosed with an SPC at the same site, representing 23.6% (4246/17970) of all SPCs, Table 2. FPC patients had an overall higher risk of cancer than the general population, SIR 1.69 (95% CI:1.64-1.75) with average diagnostic age at 69. For same site SPCs with more than 40 cases, the most common same site SPCs were breast (1190. 36.9% of total SPCs), lung (830, 58.7%), melanoma (335, 29.6%), and oral cavity (224, 30.2%), while individuals with a FPC of the vulva, SIR 135 (95% CI:97.55-181.57), oral cavity, SIR 21.2 (95% CI:18.50-24.14), and lung, SIR 6.58 (95% CI:6.14-7.05) had the highest risk of a same site SPC.
Of the 30 evaluated FPC cancer types in men, survivors with 22 different FPC were at significantly higher risk of a same site SPC than the general Kentucky population. A total of 1794 (18.0%) same site SPCs were observed for Kentucky male FPC patients. The most commonly observed same site SPCs among men were lung (370,53.6%), melanoma (220,30.3%) and oral cavity and pharynx (165,29.0%). Of the 31 evaluated FPC cancer types in women, survivors with 23 different FPC were at significantly higher risk of SPC than the general Kentucky population. 2452 (30.6%) of same site SPCs occurred for female population during study period. The most commonly observed same site SPCs among women were breast (1186,37.0%), lung (460,63.4%) and colon (103, 13.7%). Excluding gender specific cancers, lung and oral cavity and pharynx cancers were among the most common same site SPCs in both men and women. Interestingly, risk of same site SPC in the oral cavity, SIR 17.5 (95% CI: 14.9-20.4) for men versus SIR 51.7 (95% CI: 39.3-66.7) for women and lung cancer SIR 5.06 (95% CI: 4.55-5.60) for men versus SIR 8.7 (95% CI: 7.92-9.53) was significantly higher in female cancer survivors when compared to males.
3.3 Different site SPC
Among all survivors with an FPC, 9.2% (13,724/148,509) were diagnosed with an SPC at a different site and had an overall higher risk of cancer than the general population, SIR 1.11 (95% CI:1.09-1.13), Table 2. With the exception of lung cancer, survivors of an FPC were more likely to have a different site SPC. Among the 7097 individuals with lung cancer, 1415 developed a SPC, 830 (58.7%) in the same site and 585 (41.3%) at a different site. SPCs were most commonly observed in those with an FPC of prostate (3528,99%+), breast (2039,63.1%) and colon (1548,85.6%), while individuals with an FPC of the larynx, SIR 2.18 (95% CI:1.96-2.42), vulva, SIR 1.85 (95% CI:1.44-2.34), and anus, SIR 1.83 (95% CI:1.48-2.25) had the highest risk of a different site SPC. Among the 31 cancer sites evaluated, survivors with 19 different FPC were at risk of a SPC, while the remaining 11 were at the same risk as the general population.
Of the 30 evaluated FPC cancer types in men, survivors with 14 different FPC were at significantly higher risk of a different site SPC than the general Kentucky population. The most common FPCs among men with a different site SPC were prostate (3528, 99%+), colon (899,85.1%) and urinary bladder (165,84.6%), while men with a FPC of the larynx, SIR 1.97 (95% CI:1.75-2.22), Hodgkin lymphoma, SIR 1.92 (95% CI:1.44-2.51), and oral cavity and pharynx, SIR 1.58 (95% CI:1.43-1.74) had the highest risk of a different site SPC. Of the 31 evaluated FPC cancer types in women, survivors with 16 different FPC were at significantly higher risk of a different site SPC than the general Kentucky population. The most commonly FPCs among women with a different site SPC were breast (2017,63.0%), colon (649,86.3%) and uterine (447,99.3%), while women with an FPC of the larynx, SIR 3.30 (95% CI:2.65-4.07), anus, SIR 2.02 (95% CI:1.55-2.60), and vulva, SIR 1.85 (95% CI:1.44-1.34) had the highest risk of a different site SPC.
