- 1Departments of Radiology, Luxian People’s Hospital, Luzhou, Sichuan, China
- 2Departments of Radiology, West China Second Hospital of Sichuan University, Chengdu, Sichuan, China
- 3Departments of Pathology, West China Hospital of Sichuan University, Chengdu, Sichuan, China
- 4Departments of Pathology, Luxian People’s Hospital, Luzhou, Sichuan, China
- 5Departments of Radiology, West China Hospital of Sichuan University, Chengdu, Sichuan, China
Collision tumors are defined as the coexistence of two or more histologically distinct tumor types at the same anatomical site. Mediastinal collision tumors constitute an exceptionally rare subtype of these lesions. Clinically and radiologically, the preoperative diagnosis is particularly difficult due to their complex pathological composition and nonspecific manifestations. Misdiagnoses are common in clinical practice, even with preoperative needle biopsy. Radiologic identification of collision tumors is essential to ensure comprehensive sampling for guiding appropriate treatments. Herein, we report an extremely rare case of mediastinal collision tumor diagnosed by contrast-enhanced chest computed tomography (CT), with pathology confirming type AB thymoma and well-differentiated adenocarcinoma (likely of malignant foregut cyst origin). This case report aims to raise awareness of this rare tumor and to discuss the imaging features based on a systematic review of the literature.
Introduction
Collision tumors represent a rare condition in which neoplasms of different histologic origins coexist within the same organ, attached at their margins but without significant intermingling of cellular components. The different components of a collision tumor are anatomically separated from each other, usually by a thin stromal layer or their respective basement membranes (1). Collision tumors have been reported in various organs, including the esophagus (2–4), stomach (5, 6), larynx (7, 8), kidneys (9–11), intracranial region (12, 13), intraocular region (14), pancreas (15), bladder (16), ovaries (17), uterus (18) and other organs. Most collision tumors occur in the digestive or endocrine organs, whereas those located in the mediastinum are exceedingly rare. Mediastinal collision tumors are frequently misdiagnosed or underdiagnosed in clinical practice due to the differing imaging manifestations caused by the independent and primary nature of the tumor components, as well as their adjoining and mixed locations. However, CT serves as an essential tool for diagnosing and differentiating mediastinal collision tumors by precisely demonstrating the tumor location, dimensions, margin characteristics, adjacency, lymph node metastasis, tumor infiltration and providing comprehensive diagnostic information for clinical practice. Here, we present the clinical, radiological, and histopathological characteristics of a rare mediastinal collision tumor to improve recognition of this entity and to guide clinical management and postoperative follow-up.
Case description
A 49-year-old woman presented with exertional dyspnea had persisted for more than one month, without concomitant symptoms such as fever, cough, or chest pain. Her medical history included an appendectomy performed 20 years ago. Family history was notable for maternal death from lung cancer and fraternal death from hepatocellular carcinoma. Physical examination revealed no remarkable abnormalities. Hematologic analysis revealed leukopenia (leukocyte count: 3.23×109/L; reference range: 3.5-9.5×109/L) and normal tumor markers (CEA: 0.62 ng/mL [<5.00]; CA19-9: 13.50 U/mL [<30]). The endocrine profile showed decreased ACTH (<1.00 ng/L; reference: 5.00-78.00) and decreased renin activity (supine: 0.88 μIU/mL [reference: 2.80-39.90]; upright: <0.50 μIU/mL [reference: 4.40-46.10]). All catecholamine metabolites, including plasma free metanephrines and 24-hour urinary vanillylmandelic acid (VMA), were within normal ranges, with no other clinically significant abnormalities. Contrast-enhanced CT images demonstrated a well-circumscribed, round mass (8.1×7.9 cm) in the right paracardiac mediastinum, showing predominantly soft-tissue attenuation with internal hypodense septations. A markedly hyperenhancing component was observed within the lesion, while another isodense lesion was seen adjacent to the mass with no significant enhancement. The bronchus of the middle lobe of the right lung was compressed, adjacent to the right cardiac border, and the right atrium was compressed (Figures 1A-D). No destruction of the sternum or ribs was observed, and no pleural effusion was present.
