- 1Medical Oncology Department, Kuwait Cancer Control Center, Kuwait, Kuwait
- 2Division of Hematology/Oncology, American University of Beirut Medical Center (AUBMC), Beirut, Lebanon
- 3Lebanese American University (LAU) Medical Center-Rizk Hospital (LAUMC-RH), Beirut, Lebanon
- 4Department of Hematology-Oncology, Hotel Dieu De France, Beirut, Lebanon
- 5Oncology Medical Affairs, Eli Lilly U.A.E. (Suisse) SA (United Arab Emirates Division), Dubai, United Arab Emirates
Purpose: The burden of breast cancer is still growing in the Middle East and North Africa (MENA). BC patients typically present with more advanced stages than in Western countries. Limited information is available regarding the safety and efficacy of novel molecules for advanced BC in the Middle East region. The present real-world study evaluated the treatment patterns and survival outcomes of abemaciclib in patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor-2-negative (HER2-) locally advanced or metastatic BC (mBC) in Kuwait and Lebanon.
Methods: The TRACE study is an observational, retrospective, multicenter, single-arm cohort study. Medical records of HR+/HER2- locally advanced or mBC women were retrieved if they received abemaciclib as part of their treatment in Kuwait and Lebanon. Only patients who received abemaciclib monotherapy or in combination with other treatments for at least three months before data collection were included.
Results: Eighty-five patients met the eligibility criteria (Kuwait =42 patients, Lebanon =43). Nearly 57% of the patients received abemaciclib in the first-line setting, 19.8% received it in the second-line, and 16.5% received it at third or later lines of treatment. Abemaciclib 150mg twice daily was administered in combination with other treatments, mainly endocrine therapy, in 95.3% of the patients. Overall, 18 patients (21.4%) had a dose reduction at the end of the third month of abemaciclib treatment. After three months of treatment, the rates of complete response (CR) and partial response (PR) as the best response were 6.9% and 63.8%, respectively, with an objective response rate (ORR) of 70.7%. The 12-month progression-free survival (PFS) was 33.3% in the monotherapy group and 79.6% in the combination group.
Conclusion: The present real-world evidence confirms the feasibility and effectiveness, in terms of response rate and PFS, of abemaciclib in patients with HR+/HER2- patients with locally advanced or mBC from Kuwait and Lebanon in the Middle East region.
Introduction
According to the 2020 GLOBOCAN statistics, breast cancer (BC) has become the most common cancer type worldwide, accounting for 11.7% of new cancer cases and 6.9% of cancer-related deaths (1). BC is a heterogeneous disease in which the expression levels of surface and nuclear receptors are the main biomarkers of BC subtypes, prognosis, treatment, and patient outcomes (2). Nearly two-thirds of BC patients are hormone receptor-positive (HR+)/human epidermal growth factor receptor-2-negative (HER2-) (3). In locally advanced and metastatic BC (mBC) settings -collectively termed advanced BC- the treatment selection is based on subtype, history of prior treatment, and menopausal status. International guidelines recommend endocrine therapy (ET) for patients with HR+/HER2- locally advanced or mBC. Chemotherapy is used for patients with ET-resistant disease (4). Until recently, and despite the generally favorable prognosis of HR+/HER2- BC, patients with advanced or metastatic diseases still showed suboptimal survival (5, 6). Besides, limited options with modest efficacy are available for cases refractory to ET (7).
The introduction of molecular targeted therapy has revolutionized the treatment landscape and improved the outcomes of different BC subtypes, including HR+/HER2- locally advanced or mBC (8). Patients with HR+ BC usually show cyclin D overexpression, which promotes tumor cell proliferation, cell growth, anti-apoptotic activities, and genome instability via upstream activation of cyclin-dependent kinases (CDK) (9). Several CDK were identified, including CDK4 and CDK6, which were found to be involved in tumor cell proliferation in HR+ BC (10). Preclinical investigations and early clinical trials evaluated selective CDK 4/6 inhibitors in HR+ BC and showed promising antitumor activities and a well-tolerable safety profile (10–12).
Abemaciclib is an orally potent selective CDK 4/6 inhibitor that significantly improved the survival outcomes of HR+/HER2- patients with advanced BC in the first and second-line settings. In the final results of MONARCH 1 trial at 18 months, single-agent abemaciclib led to median progression-free survival (PFS) and OS of 6 and 22.3 months, respectively, with a disease control rate (DCR) of 67.4%, in refractory HR+/HER2- mBC (13). The benefits of abemaciclib were also evident in MONARCH 2, which recruited HR+/HER2- locally advanced or mBC progressed on ET. The abemaciclib plus fulvestrant had a median OS of 45.8 months (14). In the pivotal MONARCH 3 trial, abemaciclib plus ET was associated with a significantly longer PFS and increased objective response rate (ORR) than ET in treatment-naïve patients with HR+/HER2- locally advanced or mBC (15). In return, abemaciclib combined with ET is approved for treatment-naïve or pretreated women with HR+/HER2- advanced BC. At the same time, it is the only approved CDK4/6 inhibitor as a single agent for women with mBC who progressed on ET or prior chemotherapy (6). Recently, abemaciclib combined with ET was approved as adjuvant therapy for high-risk early BC (16).
The burden of breast cancer (BC) is still growing in the Middle East and North Africa (MENA). Previous reports estimated that the MENA region had witnessed the largest increase in BC incidence over the past three decades (17, 18). Data show that BC patients from the region are often diagnosed at younger ages [50% below age 50 (19)], with more aggressive clinicopathologic patterns and more advanced stages than in Western countries (20). Modern management, including CDK4/6 inhibitors, is widely practiced in many centers in the Middle East. Still, limited information is available regarding the safety and efficacy of CDK 4/6 inhibitors in the region. There is an unmet clinical need to explore the treatment patterns and outcomes of novel targeted therapy in patients with advanced BC from the MENA. Therefore, the present real-world study evaluated the treatment patterns and survival outcomes of abemaciclib in patients with HR+/HER2- locally advanced or mBC in two Middle Eastern countries, Kuwait and Lebanon, which have one of the highest incidences of BC in the region (21, 22).
Patients and methods
The present report was prepared in concordance with the STROBE statement (Supplementary Table 1) (23). All study procedures adhered to the principles of the latest version of the Declaration of Helsinki (24) and applicable local regulatory laws. The study was approved by the local ethics committee of each participating center.
