- 1Department of Endocrinology and Metabolism, Hamad General Hospital, Hamad Medical Corporation, Doha, Qatar
- 2Pharmacy Department, National Centre for Cancer Care and Research, Hamad Medical Corporation, Doha, Qatar
- 3Department of Medical Education, Weill Cornell Medicine Qatar, Doha, Qatar
- 4Health Sciences Program, Clinical and Population Health Research, College of Pharmacy, Qatar University, Doha, Qatar
- 5Department of Nursing, Hamad Medical Corporation, Doha, Qatar
- 6Department of Medicine, Unity Hospital/Rochester Regional Health, Rochester, NY, United States
- 7Department of Gastroenterology, University Hospital Southampton, Southampton, United Kingdom
- 8Department of Medical Oncology/Hematology, National Centre for Cancer Care and Research, Hamad Medical Corporation, Doha, Qatar
Tyrosine Kinase Inhibitors (TKIs) is revolutionizing the management of pediatric Chronic Myeloid Leukemia (CML), offering alternatives to Allogeneic Hematopoietic Stem Cell Transplantation (AHSCT). We conducted a comprehensive review of 16 Randomized Controlled Trials (RCTs) encompassing 887 pediatric CML patients treated with TKIs including Imatinib, Dasatinib, and Nilotinib. The median patient age ranged from 6.5 to 14 years, with a median white blood cell count of 234 x 10^9/uL, median hemoglobin level of 9.05 g/dL, and median platelet count of 431.5 x 10^9/µL. Imatinib seems to be predominant first line TKI, with the most extensive safety and efficacy data. BCR::ABL response rates below 10% ranged from 60% to 78%, CCyR at 24 months ranged from 62% to 94%, and PFS showed variability from 56.8% to 100%, albeit with differing analysis timepoints. The Safety profile of TKIs was consistent with the known safety profile in adults. With the availability of three TKIs as first line options, multiple factors should be considered when selecting first line TKI, including drug formulation, administration, comorbidities, and financial issues. Careful monitoring of adverse events, especially in growing children, should be considered in long term follow-up clinical trials.
Introduction
Chronic Myeloid Leukemia (CML) is a commonly occurring hematological neoplasm, in adults, caused by BCR::ABL1 gene mutation in the myeloid cells (1). Chromosome 22 (Philadelphia chromosome or Ph1) abnormality is seen in almost all patients (2). The median age of presentation is 50 – 60 years (3). Moreover, three clinically distinguishable phases can be seen in CML; chronic phase (CP), accelerated phase (AP), and blast phase (BP) (4). Extensive data exists for this age group concerning its molecular genetics, clinical course, and management (4). Although common in the adult population, CML is a very rare hematologic malignancy in the pediatric age group (< 2%), with knowledge gaps in the clinical behavior and management in this population group (5, 6).
Historically, allogenic stem cell transplant (SCT) has been the curative management for CML (7). Nevertheless, SCT can potentially present an array of acute and chronic severe complications, such as graft-versus-host disease, a range of cytopenias, mucositis, and persistent infectious conditions. (8, 9). In light of the significant adverse events associated with Stem Cell Transplantation (SCT), and subsequent to an extensive investigation into the genetic underpinnings of CML, the therapeutic approach to CML has witnessed a paradigm shift from invasive to non-invasive modalities. This shift, gravitating toward potentially less complex treatment strategies, has notably included the use of Tyrosine Kinase Inhibitors (TKIs) such as Imatinib. (10). TKIs inhibit the tyrosine kinase encoded by the ABL gene (1). Subsequent additions of newer TKIs such as nilotinib, dasatinib, and bosutinib have potentially made SCT a last resort in the management hierarchy, mainly reserved for CML – CP patients resistant to 2 TKIs and those with new mutations like T315I mutation (4, 11). Extensive research is now available on the use of TKI and the management of its adverse effects in adult population with CML (12–14).
TKIs work mainly by blocking the BCR::ABL pathway, resulting in the termination of cell proliferation without causing cell breakdown, causing around a 98 percent reduction in the abnormal myeloid cells, whereas leaving the normal cells undamaged (15). Imatinib, dasatinib, nilotinib, ponatinib, and bosutinib are currently used for CML in adults (6). Primary (failure to achieve optimum response) and secondary (relapse after optimum response) resistance with TKIs in CML-CP is seen in 25 and 15 percent of cases, respectively (16, 17).
