Abstract
Background:
Disparities in cancer outcomes persist between racial, ethnic, and socioeconomic groups. One potential cause is lack of appropriate representation in dose-finding clinical trials. We investigated the extent of disparities in phase I clinical trials and recent changes in the setting of institutional efforts to mitigate disparities, legislative interventions, FDA guidance for sponsors and the COVID-19 pandemic.
Methods:
We performed a retrospective review of patients enrolled in phase I clinical trials at the University of Colorado Cancer Center in 2018–2019 and 2022-2023. We collected demographics, area deprivation index (ADI), tumor type and other clinical variables. Differences between cohorts were evaluated with t-tests, chi-Square test, or Fisher exact test. Progression-free survival (PFS) and overall survival (OS) were calculated using the Kaplan-Meier method. Hazard ratios (HR), confidence intervals (CI) and p-values were derived using the Cox-proportional hazards method.
Results:
A total of 361 patients were included (209 and 152 in the 2018–2019 and 2022–2023 cohorts, respectively). The population consisted of 85.0% White, 3.3% Asian, 1.4% Black, 0.3% Native Hawaiian or Pacific Islander and no American Indian/Alaskan Native (AIAN) patients by race, and 9.1% Hispanic by ethnicity. The most common tumor type was colorectal cancer (18.3%). Compared to 2018-2019, we observed increases in non-English speakers from 1.9% (4/209) to 6.6% (10/152) (p = 0.028) and in translated informed consent forms (ICFs) from 1.4% (3/209) to 5.9% (9/152) (p = 0.033) in 2022-2023. There were no significant changes in race, ethnicity, insurance, or tumor type, although there was a moderate increase in Hispanic patients from 8.1% to 10.5%. There were no differences in clinical outcomes by race, ethnicity, or ADI scores in the overall study population. However, in the most common cancer type, colorectal cancer, higher ADI scores were associated with decreased median PFS and OS.
Conclusion:
The interventions resulted in an increase in accrual of non-English speaking patients, however, there was not yet a significant change in overall race and ethnicity. Our study confirms poorer outcomes for patients with higher ADI scores. Further research is warranted to understand disparities in clinical trial accrual, and intervention is needed to improve outcomes for disadvantaged patients.
1 Introduction
Approximately 41% of men and 39% of women in the United States are diagnosed with invasive cancer in their lifetime (1). Due to increased awareness, access to early screening and more efficacious treatments, both cancer incidence and cancer death rates have dropped since the early 1990s (1). However, not all populations in the United States have benefited equally from this progress. For example, the relative risk of death is 33% higher in non-Hispanic Black patients and 51% higher in non-Hispanic AIAN patients when compared to non-Hispanic White patients (2). Additionally, 5-year relative survival in Black patients is lower in 19 of the 23 common cancer sites reported by the American Cancer Society compared to White patients (1).
The disparities are multifactorial in nature, and include biologic factors, social determinants of health such as income, education, and health insurance, as well as access to diagnostic testing, genetic counseling, and guideline-directed care which includes clinical trial enrollment for many patients (1, 3–8).
Clinical trials leading to new drug approvals play a critical role in improving cancer outcomes. Equitable enrollment in early phase and registration clinical trials is important for many reasons, including the well-documented differences in the molecular biology of cancer and susceptibility of patients to drug toxicity according to race and ethnicity. Tumors in certain racial groups often have different molecular drivers (9–12). Examples include certain populations of Asian descent having a higher mutation rate in epidermal growth factor receptor (EGFR) in lung cancers, and Black patients having higher mutation rates in p53 in endometrial cancers (11). Differences in molecular alterations may impact presentation of disease, response to treatment, and prognosis. Additionally, differences in drug metabolism and toxicity are also observed in different racial and ethnic groups (3, 13, 14), such as the higher rates of toxicity requiring dose-reductions in Black or Hispanic patients with gastrointestinal tumors receiving Capecitabine (13).
Effect size and risk prediction may not be reliable if data is extrapolated from an unrepresentative clinical trial population onto a more diverse general population. Therefore, recruiting a diverse clinical trial population is essential to ensuring that the approved dose and schedule for new agents, based on the safety profile observed in clinical trials, is appropriate for a diverse cancer patient population. Some studies have also shown survival benefit to early enrollment in a phase I study (15), which further supports consideration of all patients.
There are a number of barriers to recruiting a representative clinical trial population (3, 16–19). Individual-level barriers include negative patient attitudes towards clinical trials, mistrust in the medical system and higher receptivity to alternative medicine in certain groups (17). There are also systemic barriers including the location of treatment accessible to patients, health insurance inadequacy, transportation and additional financial burden (20).
Despite the above barriers, studies have shown that persons from minority groups have similar willingness to participate in research once presented with the opportunity (18, 21). Clinician biases and misconceptions of persons from minority groups may also contribute to the observed accrual disparities due to perceptions and concerns about health literacy and compliance with strict study protocols (19, 22). Similarly, stringent clinical trial criteria may disproportionally exclude populations with a higher prevalence of comorbidities (23).
At the University of Colorado Cancer Center, a set of institutional initiatives were implemented in January of 2022 and included establishment of a bicultural and bilingual Spanish-speaking clinic team, partnerships with the local county hospitals, multi-lingual patient education materials, internal reviews and site assessments. Similarly, initiatives at the legislative level were also implemented both locally (24) and nationally with an FDA guidance for industry sponsors (25).
In the current study, we examined clinical trial participation demographics and treatment outcomes of patients enrolled in early phase clinical trials in two different timeframes to evaluate differences following efforts at the institutional, local and national level to increase diversity.
2 Materials and methods
2.1 Study design and participants
We performed a retrospective cohort study including patients enrolled in phase I clinical trials at the University of Colorado Cancer Center from 2018–2019 and 2022–2023 to determine the impact of recent efforts on patient demographics and treatment outcomes. If patients were enrolled in multiple phase I studies during the study period, only the first study enrollment was included. Study participants enrolled in the years 2020–2021 were excluded due to the impact of the COVID-19 pandemic on all aspects of clinical research during that time. This was also a time of transition in developing diversity initiatives.
2.2 Data collection and storage
Patient demographics and clinical characteristics collected include age, sex, race, ethnicity, language, primary insurance coverage, ADI, pre-treatment body mass index (BMI), Eastern Cooperative Oncology Group (ECOG) performance scores, smoking history, tumor type, clinical trial treatment type, dates and response.
All patient and clinical trial data was collected under an Institutional Review Board (IRB) approved protocol. Patient data was collected from electronic medical records and stored in a secure, password-protected electronic data capture system, RedCap. Data was collected until patient death, loss to follow up, or the data collection cutoff date 8/1/2023, whichever was earlier.