Study design, participants, and setting
The TRACE was an observational, retrospective, multicenter, single-arm cohort study. Medical records of HR+/HER2- locally advanced or mBC women were retrieved if they received abemaciclib as a part of routine clinical practice in Kuwait and Lebanon. Adult women (age ≥18 years old) were deemed eligible if they had a confirmed diagnosis of locally advanced or mBC, whether de-novo or progressed/recurred from early BC, histologically proven HR+/HER2- status, and received abemaciclib monotherapy or in combination with other treatments for at least three months before data collection. Eligible patients received abemaciclib at the discretion of the treating physicians only. There was no attempt to influence the prescribing patterns of any individual investigator. We excluded patients who received abemaciclib in an adjuvant setting, women who received abemaciclib as a part of a clinical trial, and/or patients who had concurrent primary malignancy.
Consecutive records of eligible patients were retrieved from three centers in Lebanon and one center in Kuwait. The data collection covered the period from the date of approval of abemaciclib in each country (June 2018 in Kuwait and January 2020 in Lebanon) to September 2022. Data covering at least three months ±30 days after initiating abemaciclib were retrieved.
Data collection
The following data were retrieved from the medical records of eligible patients: demographic characteristics, anthropometric measures, family history of cancer, associated comorbidities, disease characteristics (age at diagnosis of locally advanced or mBC, stage at diagnosis, Eastern Cooperative Oncology Group [ECOG] performance status [PS], and number and sites of metastasis), treatment patterns (age at abemaciclib initiation, dose, frequency, line of therapy, need for dose reduction, concomitant medications, and co-current radiotherapy or surgery), date of progression or death on abemaciclib line of therapy, date of last follow-up, and the recorded best response. The best response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Study endpoints
The primary endpoint of the present study was to describe the treatment patterns of abemaciclib monotherapy or in combination with other treatments for patients with HR+/HER2- locally advanced or mBC in Kuwait and Lebanon. The secondary outcomes were to describe the demographic and clinical characteristics of patients who were initiated on abemaciclib in both countries, PFS on abemaciclib, the best response on abemaciclib according to RECIST 1.1, and objective response rate (ORR), defined as the rate of patients who achieved complete response (CR) or partial response (PR).
Sample size calculation
The study was descriptive, with no formal sample size computation needed. However, with 100 participants, the study would be able to estimate the prevalence of any binary outcome within a margin of error of at most ±10% using a 95% confidence interval (CI). The study recruited 85 participants, which achieved a precision of 9% in detecting a prevalence of 25% for any binary outcomes within a margin of error of at most ±10% using a 95% confidence interval (CI).
Statistical analysis
All analyses were done using IBM SPSS Statistics for Windows (Version 27.0). Descriptive analysis was conducted using the mean ± standard deviation (SD) and counts for continuous and categorical variables, respectively. The best response rate was calculated from the total number of patients with medical records to extract the RECIST 1.1 response. The Kaplan- Meier curves were used to calculate the PFS and OS of the study cohort, which was stratified into monotherapy and combination groups. The PFS and OS were defined as the time from initiating abemaciclib to disease progression/death and death, respectively. Subgroup analyses for each country (Kuwait and Lebanon) were conducted. Besides, the treatment patterns, survival outcomes, and response rates were calculated at three timepoints: at the start of abemaciclib, whether as a single-agent treatment or combination treatment (1st timepoint); at the end of three months (± 1 month) of abemaciclib treatment (2nd timepoint); and at the earliest of death, the last medical record entry, or the end of the observation period “defined as the date of data abstraction” (3rd timepoint).
Results
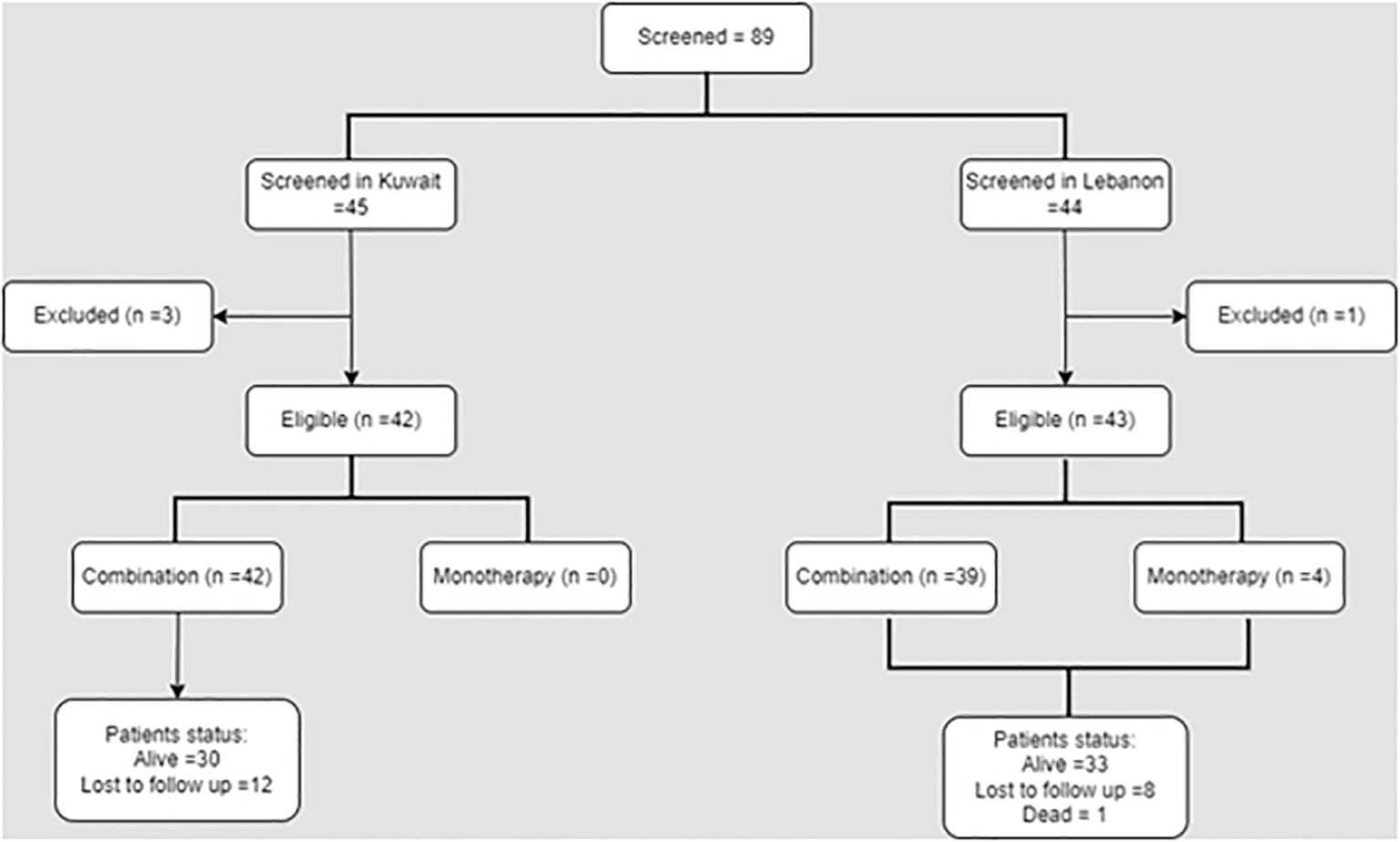
Out of the 89 patients available during the data collection period, 85 met the eligibility criteria and were retrieved in the present study. Forty-two eligible patients were from Kuwait and received abemaciclib in combination with other treatments. On the other hand, out of the 43 patients from Lebanon, four patients received abemaciclib monotherapy (Figure 1).
