Abstract
Hyoid glomus tumors represent an exceptionally rare clinical entity. This study details a case presentation of a hyoid glomus tumor accompanied by a comprehensive systematic review, aiming to expand the clinical and pathological understanding of these uncommon neoplasms while evaluating therapeutic approaches. CT imaging revealed hyoid bone destruction with features suggestive of a borderline neoplasm. Histopathological examination demonstrated local spindle-shaped cells exhibiting a chicken claw-like morphology, which showed strong immunoreactivity for SMA, calponin, and collagen type IV - findings consistent with classical glomus tumor characteristics. The patient was ultimately diagnosed with a glomus tumor of uncertain malignant potential. Postoperative recovery proceeded favorably, with serial follow-up imaging studies demonstrating no evidence of recurrence or residual disease over several months of surveillance.
Introduction
Glomus tumors (GTs) are perivascular mesenchymal neoplasms composed of modified smooth muscle cells, classified with myopericytoma, myofibroma, and angioleiomyoma. In 1812, Wood first described this disease as painful subcutaneous nodules, and Masson found that it originated from the normal glomus and named it GT in 1924. The general pathogenesis is the transformation of the arteriovenous anastomose-vascular sphere, which is believed to be formed by abnormal proliferation of the vascular sphere under the action of induction (such as trauma). It is more common in the distal limbs, sublingual and visceral organs and especially in the gastrointestinal tract, bones and mediastinum. Glomus tumors are rare, accounting for less than 2% of all benign soft tissue tumors (1). It is more common in adults aged 20 to 50 years, and half of them are aged 40 to 50 years. Subungual glomus tumors are more common in women (2), while glomus tumors outside the fingers are more common in men. It mainly occurs in the fingertip, and the treatment options are surgical resection and carbon dioxide laser treatment, which prevent relapse (3).
Case
A 60-year-old male with an 8-year history of hypertension (blood pressure maintained around 151/89 mmHg) presented with a right-sided neck mass persisting for over two weeks. Physical examination revealed asymmetrical hyoid bone enlargement (right > left) with a 3 cm firm, ill-defined, fixed mass located superior to the right hyoid body; the overlying skin remained intact with no tenderness. Comprehensive physical examination demonstrated no musculoskeletal deformities or skin tumors (including clinical appearance of neurofibromatosis type 1 (NF1)) and normal physiological reflexes. MRI revealed expansile right hyoid bone destruction (Figures 1A–D) showing hyperintense signal on fat-suppressed sequences with infiltrative margins. The lesion exhibited restricted diffusion (DWI hyperintensity with corresponding ADC hypointensity) indicating mylohyoid muscle involvement (Figures 1E–I), along with heterogeneous contrast enhancement (Figures 1J–L). Bilateral carotid sheath lymphadenopathy was noted (the largest node is about 2.1×1.0 cm) without cervical vertebral destruction. CT imaging (Figures 1M, N, the left panel) confirmed a multiloculated expansile hyoid lesion with right-sided predominance, containing punctate calcifications and osseous septations, while ultrasound identified an irregular 12×15×26 mm mass. These are imaging characteristics collectively suggested malignant etiology.
Figure 1

MR&CT imaging of head and neck masses. (A-D) Contrast-enhanced MR imaging: Expansive bone destruction of the hyoid bone to the right. (A) Coronal T2W1; (B) transverse T1W1; (C) sagittal T2W1; (D) transverse T2W1; (E-G) FS; (H) DWI; (I) ADC; (J-L) Multilocular expansive bone destruction of the hyoid bone. The tumor is approximately 3.3 cm×1.5 cm in size, with punctate calcification and a bone ridge. The bone cortex was discontinuous at the edge of the lesion, and soft tissue protrusion was observed. The lesions showed mild enhancement on contrast-enhanced scans. (M) Bone window in transverse axis view. (N) Soft window the transverse in axis position. (Left: before surgery; Right: 2 months after surgery).
The mass was located within the hyoid bone with evident destruction and deep penetration. The central and right sides of the hyoid bone were notably affected. The mass, which was yellow–white with a soft texture and had a clear boundary and no obvious film, was resected and separated along the intact left side (Figure 2).
