- 1Department of Physical Medicine and Rehabilitation and Clinic for Lymphatic Disorders, Ghent University Hospital, Ghent, Belgium
- 2Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium
- 3Department of Urology, Ghent University Hospital, Ghent, Belgium
- 4Department of Radiation Oncology and Clinic for Lymphatic Disorders, Ghent University Hospital, Ghent, Belgium
- 5Department of Radiation Oncology, Ghent University Hospital, Ghent, Belgium
- 6Department of Physical Medicine and Rehabilitation Ghent University Hospital, Ghent, Belgium
- 7Knowledge Centre for Health Ghent, Ghent University and Ghent University Hospital, Ghent, Belgium
- 8Department of Thoracic and Vascular Surgery and Clinic for Lymphatic Disorders, Ghent University Hospital, Ghent, Belgium
Introduction: As treatments in gynecological cancer improve, the number of cancer survivors increases, with more patients facing long-term side effects of their treatment. One debilitating side effect is lower limb lymphedema (LLL). Unlike upper limb lymphedema (ULL), diagnosis of LLL remains challenging due to the absence of a clear definition, bilateral presentation complicating comparison, and confusion with post-operative weight changes. This systematic review investigated incidences and risk factors for LLL.
Methods: We systematically searched PubMed, Embase, and CENTRAL databases for articles on LLL following treatment for pelvic gynecological cancer from 1979 to November 2024. Two independent researchers extracted data, based on predefined inclusion and exclusion criteria. We assessed bias using the Risk of Bias in Non-Randomized Studies (ROBINS-I) tool and adhered to PRISMA reporting guidelines.
Results: Our review included 46 studies, with incidence rates varying widely across cancer types: 7.4-55.9% in cervical cancer, 1.2-47% in endometrial cancer, 5.6-30.4% in ovarian cancer, and 10.1-43% in vulvar cancer. Several risk factors for LLL emerged. Notably, lymphadenectomy, the number of removed lymph nodes, radiotherapy, and a body mass index (BMI) exceeding 25 kg/m² were significant risk factors. Surgical technique did not impact LLL risk.
Conclusion: LLL frequently occurs following gynecological cancer treatments, emphasizing the importance of careful monitoring and proactive management in clinical settings. Overall, the findings highlight the complexity and variability in risk factors for LLL across different gynecological cancers. The significant heterogeneity in study designs, populations, and methodologies underscores the need for standardized approaches in future research to better understand and mitigate the risk of LLL in these patients.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42020198642.
1 Introduction
Gynecological cancers affect the uterus, ovaries, cervix, vulva, and vagina. The incidence of these cancers is approximately 11% in the United States and 17% worldwide (1). Treatment of those cancers often damage the lymphatic drainage due to removal of the lymphatic nodes or fibrosis following radiotherapy or systemic treatment (2). This may cause secondary LLL, a chronic and irreversible condition consisting of lymph fluid accumulation in the lower limbs or genital region. Lymphadenectomy (LA) and radiotherapy are associated with a significant risk of developing LLL. Fibrosis of the lymph nodes (LN) following radiotherapy can lead to lymphatic insufficiency and inadequate lymphatic transport (3). Obstruction of the lymphatic system results in lymphatic congestion and increased pressure in the lymphatic channels (4). Subcutaneous fluid accumulation triggers discomfort such as swelling of the limb, which can be unilateral or bilateral, a feeling of heaviness or numbness, skin changes or sometimes even pain. Indirectly it can lead to psychological or social distress, affecting the ability of these women to function in their daily activities with an additional negative impact on the patients’ quality of life (QoL) (4, 5). This review focuses on the risk factors for developing LLL after cancer treatment. Does the number of lymph nodes removed, the surgical technique used, whether in combination with radiotherapy and or chemotherapy have more impact on the development of LLL? What is the role of patient-related factors such as obesity? The development of LLL after cancer treatment is influenced by a combination of patient-related, cancer-related, and treatment-related factors. Identifying these risk factors may lead to strategies for early detection of LLL and better orientation of preventive measures. Providing accurate information about the risks of procedures is crucial for patient education and informed decision-making regarding treatment options.
Previous reviews, such as those by Biglia et al., Dessources et al., and Hu et al., have explored various aspects of LLL in gynecological cancer patients (6–8). Biglia et al. highlighted the significant impact of lymphadenectomy and radiotherapy on LLL development (6). Dessources et al. focused on the pathophysiology, incidence, and risk factors associated with LLL, emphasizing the need for more prospective studies and objective metrics (7). Hu et al. provided a comprehensive analysis of predictive factors and developed a model for identifying patients at risk of LLL following lymphadenectomy (8). This review aims to build on these findings by providing a systematic analysis of the risk factors for LLL, considering the combined effects of different treatment modalities and patient-related factors.
2 Methods
This systematic review and meta-analysis were conducted according to the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0) (9) and reported complying with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (10). The review was registered on PROSPERO, December 2020 (CRD42020198642). We performed a search of PubMed, Embase and CENTRAL databases on LLL after gynecological cancer treatment to retrieve articles from 1979 up to 01 November 2024. The search strategies are provided in Appendix 1. Additionally, the reference lists of included studies were searched manually. To identify ongoing studies, ClinicalTrials.gov and the ISRCTN registry were also searched. Inclusion criteria were original studies of women aged 18 years and older with gynecological cancer treated with surgery and/or radiotherapy, where the primary outcome measure was risk factors for LLL in relation to the incidence of LLL. Exclusion criteria were review articles and meta-analyses, articles not published in English, inaccessible full-text articles, case reports and studies with less than 15 patients. Relevant data from the eligible full texts were extracted by two authors (TD and MC) independently using pre-structured data sheets in the software program DistillerSR. The data sheets were pre-designed to extract data on risk factors in relation to LLL after gynecological cancer. Disagreements were resolved by discussion and consensus with a third researcher (CR).
We conducted an independent assessment of the risk of bias using the Risk of Bias in Non-Randomized Studies (ROBINS-I) tool. The tool covers 7 domains through which bias can occur. The first two domains deal with issues before the start of the interventions being compared (“baseline”), and the third domain deals with the classification of the interventions themselves. The other four domains address issues after the start of the interventions (11).
All analyses were performed using RStudio version 2023.06.1 Build 524 with the meta package (version 6.3-0) and the metafor package (version 4.6-0) for meta-analysis and meta-regression, respectively. A random-effects model was applied using the restricted maximum likelihood (REML) method to account for heterogeneity among studies. Odds ratios and 95 percent confidence intervals were computed, while forest plots were generated to visually display study results, including effect sizes, confidence intervals, sample sizes, and weights. Meta-regression was performed to explore the relationship between the odds ratio and the mean number of lymph nodes dissected. The regression line, along with the 95 percent confidence interval, was plotted against the mean number of lymph nodes to evaluate potential moderating effects.
3 Results
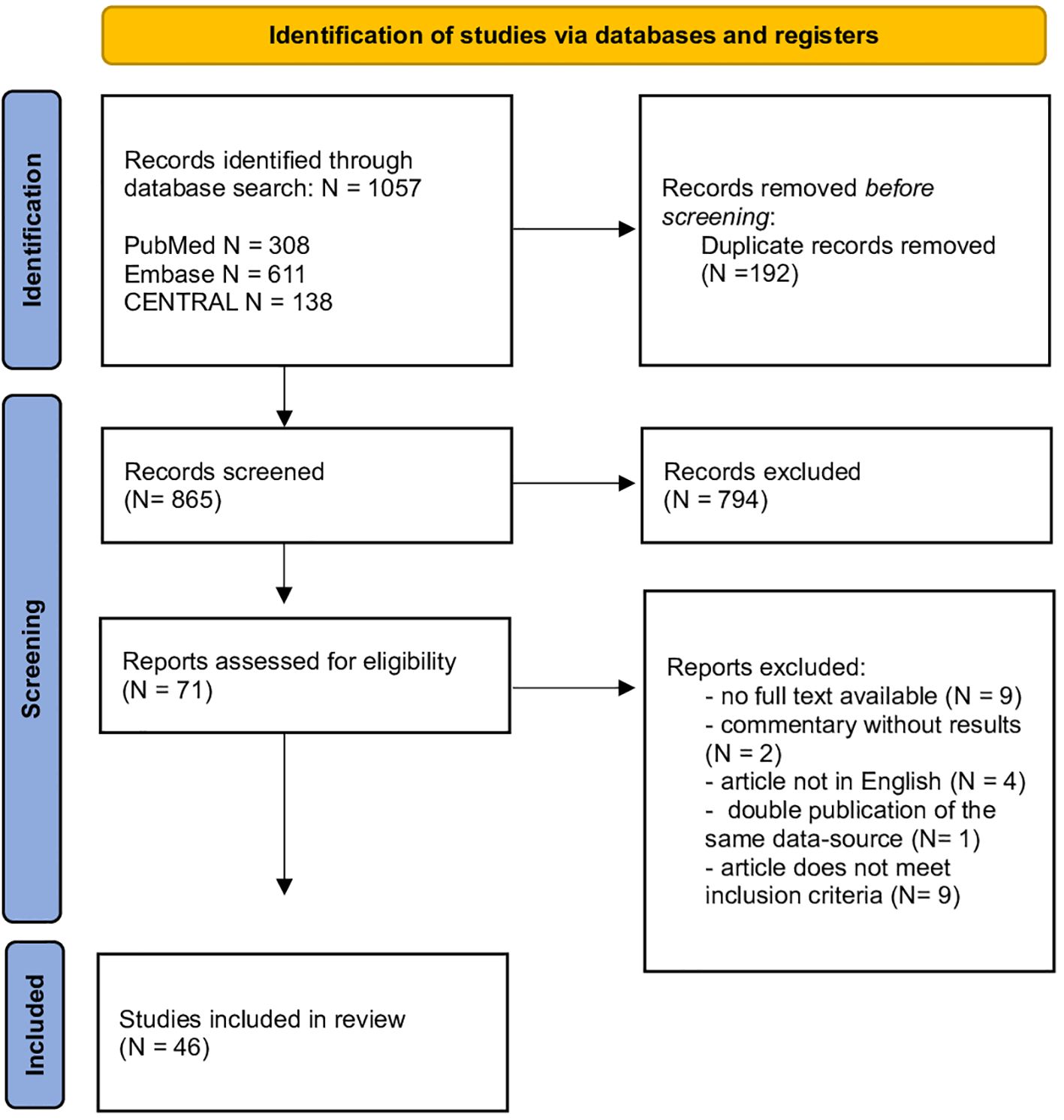
Our search yielded 1057 records of which 865 were retained after removing duplicates. After title and abstract review, 71 articles remained eligible and were reviewed for inclusion and exclusion criteria. This resulted in the inclusion of 46 studies. Figure 1 shows a flowchart of the literature screening process.