3.4 Appalachian
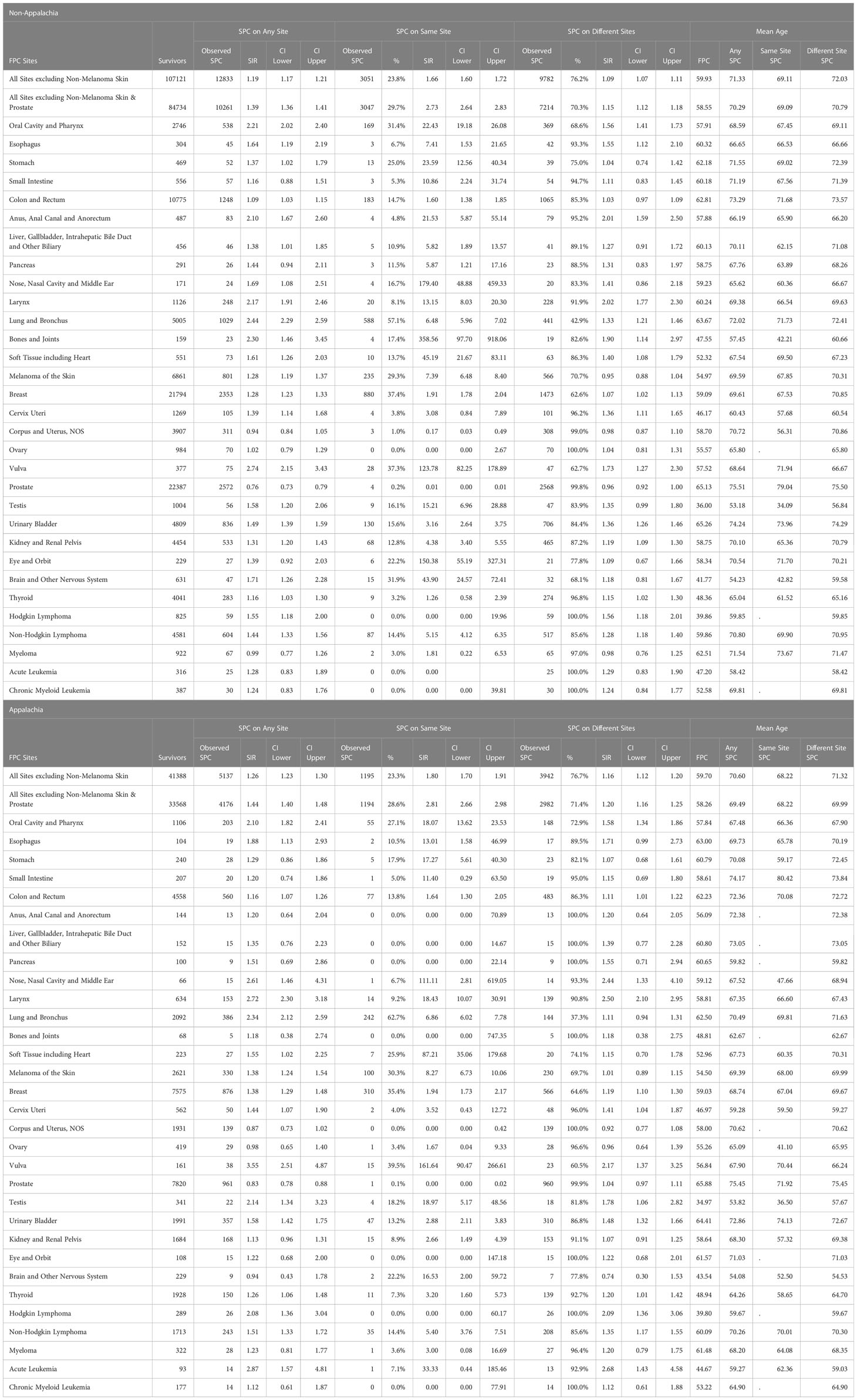
Increased risk for any site SPC among cancer survivors was consistently observed for non-Appalachian and Appalachian residents with SIR 1.19 (95%CI: 1.17-1.21) and SIR 1.26 (95%CI: 1.23-1.30), respectively, with Appalachian residents at significantly higher risk than non-Appalachian residents. (Table 3). Non-Appalachian residents were diagnosed on average at age 59.9 for FPC and 71.3 for SPC and Appalachian residents had a mean age of diagnosis of FPC at age 59.7 and 70.6 for SPC. The highest risk for any SPC in non-Appalachian survivors was seen with FPC of the vulva, SIR 2.74 (95% CI:2.15-3.43), lung and bronchus, SIR 2.44 (95% CI:2.29-2.59), and bones and joints, SIR 2.30 (95% CI:1.46-3.45). Appalachian survivors were at highest risk of SPC when the FPC was vulva, SIR 3.55 (95% CI:2.51-4.87), acute leukemia, SIR 2.87 (95% CI:1.57-4.81), and larynx, SIR 2.72 (95% CI:2.30-3.18). The most frequently observed FPC and SPC sites in both non-Appalachian and Appalachian residents were prostate, breast, and colon and rectum, Supplementary Figure 1.