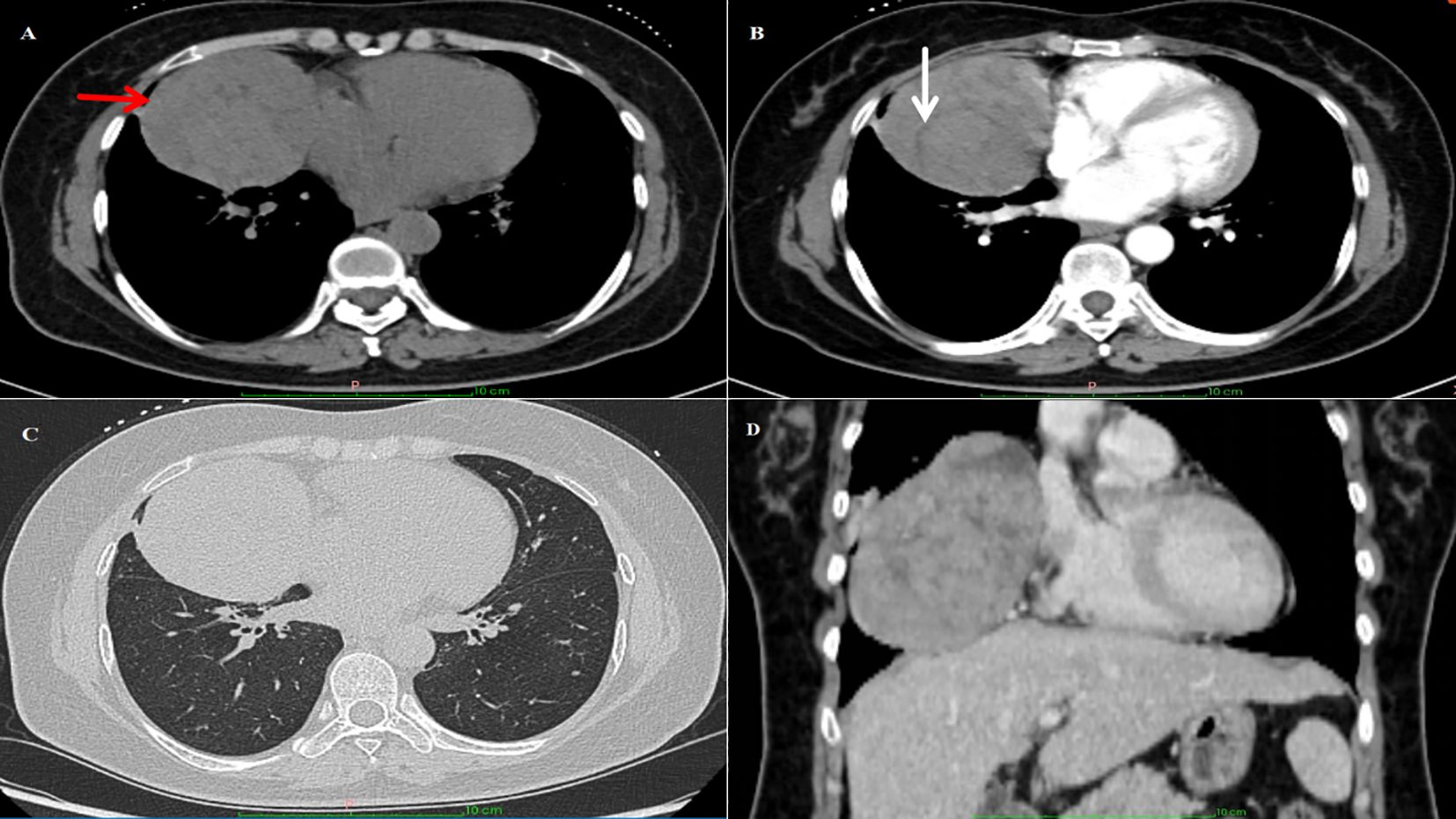
Figure 1. Imaging findings: (A) CT image of the chest showing a large irregular mass near the anterior mediastinum of the right thorax. The red arrow highlights the location of the tumor. (B) The white arrow marks the linear low-density area within the tumor. (C, D) The mass compressed the bronchi in the middle lobe of the right lung, and the right atrium was mildly compressed.
