- 1Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Hepatobiliary and Pancreatic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital and Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
- 3Department of Medical Oncology, Beijing Chaoyang District Sanhuan Cancer Hospital, Beijing, China
- 4Department of Hepatobiliary Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 5Department of Pancreatic and Gastric Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Purpose: To explore the efficacy and safety of FOLFOXIRI plus cetuximab regimen as conversion therapy for patients with unresectable RAS/BRAF wild-type colorectal liver-limited metastases (CLM).
Patients and methods: This was a dual-center, phase II trial with the rate of no evidence of disease (NED) achieved as the primary endpoint. All enrolled patients with initially unresectable left-sided RAS/BRAF wild-type colorectal liver-limited metastases received a modified FOLFOXIRI plus cetuximab regimen as conversion therapy.
Results: Between October 2019 and October 2021, fifteen patients were enrolled. Nine patients (60%) achieved NED. The overall response rate (ORR) was 92.9%, and the disease control rate (DCR) was 100%. The median relapse‐free survival (RFS) was 9 (95% CI: 0–20.7) months. The median progression-free survival (PFS) was 13.0 months (95% CI: 5.7-20.5), and the median overall survival (OS) was not reached. The most frequently occurring grade 3-4 adverse events were neutropenia (20%), peripheral neurotoxicity (13.3%), diarrhea (6.7%), and rash acneiform (6.7%).
Conclusion: The FOLFOXIRI plus cetuximab regimen displayed tolerable toxicity and promising anti-tumor activity in terms of the rate of NED achieved and response rate in patients with initially unresectable left-sided RAS/BRAF wild-type CLM. This regimen merits further investigation.
1 Introduction
Colorectal cancer (CRC) remains a major health problem, ranking second in cancer-related deaths worldwide (1). Approximately 25% of CRC patients present with liver metastases at the time of diagnosis, and half of patients who received radical resection of CRC might subsequently develop recurrence in the liver (2). Notably, patients with colorectal liver-limited metastases (CLM) are considered a specific subgroup of metastatic colorectal cancer (mCRC), as they could benefit from a curative strategy, which offers a superior 5-year survival rate than palliative chemotherapy (3–6).
The management of CLM patients is a great challenge for oncologists, as about 80% of them are initially unresectable because of the number, size, or location of liver metastases (3). However, because of the optimal integration of systemic and locoregional treatments, including surgery, thermal ablation, stereotactic ablative body radiotherapy, and embolization techniques, an increasing number of patients with CLM achieve tumor downstaging and complete tumor removal with no evidence of disease (NED) after conversion therapy.
Recently, guidelines recommended an intensified regimen as the preferable option for patients with initially unresectable or borderline resectable CLM to induce earlier and deeper tumor shrinkage (7, 8). The standard conversion therapy regimens for CLM include doublet regimens plus an anti-EGFR monoclonal antibody for left-sided RAS wild-type patients and the triplet FOLFOXIRI (fluorouracil, leucovorin, oxaliplatin, and irinotecan) plus bevacizumab for right-sided or RAS/BRAF-mutated subgroup (9–12). Several clinical trials have demonstrated that the triplet chemotherapeutic regimen, consisting of fluorouracil (5-FU)/leucovorin, irinotecan, and oxaliplatin, could bring higher response rate and resection rate for patients with mCRC compared to the doublet regimens (FOLFOX or FOLFIRI) (13–15). Besides, for left-sided RAS/BRAF wild-type mCRC, incorporating anti-EGFR agents (e.g., cetuximab) into chemotherapy regimens can enhance efficacy. Therefore, the combination of triplet chemotherapy with an anti-EGFR agent might be a feasible conversion therapy for patients with unresectable left-sided RAS/BRAF wild-type mCRC.
At the time of designing our study, it is generally acknowledged by most scholars that a higher response rate is associated with a higher conversion rate. However, due to the toxicity and uncertain benefit, only a small number of patients received intensified triplet chemotherapy with an anti-EGFR agent regimen in clinical practice. Several studies evaluated the efficacy and safety of triplet chemotherapy plus anti-EGFR antibodies as conversion therapy for mCRC patients ongoing at that time, such as the TRIPLETE and TRICE study (16, 17). There is no consensus on conversion therapy in RAS/BRAF wild-type left-sided mCRC patients.
To address this gap, we conducted a dual-center, pilot phase II study to evaluate the efficacy and safety of triplet chemotherapy plus cetuximab regimen as conversion therapy for initially unresectable left-sided RAS/BRAF wild-type mCRC patients with liver-limited metastases.
2 Patients and methods
2.1 Patients
This is a nonrandomized pilot study conducted at two centers in China, including Cancer Hospital Chinese Academy of Medical Sciences and Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
Main inclusion criteria were as follows: age between 18 and 65 years old; ECOG performance status score of 0 to 1; histologically confirmed adenocarcinoma of the left-sided colon or rectum; liver metastasis confirmed with imaging and/or histopathological examinations; liver metastasis was considered initially unresectable by multidisciplinary team (MDT), RAS and BRAF wild-type; no prior treatment for colorectal cancer and liver metastasis; imaging confirmed without other organ metastases besides liver; and adequate bone marrow, hepatic, and renal function. The main exclusion criteria were any extrahepatic metastatic disease and recurrence of primary tumors. The definition of unresectable CLM is the patients who could not achieve NED status through optimal integration of locoregional treatments, including surgery, thermal ablation, stereotactic ablative body radiotherapy, and embolization techniques, and the remnant liver volume under 30%.
The study was done in accordance with the Declaration of Helsinki, and the protocol was approved by the local ethics committees of participating sites. All patients provided their written informed consent before enrollment.
2.2 Treatments
Patients received cetuximab (500 mg/m2 on day 1) plus modified FOLFOXIRI (irinotecan 150 mg/m2, oxaliplatin 85 mg/m2, and a continuous infusion of 5-FU at a dose of 2400 mg/m2 over 46 hours). Treatment was repeated every two weeks until disease progression or unacceptable toxicity or resectability or up to a maximum of 12 cycles. Dose reductions were allowed for severe drug-associated toxicities (≥ grade 3 non-hematological or grade 4 hematological toxicity). The efficacy was evaluated every three cycles, and the resectability was also evaluated at the same time. Patients who had lesions that were radically resectable after evaluation will receive surgery. After radical resection of metastases, continuing chemotherapy as adjuvant treatment was recommended for a total of 12 perioperative cycles. However, according to the postoperative physical conditions of each patient, the maintenance regimen could be administered after surgery. For patients not achieving NED after 12 cycles of induction therapy, maintenance treatment with 5-FU and cetuximab was continued until disease progression.
2.3 Study endpoints
The primary endpoint was the rate of NED achieved. Secondary endpoints were objective response rate (ORR), disease control rate (DCR), early tumor shrinkage (ETS), depth of response (DpR), relapse‐free survival (RFS), progression-free survival (PFS), overall survival (OS), and safety.