Rates of same site SPC between Appalachia and non-Appalachia were similar, with 23% of all SPC occurring in the same site of FPC, Table 3. Among cases when the SPC count was 15 or more, the most commonly observed same site SPCs among non-Appalachian residents were breast (880), lung (588) and colon (183), while individuals with a FPC of the vulva, SIR 124 (95% CI:82-179), oral cavity, SIR 22.4 (95% CI:19.2-26.1), and CNS, SIR 43.9 (95% CI:24.5-72.4) had the highest risk of a same site SPC. Among individuals residing in Appalachia, the most commonly observed same site SPCs among Appalachian residents were breast (310), lung (242) and melanoma (100), while individuals with an FPC of the vulva, SIR 161.6 (95% CI:90-266), oral cavity, SIR 18.1 (95% CI:13.6-23.5) and melanoma, SIR 8.27 (95% CI:6.73-7.78) had the highest risk of a same site SPC.
The majority of SPCs occurred in different sites (~76%) for Appalachian and non-Appalachian populations. Increased risk for different site SPC among cancer survivors was consistently observed for non-Appalachian and Appalachian residents with SIR 1.09 (95%CI: 1.07-1.11) and SIR 1.16 (95%CI: 1.12-1.20), respectively, with Appalachian residents at significantly higher risk than non-Appalachian residents. The most commonly observed different site SPCs among non-Appalachian residents occurred in those with an FPC of prostate (2568), breast (1473), and colon (1065), while individuals with an FPC of the larynx, SIR 2.02 (95% CI:1.77-2.03), anus, SIR 2.01 (95% CI:1.59-2.02), and vulva, SIR 1.73 (95% CI:1.27-2.30) had the highest risk of a different site SPC. Among individuals residing in Appalachia, the most commonly observed different site SPCs among Appalachian residents occurred in those with a FPC of prostate (960), breast (566), and colon (483), while individuals with a FPC of the larynx, SIR 2.50 (95% CI:2.10-2.95), oral cavity and pharynx, SIR 1.58 (95% CI:1.34-1.86), and urinary bladder, SIR 1.48 (95% CI:1.32-1.66) had the highest risk of a different site SPC.
3.5 Risk by FPC
Associations between FPC and SPC for individual cancer sites are displayed in Figure 1 (24). The SIRs were compressed if values were larger than 10 and the diagonal values indicate SIRs for same site SPCs. The highest risk of SPC appeared to be a same site, with 17/31 FPC cancers having an SIR of 5 or greater for a same site SPC, however all cancers had an SIR from 1-5 for increased risk of a different site SPC, and survivors with a FPC of esophageal cancer had SIR > 5 for a SPC small intestine, cervical, eye and thyroids cancers. Similar associations were observed for men and women and Appalachian versus non-Appalachian residents and are presented in Supplementary Figures 2 and 3.