Figure 2
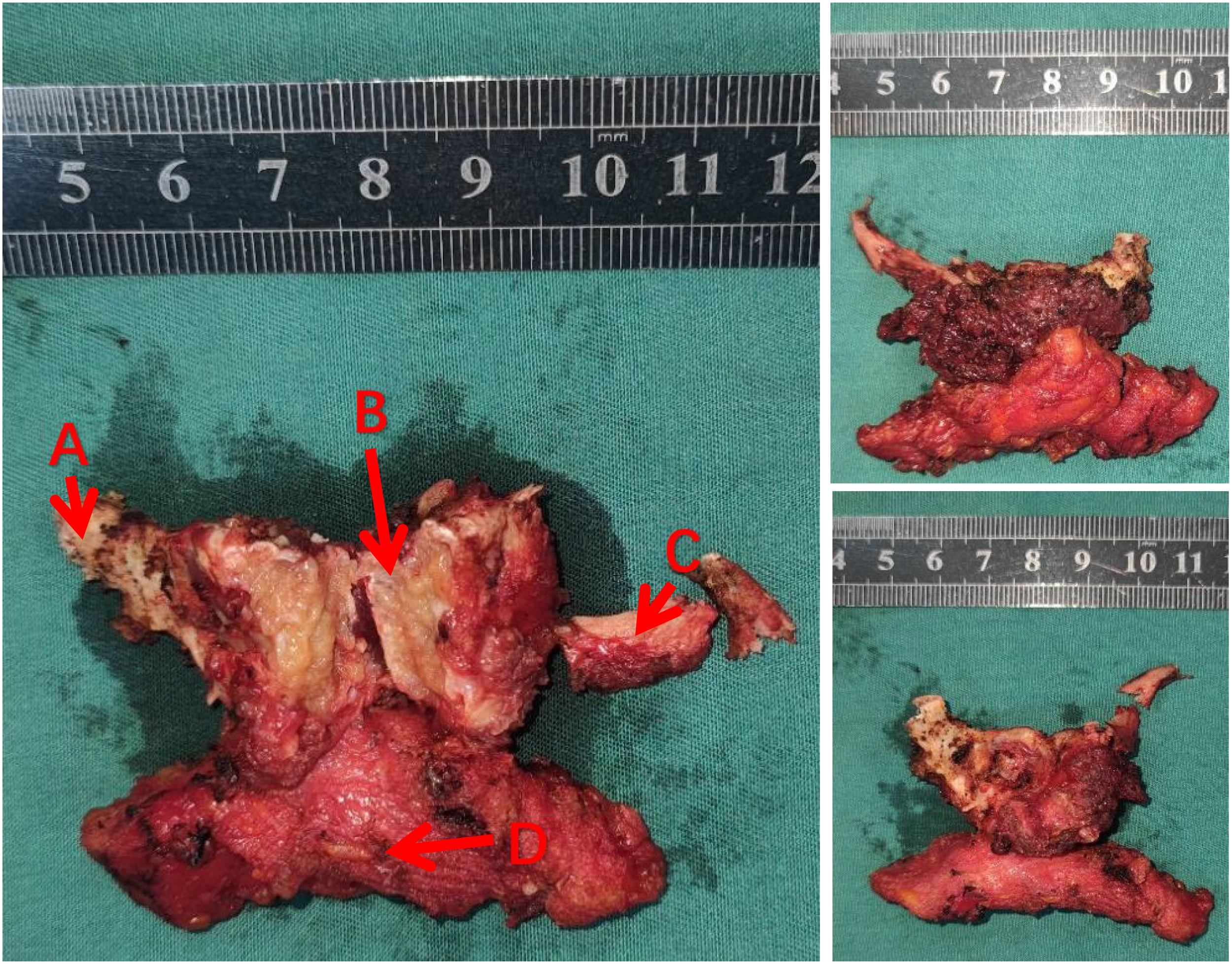
A photo of the tumor removed during surgery. (A, C) hyoid bone (B) tumors (D) lymphoid and connective tissue.
Cytologically, the tumor cells were distributed around blood vessels, and the cells were oval with fine chromatin, and no nucleoli or mitotic figures were observed; these cells tended to be mesenchymal tumors (Figure 3A). Histologically, the tumor was well demarcated from the surrounding tissue and showed a lobulated growth pattern with a richly vascularized stroma. Mitotic figures were less than 2/10 HPF, and no pathological mitotic figures were observed (Figures 3B–D). The cells were positive for myogenic markers such as SMA, calponin, and type IV collagen (the latter of which showed a chicken claw-like morphology) while they were negative for Desmin, CD34, and S-100 (Figures 3E, F).
Figure 3
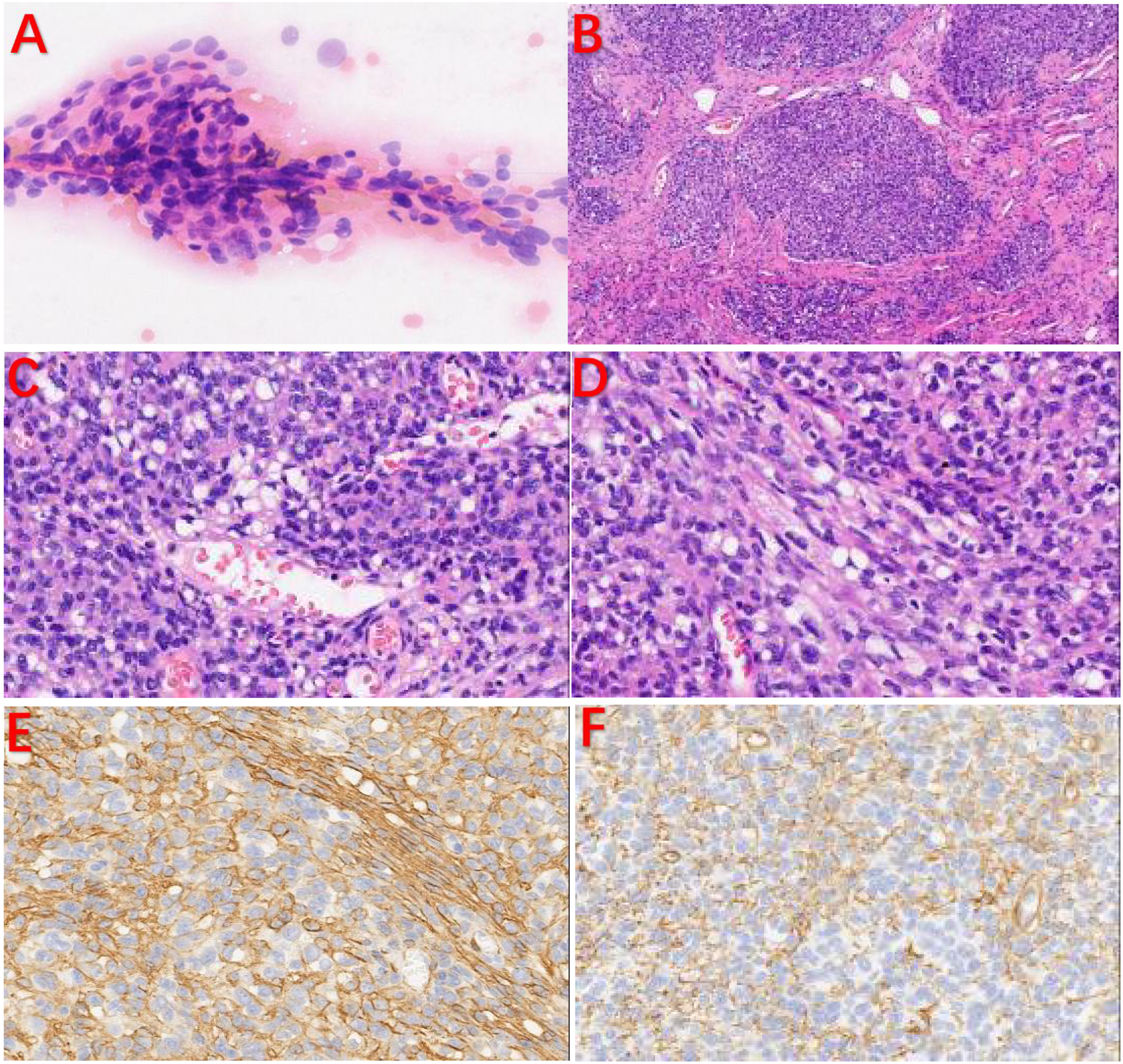
Pathological examination and IHC. (A) The tumor cells were distributed around blood vessels, and the cells were oval with fine chromatin. No nucleoli or mitotic figures were observed. (B) The tumor cells were lobulated, and the stroma was rich in blood vessels. (C, D) Some tumor cells were spindle-shaped. IHC: (E, F) The tumor cells were positive for SMA and type IV collagen in a chicken claw-like morphology.
Discussion
A literature review was conducted in Pubmed, CNKI, Medscape and other databases with the keywords “oral glomus tumor”, “tongue glomus tumor”, and “hyoid glomus tumor” from 1949 to 2024. A total of 45 relevant global cases were collected (Table 1). Male patients (n=25) were more susceptible than female patients (n=20). We found that 6 patients had tumors of the tongue (16.3%), 17 patients had tumors of the lip (37.8%), and 8 patients had tumors of the oral mucosa (17.8%). Other cases are mainly distributed in the jaw (4–45).
Table 1
| Author | Year | Age | Anatomical location | Clinical presentation | IHC | Follow-up time | Size(mm) | Outcome |
|---|---|---|---|---|---|---|---|---|
| Von Langer (4) | 1949 | 52(M) | Hard palate | NA | NA | NA | NA | NA |
| King (5) | 1954 | 32(M) | Gingiva | Tenderness | NA | NA | 6 | NA |
| Kirschner Strass-burg (6) | 1962 | 56(M) | Gingiva/alveolar mucosa | NA | NA | NA | NA | NA |
| Grande and D’Angelo (7) | 1962 | 42(M) | Hard palate | NA | NA | NA | NA | NA |
| Frankel (8) | 1965 | 13(M) | buccal mucosa | NA | NA | NA | NA | NA |
| Harris and Griffin (9) | 1965 | 35(F) | Periodontal/gum | Pain | NA | 2 years | 5*2.5 | NED |
