Abstract
Introduction:
Individuals with hematological malignancies (HMs) are at a high risk of invasive pneumococcal disease due to underlying malignancy and subsequent immunosuppressive anticancer therapy. Early management of pneumococcal infections is crucial for reducing morbidity and mortality in this vulnerable patient subgroup. In this study, we aim to review the current evidence and recommendations regarding the use of pneumococcal conjugate vaccines (PCVs) in patients with HMs and develop a consensus document on the optimal timing and patient profiles who can benefit from them.
Methods:
The modified Delphi consensus method was used for achieving consensus. The panel comprised a scientific committee of six experts from India. Questions were drafted for discussion around: (i) the risk and consequences of pneumococcal disease in HMs; (ii) barriers to pneumococcal vaccination in the hemato-oncology clinical setting; and (iii) evidence and optimal timing of pneumococcal vaccines in HMs. The questionnaire was shared with the panel through an online survey platform (Delphi round 1). The consensus level was classified as high (≥80%), moderate (60%–79%), and low (< 60%). A Delphi round 2 meeting was conducted to discuss the questions that received near or no consensus to reach an agreement. The final draft of consensus statements was circulated among the experts for approval.
Results:
Pneumonia with or without bacteremia and bacteremia without foci of infection are the most frequently reported clinical presentations of pneumococcal infections in patients with HMs. A high risk of pneumococcal disease has been observed in patients with multiple myeloma (MM), acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and chronic lymphocytic leukemia (CLL). Priming with PCV enhances the response to pneumococcal polysaccharide vaccine 23 (PPSV23) in patients with HMs. Experts agreed that PCV is beneficial and can be strongly recommended in patients with CLL, MM, and patients undergoing hematopoietic stem cell transplantation. Children with acute lymphoblastic leukemia (ALL) would benefit from systematic revaccination with PCV after chemotherapy. The evidence is inadequate to consistently recommend pneumococcal vaccination to all patients with lymphoma, AML, and adults with ALL.
Conclusion:
This expert consensus will guide clinicians on the recommended approach for administering pneumococcal vaccination to patients with HMs.
1 Introduction
Globally, pneumococcal diseases are a common cause of morbidity and mortality. The clinical spectrum of pneumococcal diseases varies from mild, noninvasive infections to severe, invasive infections (Figure 1) (1). Streptococcus pneumoniae can cause severe and potentially fatal diseases, notably pneumonia, bacteremia, and meningitis (1). Patients with hematological malignancies (HMs) have a high risk of invasive pneumococcal disease (IPD) due to underlying cancer and immunosuppressive anticancer treatments that are frequently associated with prolonged neutropenia and bone marrow suppression (2–5). These conditions usually increase the risk of serious infections requiring hospitalization (5). According to data from the Centers for Disease Control and Prevention, the rate of IPD was 129 per 100,000 population (during the period of 2013–2014) in adults (aged 18–64 years) with hematological cancer (1). The risk of IPD may vary by age, type of HM, and the time elapsed since the cancer diagnosis (2–4). Among different kinds of HMs, an increased risk of IPD was reported in patients with multiple myeloma (MM), acute lymphoblastic leukemia (ALL), and chronic lymphocytic leukemia (CLL) (2, 4). This increased susceptibility highlights the critical need for effective preventive measures, including vaccination. Anderson MA et al. reported a 3.5% annual decline in the incidence of IPD among adults following the introduction of pneumococcal vaccination in childhood vaccination programs (2). The reduction was even more pronounced in individuals with HM, showing a 9% annual decrease (2). Although pneumococcal infections are vaccine-preventable, this might often be overlooked by clinicians and patients with cancer as they may prioritize treating the cancer over vaccinating against the infection (6). Results of the global, prospective INSIGHT MM study conducted in patients with MM showed that pneumococcal vaccination in the previous 5 years impacted overall survival vs. no vaccination (p<0.0001). Furthermore, the proportion of deaths due to infections was lower among patients who were vaccinated than those not vaccinated (7). However, overall vaccination rates were low (30.2%) and varied by region, with the highest rates reported in the United States (42.83%) and the lowest in Asia (4.7%) (7). This discrepancy highlights a global challenge that needs addressing through tailored strategies and increased awareness.
Figure 1
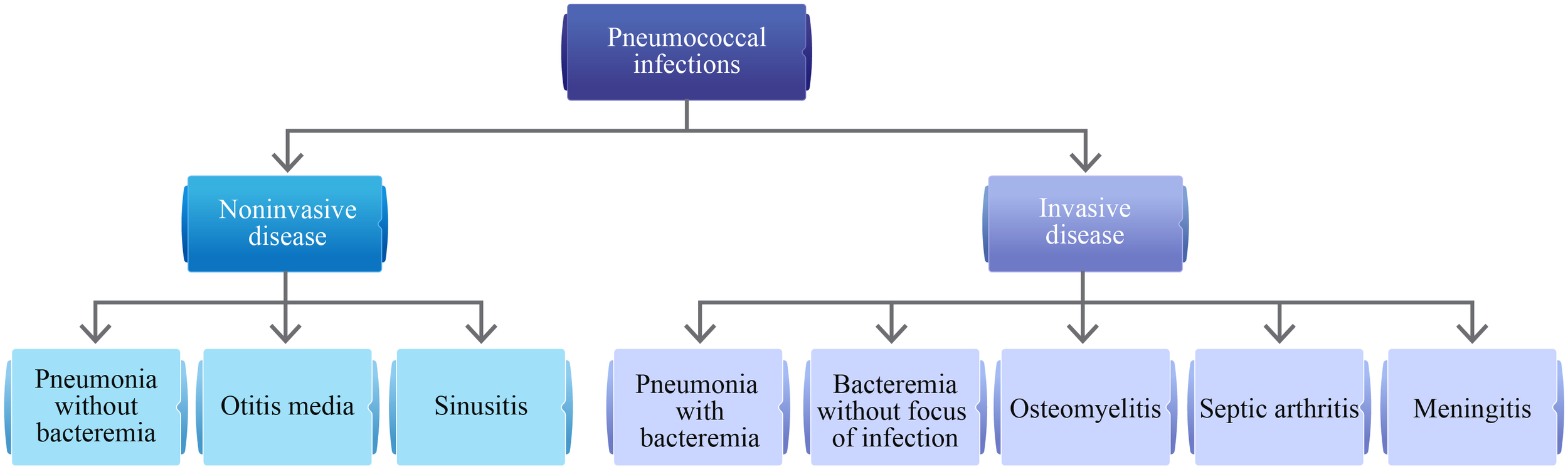
Clinical spectrum of pneumococcal infections (1).
In India, the situation is particularly concerning. Although pneumococcal vaccines (PVs) are available, vaccine uptake among patients with cancer in clinical practice is suboptimal. For example, a cross-sectional observational study conducted at the Tata Memorial Hospital between 2020 and 2023 found that only 0.68% of elderly patients with cancer (age ≥60 years) had received PV (8). Another study reported a vaccination prevalence of just 1.8% among adults with cancer (age ≥45 years) between 2017 and 2018 (9). These findings reveal the need for more rigorous measures and the adoption of specific recommendations and guidelines to improve the PV uptake in oncology clinical practice in India. Moreover, due to the paucity of well-designed randomized controlled trials (RCTs) conducted in India, hemato-oncologists rely on data from Western countries. In this study, we aim to review the current evidence and recommendations regarding the use of pneumococcal conjugate vaccines (PCVs) in patients with HMs and develop a consensus statement on the optimal timing and patient profiles who can benefit from them. We would also discuss barriers to pneumococcal vaccination in the hemato-oncology clinical setting and the recommended approach for administering pneumococcal vaccination to patients with HMs.
2 Methodology
2.1 Panel selection
A panel comprising six experts (mean age: 52.17 years; specialty: hematology) was formed based on their academic record, clinical research involvement, and experience in managing hematological diseases from all four zones of India (North, South, East, and West). The experts were required to have at least 10 years of clinical expertise in the field. A chair was identified among the panel to moderate the consensus process (Figure 2).
Figure 2
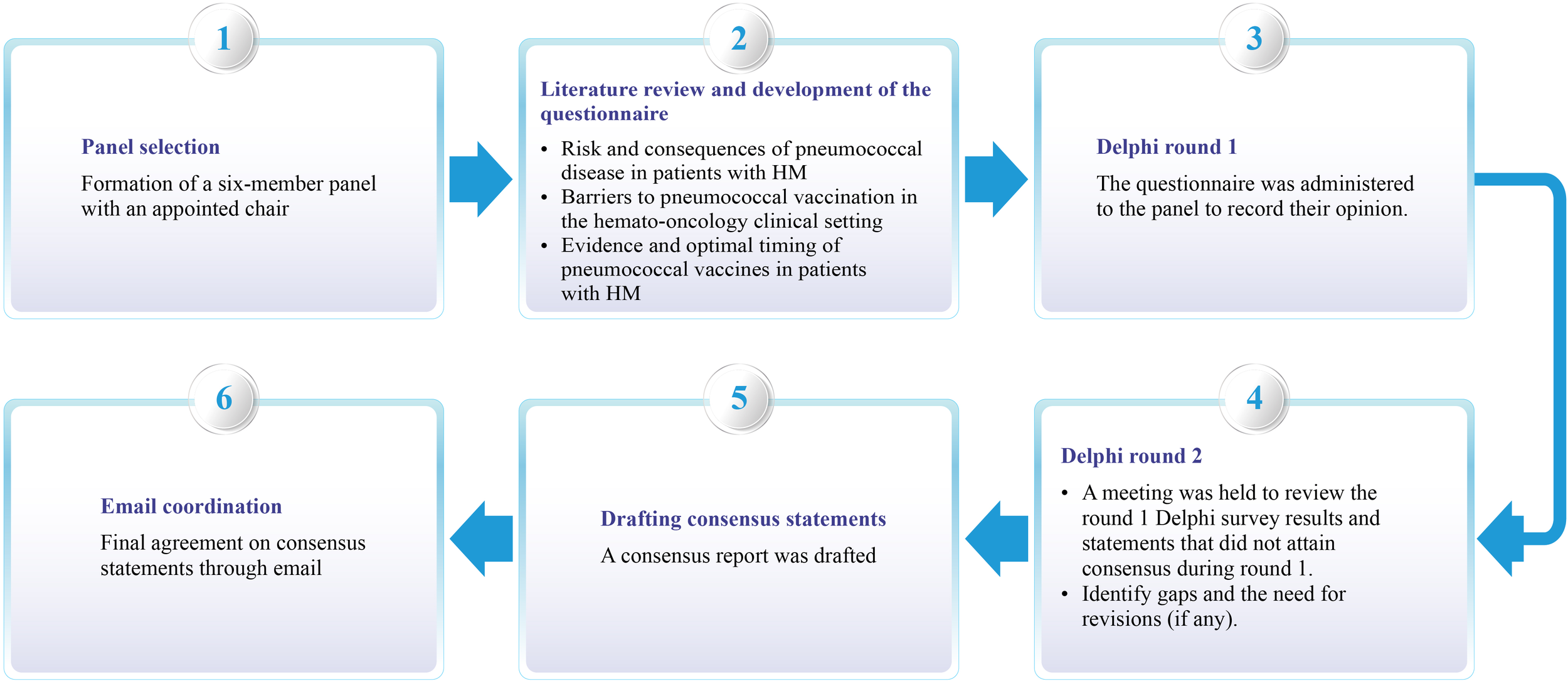
Process overview used to create the clinical consensus statement. HM, Hematological malignancy.