The patient underwent complete mass resection and thymectomy via a right-sided thoracoscopic approach. Grossly, the mass was observed as grayish-white tumor tissue with an intact capsule and firm consistency, located in the right mediastinal plane. Upon sectioning, the tumor demonstrated cystic-solid components containing gelatinous fluid, with smooth inner and outer walls, no adhesion to the adjacent pleura, and no invasion into lung lobes or pericardium. The immunohistochemistry results revealed the following: glandular component CK19 (+), CDX-2 (weakly +), PCK (+), CK7 (small foci +), CK20 (+), negative for SATB2, PAX-2, WT-1, ER, P16, TTF-1, PAX-8, and P53, with Ki-67 index of 5–10%. Thymic component P63 (+), background lymphocytes TdT (+), CD1a (partially +), and CD20 (focally +). No lower mutation peak at codon 424 of exon 15 of the GTF2I gene was not found, and codon 12 and 13 mutations of the KRAS gene were not detected. The final pathological diagnosis was collision tumor, type AB thymoma + well-differentiated adenocarcinoma (likely of malignant foregut cyst origin) (Figures 2A-D). Postoperatively, the patient experienced an uneventful recovery without recurrence. The decision to forgo adjuvant chemotherapy/radiotherapy was based on histologically confirmed R0 resection and low-risk pathological features (lymphovascular invasion-negative, pT1N0M0 stage).
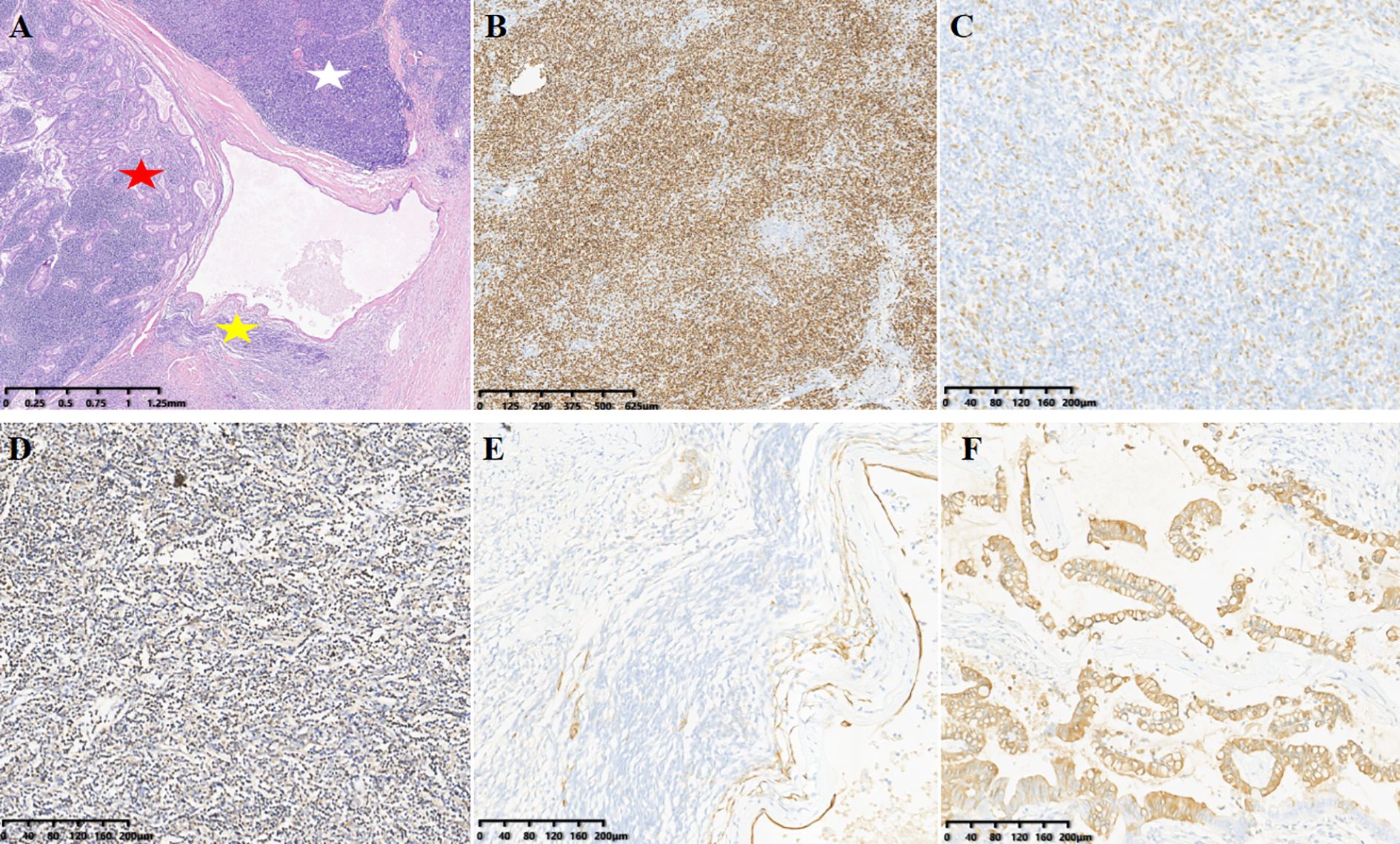
Figure 2. (A) (H&E): Magnification, x20. The white star indicates Type B thymoma, the yellow star indicates Type A thymoma, and the red star indicates adenocarcinoma. (B-F) (IHC) show specific markers: [(B–D), Type B thymoma)]. (B) background lymphocytes TdT(+), magnification, ×40. (C) Thymic component p63(+), magnification, ×100. (D) CD1a(partially +), magnification, ×100. [(E), Type A thymoma] CK(Pan)(+), magnification, ×100. [(F) adenocarcinoma] CK20(focally +), magnification, ×40.
Discussion
