- 1Gastroenterology Department, Ruijin Hospital, Affiliated with Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Nursing Department, Ruijin Hospital, Affiliated with Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Pancreatic Surgery Department, Ruijin Hospital, Affiliated with Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4Party Committee Office, Ruijin Hospital, Affiliated with Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Nutritional problems are common in patients with pancreatic cancer. However, the relationship between nutritional risk screening and the survival of patients after pancreaticoduodenectomy remains inconclusive. This study aimed to examine the association between preoperative nutritional risk and survival time among adult Chinese patients with pancreatic cancer after pancreaticoduodenectomy.
Methods: This study was conducted at Ruijin Hospital, affiliated with Shanghai Jiao Tong University School of Medicine in China. Patients aged 18 years or more who received pancreaticoduodenectomy for pancreatic cancer in our center between December 2019 and June 2022 from the follow-up database were included in the study. We retrospectively collected data on the demographics, disease, treatment, nutritional risk score, and survival time of the patients with pancreatic cancer. A Cox regression model was used to analyze the association between nutritional risk and survival time in different covariate models.
Results: A total of 656 patients were included in the study, and the median survival time was 24.0 months (95% CI:21.6-26.3). In total, 29.1% of patients had nutritional risk on admission. At the end of the follow-up, a total of 364 (55.5%) patients had died. The overall 1-, 2-, and 3-year survival rate of the 656 patients with pancreatic cancer after pancreaticoduodenectomy was 72.7%, 49.8%, and 34.4%, respectively. In the Cox regression model adjusted for age, education level, carbohydrate antigen 199 levels, neutrophil-lymphocyte ratio, tumor diameter, lymph node metastasis, distant organ metastasis, differentiation, nerve invasion, surgical margins, surgical time, intraoperative blood loss, postoperative complications, and chemotherapy, patients with nutritional risk score greater than 3 had a lower survival time compared with those without nutritional risk (HR = 1.33, 95% CI:1.06–1.67; P = 0.015).
Conclusions: Preoperative nutritional risk has a detrimental impact on survival in patients with pancreatic cancer who undergo pancreaticoduodenectomy, and this relationship is stable. Nursing staff should screen early for nutritional risk using the Nutritional Risk Screening-2002 tool in patients with pancreatic cancer at diagnosis and, in conjunction with their doctors, develop and implement a timely nutritional treatment plan for those at risk to improve the poor survival time.
Introduction
Pancreatic cancer is a malignancy with a poor prognosis and high mortality. The development of early diagnostic techniques has increased the chances of success of surgical resection (1–3). Pancreaticoduodenectomy is a classic surgical approach to treat tumors in the head or body of the pancreas. With the refinement of surgical techniques, combined with the improvement of adjuvant and neoadjuvant therapy, prolonged survival in patients with resectable pancreatic cancer has been achieved (4). The 5-year relative survival rate for pancreatic cancer has increased from 3% for diagnoses during the mid-1970s to 13% during 2013–2019 (5). Factors associated with pancreatic cancer survivor survival time in previous studies include age (6), carbohydrate antigen 199 levels, neutrophil-lymphocyte ratio (NLR), tumor diameter, lymph node metastasis, distant organ metastasis, resection margin status, vascular resection, adjuvant chemotherapy, and differentiation (7–10). However, the results of different studies vary considerably as to whether nutritional indicators have an impact on the survival of patients after pancreatic cancer surgery.
Nutritional problems, which are common in patients with advanced pancreatic cancer, not only affect disease progression but also increase mortality. Nutritional Risk Screening-2002 (NRS-2002) is an easily applied and reproducible tool to predict the nutritional risk for patients in the hospital, which has been used widely to identify the risk for surgical complications and survival outcome (11, 12). The European Society for Clinical Nutrition and Metabolism (ESPEN) also recommends the application of NRS-2002 in patients undergoing surgery and patients with cancer (13). Heckler et al. found that nutritional risk defined by NRS-2002 was not associated with worse survival in patients with resected pancreatic ductal adenocarcinoma (14), while Park found that the NRS-2002 score was associated with overall survival in patients with advanced pancreatic cancer (15). Thus, the relationship between the NRS-2002 and the survival of pancreatic cancer patients after pancreaticoduodenectomy needs more studies.
In the present study, we aimed to examine the associations between baseline nutritional risk and survival time among adult Chinese patients with pancreatic cancer after pancreaticoduodenectomy. We hope that the identification of nutritional risk indicators will contribute to the development and implementation of preoperative nutrition improvement programs and ultimately improve the survival of patients with pancreatic cancer after pancreaticoduodenectomy.
Methods and materials
Study population
This was a retrospective study. We selected patients aged 18 years or more who received pancreaticoduodenectomy treatment for pancreatic cancer at Ruijin Hospital, affiliated with Shanghai Jiao Tong University School of Medicine, between December 2019 and June 2022 from the follow-up database. Patients without a clinical data record were excluded. The study protocol was approved by the institutional review board at the authors’ affiliated hospital [Ethical Review Approval No. 293 (2023)].