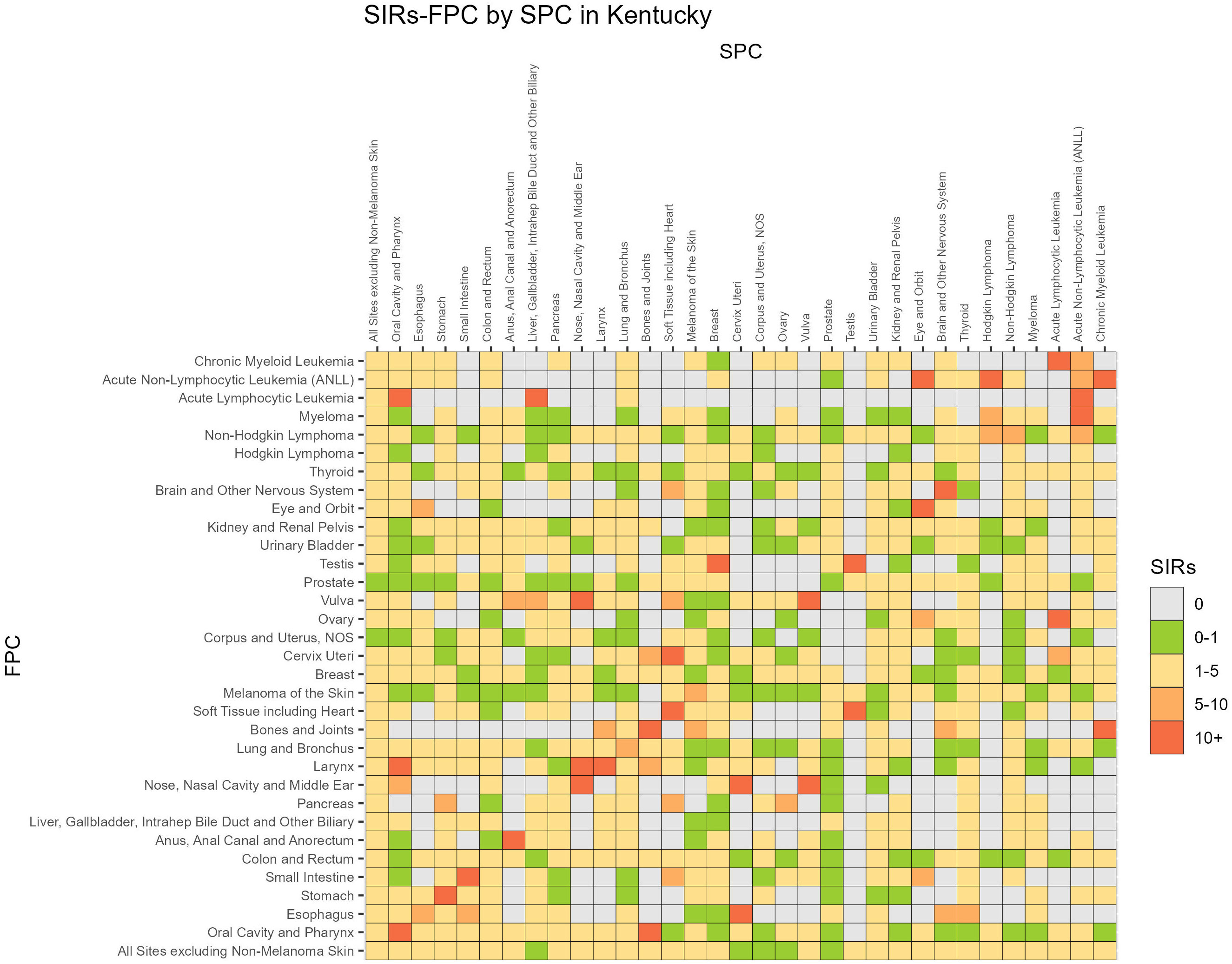
Figure 1 SIR, Standardized Incidence Ratio; FPC, First primary cancer; SPC, Subsequent primary cancer.