Collision tumors are a distinct clinicopathological entity characterized by the coexistence of two or more histologically distinct neoplastic components within a single anatomical site, maintaining their respective tissue origins and juxtaposed without transitional zones or migratory changes (7). Although collision tumors are clinically uncommon, mediastinal cases are exceptionally rare. Previously reported mediastinal collision tumor combinations have included gastric adenocarcinoma and esophageal cancer (2, 19), synovial sarcoma and arteriovenous (AV) malformation (20), embryonal rhabdomyosarcoma and benign teratoma (21), B1B2 thymoma and small lymphocytic lymphoma (22). The present case demonstrates a mediastinal collision tumor composed of type AB thymoma and well-differentiated adenocarcinoma (likely of malignant foregut cyst origin). To the best of our knowledge, this tumor combination has not been previously documented in the anterior mediastinum.
The pathogenetic mechanisms underlying the diverse clinical manifestations and nonspecific imaging features of collision tumors remain incompletely understood. Several pathogenic hypotheses have been proposed for mediastinal collision tumors: First, the tumor microenvironment theory suggests that the development of one tumor may alter the local microenvironment, facilitating the growth of a second primary or metastatic tumor. Second, the accidental concurrence theory proposes that two independent tumors may coincidentally arise at the same anatomical site. Third, the tumor genetics theory postulates that clonal cells with genetic heterogeneity can differentiate into histologically distinct tumor populations (23). Finally, prior trauma, surgical intervention, or radiation therapy has been implicated as potential etiological factors.
The clinical manifestations of mediastinal collision tumors are nonspecific and primarily depend on the tumor location and histological composition. Anterior mediastinal collision tumors typically present with cough (20), chest pain, dyspnea (21), or myasthenia (22). Tumors located at the esophagogastric junction most frequently cause dysphagia, food obstruction sensation, and retrosternal discomfort (2, 19). A proportion of cases remain asymptomatic and are detected incidentally during routine physical examination.