Data collection
We retrospectively collected patients’ clinical data from medical records and the follow-up database. The follow-up database contains the patient’s hospitalization number, age, gender, height, body weight, body mass index (BMI), comorbidities, postoperative complications, survival information, neoadjuvant therapy, and postoperative chemotherapy information. All the patients were followed up at regular intervals via telephone for routine clinical care every 3 months during the first year in our center after treatment with pancreaticoduodenectomy. The follow‐up ended when the patient died or contact was lost. Our most recent follow‐up was conducted in August 2023, and patient survival data were censored. Every patient in our study received at least 1 year of follow‐up after discharge from the hospital. We focused on the patients’ overall survival results, which were defined as the time from treatment to death due to any cause.
We then logged on to the inpatient electronic medical record system and used the patient’s hospitalization number to find out the patient’s marital status, time of entry and exit from the hospital, education level, ethnicity, smoking status, alcohol consumption, amount of weight lost and time of loss, any reduction in diet in the last week, Barthel’s index, carbohydrate antigen 199 levels on admission, neutrophil value on admission, lymphocyte value on admission, surgical margins, lymph node metastasis, distant organ metastasis, nerve invasion, maximum diameter of the tumor, and the degree of tumor differentiation. We determined the cut-off values for NLR, tumor diameter, operation time, and intraoperative blood loss based on the ROC curve, and divided these continuous variables into binary variables based on the cut-off values.
The assessments of nutritional risk score using the NRS-2002 are available from the electronic medical record system. The NRS-2002 assesses nutritional risk using the medical record data on weight loss, BMI, food intake, disease severity, and age. Patient scores in the NRS-2002 are calculated as the score of impaired nutrition status and disease severity. If the patient’s age is ≥70 years, one point is added to the total score. Patients with an NRS-2002 score <3 were classified as “without nutritional risk” and those with a score of 3 or more were classified as “with nutritional risk”.
If a patient is identified as having nutritional risk via NRS 2002, the general approach is to recommend oral nutritional supplements (ONSs) if they are able to tolerate oral intake. For patients diagnosed with malnutrition and unable to meet their nutritional requirements orally, a consultation with the nutrition support team is requested, potentially leading to the initiation of total parenteral nutrition (TPN) if necessary. All of the patients in our center received nutritional support during the postoperative hospitalization after their pancreaticoduodenectomy, but the access to and initiation of preoperative nutritional interventions can depend significantly on the individual clinician’s assessment and prioritization. Furthermore, a preoperative nutritional intervention is recommended to begin 7 to 14 days before surgery (16). However, in practice, the patients received a shorter duration of nutritional support preoperatively. So, in our study, if the patients were prescribed enteral nutrition preparations, intravenous glucose, amino acids, or fat emulsion before surgery, they were considered as having received a nutritional intervention.
Statistical analysis
The statistical software SPSS 20.0 was used in the statistical analysis. If the continuous variables were normally distributed, they are presented as mean ± standard deviation. Otherwise, they are presented as median (interquartile range). Categorical variables are presented as percentages. The survival rate and median overall survival time were calculated using the Kaplan–Meier method; then, survival curves were drawn. The log-rank test was used to compare the differences among the groups for the univariate analysis. Factors with statistical significance in the univariate analysis were included in the Cox regression model for the multivariate analysis. In addition, as nutritional support was not a standardized intervention and was also administered for an insufficient period, we used this nutritional support variable as a stratification variable in the Cox regression model to analyze the relationship between nutritional risk and the survival outcomes. A two‐sided p-value of less than 0.05 was considered to be statistically significant.
Results
General information
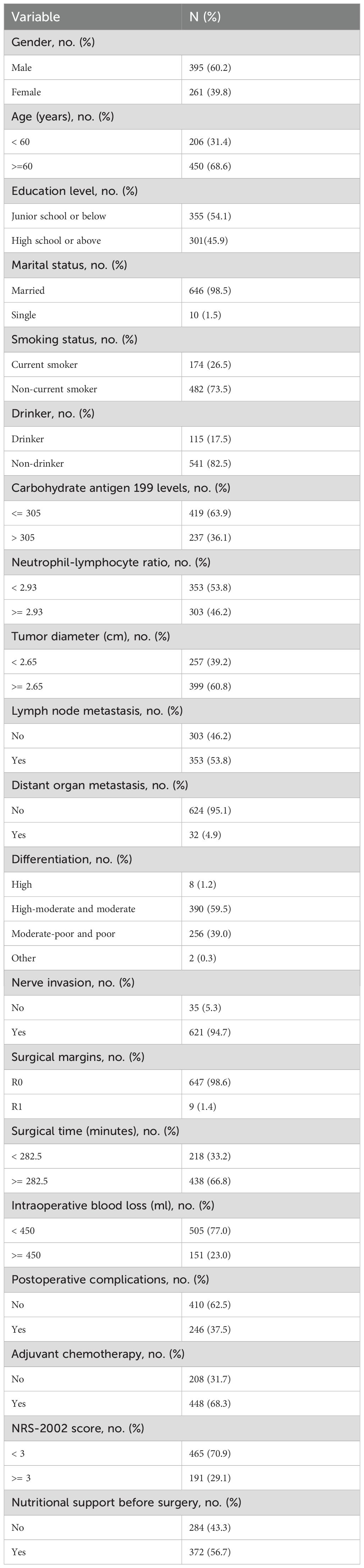
A total of 656 patients who underwent a pancreaticoduodenectomy were included in the present study, with a median age of 65 (58, 70) years old. In total, 29.1% of patients were at nutritional risk on admission. The median overall survival time was 24.0 months [95% confidence interval (CI), 21.6–26.3]. At the end of the follow-up, a total of 364 (55.5%) patients had died. The overall 1-, 2-, and 3-year survival rate of the 656 patients with pancreatic cancer after pancreaticoduodenectomy was 72.7%, 49.8%, and 34.4%, respectively (Table 1).