2.3 Statistical analysis
Patient characteristics were summarized by cohort into Cohort 1: 2018–2019 and Cohort 2: 2022–2023 using standard descriptive statistics. The differences between cohorts were evaluated with t-tests for continuous variables, the chi-square test for categorical variables, and the Fisher exact test for categorical variables with low cell counts. Median PFS and OS were calculated using the Kaplan-Meier method. Censoring occurred either at the instance of lost to follow-up or using the data cut-off date of 8/1/2023. Hazard rates and their associated p-values were derived using Cox-proportional hazards models. Additional exploratory analysis was performed on select variables and their relationship to PFS and OS. Candidate variables were assessed for multicollinearity, proportional hazards, and the best fitting model was selected utilizing the Akaike information criterion. Significance level, α, was set to 0.05. Data preparation and statistical analyses were conducted using SAS 9.4 (SAS Institute; Cary, NC), and plots were generated using R version 4.2.0 (R Core Team).
3 Results
3.1 Patient characteristics
A total of 361 patients were included (209 in the 2018–2019 Cohort 1 and 152 in the 2022–2023 Cohort 2). Baseline patient characteristics are shown in Tables 1 and 2. The most common tumor type was colorectal cancer (18.3% of the overall study population).
Table 1
| Cohort 1: 2018-2019 (n = 209) | Cohort 2: 2022-2023 (n = 152) | P-value | Both cohorts (n = 361) | |
|---|---|---|---|---|
| Age | ||||
| Mean (SD) Median (IQR) | 59.83 (11.89) 60.12 (52.61, 68.56) | 60.52 (12) 61.66 (51.85, 69.78) | 0.588 a | 60.12 (11.92) 60.94 (52.44, 69.29) |
| Sex | 0.736 b | |||
| Female | 111 (53.1%) | 78 (51.3%) | 189 (52.4%) | |
| Male | 98 (46.9%) | 74 (48.7%) | 172 (47.7%) | |
| ECOG Performance Status | 0.941 c | |||
| 0 | 90 (43.1%) | 68 (44.7%) | 158 (43.8%) | |
| 1 | 116 (55.5%) | 82 (54.0%) | 198 (54.9%) | |
| 2 | 3 (1.4%) | 2 (1.3%) | 5 (1.4%) | |
| Smoking Status | 0.787 b | |||
| Current Smoker | 7 (3.4%) | 6 (4.0%) | 13 (3.6%) | |
| Non-smoker | 115 (55.0%) | 88 (57.9%) | 203 (56.2%) | |
| Past Smoker | 87 (41.6%) | 58 (38.2%) | 145 (40.2%) | |
| BMI | 0.821 b | |||
| <18.5 | 16 (7.7%) | 12 (7.9%) | 28 (7.8%) | |
| 18.5 - 24.9 | 90 (43.1%) | 59 (38.8%) | 149 (41.3%) | |
| 25 - 29.9 | 66 (31.6%) | 49 (32.2%) | 115 (31.9%) | |
| ≥30 | 37 (17.7%) | 32 (21.1%) | 69 (19.1%) | |
| Tumor Type | 0.355 b | |||
| Gastrointestinal | 78 (37.3%) | 61 (40.1%) | 0.228 b,d | 139 (38.5%) |
| Colorectal | 32 (15.3%) | 34 (22.4%) | 66 (18.3%) | |
| Pancreatic | 29 (13.9%) | 14 (9.2%) | 43 (11.9%) | |
| Gastroesophageal | 8 (3.8%) | 4 (2.6%) | 12 (3.3%) | |
| Bile Duct/Gallbladder | 4 (1.9%) | 2 (1.3%) | 6 (1.7%) | |
| Other Gastrointestinal | 5 (2.4%) | 7 (4.6%) | 12 (3.3%) | |
| Gynecologic | 25 (12.0%) | 21 (13.8%) | 46 (12.7%) | |
| Sarcoma | 26 (12.4%) | 18 (11.8%) | 44 (12.2%) | |
| Breast | 26 (12.4%) | 13 (8.6%) | 39 (10.8%) | |
| Lung | 26 (12.4%) | 12 (7.9%) | 38 (10.5%) | |
| Head and Neck | 13 (6.2%) | 19 (12.5%) | 32 (8.9%) | |
| Skin | 6 (2.9%) | 3 (2.0%) | 9 (2.5%) | |
| Genitourinary | 6 (2.9%) | 2 (1.3%) | 8 (2.2%) | |
| Lymphoma | 1 (0.5%) | 0 (0%) | 1 (0.3%) | |
| Other | 2 (1.0%) | 3 (2.0%) | 5 (1.4%) | |
| Clinical Trial Treatment Type | 0.01 b | |||
| Immunotherapy | 103 (49.3%) | 84 (55.3%) | 187 (51.8%) | |
| Targeted Therapy | 64 (30.6%) | 40 (26.3%) | 104 (28.8%) | |
| Antibody-drug Conjugate | 8 (3.8%) | 15 (9.9%) | 23 (6.4%) | |
| Cytotoxic Therapy | 8 (3.8%) | 0 (0.0%) | 8 (2.2%) | |
| Combinations of Different Treatment Types | 26 (12.44%) | 13 (8.6%) | 39 (10.8%) | |
Patient clinical characteristics.
SD, standard deviation; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index.
T-Test.
Chi-square Test.
Fisher Exact Test.
Gastrointestinal tumor type-specific p-value.
Table 2
| Cohort 1: 2018-2019 (n = 209) | Cohort 2: 2022-2023 (n = 152) | P-value | Both cohorts (n = 361) | |
|---|---|---|---|---|
| Race | ||||
| White | 176 (84.2%) | 131 (86.2%) | 0.279 a | 307 (85.0%) |
| Asian | 7 (3.4%) | 5 (3.3%) | 12 (3.3%) | |
| Black or African American | 5 (2.4%) | 0 (0%) | 5 (1.4%) | |
| More Than One Race | 1 (0.5%) | 3 (2.0%) | 4 (1.1%) | |
| Native Hawaiian or Other Pacific Islander | 1 (0.5%) | 0 (0%) | 1 (0.3%) | |
| Unknown/Not Reported | 19 (9.1%) | 13 (8.6%) | 32 (8.9%) | |
| American Indian/Alaskan Native | 0 (0%) | 0 (0%) | 0 (0%) | |
| Ethnicity | ||||
| Not Hispanic or Latino | 192 (91.9%) | 135 (88.8%) | 0.352 a | 327 (90.6%) |
| Hispanic or Latino | 17 (8.1%) | 16 (10.5%) | 33 (9.1%) | |
| Unknown/Not Reported | 0 (0%) | 1 (0.7%) | 1 (0.3%) | |
| Preferred Language (Collapsed) | ||||
| English | 205 (98.1%) | 142 (93.4%) | 0.028 a | 347 (96.1%) |
| Other | 4 (1.9%) | 10 (6.6%) | 14 (3.9%) | |
| Preferred Language | ||||
| English | 205 (98.1%) | 142 (93.4%) | 0.070 a | 347 (96.1%) |
| Spanish | 3 (1.4%) | 5 (3.3%) | 8 (2.2%) | |
| Bosnian | 0 (0%) | 1 (0.7%) | 1 (0.3%) | |
| Mandarin Chinese | 0 (0%) | 1 (0.7%) | 1 (0.3%) | |
| Mongolian | 0 (0%) | 1 (0.7%) | 1 (0.3%) | |
| Punjabi | 1 (0.5%) | 0 (0%) | 1 (0.3%) | |
| Ukrainian | 0 (0%) | 1 (0.7%) | 1 (0.3%) | |
| Vietnamese | 0 (0%) | 1 (0.7%) | 1 (0.3%) | |
| Translated Consent Use | ||||
| No (English ICF was used) | 206 (98.6%) | 143 (94.1%) | 0.033 a | 349 (96.7%) |
| Yes | 3 (1.4%) | 9 (5.9%) | 12 (3.3%) | |
| Health Insurance Status | ||||
| Medicare | 89 (42.6%) | 76 (50.0%) | 0.368 a | 165 (45.7%) |
| Private Insurance | 101 (48.3%) | 60 (39.5%) | 161 (44.6%) | |
| Medicaid | 18 (8.6%) | 15 (9.9%) | 33 (9.1%) | |
| Uninsured | 1 (0.5%) | 1 (0.7%) | 2 (0.6%) | |
| ADI b | ||||
| 1-5 | 118 (63.4%) | 88 (62.4%) | 0.849 c | 206 (63.0%) |
| 6-10 | 68 (36.6%) | 53 (37.6%) | 121 (37.0%) | |
Patient social determinants of health.