2.2 Literature review and questionnaire development
A comprehensive literature search was conducted on the PubMed/MEDLINE database to identify pertinent articles published from January 1970 to March 2024. Diverse keyword combinations, including “burden”, “risk”,” hematologic malignancies”, “lymphoma”, “leukemia”, “multiple myeloma”, “immunocompromising”, “pneumococcal infections”, “pneumococcal pneumonia”, “invasive pneumococcal disease”, “pneumococcal conjugate vaccine”, “pneumococcal vaccine”, “efficacy”, “safety”, “pediatric”, “adult”, “prevention”, “guidelines”, and “management” were utilized. Variations in search phrases were applied, and Boolean operators (AND, OR) were used. The included sources comprised original research articles (RCTs, longitudinal studies, prospective and retrospective cohort studies, observational studies, case-control studies, and cross-sectional studies), systematic reviews, meta-analyses, practice guidelines, consensus recommendations, reviews, and surveys. Excluded sources were research studies involving animals or those published in a language other than English. Replicates were eliminated in the course of the filtering process. The questionnaire included relevant questions/statements under the following categories: (i) the risk and consequences of pneumococcal disease in patients with HM; (ii) barriers to pneumococcal vaccination in the hemato-oncology clinical setting; and (iii) evidence and optimal timing of PV in patients with HM. The questionnaire was finalized after discussions with the chair. Key articles were shortlisted and shared with the participants before the survey. The questionnaire was then rolled out through an electronic survey link to all the participants through an online survey platform (Delphi round 1).
2.3 Consensus process
A modified Delphi consensus method was used to achieve consensus (10). The level of consensus (Table 1A) was classified into three categories: (i) high (≥80%), moderate (60%–79%), and low consensus (<60%) (11). The expert participants discussed the survey results in the virtual meeting (Delphi round 2) on 9 March 2024. During the meeting, the experts discussed the questions receiving near or no consensus, discussed any differences in opinions, and modified the statements accordingly. Experts arrived at decisions based on available evidence and their current clinical practice. Table 1B lists the grades of recommendation (GOR) used during electronic voting (12). The consensus statements and recommendations were circulated to the experts for review. Another round of basic literature search was performed in PubMed/MEDLINE in July 2024 to check for any new updates. In the first week of July 2024, the final draft of the consensus statements and recommendations was circulated to the experts for their final review and approval.
Table 1A
| Level of consensus | |
|---|---|
| “High” | “When ≥80% of participants agree/strongly agree or disagree/strongly disagree with a statement”. |
| “Moderate” | “When 60%–79% of participants agree/strongly agree or disagree/strongly disagree with a statement”. |
| “Low” | “When <60% of participants agree/strongly agree or disagree/strongly disagree with a statement”. |
Level of consensus (11).
Adapted from: Jünger S et al., 2012 (11).
Table 1B
| Grade of recommendation | |
|---|---|
| ++ | “This investigation or therapeutic intervention is highly beneficial for patients, can be recommended without restriction and should be performed”. |
| + | “This investigation or therapeutic intervention is of limited benefit to patients and can be performed”. |
| +/− | “This investigation or therapeutic intervention has not shown benefit for patients and may be performed only in individual cases. According to current knowledge, a general recommendation cannot be given”. |
| − | “This investigation or therapeutic intervention can be of disadvantage to patients and might not be performed”. |
| −− | “This investigation or therapeutic intervention is of clear disadvantage to patients and should be avoided or omitted in any case”. |
Grades of recommendation (12).
Adapted from: Scharl A et al., 2013 (12).
3 Results
3.1 Risk and consequences of pneumococcal infections in patients with HMs
Patients with HMs and lymphoid malignancies are at an increased risk for IPD compared with the general population (2–4, 13). Streptococcus pneumoniae is the predominant cause of IPD and community-acquired pneumonia (CAP) in Indian adolescents and adults (14, 15). Studies report that the case fatality rate (CFR) for IPD in India ranges from 17.8% to 30% (15, 16). A population-based surveillance study (1995–2012) for IPD conducted in Canada reported CFRs of 11.8% and 22.4% in patients with IPD and HMs aged 15–64 years and ≥65 years, respectively (17). In a population-based cohort study conducted in the Netherlands from 2004 to 2016, the IPD incidence rate was 482 of 100,000 in adults with HMs vs. 15 of 100,000 without a malignancy (3). The hospital intensive care unit admission rate was 15.1% in patients with HMs and IPD. The IPD-related CFR was higher in patients with HMs vs. those without malignancies, and an increase in IPD-related CFR with age was noted (3). Another population-based cohort study (2000–2016) by Andersen MA et al. reported similar findings, with IPD incidence rates of 421.1 per 100,000 person-years in individuals with HMs vs. 12.7 per 100,000 person-years in those without HMs in Denmark (2). The study highlighted that the IPD incidence rate was highest for individuals with MM, ALL, and CLL. Incidence rates were lowest for Hodgkin lymphoma (2). Patients with chronic myeloid leukemia (CML) are usually not considered at high risk of infection unless they develop neutropenia or acute transformation. Studies mention that CML patients have an increased risk of respiratory and skin infections (pneumonia, sinusitis, bronchitis, and cellulitis) vs. healthy individuals (18, 19).
In patients with HMs, pneumococcal disease is associated with complications resulting in hospitalizations and other morbidities (1, 3–5, 7, 17, 20). Complications of pneumococcal pneumonia include bacteremia, empyema, pericarditis, and endobronchial obstruction, with atelectasis and lung abscess formation (1). Furthermore, pneumococcal bacteremia can occur with or without pneumonia, leading to arthritis, meningitis, and endocarditis (1). Chen CL et al. found that pneumonia was the primary reason for admission to the intensive care unit (ICU) among patients with HMs, accounting for 45.1% of cases, followed by septic shock (25.8%) (20). Moreover, patients with HMs in the ICU face high mortality rates, often predicted by the need for mechanical ventilation and vasopressor therapy (20–22). The vulnerability of this population makes these infections particularly significant. Early identification and prompt management of pneumococcal infections is crucial for reducing morbidity and mortality in this high-risk patient subgroup.
3.1.1 Expert opinions
Pneumonia with or without bacteremia and bacteremia without foci of infection are the most frequently reported clinical presentations of pneumococcal infections in Indian patients with HMs. On the other hand, meningitis, acute otitis media, and sinusitis are less commonly reported. Experts agreed that in patients with HMs, a majority of pneumococcal infections do not resolve in less than 2 weeks (100%), require prolonged treatment (83.3%), carry a high risk of complications (83.3%), and pose a significant mortality risk (100%). Four out of six (66.7%) experts concurred that most patients with HMs at their institutions required emergency hospitalization to manage IPD. Experts mentioned that 15%–30% of their patients with HMs required admission to intensive care units. Among Indian patients with HMs, a high risk of pneumococcal disease has been observed in patients with MM, acute myeloid leukemia (AML), ALL, and CLL. On the other hand, the risk is lower in patients with CML, Hodgkin, and non-Hodgkin lymphoma (NHL).
3.2 Evidence and optimal timing of pneumococcal vaccinations in patients with HMs
Studies suggest that the introduction of routine childhood pneumococcal vaccination programs may have an indirect effect on IPD incidence rates in patients with HMs (2, 3, 17, 23). Two population-based cohort studies showed a 9% annual decrease in IPD incidence among adult patients with HMs coinciding with the introduction of PCV7 and PCV13 and a significant 35% decline following the introduction of PCV7 and PCV10 (2, 3). A retrospective, longitudinal cohort study showed a significant 64% decline in the incidence of IPD after vaccination with PCV7 and a nonsignificant decline of 46% in the same with PCV13 vaccination in patients with HMs (23). Shigayeva A, et al. reported a significant decline in the incidence of IPD in immunocompromised adults after the introduction of pneumococcal polysaccharide vaccine 23 (PPSV23) and PCV7 vaccination programs (incidence rate ratio=0.57) (17). Routine pneumococcal vaccination as part of infection prophylaxis may offer protection against adverse outcomes in patients with HMs. Results from the INSIGHT MM study in adult patients with newly diagnosed (ND) or relapsed/refractory MM showed that pneumococcal vaccination in the prior 5 years vs. no vaccination affected overall survival (p<0.0001) (7). The proportion of infection-related deaths was significantly lower among vaccinated vs. unvaccinated patients (9.9% vs. 18.0%; p=0.032) (7). A retrospective study by Draliuk R et al, highlighted that adult patients with HMs vaccinated with PCV13 before initiating immunosuppressive therapy had significantly reduced odds of hospitalization due to pneumonia or sepsis vs. those not vaccinated (p=0.012) (5). The ability of conjugated PVs to prime for booster responses was shown in a study evaluating the effects of priming with PCV7 on the antibody responses to PPSV23 in previously treated patients with Hodgkin disease (HD) (24). Patients receiving PCV7 followed by PPSV23 showed significantly higher averaged antibody geometric mean concentrations across the six common serotypes vs. those receiving only PPSV23 (12.5 vs. 7.76 µg/mL; p=0.015). These findings supported that prior PCV immunization effectively primes for later responses to PPSV23 and may reduce the number of vaccine failures (24).
3.2.1 Pneumococcal vaccinations in patients with cancer, including HMs
Two PCV7 doses elicited protective antibody titers in 86%–100% of pediatric patients with cancer who had discontinued chemotherapy (ChT) 3–12 months before vaccination or were on maintenance ChT for ALL (25). Similarly, single-dose PCV13 elicited protective antibody titers for most serotypes in ≥70% of children with cancer who were either receiving or within 12 months of completing immunosuppressive therapy (26). Single-dose PCV13 administered to pediatric patients with cancer or three PCV13 doses ~4 weeks apart given to adult patients elicited elevated pneumococcal immunoglobulin G (IgG) and opsonophagocytic activities (OPAs) that showed persistent protection for 6 months (27). Across the studies, minimal or no serious adverse events (SAEs) were noted (25–27).