The rate of NED achieved was defined as the proportion of patients achieving R0 resection, complete remission, or macroscopically complete ablation of all tumor lesions. ORR was defined as the proportion of patients with complete response (CR) and partial response (PR) according to RECIST version 1.1, and DCR was defined as the percentage of patients experiencing CR, PR, and stable disease (SD). ETS was defined as at least a 20% decrease in the sum of the longest diameters of the RECIST target lesions at first reassessment compared to baseline, while DpR was defined as the maximum percentage of tumor shrinkage based on the sum of the longest diameters of target lesions according to RECIST v1.1 at the lowest point compared to baseline values. RFS was defined as the period from NED achieved to recurrence. PFS was defined as the time from the date of the first administration of this regimen to the date of the first documented disease progression or death due to any cause. OS was defined as the time from the date of beginning receiving this regimen to the date of death resulting from any cause.
2.4 Efficacy and safety assessments
The initial imaging was conducted within 21 days before treatment started. Tumor response was assessed every three cycles by chest and abdominopelvic computed tomography (CT) or magnetic resonance imaging (MRI) of the liver according to Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1). Experienced radiologists carried out the response evaluation. The radiologists were not involved in the study’s conduct. The resectablity was evaluated by MDT.
The surgery encompasses radical resection of primary colorectal cancer with or without concurrent removal of metastatic tumors. Complete thermal ablation was allowed for liver metastases. Surgery or thermal ablation was performed with curative intent. According to the surgical margins, surgical resection was classified as complete resection (R0), microscopic residual tumor (R1), and macroscopic residual tumor (R2). R0 resection denotes the absence of cancer cells at the margin under microscopic examination. R1 resection signifies the removal of all visible lesions but detecting cancer cells at the margin under microscopic evaluation. R2 resection refers to visible tumor tissue remaining at the margin.
Adverse events (AEs) were graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Patients receiving at least one cycle of treatment underwent safety evaluation.
2.5 Gene mutation detection
Tumor tissue specimens from the primary tumor or metastases were used to detect KRAS, NRAS, and BRAF mutations by next-generation sequencing.
2.6 Statistical analysis
The study was designed as an exploratory, pilot study. Approximately 20 patients were planned for enrollment. The ORR, DCR, ETS, and DpR analysis was performed on all patients experiencing at least one reexamination. AEs were assessed in patients who received at least one cycle of treatment. The median follow-up period with the 95% CI was calculated by the reverse Kaplan-Meier method. Survival endpoints, PFS, and OS were analyzed by the Kaplan-Meier method, expressed as medians, and compared with the log-rank test and Cox regression (with hazard ratios [HRs] and 95% CIs indicated). Moreover, the Kaplan–Meier method was used to evaluate survival endpoints (i.e., PFS and OS) and establish the survival curves. The log-rank test assessed any significant differences in PFS stratified by whether achieving NED. A two-sided p-value < 0.05 was considered statistically significant. All statistical analyses were performed using software SPSS version 29.0 (IBM, Armonk, NY, USA).
3 Results
3.1 Patient characteristics
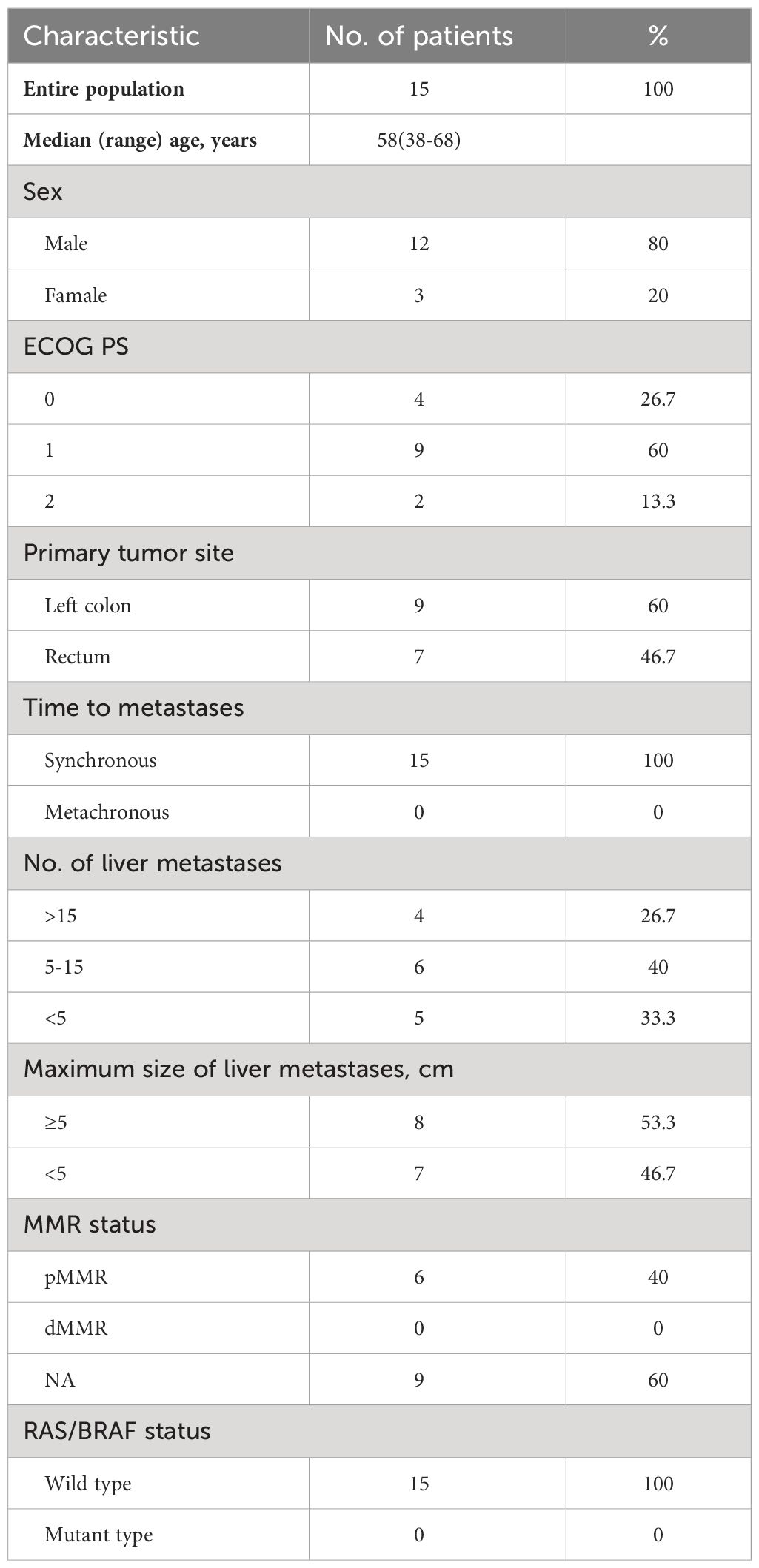
The cutoff date for the analysis was Oct 31, 2023. In all, 15 patients were enrolled between October 2019 to October 2021. The patients’ demographic and clinicopathological characteristics at baseline are summarized in Table 1. The median age was 58 (38–68), and most patients were male (80%). Four (26.7%) patients had an ECOG PS of 0, and 9 (60%) had an ECOG PS of 1. All mCRC patients presented with synchronous liver metastases and had a left-sided primary tumor. The primary tumor location was on the left-sided colon in 9 and on the rectum in 7 patients (one patient had two primary tumors). Ten patients had five or more liver metastases, and eight patients had a maximum size of liver metastasis ≥5cm. All patients were evaluated for RAS/BRAF mutation status and had RAS/BRAF wild-type tumors. The MMR status of 6 patients was pMMR, and the other nine patients were unknown. The median number of cycles of FOLFOXIRI plus cetuximab regimen administered was four (range, 1–10). Nine of 15 patients experienced primary resection in this study.