| Sidhu and Subherwal (10) | 1967 | 10(F) | Hard palate | NA | NA | NA | NA | NA |
| Charles (11) | 1976 | 17(F) | Hard palate | No | NA | NA | NA | NA |
| Lele (12) | 1977 | 35(F) | Hard palate | Bleeding | NA | 6 months | 15*10 | NED |
| Sato et al. (13) | 1979 | 29(M) | Tongue | No | NA | NA | 3 | NA |
| Tajima et al. (14) | 1981 | 63(F) | Tongue | No | NA | NA | NA | NA |
| Saku et al. (15) | 1985 | 45(M) | Buccal mucosa | No | Actin(+)smooth muscle myosin(+) | NA | 45*30*35 | NA |
| Ficarra et al. (16) | 1986 | 51(F) | Upper lip | No | NA | NA | 20 | NA |
| Moody et al. (17) | 1986 | 65(F) | Upper lip | No | NA | NA | 10*5*5 | NA |
| Stajcic and Bojic (18) | 1987 | 55(M) | Tongue | NA | NA | NA | NA | NA |
| Tokiwa et al. (19) | 1990 | 36(M) | Gingiva | NA | NA | NA | NA | NA |
| Geraghty et al. (20) | 1992 | 71(M) | Hard palate | No | NA | NA | 15 | NA |
| Kusama et al. (21) | 1995 | 57(M) | Upper lip | Tenderness | S100(+), actin(+), desmin(+),vimentin (+), factor VIII(−) |
4 years | NA | NED |
| Savaci et al. (22) | 1996 | 55 (F) | Mucosa of mouth | Pain | NA | NA | 10 | NA |
| Sakashita et al. (23) | 1997 | 54(M) | Upper lip | No | NA | NA | 12 | NA |
| Yu et al. (24) | 2000 | 54(F) | Left mandibular region, lip, mucous membrane | No | smooth muscle actin(+), S-100(−) |
NA | NA | NA |
| Kessaris et al. (25) | 2001 | 46(F) | Hard palate | No | Vimentin (+), smooth muscle actin(+), actin(−), desmin(−) chromogranin(−), neuron-specific enolase(−), epithelial membrane antigen(−) cyto-keratin(−),factor VIII(−) |
3 years | 18 | NED |
| Rallis et al. (26) | 2004 | 85(F) | Upper lip | Pain | smooth muscle actin(+), muscle specific actin(+), vimentin (+), desmin(−),S-100(−), epithelial membrane antigen(−),neuron-specific enolase(−)AE1/3(−), Leu7(−), CD3,CD31,CD34,CD45,CD20(−), cytokeratin(−) |
1.5 years | 13*10*10 | NED |
| Quesada R et al. (27) | 2004 | 61(M) | Tongue | No | NA | 7 years | 30 | Recurrence |
| Lanza et al. (28) | 2005 | 65(M) | Lower lip | NA | NA | NA | NA | NA |
| Maeda et al. (29) | 2005 | 20(M) | Jaw | Vimentin (+), smooth muscle actin(+), HHF35(+) keratin(−) S-100(−) factor VIII(−), desmin(−) |
NA | NA | NA | |
| Ide et al. (30) | 2008 | 57(M) | Upper lip | NA | NA | NA | 8 | NA |
| Ide et al. (30) | 2008 | 54(M) | Upper lip | NA | NA | NA | 12 | NA |
| Wang et al. (31) | 2008 | 58(F) | Buccal mucosa | NA | NA | NA | NA | NA |
