Abstract
Mucoepidermoid carcinoma (MEC) is a common malignancy of the salivary glands, accounting for 10-15% of salivary gland tumors. It mostly occurs in the salivary glands and very rarely in the lungs and mammary glands. The incidence of breast MEC is only 0.2% to 0.3% of all primary breast tumors. Pulmonary MEC accounts for only 0.1-0.2% of all lung tumors. This report presents two cases of MEC occurring in rare locations: the left lower lung and the breast. Both cases presented different clinical features and histopathological characteristics. The immunohistochemical analysis of Ki-67, CK5/6, P63 and other positive results also confirmed the diagnosis. Surgical resection was the primary treatment in both cases, with the patient with lung involvement additionally receiving chemotherapy and radiotherapy. These cases underscore the importance of recognizing MEC in atypical locations, as timely diagnosis and proper treatment approaches are vital for improving patient outcomes. From these cases, the complexity of MEC is highlighted and the necessity of precise histopathological diagnosis, especially in rare sites, is emphasized.
Introduction
Mucoepidermoid carcinoma (MEC) is a malignant tumor primarily found in the salivary glands, accounting for 10–15% of all salivary gland tumors and 30% of all salivary gland malignancies (1), with a higher prevalence in women (51.5%) (2). The parotid gland (56.8%) and the hard palate (18%) are typical sites of involvement (3). While MEC is more commonly found in the salivary glands, it can also occur in extra-salivary tissues, such as the lung, pancreas, thyroid, accessory lacrimal glands, and breast, though these occurrences are rare (4–8). MEC of the breast is an exceedingly rare malignancy, constituting only 0.2–0.3% of all breast cancers (9). The first case of breast MEC was reported by Patchefsky et al. in 1979 (10). By 2022, only 45 cases of breast MEC had been documented worldwide, with only four cases classified as intermediate-grade MEC. Pulmonary MEC is a subtype of non-small cell lung cancer (NSCLC) and is a small proportion (0.1–0.2%) of primary lung cancers and primarily affects younger individuals (10).
Histologically, MEC is characterized by a combination of squamous cells, mucinous cells and intermediate cells. Based on histological features, MEC is classified into low-grade, high-grade subtype and intermediate type. Low-grade MEC predominantly consists of glandular elements and mucinous cells, whereas high-grade MEC contains mostly squamous and intermediate cells, with fewer mucinous cells. Low-grade MEC is usually associated with a good prognosis, with a 5-year survival rate of 90-100% (1), while high-grade MEC is associated with a poorer prognosis. The intermediate type is somewhere in between. MEC occurring in atypical sites presents unique diagnostic challenges. These rare presentations can be misdiagnosed as more common malignancies, complicating clinical management. Accurate diagnosis in such cases relies on histopathological and immunohistochemical analysis (11).
This report adds to the growing body of knowledge regarding MEC in non-salivary gland locations and emphasizes the importance of recognizing atypical presentations to ensure accurate diagnosis and appropriate treatment (8). While most MEC cases involve the salivary glands, particularly the parotid gland, this report of MEC in a rare site underscores the need to better understand the clinical behavior, prognosis, and treatment strategies for MEC outside of traditional anatomical locations (12).
Case 1: MEC of the breast
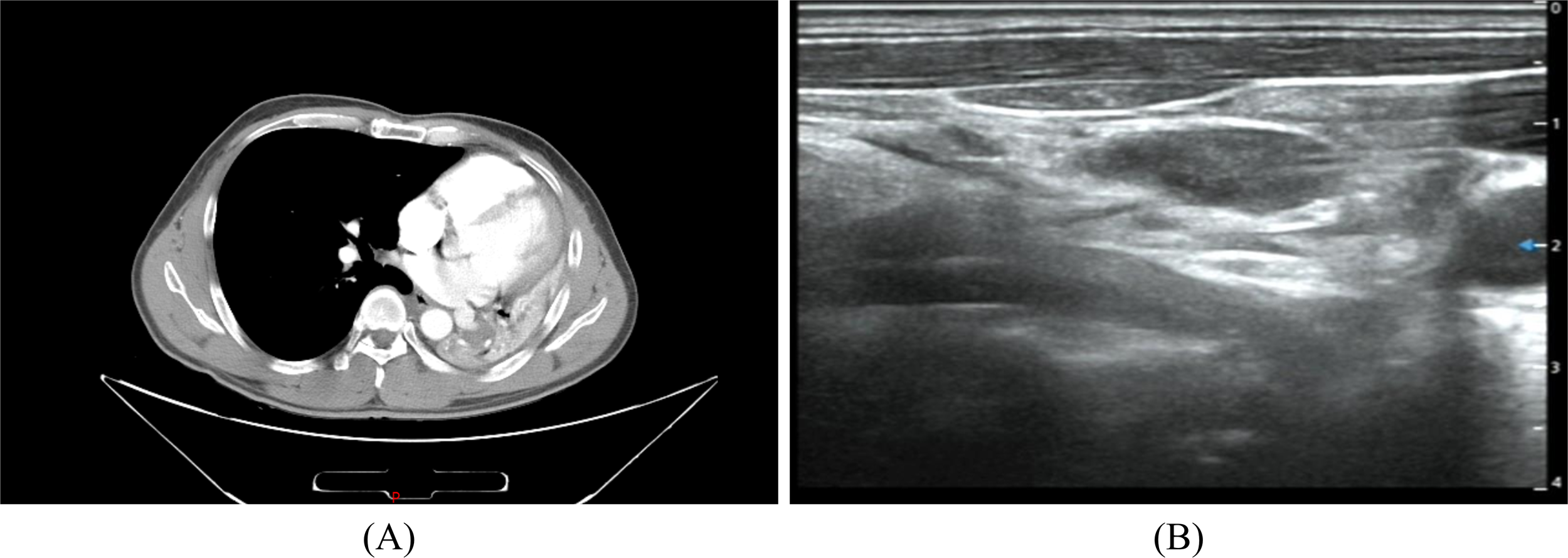
A 47-year-old female came to the hospital with a left breast mass with local pain 2 months ago. During physical examination, a mass of about 5.0cm×4.0cm in size was palpated near the edge of the breast at 2 o’clock in the upper outer quadrant of the left breast. The mass was soft, regular in shape, well bounded, with fair movement and tenderness. No obvious mass was palpable on the right breast. No obvious nipple discharge or other abnormalities were observed. Enlarged lymph node of the size 2.0×1.0cm was palpated in the left axilla, while no enlarged lymph nodes were palpated in the right axilla. In addition, the patient had no family history of breast cancer. The ultrasound examination of the breast reported that at 2 o’clock on the left breast of the patient, there was a 4.65 × 4.52 × 2.94cm mixed cystic and solid echo mass on the edge of the entry line, with a clear boundary, a long oval shape and irregular thickening of the wall. In addition, there were crisscrossed strip diaphragm echo-mass, with a low to no echo-zone in the center, and the aspect ratio was less than 1. At 2 o’clock on the right breast, a 0.61 × 0.23cm solid hypoechoic area can be seen at 2 cm from the nipple, the boundary is not clear, it is elongated, and the aspect ratio is less than 1. Several hypoechoic nodules of different sizes were seen in the left axilla, the larger one was about 2.26×0.9cm, and the central medullary structure was slightly reduced and slightly eccentric. No abnormal enlarged lymph nodes were observed in the right axilla (Figures 1A-C). Subsequently, the patient underwent a modified radical mastectomy to remove the lesion completely. According to the postoperative pathological and immunohistochemical results, the patient was diagnosed with intermediate-grade MEC. After multidisciplinary discussions, it was considered that the benefit of adjuvant chemotherapy for this patient was unclear, and radiotherapy was recommended. However, the patient did not receive any subsequent adjuvant therapy in the end. Twelve months after the operation, the patient was in good health with no signs of recurrence or metastasis. Furthermore, we developed a comprehensive patient treatment timeline that systematically delineates the therapeutic interventions and clinical progression (Figure 1G).
