- 1Department of Gastrointestinal Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Department of Nursing, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
Background: Preoperative nutritional status in patients with gastric cancer after surgery has attracted considerable interest. The nutritional risk index (NRI) has been widely used as a convenient and effective nutritional assessment index, but the relationship between preoperative NRI and postoperative complications in patients with gastric cancer has not been adequately studied. Our study aimed to investigate the effects of preoperative NRI on 30-day postoperative complications in patients with gastric cancer.
Methods: This retrospective analysis investigated 578 patients with gastric cancer. Preoperative NRI calculations were based on serum albumin levels and body weight, and receiver operating characteristic curves were used in analyzing NRI values and establishing optimal cutoff points. Patients were categorized into two groups according to cutoff value: low NRI group (NRI<96.7) and high NRI group (NRI≥96.7). The hazard ratio (HR) for postoperative complications was calculated through Cox regression analysis and adjusted for potential confounders, and the effects of NRI on postoperative complications in patients with gastric cancer were examined. In addition, we conducted subgroup analyses to examine whether there was an interaction between the effect of NRI on the cumulative incidence of postoperative complications and other confounding factors.
Results: Of the 578 patients with gastric cancer who underwent radical surgery, 120 (20.8%) experienced postoperative complications. The optimal NRI threshold of 96.7 was identified using ROC curve analysis. Cox regression analysis demonstrated that preoperative NRI was independently associated with 30-day postoperative complications after adjusting for confounding factors (HR=0.93; 95%CI: 0.90–0.96; P<0.001). Patients in the low NRI group had significantly higher rates of postoperative complications than those in the high NRI group(HR=2.89, 95%CI:1.71–4.88; P<0.001). The cumulative incidence analysis revealed a higher risk of postoperative complications over time in the low NRI group compared with the high NRI group (P<0.001). These associations remained robust in subgroup analyses.
Conclusions: NRI is an independent predictor of 30-day postoperative complications in gastric cancer patients and is a convenient and useful nutritional screening tool for identifying patients with gastric cancer at high risk of postoperative complications.
Introduction
Gastric cancer, a prevalent malignancy in oncology, poses a considerable global health threat and has an annual incidence exceeding one million worldwide, resulting in approximately 770,000 deaths (1). The incidence and mortality rates of gastric cancer are gradually increasing globally, posing formidable challenges to the diagnosis and treatment of the disease. Currently, surgical treatment is considered the primary therapeutic approach (2). However, early postoperative prognosis remains concerning because of the high incidence of complications, which considerably affect patients’ recovery and quality of life (3).
Postoperative complications are among the adverse prognostic factors in cancer care. Their occurrence prolongs hospitalization duration, increases economic burden, and leads to unfavorable prognoses (4, 5). The incidence of postoperative complications in patients with gastric cancer can be as high as 40%, and early complications appear within 30 days after surgery (6, 7). These complications may lead to systemic inflammation, potentially reducing immune response in patients with cancer (8). Therefore, effectively identifying high-risk patients prone to postoperative complications before undergoing surgery for gastric cancer is crucial.
Malnutrition potentially affects as many as 40%–80% of patients with gastric cancer, which along with weakened immunity can increase the likelihood of postoperative complications (9, 10). Thus, the preoperative assessment of the nutritional status of these patients is crucial. The nutritional risk index (NRI), designed and implemented by the Veterans Affairs Total Parenteral Nutrition Cooperative Study Group, is a nutritional assessment tool determined by serum albumin and body weight (11). The NRI enables the routine and straightforward assessment of patients’ nutritional status before surgery and can aid in predicting postoperative complications and outcomes in patients with cancer. The NRI is extensively utilized for patients with cardiac surgery (12)and breast cancer (13), but its applicability to patients with gastric cancer has not been well established. This study seeks to examine the influence of preoperative NRI on postoperative complications within 30 days for patients with gastric cancer, aiming to assess NRI values during nutritional screening for preoperative patients with gastric cancer.