3.2.2 Pneumococcal vaccinations in patients with MM
PPSV23 elicited protective antibodies in 40% of patients with MM (28). In a retrospective study by Hinge M, PPSV23 given before autologous stem cell transplantation produced responses in 33% of patients with MM, with better responses in those with good disease control (Table 2) (29–34). There were no reports of serious adverse reactions to vaccination (28, 29). Mustafa SS et al. reported that patients with MM showed similar initial response rates to PCV13 vs. controls but with a significant drop in response over time (30). Similarly, in a study largely including ND patients who received PCV13 followed by PPSV23, 85% showed responses to ≥1 antibody subtype, but there was a decrease in antibody concentrations over time (31). In patients with relapsed myeloma, two PCV7 doses given concomitantly with lenalidomide produced better responses vs. giving one dose before starting lenalidomide and the second dose while on lenalidomide (32). In a retrospective study by Palazzo M et al., a three-dose PCV13 regimen initiated at ~1 year after hematopoietic stem cell transplantation (HSCT) showed good response rates (58%), not affected by lenalidomide maintenance, in patients with MM (33). In patients with MM receiving novel agents (bortezomib, lenalidomide, ixazomib), a three-dose PCV13 regimen was associated with a 33.3% absolute risk reduction in pneumonia compared with no vaccination (34). There were no vaccine-related adverse effects (33, 34).
Table 2
| Author and year | Study design and patient population | Type of PV administered | Key results |
|---|---|---|---|
| Hinge M et al., 2012 (29) | Retrospective study Patients with MM (N=60) | A single dose of PPSV23 before autologous SCT | • Responses in 33% of patients • Significant association was noted between response and disease stage (p=0.01): ᠅ CR stage: 73% responded ᠅ PR stage: 25% responded ᠅ MR stage: 17% responded ᠅ NR stage: None responded • No serious adverse reaction to the vaccine |
| Mustafa SS et al., 2019 (30) | Prospective cohort study Patients with MM (N=7) Normal controls (N=18) | PCV13 42.9% of patients were receiving ChT during the study | • Immediate response: No difference between MM patients vs. controls • Durability of response at 6 months: 1 of 3 patients with MM vs. 7 of 7 controls (p=0.02) |
| Renaud L et al., 2019 (31) | Prospective study Patients with MM (N=28), of whom 25 were ND, 2 at first relapse, and 1 at second relapse | PCV13 and then PPSV23 Patients were allowed to receive induction ChT in between the two vaccinations. | • Response to ≥1 antibody subtype in 85% of patients, ≥2 subtypes in 65%, ≥3 subtypes in 55%, and all four subtypes in two patients • Decrease in the antibody GMC over time for all subtypes |
| Noonan K et al., 2012 (32) | Early phase, open-label, two-cohort study Patients with relapsed myeloma following 1–3 prior therapies, but lenalidomide-naïve (N=22) | Cohort A: First PCV7 dose 2 weeks before initiating lenalidomide. Second PCV7 dose during lenalidomide treatment (n=11) Cohort B: Both PCV7 doses during lenalidomide treatment (n=11) | • Cohort A: Stable or decreased antibody titers • Cohort B: Continuous increase in antibody titers • PCV-specific T-cell responses are greater in Cohort B than in Cohort A |
| Palazzo M et al., 2018 (33) | Retrospective Patients with MM on lenalidomide maintenance after autologous HSCT (N=119) | 3Ds of PCV13 at 1–3-month intervals starting at ~1 year after HSCT | Response to PCV13 series: • Response is shown in 58% of patients • No difference in response rates for those receiving vs. not receiving lenalidomide maintenance Safety: • No vaccine-related adverse effects |
| Stoma I et al., 2020 (34) | Prospective, non blinded, randomized study Patients with MM receiving novel agents (N=36): Vaccinated group (n=18) and unvaccinated matched controls (n=18) | 3Ds of PCV13 with a minimum of 1-month interval between treatment courses with novel agents | • Confirmed pneumonia (vaccinated group: 16.7%; unvaccinated group: 50%; p=0.037) • 33.3% absolute risk reduction of pneumonia in patients receiving PCV13 • No fatal outcomes were associated with pneumonia • No adverse effects of vaccination |
3Ds, Three doses; ChT, Chemotherapy; CR, Complete response; GMC, Geometric mean concentration; HSCT, Hematopoietic stem cell transplantation; MM, Multiple myeloma; MR, Minimal response; ND, Newly diagnosed; NR, No response; PCV, Pneumococcal conjugate vaccine; PPSV, Pneumococcal polysaccharide vaccine; PR, Partial response; PV, Pneumococcal vaccine; SCT, Stem cell transplantation.
3.2.3 Pneumococcal vaccinations in ALL and AML
In children with ALL, PCV13 during maintenance ChT elicited suboptimal responses, whereas vaccination at either 4 weeks or 6 months after maintenance elicited comparable protective immunity (35). Dorval S reported that PCV13 administered during maintenance ChT conferred modest seroprotection at the end of ChT. However, a dose after ChT was necessary and sufficient to attain high seroprotection (to S. pneumoniae) (36). Top et al. showed that PCV13 given 4–12 months after ChT elicited good seroprotective IgG levels (37). There were limited or no reports of vaccine-related SAEs (Supplementary Table S1) (35–37). There is a lack of robust evidence for the immune responses to pneumococcal vaccinations in exclusive AML patient populations. Patel SR et al. showed that children with AML or ALL did not have protective antibody levels against all PCV7 and additional PCV13 serotypes at 6 months after ChT (38). The results suggested that patients with AML may benefit from PCV13 vaccination at ~6 months after completing ChT (38).
3.2.4 Pneumococcal vaccinations in patients with CLL and CML
Suboptimal antibody responses to PPSV23 were noted in patients with CLL (39–41). However, responses were better in patients with a less advanced disease stage (Table 3) (39, 42–46). Single-dose PCV7 elicited lower antibody responses in patients with CLL vs. controls. However, significant responses were noted in ~40% of patients if the vaccine had been given at an early disease stage (42). A follow-up study showed persistent antibody responses to PCV7 for at least 5 years, with protective antibody levels for most serotypes in >50% of patients (47). Single-dose PCV13 induced an adequate response in 58% of treatment-naïve patients with CLL. Responders had lower clinical stages of CLL (43). In a prospective study by Mauro FR et al., single-dose PCV13 elicited adequate responses in nine patients (8%), of whom eight were treatment-naïve (44). No responses to PCV13 were noted in four patients receiving ibrutinib (45). PCV13 elicited a better immune response vs. PPSV23 at 1 and 6 months after vaccination in treatment-naïve patients (45). Svensson T et al. highlighted a longer disease burden was a negative predictor of vaccination response and PCV13 should be administered as early as possible in treatment naïve patients with CLL (46). Across studies, no serious PV-related side effects were noted in patients with CLL (39, 43, 44, 46). Patients with CML receiving tyrosine kinase inhibitors showed suboptimal immunoglobulin M humoral response to PPSV23 compared with healthy controls (48).
Table 3
| Author and year | Study design and patient population | Type of PV administered | Key results |
|---|---|---|---|
| Hartkamp A et al., 2001 (39) | Patients with CLL (N=25) | PPSV23 | Antibody responses: • Protective antibody levels in 50% of patients • Characteristics of responders: Less advanced disease stage, ChT-naïve, higher gamma globulin, total IgG, IgG2, and IgG4, and lower-soluble CD23 Safety: • No major local or systemic reactions |
| Sinisalo M et al., 2007 (42) | Prospective trial Patients with CLL (N=52) Matched controls without any immunological or hematological defect (N=25) | Single dose of PCV7 | Antibody responses: • A significant response in 20%–47% of patients with CLL vs. 75%–88% of controls • Significant response to ≥6 antigens in 39% of patients if the vaccine had been given at an early disease stage |
| Pasiarski M et al., 2014 (43) | Treatment-naïve patients with CLL (N=24) Healthy controls (N=15) | A single dose of PCV13 | Antibody responses: • Adequate response in 58.3% of patients vs. 100% of controls • Characteristics of responders: Lower CLL clinical stage, higher total IgG and IgG2 Safety: • No major side effects related to vaccination |
| Mauro FR et al., 2021 (44) | Prospective study Patients with CLL (N=112) who were either treatment-naïve (n=22) or previously treated (n=90) | Single dose of PCV13 | Immune response: • Adequate immune response in nine patients (8%), of whom eight were treatment-naïve • Factors associated with lower responses: Age ≥60 years, baseline IgG <400 mg/dL, prior treatment, and signs of disease progression Safety: • Vaccine was well tolerated |
| Andrick B et al., 2018 (45) | Prospective, nonblinded study Patients with CLL | Single dose of PCV13 in two study cohorts: • No active treatment for CLL (control cohort) • Active treatment with ibrutinib | Adequate response in all controls vs. none of the patients on ibrutinib (p=0.029) |
| Svensson T et al., 2018 (46) | Randomized, nonblinded trial Treatment-naïve patients with CLL (N=128) | PCV13 (n=63) or PPSV23 (n=65) | Immune response: • Positive immunological response in more PCV13 than in PPSV23 recipients after 1 month (p=0.034) and after 6 months (p=0.041) Safety: • No vaccine-related SAEs |
CLL, Chronic lymphocytic leukemia; ChT, Chemotherapy; IgG, Immunoglobulin G; PCV, Pneumococcal conjugate vaccine; PPSV, Pneumococcal polysaccharide vaccine; PV, Pneumococcal vaccine; SAE, Serious adverse event.
3.2.5 Pneumococcal vaccinations in patients with lymphoma
Splenectomized patients with HD or NHL showed good antibody responses to PPSV administered before splenectomy and treatment; however, nonresponder patients with NHL did not benefit from revaccination (49, 50). Significant responses to primary PPSV23 vaccination and two revaccinations were noted in splenectomized patients with HD (51). Cherif H et al. reported poor responses to PPSV23 in 28% of splenectomized patients with hematological disorders who did not benefit from revaccination (52). Overall, no severe adverse reactions to PPSV23 were noted (Supplementary Table S2) (49–54).
Previously treated patients with HD showed suboptimal responses to PCV7 vs. healthy controls or those receiving PPSV23 (53). Lee D et al. found that PCV13 given at 3 or 6 months after CD19-targeted chimeric antigen receptor-modified T cell therapy (CAR-T) did not induce humoral protection in patients with relapsed/refractory large B-cell lymphoma (54).
Table 4 lists the optimal timing of pneumococcal vaccinations in patients with HMs and patients undergoing splenectomy (19, 55–65).