Analysis of prognostic factors for pancreatic cancer patient survival after pancreaticoduodenectomy
The univariate analysis showed that age, education level, carbohydrate antigen 199 levels, NLR, tumor diameter, lymph node metastasis, distant organ metastasis, tumor tissue differentiation, nerve invasion, surgical margins, postoperative complications, operative time, intraoperative blood loss, chemotherapy, and NRS-2002 score were the relevant factors affecting the prognosis of patients with pancreatic cancer after pancreaticoduodenectomy (P < 0.05) (Table 2).
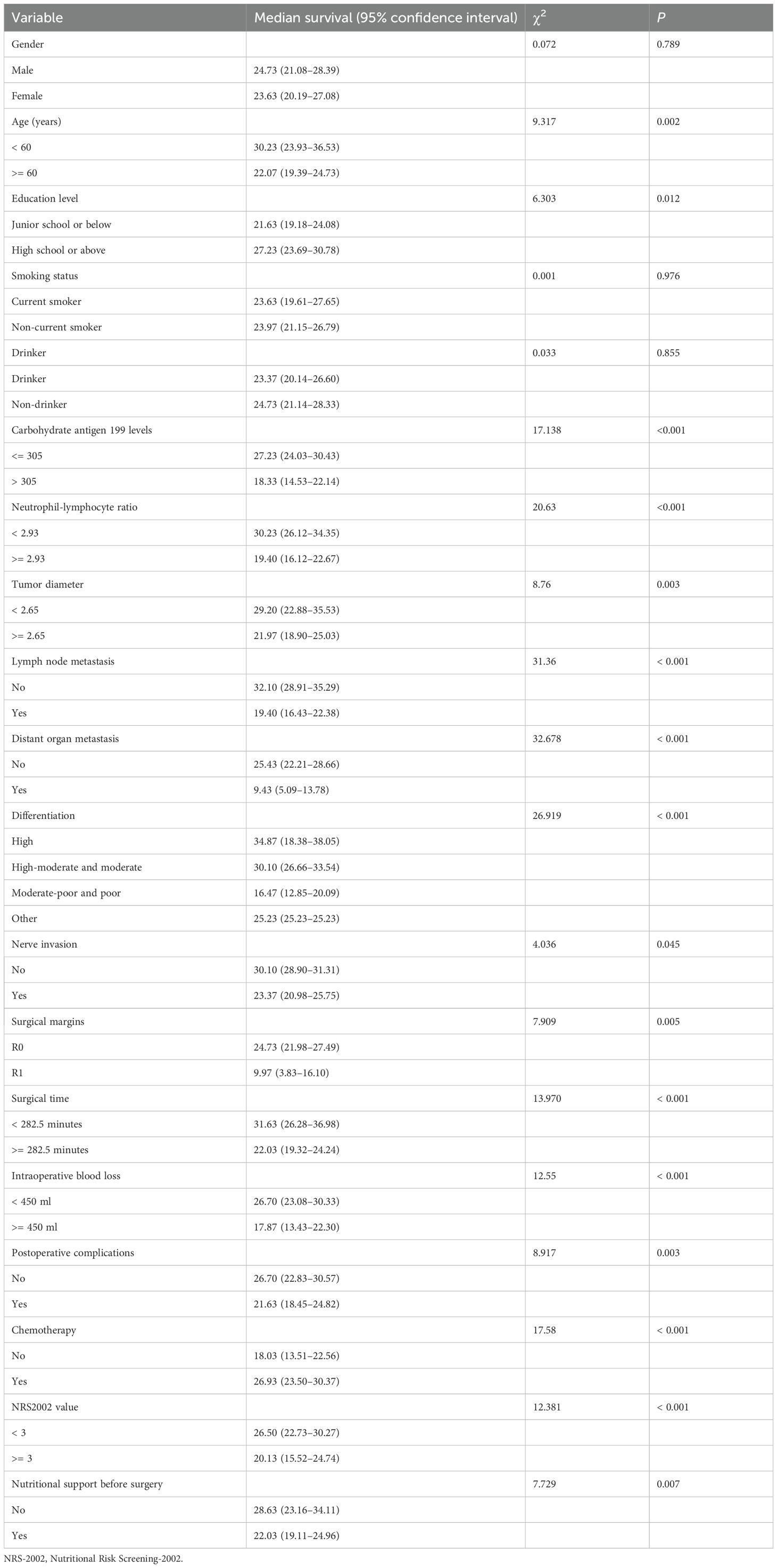
Table 2. Univariate analysis of prognostic factors for the survival of patients with pancreatic cancer.