Methods
Data source and study population
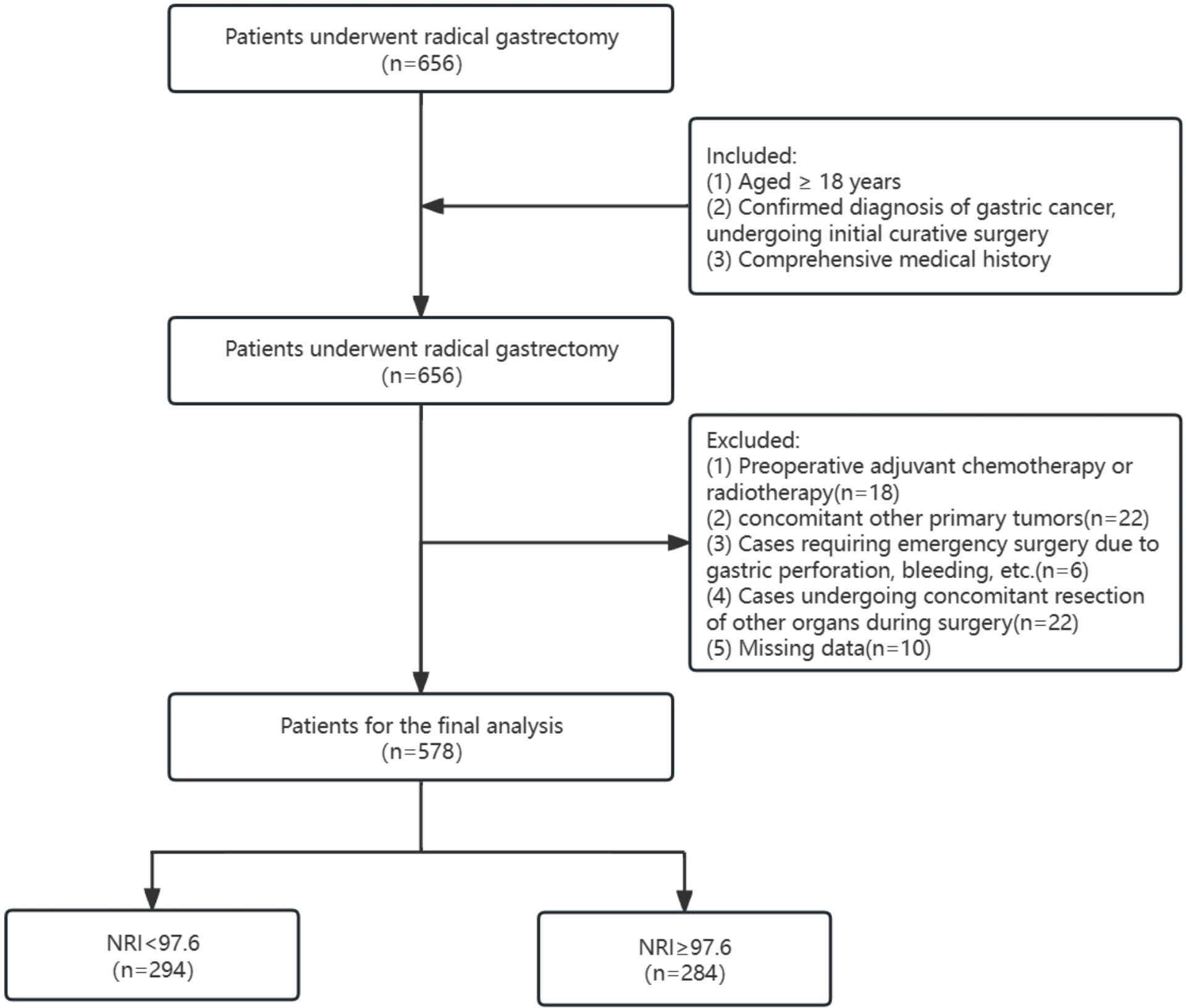
This study retrospectively included patients who underwent radical gastrectomy for gastric cancer in the Department of Gastrointestinal Surgery at the First Affiliated Hospital of Guangxi Medical University from January 2019 to June 2023. All patient data were followed up by dedicated personnel for 30 days after the surgery. The inclusion criteria were as follows: (1) age ≥ 18 years; (2) confirmed diagnosis of gastric cancer and initial curative surgery; and (3) comprehensive medical history. The exclusion criteria were (1) preoperative adjuvant chemotherapy or radiotherapy; (2) concurrent primary tumors; (3) cases requiring emergency surgery because of gastric perforation and bleeding; and (4) cases undergoing concomitant resection of other organs during surgery. A total of 578 patients were included in this study, which employed a retrospective research design and obtained a waiver for informed consent. The study was approved by the ethics committee of the First Affiliated Hospital of Guangxi Medical University (2023-k293-01).
Data collection and definitions
We collected several variables, including (1) general information: gender, age, BMI, smoking history, drinking history, comorbidities (hypertension, diabetes, coronary heart disease, and cerebral infarction); (2) laboratory parameters: albumin, hemoglobin, prealbumin, C-reactive protein, WBC, CA199, and AFP; (3) data related to surgical and tumor characteristics: gastrectomy, surgical approach, ASA class, TNM staging, tumor differentiation, intraoperative blood transfusion, operation time, intraoperative blood loss, preoperative nutritional intervention; (4) outcome variables: complications within 30 days after surgery. Use the following formula to calculate the NRI (11): NRI = 1.519 × albumin (g/L) + [41.7 × (weight/Wlo)]. Ideal weight (WLo) is calculated from height (H) and gender with the Lorenz formula (14). For men, Wlo (kg)=H (cm)−100−[(H−150)/4]. For women, Wlo (kg)=H(cm)–100−[(H−150)/2.5]. When the actual weight is greater than the ideal weight, the actual weight/ideal weight is taken as 1. The study population was divided into two groups according to the optimal cutoff value for the results of the ROC analysis: the low NRI group(NRI<96.7)and the high NRI group(NRI≥96.7). The Clavien–Dindo Classification system was used in categorizing postoperative complications into grades I, II, III, IV, and V (15). The presence of more than one complication in a patient was recorded as the highest level of complication. Frequent postoperative complications in patients with gastric cancer included pulmonary infection, gastrointestinal fistula, gastric atony, pleural effusion and others.
Statistical analysis
Continuous variables were presented as either mean ± standard deviation or median (interquartile range), whereas categorical variables were depicted as numbers and percentages (%). Intergroup comparisons were performed using t-tests, chi-square tests, and Fisher’s exact tests. The receiver operating characteristic (ROC) curve was used in determining the optimal cutoff value for NRI. Model 1 remained unadjusted, Model 2 was adjusted for age, sex, and BMI, Model 3 included adjustments for the variables in Model 2 and gastrectomy, surgical approach, intraoperative blood transfusion, operation time, and intraoperative blood loss, and Model 4 included adjustments for the variables in Model 3 and preoperative nutritional intervention. Subgroup analysis was conducted to confirm the reliability of the results, and stratification was based on gender, age (<60 and ≥60 years), BMI, gastrectomy, surgical approach, TNM staging, intraoperative blood transfusion and preoperative nutritional intervention. The cumulative incidence plot was employed, and a log-rank test was used to assess the difference in the cumulative risk of postoperative complications within 30 days between the low- and high-NRI groups. We excluded variables with missing values exceeding 20%, and for continuous variables with less than 5% missing values, the mean or median was used for replacement. All studies analyzed data using the statistical software packages R (http://www.R-project.org, R Foundation) and Free Statistics software version 1.8. For all analyses, a two-tailed P<0.05 was considered statistically significant.
