- 1School of Science, China Pharmaceutical University, Nanjing, China
- 2College of Public Health, Shanghai University of Medicine and Health Sciences, Shanghai, China
- 3Shenzhen International Graduate School, Tsinghua University, Shenzhen, China
- 4Clinical Research Center, Shenzhen Evergreen Medical Institute, Shenzhen, China
- 5Department of Neurosurgery, People’s Hospital of Lianyungang City/The First Affiliated Hospital of Kangda College of Nanjing Medical University, Lianyungang, China
- 6College of Food Sciences and Nutritional Engineering, China Agricultural University, Beijing, China
- 7Key Laboratory of Precision Nutrition and Food Quality, Department of Nutrition and Health, China Agricultural University, Beijing, China
Background: Iron is an essential element for organismal health but excessive iron is potentially toxic. However, few observational studies link plasma iron (PI) concentrations and cancer risk, and the results are inconsistent.
Objective: This study aimed to explore the associations of PI concentrations with cancer risk in Chinese adults with hypertension.
Methods: We conducted a nested, case-control study, including 223 pairs of incident cancer cases and matched controls from the China Stroke Primary Prevention Trial. The median time between blood sample collection and subsequent cancer event occurrence was 2.13 years. The odds ratio (OR) and 95% confidence interval (CI) for the risk of cancer by PI were estimated from multivariable conditional logistic regression models.
Results: There was a nonlinear association between PI concentrations and total cancer risk. When compared with participants in tertile 2 of PI, the ORs of total cancer were 2.17 (95%CI: 1.25-3.85) and 1.29 (95%CI: 0.77-2.19) in participants in PI tertiles 3 and 1, respectively. Furthermore, higher PI was associated with increased digestive system cancer risk (OR=3.25, 95%CI:1.29-8.90), while lower PI was associated with increased risk of non-digestive system cancer (OR=3.32, 95%CI: 1.39-8.71). In a sensitivity analysis, the increases in total cancer risk or digestive system cancer risk were still observed with higher PI after excluding cancer cases occurring within the first year.
Conclusion: Our results showed an increased risk of cancer related to higher PI or lower PI in Chinese adults with hypertension. Higher iron levels were linked to an increased risk of digestive system cancers, whereas lower iron levels were linked to an increased risk of non-digestive system cancers.
Introduction
Cancer, which is increasing around the world, is expected to rank as the leading cause of mortality and the main barrier to life expectancy (1). Iron plays a vital role in cell replication, metabolism, and growth and is becoming more relevant in the research of chronic disease and cancer (2). However, iron status can be a double-edged sword, for excess iron status has been determined to be harmful to human health in that it generates a lot of free radicals, such as reactive oxygen species, in reaction with hydrogen peroxide (3). Excessive free radicals are a well-known cause of cell and tissue organ damage, and these processes may affect the development of cancer (4). One study showed that patients with hereditary hemochromatosis, a genetic iron disorder characterized by elevated plasma iron (PI) levels, had an increased risk of liver cancer (5).
A large number of studies have shown that abnormal iron homeostasis is one of the markers of cancer. PI has traditionally been used as a short-term marker of body iron status (6). The results on the relationship between blood iron and cancer risk have not always been consistent and no statistically significant associations (7) or positive associations (8, 9), as well as inverse associations (10), have been indicated. A cohort of 309,443 adults in Taiwan showed that high PI (≥120 mg/dL) is a marker of increased risk for total cancers, especially liver cancer (8). In contrast, a meta-analysis in a Chinese population found that PI levels were lower in patients with cervical cancer than in controls (10). A clinical trial also found that a reduction of iron stores was associated with a modestly decreased cancer risk and mortality (7). However, a large prospective cohort from Sweden reported no association between PI and cancer risk except for postmenopausal breast cancer (11). Additionally, one prospective study reported that the relationship between PI and nonskin cancers was converse between the sexes with a negative association in males and a positive association in females (12). Taken together, the evidence that links iron status with cancer has yielded mixed results.
Different associations between nutrients and cancers may occur in individuals with or without hypertension. All the previous studies were conducted in general populations. However, to date, there is a lack of data on cancer incidence associated with PI among patients with hypertension. Given the conflicting findings from epidemiologic studies, we hypothesize that the association between iron status and cancer risk is site-dependent and varies by population characteristics. In the current study, we prospectively examined the association between PI and total cancer and its subgroups in Chinese adults with hypertension and evaluated possible effect modifiers on the iron-cancer association.
Materials and methods
Study design and participants
The present study employed a nested, case-control design based on data from the China Stroke Primary Prevention Trial (CSPPT, clinicaltrials.gov identifier: NCT00794885). A detailed description of the cohort has been reported elsewhere (13). This multi-community, randomized, double-blind clinical trial aimed to evaluate the effectiveness of daily treatment with enalapril folic acid compared to enalapril alone in preventing first strokes and other outcomes, including cancer. The trial enrolled 20,702 adults with hypertension, aged 45 to 75 years, across thirty-two communities in China’s Jiangsu and Anhui provinces. It began on 19 May 2008 and concluded on 24 August 2013.
Participant follow-up involved phone or door-to-door visits, as well as clinic appointments, conducted every three months over a median period of 4.5 years. Throughout the follow-up period, the occurrence of endpoint events was documented. Hypertension was defined as self-reported use of antihypertensive medication, history of hypertension, or seated, resting systolic blood pressure of 140 mm Hg or higher, or diastolic blood pressure of 90 mm Hg or higher. The participants in the CSPPT had no history of physician-diagnosed stroke, myocardial infarction, heart failure, coronary revascularization, congenital heart disease, or cancer at the time of recruitment.