3.2 Response to the treatment
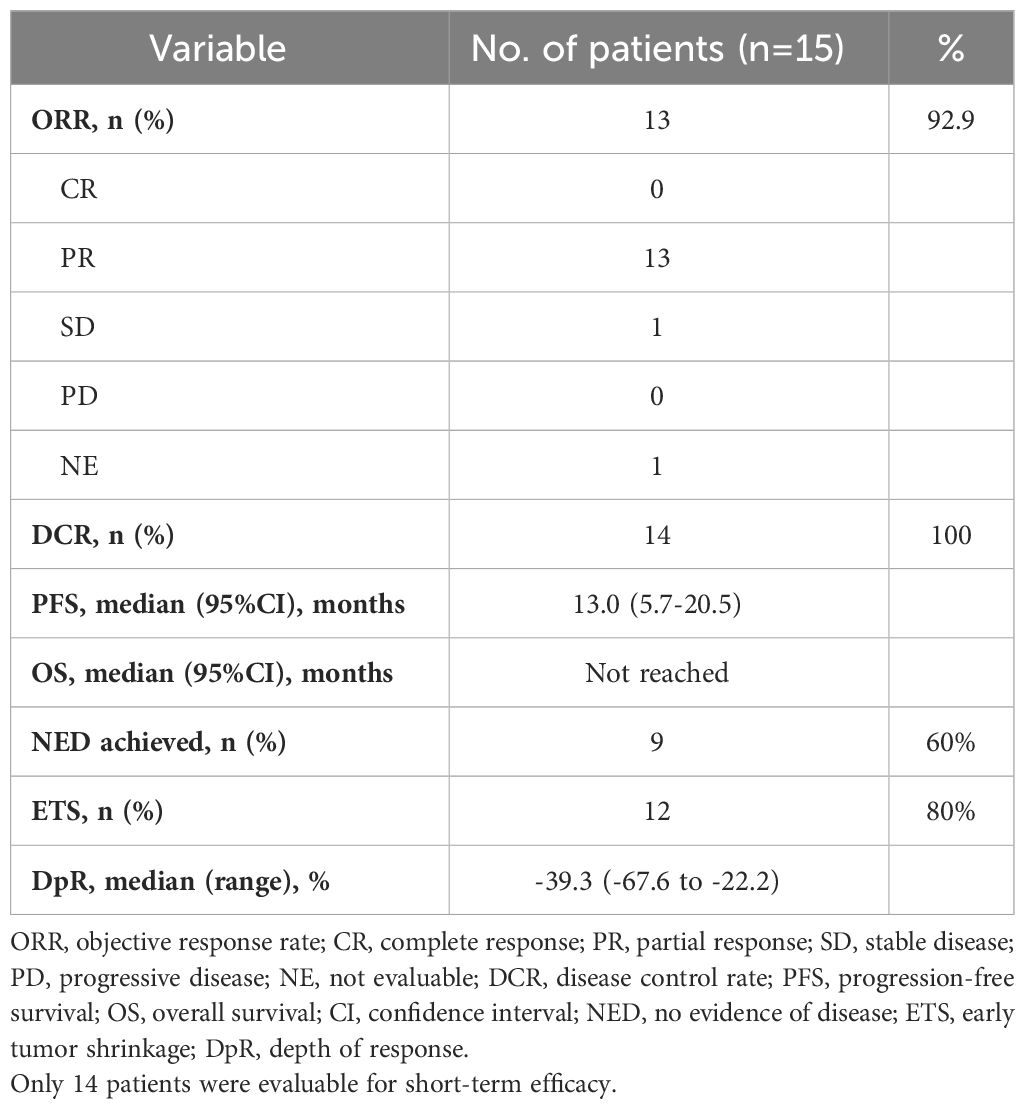
Of the 15 enrolled patients, 14 were evaluated for response and resectability. One patient could not be evaluated for the response and was excluded from the efficacy analysis because he lacked assessment of tumor response after the third cycle due to COVID-19. The median number of cycles before surgery was 4.5 (range 1-10). PR was achieved in 13 patients, and one obtained an SD. The ORR was 92.9%, and the DCR was 100%.
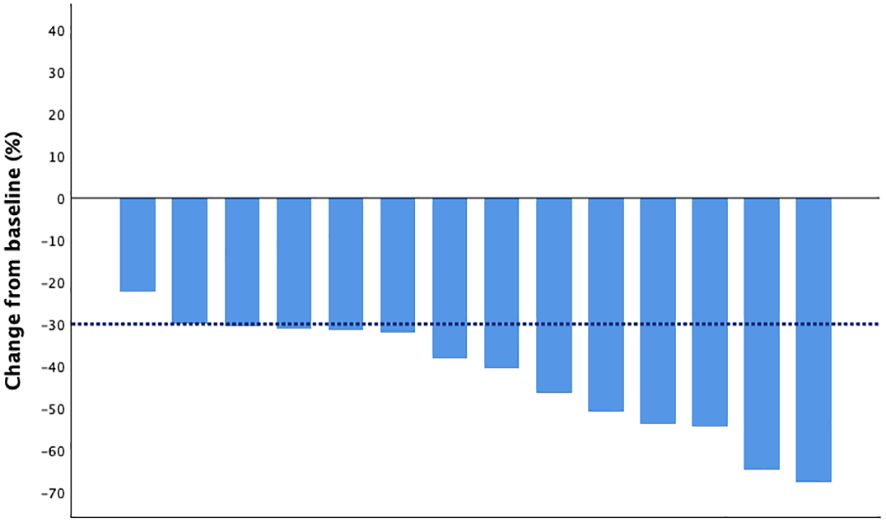
The DpR and ETS were assessed in 14 patients. The median DpR was -39.3% (range from -67.6% to -22.2%), and 12 (80%) patients achieved ETS. The waterfall plot of the depth of response is shown in Figure 1, and the indices of efficacy outcomes are listed in Table 2.
