- Department of Gastroenterology and Hepatology, General Hospital, Tianjin Medical University, National Key Clinical Specialty, Tianjin Institute of Digestive Diseases, Tianjin Key Laboratory of Digestive Diseases, Tianjin, China
Mucosa-associated lymphoid tissue (MALT) lymphoma of the small intestine is relatively rare, and the treatment guideline has not been established yet. Here we present a case of MALT lymphoma in the terminal ileum, which regressed after Helicobacter pylori (H. pylori) eradication. A 53-year-old man had complained of abdominal discomfort and underwent a gastrointestinal endoscopic examination. H. pylori-associated erosive gastritis was diagnosed, and superficial ulcerated lesions were also found in the terminal ileum. Histopathologic examination and immunohistochemical analyses of ileal biopsy specimens confirmed the diagnosis of MALT lymphoma. No distant lymph node metastasis or other organ involvement was detected in positron emission tomography/computed tomography. Surprisingly, the ileum reached mucosal healing after quadruple therapy regimens for H. pylori eradication without additional treatments. There were no signs of recurrence during the follow-up for 18 months. The unique case which located only in the ileum revealed that eradication of H. pylori might be an effective treatment and deserves further studies. Moreover, we also provide a detailed overview of recently published literature regarding the eradication treatment for intestinal MALT lymphoma.
Introduction
Mucosa-associated lymphoid tissue (MALT) lymphoma was first introduced by Isaacson and Wright in 1983 (1), which usually appeared in organs lacking lymphoid tissues, and was thought to be caused by chronic antigen stimulation induced by persistent infection and/or autoimmune processes (2). The MALT lymphoma was classified as extranodal marginal zone B cell lymphoma by the World Health Organization and is a subtype of non-Hodgkin lymphomas, accounting for approximately 5%–8% of all B-cell lymphomas (3, 4). Most MALT lymphomas occur in the gastrointestinal (GI) tract, most commonly in the stomach (50%-60%), followed by the small intestine (30%), and large intestine (10%), and also in extra-gastrointestinal organs such as the salivary gland, thyroid gland, and skin (5, 6). Gastric MALT lymphoma is related with Helicobacter pylori (H. pylori) infection, and H. pylori eradication can successfully regress low-grade gastric MALT lymphoma (7–9). However, until now, the etiology and standard treatment strategy of small intestinal MALT lymphoma have not been established due to its rarity, and most cases were treated either with chemotherapy and/or surgically (10–13). In the present report, we describe a patient with MALT lymphoma in the terminal ileum that presented with erosive lesions. The lesions were fully regressed after quadruple therapy for H. pylori eradication. A detailed literature review for different treatments regarding ileum MALT lymphoma was also summarized.
Case presentation
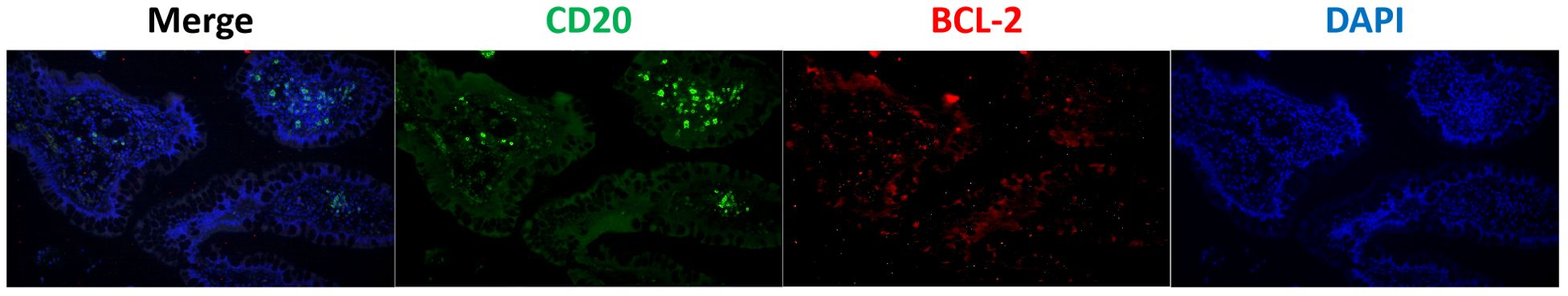
A 53-year-old man was admitted with complaints of abdominal discomfort without other symptoms, such as nausea, vomiting, diarrhea, constipation, and fever. He denied taking non-steroidal anti-inflammatory drugs. Upon gastroscopy, superficial erosive lesions in the antrum were found, and a histological evaluation of biopsy showed low-grade inflammation. The carbon-13 urea breath test for H. pylori infection was positive. Moreover, colonoscopy showed multiple superficial ulcerated lesions in the terminal ileum (Figure 1A). Hematoxylin–eosin staining revealed lymphoepithelial lesions with diffuse infiltration of atypical small lymphocytes in the lamina propria and muscularis mucosae (Figures 2A, D). Immunohistochemical staining showed diffuse positive for CD20 and Bcl-2 (Figure 2O) and negative for CD5, CD10, CD23, cyclin D1, and BCL-6 (Figures 2B–L). Ki-67 showed hot spot at about 10% (+) (Figures 2M, N). In addition, monoclonality was demonstrated by light chain restriction experiments (Figure 3). To rule out the possibility of a reactive lymphoid proliferation with co-expression of CD20 and BCL-2, we evaluated the co-expression of CD20 and BCL-2 by FISH strain (Figure 4). In addition, capsule endoscopy showed no lesions in other parts of the small intestine. The histopathological and phenotypic features were consistent with MALT lymphoma. Positron emission tomography/computed tomography showed no evidence of metastasis or any other abnormal lesions. According to the Lugano classification (12), the stage of this case was stage I. The patient received 14 days of H. pylori eradication therapy (esomeprazole, 20 mg bid; bismuth potassium citrate, 200 mg bid; amoxicillin, 1,000 mg bid; and clarithromycin, 500 mg bid) without any additional treatment. The carbon-13 urea breath test was repeated 4 weeks after treatment and confirmed successful eradication. At 3 months later, a repeated endoscopy and histological examination showed the complete disappearance of both gastric lesions and ileum MALT lymphoma (Figure 1B). The patient was recurrence-free at follow-up after 18 months.