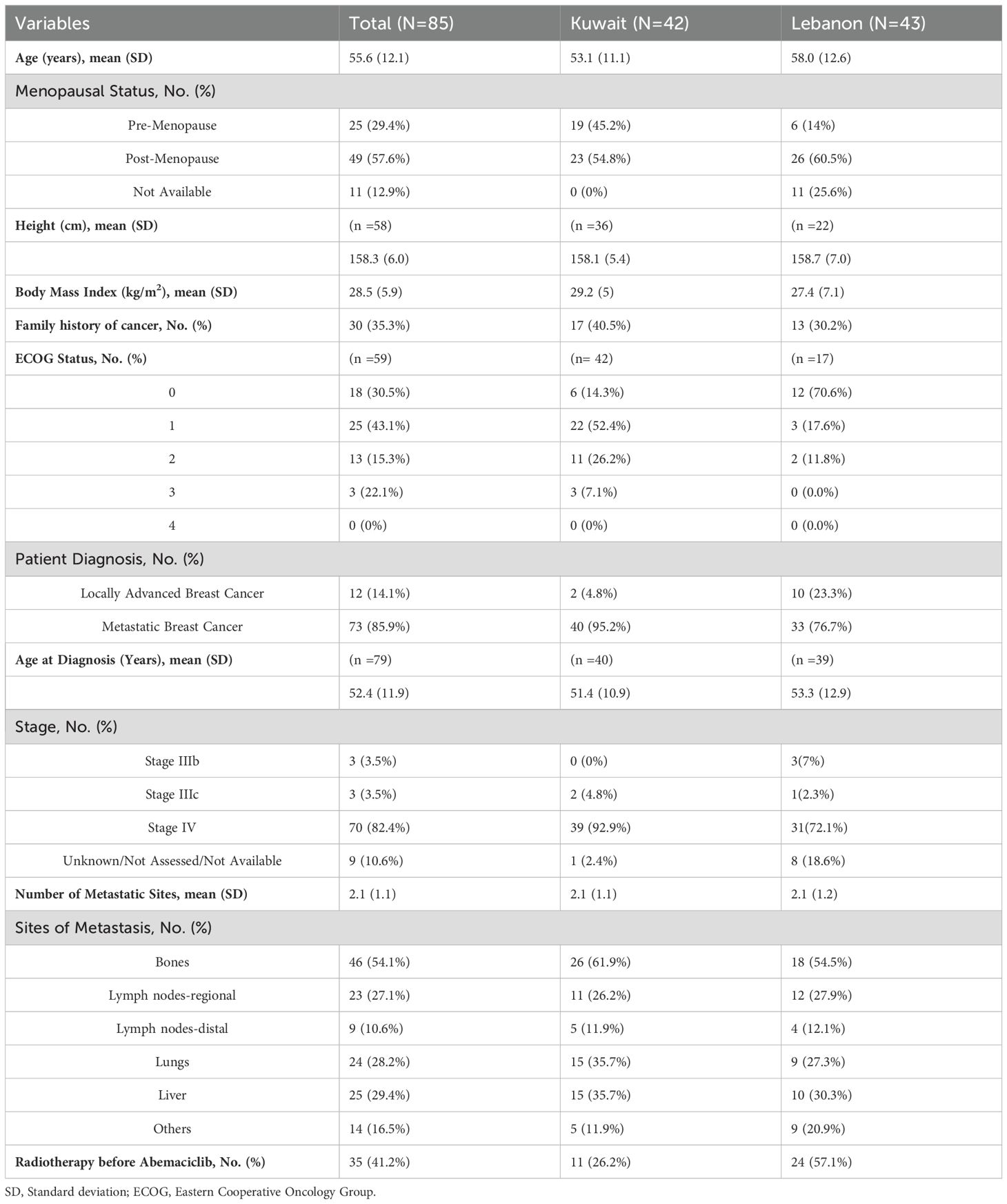
Table 1 shows the demographics and baseline characteristics of the patients at the time of the start of abemaciclib. The mean age of the included patients was 55.6 ± 12.1 years, with a slightly higher average in Lebanon (mean age =58.0 ± 12.6 years). Overall, 57.6% of patients were post-menopausal, and 35.3% had a family history of cancer. Regarding anthropometric measures, the mean body mass index (BMI) of the included patients was 28.5 ± 5.9Kg/m2, with a slightly higher average BMI in Kuwait. Nearly 73% of the patients with available ECOG PS had ECOG PS 0-1. Most patients had mBC (85.9%) at the time of abemaciclib initiation, while the mean age at the diagnosis of locally advanced or metastatic disease was 52.4 ± 11.9 years. The average number of metastatic sites was 2.1 ± 1.1 sites, and bone was the most common metastatic site (present in around 60% of cases) in the study’s cohort. Around one-third of patients had liver or lung metastases.