Figure 1

Imaging of the breast: (A, B) Ultrasonography. (C) Mammography. Histology of breast MEC: (D) ×100; (E) ×200; (F) ×400. (G) Patient Treatment Schedule.
The gross examination of the resected tumor showed that the lesions were mainly solid and clearly defined, with gray white and gray red sections, with a volume of 5×3.4×2cm. Under the microscope, the tumor is composed of a large number of atypically proliferating intermediate cells, a small number of squamous cells, and a small number of mucinous cells. The tumor cells are arranged in solid or glandular patterns, with focal necrosis visible. Mitotic figures are easily seen, and neural invasion is present. (Figures 1D-F).
Immunohistochemical results showed that tumor cells expressed CK5/6, p63 positive. Tumor cells did not express positive estrogen receptor (ER), progesterone receptor (PR) and HER-2/NEU protein. About 30% of tumor cells were positive for Ki67. Combined with histopathological findings, this case was classified as intermediate-grade breast MEC (Figure 2).
Figure 2
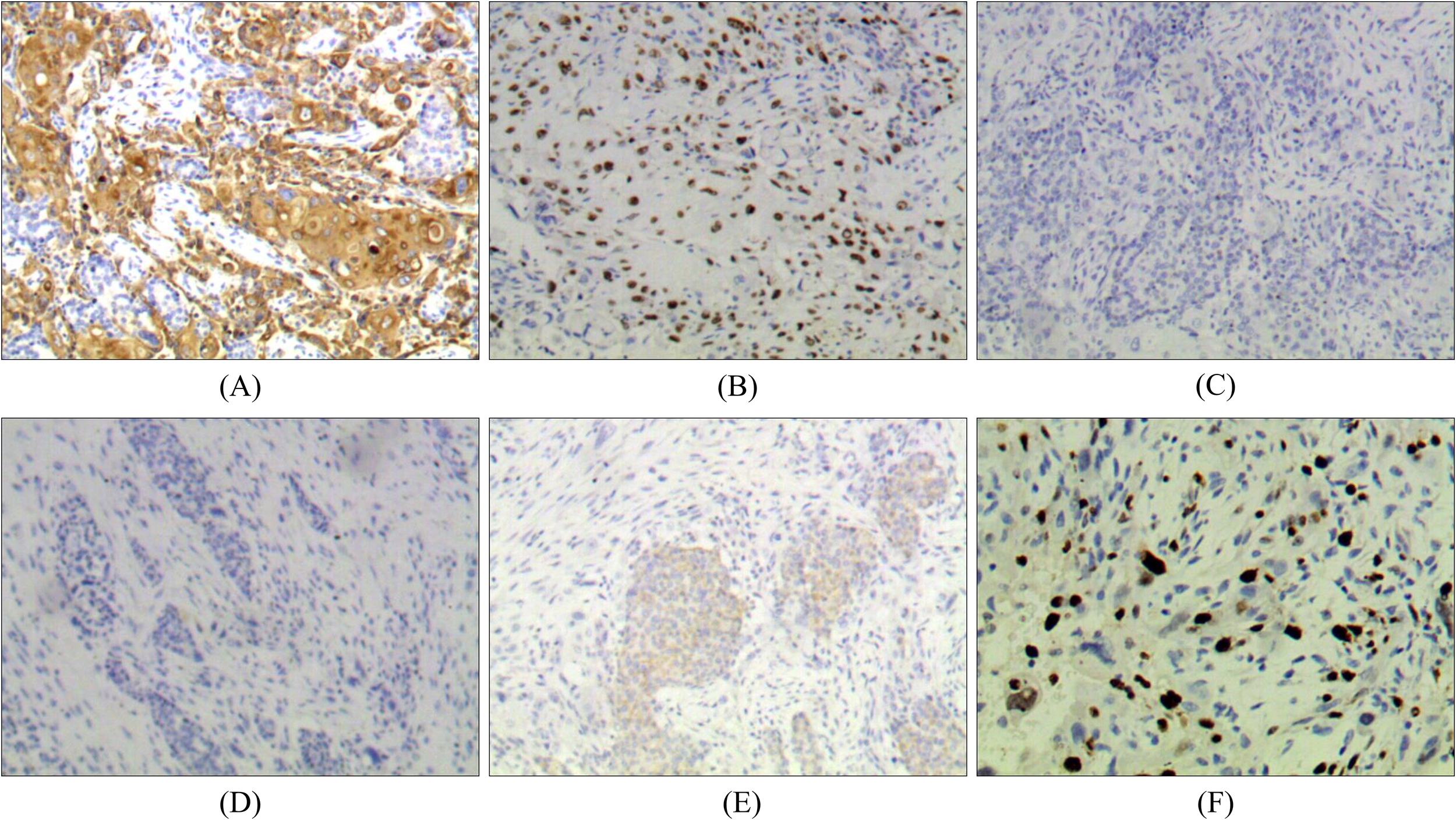
Immunohistochemistry of breast MEC: (A) Positive for CK5/6 (×100). (B) Positive for p63 (×100). (C) Negative for ER (×100). (D) Negative for PR (×100). (E) Negative for HER-2 (×100). (F) Positive for Ki67 (+, 30%).