3.1 Characteristics of the included studies
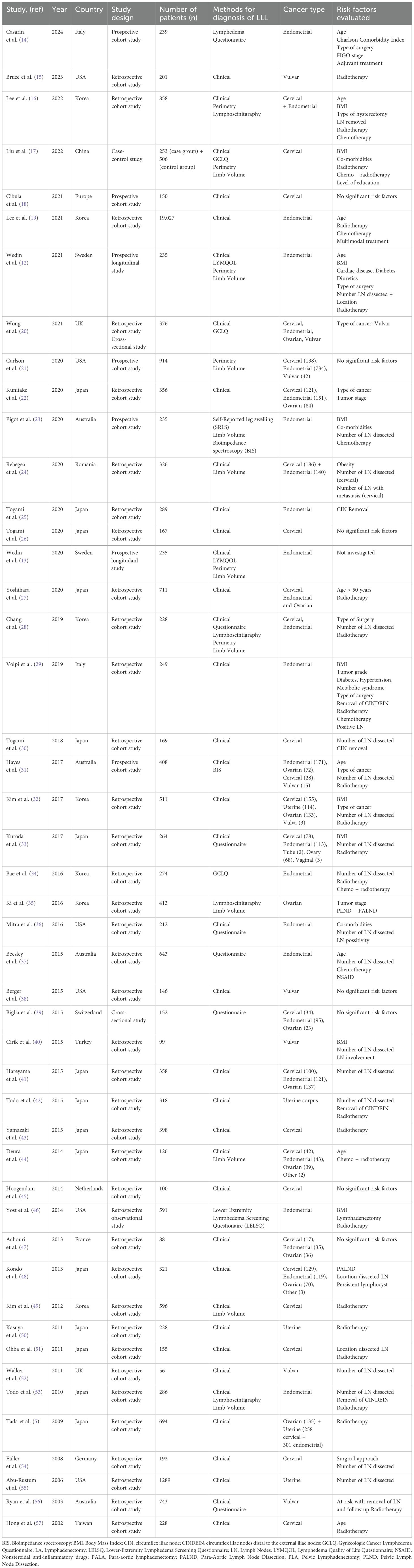
Most studies investigating risk factors following gynecological cancer treatment have been retrospective in nature, with only 6 prospective studies published to date. Two of these prospective studies, conducted by Wedin et al., included 235 patients with endometrial cancer (12, 13). The sample sizes in the studies varied significantly, ranging from 25 up to 19.027 patients. Table 1 summarizes the basic characteristics of the studies included in the review for LLL after cancer treatment.
3.2 Risk factors for gynecological cancer related lower limb lymphedema
The diagnosis of LLL was most frequently based on clinical examination using the International Society of Lymphology (ISL) grading system or questionnaires. Risk factors for LLL differ across various studies and types of cancer.
3.2.1 Endometrial cancer
Twenty-eight of the included studies investigated risk factors for LLL after endometrial cancer. The studies comprised 4 prospective cohort studies, 1 cross-sectional study, and the remainder were retrospective cohort studies. Fourteen studies focused exclusively on endometrial cancer, while the other studies in the review included various gynecological tumors. This heterogeneity complicates combining these studies into a single meta-analysis. Only the studies describing risk factors for lymphedema, limited to endometrial cancer, and applying odds ratio were considered for meta-analysis.
3.2.1.1 Patient-related risk factors
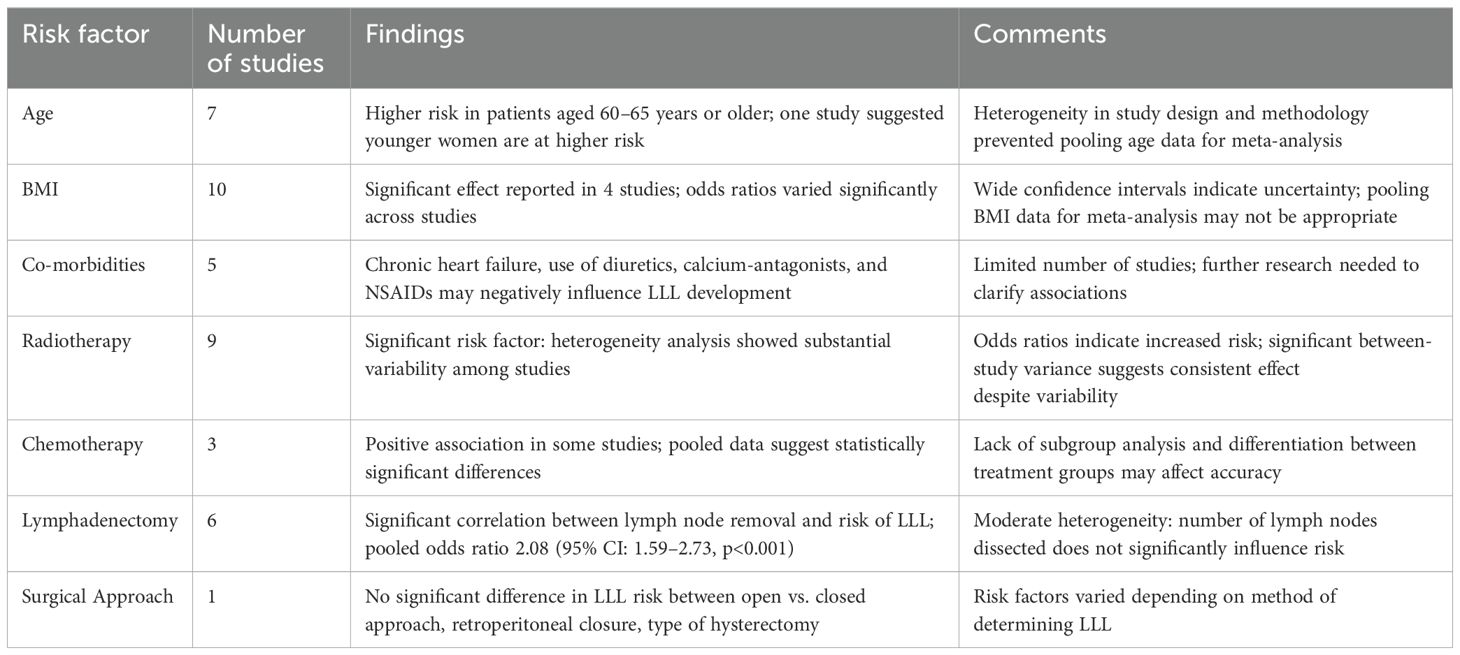
Seven studies investigated age as a risk factor, with 2 indicating that patients aged 60–65 years or older were at higher risk, contrarily to Wedin et al., who suggested younger women are at higher risk (12, 19, 37). However, due to the heterogeneity in study design, population, and methodology, pooling age-data for meta-analysis was not possible.
Among the 14 studies included for meta-analysis, 10 examined BMI as a potential risk factor. There was considerable variability in defining BMI thresholds. Only 4 studies reported a significant effect of BMI on LLL. The odds ratios for different BMI categories varied significantly across studies. For example, Yost et al. reported higher odds ratios for higher BMI categories (OR 4.69 (95% CI: 2.71-8.13) for BMI of 40.0 or higher), while Beesley et al. showed lower odds ratios (OR 0.9 (95% CI: 0.6-1.6) for BMI 25.0-29.9 and OR 0.9 (95% CI: 0.5-1.6) BMI 30.0-39.9) (37, 46). Heterogeneity among studies prevents a consistent conclusion. The wide confidence intervals (95% CI) indicate uncertainty in the estimates, with some studies including the null value (odds ratio = 1). This suggests that the effect of BMI on LLL is not consistent across studies. Given the significant variation in odds ratios and confidence intervals, pooling BMI as a risk factor for LLL may not be appropriate.
Other patient-related risk factors such as co-morbidities and smoking were only investigated in 5 studies (13, 23, 29, 36, 46). These studies suggest that certain co-morbidities, such as chronic heart failure, diuretics, calcium-antagonists, and NSAIDs, may negatively influence lymphedema development.
3.2.1.2 Treatment-related risk factors
Radiotherapy emerged as a significant risk factor in 9 studies, while 3 studies did not find any significance. The heterogeneity analysis for studies examining radiotherapy as a risk factor for LLL in endometrial cancer revealed significant variability among the included studies. The t² value was 0.2796, indicating the presence of between-study variance. The χ² test yielded a value of 25.04 with 8 degrees of freedom (df), and the associated p-value was less than 0.01, suggesting that the observed heterogeneity is statistically significant. Additionally, the I² statistic was 68%, indicating that 68% of the total variation across studies is due to heterogeneity rather than chance. Based on the statistical findings and the substantial heterogeneity observed, it is reasonable to conclude that radiotherapy is a risk factor for developing LLL among endometrial cancer patients. The odds ratios reported in the studies indicate an increased risk, and the significant between-study variance suggests that this effect is consistent across different studies, despite some variability (Figure 2A).