Significant factors in the univariate analysis were included in the Cox regression model for multivariate analysis, with nutritional support variable as a stratification variable. The results showed that carbohydrate antigen 199 levels, NLR, tumor diameter, lymph node metastasis, distant organ metastasis, tumor tissue differentiation, surgical margins, operative time, postoperative complications, chemotherapy, and NRS-2002 score were independent prognostic factors in patients with pancreatic cancer after pancreaticoduodenectomy (P < 0.05) (Table 3).
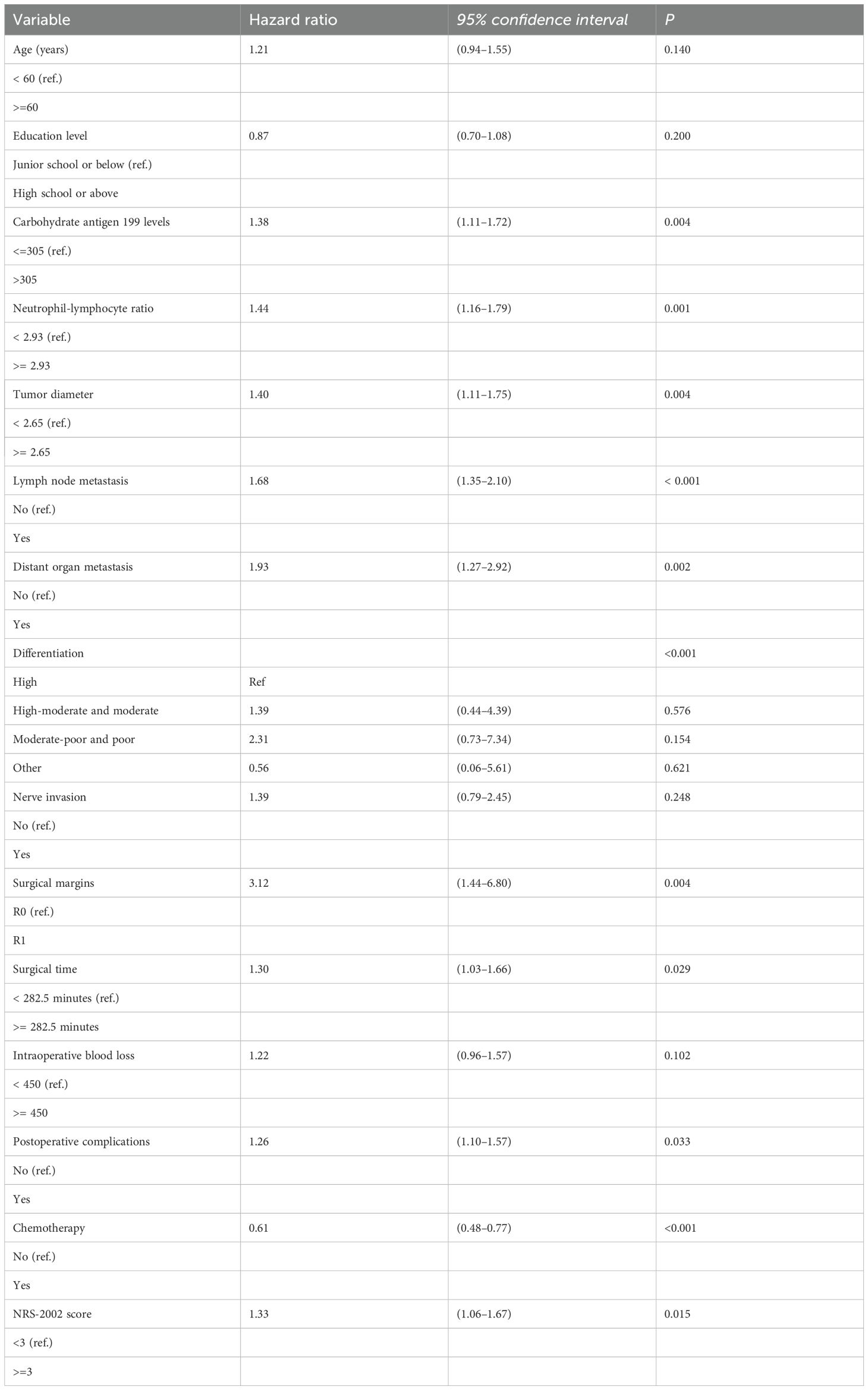
Table 3. Multivariate analysis of prognostic factors for the survival of patients with pancreatic cancer.
We also used the Bonferroni correction to address the issue of multiple hypothesis testing, thereby reducing the probability of type I errors. The significance level was adjusted to 0.0028 (0.05/18 tests) in the univariate analysis. The multivariate analysis results showed that carbohydrate antigen 199 levels, NLR, lymph node metastasis, distant organ metastasis, tumor tissue differentiation, operative time, intraoperative blood loss, chemotherapy, and NRS-2002 score were independent prognostic factors in patients with pancreatic cancer after pancreaticoduodenectomy (P < 0.05) (Supplementary Table 1).