Case 2: MEC of the lung
A 45-year-old male was admitted to the hospital for recurrence of MEC of the left lower lung more than one month after his last treatment. The patient initially visited the hospital in June 2021 because of chest and back pain, with a chest CT scan revealing several slightly weak and enhanced nodules in the left lower lobe, the largest being 2.2 cm × 1.4 cm, presenting as an irregular solid shadow (Figure 3A). A tracheoscopic biopsy revealed a poorly differentiated tumor. He subsequently underwent a left lower lobectomy with bronchial sleeve resection and mediastinal lymph node dissection. Intraoperative freezing showed negative bronchial margins and lymph nodes. Postoperative pathology confirmed MEC invading the cartilage. Histopathological examination with hematoxylin and eosin (HE) staining revealed a mixture of squamous cells, intermediate cells, and mucinous cells. The glandular structure containing mucin is obvious, with moderate nuclear pleomorphism and some hyperchromic nuclei (Figures 4A-C). Immunohistochemistry was positive for Ki-67 (+, 10%), CK5/6(+), CK7(+), P63(+), and p40(+) (Figures 4D-F), consistent with MEC. The patient was diagnosed postoperatively with stage IIB (pT3N0M0) disease and received four cycles of chemotherapy with docetaxel and cisplatin from August to November 2021.
Figure 3
Chest CT (A) The left lung is reduced in volume and multiple nodules are seen in the lower left lung. Neck ultrasound (B) Multiple swollen lymph nodes in the neck.
Figure 4
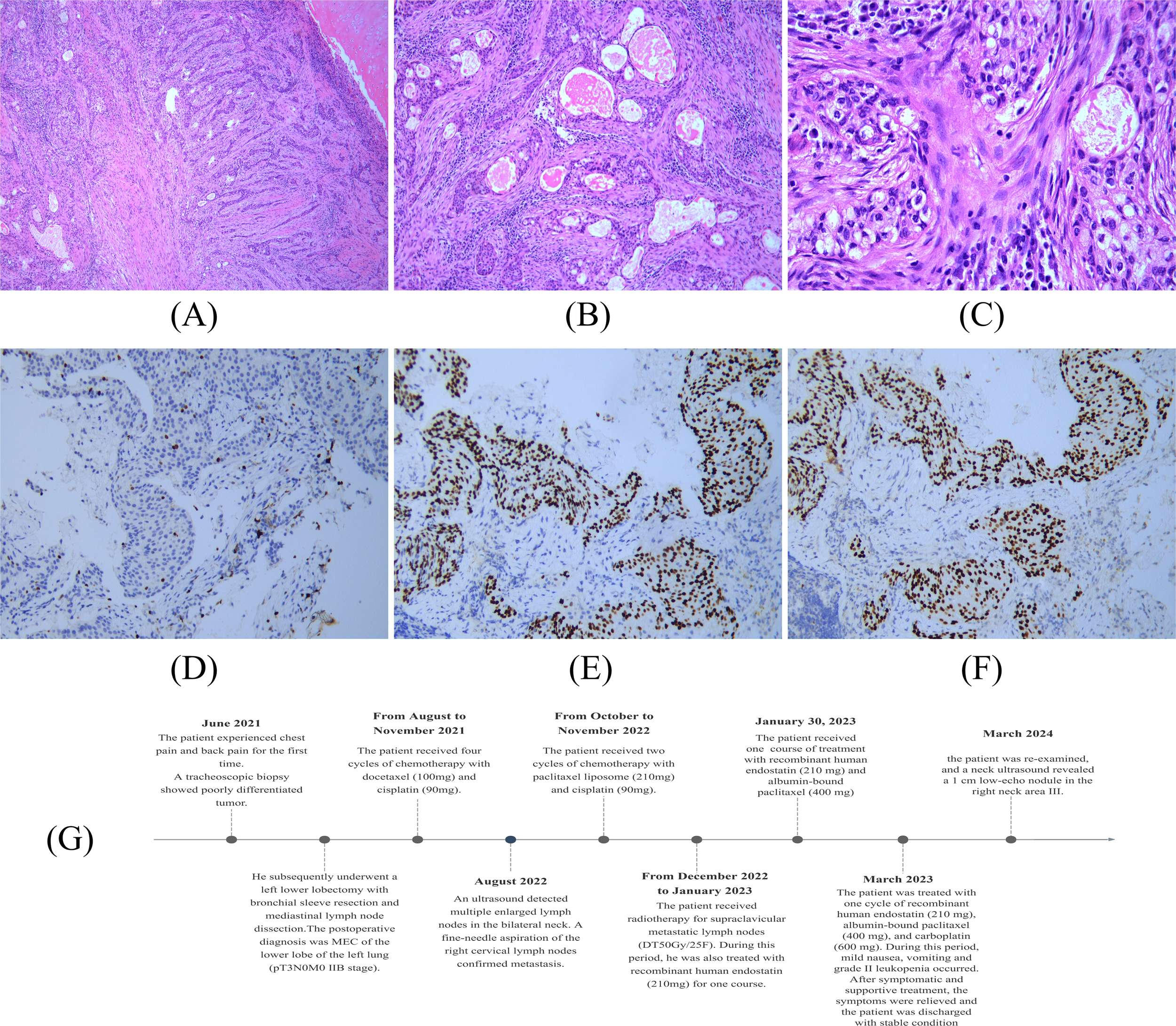
Histopathological examination of pulmonary MEC: (A) ×100; (B) ×200; (C) ×400. Immunohistochemistry of Pulmonary MEC: (D) Positive for Ki67 (+, 10%). (E) Positive for P40 (×200). (F) Positive for P63 (×200). (G) Patient Treatment Schedule.
In August 2022, an ultrasound detected multiple enlarged lymph nodes in the bilateral neck (Figure 3B). A fine-needle aspiration of the right cervical lymph nodes confirmed metastasis. From October to November 2022, he received two additional cycles of chemotherapy with paclitaxel liposome and cisplatin and then received radiotherapy for supraclavicular metastatic lymph nodes (DT50Gy/25F) between December 2022 and January 2023. During this period, he was also treated with recombinant human endostatin. In March 2023, the patient was admitted for further treatment. The patient had a history of smoking for more than 20 years, 20 cigarettes a day, and had quit smoking for one month. Physical examination revealed an old surgical scar on the chest wall, with clear lung sounds and no palpable lymphadenopathy. Auxiliary examinations showed no obvious abnormalities on brain MRI and CT scans of the chest, and upper abdomen, which indicated no significant changes compared to a previous scan. The Neck CT showed multiple small lymph nodes. The bone scan showed benign changes without evidence of bone metastasis.