ICF, informed consent form; ADI, area deprivation index.
Fisher Exact Test.
The number of patients with area deprivation index values does not add up to the total number of patients due to 34 patients (23 in Cohort 1 and 11 in Cohort 2) not having ADI scores due to being located outside the state or due to the data not being available for their home address.
Chi-square Test.
In both cohorts, the majority of patients were White (85.0%) and 9.1% were Hispanic. By comparison, The rates of new cancer cases in Colorado by race and ethnicity are reported in Table 3 (26). The majority of patients were English-speaking (96.1%) and lived in neighborhoods with ADI scores of 1-5 (63.0%).
Table 3
| Race | Number of new cases (% of total) |
|---|---|
| White | 137,102 (92.0%) |
| Black | 5,155 (3.5%) |
| Asian/Pacific Islander | 2986 (2.0%) |
| American Indian/ Native Alaskan | 1,052 (0.7%) |
| Other/Unknown | 2,697 (1.8%) |
| Ethnicity | Number of new cases (% of Total) |
| Hispanic | 17,482 (11.7%) |
| Not Hispanic | 13,1459 (88.2%) |
| Unknown | 51 (0.0%) |
State of Colorado new cancer cases by race and ethnicity (2015–2020).
Patients were enrolled in 80 clinical trials (Supplementary Materials Figure 1 and Supplementary Data 1) receiving immunotherapy (51.8%), targeted therapy (28.8%), antibody-drug conjugates (6.4%), cytotoxics (2.2%) or combination therapy (10.8%)(Table 1).
3.2 Demographic and clinical characteristics of patients in cohorts 1 and 2
Non-English-speaking patients increased from 1.9% in Cohort 1 to 6.6% (p = 0.028) in Cohort 2. The number of translated ICFs also increased from 1.4% to 4.9% (p = 0.033) and included multiple languages (Table 2). There were no significant differences in other demographic or clinical characteristics between the cohorts.
3.3 Clinical outcomes for patients by race, ethnicity and other social determinants of health
In the overall study population (Cohorts 1 and 2), we did not observe differences in PFS or OS by patient race or ethnicity (Figure 1). Patients with private insurance had a shorter median OS of 7.9 months vs. 9.5 months in patients with Medicare (HR = 1.30, 95% C.I. 1.01-1.69, p = 0.043). Patients with private insurance were younger and more likely to be non-smokers compared to patients with Medicare (Table 4). ECOG 1 was associated with shorter OS of 7.8 months vs. 11 months in ECOG 0 (HR = 1.35, 95% CI 1.06-1.73, p = 0.017).
Figure 1
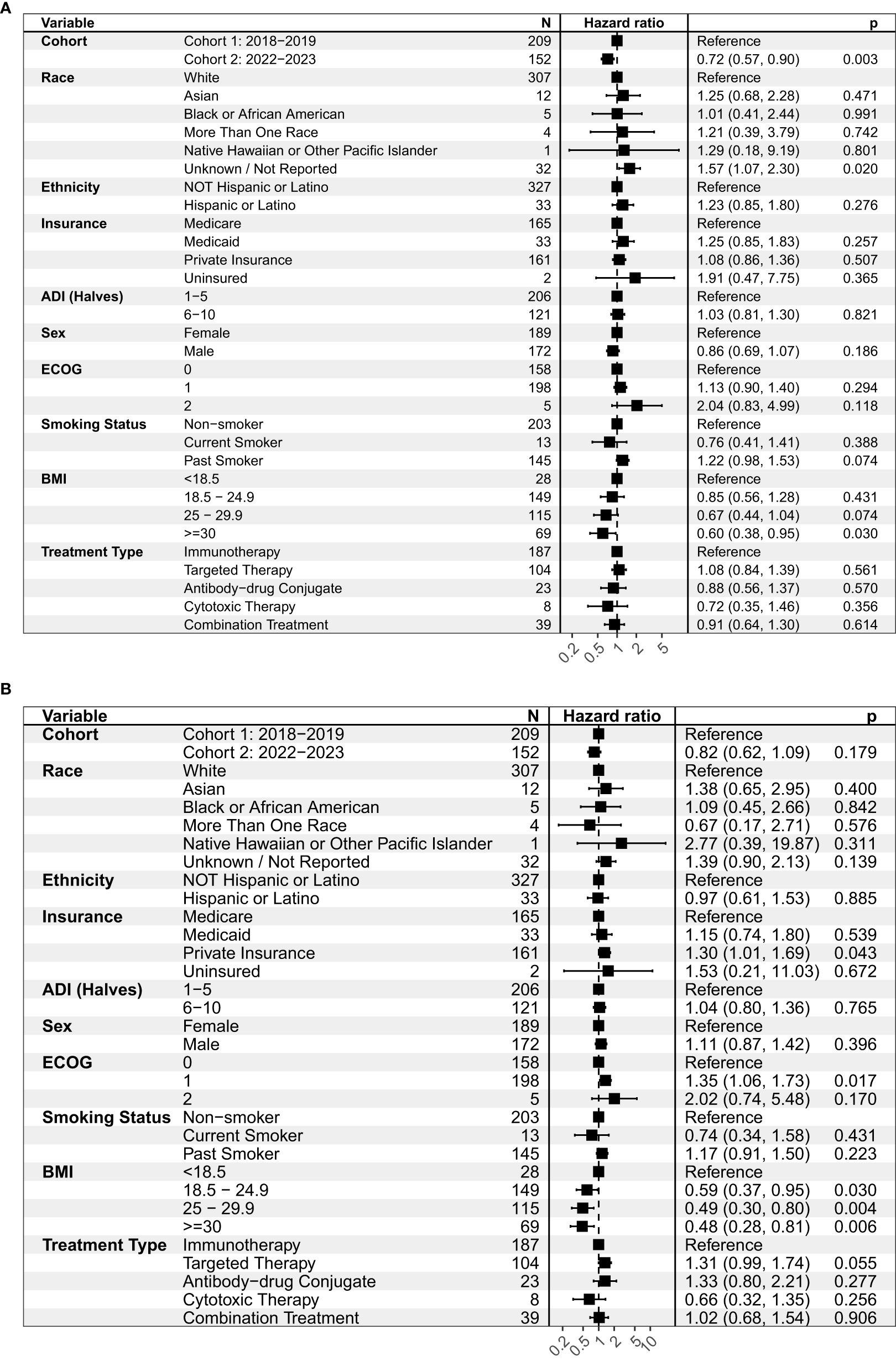
Univariate analysis on (A) progression-free survival (PFS) and (B) overall survival (OS) for the overall study population (Cohorts 1 and 2). This figure shows the hazard ratio, or the probability of an event such as a progression of disease (A) or expiration (B) relative to a reference point. ADI, Area deprivation index; ECOG, Eastern Cooperative Oncology Group; BMI, Body mass index.