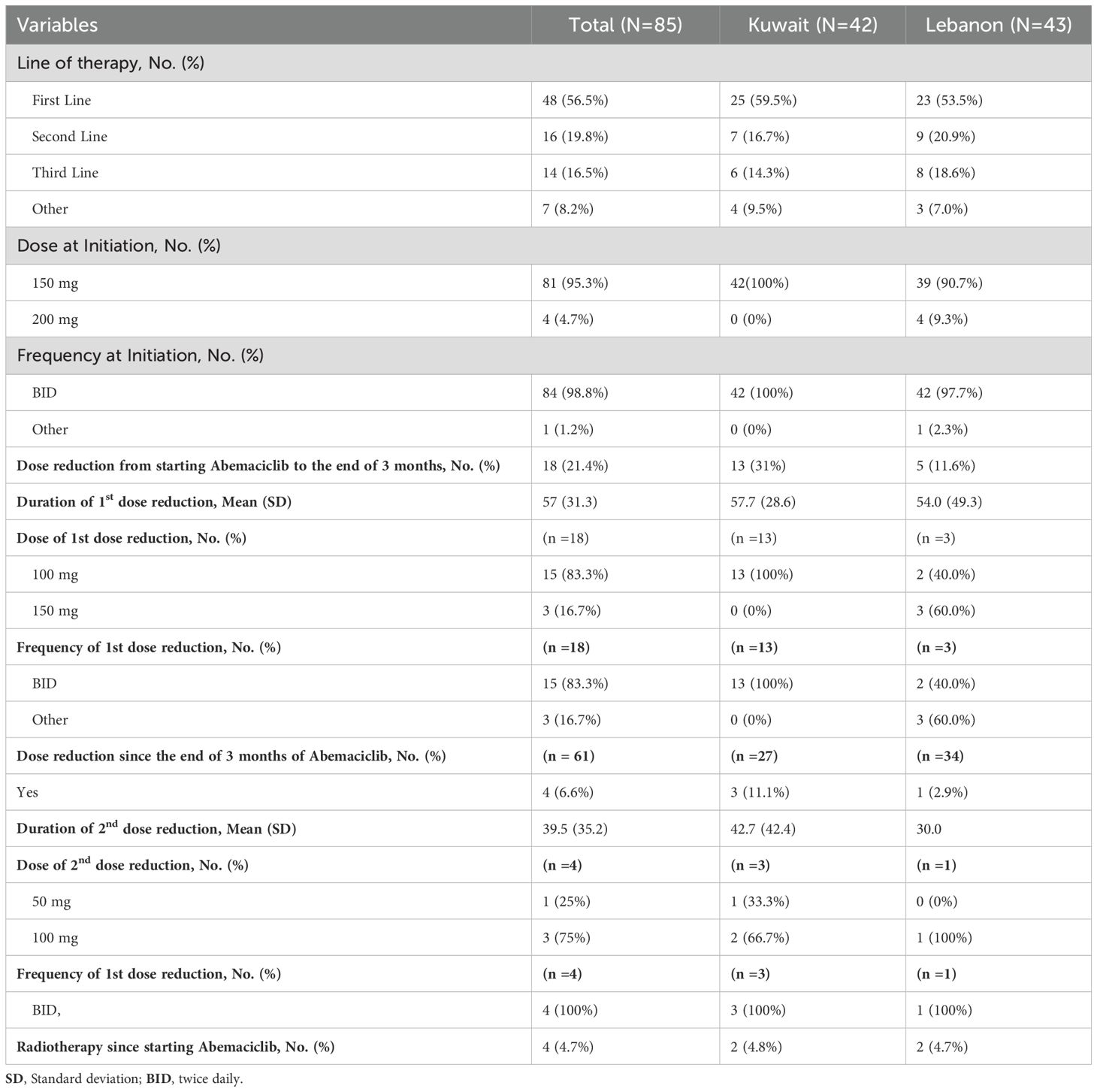
In terms of treatment patterns, 56.5% of the patients received abemaciclib in the first-line setting, 19.8% received it in the second-line, and 16.5% received it at third or later lines of treatment. The distribution of the line of abemaciclib treatment was comparable between Kuwait and Lebanon. All patients from Kuwait and 90.7% of Lebanon received abemaciclib 150mg twice daily, while the four patients from Lebanon who were on monotherapy received abemaciclib 200mg (Table 2). Regarding combination therapy, ET was the most common combined treatment among patients from Kuwait (75.4%). Letrozole was the most common agent reported to be combined with abemaciclib therapy. Bone-modulating agents were combined with abemaciclib in 24.6% of the patients from Kuwait. On the other hand, letrozole was combined with abemaciclib in 52.9% of the patients from Lebanon, followed by fulvestrant in 38.2% (Figure 2).
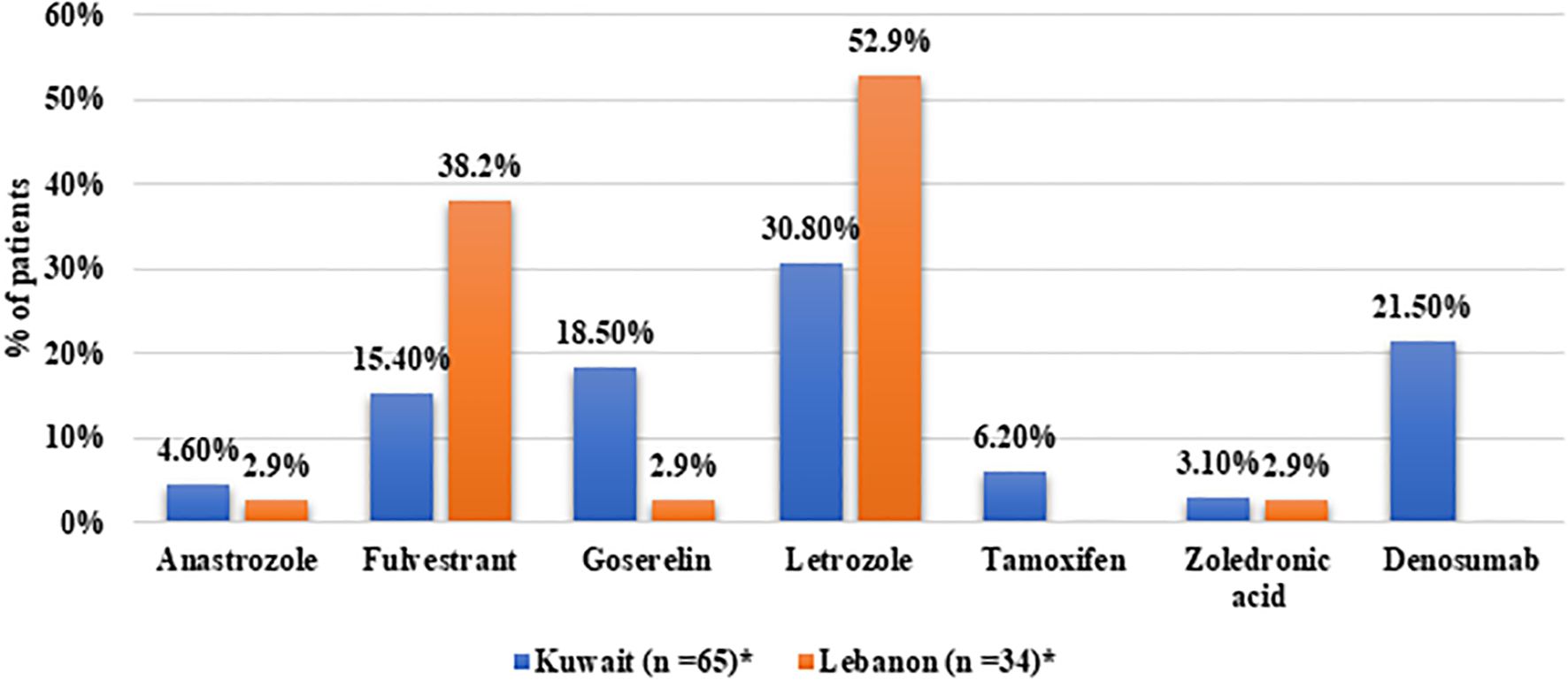
Figure 2. Distribution of medication classes in patients receiving combination therapy from Kuwait and Lebanon. *Patients may have received more than one medication.