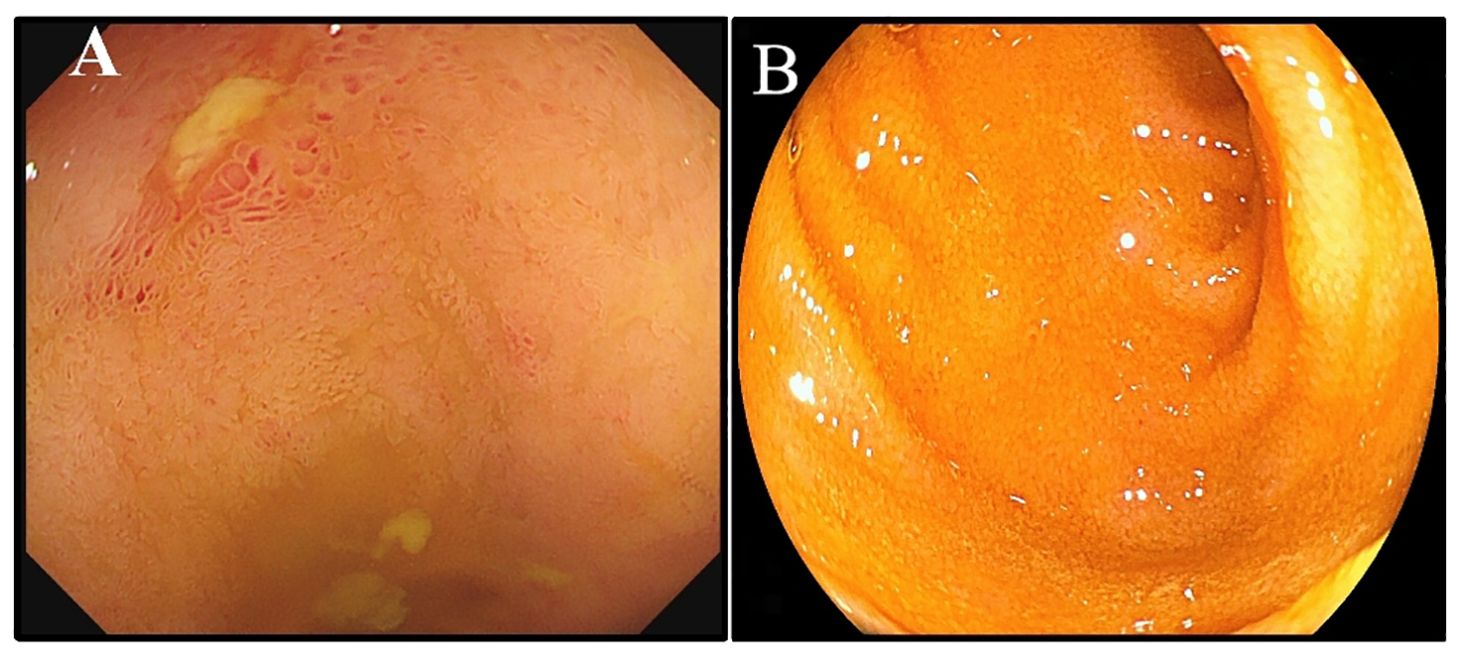
Figure 1. Endoscopic changes before and after treatment. (A) Colonoscopy performed due to multiple superficial ulcerated lesions in the terminal ileum. (B) The endoscopy result showed complete disappearance of the ileum MALT lymphoma.