The patient was treated with one cycle of recombinant human endostatin (210 mg), albumin-bound paclitaxel (400 mg), and carboplatin (600 mg) starting on March 16. During hospitalization, he developed mild nausea, vomiting, and grade II leukopenia, but these symptoms were managed with supportive care. He was discharged in a stable condition with a diagnosis of maintenance chemotherapy for left lower lobe MEC (rT0N3M0). In March 2024, the patient was re-examined, and a neck ultrasound revealed a 1 cm low-echo nodule in the right neck area III. The prognosis remains guarded given the aggressive nature and recurrent behavior of the tumor. Similarly, we have formulated a comprehensive patient treatment schedule in order to improve the readability of the report (Figure 4G).
Discussion
Case reports of MEC at rare sites highlight the complexity and aggressiveness of this rare malignancy. In the context of relevant literature, we discussed and summarized the rare occurrence sites, treatment methods and metastasis of the disease (Table 1). The aim is to provide clinicians with valuable insights into the diagnosis and management of this rare disease.
Table 1
| Author | Year | Age | Sex | Diagnosis | Organism | Metastasis | Treatment |
|---|---|---|---|---|---|---|---|
| Yuan Chen et al. (54) | 2024 | 51 | Female | Pancreatic mucoepidermoid carcinoma | Pancreas | Yes | Pancreaticoduodenectomy and chemotherapy |
| Márcio Rodrigues Costa et al. (55) | 2015 | 47 | Male | Mucoepidermoid carcinoma of the penis | Penis | Yes | Total penectomy and chemotherapy |
| Himsikhar Khataniar et al. (56) | 2022 | 51 | Female | Mucoepidermoid carcinoma of the anterior mandible | Anterior mandible | No | Segmental mandibulectomy with reconstruction |
| Janakiram T. N. et al. (57) | 2016 | 65 | Male | Primary mucoepidermoid carcinoma of the lacrimal sac | Lacrimal sac | Unknown | Radical surgery and chemoradiation |
| Zhang HY and Yang HY (58) | 2020 | 39 | Female | Mucoepidermoid carcinoma in the infratemporal fossa | Infratemporal fossa | Yes | Extended resection of primary tumor, radical neck dissection and radiotherapy |
| Mario Della Mura et al. (59) | 2023 | 58 | Female | High-grade HER2-positive breast mucoepidermoid carcinoma | Breast | No | Surgical resection, chemotherapy and targeted therapy |
| Alessandro G. Fois et al. (60) | 2017 | 46 | Male | Mucoepidermoid carcinoma of the bronchus | Bronchus | No | Left upper lobectomy and mediastinal lymphadenectomy |
| Zihan Li et al. (61) | 2024 | 67 | Male | Primary hepatobiliary mucoepidermoid carcinoma | Liver | Unknown | Surgical resection |
| Manabu Yamamoto et al. (62) | 2018 | 74 | Male | High-grade mucoepidermoid carcinoma of the anal canal | Anal canal | Yes | Local surgical resection and irradiation therapy |
Summary of case reports on MEC in rare sites.
MEC is a rare epithelial malignant tumor, usually presenting in salivary glands and was first discovered by Foote et al (13). MEC show a strong preference for the parotid gland, most commonly with low grade histology. MEC outside the salivary glands is very rare. Breast MEC is particularly rare. At present, only 45 cases of breast MEC have been reported in English literature. All patients were female, aged 27–86 years old. There were 19 cases of severe MEC, 20 cases of low MEC, 4 cases of moderate MEC, and the remaining 2 cases were not explained. Table 2 summarizes the references and publication years of these studies, as well as the clinicopathological features of these 45 patients and current cases. Pulmonary MEC is a malignant tumor originating in the bronchial glands, which was first described by Smetana in 1952 (3), with a presumed incidence of 0.1-0.2% of all lung cancers (14). Given the rarity of the disease, especially in non-salivary gland sites, MEC often has non-specific clinical features, making the diagnosis of MEC in these atypical sites challenging and often easily misdiagnosed as other common diseases at that site. For example, MECS with large cystic structure are easily mistaken for single breast cyst, while MECS with microcystic structure are easily mistaken for ductal carcinoma in situ. In this case, the breast MEC showed features associated with generally benign breast lesions, which guided our preoperative discussions with the patient and their family, focusing on a more favorable prognosis. However, the final diagnosis of MEC, a rare malignancy, presented unexpected challenges. This underscores the need for better vigilance in similar cases. In the future, even when a tumor appears benign preoperatively, clinicians must maintain a differential diagnosis that includes the possibility of malignancy, especially in cases involving atypical presentations or rare histological subtypes. The implications of underestimating such cases could lead to delays in appropriate treatment and affect patient outcomes. Therefore, preoperative planning should thoroughly consider the possibility for malignancy to ensure timely, accurate diagnosis and treatment.