While pediatric and adult CML may exhibit similar disease patterns and share certain genetic and phenotypic characteristics, variations are observed when evaluating the genetic and clinical phenotypes across these demographic cohorts. These variations span from microscopic disparities, such as the differential distribution of mutational breakpoints within the BCR::ABL1 oncogene, to macroscopic differences manifested in distinct prognostic outcomes and disease severity (18, 19). In pediatric CML, particularly during the blast phase, there is often a more pronounced lymphoid phenotype than the adult CML. This is accompanied by distinct cytogenetic aberrations that differ from those typically observed in the adult form of the disease. Such differences are critical in understanding the pathophysiology of CML across age groups and may have significant implications for diagnostic and therapeutic strategies (20). Moreover, research has revealed that in addition to the genetic aberrations differentiating pediatric and adult CML, there are also distinct variations in the signaling pathways involved. These differences in signaling mechanisms further underscore the complexity of CML pathogenesis across different age groups and highlight the need for age-specific approaches in both research and treatment of this disease (21). There are many unanswered aspects of TKI use in pediatric CML. The effectiveness of TKIs is directly related to their concentration in plasma, with better outcomes demonstrated in patients having a plasma concentration of at least 1000 ng/ml; however, this is only evidenced in the adult population and remains unexplored in pediatric CML patients (6). Additionally, prognostication criteria such as the National Comprehensive Cancer Network (NCCN) guidelines or the European Leukemia Net (ELN) are used in pediatric CML patients based on their validation for the adult CML population (6). As children have an immature skeleton and rapid growth phases, indefinite TKI therapy affects growth. Moreover, the duration of treatment with TKI in the pediatric CML population is considerably longer than in adults (5). This difference is even more clinically significant regarding drug safety, as the side effects of TKI are not well-studied beyond 15 years. The long-term side effect profile may differ from those of adults (5). TKI therapy discontinuation has been investigated in the adult CML population with favorable outcomes. However, it remains unexplored prospectively in children (22).
Rapidly accumulating data on the use of TKI in pediatric CML is emerging (23–25). Newer TKIs such as dasatinib and Nilotinib are increasingly being prescribed as upfront therapies in pediatric CML (26–28). Owing to the low prevalence of CML within the pediatric demographic, along with trials scrutinizing the impact of novel therapeutic approaches constrained to smaller cohorts, the characterization of efficacy and safety profiles of disparate TKI generations, as well as a thorough comparative analysis of the risk-benefit ratio between TKIs and SCT specifically in a pediatric context, remains nebulous. The objective of this narrative review was to discuss a balance between the effectiveness of TKIs with the adverse effect profile within the context of pediatric CML.
Methods
We used Medline, OVID, and Web of Science to identify all prospective randomized clinical trials reporting the efficacy and safety of TKI in pediatric CML (< 18 years). We excluded observational studies, non-randomized clinical trials, studies describing patients aged > 18 years; those with contraindications to the use of TKI; the presence of another active malignancy, or the use of systemic therapy for another malignancy within three years (except local/regional therapy with curative intent). We used well-defined keywords in literature search (“pediatric chronic myeloid leukemia” OR “pediatric CML” OR “chronic myeloid leukemia in children” OR “adolescent chronic myeloid leukemia” OR “adolescent CML” OR “chronic myeloid leukemia in adolescents” AND “tyrosine kinase inhibitors” OR “TKI” OR “imatinib” OR “dasatinib” OR “nilotinib” OR “ponatinib” OR “bosutinib”. We aggregated the efficacy and safety data from the included RCTs, focusing on clinical and demographic variables and outcomes relevant to the safety and efficacy of TKI in the pediatric population with CML. The narrative review highlights key aspects of patient responses and potential adverse events, offering a comprehensive view of TKI treatment profiles.