3.3 Conversion therapy
Out of 15 patients, nine (60%) achieved NED status. Seven patients achieved concurrent or staged resection of primary and metastatic lesions, and two experienced resection of primary tumor combined with thermal ablation of liver metastases.
After a median follow-up of 30 months, all these nine patients had recurrence. The median RFS was 9 (95% CI: 0–20.7) months. Eight out of 9 patients (88.9%) had disease recurrence in the liver, and only one had recurrence outside the liver (the rectal fascia). At the analysis date, eight out of the nine patients who achieved NED were alive.
3.4 PFS and OS
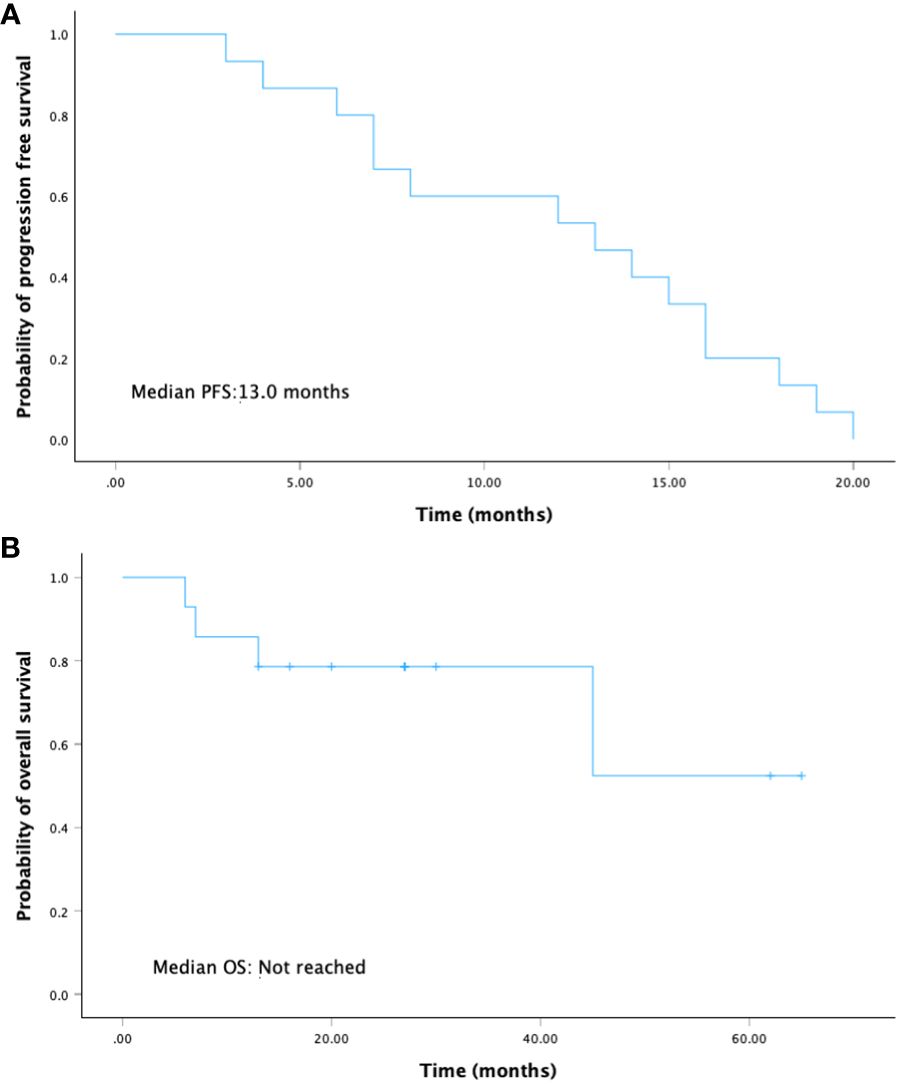
With a median follow-up of 30 months (range 13-65), there were 10 out of 15 (66.7%) patients alive and 5 out of 15 (33.3%) patients who died because of the disease progression. The median PFS was 13.0 months (95% CI: 5.7-20.5), and the median OS was not reached (Figure 2).
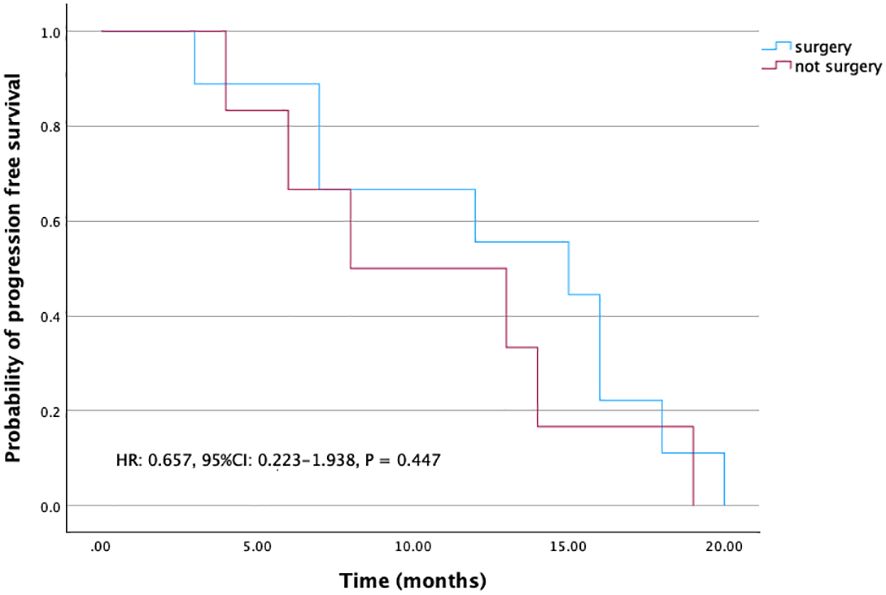
For those patients who achieved NED, PFS was 15 months (95% CI: 6.2-23.8), whereas PFS was eight months (95% CI: 0-16.4) for those patients who were not resected (Figure 3). Though this difference is not significant, patients who achieved NED tended to experience longer PFS.