Results
Baseline characteristics of patients
A total of 656 patients underwent gastric cancer surgery between January 2019 and June 2023, and 578 eligible patients were finally enrolled for analysis after the exclusion of patients with other surgical procedures and missing key data (Figure 1). The optimal cutoff value for NRI was 96.7 according to ROC curve analysis, and the study population was categorized into low-NRI (<96.7) and high-NRI (≥96.7) groups (Supplementary Figure 1).
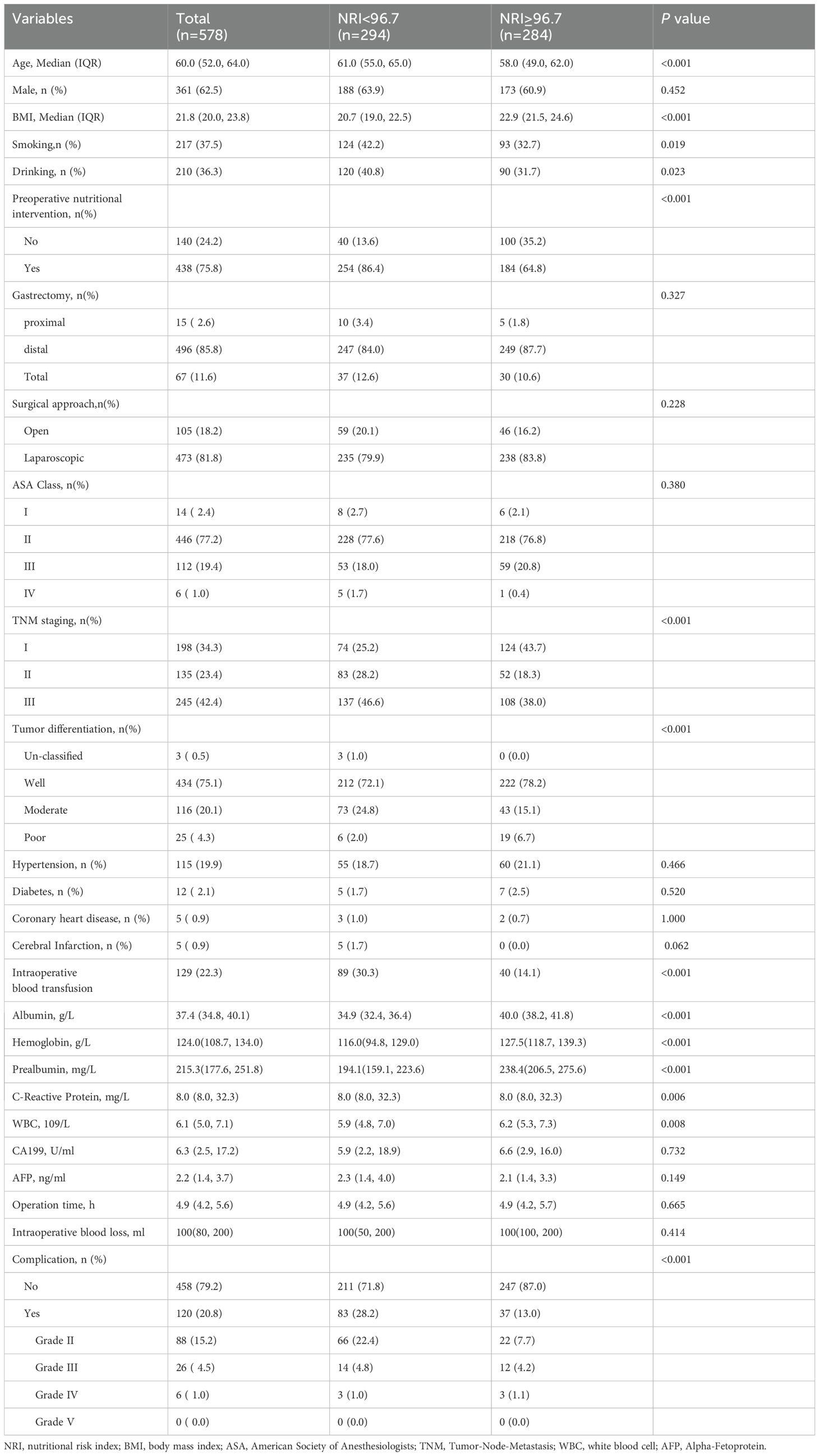
The baseline characteristics of the patients are summarized in Table 1. The median age was 60.0 (52.0, 64.0), and 361 (62.5%) were male. A total of 120 (20.8%) patients suffered from postoperative complications. There were 84 (70%) pulmonary infections, 10 (8.3%) GI fistulas, 4 (3.3%) gastroparesis, 4 (3.3%) were pleural effusions, and 18 (15%) other cases in Supplementary Table 1. The two groups were compared in terms of age, preoperative nutritional intervention, BMI, smoking, drinking, TNM staging, tumor differentiation, intraoperative blood transfusion, albumin, hemoglobin, prealbumin, C-reactive protein, and WBC. The differences were all statistically significant (P<0.05). In addition, patients in the low-NRI group presented a higher incidence of postoperative complications than those in the high-NRI group (28.2% vs. 13%), including grades II (22.4% vs. 7.7%),grade III (4.8% vs. 4.2%) and grade IV (1.0% vs. 1.1%). Additionally, the baseline characteristics of patients with and without complications are presented in Supplementary Table 2. Patients with complications had lower NRI values and were more likely to be male compared with patients without complications. Patients with complications had a higher chance of intraoperative blood transfusion and blood loss and required longer operative times than those without.