3.6 Risk factor associated cancers
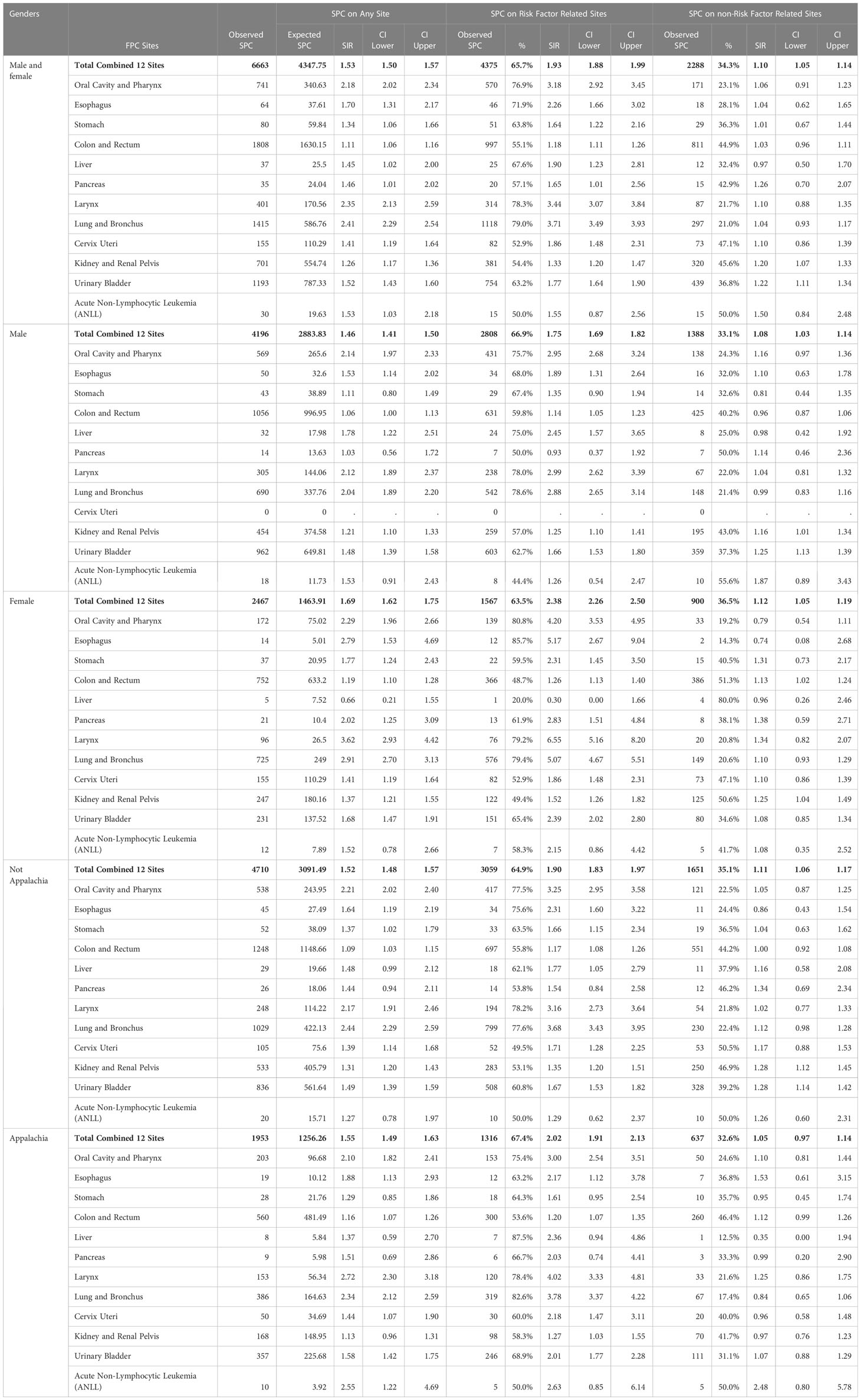
We next evaluated 12 smoking related FPCs (Table 4), which accounted for 37.1% (6663/17,970) of all SPCs. Overall, survivors with a smoking associated FPC had significantly higher risks of any site, SIR 1.53 (95% CI:1.50-1.57), same site, SIR 1.93 (95% CI:1.88-1.99) and different site SPC, SIR 1.10 (95% CI:2.1.05-1.14) when compared to the general population which was consistent across men, women, Appalachian and non-Appalachian residents. The majority 65.7% (4375/6663) of SPCs were in another smoking related site, with women having a higher risk compared to men, SIR 2.38 (95% CI:2.26-2.50) and SIR 1.75 (95% CI:1.69-1.82), respectively. Interestingly, residing in Appalachian was not associated with increased risk of a smoking associated SPC when compared to non-Appalachian residents.