Preoperative evaluation plays a crucial role in treatment planning and prognosis assessment. Contrast-enhanced CT, with its superior spatial and density resolution combined with multiplanar reformation, enables accurate lesion detection and evaluation, thus establishing a reliable basis for surgical strategy formulation. Although CT is widely used as a primary diagnostic tool, its soft-tissue resolution remains limited. Future studies should incorporate multiparametric imaging protocols to improve clinicopathological correlation, particularly by integrating contrast-enhanced MRI for neurovascular invasion assessment along with 18F-FDG PET-CT for metabolic profiling, metastasis surveillance, and TNM staging refinement.
Regarding CT characteristics of thymomas, some studies have reported that type AB thymomas typically demonstrate multiple markedly enhancing nodules, hypodense septations, and intratumoral penetrating vessels (24). Most foregut cysts are predominantly located in the middle and posterior mediastinum, with malignant transformation being exceptionally rare (25). On CT imaging, foregut cysts appear as round or oval masses with smooth margins, showing fluid/soft-tissue density on unenhanced scans without contrast enhancement. In the current case, the anterior mediastinal collision tumor presented as a rounded mass with well-defined borders, heterogeneous attenuation, predominantly showing soft-tissue attenuation, enhanced nodules and hypodense septations, findings consistent with type AB thymoma. Adjacent to this was a second soft-tissue mass that appeared isointense without enhancement, features suggestive of jelly-like fluid content. Although thymomas are the most common anterior mediastinal tumors, foregut cysts in this location remain exceedingly uncommon.
Mediastinal collision tumors exhibit diverse imaging characteristics due to the distinct origins of their tumor components. The diagnosis of mediastinal collision tumor should be considered when there is a marked disparity in boundaries and enhancement patterns between two adjacent tumors, especially when typical imaging features of two different tumor types are present without a unifying pathological explanation. Given the rarity of these tumors, accurate identification of both morphological characteristics and histological components is essential to avoid misdiagnosis. Furthermore, accumulating additional case data and conducting long-term follow-up are crucial for advancing understanding of the disease and assessing treatment outcomes.
A review of the literature combined with this case report has identified that the main features of lesions requiring imaging differentiation from mediastinal collision tumors include: 1) Noninvasive thymoma, is characterized by regular morphology, homogeneous soft-tissue attenuation, absence of mediastinal or vascular invasion, and uniform enhancement; 2) Invasive thymoma, demonstrates irregular contours, heterogeneous attenuation, marked heterogeneous enhancement, extensive cystic degeneration/necrosis, obliteration of mediastinal fat planes, and growth along the pericardial/thymic planes; 3) Non-Hodgkin lymphoma(NHL), presents with smooth margins, homogeneous density, rare calcification, variable enhancement, and small necrotic foci with sharp margins, often invading adjacent structures and accompanied by lymphadenopathy; 4) Teratoma, typically occurs in younger patients, containing calcifications/dental structures, which frequently causes hemoptysis, with well-demarcated borders and heterogeneous attenuation; 5) Yolk sac tumor, predominantly unilocular cystic lesions with ill-defined margins and local invasion; 6) Mediastinal lung carcinoma, shows spiculated margins, lobulation, and with high-grade features at the lung-tumor interface, with homogeneous or heterogeneous enhancement and metastatic propensity; 7) Paraganglioma, exhibits intermediate soft-tissue attenuation with cystic changes, heterogeneous enhancement, intralesional vascularity, mass effect without frank invasion, and requiring confirmation of biochemical catecholamine levels.