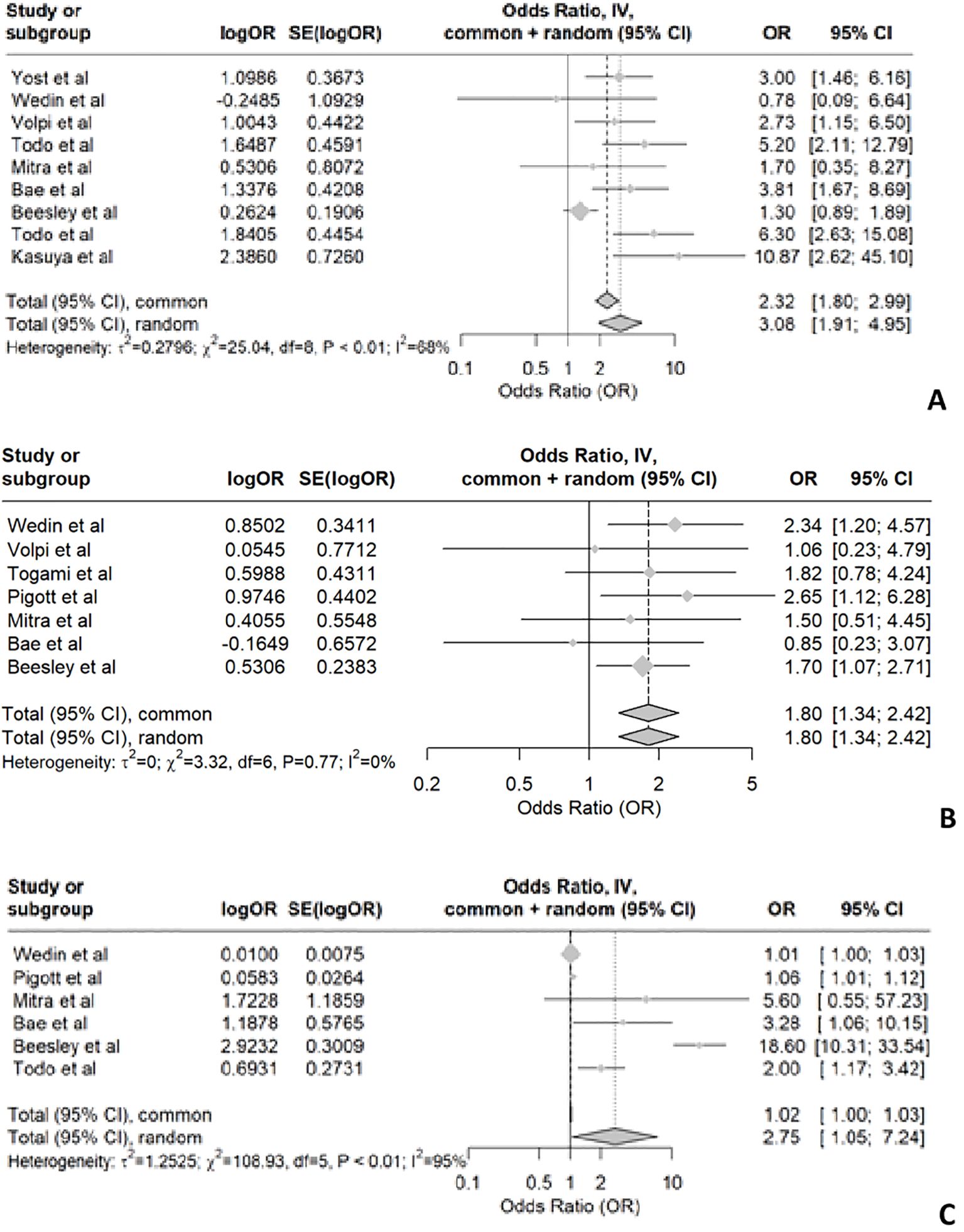
Figure 2. Shows the forest plot for radiotherapy (A), chemotherapy (B) and number of lymph nodes dissected (C).
While some studies show a positive association between chemotherapy and LLL (12, 23, 37), others do not provide conclusive evidence. In patients with endometrial cancer, the pooled data suggest that chemotherapy is associated with the development of LLL, as indicated by the forest plot (Figure 2B) showing an OR of 1.8 with a 95% confidence interval of 1.34 to 2.42. This indicates statistically significant differences. However, the meta-analysis reveals no significant heterogeneity among the included studies, suggesting a high level of consistency. It is important to note that most studies did not specify whether patients received radiotherapy in addition to chemotherapy. Only two studies described chemotherapy alone as a risk factor. Due to the lack of subgroup analysis and differentiation between treatment groups (chemotherapy alone, chemotherapy and radiotherapy, and radiotherapy alone), the pooled data may not accurately reflect the impact of chemotherapy alone on the development of LLL.
Removal of circumflex iliac nodes (CIN) may contribute to the development of lymphedema in endometrial cancer according to two retrospective cohort studies involving 289 and 286 patients, respectively (25, 53). Most studies on endometrial cancer have consistently reported two significant risk factors for LLL: the number of lymph nodes removed and radiotherapy. However, there is no uniformity in the number of lymph nodes removed across studies. Among the 15 included studies in our analysis, 6 specifically investigated endometrial cancer and found a significant correlation between lymph node removal and risk of LLL (12, 23, 34, 36, 37, 42). The forest plot for the removal of lymph nodes shows a p-value of <0.01, indicating significant heterogeneity. The I² value is 95%, with T² = 1.2525, Chi² = 108.93, and df = 5. This suggests substantial variability in the effect sizes across the studies (Figure 2C).
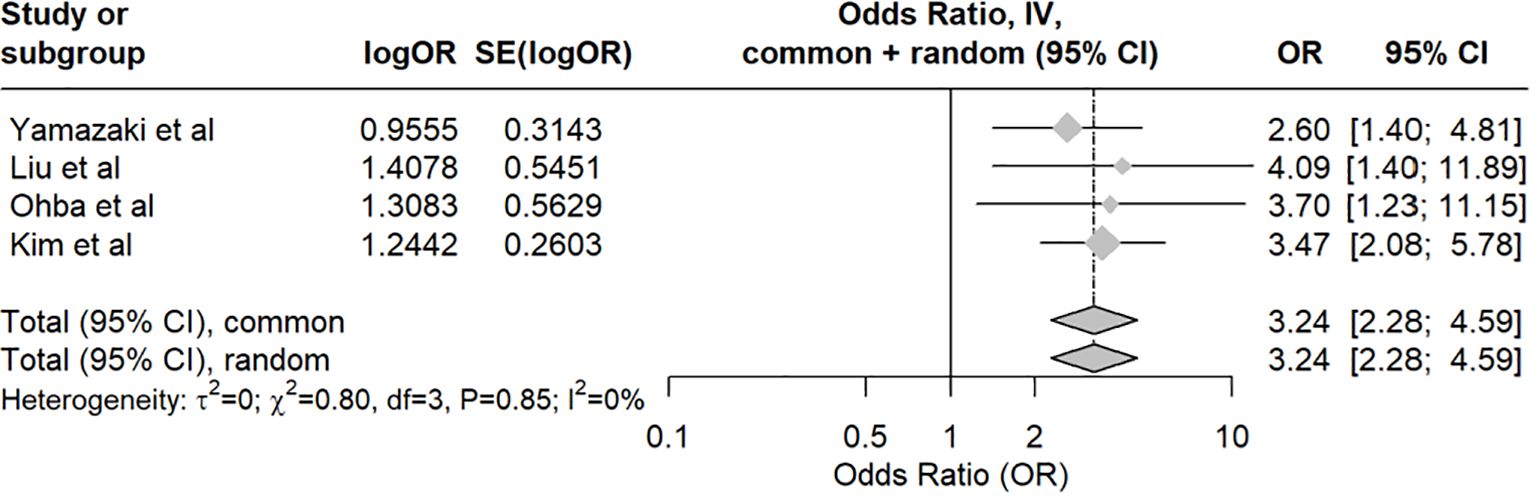
Concerning the risk of LLL followingLA, the pooled odds ratio was 2.08 (95% CI: 1.59–2.73, p<0.001). The analysis showed moderate heterogeneity, with an I-squared value of 42.6%. The meta-analysis indicates that the studies are consistent and show no significant heterogeneity, with LA (compared to no lymph node dissection) being a significant risk factor for LLL in endometrial cancer patients. (Figure 3A) The meta-regression assessing the impact of the median number of lymph nodes dissected revealed no significant moderating effect, with an estimate of -0.0195 (p=0.622). Despite consistent findings that LA increases the risk of LLL, our meta-regression analysis did not show a significant dose-response relationship based on the number of lymph nodes retrieved. This suggests that factors such as variability in surgical technique, differing anatomical dissections (e.g., inclusion of circumflex iliac nodes), and patient-related variables may confound this relationship (Figure 3B).