The independent association between nutritional risk and survival
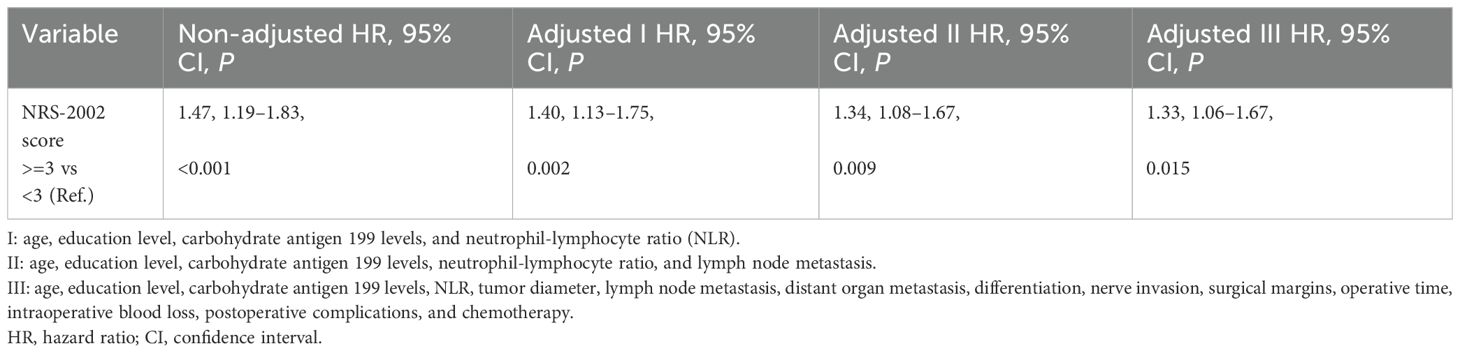
The results indicated that nutritional risk (NRS-2002 >=3) was associated with lower survival in an unadjusted model [hazard ratio (HR) = 1.47, 95% CI: 1.19–1.83]. This association was diminished after adjusting for different variables. After fully adjusting for age, education level, carbohydrate antigen 199 levels, NLR, tumor diameter, lymph node metastasis, distant organ metastasis, differentiation, nerve invasion, surgical margins, operative time, intraoperative blood loss, postoperative complications, and chemotherapy, this association remained (HR = 1.33, 95% CI: 1.06-1.67) (Figure 1). Detailed information is presented in Table 4.
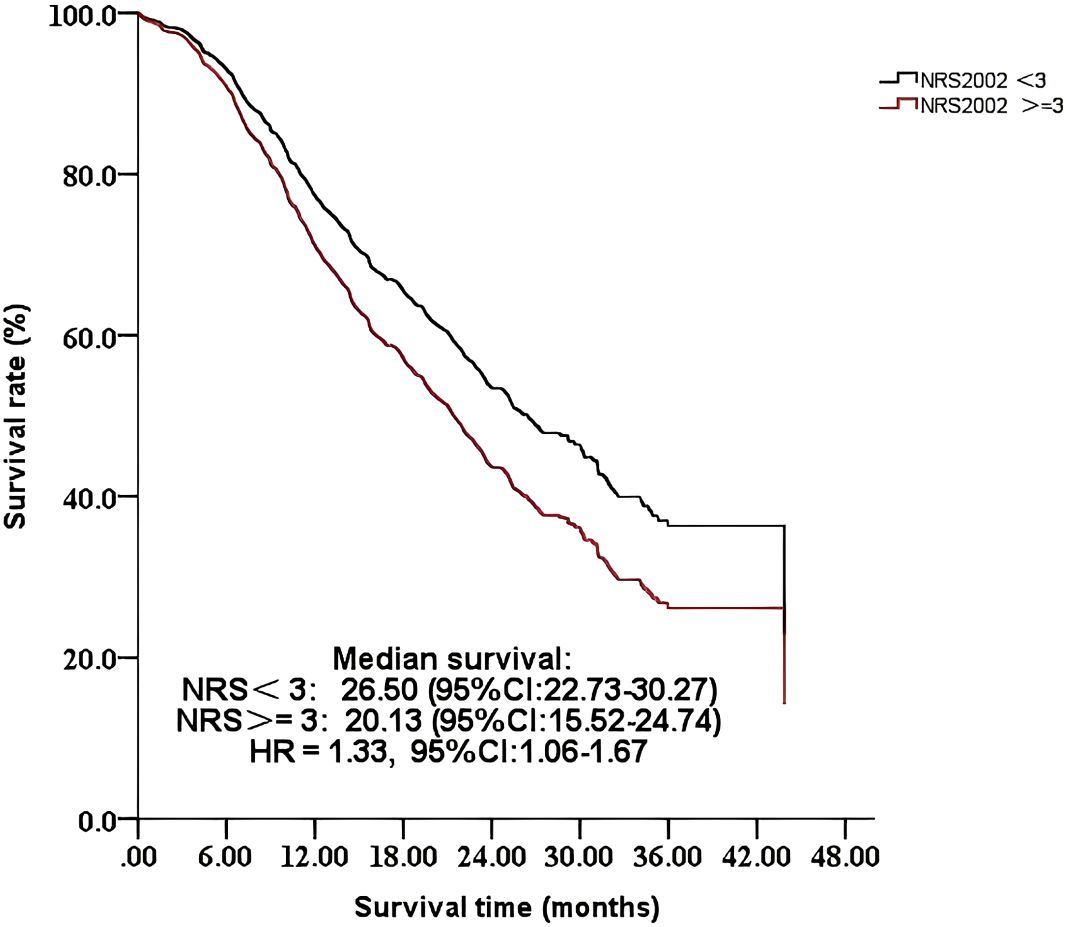
Figure 1. Comparison of the survival time of 656 patients with different NRS-2002 scores: <3 or >=3.