In summary, collision tumors in the anterior mediastinum are exceedingly rare, particularly those comprising thymoma and adenocarcinoma. Their clinical and imaging features often overlap with more common tumors in this region, potentially leading to diagnostic oversight. Definitive diagnosis ultimately requires postoperative histopathological examination. Nevertheless, comprehensive imaging evaluation provides essential information regarding tumor location, dimensions, margin characteristics, adjacency, lymph node metastasis and tumor infiltration, thereby delivers critical diagnostic and prognostic information for clinical management and follow-up.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZK: Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing, Formal Analysis, Resources, Validation. RX: Supervision, Validation, Writing – review & editing. WW: Data curation, Visualization, Writing – original draft. KY: Data curation, Visualization, Writing – original draft. YL: Investigation, Resources, Writing – original draft. LZ: Conceptualization, Data curation, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Sichuan Science and Technology Program (2023YFQ0095).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1495627/full#supplementary-material
References
1. Kim SH, Kim YJ, Park BK, Cho JY, Kim BH, and Byun JY. Collision tumors of the ovary associated with teratoma: clues to the correct preoperative diagnosis. J Comput Assist Tomogr. (1999) 23:929–33. doi: 10.1097/00004728-199911000-00017
2. Spagnolo DV and Heenan PJ. Collision carcinoma at the esophagogastric junction: report of two cases. Cancer. (1980) 46:2702–8. doi: 10.1002/1097-0142(19801215)46:12<2702::aid-cncr2820461228>3.0.co;2-m
3. Wang L, Zhan C, Ma J, Shi Y, and Wang Q. Collision tumor of esophagus: report of three cases. Ann Thorac Surg. (2014) 97:1075–7. doi: 10.1016/j.athoracsur.2013.06.090
4. Choe AR, Shim KN, Lim J, Song EM, Tae CH, Jung SA, et al. A collision tumor of the esophagus: mixed squamous cell carcinoma and neuroendocrine carcinoma. Korean J Gastroenterol. (2020) 75:207–11. doi: 10.4166/kjg.2020.75.4.207
5. Matsuno K, Kanazawa Y, Kakinuma D, Hagiwara N, Ando F, Masuda Y, et al. Preoperatively diagnosed gastric collision tumor with mixed adenocarcinoma and gastrointestinal stromal tumor: a case report and literature review. Clin J Gastroenterol. (2021) 14:494–9. doi: 10.1007/s12328-021-01343-4
6. Kitagawa H, Kaneko M, Kano M, Ibuki Y, Amatya VJ, Takeshima Y, et al. Coexistence of gastrointestinal stromal tumor and leiomyosarcoma of the stomach presenting as a collision tumor: A case report and review of literature. Pathol Int. (2018) 68:313–7. doi: 10.1111/pin.12662
7. Coca-Pelaz A, Triantafyllou A, Devaney KO, Rinaldo A, Takes RP, and Ferlito A. Collision tumors of the larynx: A critical review. Am J Otolaryngol. (2016) 37:365–8. doi: 10.1016/j.amjoto.2016.02.01
8. De Luca P, Scarpa A, Viola P, Motta G, Iacobelli A, Gencarelli A, et al. Collision tumors of the larynx: A retrospective single-center case series of an extremely rare phenomenon. Oral Oncol. (2022) 134:106096. doi: 10.1016/j.oraloncology.2022.106096
9. Lamprou S, Glykas I, Fragkoulis C, Theodoropoulou G, Koutsonikas G, and Papadopoulos G. Collision kidney tumor with clear cell renal cell carcinoma and papillary type 1 renal cell carcinoma. A case report and review of the literature. Urologia. (2022) 89:304–6. doi: 10.1177/03915603211001673
10. Lall C, Houshyar R, Landman J, Verma S, Goyenechea M, Bhargava P, et al. Renal collision and composite tumors: imaging and pathophysiology. Urology. (2015) 86:1159–64. doi: 10.1016/j.urology.2015.07.032
