- 1Department of Postgraduate, Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, China
- 2Dermatology Department, Affiliated Hospital of Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, China
Background: Skin diseases induced by Sintilimab, a programmed cell death protein-1 (PD-1) inhibitor, are rare, with only 28 cases reported. We provide a literature review on skin diseases associated with Sintilimab and summarize the patient’s primary disease, duration of Sintilimab use, treatment, and disease progression. This study aims to improve understanding of Stevens–Johnson syndrome (SJS) induced by this monoclonal antibody and its treatment strategies.
Case description: We report a case of SJS induced by Sintilimab in a patient treated at our hospital. The patient exhibited widespread erythema, papules, and vesicles, accompanied by mucosal erosion and exudation in the oral cavity, eyes, urethral orifice, and perianal region. The patient was immediately treated with intravenous methylprednisolone sodium succinate (40 mg/day), antihistamines, and supportive care, including fluid replacement and wound care. His symptoms gradually improved, and he was discharged after 20 days. At the 6-month follow-up, he remained stable, with no recurrence of symptoms.
Conclusions: Although severe drug rash, including SJS, caused by PD-1 inhibitors is relatively uncommon, its underlying molecular pathogenesis remains unclear. Physicians should remain vigilant regarding potential adverse reactions when prescribing Sintilimab. If severe reactions occur, discontinuation of chemotherapy and immediate administration of adequate corticosteroids with symptomatic support can help reduce morbidity and mortality.
Introduction
PD-1 inhibitors are a class of immune checkpoint inhibitors (ICIs) widely used in the treatment of various malignancies. These inhibitors disrupt the interaction between PD-1 and PD-L1, thereby blocking the binding of immune cells to tumor cells and activating the anti-tumor activity of lymphocytes (1). Currently, these drugs are widely used in the treatment of melanoma (2), non-small cell lung cancer (NSCLC), hepatocellular carcinoma, and other malignancies. However, their immune-modulating effects can lead to excessive immune activation, resulting in multi-system adverse reactions, particularly cutaneous toxicities (3). Skin-related adverse effects occur in approximately 40% of cases and include pruritus, vitiligo, psoriasis, capillary hyperplasia (4), erythema multiforme, SJS, and TEN. The latter two types, although rare, are severe and potentially fatal. This report presents a case of severe SJS induced by the use of a PD-1 inhibitor (Sintilimab) and provides a literature review of similar cases.
Case description
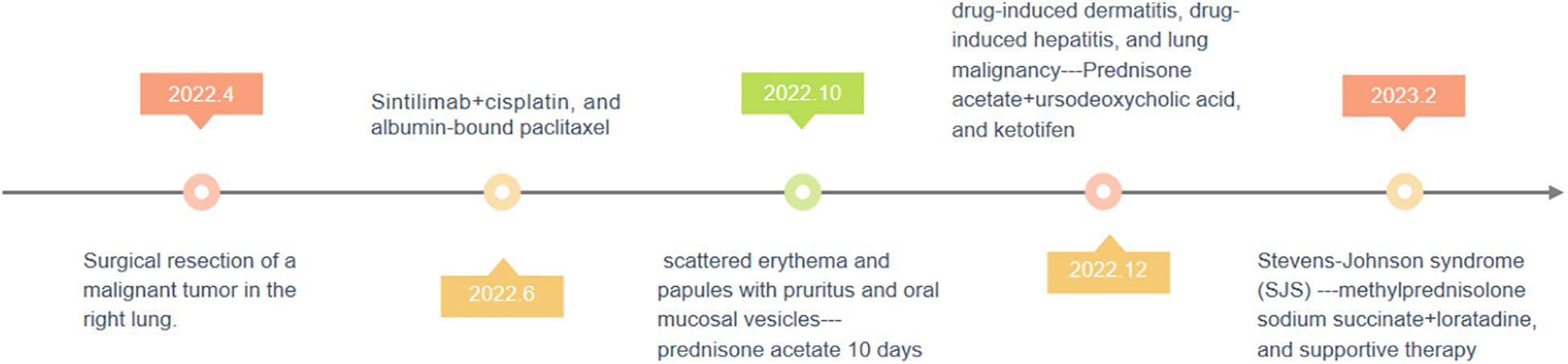
On 2 February 2023, a 49-year-old male presented with widespread erythema, blisters, itching, and pain that had persisted for 3 months and worsened over the past 3 days. In April 2022, he underwent surgery for a malignant tumor of the right lung; pathological examination confirmed adenosquamous carcinoma of the right upper lung, predominantly squamous cell carcinoma with 10% adenocarcinoma. On 8 June, 2022, he commenced chemotherapy with Sintilimab (200 mg), cisplatin (90 mg), and albumin-bound paclitaxel (300 mg). After the fourth cycle of chemotherapy in October 2022, he developed scattered erythema and papules with pruritus and oral mucosal vesicles. His oncologist recommended discontinuation of chemotherapy and initiated oral prednisone acetate (30 mg/day). Ten days later, as the presentation improved, the patient stopped the drug of prednisone, and erythema and vesicles gradually developed all over the body. In December 2022, he was hospitalized again and diagnosed with drug-induced dermatitis, drug-induced hepatitis, and lung malignancy. Prednisone acetate, ursodeoxycholic acid, and ketotifen were prescribed, yet the specific drug dose was unknown. Sintilimab was discontinued, and the patient was discharged upon symptom resolution. Three days ago, on 2 February 2023 (Figure 1), he experienced a sudden exacerbation of erythema, papules, and blisters over his entire body. The lesions exhibited a target-like appearance, predominantly affecting the limbs. Additionally, the patient presented with erosion of the oral mucosa, urethral orifice, and anal mucosa, and crusting of the lips with yellow discharge. Other symptoms included limitation of mouth opening, pharyngeal pain, dysphagia, redness and swelling of the eyelids, conjunctival congestion, photophobia, tearing, dry mouth, and a bitter taste. There were no signs of fever, joint pain, or chills. The patient had pain during urination, which suggested the involvement of a urinary tract infection. He had a history of diabetes mellitus and lung tumor surgery. Therefore, he was admitted to the Dermatology ward with a diagnosis of erythema multiforme, abnormal liver function, postoperative lung tumor, and type 2 diabetes mellitus (Figure 2).