Finally, we evaluated SPC frequency among individuals with other risk factor associated FPCs, including HPV, obesity, physical inactivity, and alcohol related cancers (Supplementary Table 1). When combining men and women, survivors of a risk related FPC were at higher risk of any site, same site, and different site SPC than the general population. Risk of same site SPC was also significantly higher than other site cancers for all risk associated cancers evaluated. Alcohol associated (6280) and obesity (6231) were the most common SPC after smoking, with HPV associated cancers conveying the highest risk, SIR 7.62 (95% CI: 3.28-15.0) and SIR 9.78 (95% CI: 7.48-12.6) for men and women, respectively.
Women with a risk factor associated FPC were at increased risk of any, same and other site FPCs when compared to the general population, while men had increased risk of any, same and other alcohol and HPV associated risk. With the exception of alcohol related FPC, there were no differences in SPC risk between men and women. However, men with an alcohol related FPC were at higher risk for an alcohol related SPC than women, SIR 2.29 (95% CI: 2.11-2.48) and SIR 1.61 (95% CI: 1.53-1.68). Similarly, when comparing Appalachian and non-Appalachian populations, both groups had increased risk of any, same and other site SPCs when compared to the general population. With the exception of alcohol related FPC, there were no differences in SPC risk between Appalachian and non-Appalachian residents. However, survivors of an alcohol related FPC living in Appalachia had an increased risk of an alcohol related SPC when compared to non-Appalachian residents, SPC 1.30 (95% CI: 1.22-1.37) and SIR 1.12 (95% CI: 1.08-1.16), respectively.
4 Discussion
SPC is a common problem for cancer survivors in Kentucky, with 10.1% of five-year survivors developing one SPC and 1.1% developing two or more in either the same or different sites. When compared to the US population (11), 8.3% of five-year survivors developed a different site SPC, while in our population the risk of different site SPC was 9.2% overall, 9.1% among non-Appalachian Kentuckians and 9.5% among Appalachian Kentuckians. While direct risk comparisons are difficult between Kentucky and US populations since our studies used different denominators (Kentucky general and US general population, respectively), it appears more Kentuckians develop SPC than the individuals residing in other parts of the US. It is also a serious problem, as the majority of survivors diagnosed with a SPC in this study were deceased (55.0%), while only 26% of those without a SPC had died.
Consistent with prior reports suggesting individuals surviving an FPC have higher risks of developing SPCs than the general population, Kentuckians with an FPC are also more likely to develop an SPC. The risk of developing SPCs was significantly higher for 20 of the 30 FPC types in men and for 20 of the 31 FPC types in women. The most common FPC in women with any site SPC were breast, colon and uterine cancers, however, the FPCs with highest risk of a SPC were larynx, lung, and vulva cancers. In men, the most common any site FPCs were prostate, colon and urinary bladder, while an FPC of the oral cavity, larynx or lung conveyed the highest risk of SPC and men with an FPC of prostate were actually less likely to have an SPC. Given that the highest risk FPCs are smoking related, and lung cancer accounts for nearly 25% of all SPC in Kentucky, smoking cessation interventions are highlighted as critical components of survivorship care, as well as careful attention to cancer screening studies (mammography, colonoscopy, CT screening) for survivors of larynx, oral cavity and pharynx cancers
Similarly, SPC frequency among individuals with a risk factor associated FPC, including HPV, obesity, physical inactivity, smoking and alcohol associated cancers was significantly higher than the general population. These effects were especially pronounced in the incidence of same site SPC, notably, male and female survivors of HPV associated FPC had same site SPC excess risks of 762% and 978%, respectively, suggesting that survivors continue high risk behaviors during cancer survivorship and again highlighting opportunities for primary prevention and cancer screening as part of survivorship care. In addition, male and female survivors of HPV associated cancers also had excess SPC of non-risk factor related SPC of 181% and 165%, suggesting factors other than risky behaviors (eg; genetic predisposition, treatment exposure, or environmental exposure) may also contribute to excess risk.