Association between NRI and 30-day postoperative complications
Univariate analysis of the factors affecting 30-day postoperative complications is displayed in Supplementary Table 3. Gender, age, NRI, albumin, hemoglobin, prealbumin, gastrectomy, preoperative nutritional intervention, intraoperative blood loss, operation time, surgical approach, and intraoperative blood transfusion may be associated with postoperative complications(P<0.05).
The results of the multivariate Cox regression analyses are presented in Table 2, including unadjusted model 1, partially adjusted model 2, partially adjusted model 3,and fully adjusted model 4. In fully adjusted model 4, NRI was treated as a continuous variable, and a negative correlation was found between NRI and the incidence of 30-day postoperative complications. Specifically, for every 1 unit increase in NRI, a 7% decrease in the risk of 30-day postoperative complications was found (HR=0.93, 95% CI:0.90–0.96, P<0.001). This negative association was maintained in unadjusted model 1, partially adjusted model 2, partially adjusted model 3,and fully adjusted model4. When NRI was used as a categorical variable in the uncorrected model 1, the risk of complications at 30 days postoperatively was higher in the low NRI group compared with the high NRI group (HR=2.63, 95% CI: 1.71–4.03; P<0.001), and this association remained significant even after full adjustments for variables (HR=2.89, 95% CI: 1.71–4.88; P<0.001).
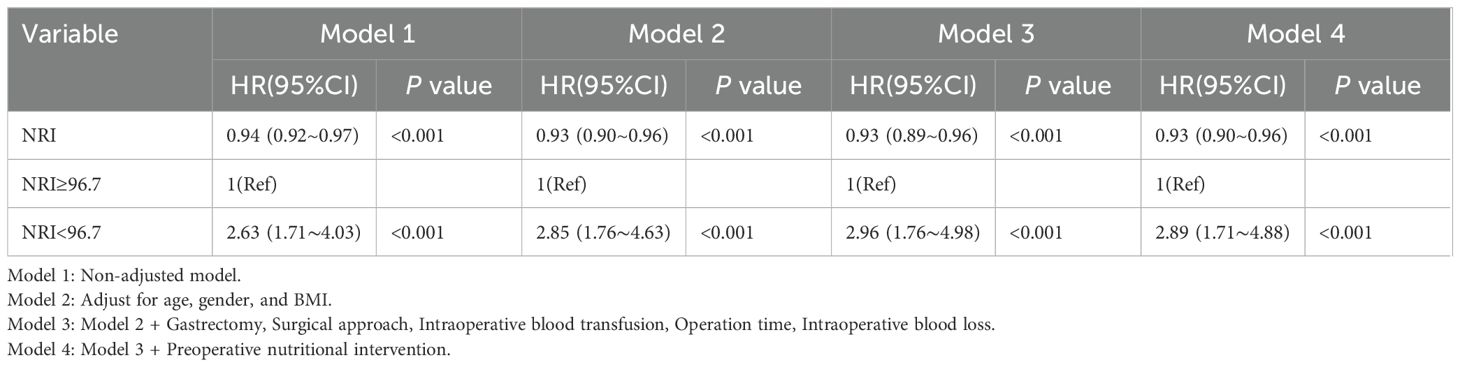
Table 2. Multivariable Cox regression to assess the association of NRI with 30-day postoperative complications.
Subgroup analysis
