- 1Department of Clinical Laboratory, The First Affiliated Hospital of Hunan Normal University, Changsha, China
- 2Department of Research and Development, Human Stem Cell National Engineering Research Center, Changsha, China
Background: Intrahepatic cholangiocarcinoma (ICC) is a highly aggressive malignancy with poor prognosis, and there is currently a lack of effective prognostic prediction models. The aim of this study was to develop a novel nomogram model based on blood tests for predicting predictors of progression free survival (PFS) in ICC patients.
Methods: A total of 99 ICC patients (70 for training, 29 for validation) were included in this study. Hematological indices and clinicopathological data were collected from ICC patients undergoing surgical resection. The independent predictors of PFS were screened by univariate and multivariate Cox regression analysis, and a nomogram model was constructed. The calibration curve was used to evaluate the consistency between the observed results and the predicted probability, and the model discrimination was evaluated by receiver operating characteristic curve (ROC). According to the risk score calculated by the constructed nomogram, patients were divided into high-risk and low-risk groups, and the predictive performance of nomogram was further tested by Kaplan Meier.
Results: The median follow-up time of this study was 7.8 months (range: 1 ~ 69 months). We found that pathological differentiation, CA19-9, neutrophil-to-lymphocyte ratio (NLR) and after-treatment Monocyte count (MON)/before-treatment MON (tMON) were independent factors affecting the PFS of postoperative ICC patients. Based on risk factors, a nomogram prediction model was constructed. ROC analysis revealed that the area under the curve (AUC) of the nomogram for predicting PFS was higher than the AJCC-TNM staging system(P<0.05). The calibration curve and decision curve analysis (DCA) showed that the nomogram had high prognostic accuracy and clinical applicability. The risk score calculated by nomogram could divide ICC patients into high-risk and low-risk groups. The median PFS of the high-risk group was significantly shorter than that of the low-risk group (P <0.05).
Conclusion: The nomogram can serve as a valuable supplementary tool for predicting PFS in ICC patients after initial surgical resection. Its performance is better than the traditional TNM staging system. The model provides clinicians with an individualized prognostic assessment tool by integrating easily available blood markers, which is helpful to optimize postoperative monitoring and adjuvant treatment strategies.
Introduction
Cholangiocarcinoma is the second most common type of liver cancer after hepatocellular carcinoma, accounting for 10-15% of all primary malignant tumors of the liver. Over the past decade, the incidence and mortality rates of cholangiocarcinoma have been increasing globally (1). Although radical surgical resection is still the only potential cure, about 65% of patients have recurrence and metastasis within two years after surgery, resulting in a 5-year overall survival rate of less than 30% (2). This critical situation underscores the importance of accurate progression-free survival (PFS) prediction for individualized postoperative management.
Current clinical practice predominantly relies on the AJCC 8th edition TNM staging system for prognostic assessment. However, this anatomy-based classification system demonstrates significant limitations, with only modest predictive accuracy (C-index ≈0.60-0.65), making it inadequate for guiding individualized treatment strategies (3, 4). Although recent studies have attempted to develop predictive models by incorporating genomic/proteomic signatures and radiomic features, their clinical translation remains limited due to either high testing costs or the requirement for tissue specimens that preclude dynamic monitoring (5).
Serum biomarkers represent a promising avenue for refining prognostic tools owing to their noninvasive nature and capacity for repeated measurements. Emerging evidence suggests that individual blood biomarkers—including CA19-9– – (6), systemic inflammation indices (e.g., NLR, PLR), and liver function markers (e.g., ALBI score)—may correlate with ICC progression (7). However, a multidimensional blood parameter-based model for predicting PFS remains to be established. In this study, we aim to establish a blood-based PFS prediction nomogram for ICC patients by analyzing the baseline characteristics of ICC patients and the levels and changes of blood markers before and after treatment.
Patients and methods
Patients
This study retrospectively included a total of 99 patients with ICC who underwent initial surgical resection treatment at Hunan Provincial People’s Hospital between January 2020 and December 2023. The inclusion criteria for patient selection were as follows: 1) Pathological confirmation of ICC; 2) No prior anti-tumor treatment before pathological diagnosis; 3) ECOG performance status score of 0 or 1. The exclusion criteria consisted of the following: 1) Prior anticancer treatment before admission; 2) History of other malignancies; 3) Patients with metastatic bile duct cancer; 4) Incomplete clinical data and incomplete laboratory examination data before and after treatment. This retrospective study was approved by the Ethics Committee of Hunan Provincial People’s Hospital, and informed consent requirements were waived.
Laboratory examination
The laboratory test indicators of liver function-related tests including total protein (TP), albumin (ALB), globulin (GLB), total bilirubin (TBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bile acids (TBA) were measured using a Hitachi LABOSPECT 008 AS fully automated biochemical analyzer. Blood routine examinations: neutrophil (NEU) count, lymphocyte (LYM) count, MON count, red blood cell (RBC) and platelet (PLT) count were measured using the Sysmex XN-9000 automated hematology analyzer. Coagulation-related tests: prothrombin time (PT), international normalized ratio of prothrombin time (INR), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen (FIB) were analyzed using the Sysmex CS-2500 automated coagulation analyzer. Alpha-fetoprotein (AFP) and CA19–9 were quantitatively measured using the Roche Cobas e601 electrochemiluminescence immunoassay analyze. The calculated values in this study were determined using the following formulas: NLR = neutrophil count/lymphocyte count, tMON = after-treatment MON/before-treatment MON.
Statistical analysis
Categorical variables were presented as the number of cases (percentage), and chi square test was used for difference analysis. All continuous variables were first evaluated for their normal distribution characteristics by Shapiro Wilk test. For variables that conform to normal distribution, we use the mean ± standard deviation (mean ± SD) to describe; For non-normally distributed variables, the median and interquartile range are used. Mann-Whitney U test was used for comparison. All potential predictive factors (including clinicopathological characteristics and blood test indicators) were first screened by univariate Cox regression analysis, and variables significantly associated with PFS (p<0.05) were selected as candidate factors. These candidate factors were then entered into the multivariate Cox regression analysis, and the variables with p<0.05 were finally determined as independent predictors. Based on the results of multivariate Cox regression analysis, a nomogram was generated using the CPH function of R package RMS. The prediction performance of the model was evaluated by ROC, calibration curve and DCA decision curve. Finally, the risk score of each patient was calculated according to the nomogram, and the patients were divided into high-risk group and low-risk group according to the score. Kaplan Meier method was used to compare PFS between high-risk group and low-risk group. P<0.05 means the difference is statistically significant. SPSS 26.0 statistical software and R language (4.4.2) software were used for statistical analysis and nomogram drawing.