Outcome assessment
Cancer was a pre-specified endpoint of the CSPPT and was the main outcome of our study. Cancer was diagnosed based on pathological findings. Original or photocopied pathological reports and original or photocopied medical records from hospitals were taken as evidence for pathological findings. In case pathological data was not available, two oncologists reviewed cases independently. Only if both of the physicians made the same clinical diagnosis based on clinical manifestations and examinations, cancer was diagnosed. An Endpoint Adjudication Committee, whose members were unaware of the treatment assignments, reviewed and adjudicated all cancer events independently.
Selection of cases and controls
To mitigate confounding factors, a nested, case-control design was adopted. As shown in Supplemental Figure 1, among the 20,702 CSPPT participants, 232 patients were identified as having new, physician-diagnosed cancer until the end of the follow-up. Those participants who were alive and never developed cancer or cardiovascular disease during the follow-up period were matched with incident cancer cases in a 1:1 ratio. Matching criteria were age ( ± 1 year), sex, residence and treatment group. After excluding participants with missing iron data (n=4) and iron concentrations above 500 ug/dL(n=5), 223 cancer case-control pairs were obtained. The median time between blood sample collection and subsequent cancer event occurrence was 2.13 years. Among the 223 cancer patients, 123 cases were digestive cancers (including esophageal cancer, stomach cancer, liver cancer, pancreatic cancer, and colorectal cancer) and 100 cases were non-digestive cancers (including lung cancer and other non-digestive cancers).
Data collection and assessment
At baseline, all study participants completed a standard questionnaire interview, including information on age, sex, education, occupation, smoking status, alcohol consumption, medical history, current medical condition, and medication intake. Height, weight, and seated blood pressure were measured by trained research staff according to standard protocol. Blood samples were collected at baseline. Serum homocysteine, fasting lipids, and glucose levels were measured using automatic clinical analyzers (Beckman Coulter) at the core laboratory of the National Clinical Research Center for Kidney Disease, Nanfang Hospital, Guangzhou, China. Serum vitamin B12 and folate levels were measured in a commercial laboratory using a chemiluminescent immunoassay (New Industrial), as published previously (13). PI, retinol, and 25-hydroxyvitamin D (25(OH)D) were measured by liquid chromatography with tandem quadrupole mass spectrometers (LC-MS/MS) in a commercial lab (Beijing DIAN Medical Laboratory) from August 2016 to July 2017 following standard lab protocol and vigorous quality control procedures.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation and compared using t-tests or median (75th percentile-25th percentile) and compared using rank-sum tests. Categorical variables were presented as number (percentage) and were compared using chi-square tests. To evaluate the association between PI and total cancer, subgroups (digestive cancers and non-digestive cancers), and four subtypes of cancers with sample sizes greater than 20 (esophageal cancer, gastric cancer, breast cancer, lung cancer), we utilized multivariate conditional logistic regression models. Here, PI was treated both as continuous variables, scaled to the standard deviation (SD), and as categorical variables (tertiles). Additionally, linear trend tests were conducted. To assess potential nonlinear associations, we employed restricted cubic spline regressions with 3 knots positioned at the 10th, 50th, and 90th percentiles. The reference point for these analyses was set at the median PI level observed in the population. If a nonlinear association had been identified, a segmented regression model was conducted to determine the breakpoint in this relationship. Subsequently, we proceed to further analyze the associations both below and above the identified breakpoints. The selection of adjustment variables was based on stepwise conditional logistic regression analysis and these were chosen based on those previously reported in the literature and included ten continuous variables: body mass index (BMI), baseline systolic blood pressure (SBP), fasting blood glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), total homocysteine, vitamin B12, plasma retinol, and 25(OH)D; and two categorical variables: smoking status (ever vs. never) and drinking status (ever vs. never). In order to conduct a sensitivity analysis, we excluded cases diagnosed within one year after blood sampling. Furthermore, a multivariate conditional logistic regression model was employed. Interactions were examined by including the interaction terms in the logistic regression models. All analyses were performed in R software version 4.2.1 and two-tailed P < 0.05 was considered statistically significant.