We further performed subgroup analysis to test whether the association between NRI and 30-day postoperative complications was stable across subgroups. As shown in Figure 2, no significant interactions were observed in the subgroups in terms of gender, age, BMI, gastrectomy, surgical approach, TNM staging, intraoperative blood transfusion and preoperative nutritional intervention after adjusting for confounders (P for interaction > 0.05). This result indicates that the results of the negative association between NRI and the risk of 30-day postoperative complications in patients with gastric cancer remain robust regardless of baseline patient characteristics.
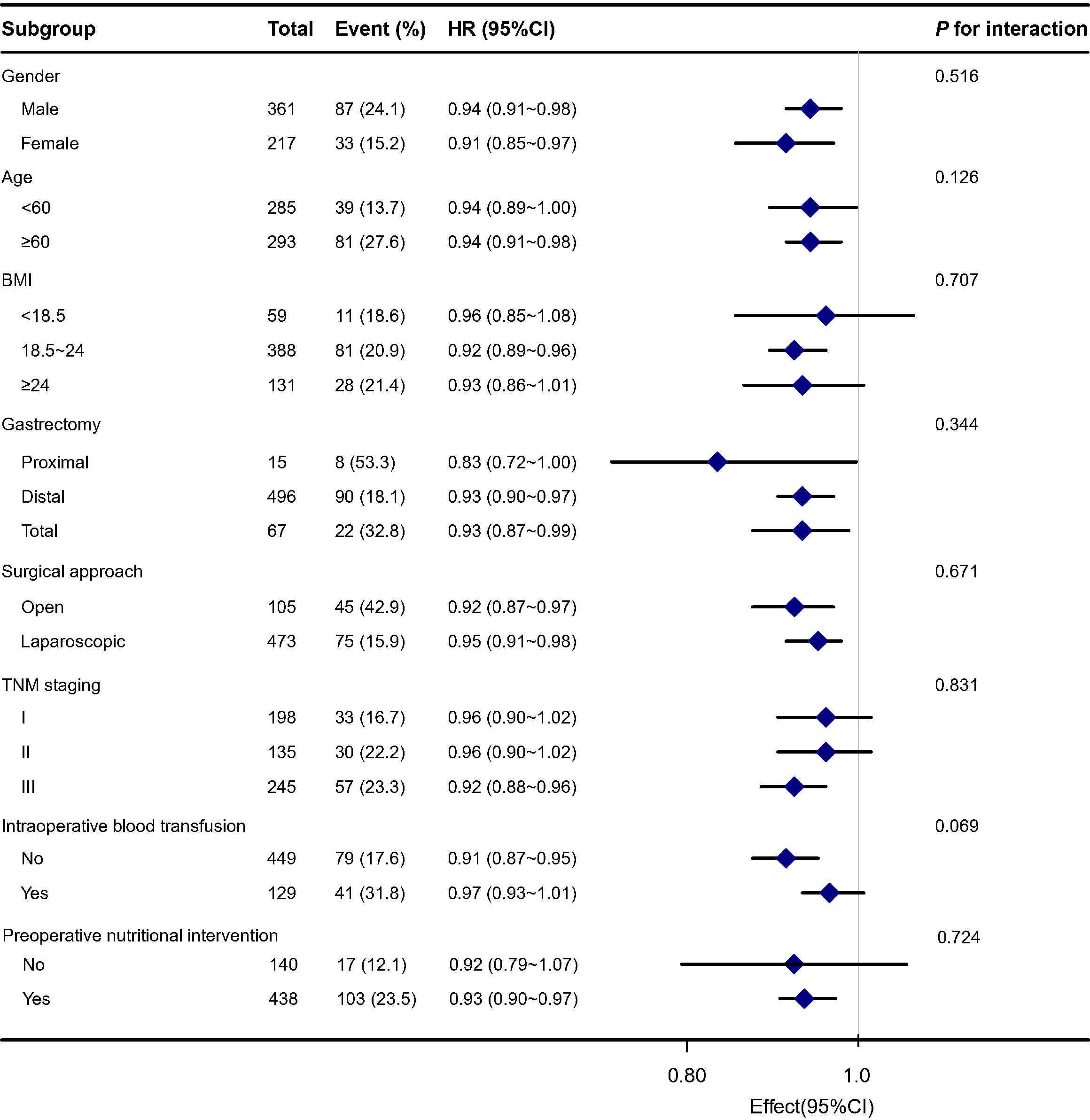
Figure 2. Subgroup analysis for the association between preoperative NRI and 30-day postoperative complications.
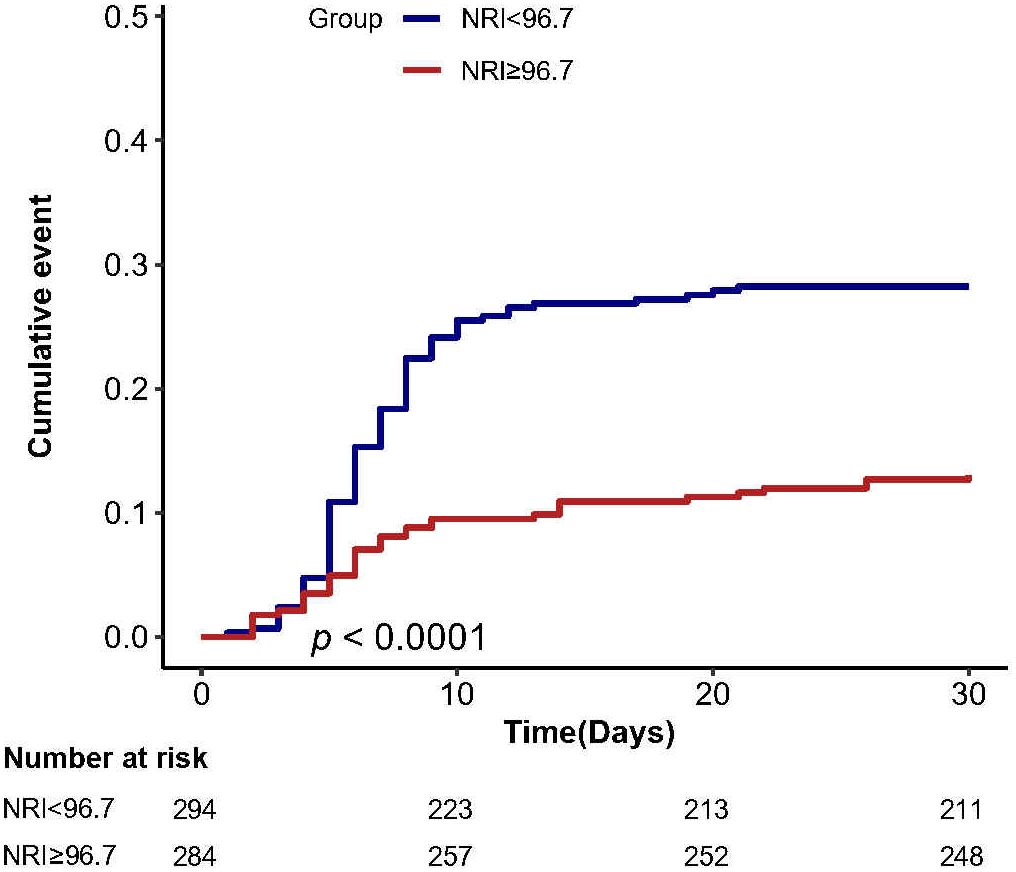
Cumulative incidence analysis
The cumulative incidence of complications at 30 days postoperatively showed that patients in the high NRI group (NRI ≥ 96.7) had a lower cumulative risk of 30-day postoperative complications compared with the low NRI group (NRI < 96.7) (Figure 3).
Discussion
Key findings
A significant correlation was found between preoperative NRI levels and the incidence of 30-day postoperative complications in patients with gastric cancer. The incidence of 30-day postoperative complications in 578 gastric cancer surgery patients was 20.8%, and the incidence of postoperative complications was significantly higher in the low-NRI group than in the high-NRI group. After adjusting for confounders, the risk of 30-day postoperative complications was 2.89 times higher in the low-NRI group than in the high-NRI group. Apparently, preoperative NRI is an independent protective factor against 30-day postoperative complications in patients with gastric cancer.