Table 2
| Study | Year | Age | Site | Size | Grade | LN | Distant | Follow-up | Status |
|---|---|---|---|---|---|---|---|---|---|
| (Years) | (cm) | Metastasis | Metastasis | (Months) | |||||
| Present study | 2024 | 48 | Left | 5 | IG | 0/4 | No | 12 | Alive |
| Wang et al (27) | 2024 | 47 | Left | 4.5 | HG | No | No | 12 | Alive |
| Mura et al (28) | 2023 | 58 | Left | 2 | HG | No | No | 61 | Alive |
| Bak et al (29) | 2022 | 47 | Right | 3.2 | IG | No | No | 37 | Alive |
| Ye Ru-Pei et al (30) | 2020 | 42 | Right | 2.6 | LG | NA | No | 12 | Alive |
| Mingfei Yan et al (31) | 2019 | 60 | Right | 1.9 | LG | NA | No | 60 | Alive |
| Burghel et al (32) | 2018 | 73 | Left | NA | LG | 0/2 | No | NA | NA |
| Sherwell-Cabello et al (7) | 2017 | 86 | Left | 6 | LG | NA | No | 3 | Alive |
| Cheng et al (33) | 2017 | 39 | Right | 1.5 | LG | 3/18 | No | 156 | Alive |
| 49 | Left | 1.5 | LG | 0/17 | No | 41 | Alive | ||
| 66 | Left | 1.3 | LG | 0/6 | No | 9 | Alive | ||
| 61 | Left | 3 | LG | 0/3 | No | 4 | Alive | ||
| Fujino et al (34) | 2016 | 71 | Right | 1.7 | IG | 0/NA | No | NA | NA |
| Palermo et al (35) | 2013 | 80 | Right | 4 | HG | 0/NA | No | NA | NA |
| Turk et al (36) | 2013 | 40 | Right | 5.5 | NA | 1/24 | No | 5 | Alive |
| Basbug et al (21) | 2011 | 69 | Left | 10 | HG | 0/12 | No | 12 | Alive |
| Camelo-Piragua et al (37) | 2009 | 49 | Right | 4 | IG | 1/3 | No | 8 | Alive |
| Hornychova et al (38) | 2007 | 62 | Right | 1.8 | HG | 0/17 | No | 18 | Alive |
| 30 | Left | 8 | LG | 0/NA | No | 60 | Alive | ||
| Horii et al (16) | 2006 | 54 | Left | 2.5 | LG | 0/NA | No | 36 | Alive |
| Gomez-Aracil et al (39) | 2006 | 69 | Right | 7.5 | HG | 24/28 | No | 54 | Alive |
| Di Tommaso et al (40) | 2004 | 80 | Left | 0.5 | LG | NA | No | 5 | Alive |
| 29 | Left | 0.8 | LG | NA | No | 90 | Alive | ||
| 54 | Left | 1.5 | LG | NA | No | 13 | Alive | ||
| 55 | Left | 1.1 | IG | NA | No | 18 | Alive | ||
| 36 | Left | 0.6 | HG | NA | No | 3 | Alive | ||
| Terzi et al (41) | 2004 | 79 | Right | 8 | HG | 4/14 | NA | NA | NA |
| Tjalma et al (42) | 2002 | 58 | Right | 3.5 | HG | 1/17 | Yes | 156 | Alive |
| Berry et al (43) | 1998 | 51 | Left | 3.5 | HG | 0/NA | No | NA | NA |
| Markopoulos et al (44) | 1998 | 40 | Right | 2 | HG | 0/NA | No | 60 | Alive |
| Chang et al (45) | 1998 | 54 | Left | 4.5 | HG | 0/9 | No | 48 | Alive |
| Luchtrath and Moll (46) | 1989 | 60 | NA | 5 | HG | 12/18 | Yes | 30 | DOD |
| Pettinato et al (47) | 1989 | 72 | Right | 7 | HG | 16/19 | Yes | 10 | DOD |
| Hanna and Kahn (48) | 1985 | 51 | Left | 2 | HG | 0/NA | No | 8 | Alive |
| 31 | NA | NA | NA | 2/18 | No | 14 | Alive | ||
| Hastrup and Sehested (49) | 1985 | 59 | Left | 1 | HG | 0/4 | Yes | 25 | DOD |
| Leong and Williams (50) | 1985 | 57 | Left | 3.5 | HG | 0/20 | Yes | 7 | DOD |
| Ratanarapee et al (51) | 1983 | 27 | NA | NA | HG | 6/15 | Yes | 14 | DOD |
| Fisher et al (52) | 1983 | 65 | Right | 2 | LG | NA | No | 60 | Alive |
| 71 | Left | 2 | LG | 0/19 | No | 48 | Alive | ||
| 57 | Right | 2.5 | LG | 0/11 | No | 120 | Alive | ||
| 49 | Right | 3.7 | LG | 0/13 | No | 108 | Alive | ||
| 60 | Left | 4 | LG | NA | No | 48 | DOR | ||
| Kovi et al (53) | 1981 | 46 | Left | 11 | HG | 17/19 | NA | NA | NA |
| Patchefsky et al (10) | 1979 | 66 | Right | 1.3 | LG | 0/20 | No | 94 | DOR |
| 70 | Right | 5 | LG | NA | No | 10 | Alive |
Summary of reported cases of breast MEC from 1979 to 2024.
This case underscores the importance of distinguishing MEC from other tumor types through histopathological and immunohistochemical analyses, both of which are crucial for an accurate diagnosis (15). Histologically, MEC consists of mucinous cells, intermediate cells, and squamous cells. Based on the degree of cytological abnormalities and the relative proportions of these cell types, MEC can be classified into three grades: low, intermediate, and high (16). Low-grade MEC accounts for the majority of cases (48%), followed by high-grade (38.7%) and intermediate-grade (13.3%) (17). Histological grade is a key prognostic factor, with low-grade tumors exhibiting a higher proportion of mucinous cells and less aggressive behavior, while high-grade tumors contain fewer mucinous cells and have a worse prognosis (18). Intermediate-grade tumors display features that fall between these two extremes. In this study, the patient with breast MEC was pathologically diagnosed with intermediate-grade MEC. Tumor cells in breast MEC usually test negative for ER, PR, and HER-2/NEU protein (19), but breast MEC generally has a better prognosis than conventional triple-negative breast cancer (TNBC) (20). The immunohistochemistry results in this case aligned with these findings. Additionally, the tumor cells expressed p63, CK5/6, and epidermal growth factor receptor (EGFR), which is consistent with previously reported cases (21). Similarly, in Pulmonary MEC, the expression of Ki-67, CK5/6, p63, and p40 confirmed the diagnosis of MEC in this study. By successfully identifying MEC through thorough histopathological and immunohistochemical analysis, this case highlights the need for increased awareness among clinicians for such atypical presentations (22). This evidence adds value to future clinical practice by encouraging a multidisciplinary diagnostic approach, including the potential use of genetic markers for more precise diagnosis and treatment planning (23). Overall, this case strengthens the existing literature by emphasizing the importance of early detection and comprehensive diagnostic evaluations, which can significantly impact patient outcomes.