Results
Patient characteristics
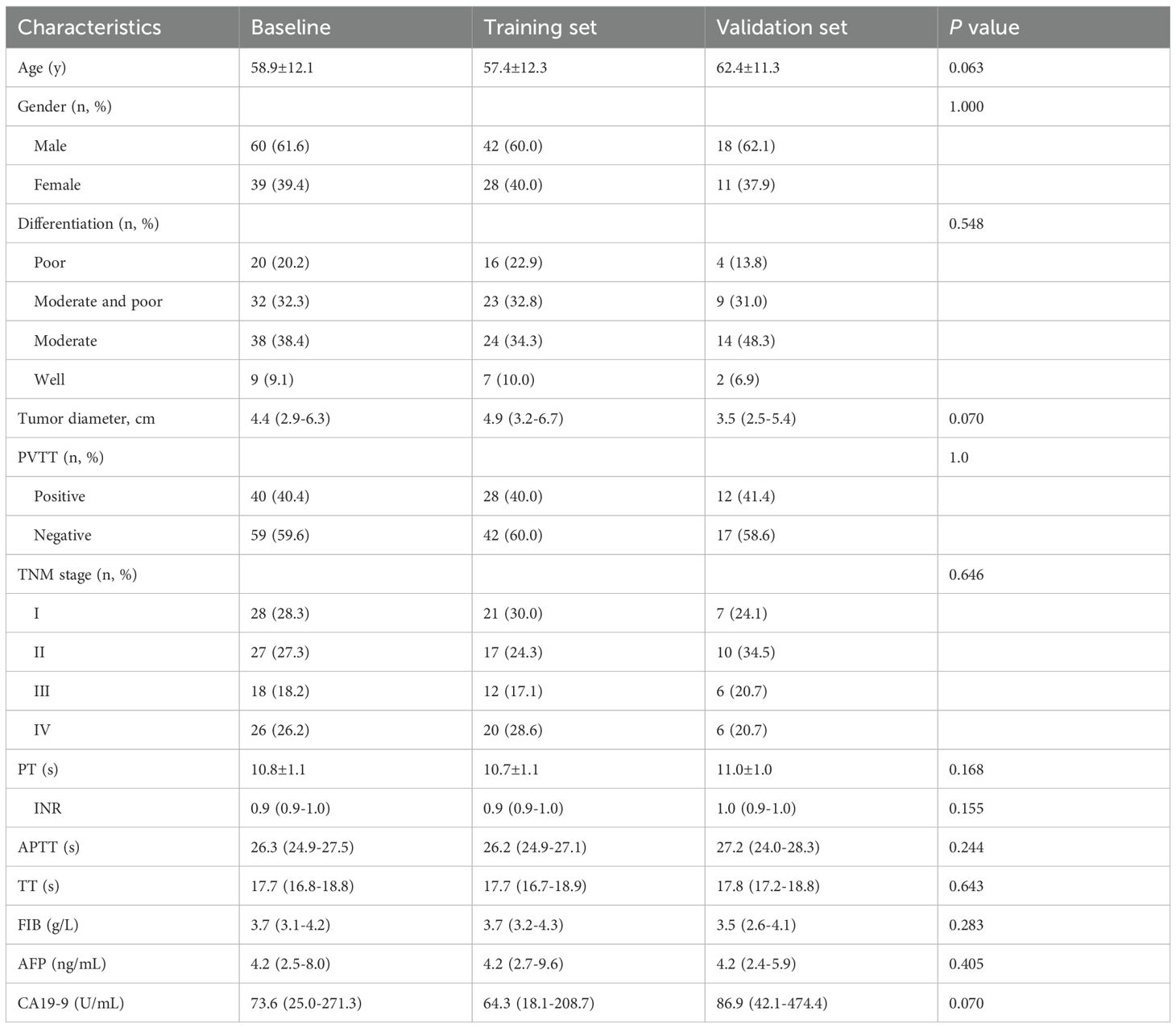
A total of 99 patients (60 males and 39 females) in this study were randomly divided into two groups (training set, n=70 cases; validation set, n=29 cases). The clinical demographics data of the training and validation sets are presented in Table 1. The mean age of the patients was 58.9 ± 12.1 years, and the tumor diameter was 4.4 (2.9-6.3) cm. Based on radiological data evaluation, 40 out of the 99 patients had portal vein tumor thrombus (PVTT). The median PFS for ICC patients in this study was 7.8 months (ranging from 1 to 69 months), and the half-year, one year, and two years PFS rates were 58.6%, 32.3%, and 13.1%, respectively.