| Boros et al. (32) | 2010 | 34(M) | Lower lip | No | smooth muscle actin(+), muscle specific actin(+), S-100(+), kerarin(−), epithelial membrane antigen(−),CD34(−), CD31(−), chromogranin(−) | 5 years | 15*15*11 | NED |
| Yoruk et al. (33) | 2010 | 30(F) | Buccal mucosa | No | smooth muscle actin(+),S-100(−), kerarin(−),p53(+) bcl2 (–)CD34(+),CD117(−)CD31(+), chromogranin(−)desmin(−) AE1/3(−) |
1 years | 20*11*5 | NED |
| Derand III et al. (34) | 2010 | 11(F) | Lower lip | No | pancytokeratin(−), vimentin (+), smooth muscle actin(+), S-100, factor VIII(−) |
7 years | 3 | NED |
| Veros et al. (35) | 2012 | 24(F) | Buccal mucosa | No | NA | 2 years | 10*10 | Recurrence |
| Chou et al. (36) | 2015 | 39(M) | Upper lip | NA | NA | NA | NA | NA |
| Kazuto et al. (37) | 2016 | 44(M) | lower jawbone | Dull pain | Vimentin (+), muscle specific actin/HHF35(+), calponin (+), typeIV collagen (+), smooth- muscle-actin(−), cytokeratin(AE1/AE3)(−), cytokeratin(CAM5.2)(−), CK19(−), CD31(−), CD34(−), CD68(−), p63(−), S-100(−), factor VIII(−), desmin(−) |
10 years | 45*30*30 | NED |
| Monaghan (38) | 2017 | 73(M) | Upper lip | No | NA | NA | 10 | NA |
| Vasconcelos et al. (39) | 2018 | 67 (F) | Upper lip mucosa | Pain | CD34(+), smooth-muscle-actin(+) Vimentin (+) S-100(−) cytokeratin(−)STAT-6 (–) |
3.3 years | 10 | NED |
| Smith et al. (40) | 2018 | 26(M) | Lower lip | Pain | HHF-35(+)SMA(+) AE1/3 (–) CD31、CD34 (–) | NA | 15*5*5 | NA |
| Smith et al. (40) | 2018 | 58(F) | Tongue | No | SMA(+), MSA/HHF35(+)S100 (–)p63 (–)GFAP (–)AE1/3 (–)CD31、CD34(+) |
1 months | 20*10 | NED |
| Zou et al. (41) | 2018 | 24(F) | Mouth floor | Pain | VIM(+)αSMA(+)AE1 (–)AE3 (–)CD31 (–)CD34 (–) S-100 (–) Ki67 (+, 5%) |
4 years | 28*18*21 | NED |
| Sánchez-Romero C et al. (42) | 2019 | 51 (F) | Upper lip mucosa | Pain | VIM(+)CD34(+),αSMA(+)HHF35(+) hCaldesmon(+)AE1/AE3(+)S-100(+)desmin(+) |
NA | 10 | NA |
| Naji Rad S et al. (43) | 2020 | 62(M) | Lower lip mucosa | No | NA | 1 year | 10 | NED |
| Chandran S et al. (44) | 2022 | 8(F) | Lowerjawbone | Pain | Vimentin (+)SMA (+)desmin (–)p63 (–)CD34 (–)CD45 (–) |
NA | 20*45*20 | NA |
| Afroozi B (45) | 2023 | 37(M) | Buccal mucosa | No | CD34(+)AE1/3 (–)S100 (–)vimentin(+)SMA(+)CD31 (–) p63 (–) |
2 years | 20*20 | NED |
| Our case | 2023 | 60(M) | Tongue bone | No | SMA(+),CD56(+),Hcald(+),Calponin (+) Collagen IV(+) Desmin (–)CK(AE1/AE3) (–), EMA (–), CD34 (–), S100 (–), Syn (–), CgA (–), Ki-67 (+,1%) |
NA | 12*15*26 | NED |
Cases of oral glomus tumor reported in the global literature.