Relation to previous research
Nutritional conditions have been recognized as an important factor affecting the prognoses of cancer patients, attracting considerable interest (16–18). Malnutrition is common in patients with gastric cancer, having prevalence rates ranging from approximately 29.1% to 80.4% (19–21), and is associated with poor outcomes (16–18). In some studies, NRI as an indicator of nutritional assessment is mostly used preoperatively for the prediction of the postoperative outcomes of related diseases (22, 23). Notably, it is implemented using serum albumin and weight to assess the nutritional status of patients and can be independent of subjective factors. NRI demonstrates strong prognostic value in various populations, such as in cardiac surgery (12), breast cancer (13), or head and neck cancer (24, 25). Its clinical outcomes in patients with gastric cancer are inconsistent. Kim et al. assessed preoperative NRI scores in 958 stage II and III gastric cancer and found that patients with higher NRI scores significantly outperformed those with lower scores in terms of survival (26). Song et al. (27)specifically investigated the prognostic ability of NRI in stage III gastric cancer patients, demonstrating that NRI had prognostic significance with an optimal cutoff value of 99, correlating with short survival times. Similar conclusions were drawn from other studies on gastric cancer (28–30). However, these studies examined patient survival and did not explore postoperative complications. A previous study (31) focused only on the relationship between low preoperative NRI levels and wound complications after gastrectomy (NRI≥97.5 vs. NRI<97.5: OR=0.653, 95% CI:0.326–0.974, P=0.014). Furthermore, Choi et al. (32)found through meta-analysis that those with gastric cancer identified as malnourished (NRI<97.5) had a higher risk of postoperative wound complications compared to those with good nutritional status (NRI≥97.5). However, these studies did not examine other common postoperative complications, such as pneumonia, pulmonary embolism, and heart failure. In this study, we assessed 30-day postoperative complications in patients with gastric cancer by adjusting for confounders, revealing a strong association between NRI and postoperative complications. The results in our subgroup analysis were consistent with the primary results, suggesting that the results of the independent effect of NRI on 30-day postoperative complications in patients with gastric cancer have high reliability and clinical applicability. Cumulative incidence analysis showed that patients with low NRI had a higher risk of postoperative complications compared with the high NRI group. In addition, we found no deaths (Complications: Grade V) within 30 days postoperatively in both the low and high NRI groups. This may be attributed to the relatively short duration of the study, the optimization of clinical management, and the limitations of the sample size. Previous study (27) suggest that the low NRI group may present a higher risk of death at longer follow-up (1, 3 and 5 years).
The NRI, a quantitative laboratory marker, integrates serum albumin levels and body weight, which reflect the nutritional status and degree of immune suppression in patients with gastric cancer (33, 34). Serum albumin is not only an indicator of nutritional status but is also strongly associated with systemic inflammatory status (35). Inflammatory factors released by tumors may affect liver function, inhibit albumin synthesis, and even lead to the structural degeneration of albumin, considerably reducing serum albumin levels (36, 37). In addition, body weight is an important indicator of nutritional status, which directly affects the tolerance of patients with gastric cancer patients (38). Body weight plays an independent predictive role in the prognoses of patients with gastric cancer (39, 40). A low NRI score implies malnutrition and immunosuppression in patients, thus leading to a low tolerance for surgery or chemotherapy and predisposed to poor clinical outcomes. In summary, malnutrition and immunosuppression are the likely main factors affecting poor clinical outcomes in patients with gastric cancer. In addition to these factors, NRI assessment may involve other unknown mechanisms affecting prognosis, which need to be explained by further in-depth studies.
Impact on clinical practice
The main significance of this study is that our findings suggest that NRI is strongly associated with 30-day postoperative complications in patients undergoing gastric cancer surgery. These findings will prompt healthcare professionals to identify high-risk patients and aid in the development of a preoperative nutritional management plan to improve the nutritional status of preoperative patients with gastric cancer and provide effective nutritional interventions. The aim was to reduce the rate of postoperative complications. In addition, our findings may contribute to future clinical studies of individualized nutritional control in gastric cancer patients to test whether the preoperative optimization of NRI can improve patients’ prognosis and adverse outcomes.
Limitations
Our study has several limitations. First, it is a retrospective study, and despite our efforts to control potential confounding factors, we were unable to assess certain covariates that may have influenced the results. Second, the study only investigated the impact of NRI on early postoperative complications in patients with gastric cancer, without evaluating long-term postoperative outcomes. Third, it failed to assess the changes in NRI values after preoperative nutritional interventions and the possible impact on the cumulative incidence of postoperative complications.Despite adjusting for preoperative nutritional intervention status in multivariate models, the lack of standardized data on preoperative nutritional support limited our ability to assess its causal role. Future intervention studies should prioritize this variable to elucidate potential treatment effects. Lastly, we only collected clinical data from a single hospital, and thus the generalizability of the study findings may be limited. Future multicenter prospective studies are necessary to confirm our findings.
Conclusion
Our study demonstrates that NRI plays a critical role in predicting adverse outcomes in gastric cancer patients, and that preoperative NRI levels correlate with the incidence of 30-day postoperative complications in patients with gastric cancer. These findings provide valuable guidance for clinical providers and emphasize the importance of accurately assessing and effectively managing populations with poor nutritional status.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The First Affiliated Hospital of Guangxi Medical University (2023-k293-01). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Retrospective studies exempt patient informed consent.