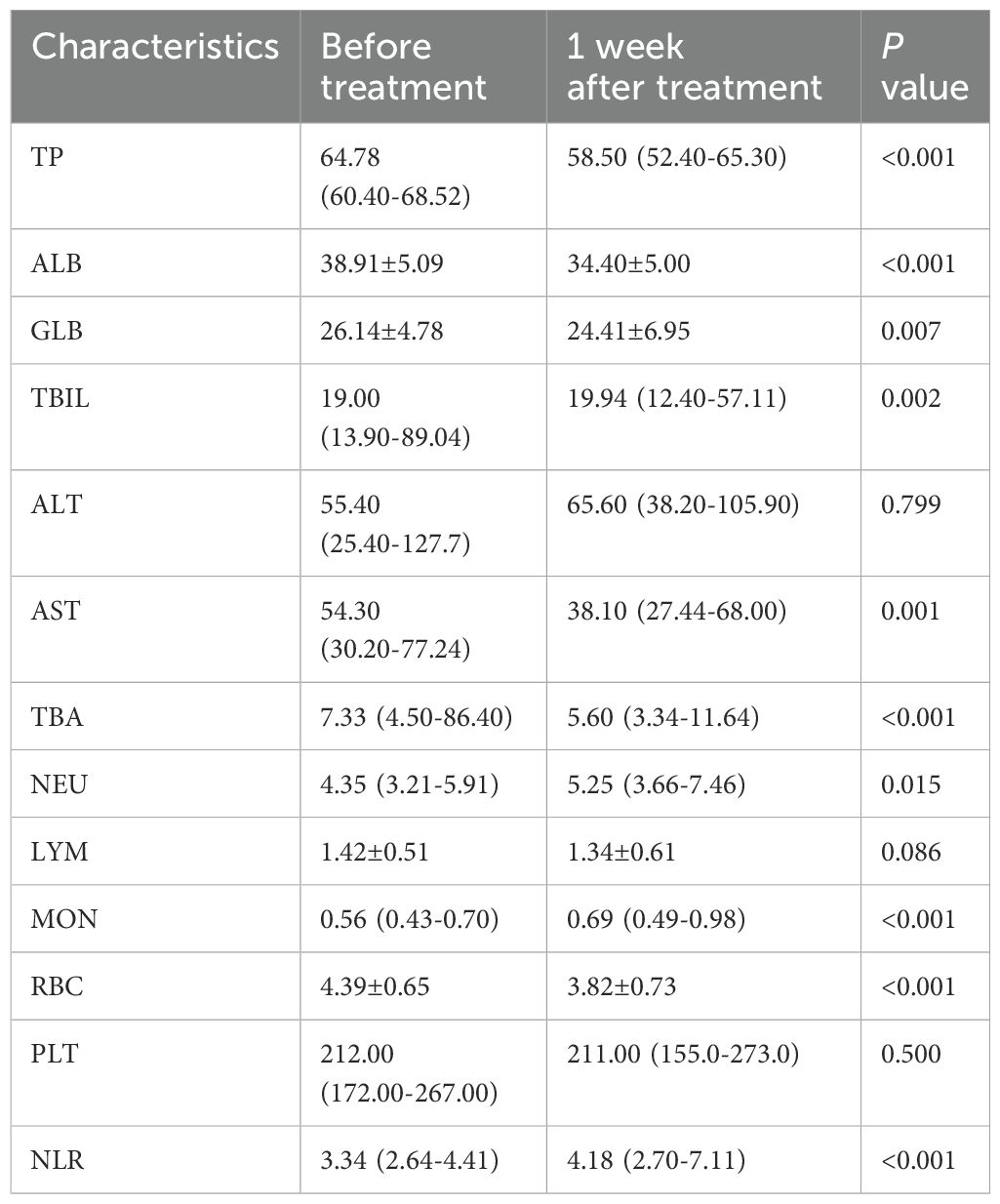
Levels of blood parameters before and after surgical treatment
We analyzed the levels and changes of blood biomarkers in ICC patients before and after surgical treatment. The results showed that most blood markers changed significantly one week after treatment compared to before treatment. Levels of TP, ALB, GLB, TBIL, AST, TBA and RBC decreased significantly after one week of treatment, while levels of NEU, MON and NLR increased significantly. Changes in ALT, LYM and PLT before and after treatment were not significant. Details of blood marker levels before and after treatment are described in Table 2.
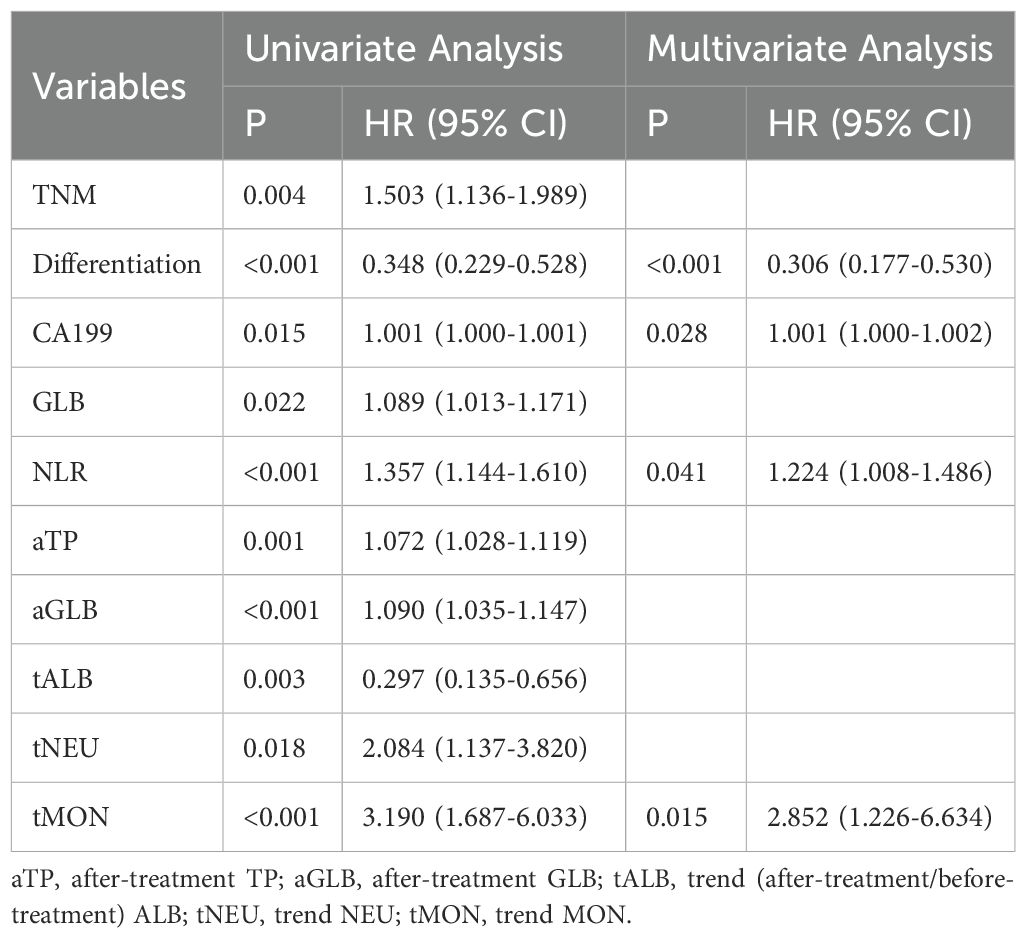
Screening for prognostic factors related to PFS in ICC patients
Univariate Cox regression analysis was used to explore the correlation between PFS and each clinical factor and blood marker in the training set. The results revealed that lower tumor pathological differentiation correlated with greater patient risk, and higher TNM staging was associated with increased patient risk. The blood markers such as CA19-9, GLB, NLR, after-treatment TP (aTP), after-treatment GLB (aGLB), trend NEU (tNEU) and tMON were identified as risk factors, while trend ALB (tALB) were identified as protective factors (Table 3).