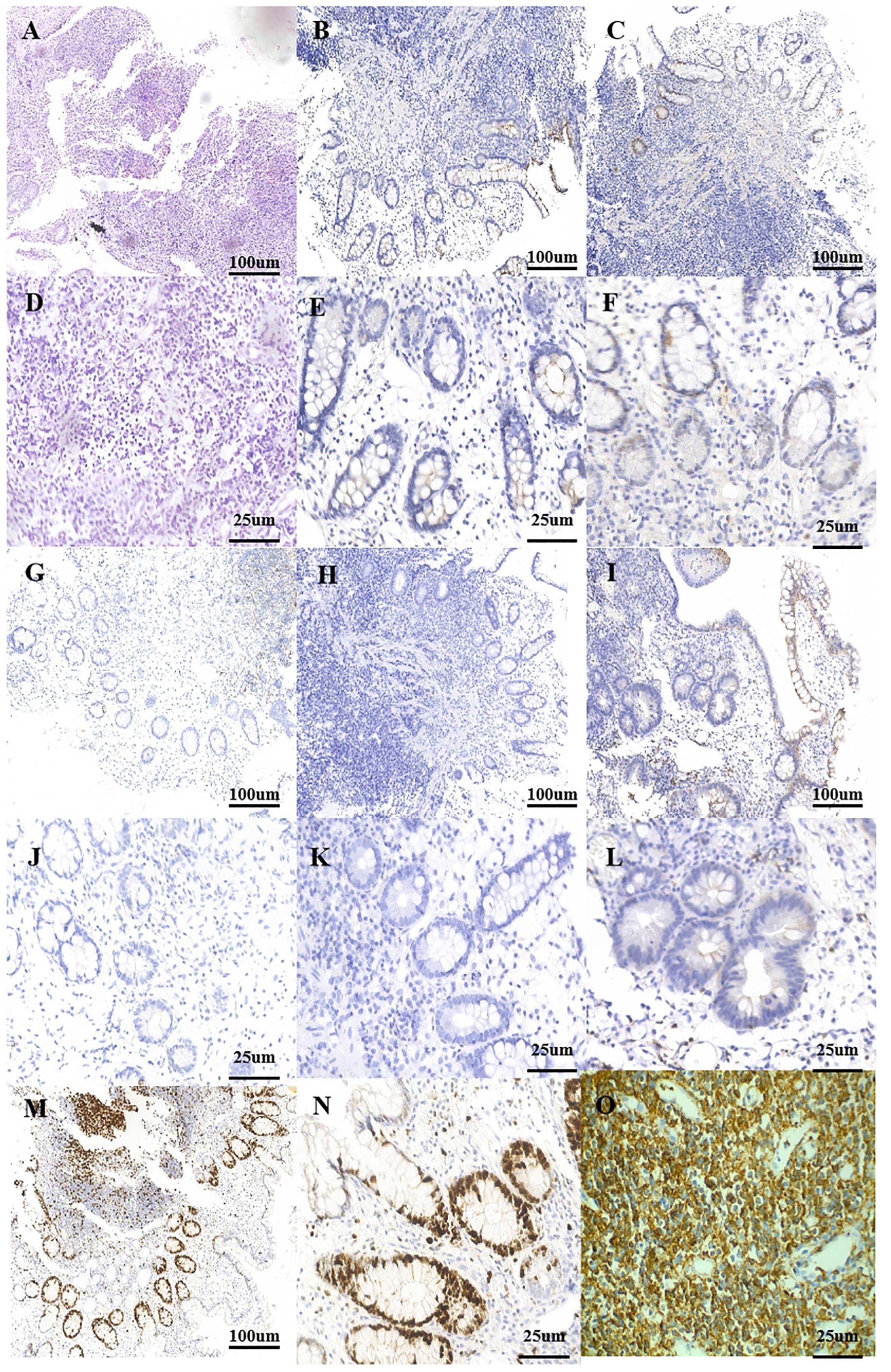
Figure 2. Postoperative pathology. (A) Small bowel with a dense and diffuse lymphocytic infiltrate (HE, ×10). (B) Typical cells are negative for the marker CD10 (IHE CD10, ×10). (C) Typical cells are negative for the marker cyclin D1 (IHE cyclin D1, ×10). (D) Small bowel with a dense and diffuse lymphocytic infiltrate (HE, ×40). (E) Typical cells are negative for the marker CD10 (IHE CD10, ×40). (F) Typical cells are negative for the marker cyclin D1 (IHE cyclin D1, ×40). (G) Typical cells are negative for the marker CD23 (IHE CD23, ×10). (H) Typical cells are negative for the marker BCL-6 (IHE BCL-6, ×10). (I) Typical cells are negative for the marker CD5 (IHE CD5, ×10). (J) Typical cells are negative for the marker CD23 (IHE CD23, ×40). (K) Typical cells are negative for the marker BCL-6 (IHE BCL-6, ×40). (L) Typical cells are negative for the marker CD5 (IHE CD5, ×40). (M) Ki-67 hot spot at about 10% (+) (IHE Ki-67, ×10). (N) Ki-67 hot spot at about 10% (+) (IHE Ki-67, ×40). (O) Typical cells are diffusely positive for the marker CD20 (IHE CD20, ×40). IHC, immunohistochemistry.