Table 4
| Medicare (N = 165) | Private insurance (N = 161) | P-value | |
|---|---|---|---|
| Age | <0.001a | ||
| Mean (SD) | 68.44 (8.29) | 53.29 (10.21) | |
| Median (IQR) | 69.29 (65.9, 73.63) | 54.89 (47.1, 59.91) | |
| Sex | 0.9008b | ||
| Male | 78 (47.27%) | 75 (46.58%) | |
| Female | 87 (52.73%) | 86 (53.42%) | |
| Area Deprivation Index | 0.9367b | ||
| 1-5 | 99 (68.28%) | 101 (68.71%) | |
| 6-10 | 46 (31.72%) | 46 (31.29%) | |
| ECOG Performance Status | 0.2475b | ||
| 0 | 67 (40.61%) | 79 (49.07%) | |
| 1 | 95 (57.58%) | 81 (50.31%) | |
| 2 | 3 (1.82%) | 1 (0.62%) | |
| Smoking Status | 0.008c | ||
| Non-smoker | 82 (49.7%) | 102 (63.35%) | |
| Past Smoker | 80 (48.48%) | 52 (32.3%) | |
| Current Smoker | 3 (1.82%) | 7 (4.35%) | |
| BMI | 0.287c | ||
| <18.5 | 15 (9.09%) | 9 (5.59%) | |
| 18.5 - 24.9 | 71 (43.03%) | 64 (39.75%) | |
| 25 - 29.9 | 53 (32.12%) | 51 (31.68%) | |
| ≥30 | 26 (15.76%) | 37 (22.98%) |
Selected comparison of patients with Medicare and private insurance.
SD, standard deviation; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index.
T-Test.
Chi-square Test.
Fisher Exact Test.
When comparing clinical outcomes of patients between cohorts, the median PFS was 1.9 months in Cohort 1 and 2.8 months in Cohort 2 (HR = 0.72, 95% CI 0.57-0.90, p = 0.003) (Supplementary Figure 2A, Supplementary Table 1). Median OS was 8.2 months in Cohort 1 and 11.1 months in Cohort 2, although this increase was not statistically significant (HR = 0.82, 95% CI 0.62-1.09, p = 0.179) (Supplementary Figure 2B, Supplementary Table 2).
In multivariable analysis, enrollment during Cohort 2 and BMI ≥ 25 was associated with improved OS (Figure 2). In contrast, ECOG 1, private insurance and treatment with a targeted therapy (versus immunotherapy) were associated with worse OS.
Figure 2
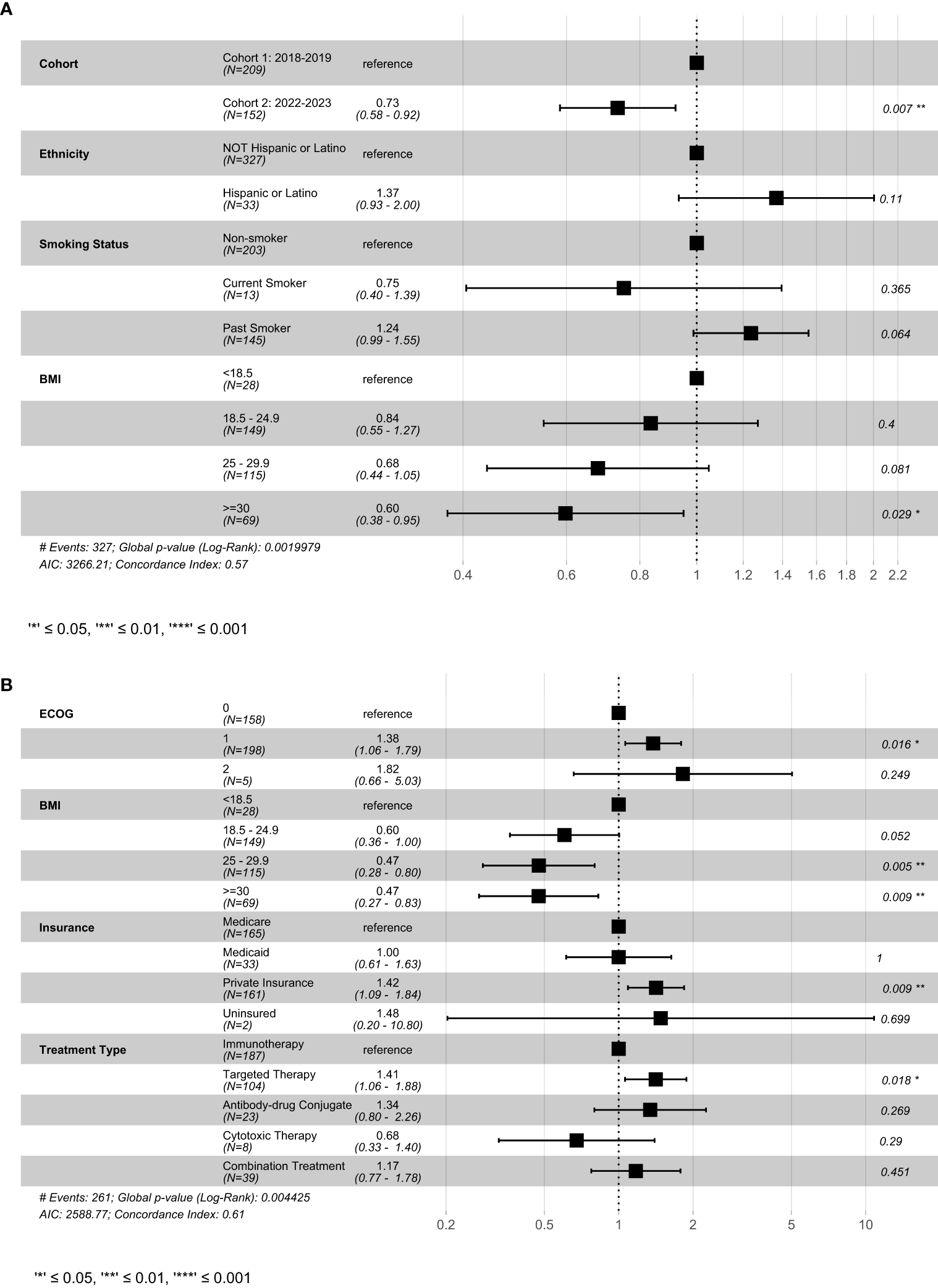
Result of cox-proportional hazard model examining select variables on (A) progression-free survival and (B) overall survival. This figure shows the best fitting models generated using the stepwise-selection algorithm relying on AIC. Variables were selected based on univariate results in previous figures. BMI, Body mass index; ECOG, Eastern Cooperative Oncology Group; AIC, Akaike information criterion.