3.5 Treatment toxicity
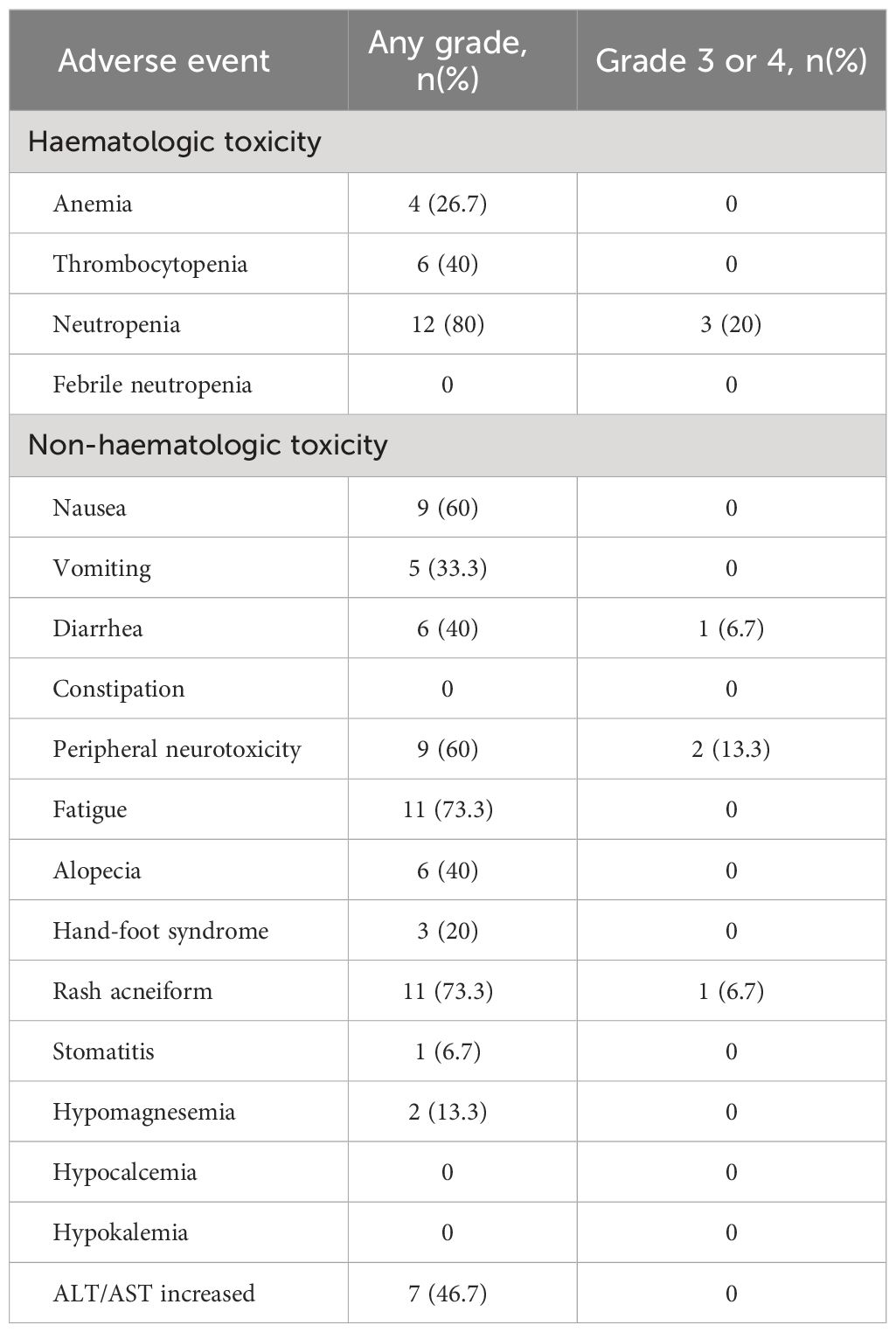
The treatment-relevant adverse events (AEs) reported during the treatment period are presented in Table 3. All 15 patients were evaluated for safety. Neutropenia (80%), fatigue (73.3%), and rash acneiform (73.3%) were the most common AEs observed in these patients, followed by nausea (60%) and peripheral neurotoxicity (60%). The most frequently occurring grade 3 or 4 AEs were neutropenia (3 patients, 20%), peripheral neurotoxicity (2 patients, 13.3%), diarrhea (1 patient, 6.7%), and rash acneiform (1 patient, 6.7%). After dose reduction, there was a significant reduction in the proportion of patients who experienced severe AEs. There was no treatment-related death in all patients.
4 Discussion
The liver is the most frequently involved organ in mCRC (18). Patients with liver-limited metastases from colorectal cancer represent a particular subgroup in which patients with surgically resectable metastatic lesions can be treated with potentially curative multidisciplinary strategies and achieve higher 5-year survival rates of 28%–39% than palliative chemotherapy (3–5). The 10-year survival rate of these patients with hepatic metastases surgically removed is about 17% (19).
However, not all CRC patients with liver metastases are candidates for surgical resection initially due to functional hepatic reserve after resection, tumor location, performance status, and comorbidities. Approximately 80% of these patients are considered initially unresectable (3). In recent years, evidence has grown that preoperative chemotherapy can downsize tumors and facilitate subsequent radical resection (3, 20–22). Because of the effective systematic therapy and local treatment (e.g., surgery, thermal ablation, stereotactic ablative body radiotherapy, embolization techniques), an increasing number of CRC patients with hepatic metastases after conversion therapy have the possibility of completely removing tumors and achieving the NED (23, 24). Patients who experience NED status will have prolonged survival time.
There is an urgent need for effective conversion therapy regimens for CRC patients with liver metastases. Chemotherapeutic doublet regimens combined with anti-VEGF or anti-EGFR monoclonal antibodies represent the standard of care in untreated mCRC (25–29). As an anti-EGFR monoclonal antibody, cetuximab blocks the binding of epidermal growth factor and other ligands, inhibiting the cellular pathways involved in cell proliferation, angiogenesis, and metastasis (30). Mutations in RAS, BRAF, and PI3K, the critical signaling effectors downstream of EGFR, are associated with resistance to cetuximab (31). Multiple retrospective studies have confirmed that patients harboring RAS and BRAF mutations could not benefit from anti-EGFR therapies. The FIRE-4.5 trial is the first prospective study to verify that FOLFOXIRI plus cetuximab did not induce a higher ORR in first-line treatment of BRAF V600E-mutant mCRC when compared with FOLFOXIRI plus bevacizumab (32). Evidence suggests that combining cetuximab with doublet chemotherapy brings survival benefits for patients with RAS/BRAF wild-type mCRC (33, 34). To further intensify efficacy, a triplet regimen, FOLFOXIRI, including 5-FU, irinotecan, and oxaliplatin, was developed and yielded higher response and resection rates (2, 13). Therefore, for RAS/BRAF wild-type mCRC patients, several trials explored the FOLFOXIRI plus anti-EGFR antibody (cetuximab or panitumumab) regimen as conversion therapy and preliminarily showed its promising efficacy (35, 36).