MEC is most effectively treated with surgery, with the extent of the procedure determined by the tumor’s location, size, and histopathological grade (18). Local resection is typically sufficient for low-grade, less aggressive tumors, whereas high-grade tumors require more extensive resections involving adjacent structures (18). Due to the rarity of breast MEC, research on this specific subtype is limited, and no standard treatment protocol exists. Consequently, breast MEC treatment is generally based on protocols used for more common breast cancers, with the surgical approach and postoperative treatment plan adjusted according to tumor location and grade. For low- and intermediate-grade breast MEC, the primary surgical options include modified radical mastectomy or mastectomy with sentinel lymph node biopsy. In contrast, high-grade breast MEC often requires more extensive procedures, such as modified radical mastectomy or radical mastectomy. Patients with high-grade MEC are recommended to receive postoperative adjuvant treatments including radiotherapy and chemotherapy to lower their chances of recurrence, and these patients should undergo closer monitoring with more frequent follow-ups. In the current case, the patient received a modified radical mastectomy, and postoperative histopathology confirmed intermediate-grade breast MEC. According to the characteristics of the tumor, the benefits of adjuvant chemotherapy were not clear. Radiotherapy was recommended, but in the end, the patient did not receive any subsequent adjuvant treatment. Twelve months after surgery, the patient remains in good health with no signs of recurrence. Surgical resection is also the standard treatment for pulmonary MEC. Low-grade pulmonary MEC generally has an excellent prognosis, with a 5-year survival rate of 95%, and adjuvant therapy is typically unnecessary. However, due to the limited number of high-grade pulmonary MEC cases, there is no established consensus about using adjuvant therapy in these patients. In the present case, the pulmonary MEC patient received a left lower lobectomy with bronchial sleeve resection and mediastinal lymph node dissection, and the surgical margins were negative. The tumor was histologically diagnosed as high-grade MEC. Despite postoperative chemotherapy, cervical lymph node metastasis occurred. Given that EGFR is often overexpressed in MEC of salivary gland origin, Han et al. identified EGFR mutations in 2 out of 5 Pulmonary MEC specimens (24). In this context, there have been several reports on the efficacy of the tyrosine kinase inhibitor gefitinib in patients with EGFR gene mutations (24, 25), and this molecular targeted therapy may improve outcomes for progressive high-grade and recurrent MEC cases. This case underscores the need for a multidisciplinary approach to diagnosis and treatment, especially in non-salivary gland cases, where the tumor’s clinical behavior and treatment options may vary from typical MEC cases (26). While surgical resection remains the primary treatment for MEC in most cases, the tumor’s grade and location can influence long-term outcomes and prognosis (12). Moreover, these two cases also highlight the importance of patient experience and compliance in the treatment of MEC in rare locations. For the breast MEC patient, attention to the breast lump and pain led to timely diagnosis and treatment of the disease. The Pulmonary MEC patient showed excellent treatment compliance. Despite having cervical lymph node metastasis, he actively cooperated with subsequent treatments.
Conclusion
This case of MEC occurring in a rare anatomical location highlights the importance of considering MEC in differential diagnoses, even in atypical sites. The primary lesson learned from this report is the critical role of comprehensive histopathological evaluation in accurately diagnosing MEC, particularly when its clinical and radiological presentation overlaps with more common malignancies. The case reinforces the need for a multidisciplinary approach to ensure timely diagnosis and appropriate treatment. Early identification of the tumor’s grade and characteristics can significantly impact patient outcomes, because high-grade MEC requires more aggressive intervention. This report emphasizes the necessity for clinicians to maintain a high index of suspicion for MEC in unusual locations, as early detection and tailored treatment strategies can improve prognosis and overall patient care.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This case report has been approved by the Ethics Committee of Jiujiang University Affiliated Hospital. Written informed consent was obtained from the participant for the publication of this case report.
Author contributions
CZ: Conceptualization, Validation, Visualization, Writing – original draft, Writing – review & editing. BL: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. HS: Conceptualization, Validation, Visualization, Writing – original draft, Writing – review & editing. ZN: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. YC: Conceptualization, Visualization, Writing – original draft, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Peraza A Gómez R Beltran J Amarista FJ . Mucoepidermoid carcinoma. An update and review of the literature. J Stomatol Oral Maxillofac Surg. (2020) 121:713–20. doi: 10.1016/j.jormas.2020.06.003
2
Limaiem F Lekkala MR Sharma S . "Mucoepidermoid lung tumor." In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. (2023).
3
Smetana HF Iverson L L.L. SWAN . Bronchogenic carcinoma; an analysis of 100 autopsy cases. Military surgeon (1952) 111:335–51.
4
Ma R Yu Y-Q Li J-T Peng S-Y . Mucoepidermoid carcinoma of the pancreas: a case report and a review of literature. J Res Med Sci. (2012) 17:886.
5
Sha AA La Fortune K Miller C Mills SE Baloch Z LiVolsi V . Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia: a clinicopathologic and molecular analysis of a distinct entity. Modern Pathology. (2017) 30:329–39. doi: 10.1038/modpathol.2016.180
6
Dithmar S Wojno TH Washington C Grossniklaus HE . Mucoepidermoid carcinoma of an accessory lacrimal gland with orbital invasion. Ophthalmic Plast Reconstr Surg. (2000) 16:162–6. doi: 10.1097/00002341-200003000-00012
7
Sherwell-Cabello S Maffuz-Aziz A Ríos-Luna NP Pozo-Romero M López-Jiménez PV Rodriguez-Cuevas S . Primary mucoepidermoid carcinoma of the breast. Breast J. (2017) 23(6):753–5. doi: 10.1111/tbj.12819
8
Bai J Wang G . Mucoepidermoid carcinoma of the breast. Radiology. (2022) 305:32. doi: 10.1148/radiol.220128
9
Bean GR Krings G Otis CN Solomon DA García JJ van Zante A . CRTC 1–MAML 2 fusion in mucoepidermoid carcinoma of the breast. Histopathology (2019) 74:463–73. doi: 10.1111/his.2019.74.issue-3
10
Patchefsky AS Frauenhoffer CM Krall RA Cooper HS . Low-grade mucoepidermoid carcinoma of the breast. Arch Pathol Lab Med. (1979) 103:196–8. doi: 10.1097/00000478-197912000-00011
11
Mimica X Yuan A Hay A Katabi N Karassawa Zanoni D Valero C . Mucoepidermoid carcinoma: Evaluating the prognostic impact of primary tumor site. Oral Oncol. (2021) 123:105602. doi: 10.1016/j.oraloncology.2021.105602
12
Li Q Wei X Wang Y Liu C Fan B Lv C . Pulmonary mucoepidermoid carcinoma in the Chinese population: A clinical characteristic and prognostic analysis. Front Oncol. (2022) 12:916906. doi: 10.3389/fonc.2022.916906
13
Stewart FW Foote FW Becker WF . Muco-epidermoid tumors of salivary glands. Ann Surg. (1945) 122:820–44. doi: 10.1097/00000658-194511000-00005
14
Leonardi HK Jung-Legg Y Legg MA Neptune WB . Tracheobronchial mucoepidermoid carcinoma: clinicopathological features and results of treatment. J Thorac Cardiovasc Surg. (1978) 76:431–8. doi: 10.1016/S0022-5223(19)41067-2
15
Kimura M Miyajima K Ishikawa R Yamada Y Kono T Okunaka T . Photodynamic therapy for pulmonary mucoepidermoid carcinoma. Respir Med Case Rep. (2021) 33:101431. doi: 10.1016/j.rmcr.2021.101431
16
Horii R Akiyama F Ikenaga M Iwase T Sakamoto G . Muco-epidermoid carcinoma of the breast. Pathol Int. (2006) 56:549–53. doi: 10.1111/j.1440-1827.2006.02004.x
17
Qureshi SM Janjua OS Janjua SM . Mucoepidermoid carcinoma: a clinico-pathological review of 75 cases. Int J Oral Maxillofac Pathol. (2012) 3:5–9. Available online at http://www.journalgateway.com.