NA, not available; NED, no evidence of disease.
GT usually presents as a solitary small red–blue nodule with obvious pain when cold and touch clinically. Approximately 10% of patients have multiple lesions, and 9% to 60% of patients have abnormal bone changes. GT in the oral cavity is rare, with an incidence of only 0.6% (32). Approximately 45 patients were identified, with a wide age of onset (8 to 85), a mean age of 45 years, and more common males. GT in the bone is most common in the phalanx, followed by the vertebral body. Imaging shows osteolytic changes with sclerotic edges, which should be differentiated from bone hemangioma, aneurysmal bone cyst, bone metastasis cancer and tuberculosis, etc (46).
The tumor cells were small, round, uniform in size, lightly eosinophilic with occasional eosinophilic or epithelioid cell morphology, hyalinization or a mucinous matrix but showed no necrosis. IHC revealed positivity for SMA, Syn, and collagen IV, while S-100 was positive. However, CK, desmin, and CD34 tested negative. A recent study revealed that BRAF V600E mutations may be associated with a malignant phenotype in glomus tumors (47); however, larger cohorts and multicenter studies are required to confirm these findings.
Differential diagnosis
-
(1) Myopericytoma: There are no uniform round cells, and the characteristic oval and spindle cells grow around the blood vessels. There was some overlap with the morphology of the glomus tumor.
-
(2) Paragangliomas: These tumors exhibit nested organ-like growth. IHC: SYN(+), CgA(+), S-100(+), and SMA(+).
-
(3) Angioleiomyoma is composed of mature smooth muscle cells arranged in fascicles lacking round cells of uniform size. IHC: SMA and Desmin(+).
-
(4) Neuroendocrine tumors: Tumor cells with speckly chromatin in the nucleus. IHC revealed CK, SYN and CgA (+) SMA (+) and Syn(+) when they occurred in the gastrointestinal tract, and these tumors were easily misdiagnosed as neuroendocrine tumors.
-
(5) Suquet-Hoyer: This structure appears as a narrow lumen lined by a single layer of endothelial cells and surrounded by 4 to 6 layers of spheroid cells, which are regarded as specialized smooth muscle cells. Sometimes, this normal structure is observed in specimens from distal limb biopsies performed for other reasons and is mistaken for GT (48).
-
(6) Aneurysmal bone cyst: CT clearly revealed peritumoral ossification and calcification. The MR plain scan signal was heterogeneous; the fluid–fluid level in the lesion is its characteristic manifestation on MR images. An enhanced scan revealed uneven progressive enhancement (49). Eccentric balloon-like expansion may be observed on X-ray, and a large amount of blood can be drawn by local puncture.
-
(7) Hyoid chondroma: the tumor is located in the upper neck of the hyoid bone plane, is surrounded by a hyoid muscle group and is imperceptible, and can slowly occur in the mouth. Subjective symptoms are not obvious and are not easy to detect early. The mass is generally hard, well-defined, and benign and moves with the hyoid bone when swallowing (50).
-
(8) Hyoid chondrosarcoma: This type of chondrosarcoma is overwhelmingly low grade and presents as a slow-growing, painless mass on the lateral side of the neck. CT shows a dilated tumor with cortical destruction and matrix calcification, and focal exophytic lesions with intimal sector features can be seen in rapidly progressing chondrosarcomas (51). T1lWI is low, T2WI shows peripheral enhancement, and T8WI is high (52).
-
(9) Radiation-induced osteonecrosis of the hyoid bone: This is a common complication after radiotherapy for tumors that are often misdiagnosed as recurrent tumors. The typical imaging manifestations are cortical fragmentation, bone fragmentation, and air filling in the bone. Some patients have soft tissue enhancement signals on PET/CT, suggesting that FDG activity is significantly enhanced and is easily mistaken for tumor recurrence (53).
-
(10) Thyrohyoid cysts: They are most common near the hyoid bone (54). Ultrasound revealed a clear boundary, regular shape, and clear fluid inside. In some cases, strip-like septa can be seen. When the course of disease is long or complicated with infection, the internal echo increases, and the floating light spot can be seen the same for the echo of a solid mass, but the posterior echo is enhanced (55). The hyoid bone is rare, and inactive thyroid tissue and cholesterol particles can be found in the cyst wall (56).