Results
Characteristics of the participants
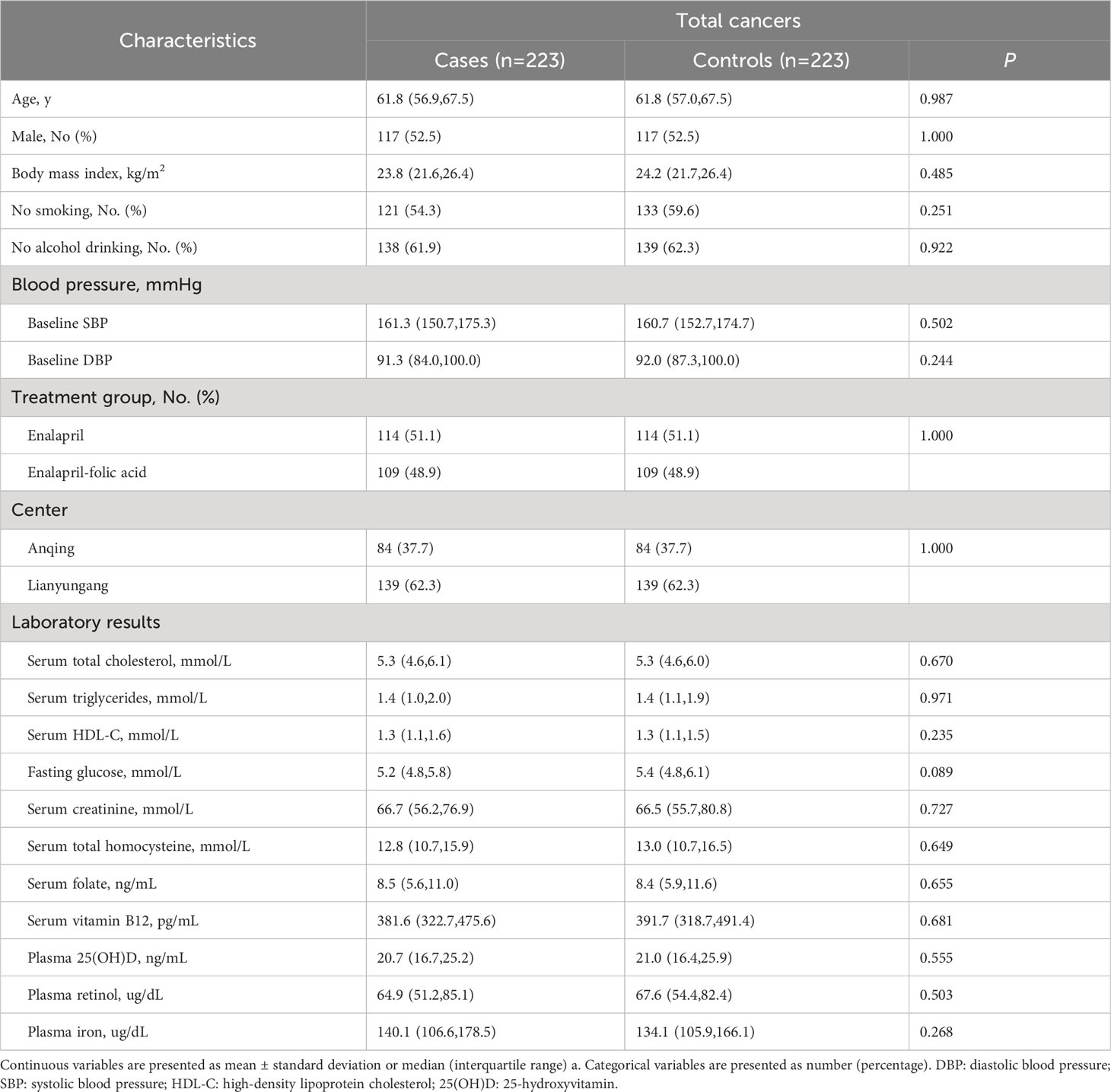
The analysis included 223 case-control pairs with PI measurements. Cancer cases included 123 cases of digestive system cancers (52 esophageal, 42 gastric, 18 colorectal, 7 liver, and 4 pancreatic cancers) and 100 cases of non-digestive system cancers (26 breast,26 lung, 9 lymphoma, 8 gynecologic, 7 bladder, and 24 cancers from other sites). The distribution of major cancer subtypes is presented in Supplemental Table 1. Table 1 shows the baseline characteristics of the total cases and controls. Median values of PI were 140.1 μg/dl in patients with cancer and 134.1 μg/dl in controls. There were no significant differences between total cancer cases and controls for any of the variables.
Association of baseline PI and the risk of total cancer and its subtypes
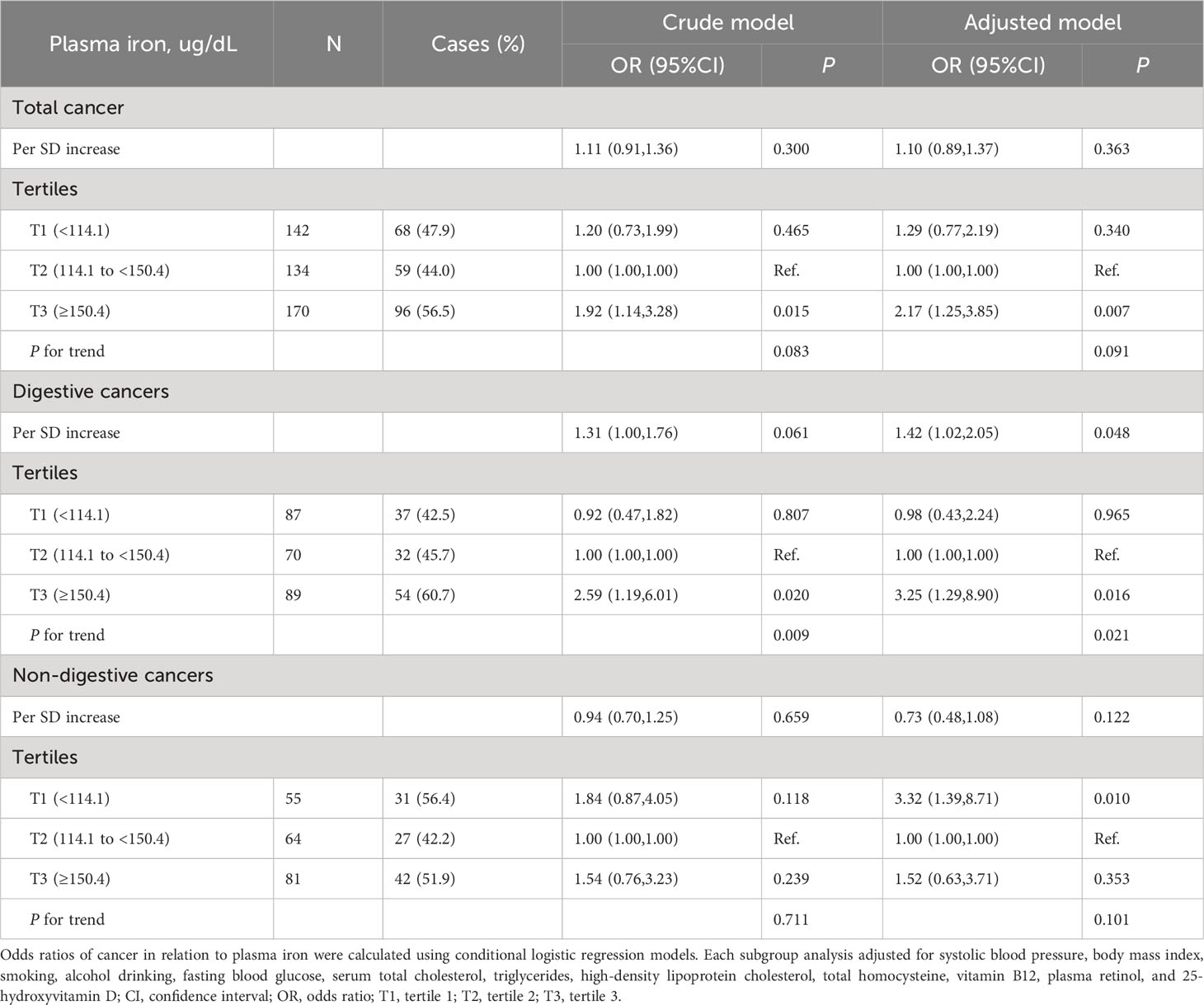
Overall, there was a nonlinear association between PI and total cancer with an increased risk of total cancer in participants who had higher or lower levels of PI (Figure 1A). Higher levels of PI were associated with an increased risk of digestive system cancers (Figure 1B), while lower levels of PI were associated with an increased risk of non-digestive system cancers (Figure 1C). Breakpoint estimates were provided by segmented regression models with digestive system cancers (breakpoint=165.6, 95%CI: 42.2-289.0) and