3.4 Impact of clinical variables on clinical outcomes in CRC patients
We performed an additional exploratory survival analysis in patients with CRC, which was the most common cancer type in our study. Analyzing a single tumor type allowed us to evaluate differences in survival without the known clinical differences between cancer types. In patients with CRC, there was no difference in PFS or OS between Cohorts 1 and 2 (Figure 3, Supplementary Figure 3). However, ADI scores of 6–10 were associated with worse median PFS of 1.7 months vs 2.8 months in ADI of 1-5 (HR = 2.09, 95% CI 1.17-3.71, p = 0.012) (Supplementary Table 3) and OS at 4.2 months vs. 15 months (HR = 2.59, 95% CI 1.38-4.87, p = 0.003) (Supplementary Table 4).
Figure 3

Univariate analysis on (A) progression-free survival and (B) overall survival in patients with colorectal cancers. This figure shows the hazard ratio, or the probability of an event such as a progression of disease (A) or expiration (B) relative to a reference point. ADI, Area deprivation index.
Cox multivariable analysis in the CRC subset of patients (Figure 4) revealed decreased PFS in patients with Medicaid (p = 0.01), and decreased PFS and OS in patients with ADI scores of 6-10 (PFS; p = 0.022) (OS; p = 0.001) (Figure 4).
Figure 4
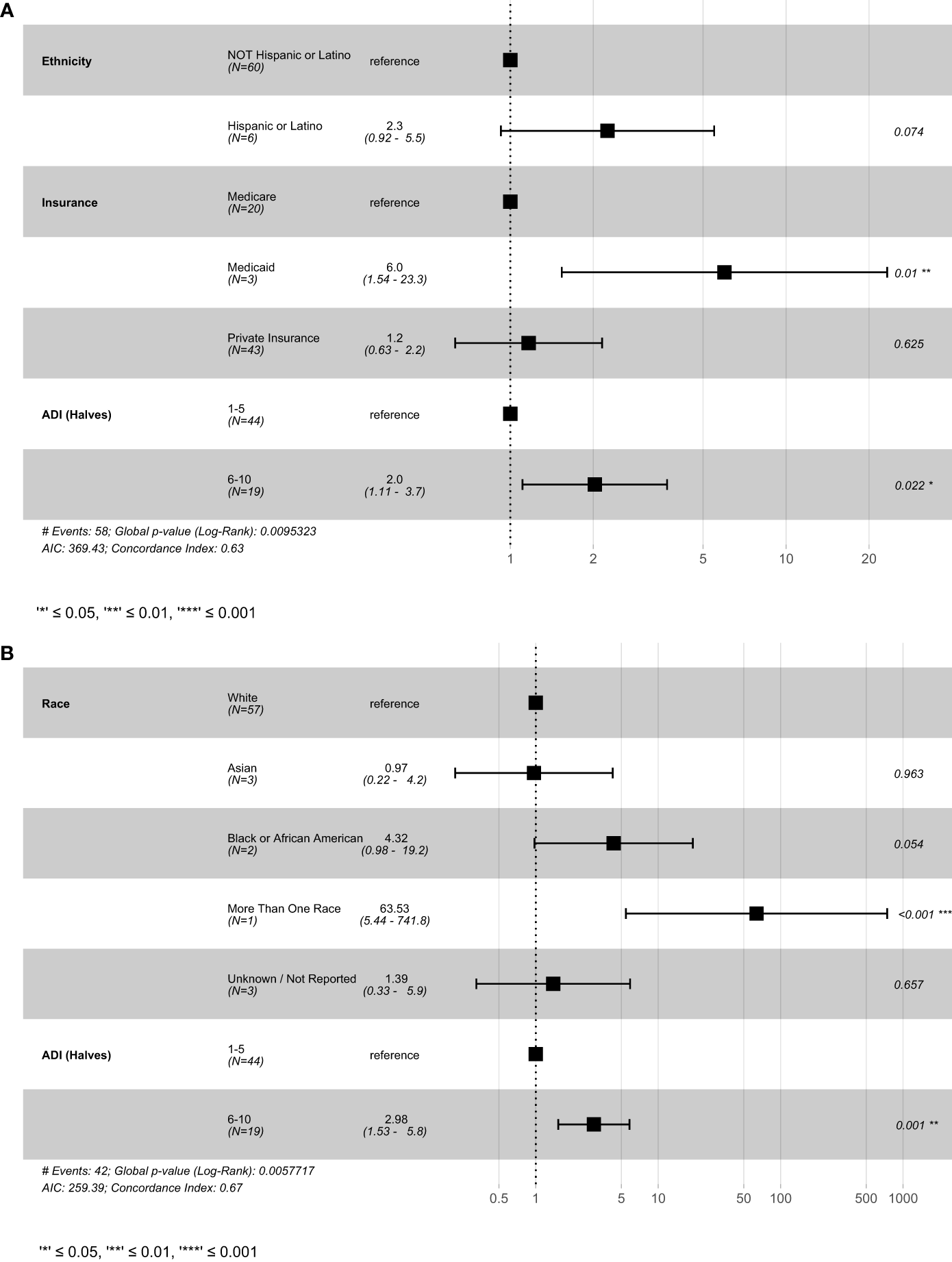
Result of cox-proportional hazard model for colorectal cancer patients (A) progression-free survival and (B) overall survival. This figure shows the best fitting models generated using the stepwise-selection algorithm relying on AIC. Variables were selected based on univariate results in previous figures. ADI, Area deprivation index; AIC, Akaike information criterion.