GT often occurs in the glomus cell-rich parts of the extremities, especially under the nail bed of the fingers and toenails, and rarely in the skin, bone or internal organs. Lingual GTs are mostly located on the back of the tongue and are rarely more than 1 cm long and have red or medium textures and clear boundaries, without the triad of subungual GTs (pain, tenderness, cold shock) (57). According to the 2013 WHO soft tissue classification criteria, the diagnostic criteria for malignant glomus tumors are (1) marked nuclear atypia and any level of mitotic figures or (2) the presence of atypical mitotic figures. When the histological appearance of the tumor does not meet the above criteria for the diagnosis of malignancy but there is at least one atypical feature (e.g. a diameter greater than 2 cm, increased mitotic count, deep location, etc.) should be called a “glomus tumor of uncertain malignant potential” (GT-UMP) (58). According to the size and location of the tumor, this patient was diagnosed with GT-UMP. As for the IHC, the tumor cells were positive for α-SMA, MSA, h-caldesmon, calponin, vimentin and collagen IV. CD34 was positive in some patients, but desmin, AE1/AE3 and S100 were negative.
At present, the most common and effective method for treating GT is surgical local resection, but there is still a possibility of recurrence. For laser treatment, a C02 laser with an output power of 2~3 W can be used to punch into the subcutaneous or nail bed for direct coagulation, or an ND: YAG laser with an output power of 3~5 W and fiber inserted directly into the lesion for coagulation can be used. The treatment is simple and easy, and no special postoperative care is needed.
Typically glomus tumors are benign, but malignant glomus tumors have high potential for recurrence and metastasis. The prognosis of patients with malignant glomus tumors is good. However, the number of follow-up cases in the literature is limited, and the follow-up time is short, so the follow-up should be strengthened in practical work.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of the Third Affiliated Hospital (KQ-YJ-2024-152). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MS: Writing – original draft. MH: Writing – review & editing. JLW: Writing – original draft, Writing – review & editing. QZ: Writing – original draft, Writing – review & editing. CR: Writing – original draft, Writing – review & editing. HL: Writing – review & editing. ZY: Writing – review & editing. JHW: Writing – review & editing. XY: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by National Natural Science Foundation of China 82173165 (XJY) and The Key Research and Development Program of Shaanxi Province (Program No. 2022SF-129).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Gopal MG Manjunath NC Kumar S Ramesh M Nandini AS . Glomangioma: a rare case report. J Evol Med Dental Sci. (2014) 3:127–33. doi: 10.14260/jemds/1800
2
Fletcher CDM Unni KK Mertens F eds. World Health Organization Classification of Tumors. In: Pathology and Genetics of Tumors of Soft Tissue and Bone. IARC Press, Lyon.
3
Zhang J Zhuang W Li Q Yin W Liu B Sang Q . Clinical analysis and etiology of glomus tumor in 80 cases. Chin J Handb Surg. (2003) 04):50–1. doi: 10.3760/cma.j.issn.1005-054X.2003.04.021
4
Langer R . Glomustumor des harten Gaumens. Monatsschr Ohrenheilkd Laryngorhinol. (1948) 82:324.
5
King ESJ . Glomus tumor. Aust New Z J Surg. (1954) 23:280–95. doi: 10.1111/j.1445-2197.1954.tb05057.x
6
Kirschner H Strassburg M . Ein am zhanlosen Alveolarfortsatz des Unterkiefers lokalisierter Glomustumor. (1962) 17:912–7.
7
Grande R D’Angelo E . Un tumore glomico del plato duro. Arch Ital Laringol. (1962) 70:89.
8
Frankel VG . Auftreten eines leimyofibromangioms (Glomangioms) im Wangen-und Jochbogenbereich. Dtsh Zahnartzl Zschr. (1965) 20:168.
9
Harris R Griffin CJ . Glomus tumor of the periodontal tissues. Aust Dent J. (1965) 10:33–7. doi: 10.1111/j.1834-7819.1965.tb01597.x
10
Sidhu SS . Glomus tumor of palate. J Indian Dental Assoc. (1967) 39:167.