Discussion
With the refinement of surgical techniques and improvements in neoadjuvant and adjuvant therapies and the application of early diagnostic techniques, the survival time of patients with pancreatic cancer has been prolonged. In this study, we found that the overall 1- and 3-year survival rates of the 656 patients with pancreatic cancer after pancreaticoduodenectomy were 72.7% and 34.4%, respectively, which were higher than the rates of 46.2% and 18.6% found in a previous study conducted in China (7). However, the reported data on survival remain lower than that from the JASPAC 01 (17) and PRODIGE-24 trials (18) in Japan and Canada, respectively. Thus, we still have a long way to go in improving the survival of patients with pancreatic cancer.
Similar to a previous study (19), our results confirmed that preoperative nutritional risk has a detrimental impact on survival in patients with pancreatic cancer who undergo a pancreaticoduodenectomy, and this relationship was stable. Conversely, Heckler et al. found that nutritional risk defined by NRS-2002 was not associated with worse survival in 116 patients with resected pancreatic ductal adenocarcinoma. The difference may be a result of different populations and sample sizes. Patients with pancreatic cancer commonly experience metabolic dysfunction, systemic inflammation, and unintentional body weight loss due to tumor-induced and treatment-associated changes in physiological function (20), which makes patients with pancreatic cancer more susceptible to nutritional risk. Nutritional risk is associated with a worse prognosis in patients with cancer (21). Pan et al. found that for cancer patients with nutritional risk, the relative risk of adverse events significantly increased compared with patients without nutritional risk (22). Moreover, nutritional risk decreases tolerance of curative cancer treatments, such as surgery and chemotherapy, leading to significant reductions in therapeutic effects (23). This adversely and severely affects cancer prognoses (24). Our results indicate that early screening for nutritional risk using the NRS-2002 and a corresponding intervention program for patients with pancreatic cancer before surgery are urgently required to improve the poor survival time. Furthermore, our survival curves show that the decline was gradual in the group without nutritional risk, while it was steeper in the group with nutritional risk. The curves appear to run parallel after approximately 18 months, indicating that the nutritional risk at baseline has a stronger influence on early rather than late mortality. Prior research has also demonstrated that the long-term survival prognosis for pancreatic cancer is more susceptible to the inherent characteristics of the tumor and the nature of the treatment (25).
Consistent with some previous reports (7–9, 19), we found that age, gender, and smoking habits had no effect on the prognosis of patients with pancreatic cancer, and higher carbohydrate antigen 199 levels, lymph node metastasis, distant organ metastasis, poor differentiation, resection margin status, postoperative complications, and absence of chemotherapy predict a poor prognosis in patients with pancreatic cancer. However, in contrast to some previous studies (7, 26), other previous studies have found that NLR, a commonly used indicator of systemic inflammation, with cutoff values ranging from 2 to 3.8, is a significant prognostic indicator for overall survival in patients undergoing pancreaticoduodenectomy (27). Similar to the previous reports, we also found that patients with NLR greater than 2.93 had significantly shorter survival than patients with NLR less than 2.93. Furthermore, nerve invasion was not associated with survival in patients after pancreaticoduodenectomy in this study. This result is different from the study of Sugimoto et al., which found that extrapancreatic nerve invasion was associated with a shorter disease-specific survival and recurrence-free survival after upfront surgery in patients with anatomically resectable pancreatic cancer (28). The difference may be due to the variation of nerve invasion diagnosis, which depends on personalized experience and on the internal protocol adopted for pathological sampling, together with its examination by experienced pancreatic pathologists (29). Future studies should focus on using standardized methods to assess nerve invasion and include larger patient cohorts to explore the interaction between nerve invasion and other prognostic factors.
Several limitations should be mentioned in our study. First, due to the limited cases with neoadjuvant chemotherapy, we analyzed the effect of chemotherapy on survival in pancreatic patients combined with adjuvant and neoadjuvant chemotherapy. We did not consider disease-free survival or cancer-specific survival in our analysis of the results, which are crucial indicators for evaluating postoperative prognosis in cancer patients. Additionally, lymphovascular invasion and vascular resection have been reported in previous studies to have a possible effect on survival after pancreatic cancer surgery, but we could not accurately obtain data on these two items in the medical record system, so they were not included as covariates in the analyses. Moreover, this is a single-center retrospective analysis, so larger multi-center studies are needed to confirm the results of this study.