Discussion
In this study, we evaluated the demographics and treatment outcomes of patients enrolled in therapeutic anti-cancer phase I clinical trials to assess for changes in the enrollment of underrepresented persons from minority groups. Our study shows several improvements in moving towards a more representative clinical trial population including increases in the number of non-English speakers, the number of translated ICFs and an increase in proportion of Hispanic or Latino patients. Overall, the current findings indicate the success of a range of efforts targeting these populations such as the bicultural clinic, which includes Spanish-speaking providers, research nurses, medical assistants, patient navigators, and scheduling staff, and bilingual patient education materials. Although no significant improvements in other markers of diversity such as race, ethnicity and ADI were seen, the inclusion of several new languages in addition to Spanish supports interventions targeting a broader population, such as the community partnerships through the Office of Community Outreach and Engagement to reach patients at county hospitals as well as quarterly internal reviews and annual presentations to address shortcomings.
Despite widespread efforts dating back to the National Institute of Health Revitalization Act of 1993 (27), representation of persons from minority groups in research has remained inadequate. Recent interventions in the literature have included the use of culturally-competent patient navigators and cultural-competency training, with positive results in different underrepresented populations (28, 29). Another study established a 5-year center-wide program consisting of outreach, marketing, partnerships with local organizations, ride-sharing, nurse navigators, and improved ICFs, which resulted in an increase of Black patients on clinical trials (30), highlighting the efficacy of a multi-faceted and targeted approach. Other interventions that have shown improvements in persons from minority groups outcomes outside of clinical trials have included automated electronic medical record alerts, and frequent institutional reviews of treatment metrics (6).
At our center, we set out to implement a multifaceted set of interventions to address the different barriers known to limit the accrual of historically underrepresented patients to phase I clinical trials and build upon interventions previously shown to be effective at other centers. One of the primary barriers targeted at our center and by FDA guidance for sponsors was the lingual and cultural barrier faced by non-English-speaking patients. Recruiting non-English-speaking patients has always been an institutional logistical challenge, due to added complexity, time and cost required to present clinical trials and translate study documents. Prior studies have shown that this is particularly evident in non-industry-sponsored studies, where the cost of document translation often falls on the investigator team (31). Nonetheless, the improvement in representation of this group suggests that this is a modifiable barrier.
In addition, our study also showed an increase in the percentage of Hispanic or Latino patients from 8.1% to 10.5%. As previously noted, this group of patients made up 9.8% of recent cancer diagnoses in the state, which indicates an appropriate current rate of clinical trial participation. However, the Hispanic or Latino demographic makes up 22.5% of the state’s population (32). The discrepancy between new cancer cases in the state and general population rates is likely due to the younger age of the Hispanic or Latino population in the state (32) as well as the lower cancer screening rates in this population (33). Lastly, changes seen in our study demographics may also reflect recent migration patterns and changes in the state demographics.
Our findings also demonstrate persistent disparities in research. For example, the ADI distribution showed that a majority of patients (63.0%) were placed in the 5 lower deprivation deciles which correspond to higher income, education, employment, housing quality among other measures of affluency (34). This may be due to more affluent patients having better access to cancer screening, diagnosis, treatment at a tertiary center and subsequently access, eligibility and willingness to phase I clinical trials. Additionally, longer distances between a patient’s residence to the treatment center, which may correlate with higher deprivation scores, likely confound the lower participation rates due to the nature of phase I clinical trials, which often require patients to travel to the treatment center multiple times a week. Interestingly, private insurance was associated with worse overall survival compared to Medicare, which may reflect tumor latency in the older Medicare population.
Discrepancies in race-reporting practices between different databases and research studies have long hindered health equity research, and consistency in methodology, improvements to current racial and ethnic categories and potentially implementing the ancestry system to better group individuals of a common lineage (35) would facilitate more accurate representation and comparisons between different sources (36).
Additionally, our clinical trial population consisted of only 0.6% uninsured patients, who make up 6.5% of the state’s population (37). It is likely that the lack of insurance limits their access to cancer screening, diagnosis, and treatment. Recent state-level legislature aims to address this barrier by reducing costs of care (24).
One of the key challenges in assessing survival was the heterogeneity of our study population, which included a wide range of tumor types, treatments, and demographics. Nonetheless, survival analysis was performed to explore overall trends. We noted an improvement in PFS in the 2022–2023 cohort, which likely reflects changes in patient composition as well as clinical trial treatments. To further assess contributions of different social factors on patient outcomes, we analyzed a smaller subset of patients with colorectal cancers. We noted worse outcomes, as shown by both PFS and OS in patients with Medicaid and patients living in areas with higher ADI scores. This association of worse outcomes with higher ADI scores has been well documented across a number of tumor types (38–41) and in phase II and III clinical trials sponsored by the SWOG Cancer Research Network (42). To our knowledge, this is the first study reporting this association across a number of phase I clinical trials However, most phase I clinical trials are not designed to evaluate efficacy outcomes, so the relevance of our observation is limited.
Beyond clinical trials, the advancement of precision medicine and multi-omics datasets has led to the identification of genetic variants that influence complex diseases and drug responses, with important differences observed between populations (43). While diversity in large datasets enhances generalizability and equity, increased heterogeneity can introduce greater variability, necessitating larger sample sizes and added statistical power to reliably detect inter-population differences. The development of advanced analytical methodologies, such as the incorporation of local ancestry, polygenic risk scores, expression quantitative trait locus mapping, and transcriptome-wide association studies, may aid in interpreting these variants in the absence of matched population reference panels, particularly in admixed populations (43).
Our study period overlapped with the COVID-19 pandemic, which had a significant impact on the healthcare system as a whole, including accrual to and conduct of oncology clinical trials (8, 44–48). Other limitations of the current study include the small sample size and single-intuition design, which may limit generalizability of the data to other regions and institutions.
In conclusion, improving the diversity of patients in phase I and registration clinical trials continues to be of utter importance to determine appropriate efficacy, dosage, the detection of accurate toxicity of new treatments. Further efforts to address the poor accrual and clinical outcomes of disadvantaged populations are warranted. To address the persistent poor accrual of some disadvantaged populations seen in our study, future efforts may include more inclusive clinical trial designs (49), and a push towards decentralizing clinical trials to combat the distance and cost barriers (50). Additionally, state-level legislature may also encourage the inclusion of underinsured patients, similar to a bill recently passed in the state of Colorado (24). The impact of such legislation remains to be seen. Lastly, improvements to race, ethnicity reporting, including standardization, grouping patients by ancestry and advancements in multi-omics may help guide future interventions (35, 43).