18
Kumar AN Nair PP Thomas S Raman PS Bhambal A . Mucoepidermoid carcinoma of sublingual gland: a Malignant neoplasm in an uncommon region. BMJ case reports (2011) 2011:bcr0220113864. doi: 10.1136/bcr.02.2011.3864
19
Jones C Ford E Gillett C Ryder K Merrett S Reis-Filho JS . Molecular cytogenetic identification of subgroups of grade III invasive ductal breast carcinomas with different clinical outcomes. Clin Cancer Res. (2004) 10:5988–97. doi: 10.1158/1078-0432.CCR-03-0731
20
Pia-Foschini M Reis-Filho JS Eusebi V Lakhani SR . Salivary gland-like tumours of the breast: surgical and molecular pathology. J Clin Pathol. (2003) 56:497–506. doi: 10.1136/jcp.56.7.497
21
Basbug M Akbulut S Arikanoglu Z Sogutcu N Firat U Kucukoner M . Mucoepidermoid carcinoma in a breast affected by burn scars: comprehensive literature review and case report. Breast care (Basel, Switzerland). (2011) 6:293–7. doi: 10.1159/000331316
22
Yamamoto T Nakajima T Suzuki H Tagawa T Iwata T Mizobuchi T . Surgical treatment of mucoepidermoid carcinoma of the lung: 20 years’ experience. Asian Cardiovasc Thorac Ann. (2016) 24:257–61. doi: 10.1177/0218492316630494
23
Zhang XP Hu PZ Shen SS Li XY . Clinical characteristics and prognostic analyses of 87 patients with pulmonary mucoepidermoid carcinoma. Zhonghua Zhong Liu Za Zhi. (2018) 40:452–5. doi: 10.3760/cma.j.issn.0253-3766.2018.06.010
24
Han S-W Kim H-P Jeon PK Oh D-Y Lee S-H Kim D-W . Mucoepidermoid carcinoma of lung: potential target of EGFR-directed treatment. Lung cancer (Amsterdam, Netherlands). (2008) 61:30–4. doi: 10.1016/j.lungcan.2007.11.014
25
Shilo K Foss RD Franks TJ DePeralta-Venturina M Travis WD . Pulmonary mucoepidermoid carcinoma with prominent tumor-associated lymphoid proliferation. Am J Surg Pathol. (2005) 29:407–11. doi: 10.1097/01.pas.0000151616.14598.e7
26
Kalhor N Moran CA . Pulmonary mucoepidermoid carcinoma: diagnosis and treatment. Expert Rev Respir Med. (2018) 12:249–55. doi: 10.1080/17476348.2018.1428563
27
Wang L Cheng D Wang H Cheng L Zhang X . A high-grade breast mucoepidermoid carcinoma without MAML2 rearrangement: A case report and literature review. Medicine. (2024) 103(8):e37163. doi:10.1097/MD.0000000000037163
28
Mura MD Clement C Foschini MP Vander Borght S Waumans L Hauben E et al . High-grade HER2-positive mucoepidermoid carcinoma of the breast: a case report and review of the literature. J Med Case Rep. (2023) 17(1):527. doi:10.1186/s13256-023-04233-0
29
Bak S Choi HY Lee JH Na JB Choi DS Cho JM et al . Mucoepidermoid carcinoma of the breast: A case report and literature review focused on radiological findings. Medicine. (2022) 101(26):e29745. doi:10.1097/MD.0000000000029745
30
Ye RP Liao YH Xia T Kuang R Long HA Xiao XL . Breast mucoepidermoid carcinoma: a case report and review of literature. Int J Clin Exp Pathol. (2020) 13(12):3192-9
31
Yan M Gilmore H Harbhajanka A . Mucoepidermoid Carcinoma of the Breast With MAML2 Rearrangement: A Case Report and Literature Review. Int J Clin Exp Pathol. (2020) 28(7):787–92. doi:10.1177/1066896920916779
32
Burghel GJ Abu-Dayyeh I Babouq N Wallace A Abdelnour A . Mutational screen of a panel of tumor genes in a case report of mucoepidermoid carcinoma of the breast from Jordan. Breast J. (2018) 24(6):1102–4. doi:10.1111/tbj.13142
33
Cheng M Geng C Tang T Song Z . Mucoepidermoid carcinoma of the breast: Four case reports and review of the literature. Medicine. (2017) 96(51):e9385. doi:10.1097/MD.0000000000009385
34
Fujino M Mori D Akashi M et al . Mucoepidermoid Carcinoma of the Breast Found during Treatment of Lymphoma. Case Rep Oncol. (2016) 9(3):806-14. doi:10.1159/000452792
35
Palermo MH Pinto MB Zanetti JS Ribeiro-Silva A . Primary mucoepidermoid carcinoma of the breast: a case report with immunohistochemical analysis and comparison with salivary gland mucoepidermoid carcinomas. Pol J Pathol. (2013) 3:210-5. doi:10.5114/pjp.2013.38141
36
Turk E Karagulle E Erinanc OH Soy EA Moray G . Mucoepidermoid carcinoma of the breast. Breast J. (2013) 19(2):206–8. doi:10.1111/tbj.12080
37
Camelo-Piragua SI Habib C Kanumuri P Lago CE Mason HS Otis CN . Mucoepidermoid carcinoma of the breast shares cytogenetic abnormality with mucoepidermoid carcinoma of the salivary gland: a case report with molecular analysis and review of the literature. Human pathology. (2009) 40(6):887–92. doi:10.1016/j.humpath.2008.11.004
38
Hornychová H Ryska A Betlach J Bohác R Cízek T Tomsová M Obermannová R . Mucoepidermoid carcinoma of the breast. Neoplasma. (2007) 54(2):168–72
39
Gómez-Aracil V Mayayo Artal E Azua-Romeo J Mayayo Alvira R Azúa-Blanco J Arraiza Goicoechea A . Fine needle aspiration cytology of high grade mucoepidermoid carcinoma of the breast: a case report. Acta cytologica. (2006) 50(3):344–8. doi:10.1159/000325967
40
Di Tommaso L Foschini MP Ragazzini T Magrini E Fornelli A Ellis IO et al . Mucoepidermoid carcinoma of the breast. Virchows Archiv. (2004) 444(1):13-9. doi:10.1007/s00428-003-0923-y