We also demonstrate that cancer survivors residing in Appalachia had a significantly increased risk of SPC compared to cancer survivors residing in non-Appalachian regions. While excess risk in this region has historically been attributed to higher rates of smoking and other cancer risk associated behaviors, in our study cancer survivors residing in Appalachia did not have increased risk of smoking associated SPCs when compared to non-Appalachians. While this could be a function of small sample size for individual cancers or not surviving longer enough (5 years) to be included in the study, genetic and environmental factors could also play a role.
Strengths of this study include a relatively large sample size, use of SEER registry data for the entire state of Kentucky and the inclusion of a Kentucky Appalachian population. This study has several limitations, first, it was conducted in a single state, which provides a robust picture of SPC in Kentucky, but may not be generalizable to other regions. However, given our standing amongst states with the highest incidence and mortality overall from cancer, it represents critical information for Kentucky. Second, only patients surviving more than five years since their first primary cancer diagnosis were included in the data analysis. The approach may provide more stable estimates, however, given the excess mortality and reduced survivorship in the Appalachian region of Kentucky, risk of SPC may have been underestimated. Third, we included same site cancers, and while this increases the risk of bias due to misclassification of recurrence, important insights related to overall excess risk were gained, and we separately reported other site SPC for reference. Fourth, effects of radiation and systemic treatment were not considered due to an inability to determine a temporal relationship between treatments and SPC development. Fifth, survivors migrating out of Kentucky are not reportable and risks may be underestimated, however less than 3% of subjects were lost to follow-up. Sixth, after initial diagnosis, survivors were likely to have increased medical surveillance and an increased likelihood of detection of SPC than the general population, although only SPCs occurring after 5 years were included in an attempt to minimize this bias.
5 Conclusions
This is the first report of the increased risk of SPCs among Appalachian cancer survivors when compared to Kentucky cancer survivors, while both are at increased risk when compared to the general Kentucky population. Intriguingly, risk of smoking related SPCs was not different between Appalachian and non-Appalachian survivors, and further study of environmental and genetic risk factors is warranted. Finally, the majority of SPC risk is associated with risk factor associated cancers which highlights important opportunities for primary prevention as part of survivorship care.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by University of Kentucky Medical Institutional Review Board (IRB). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
BH, QC, and JK contributed to conception and design of the study. QC performed the statistical analysis. JK wrote the first draft of the manuscript. QC, BH, AA, and ED wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Cancer research informatics and biostatistical and bioinformatics Shared Resource Facilities of the Markey Cancer Center, supported by the National Cancer Institute Support Grant (P30 CA177558). The authors confirm PMCID compliance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1193487/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin (2022) 72:409–36. doi: 10.3322/caac.21731
3. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev (2016) 25(7):1029–36. doi: 10.1158/1055-9965.EPI-16-0133
4. Carol Johnson B, Steve Peace BS, Peggy Adamo RHIT, April Fritz R, Antoinette Percy-Laurry MSPH, Edwards BK. Multiple Primary and Histology Coding Rules. Bethesda, MD: National Cancer Institute, Surveillance, Epidemiology and End Results Program (2007). Available at: https://seer.cancer.gov/tools/mphrules/download.html.
5. Murphy CC, Gerber DE, Pruitt SL. Prevalence of prior cancer among persons newly diagnosed with cancer: an initial report from the surveillance, epidemiology, and end results program. JAMA Oncol (2018) 4(6):832–6. doi: 10.1001/jamaoncol.2017.3605
6. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med (2016) 375(8):794–8. doi: 10.1056/NEJMsr1606602
7. World Cancer Research Fund/American Institute for Cancer Research Continuous Update Project Expert Report 2018. Alcoholic Drinks and the Risk of Cancer. (2018). World Cancer Research Fund & American Insitute for Cancer Research. Available at: https://www.wcrf.org/wp-content/uploads/2021/02/Alcoholic-Drinks.pdf.
8. Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens–Part B: biological agents. Lancet Oncol (2009) 10(4):321–2. doi: 10.1016/S1470-2045(09)70096-8
9. U.S. Department of Health and Human Services. Smoking Cessation. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health (2020).