Discussion
Patients characteristics
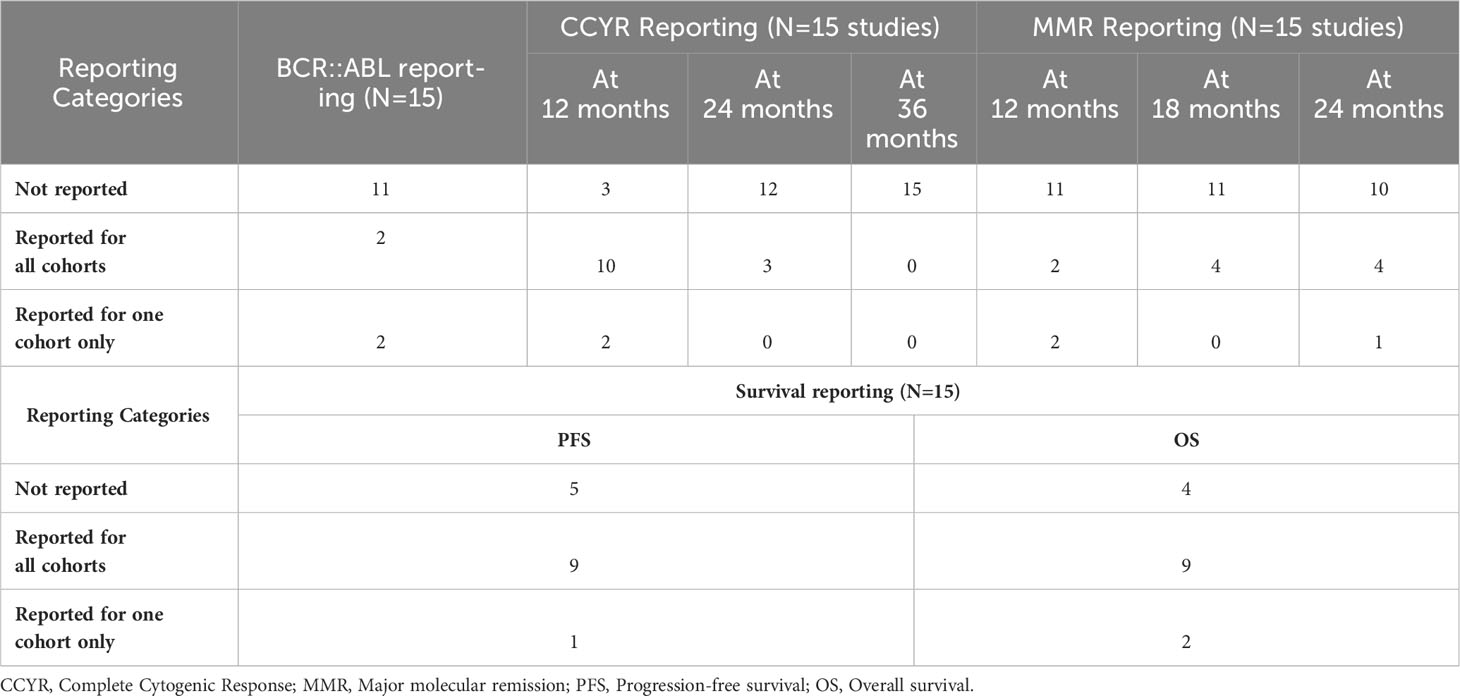
A total of 16 trials were included in this review, representing a collective of 887 pediatric CML patients. One study reported only safety outcomes and was removed from the efficacy analysis (n(efficacy studies)=15, n(efficacy patients)=718), while another reported only efficacy results and was removed from the safety analysis (n(safety studies)=15, n(safety patients)=699 patients). The median population age in the studies ranged from 6.5 to 14 years old (average 11.3 years old). Where data was available at baseline, the median white blood cell count was 234 x109/uL [range 19 to 378 x109/µL; reported in 9 of 16 studies]. The median hemoglobin level was 9.05 g/dL [range 5.6 to 10.8 g/dL; reported in 7 of 16 studies], and the median platelet count was 431.5 x109/µL, [range 31 to 594 x109/µL; reported in 6 of 16 studies]. Individual study data from the added RCTS are summarized in Table 1; Supplementary Table 1. The efficacy data is summarized in Table 2.
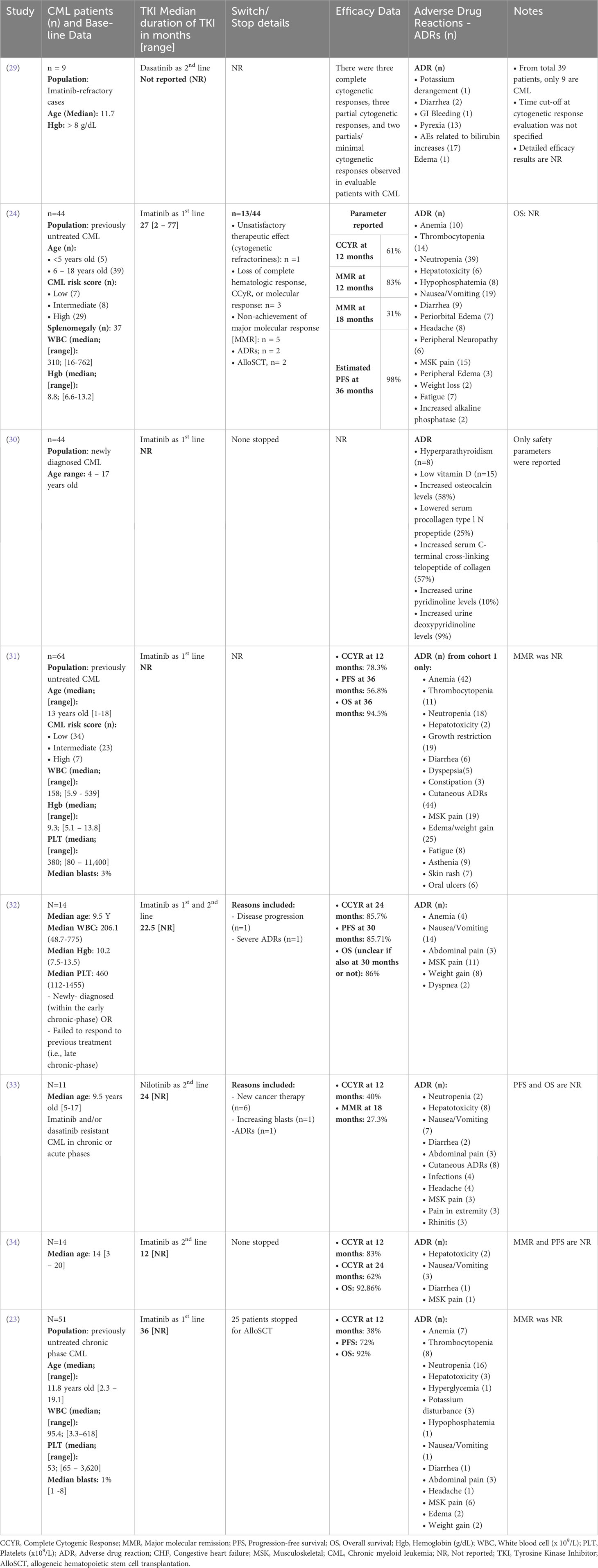
Table 1 Summary of patient demographics, Tyrosine Kinase Inhibitor (TKI) details, safety, and efficacy data from the included clinical trials with single cohorts.
Choosing initial treatment and efficacy
Tyrosine kinase inhibitors (TKIs) have revolutionized the management of CML pediatrics. Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) was considered the standard of care in pediatrics, with overall survival ranging between 60 and 80% (35). However, the approval of imatinib and second- generation TKIs dasatinib and nilotinib in pediatric CML expanded the therapeutic options for treatment in this population.
Among the included trials, TKIs were used as first-line treatment in 65% of the studies, with Imatinib being the most commonly used drug (as monotherapy in 50% of studies). Dasatinib and nilotinib were used in 17% and 11% of studies, respectively, while a small percentage of patients (6%) received a combination of Imatinib and chemotherapy. Reporting of efficacy outcomes in the studies was a major concern that challenged the accumulation of results, as shown in Table 1; Supplementary Table 1. In the studies that reported a BCR::ABL response of <10% (n= 4 studies), the percentage of patients ranged between 60% and 78%, while for complete cytogenetic response (CCyR) at 24 months (n = 3 studies), the percentage of patients ranged from 62% to 94%. Progression free survival (PFS; n= 10 studies) ranged from 56.8% to 100%; however, the timepoint for PFS analysis was not standardized among studies (ranging from 36 to 48 months).