11
Charles NC . Multiple glomus tumors of the face and eyelid. Arch Ophthalmol. (1976) 94:1283–5. doi: 10.1001/archopht.1976.03910040155005
12
Lele DN . Glomus tumor of the hard palate. Indian J Otolaryngol. (1977) 29:136–7. doi: 10.1007/BF02990683
13
Sato M Shirasuna K Sakuda M Yanagawa T Yoshida H Imai J et al . Fine structure of a glomus tumor of the tongue and expression of C type virus in its tumor cells. Int J Oral Surg. (1979) 8:199–204. doi: 10.1016/S0300-9785(79)80019-8
14
Tajima Y Weaters DR Neville BW Benoit PW Pedley DM . Glomus tumor (glomangioma) of the tongue: a light and electron microscopic study. Oral Surg Oral Med Oral Pathol. (1981) 52:288–93. doi: 10.1016/0030-4220(81)90268-1
15
Saku T Okabe H Matsutani K Sasaki M . Glomus tumor of the cheek: an immunohistochemical demonstration of actin and myosin. Oral Surg Oral Med Oral Pathol. (1985) 60:65–71. doi: 10.1016/0030-4220(85)90218-X
16
Ficarra G Merrell PW Johnston WH Hansen LS . Intraoral solitary glomus tumor (glomangioma): case report and literature review. Oral Surg Oral Med Oral Pathol. (1986) 62:306–11. doi: 10.1016/0030-4220(86)90013-7
17
Moody GH Myskow M Musgrove C . Glomus tumor of lip. A case report and immunohistochemical study. Oral Surg Oral Med Oral Pathol. (1986) 62:312–8. doi: 10.1016/0030-4220(86)90014-9
18
Stajcic Z Bojic P . Intraoral glomus tumor. A case report. J Cranio-Maxillofac Surg. (1987) 15:376–8. doi: 10.1016/s1010-5182(87)80087-2
19
Tokiwa S Sato A Sakamaki H Toba H Kimura Y Nagumo M et al . A case of glomus tumor arising in the mandibular gingiva. Jpn J Oral Maxillofac Surg. (1990) 36:2295–9. doi: 10.5794/jjoms.36.2295
20
Geraghty JM Thomas RW Robertson JM Blundell JW . Glomus tumor of the palate: case report and review of the literature. Br J Oral Maxillofac Surg. (1992) 30:398–400. doi: 10.1016/0266-4356(92)90210-A
21
Kusama K Chu L Kidokoro Y Kouzu M Uehara T Honda M et al . Glomus tumor of the upper lip. J Nihon Univ School Dentistry. (1995) 37:97–101. doi: 10.2334/josnusd1959.37.97
22
Savaci N Emiroğlu M Gümren M Güngör S . A rare case of glomus tumor; buccal localization. Br J Oral Maxillofac Surg. (1996) 34:199–200. doi: 10.1016/S0266-4356(96)90391-5
23
Sakashita H Miyata M Nagao K . Glomus tumor in the upper lip. A case report. Int J Oral Maxillofac Surg. (1997) 26:301–2. doi: 10.1016/S0901-5027(97)80876-4
24
Yu HJ Kwon SJ Bahn JY Park JM Park YW . Localized multiple glomus tumors of the face and oral mucosa. J Dermatol. (2000) 27:211–3. doi: 10.1111/j.1346-8138.2000.tb02151.x
25
Kessaris P Klimis T Zanakis S . Glomus tumor of the hard palate: case report and review. Br J Oral Maxillofac Surg. (2001) 39:478–9. doi: 10.1054/bjom.2001.0721
26
Rallis G Komis C Mahera H . Glomus tumor: a rare location in the upper lip. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2004) 98:327–36. doi: 10.1016/j.tripleo.2004.02.005
27
Quesada R González-Lagunas J Raspall G . Aggressive glomus tumor of the tongue. Rep case. (2004) 9:350. Available online at: https://www.medicinaoral.com/pubmed/medoralv9_i4_p350.pdf.
28
Lanza A Moscariello A Villani R Colella G . Glomus tumor of the lower lip. A case report. Minerva Stomatol. (2005) 54:687–90.
29
Maeda Y Irie T Yamamoto G Nagoshi Y Aida T Takarada M et al . Glomus tumor of the palate report of a case and review of the literature. Dental Med Res. (2005) 25:6–10.
30
Ide F Mishima K Yamada H Saito I Horie N Shimoyama T et al . Perivascular myoid tumors of the oral region: a clinicopathologic re-evaluation of 35 cases. J Oral Pathol Med. (2008) 37:43–9. doi: 10.1111/j.1600-0714.2007.00594.x
31
Wang B Wang J Shehan J Sarma DP . Glomus tumor of the cheek. Int J Dermatol. (2008) 6:5.
32
Boros AL Davis JP Sedghizadeh PP Yamashita DD . Glomus tumor: report of a rare case affecting the oral cavity and review of the literature. J Oral Maxillofac Surg. (2010) 68:2329–34. doi: 10.1016/j.joms.2009.10.005
33
Yoruk O Ucuncu H Aktan B Calik M Kilic K . Glomuvenous malformations in the buccal area. J craniofacial Surg. (2010) 21:2001–3. doi: 10.1097/SCS.0b013e3181f535a2
34
Dérand P Warfvinge G Thor A . Glomangioma: a case presentation. J Oral Maxillofac Surg. (2010) 68:204–7. doi: 10.1016/j.joms.2009.07.023
35
Veros K Markou K Filitatzi C Kyrmizakis DE . Glomus tumor of the cheek: a case report. Case Rep Med. (2012) 2012:307294. doi: 10.1155/2012/307294
36
Chou T Pan SC Shieh SJ Lee JW Chiu HY Ho CL . Glomus tumor: twenty-year experience and literature review. Ann Plast Surg. (2016) 76:S35–40. doi: 10.1097/SAP.0000000000000684
37