Establishment of prognostic nomogram for evaluating PFS in ICC patients
Multivariable Cox regression analysis was performed on the factors selected through univariate Cox regression analysis in the training set, including clinical factors and candidate blood indicators. Among all clinical factors, pathological differentiation was the only statistically significant independent prognostic factor. Within the hematological indicators, CA19-9, NLR and tMON were identified as independent prognostic factors for ICC patients’ PFS (Table 3).
A nomogram was established based on the results of the multivariable Cox regression analysis in the training set (Figure 1A). Calibration plots were generated to assess the agreement between predicted and actual PFS rates at half-year, one-year, and two-year intervals. The calibration curves for these intervals showed substantial overlap with the standard curve (Figure 1B). This indicates that the nomogram has good predictive efficacy. The performance of the nomogram was further evaluated using ROC curves. The AUCs of the nomogram for predicting PFS at six months, one year, and two years were 0.884, 0.913, and 0.911, respectively (Figure 1C). The nomogram showed significantly better performance compared to the commonly used AJCC TNM staging system, for the AJCC TNM system, the AUCs were 0.791, 0.704, 0.581 at the corresponding time points (Supplementary Figure 1A). Based on the established nomogram, scores were calculated for each patient, and further stratification divided patients into low-risk (below median score of 85) and high-risk groups (above median score). The median PFS was 14.3 months (429 days) in the low-risk group and 4 months (121 days) in the high-risk group (P < 0.0001, Figure 1D)
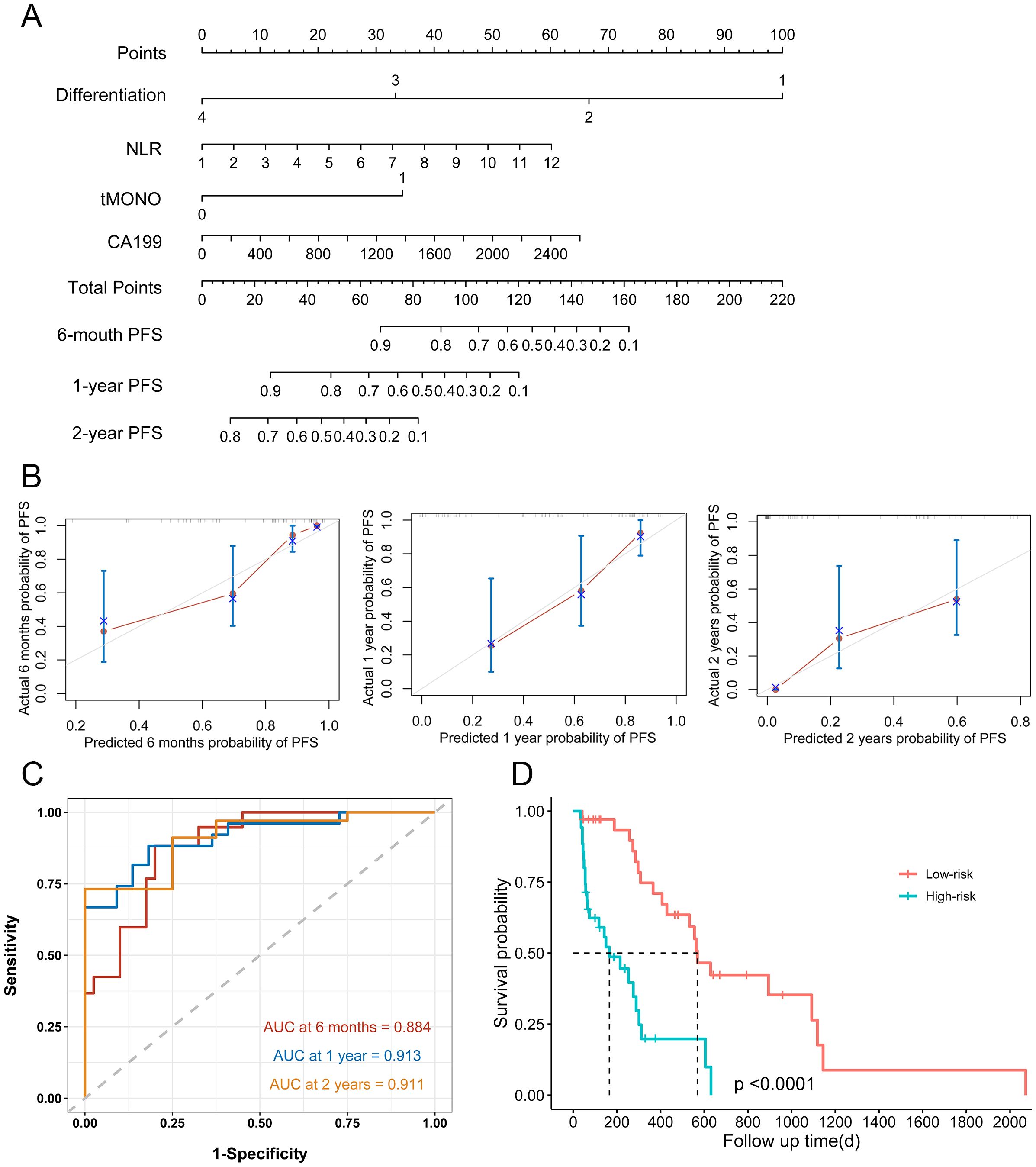
Figure 1. Nomogram for PFS in patients with ICC after surgical resection. (A) Nomogram model for half-year, 1-year, and 2-year PFS in the training set. (B) Calibration curves plots of nomogram for predicting half-year, 1-year, and 2-year probability of PFS in ICC patients after surgical resection in the training set (C) ROC curves of the nomogram in the training set. (D) Prognostic assessment and risk stratification of developed nomogram model in the training set.