Despite the approval of dasatinib and nilotinib, Imatinib remains the most studied TKI in pediatrics, leading to it being the preferred medication for first line treatment of CML in pediatric patients (35). Furthermore, a retrospective cohort study in pediatric patients (median age of 10.5 years) compared the outcomes in patients treated with HSCT prior to the introduction of Imatinib and TKIs Vs. after 2002. Post-HSCT, OS was 64% at 6 years Vs. 90% at 3 years with TKIs (statistically significant with p-value of 0.008) (36). Moreover, the results reported in the eligible trials included were comparable to results from adult studied. The 7-year-follow-up IRIS study reported 83% CCyR, 93% PFS and 88% OS among adult CML patients receiving Imatinib (37).
Safety of TKIs in pediatric CML
Lifelong TKIs administration and the associated toxicities in pediatrics remain a concern. The available data for safety included adverse events (AEs) experienced by patients, which were reported for 851 patients undergoing treatment (n(safety studies) = 15). The most common AEs were anemia (n(patients) = 225), thrombocytopenia (n(patients) = 159), and neutropenia (n(patients) = 254). Other common adverse events include hepatotoxicity (n(patients) = 77) and cutaneous side effects (n(patients) = 149).
The safety profiles of dasatinib and nilotinib were consistent with those reported in adults, except that no cases of peripheral arterial occlusive disease, ischemic heart or ischemic cerebrovascular disease, cardiac arrhythmias, pleural effusion, pulmonary arterial hypertension, pneumonitis, gonadal dysfunction, pancreatitis, loss of visual acuity, or conjunctival hemorrhage were reported. With dasatinib, the concerns for pleuro-pulmonary toxicities seen in adults are quite rare in pediatrics, with most of the few cases reported being grade 2 or lower. Some cardiovascular complications were observed with second-generation TKIs, such as QTc prolongation with nilotinib (n(patients) = 8) and congestive heart failure with dasatinib (n(patients) = 4). Gastrointestinal side effects were reported in all TKIs but occurred more frequently in imatinib patients. Nausea and vomiting were reported in 126 patients receiving Imatinib, versus 54 and 27 patients receiving dasatinib and nilotinib, respectively. Diarrhea occurred in 40 patients on Imatinib compared with 20 and 2 patients in the dasatinib and nilotinib cohorts, respectively.