11. Goyal R, Parwani AV, Gellert L, Hameed O, and Giannico GA. A collision tumor of papillary renal cell carcinoma and oncocytoma: case report and literature review. Am J Clin Pathol. (2015) 144:811–6. doi: 10.1309/AJCPQ0P1YHDBZUFL
12. Zhang Z, Yang Y, Zhang K, Zhuang J, Shao F, Liu H, et al. Collision tumor of glioblastoma and meningioma: case report and literature review. World Neurosurg. (2018) 117:137–41. doi: 10.1016/j.wneu.2018.05.246
13. Ashizawa K, Ogura K, Nagase S, Sakaguchi A, Tokugawa J, Hishii M, et al. A collision tumor of solitary fibrous tumor/hemangiopericytoma and meningioma: A case report with literature review. Pathol Int. (2021) 71:697–706. doi: 10.1111/pin.13150
14. Coupland SE, Dodson A, Liu H, Du MQ, Angi M, and Damato BE. Intraocular collision tumor: case report and literature review. Graefes Arch Clin Exp Ophthalmol. (2013) 251:1383–8. doi: 10.1007/s00417-012-2216-0
15. Serafini S, Da Dalt G, Pozza G, Blandamura S, Valmasoni M, Sperti C, et al. Collision of ductal adenocarcinoma and neuroendocrine tumor of the pancreas: a case report and review of the literature. World J Surg Oncol. (2017) 15:93. doi: 10.1186/s12957-017-1157-9
16. Jiang W, Pan C, Guo W, Xu Z, Ni Q, and Ruan Y. Pathologic collision of urinary bladder urothelial carcinoma with small cell carcinoma: a case report. Diagn Pathol. (2023) 18:80. doi: 10.1186/s13000-023-01369-x
17. Peng Y, Lin J, Guan J, Chen L, Zhang X, Li S, et al. Ovarian collision tumors: imaging findings, pathological characteristics, diagnosis, and differential diagnosis. Abdom Radiol (NY). (2018) 43:2156–68. doi: 10.1007/s00261-017-1419-6
18. Sharma S, Vasdev N, and Pandey D. Uterine collision tumor of endometrial stromal sarcoma and endometrioid adenocarcinoma: A rare case report and review of literature. Indian J Pathol Microbiol. (2021) 64:802–5. doi: 10.4103/IJPM.IJPM_701_20
19. Lim SK, Sampson CC, and Warner OG. Simultaneous primary carcinomas–a report of three cases. J Natl Med Assoc. (1981) 73:413–7.
20. Bakula A, Fiel-Gan M, Levinson M, Buckley T, Volpe J, and Lowe R. Synovial sarcoma-AV malformation collision in the anterior mediastinum. Conn Med. (2015) 79:87–91.
21. Cushing B, Bhanot PK, Watts FB Jr, Hertzler JH, and Brough AJ. Rhabdomyosarcoma and benign teratoma. Pediatr Pathol. (1983) 1:345–8. doi: 10.3109/15513818309040672
22. Szolkowska M, Langfort R, Winiarski S, Zaremba J, Prochorec-Sobieszek M, Rymkiewicz G, et al. Two neoplasms rich in small lymphocytes, B1B2 thymoma and small lymphocytic lymphoma, intermingled in one tumor mass. A case report. Pol J Pathol. (2017) 68:75–81. doi: 10.5114/pjp.2017.67620
23. Fujii H, Zhu XG, Matsumoto T, Inagaki M, Tokusashi Y, Miyokawa N, et al. Genetic classification of combined hepatocellular-cholangiocarcinoma. Hum Pathol. (2000) 31:1011–7. doi: 10.1053/hupa.2000.9782
24. Liu W, xia J, luo W, and Ge Z. CT diagnosis of type AB thymoma. J Clin Radiol. (2016) 35:1187–9. doi: 10.13437/j.cnki.jcr.2016.08.012
Keywords: collision tumor, foregut cyst, type AB thymoma, adenocarcinoma, computed tomography
Citation: Kang Z, Xu R, Wang W, Yan K, Liao Y and Zhang L (2025) Anterior mediastinal collision tumor of type AB thymoma and adenocarcinoma: a case report. Front. Oncol. 15:1495627. doi: 10.3389/fonc.2025.1495627
Received: 16 September 2024; Accepted: 25 April 2025;
Published: 16 May 2025.
Edited by:
Hussein M. El-Husseiny, Tokyo University of Agriculture and Technology, JapanReviewed by:
Mohamed Elbadawy, Benha University, EgyptYao Tian, Tianjin Medical University General Hospital, China
Copyright © 2025 Kang, Xu, Wang, Yan, Liao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lizhi Zhang, bGl0dGNoZV8yMDAwQDE2My5jb20=
 Zhongqin Kang
Zhongqin Kang Rong Xu
Rong Xu Weiya Wang3
Weiya Wang3