Table 4
| A) Cancer, Including HMs | |
|---|---|
| NCCN (55) | “PCV20 should be administered to adults who are ND with cancer and who are PV naïve. Alternatively, PCV15 can be given, followed by PPSV23 ≥8WL.” |
| ASCO (56) | “1D of PCV15 followed by PPSV23 8WL (in adults) or 1D of PCV20a (in adults)” |
| CDC (57, 58) | Children aged 2–5 years with HMs, asplenia: • “Three prior PCV doses: 1D of PCV (≥8 weeks after the most recent dose)” • “<3 prior PCV doses: 2Ds of PCV (≥8 weeks after the most recent dose and given ≥8 weeks apart)” • “Not previously received PCV20: 1D of PCV20 or 1D of PPSV23 ≥8 weeks after the most recent PCV dose” Children aged 6–18 years with HMs, asplenia: • “No prior PCV or PPSV23: 1D of PCV15 or PCV20. If received PCV15 dose, then 1D of PPSV23 ≥8WL” • “Received PCV before the age of 6 years but not PPSV23: Not previously received PCV20, then administer 1D of PCV20 or PPSV23 dose 1 ≥8 weeks after PCV and then a second dose of PPSV23” • “Received PCV13 only at or after the age of 6 years: Administer 1D of PCV20 or PPSV23 dose 1 ≥8 weeks after PCV13 and then a second dose of PPSV23” “Note: Second dose of PPSV23 to be administered ≥5 years after PPSV23 dose 1” Adults 19–49 years: • “Not previously received a PCV13, PCV15, PCV20, or PCV21 or whose previous vaccination history is unknown: 1D of PCV21 or 1D of PCV20 or 1D of PCV15 followed by 1D of PPSV23 ≥8WL” • “Received only PCV13: 1D of PCV21 or 1D of PCV20 at least 1 year later” Adults ≥50 years old: • “Not previously received a PCV13, PCV15, PCV20, or PCV21 or whose previous vaccination history is unknown: 1D of PCV21 or 1D of PCV20 or 1D of PCV15 followed by 1D of PPSV23 ≥8WL” • “Received only PCV13: 1D of PCV21 or 1D of PCV20 at least 1 year later” |
| ICS–NCCP (59) | In adults with immunocompromising conditionsb: • “19–64 years of age: 1D of PCV13 and PPSV23 ≥8WL” • “≥65 years of age: PCV13 first, followed by PPSV23 is recommended for individuals with immunocompromising conditions, functional or anatomic asplenia, or a history of IPD” |
| IMA (60) | In patients with cancer: • “PCV: 1D at not less than 3 months after cancer ChT” • “PPSV23: 1D ≥8 weeks after PCV” |
| IAP–ACVIP (61) | Children aged 24 through 71 months in high-risk conditions: • “Three prior PCV13 doses: 1D of PCV13 and 1D of PPSV23 after 8 weeks” • “<3 prior PCV13 doses: 2Ds of PCV13 ≥8 weeks apart, and PPSV23 ≥8WL” |
| ECIL (19) | • AML: “Administer PVs 3–6 months after ChT” • CML: “1D of PCV followed 2 months later by 1D of PPSV23” • MM: “1D of PCV13 followed by 1D of PPSV23 ≥8WL, preferably before treatment or during maintenance” • Lymphoma: “1D of PCV13 then 1D of PPSV23 ≥8WL, preferably before treatment or during maintenance, except in patients who are receiving high-dose ChT or who are receiving or have received anti-CD20 antibodies in the previous 6 months” • CLL: “1D of PCV13 followed by 1D of PPSV23 ≥8WL preferably before treatment” • Children with ALL: ◦ “During maintenance” ◦ “From ≥3 but preferably 6 months after ChT” |
| GSI (62) | “Recommends both PCV13 and PPSV23 for all individuals older than 50 years and immunocompromised individuals with a high risk for pneumococcal infections”. • “Consider PCV13 for elderly individuals who have previously received PPSV23” • “If previously unvaccinated, 1D of PCV13 then 1D of PPSV23 ≥8WL”. |
| IDSA (63) | • “PCV13 should be administered to ND adults with hematological or solid malignancies and children with malignancies. PPSV23 should be administered to adults and children (aged ≥2 years) ≥8 weeks after the indicated dose(s) of PCV13”. • “Vaccination timing after therapy (in patients with cancer): ≥3 months after cancer ChT and ≥6 months after regimens that include anti–B-cell antibodies”. |
| B) Patients Undergoing Splenectomy | |
| IAP (64) | “≥2 weeks before splenectomy” |
| AGIHO (65) | “Vaccinate 2 weeks before splenectomy at the latest or 14 days after surgeryc” “PCV13 first and then PPSV23 6–12 weeks later, revaccinate every 6 years” |
| IDSA (63) | “PPSV23d ≥2 weeks before surgery (and following indicated dose[s] of PCV13) or ≥2 weeks after surgery” |
Recommendations for the administration of pneumococcal vaccinations in patients with cancer, including HMs, (A) and patients undergoing splenectomy (B) (19, 55–65).
Patients who have previously received PCV13 can only receive 1D of PCV20 after 1 year.
Include HMs, asplenia, or patients undergoing HSCT.
If preoperative vaccination is not possible.
For PPSV23-naïve patients aged ≥2 years for whom splenectomy is planned.
1D, One dose; 2D, Two doses; AGIHO, Infectious Diseases Working Party of the German Society for Hematology and Medical Oncology; ALL, Acute lymphoblastic leukemia; AML, Acute myeloid leukemia; ASCO, American Society of Clinical Oncology; CDC, Centers for Disease Control and Prevention; ChT, Chemotherapy; CLL, Chronic lymphocytic leukemia; CML, Chronic myeloid leukemia; ECIL, European Conference on Infections in Leukemia; 8WL, Eight weeks later; GSI, Geriatric Society of India; HM, Hematological malignancy; IAP, Indian Academy of Pediatrics; ICS-NCCP, Indian Chest Society and the National College of Chest Physicians of India; IDSA, Infectious Diseases Society of America; IMA, Indian Medical Association; NCCN, National Comprehensive Cancer Network; ND, Newly diagnosed; PCV, Pneumococcal conjugate vaccine; PPSV, Pneumococcal polysaccharide vaccine.
3.2.6 Pneumococcal vaccinations in patients undergoing HSCT
Single-dose PPSV23 administered at a median of 756 days after allogeneic HSCT (allo-HSCT) showed positive OPA response rates of 55% at 1 year after vaccination in patients aged 20–70 years (Table 5) (66–79). In a retrospective study by Pao M, a three-dose PCV7 series from ~1 year after allo-HSCT showed response rates of 62%, with higher responses in children vs. adults (67). In pediatric patients, a three-dose PCV7 regimen starting 6–9 months after allo-HSCT elicited complete protection in 74% of patients (68). In the EBMT-IDWP01 trial, a three-dose PCV7 regimen starting at either 3 (early group) or 9 months (late group) after allo-HSCT elicited similar responses between the early and late groups, with a significant correlation between IgG and OPA titers for all PCV7 serotypes (80–82). In this trial, a dose of PPSV23 at either 12 or 18 months after HSCT improved the responses to PCV7 antigens, but this boost effect was higher in the late group vs. the early group. Regardless of the timing, the PPSV23 dose also broadened the pneumococcal serotype coverage (80–82). A 10-year follow-up of this trial revealed that antibody levels were well maintained at 8–11 years after transplant (69). Molrine DC et al. reported that among allo-HSCT recipients receiving PCV7 at 3, 6, and 12 months after transplant, those patients whose donors had received PCV7 before HSCT had improved antibody responses within the first year of HSCT (70). By 13 months, >60% of patients had protective antibody concentrations regardless of donor immunization (70). Similar findings were reported among autologous HSCT recipients receiving single-dose PCV7 before HSCT followed by the same three-dose PCV7 regimen after transplant (71). Kumar D et al., reported that in adult allo-HSCT recipients, donor immunization with PCV7 followed by recipient immunization at 6 months after HSCT showed greater responses vs. a similar strategy with PPSV23 (90.9% vs. 55.6%) (72).
Table 5
| Author and year | Study design and patient population | Type of PV administered | Key results |
|---|---|---|---|
| Okinaka K et al., 2017 (66) | Prospective, open-label, single-arm study Allo-HSCT recipients aged 20–70 years with HM (N=30) | Single dose of PPSV23 (median time from allo-HSCT to vaccination was 756 days) | Median positive response rates after PPSV23 • By IgG: 43% at 1 month and 43% at 1 year • By OPA: 72% at 1 month and 55% at 1 year Safety • No severe adverse effects due to PPSV23 |
| van der Velden AM et al., 2007 (77) | Prospective follow-up study Adult patients with NHL, MM, or amyloidosis who underwent autologous SCT (N=20) | Two PCV7 doses followed by a PPSV23 dose at 6, 8, and 14 months after transplant | Responses after the two PCV7 doses • Sufficient antibody responses in 33% of patients Response after the PPSV23 dose • Increased responders to conjugate serotypes (78%) Safety • No major adverse reactions after vaccination |
| Pao M et al., 2008 (67) | Retrospective study Pediatric and adult patients after an allo-HSCT (N=127) | Series of three PCV7 doses 4–8 weeks apart Median time to vaccination: ~1 year after HSCT | Vaccine response • Responses to PCV7 in 62% of patients (children vs. adults: 88% vs. 44%; p<0.001) Safety • No serious adverse reactions to vaccination |
| Meisel R et al., 2007 (68) | Prospective multicenter trial Pediatric patients up to 16 years of age recruited following allo-HSCT (N=53) | Three PCV7 doses in monthly intervals starting at 6–9 months after HSCT | Vaccine responses • Complete protection against all seven serotypes: 55.8% of patients after the second dose and 74.4% after the third dose Safety • Four SAEs were observed; none related to the vaccine |
| Cordonnier C et al., 2015 (69) | EBMT-IDWP01 trial (follow-up) | Three PCV7 doses given a month apart started at either ~3 months (early group) or ~9 months (late group) after HSCT A dose of PPSV23 at 12 months (early group) or 18 months (late group) after HSCT | Response rates of 40% (antibody cutoff of 0.50 μg/mL) vs. 65.5% (antibody cutoff of 0.15 μg/mL) for seven antigens of PCV7 |
| Molrine DC et al., 2003 (70) | Randomized controlled study Patients aged ≥2 years scheduled to receive allo-HSCT for HM (N=96) | Immunized donor group: Patients and donors received a dose of PCV7 at ~7–10 days before HSCT Unimmunized donor group: Patients and donors did not receive PCV7 before HSCT All study patients: PCV7 at 3, 6, and 12 months after HSCT | Antibody responses after HSCT • More patients with protective IgG levels in the immunized vs. unimmunized donor groups after the first PCV7 dose (67% vs. 36%; p=0.05) • Protective IgG levels after the third PCV7 dose in >60% of patients in both groups Safety • PCV7 was well tolerated |
| Antin JH et al., 2005 (71) | Prospective randomized study Patients >2 years old with an HM and scheduled to receive autologous HSCT (N=61) | PCV7 were given 7–10 days before stem cell collection or no vaccination Participants were given PCV7 at 3, 6, and 12 months after the transplant | Antibody responses after HSCT • Higher antibody concentrations in patients given PCV7 before HSCT • At 13 months, >60% of patients had protective concentrations regardless of preharvest vaccination Safety • No SAE related to immunization |
| Kumar D et al., 2007 (72) | Randomized, double-blind, controlled trial Adult patients undergoing allo-HSCT and their donors (N=64 donor–recipient pairs) | Strategy 1 (n=32): PCV7 in donors at least 2 weeks before stem cell collection; PCV7 in recipients at 6 months after HSCT Strategy 2 (n=32): PPSV23 in donors at least 2 weeks before stem cell collection; PPSV23 for the recipients at 6 months after HSCT | Response to ≥1 serotype after HSCT • At 12 months (after recipient vaccination): 90.9% in the PCV7 group vs. 55.6% in the PPSV23 group (p=0.02) Infections • No IPD during the study period Safety • Vaccines were well tolerated |