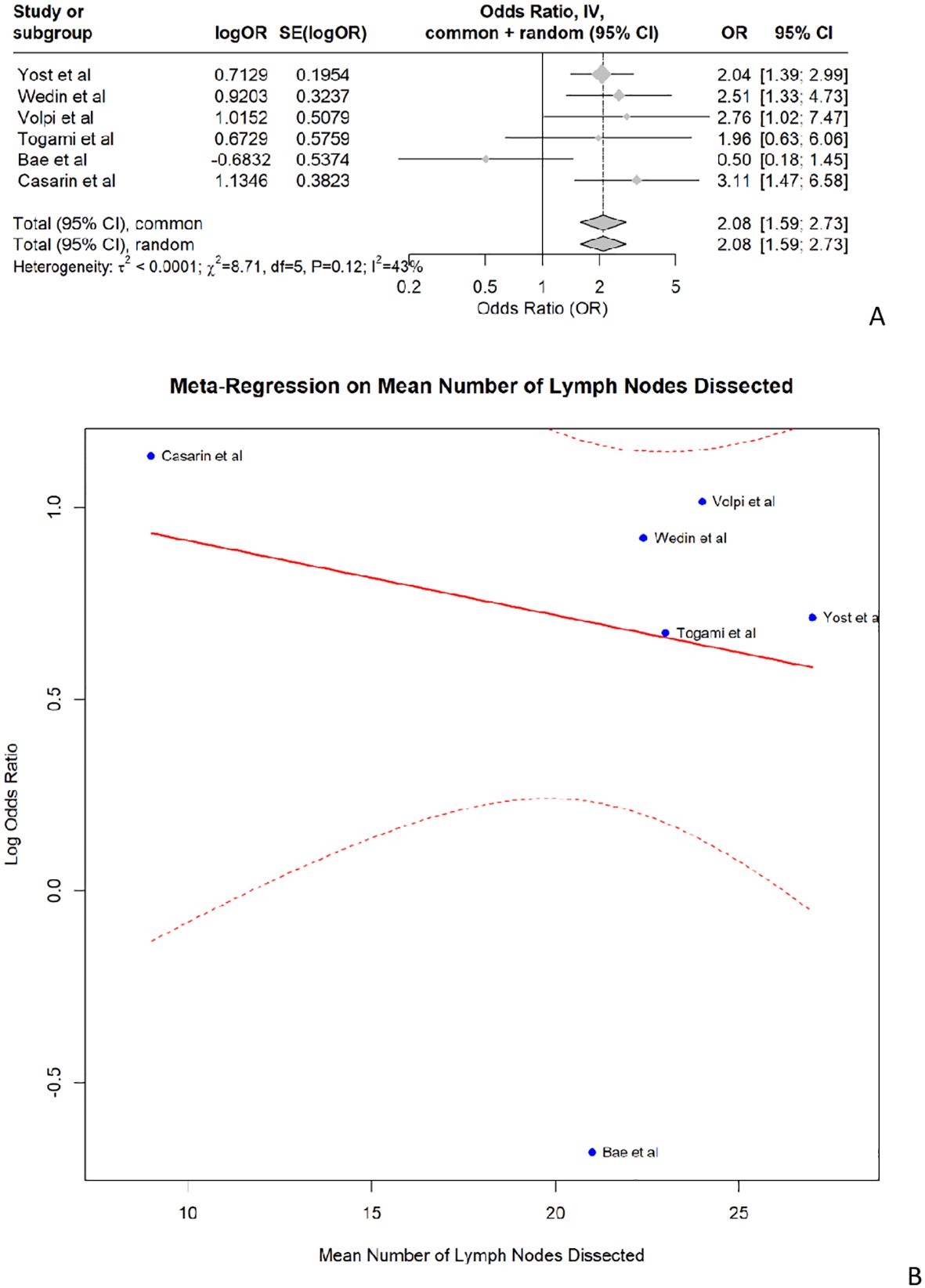
Figure 3. (A) shows the forest plot for lymphadenectomy and (B) shows the meta- regression of the median number of lymph nodes dissected.
No significant difference in LLL risk was found between surgical approach (open versus closed), application of retroperitoneal closure, type of hysterectomy: pelvic and para-aortic LA and pelvic LA alone (52.4% vs. 49.4%; p=0.630) (46). In a prospective study by Wedin et al. (12) they concluded that risk factors varied depending on the method of determining lymphedema. They found LA was a risk factor for LLL when assessed to BMI standardized volume, clinical grading, and patient-perceived swelling but not when evaluated by crude volume. Table 2 summarizes the risk factors for LLL in endometrial cancer.
The review of publications of LLL after treatment for endometrial cancer highlights several key risk factors. Age and BMI are significant patient-related factors, with older patients and those with higher BMI being at greater risk for developing LLL. However, the variability in defining BMI thresholds and the heterogeneity in study designs make it challenging to draw consistent conclusions. Radiotherapy is a significant treatment-related risk factor, with studies consistently showing an increased risk of LLL among patients who received radiotherapy. The interaction between lymph node removal and radiotherapy further complicates the risk profile, suggesting that patients undergoing extensive LA and radiotherapy are at the highest risk.
3.2.2 Cervical cancer
The review included 11 studies that investigated risk factors for LLL in patients with cervical cancer. One prospective cohort study examined risk factors for LLL after cervical cancer treatment in 150 patients and found that the development of LLL was not impacted by the number of lymph nodes removed, surgical approach, age, BMI, or adjuvant radiotherapy. However, only 12% of the included patients received radiation therapy, which may have influenced this result. Another prospective study did not identify any significant risk factors for developing LLL (45).
3.2.2.1 Patient-related risk factors
Four articles investigated whether BMI was an independent risk factor for developing LLL in patients with cervical cancer. Two of these articles did not find statistical significance (26, 49), while the other articles demonstrated a clear association, although they did not define the specific BMI values (17, 24). Co-morbidities as risk factors for LLL was investigated in only 1 article. Liu et al. found an increase in LLL risk if there was a history of coronary heart disease, vaginal disease, or abnormal menstruation (17). Interestingly, age does not appear to be a risk factor for cervical cancer according to 4 independent studies (18, 30, 43, 49). However, the study by Hong et al. suggested that age might be a risk factor for LLL in cervical cancer patients over 60 years old, although it was not significant for patients below 60 years old (57).
3.2.2.2 Treatment-related risk factors
Multiple studies have explored whether the location of LN removal in cervical cancer is a risk factor. Notably, both circumflex iliac node (CIN) and circumflex iliac nodes distal to the external iliac nodes (CINDEIN) removal appear to be significant risk factors (26, 30, 43). Additionally, 1 study identified suprafemoral node removal as a potential risk factor (51).
In the 11 studies investigating cervical cancer, radiotherapy consistently appears as a significant risk factor for LLL in 5 studies (17, 43, 49, 51, 57), while 3 studies did not find a significant association (18, 30, 45). When combining the results of these studies, the examination of the association between radiotherapy and the development of LLL in cervical cancer patients shows no significant heterogeneity among the included studies (Figure 4). This indicates that the results are highly consistent across the studies. However, it is important to note that the lack of heterogeneity does not equate to statistical significance. The pooled data indicate that radiotherapy significantly contributes to the development of LLL in cervical cancer patients.
Three studies investigated chemotherapy as an independent risk factor, but none of them found any significant association (30, 45, 54).
3.2.3 Ovarian cancer
In this review, only 2 studies specifically examined ovarian cancer to identify risk factors associated with the development of LLL. One study demonstrated an association between the number of LN removed and LLL, with an odds ratio of 1.025 (95% CI: 1.005–1.045) (35). Conversely, another study found that pelvic lymph node removal combined with radiotherapy was a risk factor for LLL in patients with ovarian or uterine cancer. No correlation was observed between surgical procedures and LLL (5).
3.2.4 Vulvar cancer
Only 8 studies investigated risk factors in vulvar cancer, 7 were retrospective and 1was prospective (20, 21, 31, 38, 40, 52, 56). Out of the 8 studies, only 4 were unique to vulvar cancer, while the remaining 4 combined various gynecological cancers but included a separate analysis for vulvar cancer. One retrospective study of 146 patients with vulvar cancer found no associated risk factors in the development of LLL due to the low incidence rate of LLL in the study population (38). The prospective study including 42 patients with vulvar cancer also showed no significant risk factor (21). Four retrospective studies with respectively 56, 68, 15 and 99 patients, showed number of LN removed as a risk factor for LLL (31, 40, 52, 56).
3.2.5 Gynecological cancer
Thirteen articles in the review described risk factors for developing LLL without distinguishing between the different types of gynecological cancer. The studies indicate varying levels of risk for developing LLL based on the different types of gynecological cancer and treatment factors, with some studies showing significant associations while others did not. This variability in findings, methodologies, and reported odds ratios complicates the analysis. Six of these articles specifically investigated whether the type of cancer influenced the development of edema. Yoshira et al. found in a study population of 711 patients that patients with cervical cancer had a greater risk of developing LLL compared to those with endometrial or ovarian cancer (OR: 1.912, p=0.001) (27). Similarly, Wong et al. reported a higher risk of LLL in patients with vulvar cancer compared to those with cervical, ovarian, and endometrial cancers, with a prevalence of 30.2% in vulvar cancer patients (OR: 11.0, CI: 3.2-38.3) (20). In contrast, studies by Kuroda et al., Kunitake et al., Hareyama et al., and Hayes et al. found no significant influence of the type of cancer on the development of LLL (22, 31, 33, 41). Additionally, differences in sample sizes, treatment modalities, and specific risk factors further complicate the ability to draw consistent conclusions across studies. Regarding patient-related risk factors, some studies identified younger age, higher BMI, and specific co-morbidities (eg. cellulitis) as significant for developing LLL (27, 33, 41). However, these results are inconsistent across studies, with some reporting no significant risk factors at all. Given the variability in findings, methodologies, and reported outcomes, conducting a meta-analysis would be challenging. The heterogeneity in the data, including differences in patient populations, risk factors assessed, and statistical methods used, makes it impractical to combine results into a single relevant meta-analysis.