Other AEs included, impaired bone and growth development reported with Imatinib (n(patients) = 70) and dasatinib (n(patients) = 5). Similar incidences of headache were reported with Imatinib (n(patients) = 36) and nilotinib (n(patients) = 31) compared with dasatinib (n(patients) = 13). A higher incidence of musculoskeletal pain was observed in imatinib patients (n(patients) = 155), followed by dasatinib (n(patients) = 19), and least with nilotinib (n(patients) = 3). Treatment AEs leading to drug discontinuation were reported in 22 patients from 6 studies, however, the exact AE leading to drug discontinuation was not reported in the studies. As the maximum follow-up period in pediatric studies thus far remains relatively short; further investigation into the chronic effects of TKIs in children is imperative before drawing definitive conclusions.
One of the challenges that remain is the assessment of long-term safety of TKI use in children with CML. As evident from data on adult CML and TKI use, some toxicities might appear early on in the course of treatment and improve over time, whereas many toxicities may appear after years and be of more clinical significance. This include but are not limited to pleural effusions and vascular events (38). Growth restriction of deceleration represent a critical area of concern for potential long-term adverse effects in pediatric CML, a phenomenon less likely to manifest in adult populations. Future longitudinal research explicitly examining the long-term safety of Tyrosine Kinase Inhibitors (TKIs) in pediatric CML populations would be invaluable. Such research is increasingly imperative, given the escalating utilization of TKIs in treating pediatric CML.
Discontinuation of TKIs in pediatrics
Discontinuation of TKI therapy in adults has been well studied, but TKI discontinuation in pediatric CML still relies on experience in adults (22). Understanding the mechanism, efficacy rates, pharmacokinetics and pharmacodynamics, short-term and long-term complications, and treatment-free remissions with TKI therapy in the pediatric CML population is essential. From a preliminary data of 102 pediatric CML patients, JT Tauer et al. identified growth retardation as a significant adverse effect associated with TKI therapy. These patients received Imatinib as an upfront therapy for a median duration of 9 months (39). The impact of TKIs on bone growth and cellular functional capacity is anticipated to be more pronounced in pediatric patients, attributed to their ongoing growth and development. This underscores the urgent need for more extensive data to thoroughly assess the long-term safety of TKIs. Such data is vital for a comprehensive evaluation of the risk-benefit profile of TKIs, especially considering these age-specific adverse effect which may guide the optimal duration of therapy (39).
Data from the International Registry of Childhood Chronic Myeloid Leukemia provides information on imatinib discontinuation in a cohort of patients under 18 years old (40). The findings of 18 patients who had sustained deep molecular response (DMR) followed by imatinib discontinuation were reported. After discontinuation, the molecular-free remission rate was 61%, 56%, and 56% at 6, 12, and 36 months, respectively. The results of 22 pediatric patients with CML were reported by Shima et al. The treatment-free remission (TFR) rate at 12 months was 50.0% (90% confidence interval: 31.7%–65.8%). However, 11 patients experienced loss of MMR within four months after TKI discontinuation and resumed TKI as originally prescribed (41). Moreover, the STOP IMAPED study reported the results of four patients who maintained DMR at 24 and 36 months after stopping TKI, whereas ten patients relapsed (42).
In a recent retrospective cohort study by Satishkumar et al., it was shown that among 11 pediatric patients who were treated with Imatinib for five years and sustained molecular response for at least two years, 8 (72%) successfully retained TFR (43). While the other three patients had disease recurrence, they achieved MMR upon restarting Imatinib. Given the chronic nature of CML and the potential impact that ongoing treatment may have on pediatrics’ quality of life, this is encouraging. The mentioned study, however, might be the only one offering insight into TFR in pediatric CML patients.
Challenges and literature gaps
There were several challenges that hindered accumulating pediatric data within a study. One of the major challenges was heterogeneity, both in population characteristics and outcome definitions. Furthermore, many studies explored the pharmacokinetics of TKIs, which, although essential, did not provide accurate results on their efficacy and long-term safety in pediatrics. A consensus on clinically significant outcome reporting in pediatric CML research, including TFR, may be useful in reducing these challenges for future systematic reviews and meta-analysis.
Conclusions
There is limited experience with CML pediatrics due to the low incidence of CML in this population. Therefore, to establish standardized guidelines for the therapeutic management of this population, information from prospective clinical trials and real-life clinical practice is needed. Compared to the other TKIs, there is more experience with imatinib efficacy and toxicity profile in pediatric patients. The safety profile of TKIs was consistent with the known safety profile in adults. With the availability of three TKIs as first line options, other factors should be considered when selecting a first- line TKI, including drug formulation, administration, comorbidities, and financial issues. Careful monitoring of adverse events, especially in growing children, should be considered with long- term follow-up clinical trials.