| Langedijk AC et al., 2019 (73) | Patients receiving immunization after allo-HSCT (N=103) | Starting at 1 year after HSCT, three PCV13 doses followed by a single PPSV23 dose | PCV13 serotype-specific antibody responses (n=39) • Sufficient seroprotection in 85% of patients across all PCV13 serotypes |
| Cordonnier C et al., 2015 (74) | Prospective, multicenter, open-label study Patients aged ≥2 years with hematological disorders who had undergone allo-HSCT (N=251) | Starting at ~3–6 months after HSCT, three doses of PCV13 at 1-month intervals, a fourth dose 6 months later, and a dose of PPSV23 1 month later | Immunogenicity: Significant increases in GMFRs of IgG GMCs across all PCV13 serotypes from baseline to post-dose 3 (GMFR: 2.99–23.85) |
| Garcia Garrido HM et al., 2022 (75) | Prospective, multicenter cohort study Adult allo-HSCT recipients (N=89) | Starting at 4–6 months after HSCT, four doses of PCV13 (at T0, T1, T2, T8*) and a dose of PPSV23 (at T10*) *T refers to months from enrollment. | Seroprotection rates at 12 months • PCV13/PPSV23 combined serotypes (33%), PCV13 serotypes (67%), and PPSV23-exclusive serotypes (19.2%) • Treatment with immunosuppressive agents at baseline was associated with less overall seroprotection (OR=0.36) and protection against PCV13 serotypes (OR=0.33) Antibody concentrations over time • Significant increase in IgG concentrations over time for all 24 serotypes Safety • A total of 14 SAEs in 12 patients; none were vaccine-related • No IPD episodes during the study period |
| Okinaka K et al., 2023 (76) | Phase 2, multicenter, open-label, randomized controlled trial Patients aged ≥2 years with HM who had undergone allo-HSCT and did not have active GVHD (N=72) | 3+1+1 group: four doses of PCV13 at 0, 1, 2, and 8 months and 1D of PPSV23 at 9 months 3+0+1 group: three doses of PCV13 at 0, 1, and 2 months and 1D of PPSV23 at 9 months | Response rate: • The overall IgG response rate at 5 months after PPSV23 did not differ between the two groups (100% vs. 93%, RR=1.07) Safety • No SAEs leading to study dropout • No IPD cases during follow-up |
| Robin C et al., 2020 (78) | Adult allo-HSCT recipients assessed at a median of 9.3 years after transplant (N=100) | PCV** • All patients received ≥1 dose • 86% of patients received ≥3 doses, of whom 11 patients received four doses PPSV23** • 94% of patients received ≥1 dose, of whom 57 patients received ≥2 doses **Different vaccination schedules according to the date of transplant. | • No difference in seroprotection rates between the different vaccination schedules |
| Roberts MB et al., 2020 (79) | Retrospective, observational study Adult (age ≥16 years) autologous HSCT and allo-HSCT recipients (800 patients with 842 HSCT events) | Pre-2010 group: PPSV23 at 12 and 24 months after HSCT followed by a 5-yearly PPSV23 booster Post-2010 group: three doses of PCV10 or PCV13 at 6, 8, and 10 months after HSCT and another PCV13 dose at 14 months if GVHD present. All patients received PPSV23 at 24 months | IPD episodes Significant reduction in IPD rates from the pre-2010 to post-2010 group. |
Allo-HSCT, Allogeneic hematopoietic stem cell transplantation; GMC, Geometric mean concentration; GMFR, Geometric mean fold rise; GVHD, Graft-versus-host disease; HM, Hematological malignancy; HSCT, Hematopoietic stem cell transplantation; IgG, Immunoglobulin G; IPD, Invasive pneumococcal disease; MM, Multiple myeloma; NHL, Non-Hodgkin lymphoma; OPA, Opsonophagocytic activity; 1D, One dose; OR, Odds ratio; PCV, Pneumococcal conjugate vaccine; PPSV, Pneumococcal polysaccharide vaccine; RR, Relative risk; SAE, Serious adverse event; SCT, Stem cell transplantation.
A three-dose PCV13 regimen starting from 1 year after allo-HSCT followed by PPSV23 at 18 months yielded response rates of 85% to PCV13 serotypes and 62% to PPSV23-only serotypes. However, ~43% of the IPD cases occurred in the first year after transplantation (73). Following a three-dose PCV13 series starting at 3–6 months after allo-HSCT, a fourth dose given 6 months later led to significantly increased antibody levels (74). A PPSV23 dose 1 month after the fourth PCV13 dose did not further increase responses to PCV13 serotypes (74). A similar four-dose PCV13 regimen starting at 4–6 months after allo-HSCT in adults followed by PPSV23 at 2 months after the fourth dose showed seroprotection rates of 67% for PCV13 serotypes but only 19% for PPSV23-exclusive serotypes (75). However, in allo-HSCT recipients without active graft-versus-host disease at 3–9 months after transplantation, the PCV13 four-dose regimen followed by PPSV23 a month later was not effective compared with a three-dose PCV13 regimen followed by PPSV23 7 months later in terms of overall IgG responses at 5 months after the PPSV23 dose (76). Table 5 lists key findings from various studies on the immune responses and safety of pneumococcal vaccinations in patients undergoing HSCT (66–79). Across the studies, PV was well tolerated in HSCT recipients, with no serious adverse reactions related to the vaccine use (66–68, 71, 76). Table 6 lists the optimal timing of pneumococcal vaccinations after HSCT as per guidelines (55, 60, 63, 65, 83–85).
Table 6
| NCCN (55) | After allogeneic or autologous HSCT: 3–4 doses of pneumococcal vaccination 3–6 months after HSCT • “If PCV20 is used, the first 3Ds are generally administered 1–2 months apart, with the fourth dose administered 6 months after the third dose”. • “If PCV15 is used, 3Ds should be administered, followed by PPSV23 6–12 months after the primary series”. |
| National Guidelines for Hematopoietic Cell Transplantation, ICMR (83) | ††Allogeneic transplant: • PCV13: “12 months after HSCT” • PPSV23: “10 months after PCV13” Autologous transplant: • PCV13: “6 months after HSCT” • PPSV23: “10 months after PCV13” |
| ECIL (84) | After allogeneic or autologous HSCT: • PCV13: “3Ds, at 1-month intervals, 3 months after the transplant” • PPSV23: “1D (≥8 weeks after the last PCV) 12 months after transplant in the absence of chronic GVHD” Note: “If there is chronic GVHD, a fourth dose of PCV at 6 months after the third PCV dose The same schedule for children and adults”. |
| IMA (60) | PCV: “3Ds at 3–6 months after HSCT” PPSV23: “1D at 12 months after HSCT” |
| AGIHO (65) AGIHO (85) | Autologous HSCT: “3Ds of PCV13, each 4–6 weeks apart, starting 3–6 months after transplant, followed by 1D of PPSV23 after ≥8 weeks”. |
| After allogeneic HSCT (3–6 months): “3Ds of PCV13, 4 weeks apart, and PPSV23 1 year after transplant” | |
| IDSA (63) | After HSCT: • PCV13: “3Ds to adults and children 3–6 months after HSCT” • PPSV23: “1D, 12 months after HSCT in the absence of chronic GVHD” |
††To be initiated once the patient is off immunosuppression (1 month) with no signs of GVHD.
1D, One dose; 3Ds, Three doses; AGIHO, Infectious Diseases Working Party of the German Society for Hematology and Medical Oncology; ECIL, European Conference on Infections in Leukemia; GVHD, Graft-versus-host disease; HSCT, Hematopoietic stem cell transplantation; ICMR, Indian Council of Medical Research; IDSA, Infectious Diseases Society of America; IMA, Indian Medical Association; NCCN, National Comprehensive Cancer Network; PCV, Pneumococcal conjugate vaccine; PPSV, Pneumococcal polysaccharide vaccine.
3.2.7 Consensus recommendations
Individuals with HMs are at an increased risk of pneumococcal disease due to underlying malignancy and subsequent immunosuppressive anticancer therapy. The experts unanimously agreed that pneumococcal vaccinations are important for patients with HMs (high consensus). PCVs induce a T-cell–dependent immune response and provide adequate protection against pneumococcal disease in patients with HMs (high consensus). Experts concurred that PCVs have an acceptable safety and tolerability profile in patients with HMs (high consensus). Priming with PCV enhances the response to PPSV23 in patients with HMs. (high consensus). Experts recommended vaccination with PCV first followed by PPSV23 8 weeks later as the optimum strategy to sequence pneumococcal vaccinations in Indian patients with HMs following assessment of their immune status (high consensus). PCV is beneficial and can be strongly recommended in patients with CLL, MM, and patients undergoing HSCT (high consensus for all; GOR: ++ for all). In patients with CLL, the experts recommended administering PCV13 as soon as possible following diagnosis or at least 2 weeks before ChT (high consensus). For patients with MM, PCV13 can be considered in ND patients or at least 2 weeks before ChT (high consensus). Based on moderate consensus, PCV13 may also be administered during maintenance in this patient population. In patients with HM undergoing HSCT, PCV13 can be administered 6–12 months after the transplant (high consensus). For patients undergoing splenectomy, the experts strongly recommend administering PCV13 at least 2 weeks before the planned procedure or 14 days after the surgery if preoperative vaccination is not possible (high consensus).
Children with ALL would benefit from systematic revaccination with PCV after ChT. PCV13 should be administered to pediatric patients with ALL 6 months after the completion of ChT. PCV13 may also be administered during maintenance therapy in this patient population (moderate consensus). There are insufficient data to consistently recommend pneumococcal vaccination to all adult patients with ALL. A case-by-case evaluation and decision on vaccination are necessary. The experts suggested administering PCV13 3–6 months after the end of ChT in this subset of the patient population (high consensus). In patients with AML, there are insufficient data to recommend pneumococcal vaccination. A case-by-case evaluation and decision on vaccination are necessary. Experts suggested that PCV13 can be administered 3–6 months after the end of ChT in patients with AML (moderate consensus). Furthermore, enough data to consistently recommend pneumococcal vaccination to all patients with lymphoma are not available. Due to insufficient data, the experts suggested a case-by-case evaluation and decision on vaccination for this patient population. Experts suggested the administration of PCV13 before treatment in ND patients or at least 2 weeks before ChT (high consensus). PCV13 may also be administered during maintenance in this patient population (moderate consensus).
3.3 Barriers to pneumococcal vaccination in the hemato-oncology clinical setting in India
Globally, barriers to pneumococcal vaccination uptake include concerns about vaccine efficacy, lack of awareness of the disease complications, and vaccination schemes (86, 87). The expert panel identified a lack of awareness about pneumococcal disease, its risk factors or vaccine availability, and lack of recommendation from healthcare practitioners (HCPs) for vaccination as the most common patient-level barriers to pneumococcal vaccination in the hemato-oncology clinical setting in India. At the HCP level, the most common perceived barriers were a lack of awareness about the burden of pneumococcal disease, uncertainty about clinical data on vaccine effectiveness, and a lack of clarity regarding the timing and schedule of pneumococcal vaccination in complex treatment schedules. Educating HCPs about vaccine guidelines and providing patient counseling about the risk of pneumococcal disease in HMs and the importance of receiving early vaccination can play an important role in reducing the risk of serious infections and adverse events among this vulnerable population.