Author contributions
YZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. LL: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – review & editing. KJ: Formal Analysis, Methodology, Project administration, Writing – review & editing. LT: Methodology, Project administration, Writing – review & editing. MH: Methodology, Project administration, Writing – review & editing. DH: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study has received funding from the National Natural Science Foundation of China (NO.82060430) and Guangxi Clinical Research Center for Critical Care Medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1475381/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Tan ZY. Recent advances in the surgical treatment of advanced gastric cancer: A review. Med Sci Monitor. (2019) 25:3537–41. doi: 10.12659/MSM.916475
3. Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, et al. Multi-institutional analysis of the prognostic significance of postoperative complications after curative resection for gastric cancer. Cancer Med. (2019) 8:5194–201. doi: 10.1002/cam4.v8.11
4. Chen QC, Zheng YL, Chen JH, Zhou J, Zhao J, Bi X, et al. Differences of intraoperative outcomes and postoperative complications between intrahepatic cholangiocarcinoma and colorectal liver metastasis in different surgical methods. Transl Cancer Res. (2021) 10:4020–32. doi: 10.21037/tcr-21-553
5. He YL, Liu HR, Ma YH, Li J, Zhang J, Ren Y, et al. Preoperative prognostic nutritional index predicts short-term complications after radical resection of distal cholangiocarcinoma. Front Surg. (2023) 9. doi: 10.3389/fsurg.2022.1091534
6. Hong QQ, Yan S, Zhao YL, Fan L, Yang L, Zhang WB, et al. Machine learning identifies the risk of complications after laparoscopic radical gastrectomy for gastric cancer. World J Gastroentero. (2024) 30. doi: 10.3748/wjg.v30.i1.79
7. Riad AM, Collaborative G, Surg NGHUG, et al. Impact of malnutrition on early outcomes after cancer surgery: an international, multicentre, prospective cohort study. Lancet Glob Health. (2023) 11:E341–E9. doi: 10.1016/S2214-109X(22)00550-2
8. Sasahara M, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al. The preoperative prognostic nutritional index predicts short-term and long-term outcomes of patients with stage II/III gastric cancer: analysis of a multi-institution dataset. Digest Surg. (2020) 37:135–44. doi: 10.1159/000497454
9. Virizuela JA, Camblor-Álvarez M, Luengo-Pérez LM, Grande E, Álvarez-Hernández J, Sendrós-Madroño MJ, et al. Nutritional support and parenteral nutrition in cancer patients: an expert consensus report. Clin Trans Oncol. (2018) 20:619–29. doi: 10.1007/s12094-017-1757-4
10. Liver EAS. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. (2019) 70:172–93. doi: 10.1016/j.jhep.2018.06.024
11. Group VATPNCS. Perioperative total parenteral nutrition in surgical patients. New Engl J Med. (1991) 325:525–32. doi: 10.1056/NEJM199108223250801
12. Barge-Caballero E, García-López F, Marzoa-Rivas R, Barge-Caballero G, Couto-Mallón D, Paniagua-Martín MJ, et al. Prognostic value of the nutritional risk index in heart transplant recipients. Rev Esp Cardiol. (2017) 70:639–45. doi: 10.1016/j.recesp.2016.11.015
13. Chen L, Qi YH, Kong XY, Su Z, Wang Z, Wang X, et al. Nutritional risk index predicts survival in patients with breast cancer treated with neoadjuvant chemotherapy. Front Nutr. (2022) 8. doi: 10.3389/fnut.2021.786742
14. Li L, Lu X, Qin S, and Huang D. Association between geriatric nutritional risk index and 28 days mortality in elderly patients with sepsis: a retrospective cohort study. Front Med. (2023) 10:1258037. doi: 10.3389/fmed.2023.1258037
15. Dindo D, Demartines N, and Clavien PA. Classification of surgical complications - A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
16. Matsuda T, Umeda Y, Matsuda T, Endo Y, Sato D, Kojima T, et al. Preoperative prognostic nutritional index predicts postoperative infectious complications and oncological outcomes after hepatectomy in intrahepatic cholangiocarcinoma. BMC Cancer. (2021) 21. doi: 10.1186/s12885-021-08424-0
17. Hirahara N, Tajima Y, Fujii Y, Kaji S, Kawabata Y, Hyakudomi R, et al. Prediction of postoperative complications and survival after laparoscopic gastrectomy using preoperative Geriatric Nutritional Risk Index in elderly gastric cancer patients. Surg Endosc. (2021) 35:1202–9. doi: 10.1007/s00464-020-07487-7
18. Yu J, Hong B, Park JY, Hwang JH, and Kim YK. Impact of prognostic nutritional index on postoperative pulmonary complications in radical cystectomy: A propensity score-matched analysis. Ann Surg Oncol. (2021) 28:1859–69. doi: 10.1245/s10434-020-08994-6
19. Seo SH, Kim SE, Kang YK, Ryoo BY, Ryu MH, Jeong JH, et al. Association of nutritional status-related indices and chemotherapy-induced adverse events in gastric cancer patients. BMC Cancer. (2016) 16. doi: 10.1186/s12885-016-2934-5
20. Guo ZQ, Yu JM, Li W, Fu ZM, Lin Y, Shi YY, et al. Survey and analysis of the nutritional status in hospitalized patients with Malignant gastric tumors and its influence on the quality of life. Support Care Cancer. (2020) 28:373–80. doi: 10.1007/s00520-019-04803-3