non-digestive system cancers (breakpoint=145.6, 95%CI: 115.1-176.2). Below the breakpoint for non-digestive system cancers, there was a negative association between PI and cancer risk (OR=0.97, 95%CI: 0.96-0.99, P=0.007, Supplemental Table 2). After adjusting for potential confounders, compared to participants in PI tertile 2 (114.1 to <150.4 ug/dL), those in tertile 3 (≥150.4 ug/dL) conferred a significantly increased risk for total cancer (OR: 2.17, 95%CI: 1.25-3.85, P=0.007) while those in tertile 1(<114.1 ug/dL) also showed an increased risk, but it was not statistically significant (OR: 1.29, 95%CI: 0.77-2.19, P=0.340). A substantially higher risk of digestive system cancer was seen for those with higher levels of PI (≥150.4 ug/dL vs 114.1 to <150.4 ug/dL, OR=3.25, 95%CI: 1.29-8.90, P=0.016). However, a substantially higher risk of non-digestive system cancer was seen for those with lower levels of PI (<114.1 ug/dL vs 114.1 to <150.4 ug/dL, OR=3.32; 95%CI: 1.39-8.71; P=0.010, Table 2). Similar results were seen after excluding cases who had been diagnosed within 1 year after the blood draw (Supplemental Table 3). Further investigation into the relationship between PI and the risk of specific cancer sites revealed that patients with higher PI levels had a significantly increased risk of gastric cancer (≥150.4 ug/dL vs 114.1 to <150.4 ug/dL, OR=22.68, 95%CI: 2.28-225.83, P=0.008, Supplemental Table 4).