4 Discussion
Pneumococcal diseases are a significant health concern for patients with HMs. Serious pneumococcal infections include pneumonia, meningitis, and febrile bacteremia; however, otitis media and sinusitis are less serious manifestations (88, 89). Two recent systematic reviews reported the effectiveness of pneumococcal vaccination for protection against vaccine type-(PCV13 type or PPSV23 type) IPD and CAP due to S. pneumoniae in adults (90, 91). In addition to reducing the medical burden, pneumococcal vaccination may provide economic benefits by lowering the risk of hospitalization due to its contribution to preventing pneumococcal disease and adverse outcomes (5, 92, 93). In India, PCV was introduced into the pediatric national immunization schedule recently in 2017 (94). Thus, the concept of herd immunity is not applicable in India. Currently, PCV10 and PCV13 are licensed and available in the private sector. PCV13 protects against three additional prevalent serotypes (3, 6A, and 19A) not included in PCV10 (95–97). Considering this, the experts recommended vaccination with PCV13 first, followed by PPSV23, as the optimum strategy to sequence pneumococcal vaccinations in patients with HMs in India. Furthermore, the expert panel strongly recommended PCV13 in CLL, MM, and patients undergoing HSCT. When higher-valency PCV20 and PCV15 become available in India, they will replace the existing PCV13 for adult vaccination. Considering the current evidence of the risk of pneumococcal disease in patients with HMs and the recommendations and consensus for the use of PCV13 and PPSV23 in these patients, it is important to consider how PCV15 and PCV20 can be utilized to extend the benefit of protection against pneumococcal disease in this high-risk patient population in Indian settings.
4.1 Strengths and limitations of consensus process
4.1.1 Strengths
Our modified Delphi study methodology represents a rigorous synthesis of expert opinions. The expert committee was formed without any selection bias. The survey was designed after an in-depth literature review to increase the study’s rigor and ensure efficient responses. Anonymity was maintained during the survey to encourage panel members to provide honest and unbiased feedback. All experts actively participated in the consensus process. The differences in opinions were also discussed during the meeting. The diverse panel helped achieve a broader perspective and generalization of consensus. These evidence-based practical consensus recommendations can guide practicing clinicians and healthcare professionals nationwide in making informed decisions about vaccine sequencing, specific patient profiles that can benefit from pneumococcal vaccination, and optimal timing of vaccination in patients with HMs.
4.1.2 Study limitation
This consensus process did not include any patient opinions or perspectives.
5 Conclusion
Preventing pneumococcal disease is paramount for high-risk individuals, and administering PV should be an important aspect of clinical care. The vaccine type and timing of vaccinations must be carefully chosen to allow for optimal immunization in patients with HMs. Prevention of pneumococcal infections by vaccination has been evaluated and found to be a viable strategy. Priming with PCV enhances the response to PPSV23 in patients with HMs. The experts recommended vaccination with PCV13 first, followed by PPSV23 8 weeks later, as the optimum strategy to sequence pneumococcal vaccinations in patients with HMs in Indian settings. In patients with CLL and MM, the experts recommended administering PCV13 as soon as possible following diagnosis or at least 2 weeks before ChT. In patients with HM undergoing HSCT, PCV13 can be administered 6–12 months after the transplant. PCV13 should be administered to pediatric patients for ALL 6 months after the completion of ChT or during maintenance. For patients undergoing splenectomy, the experts strongly recommend administering PCV13 at least 2 weeks before the planned procedure or 14 days after the surgery if preoperative vaccination is not possible. The current state of evidence is inadequate to consistently recommend pneumococcal vaccination to all patients with lymphoma, AML, and adults with ALL. The decision to administer PCV13 preceding PPSV23 should be taken jointly by the HCP and the patient on a case-by-case basis after carefully discussing the pros and cons.
Statements
Author contributions
TS: Writing – original draft, Writing – review & editing. SM: Writing – original draft, Writing – review & editing. TD: Writing – original draft, Writing – review & editing. JB: Writing – original draft, Writing – review & editing. NS: Writing – original draft, Writing – review & editing. PC: Writing – original draft, Writing – review & editing. CM: Writing – original draft, Writing – review & editing. ST: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank BioQuest Solutions Pvt Ltd for their editorial assistance.
Conflict of interest
Author PC is a consultant to Zoho Corporation. Authors CM and ST are full-time employees of Pfizer India Ltd., and hold stocks at the time of submission.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1546641/full#supplementary-material
References
1
Centers for Disease Control and Prevention. Pneumococcal disease. Available online at: https://www.cdc.gov/vaccines/pubs/pinkbook/pneumo.html (Accessed June 12, 2024).
2
AndersenMANiemannCURostgaardKDalbyTSørrigRWeinbergerDMet al. Differences and temporal changes in risk of invasive pneumococcal disease in adults with hematological Malignancies: results from a nationwide 16-year cohort study. Clin Infect Dis. (2021) 72:463–71. doi: 10.1093/cid/ciaa090
3
Garcia GarridoHMKnolMJHeijmansJVan SorgeNMSandersEAMKlümpenH-Jet al. Invasive pneumococcal disease among adults with hematological and solid organ Malignancies: A population-based cohort study. Int J Infect Dis. (2021) 106:237–45. doi: 10.1016/j.ijid.2021.03.072
4
WongAMarrieTJGargSKellnerJDTyrrellGJthe SPAT Group. Increased risk of invasive pneumococcal disease in haematological and solid-organ Malignancies. Epidemiol Infect. (2010) 138:1804–10. doi: 10.1017/S0950268810000919
5
DraliukRShadmiEPreisMDaganE. Association between PCV13 pneumococcal vaccination and risk of hospital admissions due to pneumonia or sepsis among patients with haematological Malignancies: a single-centre retrospective cohort study in Israel. BMJ Open. (2022) 12:e056986. doi: 10.1136/bmjopen-2021-056986
6
SarkarLGoliVBMenonNPatilVMNoronhaVPrabhashK. Vaccination practices, efficacy, and safety in adults with cancer: A narrative review. Cancer Res Stat Treat. (2021) 4:505–15. doi: 10.4103/crst.crst_156_21
7
ThompsonMABoccadoroMLeleuXVela-OjedaJVan RheeFWeiselKCet al. Rates of influenza and pneumococcal vaccination and correlation with survival in multiple myeloma patients. Clin Lymphoma Myeloma Leuk. (2023) 23:e171–81. doi: 10.1016/j.clml.2022.12.003
8
SabuTMNoronhaVRaoARKumarAGattaniSRamaswamyAet al. Uptake of vaccination in older Indian patients with cancer: A cross-sectional observational study. Cancer Res Stat Treat. (2023) 6:52–61. doi: 10.4103/crst.crst_29_23
9
Abbas RizviASinghA. Vaccination coverage among older adults: a population-based study in India. Bull World Health Organ. (2022) 100:375–84. doi: 10.2471/BLT.21.287390
10
NasaPJainRJunejaD. Delphi methodology in healthcare research: How to decide its appropriateness. World J Methodol. (2021) 11:116–29. doi: 10.5662/wjm.v11.i4.116
11
JüngerSPayneSBrearleySPloenesVRadbruchL. Consensus building in palliative care: A Europe-wide delphi study on common understandings and conceptual differences. J Pain Symptom Manage. (2012) 44:192–205. doi: 10.1016/j.jpainsymman.2011.09.009
12
ScharlAThomssenCHarbeckNMüllerV. AGO recommendations for diagnosis and treatment of patients with early breast cancer: update 2013. Breast Care Basel Switz. (2013) 8:174–80. doi: 10.1159/000353617
13
BackhausEBergSAnderssonROckbornGMalmströmPDahlMet al. Epidemiology of invasive pneumococcal infections: manifestations, incidence and case fatality rate correlated to age, gender and risk factors. BMC Infect Dis. (2016) 16:367. doi: 10.1186/s12879-016-1648-2
14
GhiaCJDharRKoulPARambhadGFletcherMA. Streptococcus pneumoniae as a cause of community-acquired pneumonia in Indian adolescents and adults: A systematic review and meta-analysis. Clin Med Insights Circ Respir Pulm Med. (2019) 13:1179548419862790. doi: 10.1177/1179548419862790
15
ThomasKMukkai KesavanLVeeraraghavanBJasmineSJudeJShubankarMet al. Invasive pneumococcal disease associated with high case fatality in India. J Clin Epidemiol. (2013) 66:36–43. doi: 10.1016/j.jclinepi.2012.04.006
16
JayaramanRVargheseRKumarJLNeeraviAShanmugasundaramDRalphRet al. Invasive pneumococcal disease in Indian adults: 11 years’ experience. J Microbiol Immunol Infect. (2019) 52:736–42. doi: 10.1016/j.jmii.2018.03.004
17
ShigayevaARudnickWGreenKChenDKDemczukWGoldWLet al. Invasive pneumococcal disease among immunocompromised persons: implications for vaccination programs. Clin Infect Dis. (2016) 62:139–47. doi: 10.1093/cid/civ803
18
TitmarshGJMcMullinMFMcShaneCMClarkeMEngelsEAAndersonLA. Community-acquired infections and their association with myeloid Malignancies. Cancer Epidemiol. (2014) 38:56–61. doi: 10.1016/j.canep.2013.10.009
19
MikulskaMCesaroSDe LavalladeHDi BlasiREinarsdottirSGalloGet al. Vaccination of patients with haematological Malignancies who did not have transplantations: guidelines from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. (2019) 19:e188–99. doi: 10.1016/S1473-3099(18)30601-7
20
ChenC-LWangS-TChengW-CWuB-RLiaoW-CHsuW-H. Outcomes and prognostic factors in critical patients with hematologic Malignancies. J Clin Med. (2023) 12:958. doi: 10.3390/jcm12030958
21
MongardonNMaxABougléAPèneFLemialeVCharpentierJet al. Epidemiology and outcome of severe pneumococcal pneumonia admitted to intensive care unit: a multicenter study. Crit Care. (2012) 16:R155. doi: 10.1186/cc11471