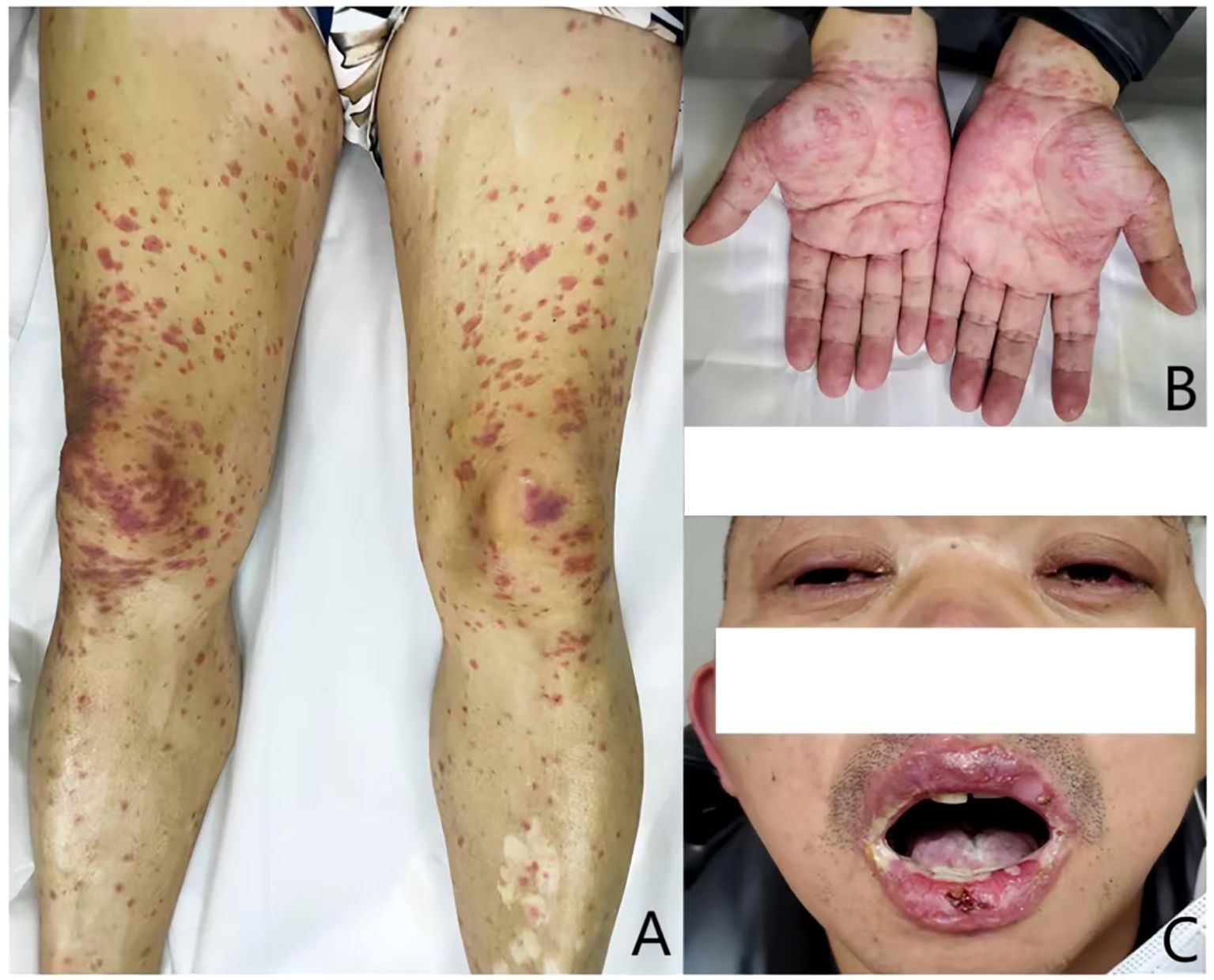
Figure 2. Skin and mucosal damage before antiallergic therapy: (A) generalized edematous erythema on limbs; (B) the skin lesions exhibited a target-like appearance; and (C) erosion of the oral mucosa and blephar.