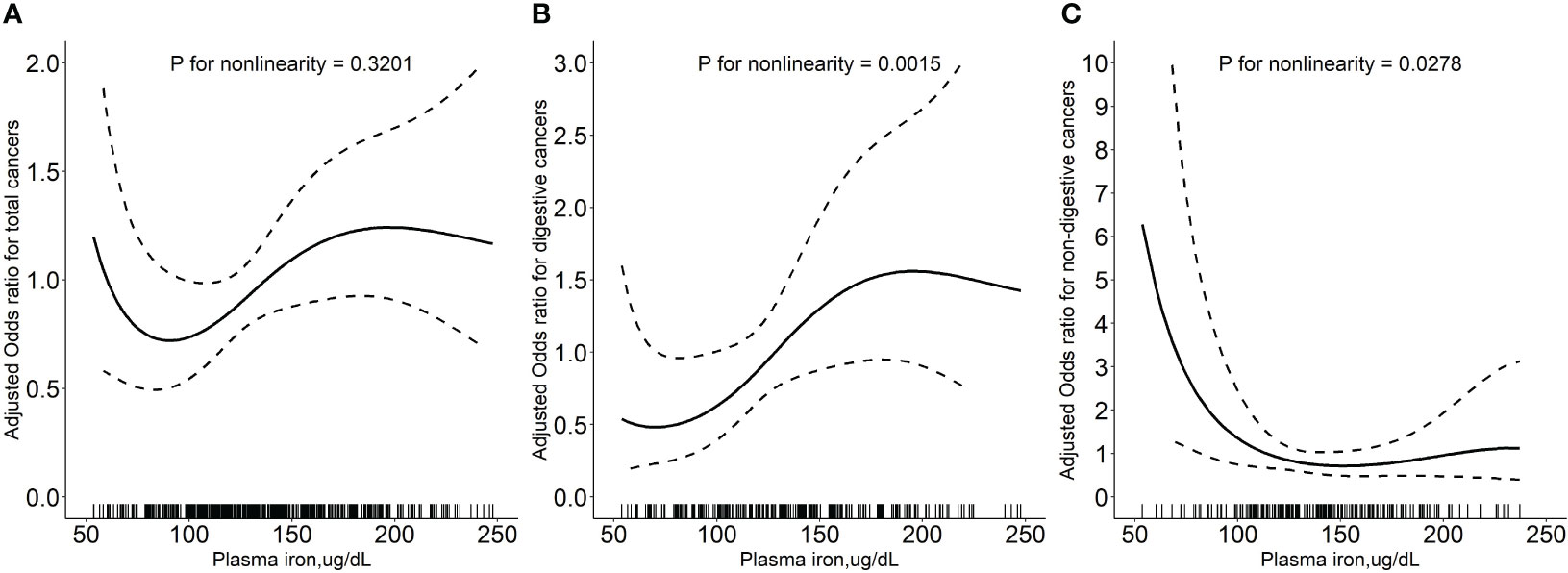
Figure 1 Relationship of plasma iron with the risk of total cancers (A), digestive system cancers (B), and non-digestive system cancers (C). Adjusted for baseline systolic blood pressure, body mass index, smoking status, drinking status, fasting blood glucose, serum total cholesterol, triglycerides, high-density lipoprotein cholesterol, total homocysteine, vitamin B12, plasma retinol, and 25-hydroxyvitamin D CI: confidence interval; OR: odds ratio.
Stratified analyses on the associations between plasma iron and cancer risk
To explore potential effect modifiers for the association between PI and cancer risk, 11 subgroup analyses were conducted stratified by age, sex, BMI, treatment group, smoking status, drinking status, total cholesterol, fasting glucose, total homocysteine, plasma retinol, and 25(OH)D (Table 3). Higher PI levels were significantly associated with an increased risk of total cancer in those with plasma retinol ≥67.0 ug/dL (median) and the P-value for the interaction and the false discovery rate (FDR) adjusted P-value were 0.047 and 0.404, respectively. None of the other variables significantly modified the association between plasma PI and total cancer.
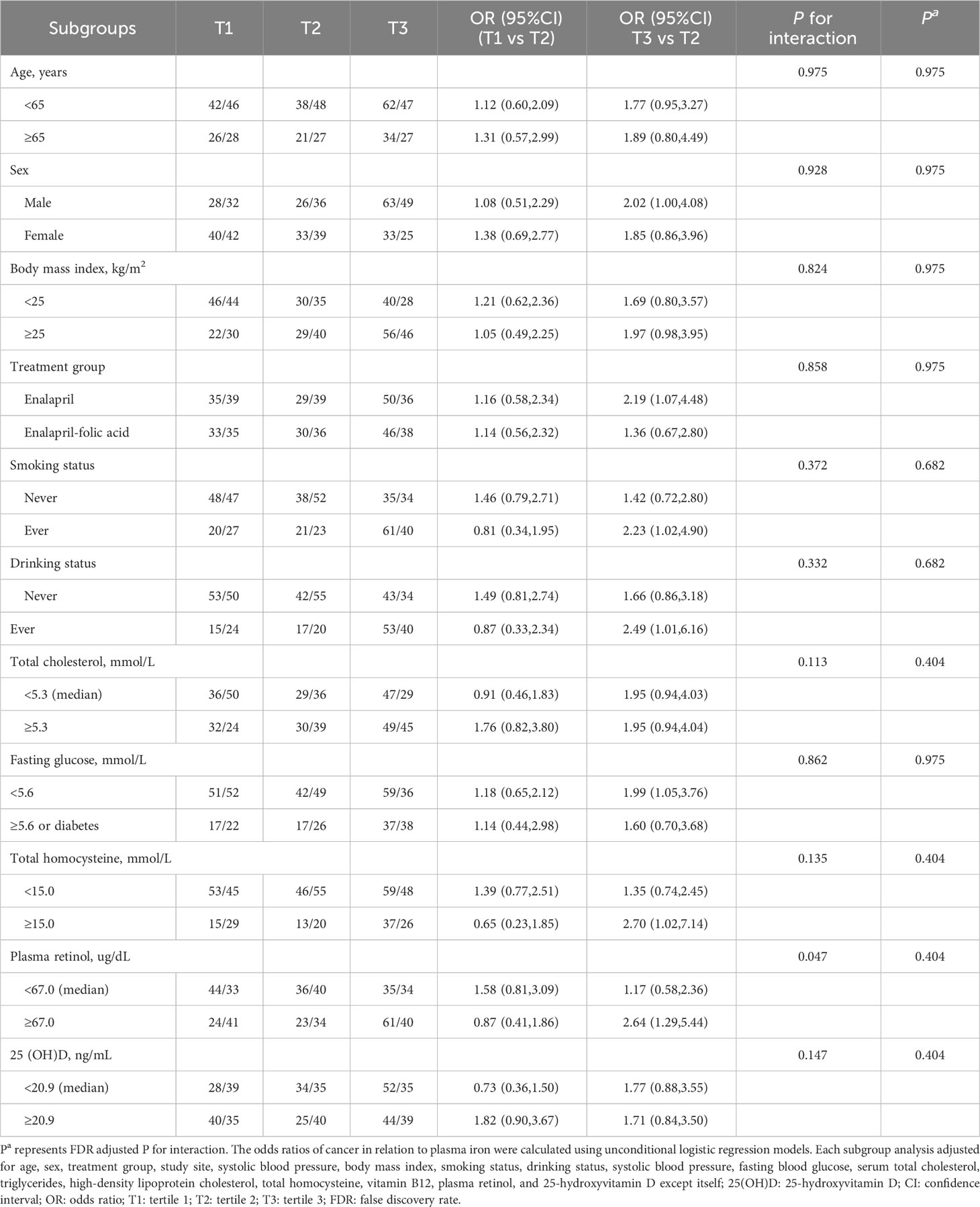
Table 3 Stratified analyses by potential effect modifiers for the association between plasma iron and risk of incident cancer.