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Colorado Anschutz Medical Campus. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
AA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SK: Data curation, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. BC: Writing – original draft, Writing – review & editing. CL: Writing – original draft, Writing – review & editing. BW: Writing – original draft, Writing – review & editing. SD: Writing – original draft, Writing – review & editing. DC: Writing – original draft, Writing – review & editing. AJ: Writing – original draft, Writing – review & editing. WM: Writing – original draft, Writing – review & editing. AN: Formal Analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. DP: Writing – original draft, Writing – review & editing. EB: Methodology, Resources, Writing – original draft, Writing – review & editing. JM: Investigation, Methodology, Writing – original draft, Writing – review & editing. JD: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the NIH and the NCI through grant numbers 5P30CA046934-25 (UCCC Support Grant).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1546500/full#supplementary-material
References
1
SiegelRLGiaquintoANJemalA. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2
JemalAWardEMJohnsonCJCroninKAMaJRyersonBet al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst. (2017) 109:djx030. doi: 10.1093/jnci/djx030
3
OwonikokoTKBusariAKKimSChenZAkintayoALewisCet al. Race-, age-, and gender-based characteristics and toxicities of targeted therapies on phase I trials. Oncology. (2018) 95:138–46. doi: 10.1159/000488763
4
BachPBSchragDBrawleyOWGalaznikAYakrenSBeggCB. Survival of blacks and whites after a cancer diagnosis. JAMA. (2002) 287:2106–13. doi: 10.1001/jama.287.16.2106
5
SprattDEChanTWaldronLSpeersCFengFYOgunwobiOOet al. Racial/ethnic disparities in genomic sequencing. JAMA Oncol. (2016) 2:1070–4. doi: 10.1001/jamaoncol.2016.1854
6
CharlotMSteinJNDamoneEWoodIForsterMBakerSet al. Effect of an antiracism intervention on racial disparities in time to lung cancer surgery. J Clin Oncol Off J Am Soc Clin Oncol. (2022) 40:1755–62. doi: 10.1200/JCO.21.01745
7
PresleyCJSoulosPRChiangACLongtineJAAdelsonKBHerbstRSet al. Disparities in next generation sequencing in a population-based community cohort of patients with advanced non-small cell lung cancer. J Clin Oncol. (2017) 35:6563–3. doi: 10.1200/JCO.2017.35.15_suppl.6563
8
PittellHCalipGSPierreARyalsCAAltomareIRoyceTJet al. Racial and ethnic inequities in US oncology clinical trial participation from 2017 to 2022. JAMA Netw Open. (2023) 6:e2322515. doi: 10.1001/jamanetworkopen.2023.22515
9
ShigematsuHLinLTakahashiTNomuraMSuzukiMWistubaIIet al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. (2005) 97:339–46. doi: 10.1093/jnci/dji055
10
BhardwajASrivastavaSKKhanMAPrajapatiVKSinghSCarterJEet al. Racial disparities in prostate cancer: a molecular perspective. Front Biosci Landmark Ed. (2017) 22:772–82. doi: 10.2741/4515
11
WhelanKDillonMStricklandKCPothuriBBae-JumpVBordenLEet al. TP53 mutation and abnormal p53 expression in endometrial cancer: Associations with race and outcomes. Gynecol Oncol. (2023) 178:44–53. doi: 10.1016/j.ygyno.2023.09.009
12
PernickNLSarkarFHPhilipPAArlauskasPShieldsAFVaitkeviciusVKet al. Clinicopathologic analysis of pancreatic adenocarcinoma in African Americans and Caucasians. Pancreas. (2003) 26:28–32. doi: 10.1097/00006676-200301000-00006
13
BrazeltonAYandeSPopeRJohnsonMLMusherBTrivediMV. Racial and ethnic differences in capecitabine toxicity in patients with gastrointestinal tract cancers. Ann Gastroenterol. (2022) 35:182–6. doi: 10.20524/aog.2022.0688
14
PetrosWPHopkinsPJSpruillSBroadwaterGVredenburghJJColvinOMet al. Associations between drug metabolism genotype, chemotherapy pharmacokinetics, and overall survival in patients with breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. (2005) 23:6117–25. doi: 10.1200/JCO.2005.06.075
15
AdashekJJLoRussoPMHongDSKurzrockR. Phase I trials as valid therapeutic options for patients with cancer. Nat Rev Clin Oncol. (2019) 16:773–8. doi: 10.1038/s41571-019-0262-9
16
HerremansKMRinerANWinnRATrevinoJG. Diversity and inclusion in pancreatic cancer clinical trials. Gastroenterology. (2021) 161:1741–1746.e3. doi: 10.1053/j.gastro.2021.06.079
17
ParieraKLMurphySTMengJMcLaughlinML. Exploring willingness to participate in clinical trials by ethnicity. J Racial Ethn Health Disparities. (2017) 4:763–9. doi: 10.1007/s40615-016-0280-6
18
WendlerDKingtonRMadansJVan WyeGChrist-SchmidtHPrattLAet al. Are racial and ethnic minorities less willing to participate in health research? PloS Med. (2006) 3:e19. doi: 10.1371/journal.pmed.0030019
19
NiranjanSJMartinMYFouadMNVickersSMWenzelJACookEDet al. Bias and stereotyping among research and clinical professionals: Perspectives on minority recruitment for oncology clinical trials. Cancer. (2020) 126:1958–68. doi: 10.1002/cncr.32755
20
FordJGHowertonMWLaiGYGaryTLBolenSGibbonsMCet al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. (2008) 112:228–42. doi: 10.1002/cncr.23157
21
UngerJMHershmanDLTillCMinasianLMOsarogiagbonRUFleuryMEet al. When offered to participate”: A systematic review and meta-analysis of patient agreement to participate in cancer clinical trials. J Natl Cancer Inst. (2021) 113:244–57. doi: 10.1093/jnci/djaa155
22
Lau-MinKSGuerraCENathansonKLBekelmanJE. From race-based to precision oncology: leveraging behavioral economics and the electronic health record to advance health equity in cancer care. JCO Precis Oncol. (2021) 5:PO.20.00418. doi: 10.1200/PO.20.00418
23
TalaricoLChenGPazdurR. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol Off J Am Soc Clin Oncol. (2004) 22:4626–31. doi: 10.1200/JCO.2004.02.175
24
Health-care billing requirements for indigent patients (2021). Available online at: https://leg.colorado.gov/bills/hb21–1198 (Accessed January 9, 2024).
25
Food and Drug Administration. Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials; draft guidance for industry; availability. Silver Spring, MD: Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH). (2022). Available online at: https://www.federalregister.gov/documents/2022/04/14/2022-07978/diversity-plans-to-improve-enrollment-of-participants-from-underrepresented-racial-and-ethnic (Accessed November 11, 2024).
26
Colorado Department of Public Health and Environment. Colorado health information dataset(CoHID), cancer cases, counts (2000). Available online at: https://cdphe.colorado.gov/center-for-health-and-environmental-data/registries-and-vital-statistics/colorado-central-cancer (Accessed January 10, 2024).
27
Studies I of M (US) C on E and LIR to the I of W in CMastroianniACFadenRFedermanD. “NIH revitalization act of 1993 public law 103-43.,”. In: Women and health research: ethical and legal issues of including women in clinical studies, vol. I. National Academies Press, US (1994). Available at: https://www.ncbi.nlm.nih.gov/books/NBK236531/.