At the end of the third month of follow-up, 18 patients (21.4%), who were mainly from Kuwait (n =13), had a dose reduction. While at the end of the follow-up, only four patients (6.6%) had a dose reduction; three of them had a dose reduction to 100mg. Four patients (4.7%) received radiotherapy during abemaciclib treatment (Table 2).
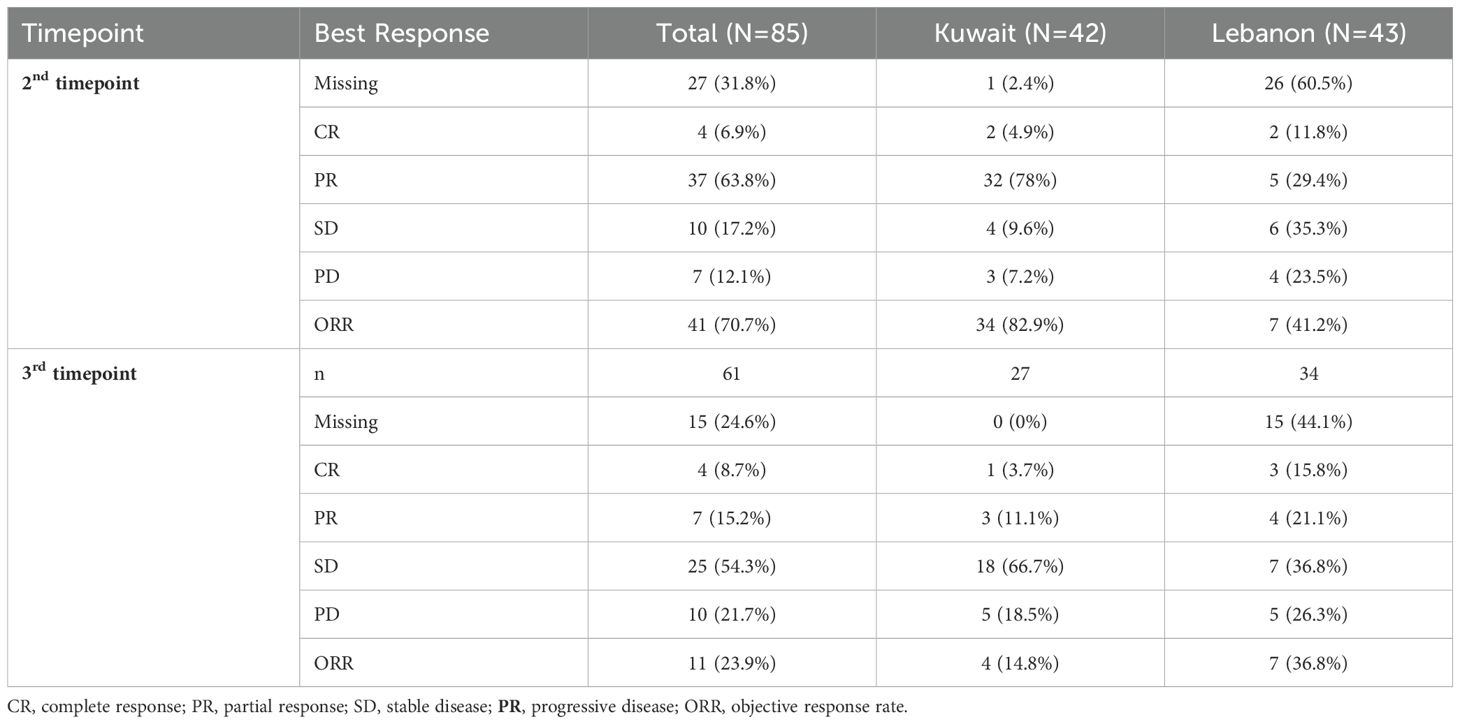
Table 3 shows the response rate to abemaciclib. Data regarding the response rate was available for 58 patients at the end of the third month of follow-up. The rates of complete response (CR) and partial response (PR) as the best response were 6.9% and 63.8%, respectively, with an ORR of 70.7%. The rate of CR was comparatively higher in Lebanon (11.8%) than in Kuwait (4.9%); however, patients from Kuwait had a notably higher PR rate (78%) than those from Lebanon (29.4%). Overall, the ORR was higher in Kuwait (82.9%) than in Lebanon (41.2%). Data from 61 patients were available beyond the three months of abemaciclib. The CR and PR rates in those patients were 8.7% and 15.2%, respectively (ORR =23.9%).
The median follow-up duration for monotherapy and combination groups were 264 (interquartile range [IQR] 248-356.5) and 226 (IQR 133-308) days, respectively. The median PFS was 275 (95% CI 239.8-310.2) days in the monotherapy group. On the other hand, the median PFS in the combination group was not reached, with a restricted mean PFS of 540.5 (35.5) days. The 12-month PFS was 33.3% in the monotherapy group and 79.6% in the combination group (Figure 3a). Concerning OS, one case died in the combination arm; thus, the mean and median time to the event were not assessed. However, the overall follow-up time from the start of treatment showed a median of 264 (IQR 248-356.5) days in the monotherapy group compared to 226 (IQR 136-307) days in the combination group (Figure 3b).