Discussion
This nested case-control study suggests a nonlinear association between PI and the risk of total cancer, with higher cancer risk associated with both higher and lower iron levels in Chinese adults with hypertension. Moreover, we found that higher iron levels (≥150.4 ug/dL) were linked to an increased risk of digestive system cancers, whereas lower iron levels (<114.1 ug/dL) were linked to an increased risk of non-digestive system cancers. Our findings demonstrate that the association of iron with cancer might differ according to tumor location.
The mean PI level in our study was 144.2 ug/dL, which was remarkably higher than that observed in the United States (83.88 ug/dL), with data from the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2016 (14). To avoid reverse causation bias and potential confounding bias, we additionally excluded cancer cases that had occurred within the first year, as well as adjusted for potential confounders, and found that the positive association was still observed. Our results support the idea that body iron plays a key role in human carcinogenesis (2). Some studies have reported sex differences in the association of PI with cancer risk (12, 15). A prospective cohort study of US adults suggested that participants with PI above 140 µg/dl had a significantly increased risk of cancer mortality (15) and this association was stronger in females. However, no sex difference was observed in our study.
The relationship between PI concentrations and digestive system cancer risk observed in this study is in line with the prevailing hypothesis that higher body iron is associated with an increased risk of liver cancer (16). A systematic review showed that PI was positively correlated with liver cancer risk (HR: 2.47; 95%CI:1.31-4.63) (16). In a large cohort study in Taiwan, it was revealed that PI levels higher than 120 µg/dL or lower than 60 µg/dL were associated with a 25% increase and 18% increase, respectively, in all cancer incidence with 60 to 79 ug/dL as the reference level (8), primarily with liver cancer. A Mendelian randomization study in a European cohort suggested that a genetically high iron status was positively associated with liver cancer (17). For other digestive tract tumors, Milde et al. observed that adenocarcinoma colorectal patients had significantly higher levels of PI as compared to the age-matched control group (18). Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) based on 481,419 individuals and 137 incident cases of esophageal adenocarcinoma, showed a statistically significant positive association of esophageal adenocarcinoma risk with heme iron and processed meat intake (19). Recent research suggests that red meat (high iron content) intake could be a risk factor for gastric cancer (20). However, one study from a large European population found that high body iron stores as measured by PI and ferritin decreased the risk of gastric cancer (21). Another study also found that high iron stores may increase the risk of colorectal cancer, whereas low iron stores may be an early sign of occult stomach cancer (22).
Data on the association between PI and non-digestive system cancers are limited and inconclusive. One study reported a dual role for iron in breast cancer where a proangiogenic environment mediated by iron deficiency and pro-oxidant conditions associated with iron accumulation could lead to a high incidence of breast cancer (23). A recent meta-analysis found that high levels of PI were associated with an increased risk of breast cancer (24). Nonetheless, some studies have reported results that were similar to ours (10, 25). A meta-analysis in a Chinese population found that PI levels were lower in patients with cervical cancer than in controls (10). A previous publication also reported that iron deficiency is known to be associated with bladder cancer (25). A study by Yuan et al. suggested that genetically high iron status was inversely associated with brain cancer based on 48,972 individuals of European-descent (17). However, a meta-analysis showed no association between PI levels and lung cancer risk (26). Epidemiological data on the association of iron status with other non-digestive system cancers are limited and scarce. More studies are needed to better elucidate this possible relation. Our study has provided some new insights. We demonstrated that the relationship between PI levels and cancer risk varies for different cancer subtypes, with a positive association for digestive system cancers and negative association for non-digestive system cancers.
Plausible reasons for the positive correlation between PI and digestive system cancers could be explained by the fact that the gastrointestinal tract is the major site of nutrient digestion and absorption, and it is prone to oxidative damage (27). Accumulated iron has been implicated in the risk of cancer through iron-catalyzed free radical-mediated oxidative stress and subsequent DNA damage, and iron also functions as a nutrient that fosters the growth and development of cancer cells. Additionally, it has been postulated that iron insufficiency could lead to oxidative deoxyribonucleic acid damage, thereby increasing the risk of cancer (28, 29). Elemental iron is crucial for the proper functioning of enzymes involved in cell respiration, energy metabolism, DNA synthesis and repair, signaling, and many more. Iron is an important trace element required for the formation of hemoglobin and myoglobin and is a key component in immune cell proliferation. Therefore, iron is both essential and potentially toxic. However, the specific mechanism of iron metabolism in different types of tumor cells is not yet fully understood and the interaction mechanism of retinol and iron should be explored further.