Author contributions
FA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MB: Data curation, Formal analysis, Writing – original draft. RG: Data curation, Formal analysis, Writing – original draft. LF: Data curation, Validation, Writing – review & editing. NO: Data curation, Writing – original draft. AN: Data curation, Writing – original draft. MA: Data curation, Writing – original draft. KM: Data curation, Writing – original draft. NK: Data curation, Writing – original draft. MY: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Financial support was received only for publication of the manuscript. The publication of this article was funded by the Qatar National Library.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
FA is a review editor for Frontiers in Medicine and Frontiers in Public Health for Clinical Diabetes.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
This manuscript is original work and has not been submitted or is not under consideration for publication elsewhere. All the authors have reviewed the manuscript and approved it before submission. None of the authors has any conflict of interest in publishing this work.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1285346/full#supplementary-material
References
1. Goldman JM, Melo JV. Targeting the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med (2001) 344:1084–6. doi: 10.1056/NEJM200104053441409
2. Verfaillie CM. Biology of chronic myelogenous leukemia. Hematol Oncol Clin North Am (1998) 12:1–29. doi: 10.1016/S0889-8588(05)70495-0
3. Siegel RL, Miller KD, Jemal A. Cancer statistic. CA Cancer J Clin (2017) 67:7–30. doi: 10.3322/caac.21387
4. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol (2018) 93:442–59. doi: 10.1002/ajh.25011
5. Hijiya N, Schultz KR, Metzler M, Millot F, Suttorp M. Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood (2016) 127:392–9. doi: 10.1182/blood-2015-06-648667
6. Hijiya N, Suttorp M. How I treat chronic myeloid leukemia in children and adolescents. Blood (2019) 133:2374–84. doi: 10.1182/blood.2018882233
7. Craddock CF. We do still transplant CML, don’t we? Hematol Am Soc Hematol Educ Program (2018) 2018:177–84. doi: 10.1182/asheducation-2018.1.177
8. Bernstein SH, Nademanee AP, Vose JM, Tricot G, Fay JW, Negrin RS, et al. A multicenter study of platelet recovery and utilization in patients after myeloablative therapy and hematopoietic stem cell transplantation. Blood (1998) 91:3509–17. doi: 10.1182/blood.V91.9.3509
9. Majhail NS. Long-term complications after hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther (2017) 10:220–7. doi: 10.1016/j.hemonc.2017.05.009
10. Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med (2001) 344:1038–42. doi: 10.1056/NEJM200104053441402
11. Radich J. Stem cell transplant for chronic myeloid leukemia in the imatinib era. Semin Hematol (2010) 47:354–61. doi: 10.1053/j.seminhematol.2010.06.008
12. Turkina A, Wang J, Mathews V, Saydam G, Jung CW, Al Hashmi HH, et al. TARGET: a survey of real-world management of chronic myeloid leukaemia across 33 countries. Br J Haematol (2020) 190:869–76. doi: 10.1111/bjh.16599
13. Yassin MA, Ghasoub RS, Aldapt MB, Abdulla MA, Chandra P, Shwaylia HM, et al. Effects of intermittent fasting on response to tyrosine kinase inhibitors (TKIs) in patients with chronic myeloid leukemia: an outcome of European leukemiaNet project. Cancer Control (2021) 28:10732748211009256. doi: 10.1177/10732748211009256
14. Kaddoura R, Dabdoob WA, Ahmed K, Yassin MA. A practical guide to managing cardiopulmonary toxicities of tyrosine kinase inhibitors in chronic myeloid leukemia. Front Med (Lausanne) (2023) 10:1163137. doi: 10.3389/fmed.2023.1163137
15. Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med (1996) 2:561–6. doi: 10.1038/nm0596-561