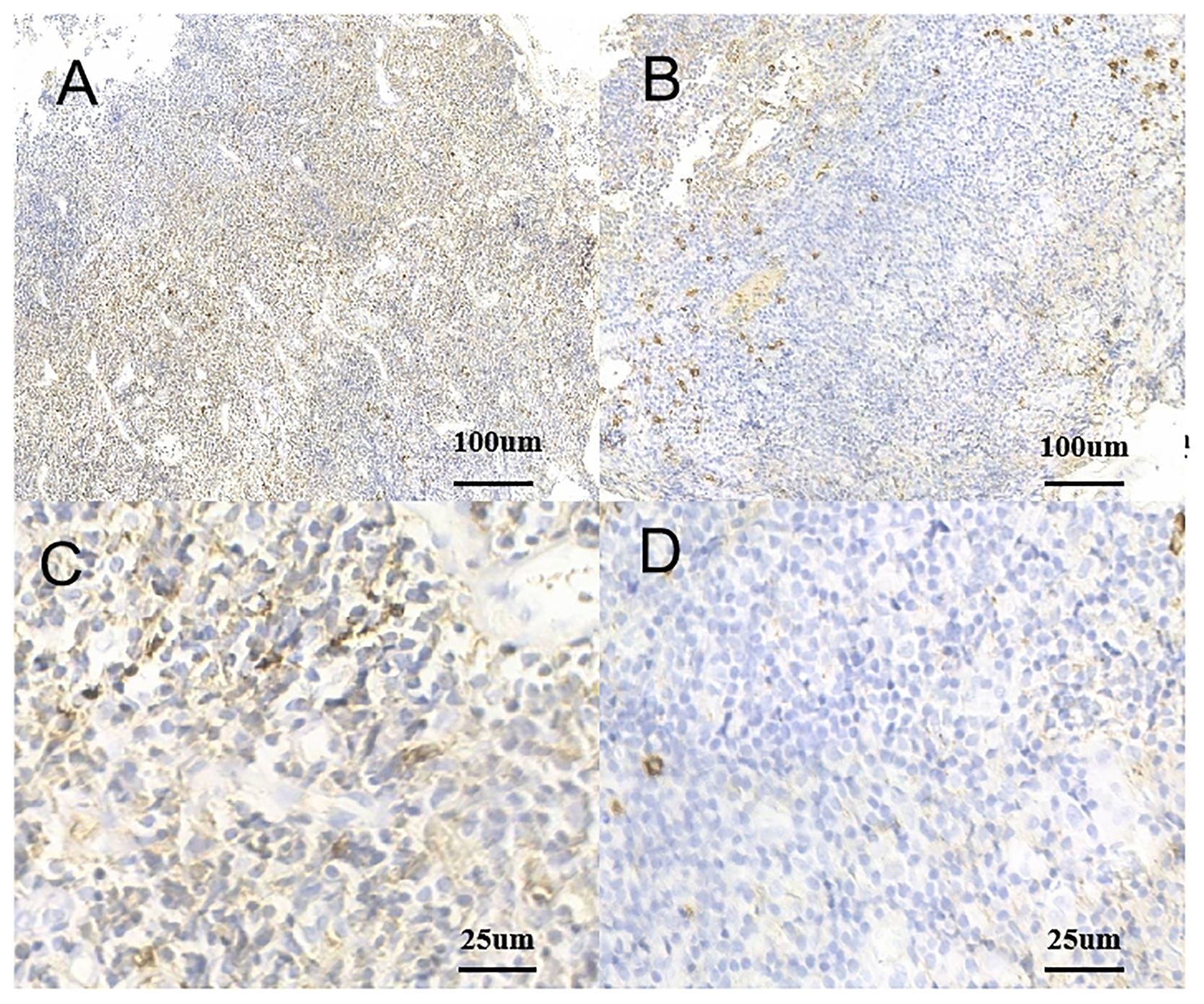
Figure 3. Monoclonality of tumor cells. (A) Positive rate of kappa light chain (IHC kappa, ×10). (B) Positive rate of lambda light chain (IHC lambda, ×40). (C) Positive rate of kappa light chain (IHC kappa, ×10). (D) Positive rate of lambda light chain (IHC lambda, ×40). IHC, immunohistochemistry.
Discussion
Although MALT lymphoma may occur in multiple parts of the gastrointestinal tract, the stomach is the predominant localization. H. pylori infection is confirmed to be closely associated with gastric MALT lymphoma, and eradication of H. pylori is widely recognized as the first-line treatment for low-grade gastric MALT lymphoma (8, 14, 15). Previously, the findings of Gong, Eun Jeong et al. have also demonstrated the efficacy of H. pylori eradication as initial therapy for gastric MALT lymphoma (16). In contrast, for MALT lymphoma of the small intestine, the controversy continues on the current therapy regarding this entity. The cases with different treatments for ileum MALT lymphoma are summarized in Supplementary Table 1 (11, 13, 17–28). In this case, the manifestation of mucosal healing of the terminal ileum was achieved after the successful eradication of H. pylori, and it could be considered a priority option.