Admission examination showed the following: T, 36.7°C; P, 126 beats/min; R, 20 breaths/min; and BP, 129/90 mmHg. Lung auscultation reveals weakened respiratory sounds. No obvious abnormalities are observed in the heart and abdomen. A 6-cm long surgical scar is visible on the right side of the chest. Neurological examinations are negative. Routine laboratory examinations revealed abnormal levels of neutrophil percentage (81.5%) and lymphocyte percentage (14.1%). Blood chemistry revealed increased gamma-glutamyl transpeptidase (212.4 U/L) and total IgE (113.4I U/mL) levels. Analysis of urine–stool samples showed the presence of ketones (+), sugar (4+), and occult blood (+). Glycated hemoglobin and general bacterial culture showed no significant abnormalities.
Using the Severity-of-Illness Score for Toxic Epidermal Necrolysis (SCORTEN) scoring system (5), the patient’s score of 4 indicated a 62% mortality risk. As this patient was at high risk, routine blood tests, liver and kidney function, blood glucose, electrolytes, and other important indicators were monitored to assess the progression. Sintilimab was suspected as the allergen, and treatment included methylprednisolone sodium succinate (40 mg qd), loratadine (10 mg qd), and supportive therapy (gastric and hepatic protection and electrolyte correction). Skin care treatment was actively applied, including extraction of blisters, mupirocin ointment, and mometasone furoate cream for external use on erythematous spots, 0.9% saline for oral cavity, tobramycin dexamethasone eye drops and erythromycin eye ointment for eye care, avoiding light; within regions of genitalia, perianal area, saline external washing, and external application of human epidermal growth factor gel and mupirocin ointment were used. The patient’s skin erythema subsided, the blisters dried, and crusts were formed, and the erosion in the oral cavity and external genitalia was relieved. As no new lesions developed and the general condition improved, the patient was discharged on 22 February 2023 (Figure 3). At the 6-month follow-up, he had not resumed Sintilimab treatment, and no recurrence of symptoms was observed. He also reported that the skin and mucosal symptoms had significantly impacted his daily life. However, after receiving treatment, his condition gradually improved. He expressed satisfaction with the treatment outcome, stating that it exceeded his expectations and helped him regain confidence in his health.
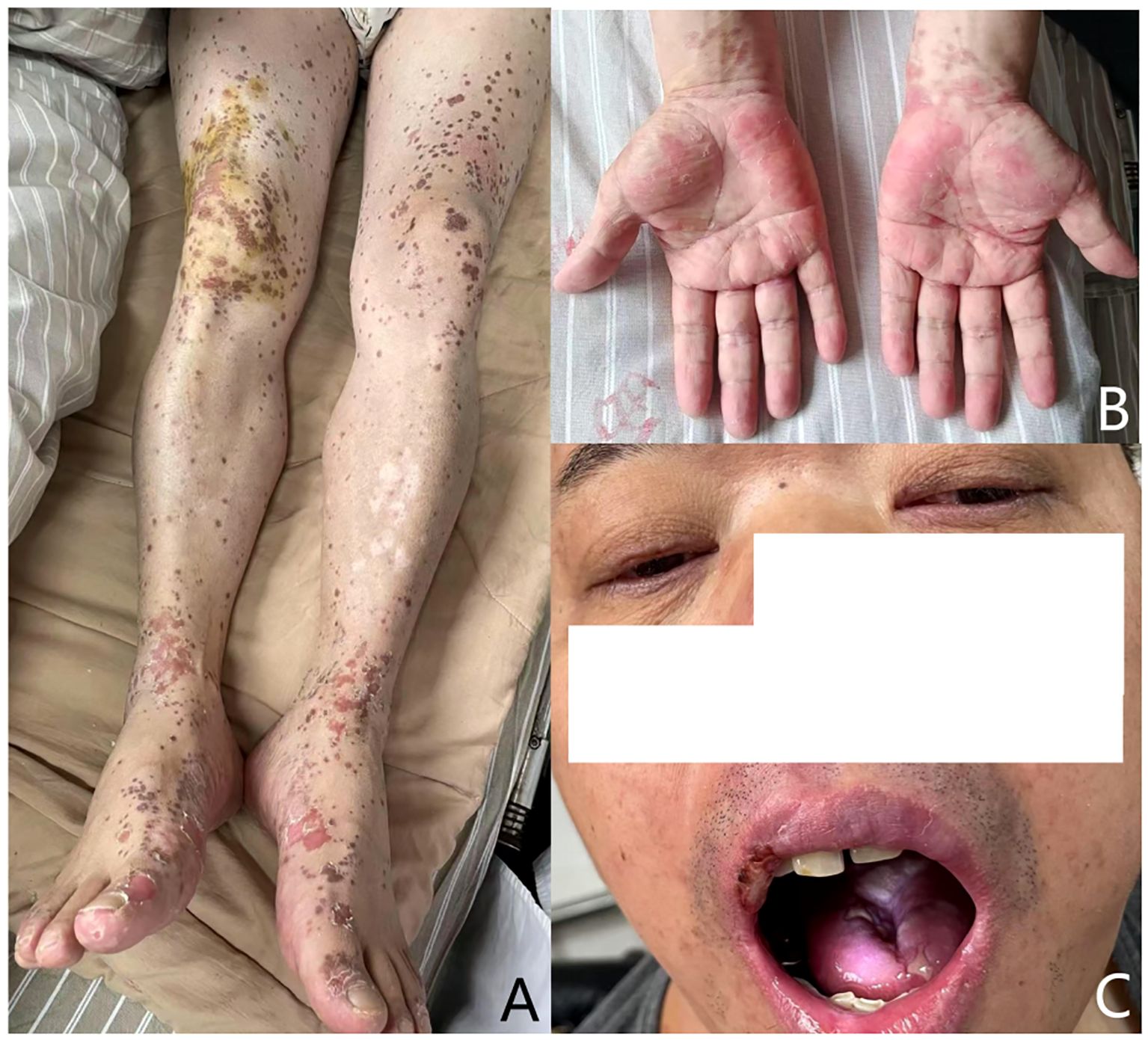
Figure 3. Lesions improved after 14 days’ therapy: (A) the vesicular and hemorrhagic lesions on the limbs and trunk had mostly resolved; (B) target-like eruption relieved; (C) significant improvement in the erosion of the lips and oral mucosa.