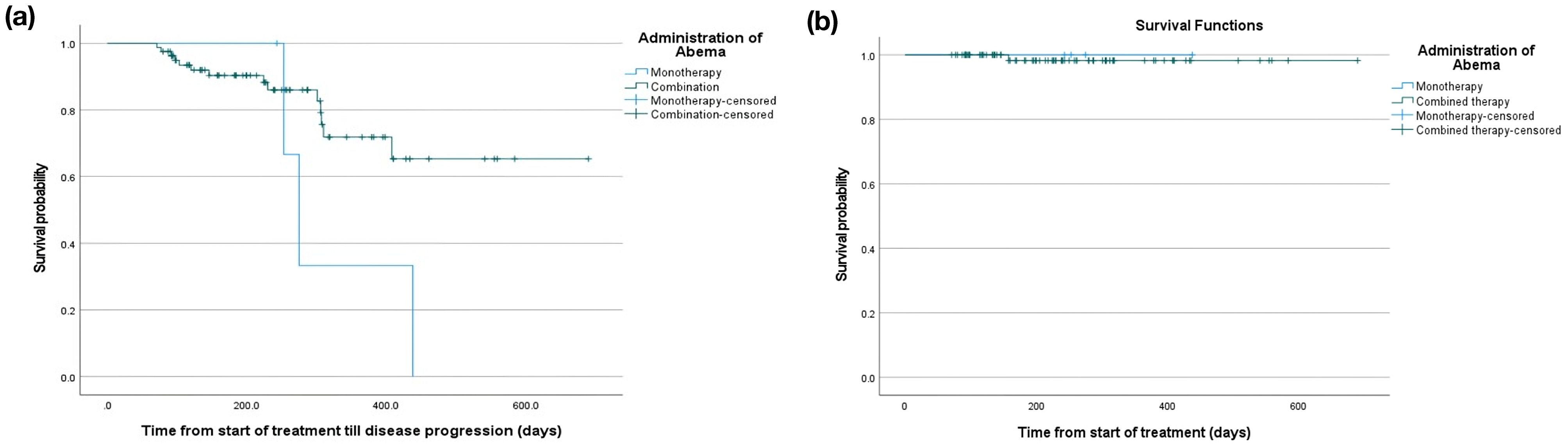
Figure 3. Kaplan Meier curve of (a) PFS and (b) OS of patients who received abemaciclib monotherapy and in combination.
We stratified the survival outcomes according to the country. In the Kuwait subgroup, five patients reported disease progression with restricted mean PFS of 563.8 (SE 8.2) days (Supplementary Figure 1a). Three progression events in the monotherapy arm versus two in the combination arm were observed in the Lebanon subgroup. The survival analysis showed that the restricted mean PFS in the monotherapy arm was 322.0 (SE 58.3) days versus 498.2 (SE 28.2) days in the combination group (Supplementary Figure 1b).
Discussion
BC is the most prevalent malignancy in Lebanon and Kuwait, with estimated incidence rates of 104.4 and 56.1 per 100,000, respectively, ranking them among the countries with the highest BC incidence in the MENA region (19, 21). Previous epidemiological studies showed that nearly 36.4% and 28% of BC patients from Lebanon and Kuwait present with stage III-IV BC at the time of diagnosis, respectively (25, 26). Despite the considerable burden of advanced disease in both countries, limited real-world data are available regarding the treatment patterns and outcomes of CDK4/6 inhibitors. Abemaciclib has recently gained approval in Lebanon and Kuwait in first and second-line advanced BC settings. The present real-world study evaluated the treatment patterns and survival outcomes of abemaciclib plus ET or as a single agent in patients with HR+/HER2- locally advanced or mBC in Kuwait and Lebanon.
Abemaciclib is an orally administered CDK4/6 inhibitor that is dosed on a twice-daily continuous schedule. The recommended starting dose in combination with ET is 150 mg twice daily; however, the recommended starting dose as monotherapy is 200 mg twice daily (27). In locally advanced or metastatic settings, abemaciclib is approved in combination with aromatase inhibitors (AI) in the first-line setting or at later lines as monotherapy following progression on ET and prior chemotherapy. The current international guidelines recommend the early introduction of CDK4/6 inhibitors, including abemaciclib, in combination with ET for HR+/HER2- locally advanced or mBC (28, 29). However, previous real-world studies noted heterogeneity between guideline recommendations and drug use during the early post-approval period (30, 31).
Nonetheless, the present real-world evidence showed that the treatment patterns at the time of starting abemaciclib align with international recommendations. The majority of the patients in Kuwait and Lebanon received abemaciclib 150mg twice daily in combination with AI as first-line therapy, while findings from two recent real-world studies from the United States (US) showing a trend towards abemaciclib initiation in patients with features of advanced stages such as later lines of treatment, prior ET or CKD 4/6 inhibitors, visceral metastases, and poor ECOG status (32, 33). The authors hypothesized these findings are attributed to the physicians’ tendency to prescribe abemaciclib once it was approved for patients with a longer duration of metastatic disease or prior lines of therapy. Besides, it should be noted that abemaciclib received the frontline indication in February 2018, while the data collection window for the two studies from the US covered the period from June 2016/September 2017 to December 2018/October 2019 (32, 33).
The survival benefits of abemaciclib have been demonstrated as a frontline therapy (15), in combination with fulvestrant in women who progressed on ET (34). Such findings were further observed in a real-life setting (32, 33). In the present study, the ORR was 70.7% after three months of abemaciclib treatment; the rate of ORR was higher in Kuwait (82.9%) than in Lebanon (41.2%), which can be explained by the higher percentage of patients with prior lines of therapy and the large number of patients with missing RECIST response in Lebanon cohort. The 12-month PFS was 33.3% in the monotherapy group and 79.6% in the combination group. Such findings confirm that the benefits of abemaciclib observed in clinical trials are reflected in the real-world setting; real-world data usually encompasses a larger pool of patients with diverse features and less strict criteria than those in clinical trials. In the MONARCH 3 trial, the ORR of frontline abemaciclib was 61%, and the median PFS was 28.18 months (35). Likewise, the ORR was 35.2% in women who progressed on ET in MONARCH 2 (36). The real-world response rate and survival benefits in the present study were notably higher than those reported in other real-world evidence from the US. In Carter et al., the ORR was 41.2%, and the 12-month PFS was 61% (33). However, as highlighted above, nearly two-thirds of the patients in Carter et al. received abemaciclib in a more advanced stage than our cohort. Besides, the assessment of tumor response in Carter et al. was based on the physician’s assessment rather than the RECIST criteria used in the present study.