3.3 Incidence of LLL in the included studies
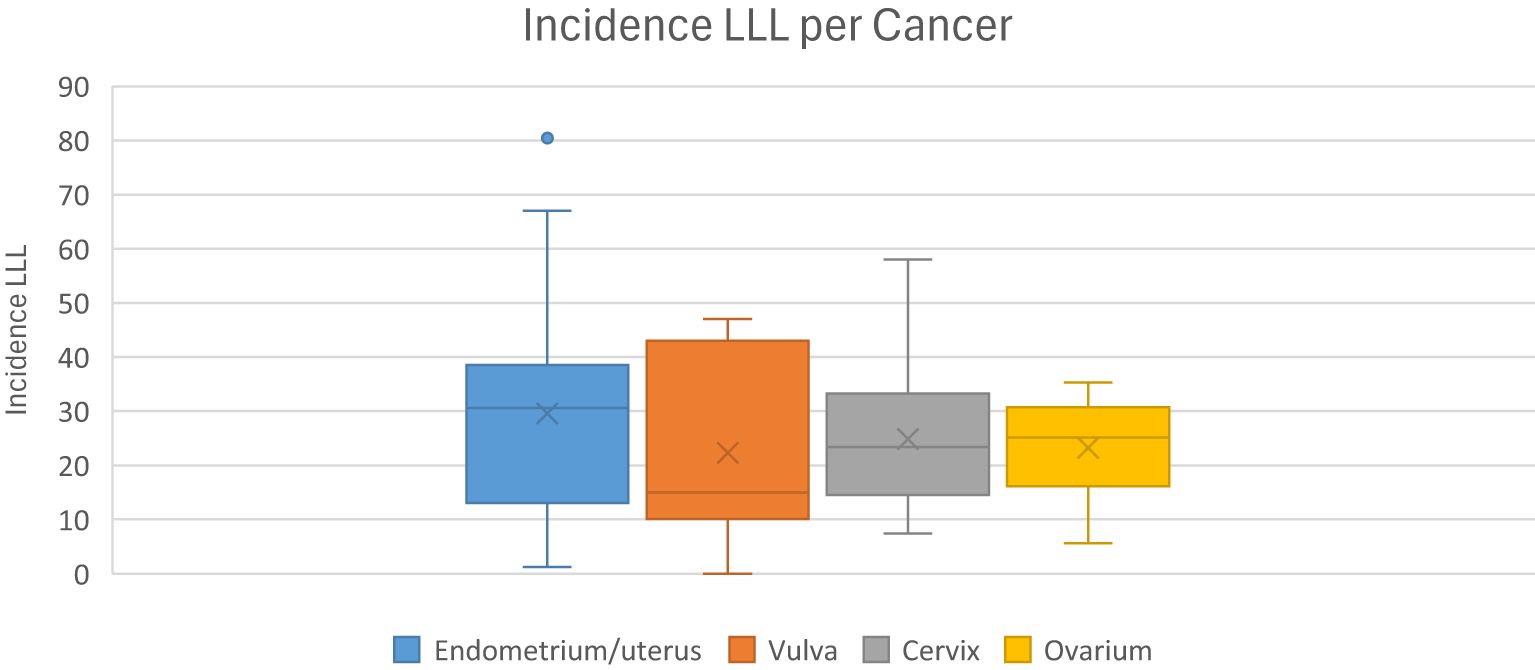
The incidence of LLL varies widely in the literature, ranging from 1-49% and depends on the tumor type (58, 59). This variation may be due to the lack of a gold standard for diagnosis of LLL. In most literature, the diagnosis is mainly based on subjective symptoms. To further explore the impact of diagnostic methods on the reported incidence rates, we calculated the incidence of LLL for each included study based on the diagnostic method used. This analysis highlights how variations in diagnostic approaches, such as the use of questionnaires and clinical control, may influence the reported incidence rates. Our study showed incidence rates of 7.8-55.9% for cervical cancer, 1.2-47% for endometrial cancer, 5.6-30.4% for ovarian cancer and 10.1-43% for vulvar/vaginal cancer. The incidence of LLL by cancer type is shown in Figure 5.
3.4 Methodological quality
Bias due to confounding was classified as critical/serious in 8/46 studies where LLL was assessed in groups that were not comparable or with different follow-up periods between groups, or where mild cases of LLL were excluded due to lack of diagnostic tools for diagnosis of LLL. Most studies were categorized as having a moderate risk of bias in participant selection (n= 24/46). This bias is due to patient characteristics observed after the start of the intervention. We found a low risk of bias in the classification of the intervention (n= 29/46). As most studies were observational and retrospective, no information on deviation from intervention was provided (n= 31/46), and if data were missing, they were excluded from the analysis resulting in a low risk of bias for missing data (n= 40/46). For the outcome measurement domain, 23/46 studies were classified as having a critical or serious risk of bias as in most studies the diagnosis of LLL was made by subjective measurements, or the physician made the diagnosis of LLL by clinical measurements using the ISL grading score, rather than using an objective measure such as volumetry or perimetry. The clinician already had knowledge of the intervention the patient received, which could lead to an overestimation of the diagnosis of LLL. In most studies the assessors were aware which intervention the patient had received, because of the retrospective nature of the trial. The overall risk of bias was dominated by moderate risk (n = 25/46) to serious risk (n = 14/46) due to selection bias, diagnostic bias of LLL and different follow-up times, sometimes unevenly distributed patient characteristics and a different approach to surgery and adjuvant therapy over the study period. Appendix B shows the of Risk of bias assessment for included studies using the ROBINS-I tool.
4 Discussion
In female patients diagnosed with gynecological cancer, the primary focus goes to treating the cancer. However, in cancer survivors the consequences of oncologic treatment have an important and often life-long impact on QoL. One of the most debilitating conditions in these cancer survivors is LLL. This study aimed to identify the main risk factors for the development of LLL following gynecological cancer treatment and to investigate its incidence. Identifying these risk factors helps to orient patient education, detection strategies and prevention. This study revealed that lymphadenectomy and radiotherapy are significant risk factors for the development of LLL in endometrial cancer patients. Additionally, some studies suggested a positive association between chemotherapy. In cervical cancer patients, radiotherapy consistently emerged as a significant risk factor for LLL, whereas chemotherapy did not show a significant association.
Risk factors have been extensively studied, and their impact is relatively clear. Several treatment-related factors, such as radiotherapy LA and the number of lymph nodes removed, influence the risk of developing LLL after gynecological cancer treatment. The most extensively studied risk factor is the number of lymph nodes removed during surgery. Although this is generally considered as the most significant risk factor, the specific cut-off value and the role of location of lymph node removal remain unclear (12, 24, 29–32, 34, 36, 37, 42, 52, 53). The impact of LA on LLL risk was only demonstrated in women with endometrial cancer. Moreover, the region most prone for developing LLL is the region of the circumflex arterial lymph nodes. Radiotherapy is described in several studies as a significant risk factor for LLL (12, 17, 23, 24, 27, 29, 32–34, 36, 37, 42, 43, 46, 49–51, 53, 57). The hypothesis is that radiotherapy may have a synergistic effect with surgery on the development of LLL (60). Surgery may directly damage the lymphatic system, disrupting lymphatic drainage, while radiotherapy may exacerbate the damage due to fibrosis and obstruction of collateral lymphatic circulation formation (54, 61). The role of other treatment-related factors was not conclusive, including type of surgery - open versus closed approach - type of hysterectomy, or application of retroperitoneal closure. No association could be demonstrated between presence of lymph node metastases and LLL (22, 28, 31, 33, 35, 41, 49, 50). Most studies also show no association between cancer type, histology or FIGO classification and lymphedema (18, 22, 28, 32, 35, 41, 49, 50, 55). Only 1 study found that lymphoedema was more common in patients with endometrial cancer than in those with ovarian cancer (5). However, there are other risk factors where the evidence remains inconclusive. These may include patient-related variables such as BMI and age. Specific patient subgroups, such as those with higher BMI or older age, may be at greater risk for developing LLL due to their overall health status and the potential for more extensive surgical interventions. Severe obesity is a recognized risk factor for secondary lymphoedema in the legs following cancer surgery (46). Studies have shown that obesity can exacerbate the risk of lymphedema by affecting lymphatic function. Further research is needed to clarify these associations and to develop targeted prevention strategies for high-risk groups. This includes understanding how BMI and age interact with other risk factors and treatment modalities to influence the risk of LLL. The role of BMI in the development of LLL is not clear. Due to significant variability in data, caution should be exercised when interpreting BMI as a risk factor for LLL. In cervical cancer, articles that demonstrated an association between BMI and lymphedema did not specifically define the BMI values but indicated a clear link between higher BMI and LLL risk (17, 24). However, the role of BMI remains equivocal, as most studies failed to find an association between BMI and LLL (7, 16–18, 27, 32, 35, 36, 39, 43, 45, 48, 51, 54). The exact mechanisms by which BMI influences lymphedema development are not fully understood. Hypotheses are that a higher BMI may lead to increased pressure on lymphatic vessels, obstructing lymph flow and impairing drainage. Obesity can alter immune function and excess adipose tissue might trigger chronic inflammation, affecting lymphatic function (62, 63). The impact of BMI on LLL risk varies across studies, and specific BMI tresholds are not well-defined. The role of age in the development of LLL post-endometrial cancer treatment remains ambiguous. While Wedin et al. suggested that younger women are at higher risk due to potentially higher activity levels leading to more damage to lymphatic vessels, other studies indicated that patients aged 60–65 years or older are more susceptible (19, 37). This discrepancy underscores the need for more targeted research to clarify age-related risks. It is important to note that the varying age cutoffs used across studies may not fully capture the impact of age on lymphedema development. In cervical cancer age was not consistently associated with cancer risk but may still play a role in LLL development. Additionally, younger women often present with more advanced cancers, requiring more invasive and hence more lympho-destructive oncologic treatment (64).