22
Grgić MedićMGornikIGašparovićV. Hematologic Malignancies in the medical intensive care unit – Outcomes and prognostic factors. Hematology. (2015) 20:247–53. doi: 10.1179/1607845414Y.0000000206
23
LeeYJHuangY-TKimSJKerpelevMGonzalezVKaltsasAet al. Trends in invasive pneumococcal disease in cancer patients after the introduction of 7-valent pneumococcal conjugate vaccine: A 20-year longitudinal study at a major urban cancer center. Clin Infect Dis. (2018) 66:244–53. doi: 10.1093/cid/cix739
24
ChanCYMolrineDCGeorgeSTarbellNJMauchPDillerLet al. Pneumococcal conjugate vaccine primes for antibody responses to polysaccharide pneumococcal vaccine after treatment of hodgkin’s disease. J Infect Dis. (1996) 173:256–8. doi: 10.1093/infdis/173.1.256
25
ChengFWTIpMChuYYLLinZLeeVShingMKet al. Humoral response to conjugate pneumococcal vaccine in paediatric oncology patients. Arch Dis Child. (2012) 97:358–60. doi: 10.1136/adc.2010.198416
26
HungTKotechaRSBlythCCSteedSKThorntonRBRyanALet al. Immunogenicity and safety of single-dose, 13-valent pneumococcal conjugate vaccine in pediatric and adolescent oncology patients. Cancer. (2017) 123:4215–23. doi: 10.1002/cncr.30764
27
TakeshitaKIshiwadaNTakeuchiNOhkusuMOhataMHinoMet al. Immunogenicity and safety of routine 13-valent pneumococcal conjugate vaccination outside recommended age range in patients with hematological Malignancies and solid tumors. Vaccine. (2022) 40:1238–45. doi: 10.1016/j.vaccine.2022.01.056
28
RobertsonJDNageshKJowittSNDougalMAndersonHMuttonKet al. Immunogenicity of vaccination against influenza, Streptococcus pneumoniae and Haemophilus influenzae type B in patients with multiple myeloma. Br J Cancer. (2000) 82:1261–5. doi: 10.1054/bjoc.1999.1088
29
HingeMIngelsHASSlotvedHMølleI. Serologic response to a 23-valent pneumococcal vaccine administered prior to autologous stem cell transplantation in patients with multiple myeloma. APMIS. (2012) 120:935–40. doi: 10.1111/j.1600-0463.2012.02922.x
30
MustafaSSShahDBressJJamshedS. Response to PCV13 vaccination in patients with multiple myeloma versus healthy controls. Hum Vaccines Immunother. (2019) 15:452–4. doi: 10.1080/21645515.2018.1534516
31
RenaudLSchraenSFouquetGGuidezSDemarquetteHNudelMet al. Response to pneumococcal vaccination in multiple myeloma. Cancer Med. (2019) 8:3822–30. doi: 10.1002/cam4.2253
32
NoonanKRudrarajuLFergusonAEmerlingAPasettiMFHuffCAet al. Lenalidomide-induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clin Cancer Res. (2012) 18:1426–34. doi: 10.1158/1078-0432.CCR-11-1221
33
PalazzoMShahGLCopelanOSeierKDevlinSMMaloyMet al. Revaccination after autologous hematopoietic stem cell transplantation is safe and effective in patients with multiple myeloma receiving lenalidomide maintenance. Biol Blood Marrow Transplant. (2018) 24:871–6. doi: 10.1016/j.bbmt.2017.12.795
34
StomaIKarpovIIskrovILendinaIUssA. Clinical efficacy of pneumococcal vaccination in multiple myeloma patients on novel agents: Results of a prospective clinical study. Vaccine. (2020) 38:4713–6. doi: 10.1016/j.vaccine.2020.05.024
35
BateJBorrowRChisholmJClarkeSCDixonEFaustSNet al. Thirteen-valent pneumococcal conjugate vaccine in children with acute lymphoblastic leukemia: protective immunity can be achieved on completion of treatment. Clin Infect Dis. (2020) 71:1271–80. doi: 10.1093/cid/ciz965
36
DorvalSGanttSLeclercJLaverdièreCOvetchkinePTapiéroB. Pneumococcal vaccination during chemotherapy in children treated for acute lymphoblastic leukemia. Pediatr Blood Cancer. (2021) 68:e28944. doi: 10.1002/pbc.28944
37
TopKAVaudryWMorrisSKPham-HuyAPernicaJMTapiéroBet al. Waning vaccine immunity and vaccination responses in children treated for acute lymphoblastic leukemia: A canadian immunization research network study. Clin Infect Dis. (2020) 71:e439–48. doi: 10.1093/cid/ciaa163
38
PatelSRBateJBorrowRHeathPT. Serotype-specific pneumococcal antibody concentrations in children treated for acute leukaemia: Table 1. Arch Dis Child. (2012) 97:46–8. doi: 10.1136/adc.2009.176271
39
HartkampAMulderAHLRijkersGTVan Velzen-BladHBiesmaDH. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B-cell chronic lymphocytic leukaemia. Vaccine. (2001) 19:1671–7. doi: 10.1016/S0264-410X(00)00409-6
40
SinisaloMAittoniemiJOivanenPKäyhtyHÖlanderRVilpoJ. Response to vaccination against different types of antigens in patients with chronic lymphocytic leukaemia. Br J Haematol. (2001) 114:107–10. doi: 10.1046/j.1365-2141.2001.02882.x
41
SafdarARodriguezGHRuedaAMWierdaWGFerrajoliAMusherDMet al. Multiple‐dose granulocyte‐macrophage–colony‐stimulating factor plus 23‐valent polysaccharide pneumococcal vaccine in patients with chronic lymphocytic leukemia: A prospective, randomized trial of safety and immunogenicity. Cancer. (2008) 113:383–7. doi: 10.1002/cncr.23561
42
SinisaloMVilpoJItäläMVäkeväinenMTaurioJAittoniemiJ. Antibody response to 7-valent conjugated pneumococcal vaccine in patients with chronic lymphocytic leukaemia. Vaccine. (2007) 26:82–7. doi: 10.1016/j.vaccine.2007.10.053
43
PasiarskiMRolinskiJGrywalskaEStelmach-GoldysAKorona-GlowniakIGozdzSet al. Antibody and plasmablast response to 13-valent pneumococcal conjugate vaccine in chronic lymphocytic leukemia patients – preliminary report. PloS One. (2014) 9:e114966. doi: 10.1371/journal.pone.0114966
44
MauroFRGiannarelliDGalluzzoCMVitaleCVisentinARiemmaCet al. Response to the conjugate pneumococcal vaccine (PCV13) in patients with chronic lymphocytic leukemia (CLL). Leukemia. (2021) 35:737–46. doi: 10.1038/s41375-020-0884-z
45
AndrickBAlwhaibiADeRemerDLQuershiSKhanRBryanLJet al. Lack of adequate pneumococcal vaccination response in chronic lymphocytic leukaemia patients receiving ibrutinib. Br J Haematol. (2018) 182:712–4. doi: 10.1111/bjh.14855
46
SvenssonTKättströmMHammarlundYRothDAnderssonP-OSvenssonMet al. Pneumococcal conjugate vaccine triggers a better immune response than pneumococcal polysaccharide vaccine in patients with chronic lymphocytic leukemia A randomized study by the Swedish CLL group. Vaccine. (2018) 36:3701–7. doi: 10.1016/j.vaccine.2018.05.012
47
LindströmVAittoniemiJSalmenniemiUKäyhtyHHuhtalaHItälä-RemesMet al. Antibody persistence after pneumococcal conjugate vaccination in patients with chronic lymphocytic leukemia. Hum Vaccines Immunother. (2018) 14:1471–4. doi: 10.1080/21645515.2018.1436424
48
De LavalladeHKhoderAHartMSarvariaASekineTAlsulimanAet al. Tyrosine kinase inhibitors impair B-cell immune responses in CML through off-target inhibition of kinases important for cell signaling. Blood. (2013) 122:227–38. doi: 10.1182/blood-2012-11-465039
49
GrimforsGSöderqvistMHolmGLefvertAKBjörkholmM. A longitudinal study of class and subclass antibody response to pneumococcal vaccination in splenectomized individuals with special reference to patients with Hodgkin’s disease. Eur J Haematol. (1990) 45:101–8. doi: 10.1111/j.1600-0609.1990.tb00426.x
50
PetraschSKühnemundOReinacherAUppenkampMReinertRSchmiegelWet al. Antibody responses of splenectomized patients with non-Hodgkin’s lymphoma to immunization with polyvalent pneumococcal vaccines. Clin Diagn Lab Immunol. (1997) 4:635–8. doi: 10.1128/cdli.4.6.635-638.1997
51
LandgrenOBjörkholmMKonradsenHBSöderqvistMNilssonBGustavssonAet al. A prospective study on antibody response to repeated vaccinations with pneumococcal capsular polysaccharide in splenectomized individuals with special reference to Hodgkin’s lymphoma. J Intern Med. (2004) 255:664–73. doi: 10.1111/j.1365-2796.2004.01312.x
52
CherifHLandgrenOKonradsenHBKalinMBjörkholmM. Poor antibody response to pneumococcal polysaccharide vaccination suggests increased susceptibility to pneumococcal infection in splenectomized patients with hematological diseases. Vaccine. (2006) 24:75–81. doi: 10.1016/j.vaccine.2005.07.054
53
MolrineDC. Antibody responses to polysaccharide and polysaccharide-conjugate vaccines after treatment of hodgkin disease. Ann Intern Med. (1995) 123:828. doi: 10.7326/0003-4819-123-11-199512010-00003
54
LeeDJordanAIMengesMALazaryanANishihoriTGaballaSRet al. Pneumococcal conjugate vaccine does not induce humoral response when administrated within the six months after CD19 CAR T-cell therapy. Transplant Cell Ther. (2023) 29:277.e1–9. doi: 10.1016/j.jtct.2022.08.011
55
National Comprehensive Cancer Network. NCCN clinical practice guidelines for prevention and treatment of cancer-related infections (version 3, 2024)(2024). Available online at: https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1457 (Accessed June 14, 2024).
56
KambojMBohlkeKBaptisteDMDunleavyKFuegerAJonesLet al. Vaccination of adults with cancer: ASCO guideline. J Clin Oncol. (2024) 42:1699–721. doi: 10.1200/JCO.24.00032
57
Centers for Disease Control and Prevention. Recommended child and adolescent immunization schedule for ages 18 years or younger. Available online at: https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf (Accessed July 29, 2024).
58
Centers for Disease Control and Prevention. Pneumococcal vaccine timing for adults. Available online at: https://www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf (Accessed July 29, 2024).
59
DharRGhoshalAGuleriaRSharmaSKulkarniTSwarnakarRet al. Clinical practice guidelines 2019: Indian consensus-based recommendations on pneumococcal vaccination for adults. Lung India. (2020) 37:S19. doi: 10.4103/lungIndia.lungIndia_272_20
60
Indian Medical Association. Indian medical association immunisation guideline book (2018). Available online at: https://www.ima-India.org/ima/important-news.php?id=8 (Accessed June 18, 2024).
61
Indian Academy of Pediatrics Advisory Committee on Vaccines and Immunization Practices. Pneumococcal conjugate vaccines. Available online at: https://acvip.org/professional/columns/pcvs-vaccines (Accessed July 29, 2024).
62
Geriatric Society of India. Indian guidelines for vaccination in older adults. Available online at: https://www.geriatricIndia.com/Indian_vaccination_guidelines.html (Accessed August 7, 2024).