In MONARCH trials, abemaciclib was associated with manageable safety profiles and a low incidence of severe adverse events leading to treatment discontinuation; neutropenia, diarrhea, and leukopenia were the most common adverse events in MONARCH trials (35). Although the present real-world study did not capture the incidence of adverse events, we found that the rate of dose reduction, as a measure of treatment tolerability, was 21.4% within the first three months of abemaciclib treatment. The mean duration of dose reduction was 57 ± 31.3 days. In a real-world study from the US, the rate of dose reduction in mBC patients receiving abemaciclib was 30.8% (32). These rates are also consistent with the real-world evidence evaluating other CKD 4/6 inhibitors, palbociclib, and ribociclib, in patients with mBC (37–39). The rate of dose reduction in the present study was lower than the reported figures from MONARCH 2 and 3 trials, 42.9% and 43.4%, respectively (40). Such heterogeneity is expected due to the strict dosing protocols followed in the clinical trials, which are usually lacking in the real-world setting. Nonetheless, the impact of dose reduction on the outcomes of abemaciclib appears to be insignificant, as recently reported in a subgroup analysis of MONARCH 2 and MONARCH 3 data (40).
Limitations
To our knowledge, the present study is the first real-world evidence to investigate the treatment patterns and survival outcomes of CKD 4/6 inhibitors in women with HR+/HER2- locally advanced or mBC in the MENA. However, we acknowledge that the study has certain limitations. The retrospective nature of the study can increase the risk of misclassification and recall bias. The sample size was relatively small, and the median duration of follow-up was limited, as the study was initiated within one year of the approval of abemaciclib in Kuwait and Lebanon. The small sample size and short follow-up duration limited the maturity of the time-to-event analyses and the feasibility of conducting subgroup analysis according to the treatment regimen (monotherapy versus combination). Data regarding the response rates and survival were not available for all patients, especially the cohort from Lebanon. As a real-world retrospective study, PFS was not assessed through standardized, prospective serial imaging. Instead, radiographic progression was determined based on the available imaging studies conducted as part of routine clinical care, which may have introduced variability in assessment intervals across patients. The timing and frequency of imaging were not protocol-driven, and there may have been inconsistencies in follow-up imaging due to variations in clinical practice, patient condition, or physician discretion. Furthermore, data were not available to stratify PFS according to the line of treatment. The present study also did not capture the toxicity profile of abemaciclib. Although we have collected several important variables, we acknowledge that, due to the retrospective nature of the study, our dataset was limited to the routinely monitored data in real-world practice. Future prospective studies from the Middle East, with larger sample sizes and longer follow-ups, are needed to understand the toxicity profile of abemaciclib and provide a more comprehensive analysis of the region.
Conclusion
The present real-world evidence confirms the feasibility and effectiveness, in terms of response rate and PFS, of abemaciclib in patients with HR+/HER2- patients with locally advanced or mBC from Kuwait and Lebanon. In both countries, abemaciclib was usually used in the frontline setting in combination with AI or as a part of a combination regimen for women who progressed on ET. The observed real-world effectiveness in the present study was comparable to the results of pivotal MONARCH trials, further complementing clinical trial results. Further research is warranted to compare the real-world response and survival outcomes of abemaciclib according to the line of therapy.
Data availability statement
As this is an observational study that uses data previously collected and does not impose any form of intervention, the data has been de-identified to protect subject privacy. Therefore, a formal consent to release information form was not required, and a waiver of informed consent was requested from the IRB/IEC by the PI. Requests to access these datasets should be directed to cm9zY2FfYWxleGFuZHJ1QGxpbGx5LmNvbQ==.
Ethics statement
The studies involving humans were approved by the local ethics committee of each participating center. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the retrospective nature of the research study and considering that no risk is associated with the study.
Author contributions
FA: Investigation, Writing – original draft, Writing – review & editing. AAN: Investigation, Writing – original draft, Writing – review & editing. HG: Investigation, Writing – original draft, Writing – review & editing. JK: Investigation, Writing – original draft, Writing – review & editing. NE: Investigation, Writing – original draft, Writing – review & editing. CB: Supervision, Writing – original draft, Writing – review & editing. SR: Supervision, Writing – original draft, Writing – review & editing. AR: Supervision, Writing – original draft, Writing – review & editing. JD: Supervision, Project administration, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The present study was funded by Eli Lilly and Company. The funding body had no role in the analysis and interpretation of data, as well as the decision to submit the manuscript for publication.
Acknowledgments
We would like to thank CTI Clinical Trial and Consulting Services, MEA office, for their support in the clinical operations, data collection, analysis, medical writing, and editorial support.
Conflict of interest
Authors CB, SR, and AR were employed by the company Eli Lilly U.A.E. Suisse SA.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views and opinions expressed in this study are those of the authors.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1437380/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Parise CA and Caggiano V. Breast cancer survival defined by the ER/PR/HER2 subtypes and a surrogate classification according to tumor grade and immunohistochemical biomarkers. J Cancer Epidemiol. (2014). doi: 10.1155/2014/469251
4. Gradishar WJ. NCCN guidelines updates: management of patients with HER2-negative breast cancer. J Natl Compr Cancer Netw. (2022) 20:561–5. doi: 10.6004/jnccn.2022.5016
5. Tryfonidis K, Senkus E, Cardoso MJ, et al. Management of locally advanced breast cancer-perspectives and future directions. Nat Rev Clin Oncol. (2015) 12:147–62. doi: 10.1038/nrclinonc.2015.13
6. Lee KA, Shepherd ST, and Johnston SR. Abemaciclib, a potent cyclin-dependent kinase 4 and 6 inhibitor, for treatment of ER-positive metastatic breast cancer. Futur Oncol. (2019) 15:3309–26. doi: 10.2217/fon-2019-0169
7. Zhang H and Peng Y. Current biological, pathological and clinical landscape of HER2-low breast cancer. Cancers (Basel). 15. doi: 10.3390/CANCERS15010126
8. An J, Peng C, Xie X, et al. New advances in targeted therapy of HER2-negative breast cancer. Front Oncol. (2022) 12:757. doi: 10.3389/fonc.2022.828438
9. Cicenas J, Kalyan K, Sorokinas A, et al. Highlights of the latest advances in research on CDK inhibitors. Cancers (Basel). (2014) 6:2224–42. doi: 10.3390/cancers6042224
10. Niu Y, Xu J, and Sun T. Cyclin-dependent kinases 4/6 inhibitors in breast cancer: Current status, resistance, and combination strategies. J Cancer. (2019) 10:5504–17. doi: 10.7150/jca.32628
11. Pernas S, Tolaney SM, Winer EP, et al. CDK4/6 inhibition in breast cancer: current practice and future directions. Ther Adv Med Oncol. 10. doi: 10.1177/1758835918786451
12. Messina C, Cattrini C, Buzzatti G, et al. CDK4/6 inhibitors in advanced hormone receptor-positive/HER2-negative breast cancer: a systematic review and meta-analysis of randomized trials. Breast Cancer Res Treat. (2018) 172:9–21. doi: 10.1007/s10549-018-4901-0
13. Rugo HS, Tolaney SM, Cortés J, et al. Abstract CT044: MONARCH 1: Final overall survival analysis of a phase 2 study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. Cancer Res. (2017) 77:CT044–4. doi: 10.1158/1538-7445.AM2017-CT044
14. Sledge G, Toi M, Neven P, et al. SABCS 2022: Final overall survival analysis of Monarch 2 : A phase 3 trial of Abemaciclib plus Fulvestrant in patients with hormone receptor-positive, HER2-negative advanced breast cancer. SABCS (2022).