41
Terzi A Saglam A Uner A . A 79 year-old woman with a mass in the right breast. Turk J Cancer. (2004) 34:38-9
42
Tjalma WA Verslegers IO De Loecker PA Van Marck EA . Low and high grade mucoepidermoid carcinomas of the breast. Eur J Gynaecol Oncol. (2002) 23(5):423–5
43
Berry MG Caldwell C Carpenter R . Mucoepidermoid carcinoma of the breast: a case report and review of the literature. Eur J Surg Oncol. (1998) 24(1):78-80. doi:10.1016/s0748-7983(98)80135-2
44
Markopoulos C Gogas H Livaditou A Floros D . Mucoepidermoid carcinoma of the breast. Eur J Gynaecol Oncol. (1998) 19(3):291–3
45
Chang LC Lee N Lee CT Huang JS . High-grade mucoepidermoid carcinoma of the breast: case report. Changgeng yi xue za zhi. (1998) 21(3):352-7
46
Lüchtrath H Moll R . Mucoepidermoid mammary carcinoma. Immunohistochemical and biochemical analyses of intermediate filaments. Virchows Arch A Pathol Anat Histopathol. (1989) 416(2):105-13. doi:10.1007/BF01606314
47
Pettinato G Insabato L De Chiara A Manco A Petrella G . High-grade mucoepidermoid carcinoma of the breast. Fine needle aspiration cytology and clinicopathologic study of a case. Acta cytologica. (1989) 33(2):195–200.
48
Hanna W Kahn HJ . Ultrastructural and immunohistochemical characteristics of mucoepidermoid carcinoma of the breast. Human pathology. (1985) 16(9):941–6. doi:10.1016/s0046-8177(85)80133-7
49
Hastrup N Sehested M . High-grade mucoepidermoid carcinoma of the breast. Histopathology. (1985) 9(8):887–92. doi:10.1111/j.1365-2559.1985.tb02873.x
50
Leong AS Williams JA . Mucoepidermoid carcinoma of the breast: high grade variant. Pathology. (1985) 17(3):516–21. doi:10.3109/00313028509105513
51
Ratanarapee S Prinyar-Nussorn N Chantarakul N Pacharee P . High-grade mucoepidermoid carcinoma of the breast. A case report. J Med Assoc Thai. (1983) 66(10):642–8.
52
Fisher ER Palekar AS Gregorio RM Paulson JD . Mucoepidermoid and squamous cell carcinomas of breast with reference to squamous metaplasia and giant cell tumors. Am J Surg Pathol. (1983) 7(1):15–27. doi:10.1097/00000478-198301000-00002
53
Kovi J Duong HD Leffall LS Jr . High-grade mucoepidermoid carcinoma of the breast. Arch Pathol Lab Med. (1981) 105(11):612–614.
54
Chen Y Zhu C Xu P Yao J . A case report of pancreatic mucoepidermoid carcinoma responded to gemcitabine and paclitaxel. Heliyon. (2024) 10(10):e31673. doi: 10.1016/j.heliyon.2024.e31673
55
Costa MR Sugita DM Vilela MH da Silva Mendonça RP de Morais DT Júnior PC et al . Mucoepidermoid carcinoma of the penis: case report and literature review. Can Urol Assoc J. 9(1–2):E27–9. doi: 10.5489/cuaj.2126
56
Khataniar H Senthil S Deep SS Ramesh R Y K I . Intraosseous Mucoepidermoid Carcinoma of the anterior mandible: A Case Report. Cureus. 14(5):e25036. doi: 10.7759/cureus.25036
57
Janakiram TN Sagar S Sharma SB Subramaniam V . Primary Mucoepidermoid Carcinoma of the Lacrimal Sac - a Case Report and Literature Review. Klinicka onkologie : casopis Ceske a Slovenske onkologicke spolecnosti. 29(4):1–4. doi: 10.14735/amko2016291
58
Zhang HY Yang HY . Mucoepidermoid carcinoma in the infratemporal fossa: A case report. World J Clin Cases. 8(14):3090–6. doi: 10.12998/wjcc.v8.i14.3090
59
Mura MD Clement C Foschini MP Vander Borght S Waumans L Van Eyken P et al . High-grade HER2-positive mucoepidermoid carcinoma of the breast: a case report and review of the literature. J Med Case Rep. 17(1):527. doi: 10.1186/s13256-023-04233-0
60
Fois AG Diana G Arcadu A Maras V Crivelli P Putzu C et al . Bronchial mucoepidermoid carcinoma: A case report. Int J Surg Case Rep. 31:159–62. doi: 10.1016/j.ijscr.2017.01.042
61
Li Z Nguyen Canh H Nguyen Thi K Takahashi K Nguyen Thi Q Le Thanh D et al . Primary hepatobiliary mucoepidermoid carcinoma: a case report and review of literature. Med Mol Morphol. 57(3):233–43. doi: 10.1007/s00795-024-00390-3
62
Yamamoto M Hirata K Tuneyoshi M Yoshida Y Matsuda H Gion T et al . Mucoepidermoid carcinoma of the anal canal: A case report and review of the literature. Mol Clin Oncol. 9(5):504–6. doi: 10.3892/mco.2018.1706
Summary
Keywords
mucoepidermoid carcinoma, lung, breast, histopathology, immunohistochemistry
Citation
Zhang C, Lu B, Shi H, Ni Z and Cao Y (2025) Case Report: Mucoepidermoid carcinoma in rare locations: a report of two cases. Front. Oncol. 15:1522968. doi: 10.3389/fonc.2025.1522968
Received
05 November 2024
Accepted
22 May 2025
Published
19 June 2025
Volume
15 - 2025
Edited by
Jun-ichi Tanuma, Niigata University, Japan
Reviewed by
Nektarios I. Koufopoulos, University General Hospital Attikon, Greece
Xuhui Liu, Lanzhou University Second Hospital, China
Updates
Copyright
© 2025 Zhang, Lu, Shi, Ni and Cao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuyan Cao, abraham72@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.