There are several strengths of this study. The data are directly derived from a large cohort study, with strict quality control measures. Furthermore, our study utilized a matched design to minimize potential data bias as much as possible. First, we focused our analysis on PI, one of the biomarkers of iron status, and the analysis did not include other measures that are thought to more closely reflect levels of iron in the body, such as ferritin levels. As PI is a relatively inexpensive and widely available test, tests for high PI status could be routinely conducted and interpreted in daily practice, making our results clinically relevant. Moreover, Chua et al. suggest that circulating iron may be more related to breast carcinogenesis than stored iron (ferritin) (12). A second study limitation was that the average follow-up time from the time of PI testing was 2.13 years, which may be too short a time for assessing cancer development. A third limitation is that the conclusions from our study may not apply to other populations as the participants were patients with hypertension. The differences in findings might be attributed to residual confounding by other confounders, such as unhealthy dietary patterns or other lifestyle behaviors.
In conclusion, our study provides evidence of a nonlinear relationship between iron status and overall cancer. Considering the small number of cancer cases, additional studies with larger sample sizes are warranted to verify our findings. Furthermore, the effects of iron on different cancer types need to be explored. The results of this study have important clinical implications with regard to the excessive supplement of iron and iron deficiency.
Data availability statement
The datasets presented in this article are not readily available because Data described in the article, code book, and analytic code will be made available upon request pending approval. Requests to access the datasets should be directed to Zhu Hehao, emh1aGVoYW9AZm94bWFpbC5jb20=.
Ethics statement
The studies involving humans were approved by The CSPPT was approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China (FW A assurance number: FW A00001263). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
The authors’ responsibilities were as follows— HeZ and YW: data curation, writing original draft; QH: supervision; YuS and LL: audited the data; XX: conception and conducted research; HG, YoS and HaZ: helped with copyediting; BW: had primary responsibility for the final content of the manuscript. All authors read and approved the final manuscript.
Funding
This project was funded by the National Key Research and Development Program [2016YFE0205400, 2018ZX09739010, 2018ZX09301034003]; the Department of Science and Technology of Guangdong Province [2020B121202010]; the Science, Technology and Innovation Committee of Shenzhen [JSGG20180703155802047, JSGG20201103153807021].
Acknowledgments
We thank all the participants in this study. We thank all members for their contribution, time and patience. We also thank Ms. Emmy Graham for her help with language polishing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1223579/full#supplementary-material
Abbreviations
BMI, body mass index; CI, confidence interval; CSPPT, the China Stroke Primary Prevention Trial; DBP, diastolic blood pressure; FDR, false discovery rate; HDL-C, high-density lipoprotein cholesterol; OR, odds ratio; PI, plasma iron; SBP, systolic blood pressure; T1, tertile 1; T2, tertile 2; T3, tertile 3; 25(OH)D, 25-hydroxyvitamin D.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Torti SV, Manz DH, Paul BT, Blanchette-Farra N, Torti FM. Iron and cancer. Annu Rev Nutr (2018) 38:97–125. doi: 10.1146/annurev-nutr-082117-051732
3. Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology (2011) 283:65–87. doi: 10.1016/j.tox.2011.03.001
4. Chang VC, Cotterchio M, Bondy SJ, Kotsopoulos J. Iron intake, oxidative stress-related genes and breast cancer risk. Int J Cancer (2020) 147:1354–73. doi: 10.1002/ijc.32906
5. Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med (2008) 358:221–30. doi: 10.1056/NEJMoa073286
6. Gutierrez-Bedmar M, Olmedo P, Gil F, Ruiz-Canela M, Martínez-González MA, Salas-Salvadó J, et al. Low plasma iron levels and risk of cardiovascular disease in high risk elderly population: Nested case-control study in the PREvención con DIeta MEDiterránea (PREDIMED) trial. Clin Nutr (2021) 40:496–504. doi: 10.1016/j.clnu.2020.05.044
7. Zacharski LR, Chow BK, Howes PS, Shamayeva G, Baron JA, Dalman RL, et al. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst (2008) 100:996–1002. doi: 10.1093/jnci/djn209
8. Wen CP, Lee JH, Tai YP, Wen C, Wu SB, Tsai MK, et al. High plasma iron is associated with increased cancer risk. Cancer Res (2014) 74:6589–97. doi: 10.1158/0008-5472.CAN-14-0360