Literature review
We searched PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase, Web of Science, Google Scholar (http://scholar.google.com/), WanFang Database, and Chinese National Knowledge Infrastructure (CNKI). Keywords included “sintilimab,” “sintilizumab,” “PD-1 inhibitor,” “tumor,” “tumour,” “skin disease,” “Dermatological disease,” and “Stevens–Johnson syndrome” published in English or Chinese language. Inclusion criteria included the following: (1) study types include case reports, case series, retrospective studies, prospective studies, systematic reviews, and meta-analyses; (2) literature published in English and Chinese to ensure coverage of relevant studies from major medical databases; and (3) studies focusing on cutaneous adverse reactions induced by Sintilimab. Exclusion criteria included the following: (1) conference abstracts or discussions; (2) literature for which full text is unavailable; (3) case reports or studies unrelated to Sintilimab; and (4) cases without a confirmed diagnosis of cutaneous adverse reactions.
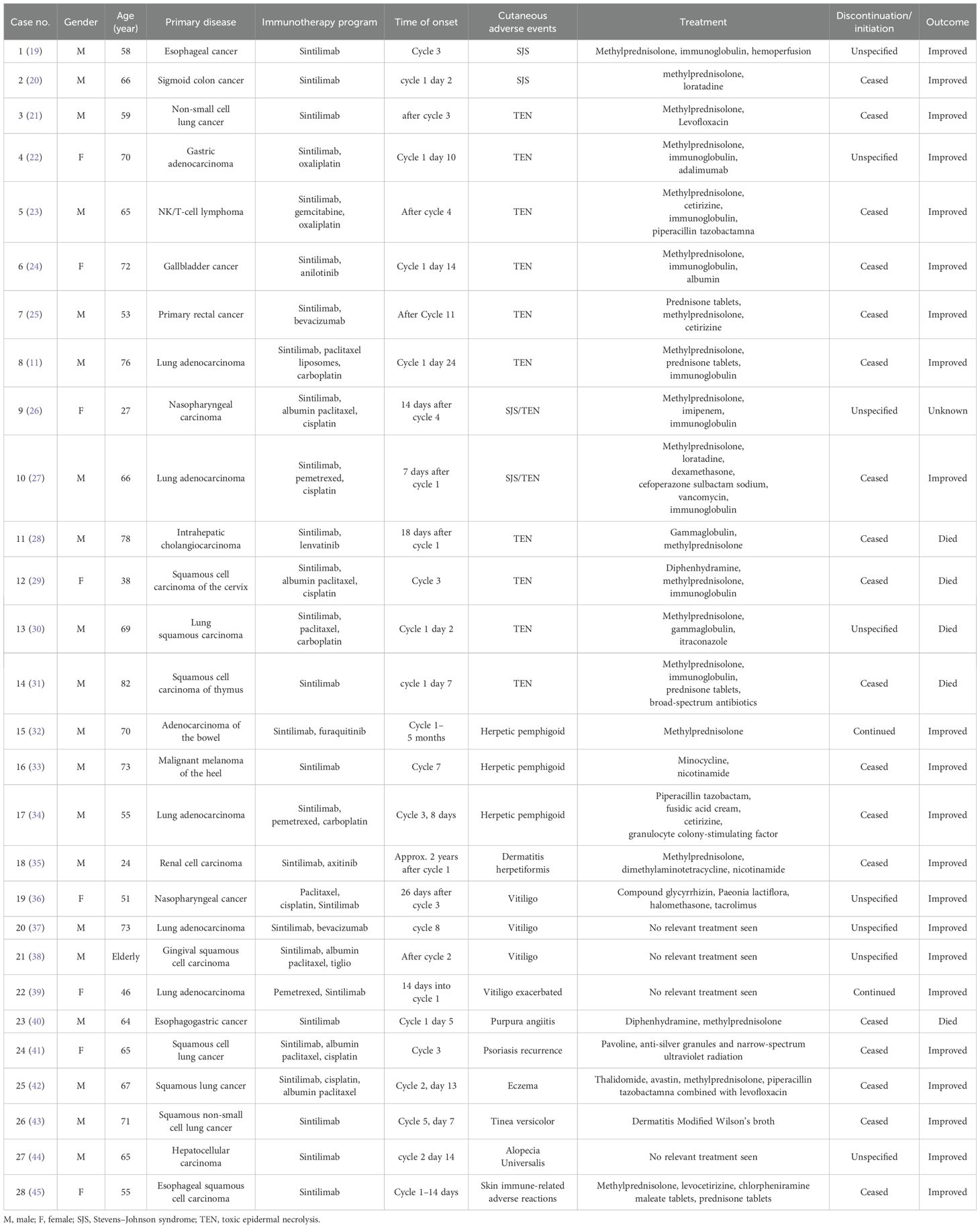
A total of 28 cases of Sintilimab-induced cutaneous adverse reactions were documented (Table 1), including 14 cases of TEN and severe erythematous drug eruptions, three herpetic pemphigoid, one herpetic dermatitis, four vitiligo, one purpura-like vasculitis, one eczema, one aggravated psoriasis, one lichen-like dermatitis, one pruritus, and one unexplained skin immune-related adverse reactions. There were 20 male patients (71.4%) with the age of 24–82 (64.7 ± 12.2) years, and eight women (28.6%) with 27–72 (53 ± 15.8) years. The most cases with primary disease were lung cancer (10 cases), followed by esophageal cancer (three cases), intestinal cancer (three cases), hepatocellular carcinoma (two cases), nasopharyngeal carcinoma (two cases), and one case of gastric cancer, lymphoma, gallbladder cancer, cervical cancer, malignant melanoma, renal carcinoma, thymus carcinoma, and gingival carcinoma, respectively. Most patients (19/28, 67.86%) had adverse reactions within 50 days after the first administration, and the longest incubation period is 2 years. More than half of the patients had erythema and blisters as skin lesions (64.3%). As treatment, glucocorticoids were routinely used, besides immunoglobulins (37.5%), antibiotics (41.7%), and antihistamines (33.3%) were commonly used (Table 1). The administration of the antitumor regimen was ceased in a total of 18 patients, continued in two cases, and information unknown in eight cases. After treatment, 22 patients’ skin damage improved or cured, five patients died, and one patient was lost. Of the five died cases, three died from SJS and toxic epidermal necrolysis.
Discussion
SJS is a rare severe drug-induced hypersensitivity reaction affecting the skin and mucous membranes (6). The disease usually starts with flu-like symptoms, i.e., fever, cough, epipephysitis, rhinitis, oral ulcers, and arthralgia (7), followed by a painful rash of erythema, papules, urticaria, and purpura. Skin lesions can occur on any part of the body and are often associated with mucosal lesions, including mouth, lips, eyes, respiratory system, digestive system, and urogenital tracts (8). Diffuse purplish-red or dark-red plaques, and the erythema gradually merge into a patch, further forming loose blisters and massive epidermal exfoliation. Severe infection, i.e., septicemia is the most cause