Several studies have been conducted on anti-EGFR monoclonal antibody plus triplet chemotherapy regimen as first-line or conversion therapy for RAS/BRAF wild-type mCRC patients. In these studies, the ORR ranged from 66.7% to 95.5% (36, 37). The median PFS ranged from 9.3 months to 16.0 months (38, 39), and the median OS ranged from 24.7 months to 55 months (40, 41). In our study, the ORR was 92.9%, and the median PFS was 13.0 months, conformed to the previous studies. A triplet regimen combined with anti-EGFR monoclonal antibody is more applicable for conversion therapy in mCRC patients.
However, whether the FOLFOXIRI plus anti-EGFR antibody regimen can be used as an upfront treatment for RAS/BRAF wild-type mCRC patients remains controversial due to negative results from several clinical trials. TRIPLETE study, a prospective phase III trial, investigated the efficacy of FOLFOXIRI plus panitumumab regimen compared to FOLFOX plus panitumumab regimen in untreated patients with unresectable RAS/BRAF wild type mCRC. However, FOLFOXIRI plus panitumumab regimen did not bring additional survival benefits compared to doublet chemotherapy plus panitumumab (42). Another phase II randomized controlled trial, the TRICE study (NCT03493048), compared the efficacy and safety of cetuximab plus FOLFOXIRI regimen versus cetuximab plus FOLFOX regimen in the first-line treatment of patients with RAS wild-type initially unresectable colorectal cancer liver metastasis. From January 2018 to December 2022, 146 patients were recruited. Updated results were presented at the 2023 ESMO Congress (17). It is reported that though intensified systemic chemotherapy in RAS wild-type metastatic CRC patients offered a better DpR, there was no significant difference in ORR, PFS, and R0 resection rate with a higher incidence of grade 3-4 neutropenia and diarrhea. The unselected patient population may have contributed to the negative results. Several clinical trials are ongoing to continue exploring this regimen’s clinical value.
Conversion therapy is necessary for mCRC patients, especially for patients with CLM. CLM is heterogeneous to other mCRC, with distinct biological behaviors (43). Several studies have confirmed that the R0 resection of CLM can significantly prolong the survival time, highlighting the importance of designing optimal conversion therapy for CLM patients (44). According to a meta-analysis, the pooled R0 resection rate in CLM patients was 60%. Among all included studies, five were conducted to evaluate the R0 resection rate of CLM after triplet chemotherapy plus an anti-EGFR antibody regimen, and the R0 resection rate ranged from 60% to 84% (45). In our study, the rate of NED achieved is 60%. Patients who achieved NED had a numerically longer PFS compared with those who did not achieve NED, and their median OS did not reach. Other indices for conversion therapy, such as DpR and ETS, also reflect the rate and magnitude of tumor downsizing. We gained promising DpR and ETS from our study, which demonstrated that triplet chemotherapy plus cetuximab might become a preferable choice as conversion therapy for CLM patients.
Our final goal is to prolong overall survival, and only by converting tumor shrinkage to R0 resection can patients experience longer survival time. Besides designing optimal treatment, it is also necessary to identify the patients who may benefit most from this regimen. Considering the favorable survival outcomes in patients with left-sided mCRC tumors receiving anti-EGFR agents, it could be reasonably inferred that RAS/BRAF wild-type left-sided CLM patients may benefit most from triplet chemotherapy plus anti-EGFR agent regimen (46). The DEEPER trial also indicated that compared to mFOLFOXIRI plus bevacizumab regimen, mFOLFOXIRI plus cetuximab regimen could be an ideal option for first-line chemotherapy with higher DpR and longer PFS in left-sided mCRC patients with RAS/BRAF wild-type (47). However, in the 2023 ESMO congress, the CAIRO5 study indicated that OS was not different between adding panitumumab versus bevacizumab to FOLFOX/FOLFIRI for initially unresectable left-sided RAS/BRAF V600E wild-type CRLM. Compared with chemotherapy plus bevacizumab, there was neither no improvement in PFS (10.8 months versus 10.4 months; p = 0.46)) or R0/R1 resection and/or ablation rate (58% versus 58%; p = 1.0) from chemotherapy plus anti-EGFR therapy in this population, but was associated with more toxicity (48). Further research needs to be conducted to identify the patients who may benefit most from this regimen.
Furthermore, we believe a strong relationship exists between the number of liver metastases and prognosis. In our study, 4 (26.7%) patients with liver metastases above 15, 6 patients (40%) with liver metastases between 5 and 15, 5 patients (33.3%) with liver metastases under 5. Neither of the patients with liver metastases above 15 achieved NED status. Four of the 6 (66.7%) patients with liver metastases between 5 and 15 achieved NED status. All patients (100%) with liver metastases under 5 achieved NED status. These data preliminarily verified this opinion. We also hold the opinion that liver metastases could be divided into two groups: huge mass type liver metastases with fewer numbers and multiple distributed metastases in both lobes of the liver. Patients with huge mass type liver metastases might be suitable for this intensified regimen with a better prognosis. Further investigations on screening appropriate patients for benefitting from this regimen s are needed.
Concerning safety, even though the dose of FOLFOXIRI chemotherapy was reduced to decrease toxicity, the triplet was still associated with increased toxicity compared with doublet regimens. In our study, the most common AEs were neutropenia (80%), fatigue (73.3%), and rash acneiform (73.3%). Neutropenia and peripheral neurotoxicity were the major grade ≥ 3 AEs reported in 3 and 2 patients, respectively. Grade ≥ 3 diarrhea and rash acneiform were both reported in 1 patient (6.7%), respectively. A similar incidence of AEs was documented in previous studies. The rate of grade 3 or 4 neutropenia ranged from 0 to 48.6% (37, 49). Grade≥ 3 diarrhea incidence ranged from 7.5% to 53.3% (36, 50). The reported incidence of grade 3/4 skin toxicity in patients with FOLFOXIRI plus anti-EGFR agent ranges from 0 to 33.3% (36, 37, 40). However, these AEs were generally manageable based on dose reduction. Overall, these studies indicated that, for triplet chemotherapy plus anti-EGFR antibodies regimen, appropriate management and supportive treatment are required for good tolerability.
Several limitations of our study should be considered. Firstly, the sample size of this study was relatively small. Secondly, this is a non-comparative study and only demonstrated the efficacy of FOLFOXIRI plus cetuximab regimen compared with historical controls rather than the control arm. A randomized controlled trial is needed to determine whether this regimen could bring patients survival benefits.
The best regimen for left-sided RAS/BRAF wild-type CLM remains a question. Some scholars suggest that the efficacy of doublet chemotherapy plus anti-EGFR agent regimen is enough for RAS/BRAF wild-type mCRC patients in first-line treatment, which enables them to have more chemotherapy regimen options in second or later-line treatments. Nevertheless, triplet chemotherapy plus anti-EGFR antibody is not without its applicable population. This regimen could be used chiefly for conversion therapy, and the target population is refined to younger patients with overt symptoms and a high tumor load or metastatic burden. In clinical practice, an appropriate individualized chemotherapy regimen should be designed based on both patient and tumor characteristics. The efficacy and safety of cetuximab combined with FOLFOXIRI for CLM are under active investigation. We look forward to these studies providing further insights.