The reported incidence of LLL following gynecological cancer treatment varies significantly across studies and appears strongly influenced by the diagnostic method and threshold applied. For instance, using a diagnostic cut-off of a 5–10% increase in leg circumference may underestimate incidence particularly in patients with bilateral LLL, where asymmetry is less pronounced. In 2017, Biglia et al. reported LLL incidences of 11-24.1% for cervical cancer, 1.2-47% for endometrial cancer, 4.7-40% for ovarian cancer and 30-70% for vulvar cancer following treatment (6). Similarly Dessources et al. documented ranges from 7.4% to 58% in cervical cancer, 1.2% to 80.4% in endometrial cancer, 5.6% to 35.3% in ovarian cancer and 0% to 58.3% for vulvar/vaginal cancer (7). In our current review, incidence rates ranged from 7.8–55.9% for cervical cancer, 1.2–47% for endometrial cancer, 5.6–30.4% for ovarian cancer, and 10.1–43% for vulvar/vaginal cancer. These variations underscore the absence of a standardized diagnostic approach. Methods used across studies included patient-reported questionnaires, clinical examinations, and imaging techniques, each with different sensitivity and specificity. For example, studies relying solely on questionnaires may overestimate incidence due to subjective bias, while more objective volumetric methods although more specific might miss subtler cases, especially bilateral ones. Diagnostic methods varied widely, including questionnaires, clinical examinations, and imaging techniques. This analysis highlights how variations in diagnostic approaches can influence the reported incidence rates. For instance, studies using only questionnaires may report higher subjective incidence rates compared to those using clinical examinations or imaging, which might provide more objective measurements. The lack of a standardized diagnostic approach for LLL contributes to the wide range of reported incidence rates. Establishing a gold standard for diagnosis could help in obtaining more consistent and comparable data across studies. Our findings underscore the need for standardized diagnostic criteria in future research to reduce variability and improve the accuracy of LLL incidence reporting. Although volumetric measures are more objective, they are less sensitive and may underestimate the incidence of LLL, particularly for grade I LLL. Other complicating factors are its frequent bilateral occurrence, which hinders limb comparison, and central manifestation in the groin or pubic region, where circumference measurement is impractical. The most objective tool for objective diagnosis of impaired lymphatic drainage is lymphoscintigraphy, a time-consuming and ionizing technical exam, requiring advanced technological infrastructure. It is valuable in detecting advanced LLL, but seems less sensitive in early stage LLL or in LLL of the pelvic region (65). In many studies, diagnosis was based on scoring questionnaires to detect LLL, such as the Gynecologic Cancer Lymphedema Questionnaire (GCLQ) and the Self-Report Lower-Extremity Lymphedema Screening Questionnaire (LELSQ). Another frequent strategy is clinical assessment using the ISL grading system. Only few studies performed imaging techniques for validation of diagnosis. Circumference and volumetric measurements are more frequently used, with some studies applying a volume increase of 5%, while others use a 20% difference between both legs to define LLL (58). No metachronous evaluations were found. Additionally, the heterogeneity in treatment approaches—ranging from surgery alone, surgery with LA, surgery combined with brachytherapy, surgery with sentinel lymph node biopsy, to surgery with sentinel lymph node biopsy and adjuvant radiotherapy—adds another layer of complexity. Our meta-analysis did not find a statistically significant association between the number of lymph nodes retrieved, surgical approach (open vs. minimal invasive surgery (MIS)), and the incidence of LLL in endometrial cancer patients. This finding contrasts with several previous meta-analyses and reviews, including Desources et al., who reported that a greater number of lymph nodes removed was associated with increased risk of lymphedema (6, 7). Several factors may explain this discrepancy. First, there was substantial heterogeneity across studies in our meta-analysis, both in terms of methodology and outcome definitions. For example, the number of lymph nodes retrieved was inconsistently reported, and thresholds for “extensive” dissection varied between studies, which may have masked a potential dose-response effect. Second, data on surgical approach (open vs. MIS) were limited in the included studies and often not stratified or analyzed separately, restricting our ability to draw firm conclusions on this variable. Third, unlike some broader reviews, our meta-analysis included only studies that specifically analyzed lymphedema as an outcome in endometrial cancer and reported effect sizes (e.g., odds ratios), which may have led to the exclusion of more general studies that reported on surgical factors but did not focus on lymphedema risk. Lastly, our meta-regression did not identify a significant moderating effect of the median number of lymph nodes removed, suggesting that while lymphadenectomy overall increases risk, the extent of dissection may not be a linear predictor, or the signal may be diluted by confounding factors such as radiotherapy. These methodological and reporting differences likely contribute to the divergence from prior findings. Although sentinel lymph node (SLN) biopsy is increasingly adopted in the surgical management of endometrial cancer to reduce morbidity, our meta-analysis did not include studies that specifically evaluated LLL risk in patients who underwent SLN mapping alone. Most studies included in our review focused on patients treated with full pelvic and/or para-aortic LA, and data distinguishing SLN-only procedures were either absent or not reported separately. Given the growing clinical relevance of SLN biopsy, future prospective studies are needed to assess the incidence and risk factors of LLL in this specific subgroup. The variability in both diagnostic and treatment protocols underscore the need for standardized guidelines to accurately assess and manage LLL in gynecological cancer patients.
A limitation of our review is that most of the included studies are retrospective and vary significantly in design, population, and methodology. Only for endometrial and cervical cancer were the data sufficient and consistent enough to allow pooling for meta-analysis. The large variability between studies in type of cancer, diagnostic strategy, population size, and trial methodology may introduce bias and limit the ability to draw consistent conclusions. Effectively, there is no consensus on the diagnosis of LLL, with different studies using various criteria and methods for diagnosis, hence undermining the comparability of the results. Not all studies investigated the same risk factors, and those that did often used different definitions and thresholds. Additionally, most of the included studies are from Asia, which may not be directly applicable to Western populations due to potential cultural differences. Another difficulty is the variation in therapeutic approaches between regions and over time. Another difficulty is the variation in therapeutic approaches between regions and over time. Specifically, data on SLN biopsy alone were limited or not reported separately in most studies, making it difficult to assess the risk of LLL in patients undergoing SLN mapping without full LA. This represents an important gap, especially given the increasing adoption of SLN in clinical practice. Moreover, the presence of moderate statistical heterogeneity indicates variability in the effect estimates across studies. Clinical heterogeneity, arising from variations in patient characteristics, disease stages, and treatment protocols, complicates the pooling of data. This inherent variability among studies remains a significant limitation. We also combined patients with early-stage (I-II) and advanced-stage (III) disease, as well as those with and without lymph node dissection and involvement. Although subgroup analyses supported the pooling of these groups, the potential differences in baseline risks for lymphedema may introduce bias.
LA and radiotherapy remain the most clearly established risk factors for LLL following treatment for gynecological cancers. However, the evidence for other factors such as BMI, age, and chemotherapy is inconsistent and often confounded by methodological variability. The wide variation in diagnostic criteria and treatment protocols further complicates comparison across studies. Notably, data on SLN biopsy—a procedure increasingly used to minimize morbidity—are scarce, underscoring an important gap in the current literature. Our findings highlight the need for future prospective studies with standardized diagnostic tools, consistent definitions of risk factors, and focused analyses on emerging treatment modalities such as SLN. Ultimately, improving consistency in study design and reporting is essential for developing targeted prevention strategies and improving long-term outcomes for cancer survivors.
5 Conclusion
This systematic review confirms that LA, particularly involving the circumflex arterial lymph nodes and radiotherapy are the most consistently reported treatment-related risk factors for the development of LLL in gynecological cancer patients, especially in those with endometrial and cervical cancer. In contrast, the role of other variables such as BMI, age, cancer histology, and surgical approach remains inconclusive due to inconsistent study designs, heterogeneous reporting, and lack of standardized definitions. A major challenge in interpreting LLL incidence across studies lies in the absence of a clear diagnostic standard. The variability in diagnostic tools, thresholds for defining LLL, and methods of assessment has led to a wide range of reported incidence rates across tumor types. This diagnostic heterogeneity significantly limits comparability and hinders the ability to draw firm conclusions. Our meta-analysis did not identify a significant association between the number of lymph nodes retrieved or surgical approach (open vs. MIS) and the incidence of LLL, which may reflect methodological limitations, variability in reporting, and the exclusion of studies not specifically focused on lymphedema. Moreover, the lack of data on patients treated solely with SLN biopsy represents a critical gap in the literature, particularly as SLN mapping gains prominence in clinical practice. Despite these limitations, this review represents the most comprehensive synthesis of current evidence on LLL risk to date. It underscores the need for standardized diagnostic tools, clearer treatment variable definitions, and inclusion of both early and advanced disease stages in future prospective trials. Improved understanding of how risk factors both treatment-related and patient-specific interact will be essential for developing targeted prevention and management strategies for high-risk subgroups. Ultimately, such efforts will enhance clinical decision-making and improve outcomes for gynecologic cancer survivors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
TD: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MC: Data curation, Investigation, Methodology, Writing – review & editing. GK: Formal Analysis, Software, Writing – review & editing. CM: Funding acquisition, Supervision, Writing – review & editing. KV: Writing – review & editing. LV: Writing – review & editing. NP: Conceptualization, Project administration, Software, Writing – review & editing. CR: Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Kom op Tegen Kanker (Stand up to Cancer), the Flemish cancer society (project ID: 12149).
Acknowledgments
We would like to thank Nina Vermassen, MD, for participating in the initial phase of the review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1561836/full#supplementary-material
References
1. Carter J, Stabile C, Gunn A, and Sonoda Y. The physical consequences of gynecologic cancer surgery and their impact on sexual, emotional, and quality of life issues. J Sex Med. (2013) 10 Suppl 1:21–34. doi: 10.1111/jsm.12002
2. Baiocchi Neto G, Makdissi FBA, and Cagnacci Neto R. Gynecological Cancer and Breast Cancer. In: Zerati AE, Nishinari K, and Wolosker N, editors. Vascular Surgery in Oncology. Springer International Publishing, Cham (2022). p. 163–200.