MALT lymphomas arise from post-germinal center marginal zone B cells, which are positive for superficial Ig and pan B antigens CD19, CD20, CD79, and Bcl-2 and negative for CD5, CD10, CD23, cyclin D1, and Bcl-6 (29). In the present case, the immunohistochemical results were positive for CD20 and Bcl-2 and negative for CD10 and cyclin D1. Bacterial infection has been postulated to play a critical role in the pathogenesis of MALT lymphomas, such as H. pylori, Borrelia afzelii, and Campylobacter jejuni (30–33). Moreover, Du MQ et al. (34) reported that the intestinal lesions could be secondary to H. pylori-induced gastric MALT lymphoma. This suggested that antigen stimulation may play a critical role in the clonal expansion of low-grade MALT lymphomas. The present case was positive for H. pylori infection; however, MALT lymphoma was found only in the terminal ileum, but not in the stomach. The regression of the lesion was achieved after the successful eradication of H. pylori, and regular follow-up has, so far, not detected any evidence of recurrence. Thus, we speculated that the MALT lymphoma in our case was secondary to some infection, most likely H. pylori. Although controversy exists with regard to the antibiotic treatment for extra-gastric MALT lymphoma, some reports have described the effective remission of intestinal MALT lymphoma by eradication therapy in H. pylori-positive patients, even in negative cases (2, 23, 35–47) (Supplementary Table 2). These suggested that H. pylori or unknown antibiotic-sensitive microorganisms may play a critical role in the development of intestinal MALT lymphoma.
Notably, while our case and some literature highlight successful mucosal healing post-eradication, other reports emphasize that ileal MALT lymphoma may not always respond to H. pylori-targeted therapy, underscoring the need for individualized management—for instance, Ohashi et al. (48) described a rare case of terminal ileum MALT lymphoma without Helicobacter pylori infection in a patient; thus, antibiotic treatment targeting H. pylori might be ineffective in this scenario. Similarly, Hirata et al. (12) reported a case of small intestine MALT lymphoma presenting as multiple lymphoma polyps, where the lymphoma persisted despite the successful eradication of H. pylori. These examples illustrate that variations in treatment outcomes for anti-Helicobacter therapy can arise due to differences in disease location, lymphoma type, or whether H. pylori is the primary driving factor. These contrasting outcomes highlight the importance of comprehensive diagnostic workups to identify patients unlikely to benefit from antibiotic therapy alone. Further studies are needed to clarify the predictors of treatment response in ileal MALT lymphoma.
C. jejuni is increasingly recognized as a pathogen associated with immunoproliferative disorders, particularly mucosa-associated lymphoid tissue (MALT) lymphoma of the small intestine. Chronic infection with C. jejuni can induce persistent antigenic stimulation and chronic inflammation, fostering a microenvironment conducive to lymphoid hyperplasia and eventual malignant transformation. Studies such as those by Lecuit et al. (49) and Isaacson et al. (50) have identified C. jejuni in small intestinal MALT lymphoma tissues, with molecular evidence linking bacterial presence to clonal B-cell proliferation. Antibiotic eradication of C. jejuni has shown promising therapeutic potential in early-stage disease, akin to H. pylori eradication in gastric MALT lymphoma—for instance, regimens combining macrolides (e.g., clarithromycin) or fluoroquinolones (e.g., ciprofloxacin) with proton pump inhibitors have led to complete histological remission in some patients. This underscores the importance of identifying and targeting bacterial triggers, as antibiotic therapy may obviate the need for aggressive interventions in susceptible cases (12). However, treatment efficacy depends on early diagnosis, highlighting the clinical relevance of routine C. jejuni screening in suspected small intestinal lymphoproliferative disorders. Regrettably, our study was unable to test for C. jejuni due to technical constraints. Specifically, the detection of C. jejuni requires specialized methodologies, such as microaerobic bacterial culture, species-specific PCR, or fluorescence in situ hybridization (FISH), which were unavailable in our laboratory during the study period. Additionally, archival tissue samples lacked sufficiently fresh material for optimal molecular analysis, further limiting our capacity to assess bacterial presence. We acknowledge this as a critical limitation, as undetected C. jejuni infection could influence disease progression or treatment responsiveness in subsets of patients. Future studies incorporating multiplex PCR, metagenomic sequencing, or serological assays would help address this gap.