15. Goetz M, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. (2017) 35:JCO2017756155. doi: 10.1200/JCO.2017.75.6155
17. Li N, Deng Y, Zhou L, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: Results from the Global Burden of Disease Study 2017. J Hematol Oncol. (2019) 12:140. doi: 10.1186/s13045-019-0828-0
18. Zahedi R, Molavi Vardanjani H, Baneshi MR, et al. Incidence trend of breast Cancer in women of eastern Mediterranean region countries from 1998 to 2019: A systematic review and meta-analysis. BMC Womens Health. (2020) 20:53. doi: 10.1186/s12905-020-00903-z
19. El Saghir NS, Khalil MK, Eid T, et al. Trends in epidemiology and management of breast cancer in developing Arab countries: A literature and registry analysis. Int J Surg. (2007) 5:225–33. doi: 10.1016/j.ijsu.2006.06.015
20. Albeshan SM, Mackey MG, Hossain SZ, et al. Breast cancer epidemiology in gulf cooperation council countries: A regional and international comparison. Clin Breast Cancer. doi: 10.1016/j.clbc.2017.07.006
21. Fares MY, Salhab HA, Khachfe HH, et al. Breast cancer epidemiology among lebanese women: An 11-year analysis. Med. 55. doi: 10.3390/medicina55080463
22. Elbasmi A, Al-Asfour A, Al-Nesf Y, et al. Cancer in Kuwait: magnitude of the problem. Gulf J Oncolog. (2010) 1:7–14.
23. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg. doi: 10.1016/j.ijsu.2014.07.013
24. JAVA. Declaration of helsinki world medical association declaration of helsinki. Bull World Heal Organ. (2013) 79:373–4.
25. Chahine G, El Rassy E, Khazzaka A, et al. Characteristics of incident female breast cancer in Lebanon, 1990–2013: Descriptive study of 612 cases from a hospital tumor registry. Cancer Epidemiol. (2015) 39:303–6. doi: 10.1016/j.canep.2015.03.008
26. El Saghir NS, Assi HA, Jaber SM, et al. Outcome of breast cancer patients treated outside of clinical trials. J Cancer. (2014) 5:491–8. doi: 10.7150/jca.9216
27. Martin M, Garcia-Saenz JA, Manso L, et al. Abemaciclib, a CDK4 and CDK6 inhibitor for the treatment of metastatic breast cancer. Future Oncol. (2020) 16:2763–78. doi: 10.2217/fon-2020-0604
28. Gennari A, André F, Barrios CH, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer ☆. Ann Oncol. (2021) 32:1475–95. doi: 10.1016/j.annonc.2021.09.019
29. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. (2020) 31:1623–49. doi: 10.1016/j.annonc.2020.09.010
30. Schneeweiss S, Gagne JJ, Glynn RJ, et al. Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther. (2011) 90:777–90. doi: 10.1038/clpt.2011.235
31. Rassen JA and Schneeweiss S. Newly marketed medications present unique challenges for nonrandomized comparative effectiveness analyses. J Comp Eff Res. (2012) 1:109–11. doi: 10.2217/cer.12.12
32. Smyth EN, Beyrer J, Saverno KR, et al. Real-world patient characteristics, utilization patterns, and outcomes of US patients with HR+, HER2- metastatic breast cancer treated with abemaciclib. Drugs - real World outcomes. (2022) 9:681–93. doi: 10.1007/s40801-022-00327-1
33. Cuyun Carter G, Sheffield KM, Gossai A, et al. Real-world treatment patterns and outcomes of abemaciclib for the treatment of HR+, HER2- metastatic breast cancer. Curr Med Res Opin. (2021) 37:1179–87. doi: 10.1080/03007995.2021.1923468
34. Sledge GW Jr., Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: A randomized clinical trial. JAMA Oncol. (2020) 6:116–24. doi: 10.1001/jamaoncol.2019.4782
35. Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. Breast Cancer. (2019) 5:5.
36. Sledge GW, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. (2017) 35:2875–84. doi: 10.1200/JCO.2017.73.7585
37. Balu S, O’Shaughnessy J, Paul ML, et al. EARLY real-world treatment and dosing patterns of ribociclib for metastatic breast cancer (mBC): A retrospective observational study. (2020) 38:e13059–9. doi: 10.1200/JCO20203815_suppl.e13059
38. Varella L, Eziokwu AS, Jia X, et al. Real-world clinical outcomes and toxicity in metastatic breast cancer patients treated with palbociclib and endocrine therapy. Breast Cancer Res Treat. 176. doi: 10.1007/S10549-019-05176-1
39. Li N, Du EX, Chu L, et al. Real-world palbociclib dosing patterns and implications for drug costs in the treatment of HR+/HER2- metastatic breast cancer. Expert Opin Pharmacother. (2017) 18:1167–78. doi: 10.1080/14656566.2017.1351947
Keywords: breast neoplasms, disease-free survival, CDK inhibitor, abemaciclib, metastatic breast cancer
Citation: Al Nouri A, Al Terkait F, El Saghir N, Ghanem H, Kattan J, Bishouti C, Dreyer J, Rai S and Rosca A (2025) Abemaciclib treatment patterns and outcome in HR+/HER2- locally advanced or metastatic breast cancer: a real-world study from Kuwait and Lebanon. Front. Oncol. 15:1437380. doi: 10.3389/fonc.2025.1437380
Received: 31 May 2024; Accepted: 10 March 2025;
Published: 17 June 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesCopyright © 2025 Al Nouri, Al Terkait, El Saghir, Ghanem, Kattan, Bishouti, Dreyer, Rai and Rosca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandru Rosca, cm9zY2FfYWxleGFuZHJ1QGxpbGx5LmNvbQ==
 Anwar Al Nouri1
Anwar Al Nouri1 Faisal Al Terkait
Faisal Al Terkait Nagi El Saghir
Nagi El Saghir Hady Ghanem
Hady Ghanem Joseph Kattan
Joseph Kattan Alexandru Rosca
Alexandru Rosca