of death (9). The SCORTEN scoring is used to predict the risk of mortality, and this patient got a score of 4 and had a high death risk (62%) (10). Luckily, with active treatment of systemic glucocorticoids and adjuvant therapy, he was discharged from the hospital with a full recovery. In comparison to Li et al. (11), which reported a 76-year-old lung adenocarcinoma patient with SJS following Sintilimab treatment, our case involves a younger (49-year-old) patient with adenosquamous carcinoma. Notably, they identified PD-L1 expression in the glands and basal layer of the skin, which may have contributed to localized immune activation leading to SJS. Our case, although lacking immunohistochemical analysis, suggests that tumor histology and patient-specific factors might influence the clinical presentation and severity of SJS induced by PD-1 inhibitors. This highlights the need for further studies examining the immunopathological mechanisms underlying SJS across different tumor types.
The pathogenesis of severe SJS is not yet fully understood and T-lymphocyte-mediated immune reaction causing destruction of the keratinocytes-expressing foreign antigens is regarded as the primary cause of blistering and edematous erythema (12). Drugs are the most common causes, with penicillins, sulfonamides, non-steroidal anti-inflammatory drugs (NSAIDs), carbamazepine, allopurinol, and immunosuppressants as the commonest drugs for SJS (13). Genetic studies have demonstrated significant associations between specific human leukocyte antigen (HLA) alleles and an increased risk of severe cutaneous adverse reactions. For example, HLA-B15:02 is linked to carbamazepine-induced SJS/TEN in Han Chinese populations, while HLA-B58:01 is associated with allopurinol-induced severe cutaneous reactions. Although genetic testing was not performed in our case, the potential role of HLA polymorphisms in PD-1 inhibitor-induced SJS should not be overlooked. Further research into the immunogenetic mechanisms underlying SJS/TEN could pave the way for predictive screening strategies to enhance patient safety (14). Anticancer drugs like immune checkpoint inhibitors are also reported to cause SJS/TEN. As PD-1/PD-L1 inhibitors are increasingly used in clinical, and their potential side effects should be of concern. Cancer patients might be at a higher risk of developing severe blistering and toxic reactions, due to the nature of neoplastic diseases more exposure to a line of anticancer drugs, and disruption of the immune system (15). These anticancer drugs may trigger an abnormal cytotoxic T-lymphocyte response, predisposing them to SJS/TEN.
While our study focuses on the acute immune-mediated pathogenesis of SJS, it is important to differentiate this from chronic epigenetic-driven skin stress responses, such as ultraviolet (UV)-induced photoaging. Song et al. (16) demonstrated that carnitine acetyltransferase (CRAT) downregulation via promoter hypermethylation leads to increased matrix metalloproteinase-1 (MMP-1) expression, which contributes to collagen degradation and chronic skin damage. In contrast, SJS is characterized by rapid, immune-mediated keratinocyte apoptosis rather than gradual fibroblast dysfunction. This distinction underscores the diverse molecular mechanisms governing skin injury—one being a slow, epigenetically driven process (photoaging), and the other a swift, cytotoxic event mediated by immune activation (SJS).