3. Tiwari P, Coriddi M, Salani R, and Povoski SP. Breast and gynecologic cancer-related extremity lymphedema: a review of diagnostic modalities and management options. World J Surg Oncol. (2013) 11:237. doi: 10.1186/1477-7819-11-237
4. Tashiro K, Feng J, Wu SH, Mashiko T, Kanayama K, Narushima M, et al. Pathological changes of adipose tissue in secondary lymphoedema. Br J Dermatol. (2017) 177:158–67. doi: 10.1111/bjd.15238
5. Tada H, Teramukai S, Fukushima M, and Sasaki H. Risk factors for lower limb lymphedema after lymph node dissection in patients with ovarian and uterine carcinoma. BMC Cancer. (2009) 9:47. doi: 10.1186/1471-2407-9-47
6. Biglia N, Zanfagnin V, Daniele A, Robba E, and Bounous VE. Lower body lymphedema in patients with gynecologic cancer. Anticancer Res. (2017) 37:4005–15. doi: 10.21873/anticanres.11785
7. Dessources K, Aviki E, and Leitao MM Jr. Lower extremity lymphedema in patients with gynecologic Malignancies. Int J Gynecol Cancer. (2020) 30:252–60. doi: 10.1136/ijgc-2019-001032
8. Hu H, Fu M, Huang X, Huang J, and Gao J. Risk factors for lower extremity lymphedema after cervical cancer treatment: a systematic review and meta-analysis. Trans Cancer Res. (2022) 11:1713–21. doi: 10.21037/tcr-22-1256
9. Chandler J, Cumpston M, Li T, Page MJ, and Welch V. Cochrane handbook for systematic reviews of interventions. Hoboken: Wiley (2019).
10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372. doi: 10.1136/bmj.n71
11. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj. (2016) 355:i4919. doi: 10.1136/bmj.i4919
12. Wedin M, Stalberg K, Marcickiewicz J, Ahlner E, Ottander U, Åkesson Å, et al. Risk factors for lymphedema and method of assessment in endometrial cancer: a prospective longitudinal multicenter study. Int J Gynecol Cancer. (2021) 31:1416–27. doi: 10.1136/ijgc-2021-002890
13. Wedin M, Stålberg K, Marcickiewicz J, Ahlner E, Åkesson Å, Lindahl G, et al. Incidence of lymphedema in the lower limbs and lymphocyst formation within one year of surgery for endometrial cancer: A prospective longitudinal multicenter study. Gynecol Oncol. (2020) 159:201–8. doi: 10.1016/j.ygyno.2020.07.014
14. Casarin J, Schivardi G, Artuso V, Giudici A, Meschini T, De Vitis L, et al. Laparoscopic treatment of early-stage endometrial cancer: benefits of sentinel lymph node mapping and impact on lower extremity lymphedema. Int J Gynecol Cancer. (2024) ijgc-2024-005670. doi: 10.1136/ijgc-2024-005670
15. Bruce KH, Alabaster A, and Tran AM. Vulvar cancer survival by primary treatment modality: A retrospective cohort study. Gynecol Oncol. (2023) 176:173–8. doi: 10.1016/j.ygyno.2023.07.013
16. Lee J, Byun HK, Im SH, Son WJ, Roh YH, and Kim YB. Risk factors for lower extremity lymphedema after surgery in cervical and endometrial cancer. J Gynecol Oncol. (2023) 34:e28. doi: 10.3802/jgo.2023.34.e28
17. Liu G, Hu J, Liu Y, and Yuan M. Factors influencing lower limb lymphedema after cervical cancer surgery: A case-control study. Lymphat Res Biol. (2023) 21(2):169–78. doi: 10.1089/lrb.2022.0025
18. Cibula D, Borčinová M, Marnitz S, Jarkovský J, Klát J, Pilka R, et al. Lower-limb lymphedema after sentinel lymph node biopsy in cervical cancer patients. Cancers (Basel). (2021) 13(10):2360. doi: 10.3390/cancers13102360
19. Lee SJ, Myong JP, Lee YH, Cho EJ, Lee SJ, Kim CJ, et al. Lymphedema in endometrial cancer survivor: A nationwide cohort study. J Clin Med. (2021) 10(20):4647. doi: 10.3390/jcm10204647
20. Wong M, Eaton PK, Zanichelli C, Moore C, Hegarty C, and MacDonald N. The prevalence of undiagnosed postoperative lower limb lymphedema among gynecological oncology patients. Eur J Surg Oncol. (2022) 48:1167–72. doi: 10.1016/j.ejso.2021.12.464
21. Carlson JW, Kauderer J, Hutson A, Carter J, Armer J, Lockwood S, et al. GOG 244-The lymphedema and gynecologic cancer (LEG) study: Incidence and risk factors in newly diagnosed patients. Gynecol Oncol. (2020) 156:467–74. doi: 10.1016/j.ygyno.2019.10.009
22. Kunitake T, Kakuma T, and Ushijima K. Risk factors for lower limb lymphedema in gynecologic cancer patients after initial treatment. Int J Clin Oncol. (2020) 25:963–71. doi: 10.1007/s10147-019-01608-6
23. Pigott A, Obermair A, Janda M, Vagenas D, Ward LC, Reul-Hirche H, et al. Incidence and risk factors for lower limb lymphedema associated with endometrial cancer: Results from a prospective, longitudinal cohort study. Gynecol Oncol. (2020) 158:375–81. doi: 10.1016/j.ygyno.2020.04.702
24. Rebegea LF, Stoleriu G, Manolache N, Serban C, Craescu M, Lupu MN, et al. Associated risk factors of lower limb lymphedema after treatment of cervical and endometrial cancer. Exp Ther Med. (2020) 20:181. doi: 10.3892/etm.2020.9311
25. Togami S, Kubo R, Kawamura T, Yanazume S, Kamio M, and Kobayashi H. Risk factors for lymphatic complications following lymphadenectomy in patients with endometrial cancer. Taiwan J Obstet Gynecol. (2020) 59:420–4. doi: 10.1016/j.tjog.2020.03.015
26. Togami S, Kubo R, Kawamura T, Yanazume S, Kamio M, and Kobayashi H. Comparison of lymphatic complications between sentinel node navigation surgery and pelvic lymphadenectomy in patients with cervical cancer. Jpn J Clin Oncol. (2020) 50:543–7. doi: 10.1093/jjco/hyaa001
27. Yoshihara M, Shimono R, Tsuru S, Kitamura K, Sakuda H, Oguchi H, et al. Risk factors for late-onset lower limb lymphedema after gynecological cancer treatment: A multi-institutional retrospective study. Eur J Surg Oncol. (2020) 46:1334–8. doi: 10.1016/j.ejso.2020.01.033
28. Chang WI, Kang HC, Wu HG, Kim HJ, Jeon SH, Lee M, et al. Lower extremity lymphedema in gynecologic cancer patients: Propensity score matching analysis of external beam radiation versus brachytherapy. Cancers (Basel). (2019) 11(10):1471. doi: 10.3390/cancers11101471
29. Volpi L, Sozzi G, Capozzi VA, Ricco M, Merisio C, Di Serio M, et al. Long term complications following pelvic and para-aortic lymphadenectomy for endometrial cancer, incidence and potential risk factors: a single institution experience. Int J Gynecol Cancer. (2019) 29:312–9. doi: 10.1136/ijgc-2018-000084
30. Togami S, Kawamura T, Fukuda M, Yanazume S, Kamio M, and Kobayashi H. Risk factors for lymphatic complications following lymphadenectomy in patients with cervical cancer. Jpn J Clin Oncol. (2018) 48:1036–40. doi: 10.1093/jjco/hyy151
31. Hayes SC, Janda M, Ward LC, Reul-Hirche H, Steele ML, Carter J, et al. Lymphedema following gynecological cancer: Results from a prospective, longitudinal cohort study on prevalence, incidence and risk factors. Gynecol Oncol. (2017) 146:623–9. doi: 10.1016/j.ygyno.2017.06.004
32. Kim M, Suh DH, Yang EJ, Lim MC, Choi JY, Kim K, et al. Identifying risk factors for occult lower extremity lymphedema using computed tomography in patients undergoing lymphadenectomy for gynecologic cancers. Gynecol Oncol. (2017) 144:153–8. doi: 10.1016/j.ygyno.2016.10.037
33. Kuroda K, Yamamoto Y, Yanagisawa M, Kawata A, Akiba N, Suzuki K, et al. Risk factors and a prediction model for lower limb lymphedema following lymphadenectomy in gynecologic cancer: a hospital-based retrospective cohort study. BMC Womens Health. (2017) 17:50. doi: 10.1186/s12905-017-0403-1
34. Bae HS, Lim MC, Lee JS, Lee Y, Nam BH, Seo SS, et al. Postoperative lower extremity edema in patients with primary endometrial cancer. Ann Surg Oncol. (2016) 23:186–95. doi: 10.1245/s10434-015-4613-1
35. Ki EY, Park JS, Lee KH, and Hur SY. Incidence and risk factors of lower extremity lymphedema after gynecologic surgery in ovarian cancer. Int J Gynecol Cancer. (2016) 26:1327–32. doi: 10.1097/IGC.0000000000000757
36. Mitra D, Catalano PJ, Cimbak N, Damato AL, Muto MG, and Viswanathan AN. The risk of lymphedema after postoperative radiation therapy in endometrial cancer. J Gynecol Oncol. (2016) 27:e4. doi: 10.3802/jgo.2016.27.e4
37. Beesley VL, Rowlands IJ, Hayes SC, Janda M, O'Rourke P, Marquart L, et al. Incidence, risk factors and estimates of a woman's risk of developing secondary lower limb lymphedema and lymphedema-specific supportive care needs in women treated for endometrial cancer. Gynecol Oncol. (2015) 136:87–93. doi: 10.1016/j.ygyno.2014.11.006