Validation the prognostic nomogram model
To validate the nomogram model, we introduced a validation set. The calibration plot demonstrated moderate agreement between predicted and observed outcomes (Figure 2A). For predicting progression-free survival (PFS), the nomogram achieved AUCs of 0.900, 0.768, and 0.885 at six months, one year, and two years, respectively, in the validation cohort (Figure 2B). For the AJCC TNM system, the AUCs were 0.591, 0.649, 0.730 at the corresponding time points (Supplementary Figure 1B). Using the same cutoff value as in the training set, we stratified the validation cohort into low- and high-risk groups. The median PFS was significantly longer in the low-risk group (14.3 months) than in the high-risk group (4 months; P = 0.0073), confirming robust risk stratification (Figure 2C).
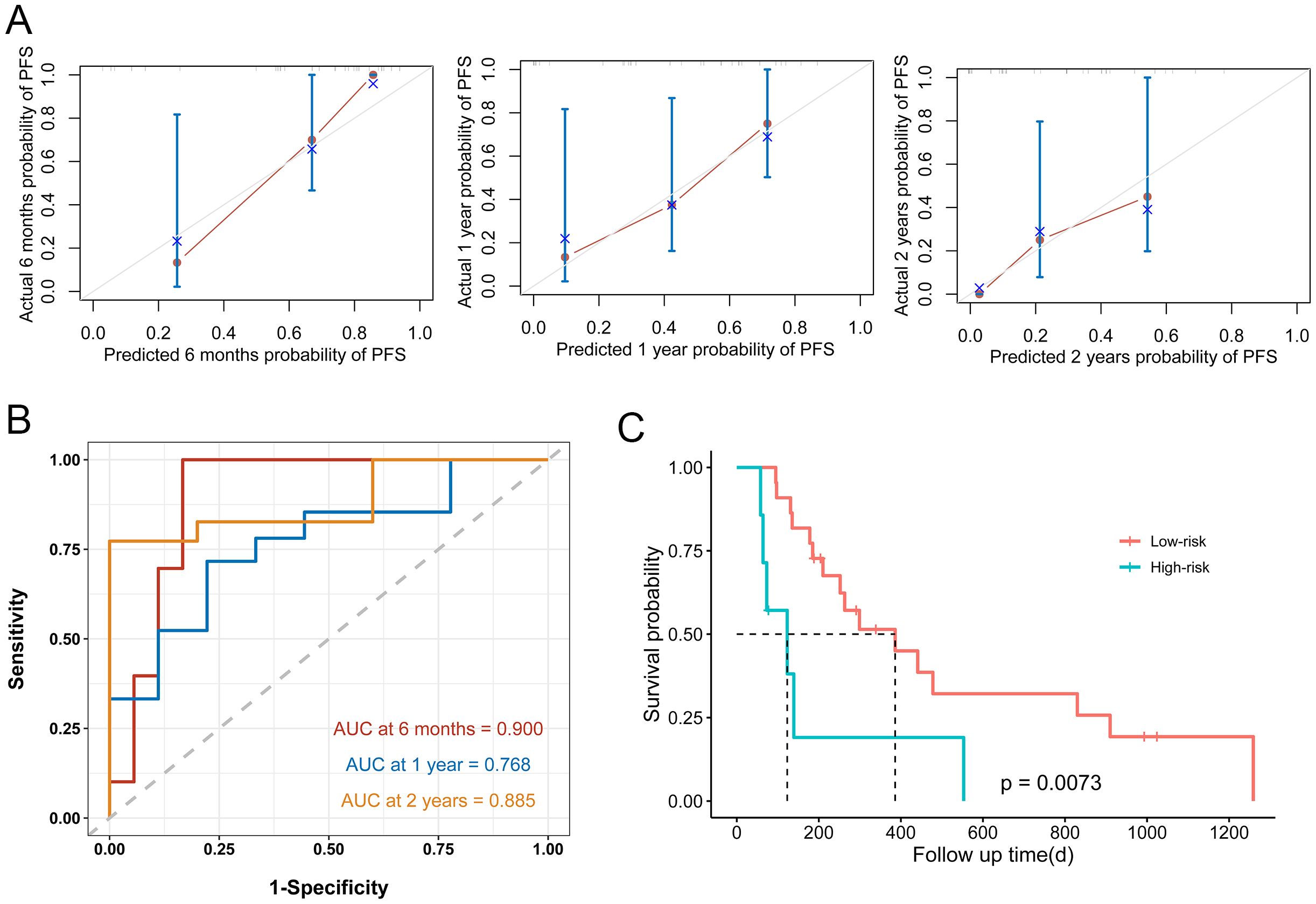
Figure 2. Nomogram model performance in the validation set. (A) Calibration curves plots of nomogram for predicting half-year, 1-year, and 2-year probability of PFS in ICC patients after surgical resection in the validation set (B) ROC curves of the nomogram in the validation set. (C) Prognostic assessment and risk stratification of developed nomogram model in the validation set.
DCA for clinical utility of the nomogram
The DCA curve, which is employed for the purpose of evaluating the clinical utility of the nomogram, is illustrated in Figure 3. The DCA analysis demonstrated that the nomogram model has the potential to enhance net benefits and exhibited a broader range of threshold probabilities in the prediction of PFS in ICC patients.