Based on our analysis, when patients receiving anticancer therapy with Sintilimab develop erythema, and maculopapular rashes, particularly vesicular and mucosal lesions, clinicians should strongly suspect SJS and immediately discontinue the medication. Extensive epidermolysis can lead to multi-organ damage, including fluid and electrolyte imbalance, thermoregulatory dysfunction, hepatic and renal impairment, and secondary infections. In addition to anti-allergy medications, comprehensive and supportive treatment is essential, including patient isolation, continuous monitoring of vital signs, meticulous wound care (cleaning necrotic vesicles and scabs), and appropriate anti-infective therapy. Furthermore, rehydration and nutritional support are crucial for recovery. Among the systemic treatment options are intravenous immunoglobulins, glucocorticoids, and cyclosporine; however, glucocorticoids remain the first-line treatment (17). Studies have demonstrated that early and adequate systemic glucocorticoid administration effectively controls disease progression and significantly reduces mortality compared to non-users (18). In this case, the patient was treated with methylprednisolone sodium succinate symptomatically after being admitted to the hospital, and the clinical outcome was remarkable. Moreover, in our review analysis, all the patients received glucocorticoid therapy.
Conclusion
Sintilimab, a PD-1 inhibitor, was launched in China in December 2018 with a relatively short period of use. There are few reports of its adverse effects up to now, and therefore, more caution should be exercised with these new drugs. In this review, we analyze the significant association between “skin eruption” and “Sintilimab” and find that more than half of the cases presented as SJS/TEN. Therefore, clinicians should be aware of the potential for severe immune-related dermatological toxicity when administering PD-1 inhibitor reactions. If skin erythema, papules, or vesicles occur during anticancer therapy with Sintilimab, the chemotherapy regimen should be immediately discontinued, and an alternative treatment strategy should be promptly considered. Adequate systemic glucocorticosteroid therapy should be initiated to mitigate inflammatory and toxic reactions, which is essential for infection prevention and disease control. Supportive care also plays a crucial role in reducing morbidity and mortality associated with severe adverse reactions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HK: Investigation, Writing – review & editing. DH: Conceptualization, Writing – original draft, Writing – review & editing. CH: Writing – original draft. LG: Writing – original draft. ZY: Writing – original draft. XZ: Writing – original draft. HL: Writing – original draft. GH: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The funding for this study was supported by the Natural Science Foundation of Jiangxi Provincial (#20212BAB206064), the National Administration of Traditional Chinese Medicine Talent Education (2022), and Jiangxi University of Chinese Medicine Science and Technology Innovation Team Development Program.
Acknowledgments
During the preparation of this article, I would like to express my sincere gratitude to the patient mentioned in this case report and to all individuals who have provided me with assistance and support. Their contributions have enabled the successful completion of this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1568316/full#supplementary-material
References
1. Bhardwaj M, Chiu MN, and Pilkhwal Sah S. Adverse cutaneous toxicities by Pd-1/Pd-L1 immune checkpoint inhibitors: pathogenesis, treatment, and surveillance. Cutan Ocular Toxicol. (2022) 41:73–90. doi: 10.1080/15569527.2022.2034842
2. Tang B, Chi Z, and Guo J. Toripalimab for the treatment of melanoma. Expert Opin Biol Ther. (2020) 20:863–9. doi: 10.1080/14712598.2020.1762561
3. Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, and Chandra AB. Current diagnosis and management of immune related adverse events (Iraes) induced by immune checkpoint inhibitor therapy. Front Pharmacol. (2017) 8:49. doi: 10.3389/fphar.2017.00049
4. Wang F, Qin S, Sun X, Ren Z, Meng Z, Chen Z, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol. (2020) 13:47. doi: 10.1186/s13045-020-00886-2
5. Dobry AS, Himed S, Waters M, and Kaffenberger BH. Scoring assessments in Stevens-Johnson syndrome and toxic epidermal necrolysis. Front Med. (2022) 9:883121. doi: 10.3389/fmed.2022.883121
6. Stern RS and Divito SJ. Stevens-Johnson syndrome and toxic epidermal necrolysis: associations, outcomes, and pathobiology-thirty years of progress but still much to be done. J Invest Dermatol. (2017) 137:1004–8. doi: 10.1016/j.jid.2017.01.003
7. de Arruda JA, Silva P, Amaral MB, Cotta F, Avendanho R, and Mesquita R. Erythema multiforme induced by alendronate sodium in a geriatric patient: A case report and review of the literature. J Clin Exp Dent. (2017) 9:e929–e33. doi: 10.4317/jced.53653
8. Siedner-Weintraub Y, Gross I, David A, Reif S, and Molho-Pessach V. Paediatric erythema multiforme: epidemiological, clinical and laboratory characteristics. Acta Derm Venereol. (2017) 97:489–92. doi: 10.2340/00015555-2569
9. Chen CB and Chung WH. Current agreement on the management of Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. (2021) 185:484–6. doi: 10.1111/bjd.20515
10. Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, and Wolkenstein P. Scorten: A severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. (2000) 115:149–53. doi: 10.1046/j.1523-1747.2000.00061.x
11. Li X, Li G, Chen D, Su L, Wang RP, and Zhou Y. Case report: sintilimab-induced Stevens-Johnson syndrome in a patient with advanced lung adenocarcinoma. Front Oncol. (2023) 13:912168. doi: 10.3389/fonc.2023.912168
12. Mockenhaupt M. Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management. Semin Cutan Med Surg. (2014) 33:10–6. doi: 10.12788/j.sder.0058
13. Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, and Harr T. Current perspectives on Stevens-Johnson syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol. (2018) 54:147–76. doi: 10.1007/s12016-017-8654-z
14. Chen CB, Lee CC, Wang CW, Hung WK, and Chung W. Genetic associations of human leukocyte antigen alleles in cutaneous delayed drug hypersensitivity reactions: An updated review. Dermatol Sin. (2023) 41:183–98. doi: 10.4103/ds.DS-D-23-00082
15. Dika E, Ravaioli GM, Fanti PA, Piraccini BM, Lambertini M, Chessa MA, et al. Cutaneous adverse effects during ipilimumab treatment for metastatic melanoma: A prospective study. Eur J Dermatol: EJD. (2017) 27:266–70. doi: 10.1684/ejd.2017.3023
16. Song MJ, Kim MK, Park CH, Kim H, Lee SH, Lee DH, et al. Downregulation of carnitine acetyltransferase by promoter hypermethylation regulates ultraviolet-induced matrix metalloproteinase-1 expression in human dermal fibroblasts. J Dermatol Sci. (2024) 116:70–7. doi: 10.1016/j.jdermsci.2024.09.005
17. Kridin K, Brüggen MC, Chua SL, Bygum A, Walsh S, Nägeli MC, et al. Assessment of treatment approaches and outcomes in Stevens-Johnson syndrome and toxic epidermal necrolysis: insights from a Pan-European multicenter study. JAMA Dermatol. (2021) 157:1182–90. doi: 10.1001/jamadermatol.2021.3154
18. Ye YJ, Zhu Y, and Xu AE. Clinical analysis of 73 cases of severe erythema multiform and toxic epidermal necrolysis. Chinese Journal of General Practice. (2017) 15(10):1691-4,1794. doi: 10.16766/j.cnki.issn.1674-4152.2017.10.016
19. Zha W, Wen W, Wang Y, and Shan JJ. A case of toxic epidermal necrolysis caused by cindilizumab and treatment countermeasures. Practical Pharmacy and Clinical Remedies. (2023) 26(9):843–6. doi: 10.14053/j.cnki.ppcr.202309016
20. Zhang M, Wu R, Jia M, Sun S, Zhang L, and Tang T. Sintilimab-induced erythema multiforme drug eruption in the treatment of sigmoid colon cancer: A case report and literature review. Medicine. (2023) 102:e35659. doi: 10.1097/md.0000000000035659
21. Li G, Gong S, Wang N, and Yao X. Toxic epidermal necrolysis induced by sintilimab in a patient with advanced non-small cell lung cancer and comorbid pulmonary tuberculosis: A case report. Front Immunol. (2022) 13:989966. doi: 10.3389/fimmu.2022.989966
22. Zhang L and Wu Z. Adalimumab for sintilimab-induced toxic epidermal necrolysis in a patient with metastatic gastric Malignancy: A case report and literature review. Clin Cosmet Investig Dermatol. (2023) 16:457–61. doi: 10.2147/ccid.S401286
23. Yang W, Xu X, Xia D, Wang H, Jiang J, and Yang G. Toxic epidermal necrolysis associated with chemoimmunotherapy for lymphoma: case report and literature review. Immunotherapy. (2022) 14:275–82. doi: 10.2217/imt-2021-0074
24. Zhao Y, Cao Y, Wang X, and Qian T. Treatment of Pd-1 inhibitor-associated toxic epidermal necrolysis: A case report and brief review. OncoTargets Ther. (2022) 15:345–51. doi: 10.2147/ott.S353743
25. Tian XM, Lu LS, Li JY, Shi CF, Luo K, Liu M, et al. A case of severe skin immunotoxicity and the literature. China Journal of Rational Drug Use. (2022) 19(9):94–101. doi: 10.3969/j.issn.2096-3327.2022.09.012
26. Huang Y, Zhu L, Ma X, Hong Y, Su X, Lai W, et al. A case of sintilimab-induced Sjs/Ten: dermatologic adverse reactions associated with programmed cell death protein-1 inhibitors. Dermatol Ther. (2022) 35:e15663. doi: 10.1111/dth.15663
27. Jiang Z, Chen X, Sun Z, Shen X, Huang Y, and Liu J. Toxic epidermal necrolysis and Stevens - Johnson syndrome following sintilimab administration in a non-small cell lung cancer patient: A case report. J Inflammation Res. (2023) 16:5061–7. doi: 10.2147/jir.S427336
28. Gong Y, Mao J, Liu M, and Gao J. A case of toxic epidermal necrolysis associated with lenvatinib and sintilimab therapy for intrahepatic cholangiocarcinoma. J Int Med Res. (2023) 51:3000605231173556. doi: 10.1177/03000605231173556
29. Li X, Qu LX, Ren YM, and Hu C. Case report: A case report and literature review on severe bullous skin reaction induced by anti-Pd-1 immunotherapy in a cervical cancer patient. Front Pharmacol. (2021) 12:707967. doi: 10.3389/fphar.2021.707967
30. Li LM and Zhang SP. Two cases of toxic epidermal necrolysis caused by PD-1 inhibitor and literature review. Chinese Journal of Dermatovenereology. (2022) 36(7):815–8. doi: 10.13735/j.cjdv.1001-7089.202109096
31. Yang H, Ma Q, Sun Y, Zhang K, Xing Y, and Li H. Case report: toxic epidermal necrolysis associated with sintilimab in a patient with relapsed thymic carcinoma. Front Oncol. (2022) 12:1065137. doi: 10.3389/fonc.2022.1065137
32. Wang T, Shao Q, Xiao C, and Liu L. Case report: bullous pemphigoid associated with sintilimab therapy for Pmmr/Mss colorectal cancer. Front Oncol. (2023) 13:1124730. doi: 10.3389/fonc.2023.1124730
33. Sun J and Gong C. PD-1 inhibitor causes bullous pemphigoid in 3 cases reports and literature analysis. Chin J New Drug. (2022) 31:509–12.