16. Kantarjian H, Talpaz M, O’brien S, Giles F, Rios MB, White K, et al. Prediction of initial cytogenetic response for subsequent major and complete cytogenetic response to imatinib mesylate therapy in patients with Philadelphia chromosome-positive chronic myelogenous leukemia. Cancer (2003) 97:2225–8. doi: 10.1002/cncr.11381
17. Shah NP. Medical management of CML. Hematol Am Soc Hematol Educ Program (2007) 2007(1), 371–5. doi: 10.1182/asheducation-2007.1.371
18. Krumbholz M, Karl M, Tauer JT, Thiede C, Rascher W, Suttorp M, et al. Genomic BCR-ABL1 breakpoints in pediatric chronic myeloid leukemia. Genes Chromosomes Cancer (2012) 51:1045–53. doi: 10.1002/gcc.21989
19. Kalmanti L, Saussele S, Lauseker M, Proetel U, Muller MC, Hanfstein B, et al. Younger patients with chronic myeloid leukemia do well in spite of poor prognostic indicators: results from the randomized CML study IV. Ann Hematol (2014) 93:71–80. doi: 10.1007/s00277-013-1937-4
20. Sembill S, Ampatzidou M, Chaudhury S, Dworzak M, Kalwak K, Karow A, et al. Management of children and adolescents with chronic myeloid leukemia in blast phase: International pediatric CML expert panel recommendations. Leukemia (2023) 37:505–17. doi: 10.1038/s41375-023-01822-2
21. Chae H-D, Murphy LC, Donato M, Lee AG, Sweet-Cordero EA, Abidi P, et al. Comparison of the transcriptomic signature of pediatric vs. Adult CML and normal bone marrow stem cells. Blood (2018) 132:4246. doi: 10.1182/blood-2018-99-119974
22. Mahon FX. Treatment-free remission in CML: who, how, and why? Hematol Am Soc Hematol Educ Program (2017) 2017:102–9. doi: 10.1182/asheducation.V2017.1.102.00014
23. Champagne MA, Fu CH, Chang M, Chen H, Gerbing RB, Alonzo TA, et al. Higher dose imatinib for children with de novo chronic phase chronic myelogenous leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer (2011) 57:56–62. doi: 10.1002/pbc.23031
24. Millot F, Baruchel A, Guilhot J, Petit A, Leblanc T, Bertrand Y, et al. Imatinib is effective in children with previously untreated chronic myelogenous leukemia in early chronic phase: results of the French national phase IV trial. J Clin Oncol (2011) 29:2827–32. doi: 10.1200/JCO.2010.32.7114
25. Suttorp M, Schulze P, Glauche I, Göhring G, Von Neuhoff N, Metzler M, et al. Front-line imatinib treatment in children and adolescents with chronic myeloid leukemia: results from a phase III trial. Leukemia (2018) 32:1657–69. doi: 10.1038/s41375-018-0179-9
26. Gore L, Kearns PR, De Martino ML, Lee, De Souza CA, Bertrand Y, Hijiya N, et al. Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol (2018) 36:1330–8. doi: 10.1200/JCO.2017.75.9597
27. Hijiya N, Maschan A, Rizzari C, Shimada H, Dufour C, Goto H, et al. Phase 2 study of nilotinib in pediatric patients with Philadelphia chromosome–positive chronic myeloid leukemia. Blood (2019) 134:2036–45. doi: 10.1182/blood.2019000069
28. Hijiya N, Maschan A, Rizzari C, Shimada H, Dufour C, Goto H, et al. Phase 2 study of nilotinib in pediatric patients with Philadelphia chromosome-positive chronic myeloid leukemia. Blood (2019) 134:2036–45. doi: 10.1182/blood.2019000069
29. Aplenc R, Blaney SM, Strauss LC, Balis FM, Shusterman S, Ingle AM, et al. Pediatric phase I trial and pharmacokinetic study of dasatinib: a report from the children’s oncology group phase I consortium. J Clin Oncol (2011) 29:839–44. doi: 10.1200/JCO.2010.30.7231
30. Jaeger BA, Tauer JT, Ulmer A, Kuhlisch E, Roth HJ, Suttorp M. Changes in bone metabolic parameters in children with chronic myeloid leukemia on imatinib treatment. Med Sci Monit (2012) 18:Cr721–728. doi: 10.12659/MSM.883599
31. Linga VG, Ganta RR, Kalpathi KI, Gundeti S, Rajappa SJ, Digumarti R, et al. Response to imatinib mesylate in childhood chronic myeloid leukemia in chronic phase. South Asian J Cancer (2014) 3:203–5. doi: 10.4103/2278-330X.142961
32. Gholamreza B, Mardavizh A, Parvaneh V. Imatinib mesylate (Glivec) in pediatric chronic myelogenous leukemia. Int J Hematology-Oncol Stem Cell Res (1970) 3:8–13.