21. Na BG, Han SS, Cho YA, Wie GA, Kim JY, Lee JM, et al. Nutritional status of patients with cancer: A prospective cohort study of 1,588 hospitalized patients. Nutr Cancer. (2018) 70:1228–36. doi: 10.1080/01635581.2019.1578392
22. Özbilgin S, Hanci V, Ömür D, Özbilgin M, Tosun M, Yurtlu S, et al. Morbidity and mortality predictivity of nutritional assessment tools in the postoperative care unit. Medicine. (2016) 95. doi: 10.1097/MD.0000000000005038
23. Shinkawa H, Takemura S, Uenishi T, Sakae M, Ohata K, Urata Y, et al. Nutritional risk index as an independent predictive factor for the development of surgical site infection after pancreaticoduodenectomy. Surg Today. (2013) 43:276–83. doi: 10.1007/s00595-012-0350-2
24. Jiao Z, Liang CC, Luo GF, Liu M, Jiang K, Yang A, et al. Prognostic utility of nutritional risk index in patients with head and neck soft tissue sarcoma. Nutrients. (2023) 15. doi: 10.3390/nu15030641
25. Oh J, Liu A, Tran E, Berthelet E, Wu J, Olson RA, et al. Association between nutritional risk index and outcomes for head and neck cancer patients receiving concurrent chemo-radiotherapy. Head Neck-J Sci Spec. (2020) 42:2560–70. doi: 10.1002/hed.26315
26. Kim KW, Lee K, Lee JB, Park T, Khang S, Jeong H, et al. Preoperative nutritional risk index and postoperative one-year skeletal muscle loss can predict the prognosis of patients with gastric adenocarcinoma: a registry-based study. BMC Cancer. (2021) 21. doi: 10.1186/s12885-021-07885-7
27. Song HB, Sun HK, Yang LS, Gao H, Cui Y, Yu C, et al. Nutritional risk index as a prognostic factor predicts the clinical outcomes in patients with stage III gastric cancer. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.880419
28. Li GM, He LJ, and Sun H. Nutritional risk index predicts the prognosis of gastric cancer patients with pyloric stenosis who received preoperative parenteral nutrition. Oncol Lett. (2023) 26. doi: 10.3892/ol.2023.13988
29. Fujiya K, Kawamura T, Omae K, Makuuchi R, Irino T, Tokunaga M, et al. Impact of malnutrition after gastrectomy for gastric cancer on long-term survival. Ann Surg Oncol. (2018) 25:974–83. doi: 10.1245/s10434-018-6342-8
30. Karabulut S, Dogan I, Usul Afsar C, Karabulut M, Ak N, Duran A, et al. Does nutritional status affect treatment tolerability, chemotherapy response and survival in metastatic gastric cancer patients? Results of a prospective multicenter study in Turkey. J Oncol Pharm Pract. (2022) 28:127–34. doi: 10.1177/1078155220987291
31. Oh CA, Kim DH, Oh SJ, Choi MG, Noh JH, Sohn TS, et al. Nutritional risk index as a predictor of postoperative wound complications after gastrectomy. World J Gastroentero. (2012) 18:673–8. doi: 10.3748/wjg.v18.i7.673
32. Choi WJ and Kim J. Nutritional care of gastric cancer patients with clinical outcomes and complications: A review. Clin Nutr Res. (2016) 5:65–78. doi: 10.7762/cnr.2016.5.2.65
33. Urakawa S, Yamasaki M, Goto K, Haruna M, Hirata M, Morimoto-Okazawa A, et al. Peri-operative monocyte count is a marker of poor prognosis in gastric cancer: increased monocytes are a characteristic of myeloid-derived suppressor cells. Cancer Immunol Immun. (2019) 68:1341–50. doi: 10.1007/s00262-019-02366-0
34. Lu W, Shen J, Zou DH, Li P, Liu X, and Jian Y. Predictive role of preoperative geriatric nutritional risk index for clinical outcomes in surgical gastric cancer patients: A meta-analysis. Front Surg. (2022) 9. doi: 10.3389/fsurg.2022.1020482
35. Evans DC, Corkins MR, Malone A, Miller S, Mogensen KM, et al. The use of visceral proteins as nutrition markers: an ASPEN position paper. Nutr Clin Pract. (2021) 36:22–8. doi: 10.1002/ncp.10588
36. Zhang QQ, Zhang LL, Jin Q, He Y, Wu M, Peng H, et al. The prognostic value of the GNRI in patients with stomach cancer undergoing surgery. J Pers Med. (2023) 13. doi: 10.3390/jpm13010155
37. Alwarawrah Y, Kiernan K, and MacIver NJ. Changes in Nutritional Status impact immune Cell Metabolism and Function. Front Immunol. (2018) 9. doi: 10.3389/fimmu.2018.01055
38. Zhang R, Li H, Li N, Shi JF, Li J, Chen HD, et al. Risk factors for gastric cancer: a large-scale, population-based case-control study. Chin Med J-Peking. (2021) 134:1952–8. doi: 10.1097/CM9.0000000000001652
39. Ahmed M, von Itzstein MS, Sheffield T, Khan S, Fattah F, Park JY, et al. Association between body mass index, dosing strategy, and efficacy of immune checkpoint inhibitors. J immunotherapy Cancer. (2021) 9. doi: 10.1136/jitc-2021-002349
Keywords: gastric cancer, nutritional risk index, postoperative complication, surgery, cancer
Citation: Zou Y, Li L, Jia K, Tian L, He M and Huang D (2025) Effect of preoperative nutritional risk index on 30-day postoperative complications in patients with gastric cancer: a retrospective cohort study. Front. Oncol. 15:1475381. doi: 10.3389/fonc.2025.1475381
Received: 03 August 2024; Accepted: 28 May 2025;
Published: 16 June 2025.
Edited by:
Stefano Turi, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Dina Keumala Sari, Universitas Sumatera Utara, IndonesiaEster Oneda, Fondazione Poliambulanza Istituto Ospedaliero, Italy
Copyright © 2025 Zou, Li, Jia, Tian, He and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Debin Huang, aGRlYkBzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Yingfeng Zou
Yingfeng Zou Ling Li
Ling Li Kui Jia1
Kui Jia1