34. Yan QX, Fang C, Pan Y, Mei ZX, and Tang WJ. Sindilizumab treated advanced lung adenocarcinoma causing bullous dermatitis in a case. Chinese Journal of New Drugs and Clinical Remedies. (2022) 41(4):253–6. doi: 10.14109/j.cnki.xyylc.2022.04.13
35. He J, Duan X, Liu T, Yang H, Jiang J, and Mu Y. A case of systemic severe bullous pemphigoid caused by long-term sintilimab treatment for renal cell carcinoma. Clin Cosmet Investig Dermatol. (2022) 15:1611–4. doi: 10.2147/ccid.S374449
36. Yu ZY, Lou YN, Jia LQ, Zhang ZH, Qu J, Liu F, et al. Vitiligo-like depigmentation caused by cindilizumab in one case and literature review. Central South Pharmacy. (2022) 20(9):2204–7. doi: 10.7539/j.issn.1672-2981.2022.09.043
37. Wang D, Tang T, Gao J, Wang N, Fan YX, Wu L, et al. PD-1-antibody treatment of lung cancer showed a case of vitiligo-like skin reaction and immune checkpoint inhibitor-related pneumonia. Journal of Practical Oncology. (2023) 38(5):480–6. doi: 10.13267/j.cnki.syzlzz.2023.075
38. Zhang C, Tao Y, Hu JL, Zhang TT, and Gao WC. A case of vitiligo caused by Sindilizumab injection. Chinese Journal of Modern Applied Pharmacy. (2021) 38(19):2431–2. doi: 10.13748/j.cnki.issn1007-7693.2021.19.015
39. Rao H, Guo Z, Wen X, Zeng X, Wu L, and Huang L. Case report: immune checkpoint inhibitor-related vitiligo-like depigmentation in non-melanoma advanced cancer: A report of three cases and a pooled analysis of individual patient data. Front Oncol. (2022) 12:1099108. doi: 10.3389/fonc.2022.1099108
40. Wu YP, Zhang LN, and Yin YS. Rare purpuroid cutaneous vasculitis caused by cindilizumab. Chinese Journal of Hospital Pharmacy. (2022) 42(12):1290–2. doi: 10.13286/j.1001-5213.2022.12.22
41. Huang W, Liu Y, Li M, Xue Y, Bao W, and Guo Y. Sintilimab-related diabetes mellitus and psoriasis: A case report and literature review. Medicine. (2023) 102:e35946. doi: 10.1097/md.0000000000035946
42. Yan J, Ma N, Qiao WL, Liu KQ, Liu DW, Wang Y, et al. Adverse skin reactions induced by sintilimab in advanced lung squamous carcinoma: A case report and review of the literature. Ann Trans Med. (2022) 10:1411. doi: 10.21037/atm-22-5925
43. Liu Y, Tang J, Yu LY, and Jiang Q. Successful treatment of immune-related lichenoid dermatitis by weiling decoction in a patient with non-small cell lung cancer: A case report and review of literature. Explore (New York NY). (2023) 19:730–5. doi: 10.1016/j.explore.2023.02.008
44. Wen L, Zhao J, Yang Y, Chen W, Bao Y, Zhang J, et al. Sintilimab-induced alopecia universalis in a patient with the anti-tumor effect of complete remission after hepatectomy. J Immunother (Hagerstown Md: 1997). (2023) 46:232–5. doi: 10.1097/cji.0000000000000473
Keywords: Stevens-Johnson syndrome, PD-1 inhibitor, Sintilimab, case report, literature review
Citation: Kuang H, Huang D, Hu C, Gong L, Yu Z, Zhu X, Lan H and Huang G (2025) Stevens–Johnson syndrome induced by Sintilimab: a case report and literature review. Front. Oncol. 15:1568316. doi: 10.3389/fonc.2025.1568316
Received: 29 January 2025; Accepted: 23 April 2025;
Published: 22 May 2025.
Edited by:
Paul Takam Kamga, Université de Versailles Saint-Quentin-en-Yvelines, FranceCopyright © 2025 Kuang, Huang, Hu, Gong, Yu, Zhu, Lan and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Huang, Y2hpbmFwb3J0NzhAMTI2LmNvbQ==
 Huan Kuang1
Huan Kuang1 Gang Huang
Gang Huang