9. Stevens RG, Graubard BI, Micozzi MS, Neriishi K, Blumberg BS. Moderate elevation of body iron level and increased risk of cancer occurrence and death. Int J Cancer (1994) 56:364–9. doi: 10.1002/ijc.2910560312
10. Chen S, Shen L, Luo S, Lan X, Wang L. Association between plasma iron levels and the risk of cervical cancer in Chinese: a meta-analysis. J Int Med Res (2020) 48:300060519882804. doi: 10.1177/0300060519882804
11. Gaur A, Collins H, Wulaningsih W, Holmberg L, Garmo H, Hammar N, et al. Iron metabolism and risk of cancer in the Swedish AMORIS study. Cancer Causes Control (2013) 24:1393–402. doi: 10.1007/s10552-013-0219-8
12. Chua AC, Knuiman MW, Trinder D, Divitini ML, Olynyk JK. Higher concentrations of plasma iron and transferrin saturation but not serum ferritin are associated with cancer outcomes. Am J Clin Nutr (2016) 104:736–42. doi: 10.3945/ajcn.115.129411
13. Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. Jama (2015) 313:1325–35. doi: 10.1001/jama.2015.2274
14. Xu J, Xu G, Fang J. Association between iron exposures and stroke in adults: Results from National Health and Nutrition Examination Survey during 2007-2016 in United States. Int J Environ Health Res (2021) 32:1925–34. doi: 10.1080/09603123.2021.1926440
15. Wu T, Sempos CT, Freudenheim JL, Muti P, Smit E. Plasma iron, copper and zinc concentrations and risk of cancer mortality in US adults. Ann Epidemiol (2004) 14:195–201. doi: 10.1016/S1047-2797(03)00119-4
16. Tran KT, Coleman HG, McCain RS, Cardwell CR. Serum biomarkers of iron status and risk of primary liver cancer: A systematic review and meta-analysis. Nutr Cancer (2019) 71:1365–73. doi: 10.1080/01635581.2019.1609053
17. Yuan S, Carter P, Vithayathil M, Kar S, Giovannucci E, Mason AM, et al. Iron status and cancer risk in UK biobank: A two-sample mendelian randomization study. Nutrients (2020) 12:526. doi: 10.3390/nu12020526
18. Milde D, Novák O, Stu ka V, Vyslou il K, Machá ek J. Serum levels of selenium, manganese, copper, and iron in colorectal cancer patients. Biol Trace Elem Res (2001) 79:107–14. doi: 10.1385/BTER:79:2:107
19. Jakszyn P, Luján-Barroso L, Agudo A, Bueno-de-Mesquita HB, Molina E, Sánchez MJ, et al. Meat and heme iron intake and esophageal adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer (2013) 133:2744–50. doi: 10.1002/ijc.28291
20. González CA, Jakszyn P, Pera G, Agudo A, Bingham S, Palli D, et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst (2006) 98:345–54. doi: 10.1093/jnci/djj071
21. Fonseca-Nunes A, Agudo A, Aranda N, Arija V, Cross AJ, Molina E, et al. Body iron status and gastric cancer risk in the EURGAST study. Int J Cancer (2015) 137:2904–14. doi: 10.1002/ijc.29669
22. Knekt P, Reunanen A, Takkunen H, Aromaa A, Heliövaara M, Hakulinen T. Body iron stores and risk of cancer. Int J Cancer (1994) 56:379–82. doi: 10.1002/ijc.2910560315
23. Jian J, Yang Q, Dai J, Eckard J, Axelrod D, Smith J, et al. Effects of iron deficiency and iron overload on angiogenesis and oxidative stress-a potential dual role for iron in breast cancer. Free Radic Biol Med (2011) 50:841–7. doi: 10.1016/j.freeradbiomed.2010.12.028
24. Sanagoo A, Kiani F, Saei Gharenaz M, Sayehmiri F, Koohi F, Jouybari L, et al. A systematic review and meta-analysis on the association of serum and tumor tissue iron and risk of breast cancer. Caspian J Intern Med (2020) 11:1–11. doi: 10.22088/cjim.11.1.1
25. Mazdak H, Yazdekhasti F, Movahedian A, Mirkheshti N, Shafieian M. The comparative study of plasma iron, copper, and zinc levels between bladder cancer patients and a control group. Int Urol Nephrol (2010) 42:89–93. doi: 10.1007/s11255-009-9583-4
26. Chen HF, Wu LX, Li XF, Zhu YC, Wang WX, Xu CW, et al. A meta-analysis of association between plasma iron levels and lung cancer risk. Cell Mol Biol (Noisy-le-grand) (2018) 64:33–7. doi: 10.14715/cmb/2018.64.13.7
27. Aviello G, Knaus UG. ROS in gastrointestinal inflammation: Rescue Or Sabotage? Br J Pharmacol (2017) 174:1704–18. doi: 10.1111/bph.13428
28. Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer (2013) 13:342–55. doi: 10.1038/nrc3495
Keywords: plasma iron, cancer, hypertension, nested case-control study, digestive cancer
Citation: Zhu H, Wei Y, He Q, Song Y, Liu L, Sun Y, Zhang H, Guo H, Xu X and Wang B (2023) Association of plasma iron with the risk of incident cancer in Chinese adults with hypertension: a nested case-control study. Front. Oncol. 13:1223579. doi: 10.3389/fonc.2023.1223579
Received: 16 May 2023; Accepted: 18 September 2023;
Published: 04 October 2023.
Edited by:
Lin Qi, Central South University, ChinaCopyright © 2023 Zhu, Wei, He, Song, Liu, Sun, Zhang, Guo, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Binyan Wang, YmlueWFud2FuZzE2M0AxNjMuY29t
†These authors have contributed equally to this work
 Hehao Zhu
Hehao Zhu Yaping Wei
Yaping Wei Qiangqiang He
Qiangqiang He Yun Song
Yun Song Lishun Liu
Lishun Liu Yong Sun5
Yong Sun5 Hao Zhang
Hao Zhang Huiyuan Guo
Huiyuan Guo Xiping Xu
Xiping Xu Binyan Wang
Binyan Wang