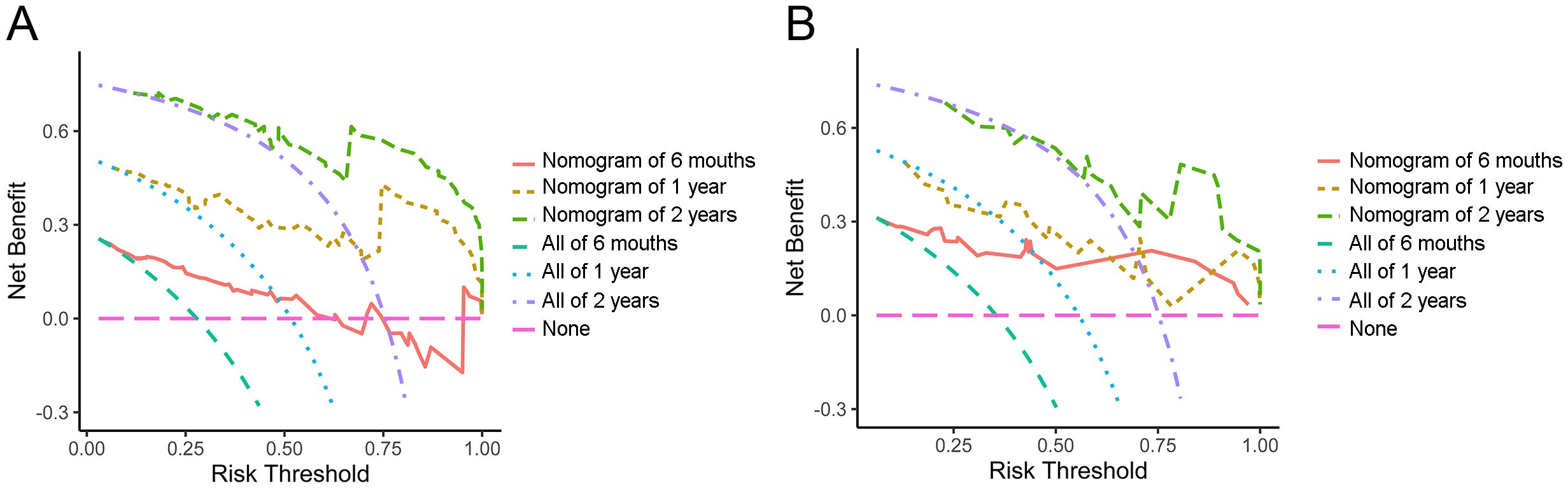
Figure 3. Decision curve analysis of half-year, 1-year and 2-year PFS in the training set (A) and validation set (B).
Discussion
ICC is characterized by a high overall malignancy and a high recurrence rate, leading to poor prognosis for patients. Even after radical resection, the 5-year survival rate remains below 20% (8).Although various treatment modalities have shown differences in OS, the overall OS remains limited (9). It has been reported that the average weighted OS for ICC patients receiving local treatment is only 15.7 months, while those receiving first-line treatment with concurrent systemic chemotherapy have an OS of 25.2 months (10). Although multiple treatment options are currently available for intrahepatic cholangiocarcinoma (ICC) patients, surgical resection remains the only potentially curative approach (11). Predictive models can assist clinicians in evaluating disease progression risk and prognosis, thereby facilitating personalized treatment strategies. Furthermore, by predicting patient outcomes, these models enable prioritization of more aggressive therapies for high-risk patients while avoiding overtreatment of low-risk individuals.
In this study, a nomogram model for predicting postoperative PFS of ICC patients was constructed by integrating clinicopathological parameters and dynamic hematological biomarkers. The nomogram showed significantly better performance compared to the commonly used AJCC TNM staging system, with AUCs of 0.884, 0.913, and 0.911 in the training set and 0.900, 0.768 and 0.885 in the validation set at six months, one year, and two years, respectively, while the AUCs for the AJCC TNM system in the training set were 0.791, 0.704, 0.581, and 0.591, 0.649, 0.730 in the validation set at the corresponding time points. Recent years have witnessed remarkable advancements in cancer diagnosis and prognosis, driven by technological innovations and scientific breakthroughs. An array of novel methodologies has emerged for prognostic evaluation, including liquid biopsy (encompassing ctDNA/CTC analysis) (12), multi-omics profiling (spanning genomic, transcriptomic, and epigenomic dimensions) (13), radiomics (leveraging AI-based imaging feature extraction) (14), and comprehensive tumor microenvironment characterization (e.g., Immunoscore systems) (15). However, research on predictive models for postoperative outcomes in intrahepatic cholangiocarcinoma (ICC) remains limited (11, 16, 17). Existing studies have incorporated various factors including immune-inflammatory indices (7, 18), gene expression and modification profiles (19, 20), treatment modalities (21, 22), pathological parameters and imaging characteristics (23, 24). While current models demonstrate varying degrees of prognostic capability for ICC patients, they predominantly focus on overall survival while inadequately addressing the dynamic disease progression. In this study, we incorporated not only baseline clinicopathological parameters but also serial hematological biomarkers measured before treatment and two weeks postoperatively, with their dynamic changes included in the analysis to highlight the predictive value of longitudinal monitoring. Through multivariate Cox regression analysis, we identified pathological differentiation, CA19–9 levels, neutrophil-to-lymphocyte ratio (NLR), and temporal monocyte counts (tMONO) as independent prognostic factors for progression-free survival (PFS) in ICC patients following resection. Pathological differentiation and AJCC TNM classification are among the main factors affecting the development and prognosis of ICC (25, 26). Our results showed that the lower the degree of differentiation, the higher the risk of patients (HR:0.306, 95% CI:0.177~0.530, P<0.001), which is consistent with mainstream reports (27, 28).Hematological indicators have unparalleled advantages for tumor follow-up due to their ease of acquisition, ability for repeated sampling, and potential for early detection before imaging changes occur. Serum tumor marker CA19–9 is the most widely used diagnostic and prognostic indicator for ICC patients (29, 30). Consistent with the findings of Sanchez L et al. our study confirms that CA19–9 serves as an independent prognostic factor in patients with ICC– (1). Additionally, The NLR and platelet-to-lymphocyte ratio (PLR) serve as indicators of systemic inflammatory status, where chronic inflammation promotes immunosuppression and angiogenesis within the tumor microenvironment (31, 32). Our study validated NLR as an independent prognostic factor, highlighting the role of inflammatory markers in hepatobiliary malignancies. To evaluate the prognostic value of dynamic parameter changes following surgery, we analyzed postoperative hematological trends and identified the preoperative-to-postoperative monocyte ratio (tMON) as an independent prognostic factor. To our knowledge, this is the first study to validate tMON for ICC outcome prediction. The incorporation of this novel biomarker may enable earlier risk stratification and optimized patient surveillance.