28
NouviniRParkerPAMallingCDGodwinKCostas-MuñizR. Interventions to increase racial and ethnic minority accrual into cancer clinical trials: A systematic review. Cancer. (2022) 128:3860–9. doi: 10.1002/cncr.34454
29
WellsJSPughSBoparaiKReardenJYeagerKABrunerDW. Cultural competency training to increase minority enrollment into radiation therapy clinical trials-an NRG oncology RTOG study. J Cancer Educ Off J Am Assoc Cancer Educ. (2017) 32:721–7. doi: 10.1007/s13187-016-1051-0
30
GuerraCESalleeVHwangW-TBryantBWashingtonALTakvorianSUet al. Accrual of Black participants to cancer clinical trials following a five-year prospective initiative of community outreach and engagement. J Clin Oncol. (2021) 39:100–0. doi: 10.1200/JCO.2021.39.15_suppl.100
31
VelezMAGlennBAGarcia-JimenezMCummingsALLisbergANañezAet al. Consent document translation expense hinders inclusive clinical trial enrolment. Nature. (2023) 620:855–62. doi: 10.1038/s41586-023-06382-0
32
American Community Survey. (2020). Available online at: https://www.census.gov/programs-surveys/acs (Accessed September 28, 2023).
33
MillerKDOrtizAPPinheiroPSBandiPMinihanAFuchsHEet al. Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J Clin. (2021) 71:466–87. doi: 10.3322/caac.21695
34
KindAJHBuckinghamW. Making neighborhood disadvantage metrics accessible: the neighborhood atlas. New Engl J Med. (2018) 378:2456–8. doi: 10.1056/NEJMp1802313
35
BorrellLNElhawaryJRFuentes-AfflickEWitonskyJBhaktaNWuAHBet al. Race and genetic ancestry in medicine - A time for reckoning with racism. N Engl J Med. (2021) 384:474–80. doi: 10.1056/NEJMms2029562
36
KaderFÐoànLNChinMKSchererMCárdenasLFengLet al. IDEAL: A community-academic-governmental collaboration toward improving evidence-based data collection on race and ethnicity. Prev Chronic Dis. (2023) 20:E90. doi: 10.5888/pcd20.230029
37
2019 colorado health access survey: health insurance coverage (2020). Available online at: www.coloradohealthinstitute.org (Accessed September 28, 2023).
38
HufnagelDHKhabeleDYullFEHullPCSchildkrautJCrispensMAet al. Increasing Area Deprivation Index negatively impacts ovarian cancer survival. Cancer Epidemiol. (2021) 74:102013. doi: 10.1016/j.canep.2021.102013
39
GoelNHernandezAThompsonCChoiSWestrickAStolerJet al. Neighborhood disadvantage and breast cancer-specific survival. JAMA Netw Open. (2023) 6:e238908. doi: 10.1001/jamanetworkopen.2023.8908
40
YuK-XYuanW-JHuangC-HXiaoLXiaoR-SZengP-Wet al. Socioeconomic deprivation and survival outcomes in patients with colorectal cancer. Am J Cancer Res. (2022) 12:829–38.
41
FairfieldKMBlackAWZillerECMurrayKLucasFLWaterstonLBet al. Area deprivation index and rurality in relation to lung cancer prevalence and mortality in a rural state. JNCI Cancer Spectr. (2020) 4:pkaa011. doi: 10.1093/jncics/pkaa011
42
UngerJMMoseleyABCheungCKOsarogiagbonRUSymingtonBRamseySDet al. Persistent disparity: socioeconomic deprivation and cancer outcomes in patients treated in clinical trials. J Clin Oncol Off J Am Soc Clin Oncol. (2021) 39:1339–48. doi: 10.1200/JCO.20.02602
43
YangGMishraMPereraMA. Multi-omics studies in historically excluded populations: the road to equity. Clin Pharmacol Ther. (2023) 113:541–56. doi: 10.1002/cpt.2818
44
BougheyJCSnyderRAKantorOZhengLChawlaANguyenTTet al. Impact of the COVID-19 pandemic on cancer clinical trials. Ann Surg Oncol. (2021) 28:7311–6. doi: 10.1245/s10434-021-10406-2
45
BakounyZLabakiCBhallaSSchmidtALSteinharterJACoccoJet al. Oncology clinical trial disruption during the COVID-19 pandemic: a COVID-19 and cancer outcomes study. Ann Oncol Off J Eur Soc Med Oncol. (2022) 33:836–44. doi: 10.1016/j.annonc.2022.04.071
46
YeboaDNAkinfenwaCANguyenJAmayaDde GraciaBNingMet al. Effectively conducting oncology clinical trials during the COVID-19 pandemic. Adv Radiat Oncol. (2021) 6:100676. doi: 10.1016/j.adro.2021.100676
47
NodoraJ. “Overcoming racial and ethnic disparities in cancer clinical trial accrual during the COVID_19 pandemic: A partnership between a cancer center's clinical trials office and community outreach and engagement [abstract]”. In: Proceedings of the 15th AACR Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved. Philadelphia, PA: American Association for Cancer Research. (2023). p. A090. doi: 10.1158/1538-7755.DISP22-A090
48
BundorfMKGuptaSKimC. Trends in US health insurance coverage during the COVID-19 pandemic. JAMA Health Forum. (2021) 2:e212487. doi: 10.1001/jamahealthforum.2021.2487
49
UngerJMHershmanDLFleuryMEVaidyaR. Association of patient comorbid conditions with cancer clinical trial participation. JAMA Oncol. (2019) 5:326–33. doi: 10.1001/jamaoncol.2018.5953
50
ApostolarosMBabaianDCorneliAForrestAHamreGHewettJet al. Legal, regulatory, and practical issues to consider when adopting decentralized clinical trials: recommendations from the clinical trials transformation initiative. Ther Innov Regul Sci. (2020) 54:779–87. doi: 10.1007/s43441-019-00006-4
Summary
Keywords
clinical trials, phase I, cancer, social determinants of health, health disparities
Citation
Alsafar A, Kareem SL, Corr BR, Lieu CH, Wilky B, Davis SL, Camidge DR, Jimeno A, Messersmith WA, Nicklawsky A, Pacheco D, Borrayo EA, McDermott JD and Diamond JR (2025) Increased accrual of diverse patient populations in oncology phase I clinical trials at the University of Colorado Cancer Center. Front. Oncol. 15:1546500. doi: 10.3389/fonc.2025.1546500
Received
17 December 2024
Accepted
18 June 2025
Published
15 July 2025
Volume
15 - 2025
Edited by
Ana Maria Lopez, Sidney Kimmel Cancer Center, United States
Reviewed by
Jared C Roach, Institute for Systems Biology (ISB), United States
Camille Ragin, Fox Chase Cancer Center, United States
Updates
Copyright
© 2025 Alsafar, Kareem, Corr, Lieu, Wilky, Davis, Camidge, Jimeno, Messersmith, Nicklawsky, Pacheco, Borrayo, McDermott and Diamond.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer R. Diamond, Jennifer.Diamond@CUAnschutz.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.