Conclusion
We reported a rare case of MALT lymphoma in the terminal ileum, which completely resolved after H. pylori eradication. The eradication of treatable infectious organisms seems to be the first-step treatment option in patients with ileum MALT lymphoma, especially for H. pylori-positive patients. Large-scale clinical trials in multiple centers are required to investigate the etiology of ileum MALT lymphomas and establish a standard treatment strategy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YH: Writing – original draft. JJ: Writing – original draft. KJ: Writing – review & editing. BW: Writing – review & editing. TL: Writing – review & editing. HC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study is supported by a grant (82100574) from National Natural Science Foundation of China and a grant (21JCQNJC01240) from Diversified Fund Project of the Natural Science Foundation of Tianjin, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1544858/full#supplementary-material
References
1. Isaacson P and Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. (1983) 52:1410–6. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3
2. Raderer M, Pfeffel F, Pohl G, Mannhalter C, Valencak J, and Chott A. Regression of colonic low grade B cell lymphoma of the mucosa associated lymphoid tissue type after eradication of helicobacter pylori. Gut. (2000) 46:133–5. doi: 10.1136/gut.46.1.133
3. Cascione L, Rinaldi A, Bruscaggin A, Tarantelli C, Arribas AJ, Kwee I, et al. Novel insights into the genetics and epigenetics of malt lymphoma unveiled by next generation sequencing analyses. Haematologica. (2019) 104:e558–e61. doi: 10.3324/haematol.2018.214957
4. Thieblemont C and Zucca E. Clinical aspects and therapy of gastrointestinal malt lymphoma. Best Pract Res Clin Haematol. (2017) 30:109–17. doi: 10.1016/j.beha.2017.01.002
5. Sugizaki K, Tari A, Kitadai Y, Oda I, Nakamura S, Yoshino T, et al. Anti-helicobacter pylori therapy in localized gastric mucosa-associated lymphoid tissue lymphoma: A prospective, nationwide, multicenter study in Japan. Helicobacter. (2018) 23:e12474. doi: 10.1111/hel.12474
6. Zucca E, Roggero E, Bertoni F, and Cavalli F. Primary extranodal non-hodgkin’s lymphomas. Part 1: gastrointestinal, cutaneous and genitourinary lymphomas. Ann Oncol. (1997) 8:727–37. doi: 10.1023/a:1008282818705
7. Mentis AA, Boziki M, Grigoriadis N, and Papavassiliou AG. Helicobacter pylori infection and gastric cancer biology: tempering a double-edged sword. Cell Mol Life Sci. (2019) 76:2477–86. doi: 10.1007/s00018-019-03044-1
8. Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of helicobacter pylori. Lancet. (1993) 342:575–7. doi: 10.1016/0140-6736(93)91409-f
9. Zucca E and Bertoni F. The spectrum of malt lymphoma at different sites: biological and therapeutic relevance. Blood. (2016) 127:2082–92. doi: 10.1182/blood-2015-12-624304
10. Al-Shemmari SH, Sajnani KP, Ameen RM, and Ragheb AM. Primary gastrointestinal non-hodgkin’s lymphoma: treatment outcome. Clin Lymphoma. (2003) 4:99–103. doi: 10.3816/clm.2003.n.018
11. Dhull AK, Kaushal V, Singh S, Pal M, and Lathwal A. A journey into insidious world of malt lymphoma of the ileum: from the beginning to the end. J Gastrointest Oncol. (2014) 5:E125–7. doi: 10.3978/j.issn.2078-6891.2014.063
12. Hirata N, Tominaga K, Ohta K, Kadouchi K, Okazaki H, Tanigawa T, et al. A case of mucosa-associated lymphoid tissue lymphoma forming multiple lymphomatous polyposis in the small intestine. World J Gastroenterol. (2007) 13:1453–7. doi: 10.3748/wjg.v13.i9.1453
13. Terada T. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Malt lymphoma) of the ileum in a 35-year-old Japanese woman. Int J Clin Exp Pathol. (2013) 6:951–6.
14. Fischbach W, Goebeler-Kolve ME, Dragosics B, Greiner A, and Stolte M. Long term outcome of patients with gastric marginal zone B cell lymphoma of mucosa associated lymphoid tissue (Malt) following exclusive helicobacter pylori eradication therapy: experience from a large prospective series. Gut. (2004) 53:34–7. doi: 10.1136/gut.53.1.34
15. Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, and Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. (1991) 338:1175–6. doi: 10.1016/0140-6736(91)92035-z
16. Gong EJ, Ahn JY, Jung HY, Park H, Ko YB, Na HK, et al. Helicobacter pylori Eradication Therapy Is Effective as the Initial Treatment for Patients with H. pylori-Negative and Disseminated Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. Gut Liver. (2016) 10:706–13. doi: 10.5009/gnl15510