It should be noted, however, that the model established in this study is not without limitations. Firstly, the optimal endpoint for follow-up should be OS; Nevertheless, given the brief period of patient enrolment, PFS was selected as a surrogate endpoint. Secondly, this is a single center, retrospective study. It would be beneficial to validate the results further with data from other centers. Finally, due to data accessibility constraints, our model did not account for potential influences of postoperative adjuvant therapies on PFS outcomes.
Conclusion
This study developed a novel nomogram model for predicting postoperative progression-free survival (PFS) in patients with intrahepatic cholangiocarcinoma (ICC). The nomogram demonstrated excellent discriminative ability and calibration in both training and validation cohorts. Compared with the conventional AJCC-TNM staging system, our model showed superior performance in predicting postoperative PFS for ICC patients. This tool not only provides a reliable basis for clinical risk stratification but also opens new avenues for treatment monitoring and personalized therapeutic decision-making, representing a potentially valuable clinical instrument for individualized prognosis assessment in ICC management.
Data availability statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request. In order to protect study participants’ privacy, our data cannot be shared openly. Requests to access these datasets should be directed to LP, ODM2NTMzMDcxQHFxLmNvbQ==.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Hunan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LP: Conceptualization, Funding acquisition, Writing – original draft. YS: Data curation, Formal Analysis, Methodology, Software, Writing – original draft. SY: Investigation, Software, Writing – review & editing. CL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Hunan Provincial Natural Science Foundation of China (No. 2022JJ40223), the Project of Hunan Provincial Department of Education of China (No. 22B0057), and the Doctoral Research Fund of Hunan Provincial People’s Hospital (No. BSJJ202110). Scientific Research Project of Hunan Provincial Health and Wellness Commission (20231161), the Hunan Provincial Natural Science Foundation of China (No. 2022JJ50023); the Project of Hunan Provincial Department of Education of China (No. 22A0065).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1507602/full#supplementary-material
References
1. Izquierdo-Sanchez L, Lamarca A, La Casta A, Buettner S, Utpatel K, Klümpen HJ, et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J Hepatol. (2022) 76:1109–21. doi: 10.1016/j.jhep.2021.12.010
2. Mavros MN, Economopoulos KP, Alexiou VG, and Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. (2014) 149:565–74. doi: 10.1001/jamasurg.2013.5137
3. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA: Cancer J Clin. (2017) 67:93–9. doi: 10.3322/caac.21388
4. Ruzzenente A, Bagante F, Ardito F, Campagnaro T, Scoleri I, Conci S, et al. Comparison of the 7th and 8th editions of the American Joint Committee on Cancer Staging Systems for perihilar cholangiocarcinoma. Surgery. (2018) 164:244–50. doi: 10.1016/j.surg.2018.03.012
5. Ma WH, Lei ZQ, Yu QS, Xiao QR, Tang HL, Si AF, et al. A novel nomogram for individualized preoperative prediction of lymph node metastasis in patients with intrahepatic cholangiocarcinoma. Zhonghua wai ke za zhi [Chinese J surgery]. (2022) 60:363–71. doi: 10.3760/cma.j.cn112139-20220105-00008
6. Macias RIR, Cardinale V, Kendall TJ, Avila MA, Guido M, Coulouarn C, et al. Clinical relevance of biomarkers in cholangiocarcinoma: critical revision and future directions. Gut. (2022) 71:1669–83. doi: 10.1136/gutjnl-2022-327099
7. Zhu J, Wang D, Liu C, Huang R, Gao F, Feng X, et al. Development and validation of a new prognostic immune-inflammatory-nutritional score for predicting outcomes after curative resection for intrahepatic cholangiocarcinoma: A multicenter study. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1165510
8. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. (2020) 17:557–88. doi: 10.1038/s41575-020-0310-z
9. Khan SA, Tavolari S, and Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver international: Off J Int Assoc Study Liver. (2019) 39 Suppl 1:19–31. doi: 10.1111/liv.14095
10. Edeline J, Lamarca A, Mcnamara MG, Jacobs T, Hubner RA, Palmer D, et al. Locoregional therapies in patients with intrahepatic cholangiocarcinoma: A systematic review and pooled analysis. Cancer Treat Rev. (2021) 99:102258. doi: 10.1016/j.ctrv.2021.102258
11. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. (2014) 60:1268–89. doi: 10.1016/j.jhep.2014.01.021
12. Nakamura Y, Watanabe J, Akazawa N, Hirata K, Kataoka K, Yokota M, et al. ctDNA-based molecular residual disease and survival in resectable colorectal cancer. Nat Med. (2024) 30:3272–83. doi: 10.1038/s41591-024-03254-6
13. Ye B, Fan J, Xue L, Zhuang Y, Luo P, Jiang A, et al. iMLGAM: Integrated Machine Learning and Genetic Algorithm-driven Multiomics analysis for pan-cancer immunotherapy response prediction. iMeta. (2025) 4:e70011. doi: 10.1002/imt2.70011
14. Zhao J, He Y, Yang X, Tian P, Zeng L, Huang K, et al. Assessing treatment outcomes of chemoimmunotherapy in extensive-stage small cell lung cancer: an integrated clinical and radiomics approach. J immunotherapy Cancer. (2023) 11:e007492-504. doi: 10.1136/jitc-2023-007492