38. Berger J, Scott E, Sukumvanich P, Smith A, Olawaiye A, Comerci J, et al. The effect of groin treatment modality and sequence on clinically significant chronic lymphedema in patients with vulvar carcinoma. Int J Gynecol Cancer. (2015) 25:119–24. doi: 10.1097/IGC.0000000000000311
39. Biglia N, Librino A, Ottino MC, Panuccio E, Daniele A, and Chahin A. Lower limb lymphedema and neurological complications after lymphadenectomy for gynecological cancer. Int J Gynecol Cancer. (2015) 25:521–5. doi: 10.1097/IGC.0000000000000341
40. Cirik DA, Karalok A, Ureyen I, Tasci T, Kalyoncu R, Turkmen O, et al. Early and late complications after inguinofemoral lymphadenectomy for vulvar cancer. Asian Pac J Cancer Prev. (2015) 16:5175–9. doi: 10.7314/APJCP.2015.16.13.5175
41. Hareyama H, Hada K, Goto K, Watanabe S, Hakoyama M, Oku K, et al. Prevalence, classification, and risk factors for postoperative lower extremity lymphedema in women with gynecologic Malignancies: a retrospective study. Int J Gynecol Cancer. (2015) 25:751–7. doi: 10.1097/IGC.0000000000000405
42. Todo Y, Yamazaki H, Takeshita S, Ohba Y, Sudo S, Minobe S, et al. Close relationship between removal of circumflex iliac nodes to distal external iliac nodes and postoperative lower-extremity lymphedema in uterine corpus Malignant tumors. Gynecol Oncol. (2015) 139:160–4. doi: 10.1016/j.ygyno.2015.07.003
43. Yamazaki H, Todo Y, Takeshita S, Ohba Y, Sudo S, Minobe S, et al. Relationship between removal of circumflex iliac nodes distal to the external iliac nodes and postoperative lower-extremity lymphedema in uterine cervical cancer. Gynecol Oncol. (2015) 139:295–9. doi: 10.1016/j.ygyno.2015.09.007
44. Deura I, Shimada M, Hirashita K, Sugimura M, Sato S, Sato S, et al. Incidence and risk factors for lower limb lymphedema after gynecologic cancer surgery with initiation of periodic complex decongestive physiotherapy. Int J Clin Oncol. (2015) 20:556–60. doi: 10.1007/s10147-014-0724-0
45. Hoogendam JP, Verheijen RH, Wegner I, and Zweemer RP. Oncological outcome and long-term complications in robot-assisted radical surgery for early stage cervical cancer: an observational cohort study. Bjog. (2014) 121:1538–45. doi: 10.1111/bjo.2014.121.issue-12
46. Yost KJ, Cheville AL, Al-Hilli MM, Mariani A, Barrette BA, McGree ME, et al. Lymphedema after surgery for endometrial cancer: prevalence, risk factors, and quality of life. Obstet Gynecol. (2014) 124:307–15. doi: 10.1097/AOG.0000000000000372
47. Achouri A, Huchon C, Bats AS, Bensaid C, Nos C, and Lécuru F. Complications of lymphadenectomy for gynecologic cancer. Eur J Surg Oncol. (2013) 39:81–6. doi: 10.1016/j.ejso.2012.10.011
48. Kondo E, Tabata T, Shiozaki T, Motohashi T, Tanida K, Okugawa T, et al. Large or persistent lymphocyst increases the risk of lymphedema, lymphangitis, and deep vein thrombosis after retroperitoneal lymphadenectomy for gynecologic Malignancy. Arch Gynecol Obstet. (2013) 288:587–93. doi: 10.1007/s00404-013-2769-0
49. Kim JH, Choi JH, Ki EY, Lee SJ, Yoon JH, Lee KH, et al. Incidence and risk factors of lower-extremity lymphedema after radical surgery with or without adjuvant radiotherapy in patients with FIGO stage I to stage IIA cervical cancer. Int J Gynecol Cancer. (2012) 22:686–91. doi: 10.1097/IGC.0b013e3182466950
50. Kasuya G, Ogawa K, Iraha S, Nagai Y, Shiraishi M, Hirakawa M, et al. Severe late complications in patients with uterine cancer treated with postoperative radiotherapy. Anticancer Res. (2011) 31(10):3527–33.
51. Ohba Y, Todo Y, Kobayashi N, Kaneuchi M, Watari H, Takeda M, et al. Risk factors for lower-limb lymphedema after surgery for cervical cancer. Int J Clin Oncol. (2011) 16:238–43. doi: 10.1007/s10147-010-0171-5
52. Walker KF, Day H, Abu J, Nunns D, Williamson K, and Duncan T. Do surgical techniques used in groin lymphadenectomy for vulval cancer affect morbidity rates? Int J Gynecol Cancer. (2011) 21:1495–9. doi: 10.1097/IGC.0b013e318228f314
53. Todo Y, Yamamoto R, Minobe S, Suzuki Y, Takeshi U, Nakatani M, et al. Risk factors for postoperative lower-extremity lymphedema in endometrial cancer survivors who had treatment including lymphadenectomy. Gynecol Oncol. (2010) 119:60–4. doi: 10.1016/j.ygyno.2010.06.018
54. Füller J, Guderian D, Köhler C, Schneider A, and Wendt TG. Lymph edema of the lower extremities after lymphadenectomy and radiotherapy for cervical cancer. Strahlenther Onkol. (2008) 184:206–11. doi: 10.1007/s00066-008-1728-3
55. Abu-Rustum NR, Alektiar K, Iasonos A, Lev G, Sonoda Y, Aghajanian C, et al. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus Malignancies: a 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol Oncol. (2006) 103:714–8. doi: 10.1016/j.ygyno.2006.03.055
56. Ryan M, Stainton MC, Slaytor EK, Jaconelli C, Watts S, and Mackenzie P. Aetiology and prevalence of lower limb lymphoedema following treatment for gynaecological cancer. Aust New Z J Obstetrics Gynaecology. (2003) 43:148–51. doi: 10.1046/j.0004-8666.2003.00040.x
57. Hong JH, Tsai CS, Lai CH, Chang TC, Wang CC, Lee SP, et al. Postoperative low-pelvic irradiation for stage I-IIA cervical cancer patients with risk factors other than pelvic lymph node metastasis. Int J Radiat Oncol Biol Phys. (2002) 53:1284–90. doi: 10.1016/S0360-3016(02)02831-6
58. Lu Q, Li Y, Chen TW, Yao Y, Zhao Z, Li Y, et al. Validity of soft-tissue thickness of calf measured using MRI for assessing unilateral lower extremity lymphoedema secondary to cervical and endometrial cancer treatments. Clin Radiol. (2014) 69:1287–94. doi: 10.1016/j.crad.2014.08.011
59. Beesley V, Janda M, Eakin E, Obermair A, and Battistutta D. Lymphedema after gynecological cancer treatment: prevalence, correlates, and supportive care needs. Cancer. (2007) 109:2607–14. doi: 10.1002/cncr.v109:12
60. Oonk MHM, Slomovitz B, Baldwin PJW, van Doorn HC, van der Velden J, de Hullu JA, et al. Radiotherapy versus inguinofemoral lymphadenectomy as treatment for vulvar cancer patients with micrometastases in the sentinel node: results of GROINSS-V II. J Clin Oncol. (2021) 39:3623–32. doi: 10.1200/JCO.21.00006
61. Kim SI, Lim MC, Lee JS, Kim YJ, Seo SS, Kang S, et al. Comparison of lower extremity edema in locally advanced cervical cancer: pretreatment laparoscopic surgical staging with tailored radiotherapy versus primary radiotherapy. Ann Surg Oncol. (2016) 23:203–10. doi: 10.1245/s10434-015-4653-6
62. Sudduth CL and Greene AK. Current overview of obesity-induced lymphedema. Adv Wound Care (New Rochelle). (2022) 11:392–8. doi: 10.1089/wound.2020.1337
63. Sudduth CL and Greene AK. Lymphedema and obesity. Cold Spring Harb Perspect Med. (2022) 12(5). doi: 10.1101/cshperspect.a041176
64. Guliyeva G, Huayllani MT, Boczar D, Avila FR, Lu X, and Forte AJ. Age as a risk factor for breast cancer-related lymphedema: a systematic review. J Cancer Surviv. (2023) 17:246–53. doi: 10.1007/s11764-021-00994-z
Keywords: gynecological cancer, risk factors, lower extremity lymphedema, review, incidence
Citation: Decorte T, Cerckel M, Kheir GB, Monten C, Vandecasteele K, Vanden Bossche L, Pauwels NS and Randon C (2025) Risk factors for lower limb lymphedema after gynecological cancer treatment: a systematic review. Front. Oncol. 15:1561836. doi: 10.3389/fonc.2025.1561836
Received: 16 January 2025; Accepted: 25 April 2025;
Published: 20 May 2025.
Edited by:
Liliana Mereu, AOU Policlinico “G.Rodolico-San Marco”, ItalyReviewed by:
Gabriele Centini, University of Siena, ItalyRamon Rovira Negre, Universitat Autònoma de Barcelona, Spain
Copyright © 2025 Decorte, Cerckel, Kheir, Monten, Vandecasteele, Vanden Bossche, Pauwels and Randon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tina Decorte, dGluYS5kZWNvcnRlQHV6Z2VudC5iZQ==
 Tina Decorte
Tina Decorte Marie Cerckel2
Marie Cerckel2 Katrien Vandecasteele
Katrien Vandecasteele