17. Adams KM and Roe NM. A rare case of malt lymphoma underlying ileocecal intussusception. J Am Osteopath Assoc. (2016) 116:e37–40. doi: 10.7556/jaoa.2016.108
18. Álvarez-Nava Torrego MT, Ballesteros de Diego L, García Pérez JL, and de Las Heras de la Cadena Páez B. Pseudotumor lymphomatous polyposis of the ileum in a patient with gastrointestinal malt lymphoma. Rev Esp Enferm Dig. (2019) 111:483–4. doi: 10.17235/reed.2019.5772/2018
19. Badwaik N, Gharde P, Lamture Y, Singh S, and Shukla R. A rare case of mucosa-associated lymphoid tissue lymphoma in the ileum. Cureus. (2022) 14:e32851. doi: 10.7759/cureus.32851
20. Bennani A, Kharrasse G, Achraf M, Wafa K, Zahi I, Imane K, et al. Synchronous colonic adenoma and intestinal marginal zone B-cell lymphoma associated with crohn’s disease: A case report and literature review. BMC Cancer. (2019) 19:966. doi: 10.1186/s12885-019-6224-x
21. Da B, Zhang J, Zhu F, Wang Z, and Diao Y. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue of the ileum in an adult presenting with intussusception: A case report and literature review. Front Oncol. (2024) 14:1395144. doi: 10.3389/fonc.2024.1395144
22. de Figueiredo VLP, Ribeiro IB, de Moura DTH, Oliveira CC, and de Moura EGH. Mucosa-associated lymphoid tissue lymphoma in the terminal ileum: A case report. World J Gastrointest Endosc. (2022) 14:176–82. doi: 10.4253/wjge.v14.i3.176
23. Kawasaki K, Eizuka M, Nakamura S, Sugai T, and Matsumoto T. Gastrointestinal: discordant lymphoma consisting of ileal follicular lymphoma and colonic mucosa-associated lymphoid tissue lymphoma. J Gastroenterol Hepatol. (2019) 34:1894. doi: 10.1111/jgh.14723
24. Kikuchi Y, Matsui T, Hisabe T, Wada Y, Hoashi T, Tsuda S, et al. Deep infiltrative low-grade malt (Mucosal-associated lymphoid tissue) colonic lymphomas that regressed as a result of antibiotic administration: endoscopic ultrasound evaluation. J Gastroenterol. (2005) 40:843–7. doi: 10.1007/s00535-005-1639-3
25. Kinkade Z, Esan OA, Rosado FG, Craig M, and Vos JA. Ileal mucosa-associated lymphoid tissue lymphoma presenting with small bowel obstruction: A case report. Diagn Pathol. (2015) 10:105. doi: 10.1186/s13000-015-0353-6
26. Makino Y, Suzuki H, Nishizawa T, Kameyama K, Hisamatsu T, Imaeda H, et al. Ileal mucosa-associated lymphoid tissue (Malt) lymphoma with a large-cell component that regressed spontaneously. Gut Liver. (2010) 4:117–21. doi: 10.5009/gnl.2010.4.1.117
27. Rosat A and Sánchez JM. Perforated ileal phytobezoar revealed a malt lymphoma. Pan Afr Med J. (2016) 25:16. doi: 10.11604/pamj.2016.25.16.10361
28. Srinivasan AP, Parijatham BO, and Ganapathy H. A case of intestinal maltoma with co-existent tuberculosis and peutz-jeghers polyp. J Postgrad Med. (2015) 61:134–6. doi: 10.4103/0022-3859.150900
29. Seo SW, Lee SH, Lee DJ, Kim KM, Kang JK, Kim DW, et al. Colonic mucosa-associated lymphoid tissue lymphoma identified by chromoendoscopy. World J Gastroenterol. (2014) 20:18487–94. doi: 10.3748/wjg.v20.i48.18487
30. Al-Saleem T and Al-Mondhiry H. Immunoproliferative small intestinal disease (Ipsid): A model for mature B-cell neoplasms. Blood. (2005) 105:2274–80. doi: 10.1182/blood-2004-07-2755
31. Goodlad JR, Davidson MM, Hollowood K, Ling C, MacKenzie C, Christie I, et al. Primary cutaneous B-cell lymphoma and borrelia burgdorferi infection in patients from the highlands of scotland. Am J Surg Pathol. (2000) 24:1279–85. doi: 10.1097/00000478-200009000-00012
32. Husain A, Roberts D, Pro B, McLaughlin P, and Esmaeli B. Meta-analyses of the association between chlamydia psittaci and ocular adnexal lymphoma and the response of ocular adnexal lymphoma to antibiotics. Cancer. (2007) 110:809–15. doi: 10.1002/cncr.22843
33. Lugton I. Mucosa-associated lymphoid tissues as sites for uptake, carriage and excretion of tubercle bacilli and other pathogenic mycobacteria. Immunol Cell Biol. (1999) 77:364–72. doi: 10.1046/j.1440-1711.1999.00836.x
34. Du MQ, Xu CF, Diss TC, Peng HZ, Wotherspoon AC, Isaacson PG, et al. Intestinal dissemination of gastric mucosa-associated lymphoid tissue lymphoma. Blood. (1996) 88:4445–51. doi: 10.1182/blood.V88.12.4445.bloodjournal88124445
35. Ahlawat S, Haddad N, Kanber Y, Cohen P, Ozdemirli M, and Benjamin S. Primary mucosa-associated lymphoid tissue lymphoma occurring in the rectum. Gastrointest Endosc. (2005) 62:443–4. doi: 10.1016/s0016-5107(05)01590-7