63
RubinLGLevinMJLjungmanPDaviesEGAveryRTomblynMet al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. (2014) 58:309–18. doi: 10.1093/cid/cit684
64
MoulikNRMandalPChandraJBansalSJogPSanjaySet al. Immunization of children with cancer in India treated with chemotherapy - consensus guideline from the pediatric hematology-oncology chapter and the advisory committee on vaccination and immunization practices of the Indian academy of pediatrics. Indian Pediatr. (2019) 56:1041–8. doi: 10.1007/s13312-019-1678-0
65
RiegerCTLissBMellinghoffSBuchheidtDCornelyOAEgererGet al. Anti-infective vaccination strategies in patients with hematologic Malignancies or solid tumors-Guideline of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO). Ann Oncol. (2018) 29:1354–65. doi: 10.1093/annonc/mdy117
66
OkinakaKAkedaYKurosawaSFujiSTajimaKOishiKet al. Pneumococcal polysaccharide vaccination in allogeneic hematopoietic stem cell transplantation recipients: a prospective single-center study. Microbes Infect. (2017) 19:553–9. doi: 10.1016/j.micinf.2017.08.005
67
PaoMPapadopoulosEBChouJGlennHCastro-MalaspinaHJakubowskiAAet al. Response to pneumococcal (PNCRM7) and haemophilus influenzae conjugate vaccines (HIB) in pediatric and adult recipients of an allogeneic hematopoietic cell transplantation (alloHCT). Biol Blood Marrow Transplant. (2008) 14:1022–30. doi: 10.1016/j.bbmt.2008.06.012
68
MeiselRKuypersLDirksenUSchubertRGruhnBStraussGet al. Pneumococcal conjugate vaccine provides early protective antibody responses in children after related and unrelated allogeneic hematopoietic stem cell transplantation. Blood. (2007) 109:2322–6. doi: 10.1182/blood-2006-06-032284
69
CordonnierCLabopinMRobinCRibaudPCabanneLChadelatCet al. Long-term persistence of the immune response to antipneumococcal vaccines after Allo-SCT: 10-year follow-up of the EBMT-IDWP01 trial. Bone Marrow Transplant. (2015) 50:978–83. doi: 10.1038/bmt.2015.42
70
MolrineDCAntinJHGuinanECSoifferRJMacDonaldKMalleyRet al. Donor immunization with pneumococcal conjugate vaccine and early protective antibody responses following allogeneic hematopoietic cell transplantation. Blood. (2003) 101:831–6. doi: 10.1182/blood-2002-03-0832
71
AntinJHGuinanECAviganDSoifferRJJoyceRMMartinVJet al. Protective antibody responses to pneumococcal conjugate vaccine after autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2005) 11:213–22. doi: 10.1016/j.bbmt.2004.12.330
72
KumarDChenMHWelshBSiegalDCobosIMessnerHAet al. A randomized, double-blind trial of pneumococcal vaccination in adult allogeneic stem cell transplant donors and recipients. Clin Infect Dis. (2007) 45:1576–82. doi: 10.1086/523583
73
LangedijkACVan AalstMMeekBVan LeeuwenEMMZeerlederSMeijerEet al. Long-term pneumococcal vaccine immunogenicity following allogeneic hematopoietic stem cell transplantation. Vaccine. (2019) 37:510–5. doi: 10.1016/j.vaccine.2018.11.053
74
CordonnierCLjungmanPJuergensCMaertensJSelleslagDSundaraiyerVet al. Immunogenicity, safety, and tolerability of 13-valent pneumococcal conjugate vaccine followed by 23-valent pneumococcal polysaccharide vaccine in recipients of allogeneic hematopoietic stem cell transplant aged ≥2 years: an open-label study. Clin Infect Dis. (2015) 61:313–23. doi: 10.1093/cid/civ287
75
Garcia GarridoHMHaggenburgSSchoordijkMCEMeijerETanckMWTHazenbergMDet al. Immunogenicity of a 5‐dose pneumococcal vaccination schedule following allogeneic hematopoietic stem cell transplantation. Am J Hematol. (2022) 97:592–602. doi: 10.1002/ajh.26493
76
OkinakaKAkedaYInamotoYFujiSItoATanakaTet al. Immunogenicity of three versus four doses of 13-valent pneumococcal conjugate vaccine followed by 23-valent pneumococcal polysaccharide vaccine in allogeneic haematopoietic stem cell transplantation recipients: a multicentre, randomized controlled trial. Clin Microbiol Infect. (2023) 29:482–9. doi: 10.1016/j.cmi.2022.12.007
77
Van Der VeldenAMTClaessenAMEVan Velzen-BladHDe GrootMRKramerMHHBiesmaDHet al. Vaccination responses and lymphocyte subsets after autologous stem cell transplantation. Vaccine. (2007) 25:8512–7. doi: 10.1016/j.vaccine.2007.10.008
78
RobinCBahuaudMRedjoulRJeljeliMLeclercMCabanneLet al. Antipneumococcal seroprotection years after vaccination in allogeneic hematopoietic cell transplant recipients. Clin Infect Dis. (2020) 71:e301–7. doi: 10.1093/cid/ciz1168
79
RobertsMBBakNWeeLYAChhetriRYeungDTLewisIet al. Clinical effectiveness of conjugate pneumococcal vaccination in hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. (2020) 26:421–7. doi: 10.1016/j.bbmt.2019.10.006
80
CordonnierCLabopinMChesnelVRibaudPDe La CamaraRMartinoRet al. Randomized Study of Early versus Late Immunization with Pneumococcal Conjugate Vaccine after Allogeneic Stem Cell Transplantation. Clin Infect Dis. (2009) 48:1392–401. doi: 10.1086/598324
81
CordonnierCLabopinMJansenKUPrideMChesnelVBonnetEet al. Relationship between IgG titers and opsonocytophagocytic activity of anti-pneumococcal antibodies after immunization with the 7-valent conjugate vaccine in allogeneic stem cell transplant. Bone Marrow Transplant. (2010) 45:1423–6. doi: 10.1038/bmt.2009.364
82
CordonnierCLabopinMChesnelVRibaudPCamaraRDLMartinoRet al. Immune response to the 23-valent polysaccharide pneumococcal vaccine after the 7-valent conjugate vaccine in allogeneic stem cell transplant recipients: Results from the EBMT IDWP01 trial. Vaccine. (2010) 28:2730–4. doi: 10.1016/j.vaccine.2010.01.025
83
Indian Council of Medical Research. National guidelines for hematopoietic cell transplantation. Available online at: https://main.icmr.nic.in/sites/default/files/upload_documents/Nat_Guide_HCT.pdf (Accessed June 18, 2024).
84
CordonnierCEinarsdottirSCesaroSDi BlasiRMikulskaMRiegerCet al. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. (2019) 19:e200–12. doi: 10.1016/S1473-3099(18)30600-5
85
UllmannAJSchmidt-HieberMBertzHHeinzWJKiehlMKrügerWet al. Infectious diseases in allogeneic haematopoietic stem cell transplantation: prevention and prophylaxis strategy guidelines 2016. Ann Hematol. (2016) 95:1435–55. doi: 10.1007/s00277-016-2711-1
86
HuangJMakF-YWongY-YKoSChongMKCWangZet al. Enabling factors, barriers, and perceptions of pneumococcal vaccination strategy implementation: A qualitative study. Vaccines. (2022) 10:1164. doi: 10.3390/vaccines10071164
87
MieczkowskiTAWilsonSA. Adult pneumococcal vaccination: a review of physician and patient barriers. Vaccine. (2002) 20:1383–92. doi: 10.1016/s0264-410x(01)00463-7
88
Centers for Disease Control and Prevention. Pneumococcal vaccination. Available online at: https://www.cdc.gov/pneumococcal/vaccines/index.html (Accessed August 2, 2024).
89
World Health Organization. Pneumococcal disease. Available online at: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/pneumococcal-disease (Accessed August 2, 2024).
90
BerildJDWinjeBAVestrheimDFSlotvedH-CValentiner-BranthPRothAet al. A systematic review of studies published between 2016 and 2019 on the effectiveness and efficacy of pneumococcal vaccination on pneumonia and invasive pneumococcal disease in an elderly population. Pathog Basel Switz. (2020) 9:259. doi: 10.3390/pathogens9040259
91
FarrarJLChildsLOuattaraMAkhterFBrittonAPilishviliTet al. Systematic review and meta-analysis of the efficacy and effectiveness of pneumococcal vaccines in adults. Pathogens. (2023) 12:732. doi: 10.3390/pathogens12050732
92
KrishnamoorthyYEliyasSKNairNPSakthivelMSarveswaranGChinnakaliP. Impact and cost effectiveness of pneumococcal conjugate vaccine in India. Vaccine. (2019) 37:623–30. doi: 10.1016/j.vaccine.2018.12.004
93
SmithKJWateskaARNowalkMPRaymundMNuortiJPZimmermanRK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. (2012) 307:804–12. doi: 10.1001/jama.2012.169
94
National Health Mission. National operational guidelines on the introduction of pneumococcal conjugate vaccine, India(2017). Available online at: https://nhm.gov.in/New_Updates_2018/NHM_Components/Immunization/Guildelines_for_immunization/Operational_Guidelines_for_PCV_introduction.pdf (Accessed August 2, 2024).
95
NagarajGGovindanVGanaieFVenkateshaVTHawkinsPAGladstoneRAet al. Streptococcus pneumoniae genomic datasets from an Indian population describing pre-vaccine evolutionary epidemiology using a whole genome sequencing approach. Microb Genomics. (2021) 7:645. doi: 10.1099/mgen.0.000645
96
Indian Council of Medical Research. ICMR Annual Report 2021 (Antimicrobial resistance research and surveillance network). Available online at: https://main.icmr.nic.in/sites/default/files/upload_documents/AMR_Annual_Report_2021.pdf (Accessed August 2, 2024).
97
SinghJSundaresanSManoharanAShetA. Serotype distribution and antimicrobial susceptibility pattern in children ≤ 5 years with invasive pneumococcal disease in India – A systematic review. Vaccine. (2017) 35:4501–9. doi: 10.1016/j.vaccine.2017.06.079
Summary
Keywords
hematological malignancies, hematopoietic stem cell transplant, pneumococcal Infections, risk, vaccines, consensus, delphi
Citation
Seth T, Melinkeri S, Dolai TK, Bhattacharyya J, Sidharthan N, Chakrabarti P, Malalur C and Taur S (2025) Preventing pneumococcal infections in patients with hematological malignancies: a review of evidence and recommendations based on modified Delphi consensus. Front. Oncol. 15:1546641. doi: 10.3389/fonc.2025.1546641
Received
17 December 2024
Accepted
07 April 2025
Published
01 May 2025
Volume
15 - 2025
Edited by
Adam Finn Binder, Thomas Jefferson University, United States
Reviewed by
Srinivas Devarakonda, The Ohio State University, United States
Elisa Buzzatti, University of Rome Tor Vergata, Italy
Updates
Copyright
© 2025 Seth, Melinkeri, Dolai, Bhattacharyya, Sidharthan, Chakrabarti, Malalur and Taur.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaithanya Malalur, chaithanya.malalur@pfizer.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.