Kurohara K Michi Y Yukimori A Yamaguchi S . The glomus tumor resorbed bone and teeth in the mandible: a case report. Head Face Med. (2018) 14:18. doi: 10.1186/s13005-018-0175-3
38
Monaghan L . Glomus tumor presenting in the upper lip - a case report. Br J Oral Maxillofac Surg. (2017) 55:e156–7. doi: 10.1016/j.bjoms.2017.08.205
39
Vasconcelos ACU Loyola AM Gomes APN de Araújo VC Tarquínio SBC Silveira FM et al . A symptomatic swelling of the upper lip. Oral Surg Oral Med Oral Pathol Oral Radiol. (2018) 125:107–11. doi: 10.1016/j.oooo.2017.10.012
40
Smith MH Bhattacharyya I Cohen DM Hinze SR Islam MN . Glomus tumor: a comprehensive review of the clinical and histopathologic features with report of two intraoral cases. Oral Surgery Oral Medicine Oral Pathol Oral Radiol. (2019) 127(1):62–70. doi: 10.1016/j.oooo.2018.07.056
41
Zou H Song L Jia M Wang L Sun Y . Glomus tumor in the floor of the mouth: a case report and review of the literature. World J Surg Oncol. (2018) 16:201. doi: 10.1186/s12957-018-1503-6
42
Sánchez-Romero C Pérez de Oliveira ME de Castro JFL de Amorim CEJ de Almeida OP da Cruz Perez DE . Glomus tumor of the oral cavity: report of a rare case and literature review. Braz Dent. J. (2019) 30(2):185–90. doi: 10.1590/0103-6440201902222
43
Naji Rad S Najirad S Rafiei R . A rare case of glomus tumor on the mucosal surface of lower lip. J Investig Med High Impact Case Rep. (2020) 8:2324709620936159. doi: 10.1177/2324709620936159
44
Chandran S Elangovan A Vijayakumar S Kumar KSS . Intraoral Malignant glomus tumor. J Oral Maxillofac Pathol. (2022) 26:259–62. doi: 10.4103/jomfp.jomfp_444_21
45
Afroozi B Rezazadeh F Jaafari-Ashkavandi Z Tavanafar S . Glomus tumor in the buccal mucosa: A case report and review of the literature. J Oral Maxillofac Pathol. (2023) 27:S15–9. doi: 10.4103/jomfp.jomfp_232_22
46
Chen Z Zhao W Liu A . Rare oncology. Beijing: Science Press (2022). p. 281.
47
Karamzadeh ND Bahrami A Lee SJ Jenkins SM Rodriguez FJ Folpe AL et al . BRAF V600E mutations occur in a subset of glomus tumors, and are associated with Malignant histologic characteristics. Am J Surg Pathol. (2017) 41:1532–41. doi: 10.1097/PAS.0000000000000913
48
Stacey E Mills J Greenson K .Sternberg J . Diagnostic Surgical Pathology Vol. 68. . Beijing: Peking University Medical Press (2017).
49
Fu Z . CT and MRI diagnosis of aneurysmal bone cyst. Chin Health Standards Manage. (2014) 5:39–40.
50
Wang Y Zhou Z Xu X . Chondroma of hyoid bone: a case report. J Second Military Med Univ. (1998) (03):49. doi: 10.16781/j.0258-879x.1998.03.023
51
Murphey MD Walker EA Wilson AJ Kransdorf MJ Temple HT Gannon FH . From the archives of theAFIP: imaging primary chondro sarcoma: radiologic-pathologic correlation. Radiographics. (2003) 23:1245–1278. doi: 10.1148/rg.235035134
52
Ollivier L Vanel D Leclère J . Imaging of chondrosarcomas. Cancer Imaging. (2004) 4:36–38. doi: 10.1102/1470-7330.2003.0022
53
Yoo JS Rosenthal DI Mitchell K Ginsberg LE . Osteoradionecrosis of the hyoid bone: imaging findings. AJNR Am J Neuroradiol. (2010) 31:761–6. doi: 10.3174/ajnr.A1892
54
Kovacic M Bavesic K . Thyroglossal duct cyst with laryngeal ex 一 tension. Acta Med croatic. (2007) 61:191—193.
55
Li B Gao S . Ultrasonographic diagnosis of thyrohyoid cyst (analysis of 40 cases). J Pract Med Imaging. (2008) (02):108–9.
56
Tas A Karasalihoglu AR Yagiz R Doğanay L Guven S . Thyroglossal duct cyst in hyoid bone: unusual location. J Laryngol Otol. (2003) 117:656–7. doi: 10.1258/002221503768200039
57
Guo X Zhu X Dong C . Analysis of 36 cases of intractable pain at the end of limb caused by glomus tumor. Chin J Misdiagnosis. (2011) 11:8167.
58
Folpe AL Fanburg-Smith JC Miettinen M Weiss SW . Atypical and Malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. (2001) 25:1–12. doi: 10.1097/00000478-200101000-00001
Summary
Keywords
glomus tumor, hyoid bone, case report, literature review, differential diagnosis
Citation
Shi M, Han M, Wang J, Zhao Q, Ren C, Li H, Yang Z, Wei J and Yang X (2025) Glomus tumor of the hyoid bone: a case report and literature review. Front. Oncol. 15:1512465. doi: 10.3389/fonc.2025.1512465
Received
16 October 2024
Accepted
13 March 2025
Published
04 April 2025
Volume
15 - 2025
Edited by
Yi Li, Sichuan University, China
Reviewed by
Guiquan Zhu, Sichuan University, China
Jing Zhou Hu, Shanghai Jiao Tong University, China
Updates
Copyright
© 2025 Shi, Han, Wang, Zhao, Ren, Li, Yang, Wei and Yang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinjie Yang, yangxinjie@fmmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.