36. De Sanctis V, Marignani M, Angeletti S, Assisi D, Armosini V, Valeriani M, et al. Anti-helicobacter pylori therapy in primary malt lymphoma of rectum. Tumori. (2012) 98:e105–10. doi: 10.1177/030089161209800423
37. Dohden K, Kaizaki Y, Hosokawa O, Hayashi H, and Hattori M. Regression of Rectal Mucosa-Associated Lymphoid Tissue Lymphoma but Persistence of Helicobacter Pylori Infection of Gastric Mucosa after Administration of Levofloxacin: Report of a Case. Dis Colon Rectum. (2004) 47:1544–6. doi: 10.1007/s10350-004-0575-2
38. Hisabe T, Imamura K, Furukawa K, Tsuda S, Matsui T, Yao T, et al. Regression of cd5-positive and helicobacter pylori-negative mucosa-associated lymphoid tissue lymphoma of the rectum after administration of antibiotics: report of a case. Dis Colon Rectum. (2002) 45:1267–70. doi: 10.1007/s10350-004-6404-9
39. Hori K, Suguro M, Koizuka H, Sakagami T, Tomita T, Kosaka T, et al. Disappearance of rectal mucosa-associated lymphoid tissue lymphoma following antibiotic therapy. Dig Dis Sci. (2004) 49:413–6. doi: 10.1023/b:ddas.0000020495.83052.74
40. Inoue F and Chiba T. Regression of malt lymphoma of the rectum after anti-H. Pylori therapy in a patient negative for H. Pylori. Gastroenterology. (1999) 117:514–5. doi: 10.1053/gast.1999.0029900514b
41. Matsumoto T, Iida M, and Shimizu M. Regression of mucosa-associated lymphoid-tissue lymphoma of rectum after eradication of helicobacter pylori. Lancet. (1997) 350:115–6. doi: 10.1016/s0140-6736(05)61818-1
42. Matsumoto T, Inokuma T, and Imai Y. Education and imaging. Gastrointestinal: colonic mucosa-associated lymphoid tissue lymphoma regressed by levofloxacin. J Gastroenterol Hepatol. (2013) 28:750. doi: 10.1111/jgh.12130
43. Nakamura S, Matsumoto T, Nakamura S, Kusano Y, Esaki M, Kurahara K, et al. Duodenal mucosa-associated lymphoid tissue lymphoma treated by eradication of helicobacter pylori: report of 2 cases including eus findings. Gastrointest Endosc. (2001) 54:772–5. doi: 10.1067/mge.2001.119602
44. Nakase H, Okazaki K, Ohana M, Ikeda K, Uchida K, Uose S, et al. The possible involvement of micro-organisms other than helicobacter pylori in the development of rectal malt lymphoma in H. Pylori-Negative Patients Endoscopy. (2002) 34:343–6. doi: 10.1055/s-2002-23643
45. Nam MJ, Kim BC, Park SC, Hong CW, Han KS, Sohn DK, et al. Mucosa-associated lymphoid-tissue lymphoma of the cecum and rectum: A case report. Ann Coloproctol. (2017) 33:35–8. doi: 10.3393/ac.2017.33.1.35
46. Niino D, Yamamoto K, Tsuruta O, Maeda T, Yakushijin Y, Aoki R, et al. Regression of rectal mucosa-associated lymphoid tissue (Malt) lymphoma after antibiotic treatments. Pathol Int. (2010) 60:438–42. doi: 10.1111/j.1440-1827.2010.02538.x
47. Ohara E, Kitadai Y, Onoyama M, Ohnishi M, Shinagawa K, Oka S, et al. Regression of rectal malt lymphoma after antibiotic treatment in a patient negative for helicobacter pylori. Clin J Gastroenterol. (2012) 5:59–63. doi: 10.1007/s12328-011-0270-5
48. Ohashi S, Yazumi S, Watanabe N, Matsumoto S, Fukui T, Nishio A, et al. Education and imaging. Gastrointestinal: malt lymphoma of the terminal ileum. J Gastroenterol Hepatol. (2006) 21:1495. doi: 10.1111/j.1440-1746.2006.04634.x
49. Lecuit M, Abachin E, Martin A, Poyart C, Pochart P, Suarez F, et al. Immunoproliferative small intestinal disease associated with campylobacter jejuni. N Engl J Med. (2004) 350:239–48. doi: 10.1056/NEJMoa031887
Keywords: mucosa-associated lymphoid tissue, lymphoma, ileum, Helicobacter pylori eradication, case report
Citation: Huang Y, Jiang J, Jiang K, Wang B, Liu T and Cao H (2025) Mucosal healing of ileum-mucosa-associated lymphoid tissue lymphoma after Helicobacter pylori eradication: a case report and literature review. Front. Oncol. 15:1544858. doi: 10.3389/fonc.2025.1544858
Received: 13 December 2024; Accepted: 31 July 2025;
Published: 21 August 2025.
Edited by:
Duygu Agagündüz, Gazi University, TürkiyeReviewed by:
Yuhao Jiao, Peking Union Medical College Hospital (CAMS), ChinaAli Alshiekh, Syrian Private University, Syria
Copyright © 2025 Huang, Jiang, Jiang, Wang, Liu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bangmao Wang, dGptdWdoZ2lAaG90bWFpbC5jb20=; Tianyu Liu, bGl1dGlhbnl1QHRtdS5lZHUuY24=; Hailong Cao, Y2FvaGFpbG9uZ0B0bXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yihan Huang
Yihan Huang Jiaying Jiang†
Jiaying Jiang† Kui Jiang
Kui Jiang Bangmao Wang
Bangmao Wang Tianyu Liu
Tianyu Liu Hailong Cao
Hailong Cao