5 Conclusion
In conclusion, the FOLFOXIRI plus cetuximab regimen offered a promising rate of NED achieved and response rate to initially unresectable left-sided RAS/BRAF wild-type CLM patients with tolerable toxicity, which might become an option for patients with initially unresectable CLM. This approach remains investigational at this stage, and its potential needs to be further studied.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WY: Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. DC: Data curation, Methodology, Validation, Writing – original draft, Writing – review & editing. YN: Data curation, Validation. GW: Writing – review & editing. ZH: Writing – review & editing. XB: Writing – review & editing. HZ: Writing – review & editing. XC: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. YS: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Sanming Project of Medicine in Shenzhen (No. SZSM202011010)and Beijing Bethune Charitable Foundation (No. 2019-052-ZZ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1375906/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Tomasello G, Petrelli F, Ghidini M, Russo A, Passalacqua R, Barni S. FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: A systematic review and pooled analysis. JAMA Oncol. (2017) 3:e170278. doi: 10.1001/jamaoncol.2017.0278
3. Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. (2004) 240:644–57. doi: 10.1097/01.sla.0000141198.92114.f6
4. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. (1999) 230:309–18. doi: 10.1097/00000658-199909000-00004
5. Symonds LK, Cohen SA. Use of perioperative chemotherapy in colorectal cancer metastatic to the liver. Gastroenterol Rep (Oxf). (2019) 7:301–11. doi: 10.1093/gastro/goz035
6. Adam R, Wicherts DA, de Haas RJ, Ciacio O, Lévi F, Paule B, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. (2009) 27:1829–35. doi: 10.1200/JCO.2008.19.9273
7. National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology. In: Colon cancer, United States National Comprehensive Cancer Network (2024). Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428.
8. Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2023) 34:10–32. doi: 10.1016/j.annonc.2022.10.003
9. Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. (2010) 11:38–47. doi: 10.1016/S1470-2045(09)70330-4
10. Pietrantonio F, Cremolini C, Petrelli F, Di Bartolomeo M, Loupakis F, Maggi C, et al. First-line anti-EGFR monoclonal antibodies in panRAS wild-type metastatic colorectal cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. (2015) 96:156–66. doi: 10.1016/j.critrevonc.2015.05.016
11. Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. (2015) 16:1306–15. doi: 10.1016/S1470-2045(15)00122-9
12. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. (2016) 27:1386–422. doi: 10.1093/annonc/mdw235
13. Gruenberger T, Bridgewater J, Chau I, García Alfonso P, Rivoire M, Mudan S, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. (2015) 26:702–8. doi: 10.1093/annonc/mdu580
14. Ychou M, Rivoire M, Thezenas S, Quenet F, Delpero JR, Rebischung C, et al. A randomized phase II trial of three intensified chemotherapy regimens in first-line treatment of colorectal cancer patients with initially unresectable or not optimally resectable liver metastases. METHEP trial. Ann Surg Oncol. (2013) 20:4289–97. doi: 10.1245/s10434-013-3217-x
15. Marques RP, Duarte GS, Sterrantino C, Pais HL, Quintela A, Martins AP, et al. Triplet (FOLFOXIRI) versus doublet (FOLFOX or FOLFIRI) backbone chemotherapy as first-line treatment of metastatic colorectal cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. (2017) 118:54–62. doi: 10.1016/j.critrevonc.2017.08.006
16. Borelli B, Moretto R, Lonardi S, Bonetti A, Antoniotti C, Pietrantonio F, et al. TRIPLETE: a randomised phase III study of modified FOLFOXIRI plus panitumumab versus mFOLFOX6 plus panitumumab as initial therapy for patients with unresectable RAS and BRAF wild-type metastatic colorectal cancer. ESMO Open. (2018) 3:e000403. doi: 10.1136/esmoopen-2018-000403
17. Li Y, Wang D, Ren C, Li SS, Pat Fong W, Wu X, et al. 554MO Cetuximab plus FOLFOXIRI versus cetuximab plus FOLFOX in RAS wild-type patients with initially unresectable colorectal liver metastases: The TRICE randomized clinical trial. Ann Oncol. (2023) 34:S413–S4. doi: 10.1016/j.annonc.2023.09.1745
18. Siriwardena AK, Mason JM, Mullamitha S, Hancock HC, Jegatheeswaran S. Management of colorectal cancer presenting with synchronous liver metastases. Nat Rev Clin Oncol. (2014) 11:446–59. doi: 10.1038/nrclinonc.2014.90
19. Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. (2007) 25:4575–80. doi: 10.1200/JCO.2007.11.0833
20. Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. (1996) 224:509–20. doi: 10.1097/00000658-199610000-00009
21. Folprecht G, Grothey A, Alberts S, Raab HR, Köhne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. (2005) 16:1311–9. doi: 10.1093/annonc/mdi246
22. Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. (2005) 23:9243–9. doi: 10.1200/JCO.2005.07.740
23. Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. (2017) 109(9):djx015. doi: 10.1093/jnci/djx015
24. Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. (2009) 27:1572–8. doi: 10.1200/JCO.2008.19.6329
25. Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. (2008) 26:2013–9. doi: 10.1200/JCO.2007.14.9930
26. Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. (2013) 369:1023–34. doi: 10.1056/NEJMoa1305275
27. Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. (2014) 15:1065–75. doi: 10.1016/S1470-2045(14)70330-4
28. Van Cutsem E, Lenz HJ, Köhne CH, Heinemann V, Tejpar S, Melezínek I, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. (2015) 33:692–700. doi: 10.1200/JCO.2014.59.4812
29. Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A randomized clinical trial. JAMA. (2017) 317:2392–401. doi: 10.1001/jama.2017.7105
30. Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. (2008) 358:1160–74. doi: 10.1056/NEJMra0707704
31. Siena S, Sartore-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst. (2009) 101:1308–24. doi: 10.1093/jnci/djp280