15. Deng F, Xiao G, Tanzhu G, Chu X, Ning J, Lu R, et al. Predicting survival rates in brain metastases patients from non-small cell lung cancer using radiomic signatures associated with tumor immune heterogeneity. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2025) 12:e2412590. doi: 10.1002/advs.202412590
16. Ye YH, Xin HY, Li JL, Li N, Pan SY, Chen L, et al. Development and validation of a stromal-immune signature to predict prognosis in intrahepatic cholangiocarcinoma. Clin Mol Hepatol. (2024) 30:914–28. doi: 10.3350/cmh.2024.0296
17. Xin HY, Zou JX, Sun RQ, Hu ZQ, Chen Z, Luo CB, et al. Characterization of tumor microbiome and associations with prognosis in intrahepatic cholangiocarcinoma. J Gastroenterol. (2024) 59:411–23. doi: 10.1007/s00535-024-02090-2
18. Wu H, Wei Y, Jian M, Lu H, Song Q, Hao L, et al. Clinicopathological and prognostic significance of immunoscore and PD-L1 in intrahepatic cholangiocarcinoma. OncoTargets Ther. (2021) 14:39–51. doi: 10.2147/OTT.S288982
19. Liu S, Weng J, Cao M, Zhou Q, Xu M, Xu W, et al. FGFR2 fusion/rearrangement is associated with favorable prognosis and immunoactivation in patients with intrahepatic cholangiocarcinoma. oncologist. (2024) 29:e1734–e47. doi: 10.1093/oncolo/oyae170
20. Chen X, Dong L, Chen L, Wang Y, Du J, Ma L, et al. Epigenome-wide development and validation of a prognostic methylation score in intrahepatic cholangiocarcinoma based on machine learning strategies. Hepatobiliary Surg Nutr. (2023) 12:478–94. doi: 10.21037/hbsn-21-424
21. Liu G, Guo W, Wang H, Liu W, Lei L, Xie Q, et al. Influence of postoperative adjuvant transarterial chemoembolization on the prognosis of early-stage intrahepatic cholangiocarcinoma: a single center study. Ann palliative Med. (2021) 10:3673–83. doi: 10.21037/apm-20-1337
22. Zhu C, Li H, Yang X, Wang S, Wang Y, Zhang N, et al. Efficacy, safety, and prognostic factors of PD-1 inhibitors combined with lenvatinib and Gemox chemotherapy as first-line treatment in advanced intrahepatic cholangiocarcinoma: a multicenter real-world study. Cancer immunology immunotherapy: CII. (2023) 72:2949–60. doi: 10.1007/s00262-023-03466-8
23. Zhou Q, Cai H, Xu MH, Ye Y, Li XL, Shi GM, et al. Do the existing staging systems for primary liver cancer apply to combined hepatocellular carcinoma-intrahepatic cholangiocarcinoma? HBPD INT. (2021) 201:13–20. 10.1016/j.hbpd.2020.10.002
24. Li MD, Lu XZ, Liu JF, Chen B, Xu M, Xie XY, et al. Preoperative survival prediction in intrahepatic cholangiocarcinoma using an ultrasound-based radiographic-radiomics signature. J ultrasound medicine: Off J Am Institute Ultrasound Med. (2022) 41:1483–95. doi: 10.1002/jum.15833
25. Razumilava N and Gores GJ. Cholangiocarcinoma. Lancet (London England). (2014) 383:2168–79. doi: 10.1016/S0140-6736(13)61903-0
26. Fiz F, Masci C, Costa G, Sollini M, Chiti A, Ieva F, et al. PET/CT-based radiomics of mass-forming intrahepatic cholangiocarcinoma improves prediction of pathology data and survival. Eur J Nucl Med Mol Imaging. (2022) 49:3387–400. doi: 10.1007/s00259-022-05765-1
27. Hau HM, Meyer F, Jahn N, Rademacher S, Sucher R, and Seehofer D. Prognostic relevance of the eighth edition of TNM classification for resected perihilar cholangiocarcinoma. J Clin Med. (2020) 9:3152–77. doi: 10.3390/jcm9103152
28. Cheng Z, Lei Z, and Shen F. Coming of a precision era of the staging systems for intrahepatic cholangiocarcinoma? Cancer Lett. (2019) 460:10–7. doi: 10.1016/j.canlet.2019.114426
29. Lee JW, Lee JH, Park Y, Kwon J, Lee W, Song KB, et al. Prognostic impact of perioperative CA19–9 levels in patients with resected perihilar cholangiocarcinoma. J Clin Med. (2021) 10:1345–57. doi: 10.3390/jcm10071345
30. Moro A, Mehta R, Sahara K, Tsilimigras DI, Paredes AZ, Farooq A, et al. The impact of preoperative CA19–9 and CEA on outcomes of patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. (2020) 27:2888–901. doi: 10.1245/s10434-020-08350-8
31. Zhang D, Zeng H, Pan Y, Zhao Y, Wang X, Chen J, et al. Liver tumor markers, HALP score, and NLR: simple, cost-effective, easily accessible indexes for predicting prognosis in ICC patients after surgery. J personalized Med. (2022) 12:2041–55. doi: 10.3390/jpm12122041
32. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer (Amsterdam Netherlands). (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
Keywords: intrahepatic cholangiocarcinoma, progression free survival, nomogram, blood biomarkers, prognostic model
Citation: Peng L, Shi Y, Yang S and Li C (2025) A blood test-based nomogram to predict the progression-free survival of patients with intrahepatic cholangiocarcinoma after surgical resection. Front. Oncol. 15:1507602. doi: 10.3389/fonc.2025.1507602
Received: 16 November 2024; Accepted: 19 May 2025;
Published: 09 June 2025.
Edited by:
Pengpeng Zhang, Nanjing Medical University, ChinaReviewed by:
Bharath Kanakapura Sundararaj, Boston University, United StatesQiuxia Zhao, The University of Texas at Austin, United States
Ökkeş Zortuk, Ministry of Health, Türkiye
Copyright © 2025 Peng, Shi, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lirong Peng, cGVuZ2xpcm9uZ2NzQGh1bm51LmVkdS5jbg==
 Lirong Peng
Lirong Peng Yang Shi
Yang Shi Shuang Yang1
Shuang Yang1 Cunyan Li
Cunyan Li