Conclusion
In conclusion, the prognosis of patients with pancreatic cancer who undergo pancreaticoduodenectomy is affected by many factors. Higher carbohydrate antigen 199 levels, lymph node metastasis, distant organ metastasis, poor differentiation, NLR, resection margin status, operation time, postoperative complications, and preoperative nutritional risk predict a poor prognosis in patients with pancreatic cancer after surgery. Our findings confirmed that this relationship between nutritional risk and poor survival is stable. Thus, in addition to early detection, timely surgery, and aggressive postoperative treatment, nursing staff should screen early for nutritional risk using the NRS-2002 in patients with pancreatic cancer at diagnosis and, in conjunction with their doctors, develop and implement a timely nutritional treatment plan for those at risk to improve the poor survival time.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ruijin Hospital Ethics Committee Shanghai Jiao Tong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective study.
Author contributions
QT: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Writing – original draft. JS: Formal analysis, Methodology, Writing – original draft. LC: Data curation, Investigation, Methodology, Writing – review & editing. MZ: Data curation, Investigation, Project administration, Writing – review & editing. BW: Conceptualization, Supervision, Writing – review & editing. BS: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Shanghai Hospital Development Centre’s “Three-Year Action Plan to Promote Clinical Skills and Clinical Innovation in Municipal Hospitals Training Programme” (SHDC2023CRS017) and the Nursing Discipline Construction Project of Shanghai Jiaotong University School of Medicine (SJTUHLXK2021). Author Tian Qiuju received research support. The funders played no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Acknowledgments
The authors thank all the patients and the hospital staff.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1539215/full#supplementary-material
References
1. Ren S, Song L, Tian Y, Zhu L, Guo K, Zhang H, et al. Emodin-conjugated PEGylation of Fe(3)O(4) nanoparticles for FI/MRI dual-modal imaging and therapy in pancreatic cancer. Int J Nanomed. (2021) 16:7463–78. doi: 10.2147/ijn.S335588
2. Cao YY, Guo K, Zhao R, Li Y, Lv XJ, Lu ZP, et al. Untargeted metabolomics characterization of the resectable pancreatic ductal adenocarcinoma. Digital Health. (2023) 9:20552076231179007. doi: 10.1177/20552076231179007
3. Ren S, Qian LC, Cao YY, Daniels MJ, Song LN, Tian Y, et al. Computed tomography-based radiomics diagnostic approach for differential diagnosis between early- and late-stage pancreatic ductal adenocarcinoma. World J Gastrointest Oncol. (2024) 16:1256–67. doi: 10.4251/wjgo.v16.i4.1256
4. Torphy RJ, Fujiwara Y, and Schulick RD. Pancreatic cancer treatment: better, but a long way to go. Surg Today. (2020) 50:1117–25. doi: 10.1007/s00595-020-02028-0
5. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA: Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
6. Aziret M, Aşıkuzunoğlu F, Altıntoprak F, Tozlu M, Demirci A, Ercan M, et al. Early and long-term morbidity and mortality following pancreaticoduodenectomy for periampullary tumors in elderly patients. Annali italiani di chirurgia. (2024) 95:235–45. doi: 10.62713/aic.3380
7. Li Q, Feng Z, Miao R, Liu X, Liu C, and Liu Z. Prognosis and survival analysis of patients with pancreatic cancer: retrospective experience of a single institution. World J Surg Oncol. (2022) 20:11. doi: 10.1186/s12957-021-02478-x
8. Serrano PE, Kim D, Kim PT, Greig PD, Moulton CA, Gallinger S, et al. Effect of pancreatic fistula on recurrence and long-term prognosis of periampullary adenocarcinomas after pancreaticoduodenectomy. Am Surgeon. (2016) 82:1187–95. doi: 10.1177/000313481608201225
9. Fang LP, Xu XY, Ji Y, and Huang PW. Factors influencing survival of patients with pancreatic adenocarcinoma after surgical resection. Zhonghua yi xue za zhi. (2018) 98:606–11. doi: 10.3760/cma.j.issn.0376-2491.2018.08.011
10. Guarneri G, Pecorelli N, Bettinelli A, Campisi A, Palumbo D, Genova L, et al. Prognostic value of preoperative CT scan derived body composition measures in resected pancreatic cancer. Eur J Surg Oncol: J Eur Soc Surg Oncol Br Assoc Surg Oncol. (2024) 50:106848. doi: 10.1016/j.ejso.2023.02.005
11. Limbu Y, Raut S, Pudasaini P, Regmee S, Ghimire R, Maharjan DK, et al. Correlation of the nutritional risk screening 2002 score with post-operative complications in gastrointestinal and hepatopancreatobiliary oncosurgeries. Cureus. (2024) 16:e58514. doi: 10.7759/cureus.58514
12. Sun Z, Kong XJ, Jing X, Deng RJ, and Tian ZB. Nutritional risk screening 2002 as a predictor of postoperative outcomes in patients undergoing abdominal surgery: A systematic review and meta-analysis of prospective cohort studies. PloS One. (2015) 10:e0132857. doi: 10.1371/journal.pone.0132857
13. Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin Nutr (Edinburgh Scotland). (2021) 40:2898–913. doi: 10.1016/j.clnu.2021.02.005
14. Heckler M, Klaiber U, Hüttner FJ, Haller S, Hank T, Nienhüser H, et al. Prospective trial to evaluate the prognostic value of different nutritional assessment scores for survival in pancreatic ductal adenocarcinoma (NURIMAS Pancreas SURVIVAL). J Cachexia Sarcopenia Muscle. (2021) 12:1940–7. doi: 10.1002/jcsm.12796
15. Park JS, Kim HM, Jeung HC, and Kang SA. Association between early nutritional risk and overall survival in patients with advanced pancreatic cancer: A single-center retrospective study. Clin Nutr ESPEN. (2019) 30:94–9. doi: 10.1016/j.clnesp.2019.01.012
16. Nutrition CSfPaE and Association ERASCoCME. Chinese expert consensus on perioperative nutritional support in enhanced recovery after surgery (2019 edition). Chin J Dig Surg. (2019) 18:897–902. doi: 10.3760/cma.j.issn.1673-9752.2019.10.001
17. Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet (London England). (2016) 388:248–57. doi: 10.1016/s0140-6736(16)30583-9
18. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. New Engl J Med. (2018) 379:2395–406. doi: 10.1056/NEJMoa1809775
19. Trestini I, Paiella S, Sandini M, Sperduti I, Elio G, Pollini T, et al. Prognostic impact of preoperative nutritional risk in patients who undergo surgery for pancreatic adenocarcinoma. Ann Surg Oncol. (2020) 27:5325–34. doi: 10.1245/s10434-020-08515-5
20. Mitsunaga S, Kasamatsu E, and Machii K. Incidence and frequency of cancer cachexia during chemotherapy for advanced pancreatic ductal adenocarcinoma. Supportive Care Cancer: Off J Multinational Assoc Supportive Care Cancer. (2020) 28:5271–9. doi: 10.1007/s00520-020-05346-8
21. Zang Y, Xu W, Qiu Y, Gong D, and Fan Y. Association between risk of malnutrition defined by the nutritional risk screening 2002 and postoperative complications and overall survival in patients with cancer: A meta-analysis. Nutr Cancer. (2023) 75:1600–9. doi: 10.1080/01635581.2023.2227402
22. Pan H, Cai S, Ji J, Jiang Z, Liang H, Lin F, et al. The impact of nutritional status, nutritional risk, and nutritional treatment on clinical outcome of 2248 hospitalized cancer patients: a multi-center, prospective cohort study in Chinese teaching hospitals. Nutr Cancer. (2013) 65:62–70. doi: 10.1080/01635581.2013.741752
23. Reber E, Schönenberger KA, Vasiloglou MF, and Stanga Z. Nutritional risk screening in cancer patients: the first step toward better clinical outcome. Front Nutr. (2021) 8:603936. doi: 10.3389/fnut.2021.603936
24. Watanabe H and Oshima T. The latest treatments for cancer cachexia: an overview. Anticancer Res. (2023) 43:511–21. doi: 10.21873/anticanres.16188
25. Yamamoto T, Yagi S, Kinoshita H, Sakamoto Y, Okada K, Uryuhara K, et al. Long-term survival after resection of pancreatic cancer: a single-center retrospective analysis. World J Gastroenterol. (2015) 21:262–8. doi: 10.3748/wjg.v21.i1.262
26. Sierzega M, Lenart M, Rutkowska M, Surman M, Mytar B, Matyja A, et al. Preoperative neutrophil-lymphocyte and lymphocyte-monocyte ratios reflect immune cell population rearrangement in resectable pancreatic cancer. Ann Surg Oncol. (2017) 24:808–15. doi: 10.1245/s10434-016-5634-0
27. Wang D, Wang Y, Dong X, Yu M, and Cai H. The significance of preoperative neutrophil-to-lymphocyte ratio in predicting short-term complications and survival benefits of pancreaticoduodenectomy: A systematic review and meta-analysis. Am J Surg. (2024) 229:76–82. doi: 10.1016/j.amjsurg.2023.11.030
28. Sugimoto M, Kobayashi T, Kobayashi S, Takahashi S, Konishi M, Mitsunaga S, et al. Extrapancreatic nerve plexus invasion on imaging predicts poor survival after upfront surgery for anatomically resectable pancreatic cancer. Pancreas. (2020) 49:675–82. doi: 10.1097/mpa.0000000000001547
Keywords: nutritional risk, screening, pancreatic cancer, pancreaticoduodenectomy, survival
Citation: Tian Q, Su J, Chen L, Zhang M, Wu B and Shen B (2025) The association between nutritional risk and survival time among patients with pancreatic cancer following pancreaticoduodenectomy: a retrospective cohort study. Front. Oncol. 15:1539215. doi: 10.3389/fonc.2025.1539215
Received: 04 December 2024; Accepted: 30 May 2025;
Published: 01 July 2025.
Edited by:
Mats Lukas Wiese, Münster University of Applied Sciences, GermanyReviewed by:
Rodrigo Erick Escartín-Pérez, National Autonomous University of Mexico, MexicoShuai Ren, Affiliated Hospital of Nanjing University of Chinese Medicine, China
Giulliana Moralez, Hospital Copa Star, Brazil
Copyright © 2025 Tian, Su, Chen, Zhang, Wu and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beiwen Wu, Z2FvYW4yMDA1bmV3QDE2My5jb20=; Baiyong Shen, c2hlbmJ5QHNoc211LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Qiuju Tian
Qiuju Tian Jing Su2†
Jing Su2† Baiyong Shen
Baiyong Shen