33. Hijiya N, Zwaan CM, Rizzari C, Foà R, Abbink F, Lancaster D, et al. Pharmacokinetics of nilotinib in pediatric patients with philadelphia chromosome-positive chronic myeloid leukemia or acute lymphoblastic leukemia. Clin Cancer Res (2020) 26:812–20. doi: 10.1158/1078-0432.CCR-19-0090
34. Champagne MA, Capdeville R, Krailo M, Qu W, Peng B, Rosamilia M, et al. Imatinib mesylate (STI571) for treatment of children with Philadelphia chromosome-positive leukemia: results from a Children’s Oncology Group phase 1 study. Blood (2004) 104:2655–60. doi: 10.1182/blood-2003-09-3032
35. De La Fuente J, Baruchel A, Biondi A, De Bont E, Dresse M-F, Suttorp M, et al. Managing children with chronic myeloid leukaemia (CML). Br J Haematol (2014) 167:33–47. doi: 10.1111/bjh.12977
36. Egan G, Athale U, Johnston D, Pole JD, Silva M, Zorzi A, et al. Outcomes of children with chronic myeloid leukemia: A population-based cohort study. Pediatr Blood Cancer (2020) 67:e28491. doi: 10.1002/pbc.28491
37. Hochhaus A, O’brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia (2009) 23:1054–61. doi: 10.1038/leu.2009.38
38. Liu H-C, Kuo M-C, Wu K-H, Chen T-Y, Chen J-S, Wang M-C, et al. Children with chronic myeloid leukaemia treated with front-line imatinib have a slower molecular response and comparable survival compared with adults: a multicenter experience in Taiwan. Br J Cancer (2023) 128:1294–300. doi: 10.1038/s41416-023-02162-9
39. Tauer JT, Nowasz C, Sedlacek P, De Bont ESJM, Aleinikova OV, Suttorp M, et al. Impairment of longitudinal growth by tyrosine kinase inhibitor (TKI) treatment - data from a large pediatric cohort with chronic myeloid leukemia (CML). Blood (2014) 124:522–2. doi: 10.1182/blood.V124.21.522.522
40. Millot F, Suttorp M, Ragot S, Leverger G, Dalle JH, Thomas C, et al. Discontinuation of imatinib in children with chronic myeloid leukemia: A study from the international registry of childhood CML. Cancers (Basel) (2021) 13. doi: 10.3390/cancers13164102
41. Shima H, Kada A, Tanizawa A, Sato I, Tono C, Ito M, et al. Discontinuation of tyrosine kinase inhibitors in pediatric chronic myeloid leukemia. Pediatr Blood Cancer (2022) 69:e29699. doi: 10.1002/pbc.29699
42. De Bruijn CMA, Millot F, Suttorp M, Borisevich M, Brons P, Lausen B, et al. Discontinuation of imatinib in children with chronic myeloid leukaemia in sustained deep molecular remission: results of the STOP IMAPED study. Br J Haematol (2019) 185:718–24. doi: 10.1111/bjh.15826
Keywords: Tyrosine Kinase Inhibitors, TKI, pediatric chronic myeloid leukemia, CML, Imatinib
Citation: Ata F, Benkhadra M, Ghasoub R, Fernyhough LJ, Omar NE, Nashwan AJ, Aldapt MB, Mushtaq K, Kassem NA and Yassin MA (2023) Tyrosine Kinase Inhibitors in pediatric chronic myeloid leukemia: a focused review of clinical trials. Front. Oncol. 13:1285346. doi: 10.3389/fonc.2023.1285346
Received: 29 August 2023; Accepted: 29 November 2023;
Published: 20 December 2023.
Edited by:
Gianantonio Rosti, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Fabio Stagno, University Hospital Polyclinic Vittorio Emanuele, ItalyConcetta Micalizzi, Giannina Gaslini Institute (IRCCS), Italy
Szymon Skoczen, Jagiellonian University Medical College, Poland
Copyright © 2023 Ata, Benkhadra, Ghasoub, Fernyhough, Omar, Nashwan, Aldapt, Mushtaq, Kassem and Yassin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fateen Ata, ZG9jZmF0ZWVuYXRhQGdtYWlsLmNvbQ==; RkF0YUBoYW1hZC5xYQ==
†These authors have contributed equally to this work
‡ORCID: Fateen Ata, orcid.org/0000-0001-7121-8574
Maria Benkhadra, orcid.org/0000-0002-2382-3021
Rola Ghasoub, orcid.org/0000-0002-1392-8831
Liam J. Fernyhough, orcid.org/0000-0002-5505-2380
Nabil E. Omar, orcid.org/0000-0001-8291-7987
Abdulqadir J. Nashwan, orcid.org/0000-0003-4845-4119
Kamran Mushtaq, orcid.org/0000-0002-1787-640X
Mohamed A. Yassin, orcid.org/0000-0002-1144-8076
 Fateen Ata
Fateen Ata Maria Benkhadra
Maria Benkhadra Rola Ghasoub
Rola Ghasoub Liam J. Fernyhough
Liam J. Fernyhough Nabil E. Omar
Nabil E. Omar Abdulqadir J. Nashwan
Abdulqadir J. Nashwan Mahmood B. Aldapt
Mahmood B. Aldapt Kamran Mushtaq7‡
Kamran Mushtaq7‡ Mohamed A. Yassin
Mohamed A. Yassin