10. Demandante CG, Troyer DA, Miles TP. Multiple primary malignant neoplasms: case report and a comprehensive review of the literature. Am J Clin Oncol (2003) 26(1):79–83. doi: 10.1097/00000421-200302000-00015
11. Sung H, Hyun N, Leach CR, Yabroff KR, Jemal A. Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. JAMA (2020) 324(24):2521–35. doi: 10.1001/jama.2020.23130
12. Coyte A, Morrison DS, McLoone P. Second primary cancer risk - the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer (2014) 14:272. doi: 10.1186/1471-2407-14-272
13. Yao N, Alcalá HE, Anderson R, Balkrishnan R. Cancer disparities in rural Appalachia: incidence, early detection, and survivorship. J Rural Health (2017) 33(4):375–81. doi: 10.1111/jrh.12213
14. Dong W, Bensken WP, Kim U, Rose J, Fan Q, Schiltz NK, et al. Variation in and factors associated with US county-level cancer mortality, 2008-2019. JAMA Netw Open (2022) 5(9):e2230925. doi: 10.1001/jamanetworkopen.2022.30925
15. SEER*Stat Database. Incidence - SEER Research Plus Data, 17 Registries (excl AK), Nov 2021 Sub (2000-2019) - Linked To County Attributes - Total U.S., 1969-2020 Counties. National Cancer Institute, DCCPS, Surveillance Research Program (2021). Available at: www.seer.cancer.gov.
16. SEER. Site Recode ICD-O-3/WHO 2008 - SEER Data Reporting Tools n.d . Available at: https://seer.cancer.gov/siterecode/icdo3_dwhoheme/index.html.
17. SEER. Multiple Primary and Histology Coding Rules (2012). Available at: https://seer.cancer.gov/tools/mphrules/index.html.
18. SEER. MP-SIR Events Tab n.d . Available at: https://seer.cancer.gov/seerstat/WebHelp/MP-SIR_Events_Tab.htm.
19. SEER. Race and Hispanic Ethnicity Changes - SEER Documentation (2021). Available at: https://seer.cancer.gov/seerstat/variables/seer/race_ethnicity/index.html.
20. SEER. Localized/Regional/Distant Stage Adjustments - SEER Documentation n.d. Available at: https://seer.cancer.gov/seerstat/variables/seer/lrd-stage/index.html.
21. Appalachian Regional Commission. Appalachian Regional Commission - Investing in Appalachia’s economic future n.d . Available at: https://www.arc.gov/.
22. Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, et al. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. U.S. Bethesda, MD: National Institutes of Health. (2006).
23. Centers for Disease Control and Prevention. Definitions of Risk Factor-Associated Cancers for U.S. Data. U.S. Cancer Statistics Public Use Databases (2022). Available at: https://www.cdc.gov/cancer/uscs/public-use/predefined-seer-stat-variables.htm.
Keywords: subsequent primary cancer, first primary cancer, cancer survivors, Appalachian Kentucky, surveillance, cancer disparities, cancer associated risk factors
Citation: Chen Q, Huang B, Anderson AM, Durbin EB, Arnold SM and Kolesar JM (2023) Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in Kentucky and Appalachian Kentucky. Front. Oncol. 13:1193487. doi: 10.3389/fonc.2023.1193487
Received: 25 May 2023; Accepted: 31 July 2023;
Published: 17 August 2023.
Edited by:
Jinyan Huang, Zhejiang University, ChinaReviewed by:
Reda Wilson, Centers for Disease Control and Prevention (CDC), United StatesDeborah Vollmer Dahlke, Texas A&M School of Public Health, United States
Copyright © 2023 Chen, Huang, Anderson, Durbin, Arnold and Kolesar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jill M. Kolesar, amlsbC5rb2xlc2FyQHVreS5lZHU=
 Quan Chen
Quan Chen Bin Huang1,2
Bin Huang1,2 Abigail M. Anderson
Abigail M. Anderson Susanne M. Arnold
Susanne M. Arnold Jill M. Kolesar
Jill M. Kolesar