32. Stintzing S, Heinrich K, Tougeron D, Modest DP, Schwaner I, Eucker J, et al. FOLFOXIRI plus cetuximab or bevacizumab as first-line treatment of BRAF(V600E)-mutant metastatic colorectal cancer: the randomized phase II FIRE-4.5 (AIO KRK0116) study. J Clin Oncol. (2023) 41:4143–53. doi: 10.1200/JCO.22.01420
33. Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. (2009) 27:663–71. doi: 10.1200/JCO.2008.20.8397
34. Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. (2009) 360:1408–17. doi: 10.1056/NEJMoa0805019
35. Modest DP, Martens UM, Riera-Knorrenschild J, Greeve J, Florschütz A, Wessendorf S, et al. FOLFOXIRI plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: the randomized, open-label, phase II VOLFI study (AIO KRK0109). J Clin Oncol. (2019) 37:3401–11. doi: 10.1200/JCO.19.01340
36. Hu H, Wang K, Huang M, Kang L, Wang W, Wang H, et al. Modified FOLFOXIRI with or without cetuximab as conversion therapy in patients with RAS/BRAF wild-type unresectable liver metastases colorectal cancer: the FOCULM multicenter phase II trial. Oncologist. (2021) 26:e90–e8. doi: 10.1634/theoncologist.2020-0563
37. Satake H, Tsuji A, Nakamura M, Ogawa M, Kotake T, Hatachi Y, et al. Phase I study of primary treatment with 5-FU, oxaliplatin, irinotecan, levofolinate, and panitumumab combination chemotherapy in patients with advanced/recurrent colorectal cancer involving the wild-type RAS gene: the JACCRO CC-14 study. Int J Clin Oncol. (2018) 23:490–6. doi: 10.1007/s10147-017-1228-5
38. Folprecht G, Hamann S, Schütte K, Trarbach T, Stoehlmacher-Williams J, Ehninger G. Dose escalating study of cetuximab and 5-FU/folinic acid (FA)/oxaliplatin/irinotecan (FOLFOXIRI) in first line therapy of patients with metastatic colorectal cancer. BMC Cancer. (2014) 14:521. doi: 10.1186/1471-2407-14-521
39. Cremolini C, Antoniotti C, Lonardi S, Aprile G, Bergamo F, Masi G, et al. Activity and safety of cetuximab plus modified folfoxiri followed by maintenance with cetuximab or bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer a randomized phase 2 clinical trial. JAMA Oncol. (2018) 4:529–36. doi: 10.1001/jamaoncol.2017.5314
40. Assenat E, Desseigne F, Thezenas S, Viret F, Mineur L, Kramar A, et al. Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: A phase II trial. Oncologist. (2011) 16:1557–64. doi: 10.1634/theoncologist.2011-0141
41. Folprecht G, Mende M, Liersch T, Bechstein WO, Kohne C-H, Stein A, et al. Cetuximab/irinotecan/5-FU +/-oxaliplatin or FOLFOXIRI +/- bevacizumab in patients with colorectal cancer and nonresectable liver metastases (AIO CELIM2-study). J Clin Oncol. (2020) 38:4024–. doi: 10.1200/JCO.2020.38.15_suppl.4024
42. Cremolini C, Rossini D, Lonardi S, Antoniotti C, Pietrantonio F, Marmorino F, et al. Modified FOLFOXIRI plus panitumumab (mFOLFOXIRI/PAN) versus mFOLFOX6/PAN as initial treatment of patients with unresectable RAS and BRAF wild-type metastatic colorectal cancer (mCRC): Results of the phase III randomized TRIPLETE study by GONO. J Clin Oncol. (2022) 40:LBA3505–LBA. doi: 10.1200/JCO.2022.40.17_suppl.LBA3505
43. Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. (2017) 28:1713–29. doi: 10.1093/annonc/mdx175
44. Folprecht G, Gruenberger T, Bechstein W, Raab HR, Weitz J, Lordick F, et al. Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann Oncol. (2014) 25:1018–25. doi: 10.1093/annonc/mdu088
45. Wu Q, Wang H, Zhang S, Zeng Y, Yang W, Pan W, et al. Efficacy and safety of triplet chemotherapy plus anti-EGFR agents in metastatic colorectal cancer: a systematic review and meta-analysis. World J Surg Oncol. (2022) 20:258. doi: 10.1186/s12957-022-02707-x
46. Lee SF, Choi HCW, Chan SK, Lam KO, Lee VHF, Wong IOL, et al. Cost-effectiveness of anti-epidermal growth factor receptor therapy versus bevacizumab in KRAS wild-type (WT), Pan-RAS WT, and Pan-RAS WT left-sided metastatic colorectal cancer. Front Oncol. (2021) 11:651299. doi: 10.3389/fonc.2021.651299
47. Satake H, Tsuji A, Tanaka C, Takahashi T, Wakamura K, Yoshida T, et al. Tumor response of FOLFOXIRI plus cetuximab versus bevacizumab in RAS wild-type metastatic colorectal cancer: The subgroup-analysis of DEEPER trial (JACCRO CC-13). J Clin Oncol. (2022) 40:109–. doi: 10.1200/JCO.2022.40.4_suppl.109
48. Bond MJG, Bolhuis K, Loosveld OJL, de Groot JWB, Droogendijk H, Helgason HH, et al. Dutch Colorectal Cancer Study Group. First-line systemic treatment strategies in patients with initially unresectable colorectal cancer liver metastases (CAIRO5): an open-label, multicentre, randomised, controlled, phase 3 study from the Dutch Colorectal Cancer Group. Lancet Oncol. (2023) 24(7):757–71. doi: 10.1016/S1470-2045(23)00219-X
49. Fornaro L, Lonardi S, Masi G, Loupakis F, Bergamo F, Salvatore L, et al. FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol. (2013) 24:2062–7. doi: 10.1093/annonc/mdt165
50. Saridaki Z, Androulakis N, Vardakis N, Vamvakas L, Kabouraki E, Kalbakis K, et al. A triplet combination with irinotecan (CPT-11), oxaliplatin (LOHP), continuous infusion 5-fluorouracil and leucovorin (FOLFOXIRI) plus cetuximab as first-line treatment in KRAS wt, metastatic colorectal cancer: a pilot phase II trial. Br J Cancer. (2012) 107:1932–7. doi: 10.1038/bjc.2012.509
Keywords: metastatic colorectal cancer, FOLFOXIRI, cetuximab, liver metastases, conversion therapy
Citation: Yang W, Chen D, Niu Y, Wu G, Huang Z, Bi X, Zhao H, Che X and Sun Y (2024) FOLFOXIRI plus cetuximab as conversion therapy for unresectable RAS/BRAF wild-type left-sided colorectal cancer with liver-limited metastases: a prospective dual-center pilot study. Front. Oncol. 14:1375906. doi: 10.3389/fonc.2024.1375906
Received: 24 January 2024; Accepted: 20 March 2024;
Published: 04 April 2024.
Edited by:
Zhaohui Jin, Mayo Clinic, United StatesCopyright © 2024 Yang, Chen, Niu, Wu, Huang, Bi, Zhao, Che and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongkun Sun, aHN1bnlrQGNpY2Ftcy5hYy5jbg==; Xu Che, ZHIuY2hleEBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share the first authorship
 Wenwei Yang
Wenwei Yang Dong Chen
Dong Chen Yaru Niu1†
Yaru Niu1† Xinyu Bi
Xinyu Bi Xu Che
Xu Che Yongkun Sun
Yongkun Sun