- 1Department of General Surgery, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 2Department of Pharmacy, Guangxi Hospital Division of The First Affiliated Hospital, Sun Yat-sen University, Nanning, Guangxi, China
- 3School of Pharmaceutical Science, Guangxi Medical University, Nanning, Guangxi, China
- 4State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, Jiangsu, China
- 5Center for New Drug Safety Evaluation and Research, China Pharmaceutical University, Nanjing, Jiangsu, China
- 6School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu, China
Background: The relationship between the triglyceride-glucose (TyG) index and the risk of colorectal carcinogenesis, encompassing both colorectal adenomas and carcinoma, is not yet definitively established. This meta-analysis aims to synthesize available evidence to provide a comprehensive estimate of the association between the TyG index and the likelihood of this disease spectrum.
Methods: A systematic search was conducted in PubMed, Embase, and Scopus to identify studies examining the TyG index and colorectal carcinogenesis risk. Meta-analysis was performed to calculate effect sizes (ESs) and their corresponding 95% confidence intervals (CIs) to obtain a summary estimate.
Results: A total of nine observational studies involving 1,056,401 participants were included in the analysis. The meta-analysis revealed that a continuous increase in the TyG index was associated with an elevated risk of colorectal carcinogenesis (ES, 1.19; 95% CI, 1.05-1.34). Similarly, a one-unit increase in the TyG index was linked to an increased risk of colorectal carcinogenesis (ES, 1.24; 95% CI, 1.15-1.32). Additionally, compared to the lowest quartile, the second (ES, 1.07; 95% CI, 1.02-1.13), third (ES, 1.12; 95% CI, 1.06-1.18), and highest quartile (ES, 1.19; 95% CI, 1.13-1.24) of the TyG index exhibited a significantly higher risk of colorectal carcinogenesis.
Conclusion: This meta-analysis demonstrates a significant association between the TyG index and colorectal carcinogenesis, suggesting that the TyG index could serve as a convenient and reliable surrogate marker for identifying individuals at risk. These findings may inform screening and prevention strategies targeting the full spectrum of colorectal neoplasia. Standardization of TyG index cutoffs and validation in diverse populations are warranted.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024605775.
Introduction
Colorectal cancer (CRC) represents a major global health challenge, ranking among the leading causes of cancer-related mortality worldwide and imposing a substantial burden on public health systems (1, 2). Although genetic factors account for a minority of CRC cases, most are sporadic, arising from a complex interplay between environmental exposures and lifestyle factors (3). Accumulating evidence indicates that the majority of CRCs evolve from pre-existing colorectal adenomas through the well-established adenoma–carcinoma sequence, underscoring adenomas as critical precancerous lesions. Therefore, identifying reliable risk factors and developing effective preventive strategies for colorectal carcinogenesis remain of paramount importance.
Insulin resistance (IR), a metabolic disorder characterized by reduced responsiveness to insulin, has been increasingly recognized as a key factor in the pathogenesis of CRC (4, 5). The hyperinsulinemic-euglycemic glucose clamp technique is considered the gold standard for directly quantifying insulin sensitivity and estimating IR (6). However, this method is not only time-consuming and costly but also labor-intensive and technically complex, which limits its feasibility for large-scale epidemiological studies, particularly in resource-limited settings. The homeostatic model assessment of IR (HOMA-IR), on the other hand, offers an indirect means of evaluating IR through a mathematical model (7). However, its clinical utility is limited, as it requires the measurement of fasting insulin—an assessment that is not routinely performed in clinical practice unless there is a specific need to evaluate the endocrine system.
Given the challenges associated with both direct and indirect methods for assessing IR, there is a growing demand for more accessible and practical tools for its evaluation. In response, researchers have focused on developing surrogate markers that can simplify the screening process for IR. Among these, the triglyceride-glucose (TyG) index has emerged as a promising and practical surrogate marker (8). The TyG index is calculated using a simple formula: the natural logarithm of the product of fasting triglycerides and fasting plasma glucose levels divided by 2 (ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)]/2). This approach offers significant advantages, as it bypasses the need for direct insulin measurement and presents a more feasible alternative to other indirect assessment methods. The simplicity and practicality of the TyG index have made it a focal point in colorectal carcinogenesis research, offering a more accessible means of identifying individuals at risk for colorectal carcinogenesis.
Colorectal carcinogenesis is a multistep process that encompasses both the formation of colorectal adenomas and their potential malignant transformation into CRC. Although several studies have explored the association between the TyG index and the risk of colorectal carcinogenesis, the results remain inconclusive. In light of this uncertainty, a comprehensive meta-analysis is warranted to integrate the available studies. Therefore, we conducted a meta-analysis to comprehensively assess the association between the TyG index and the risk of colorectal carcinogenesis, with the goal of informing future clinical practices and preventive strategies.
Methods
Protocol and guideline
This study was registered in the International Prospective Register for Systematic Reviews under the identifier CRD42024605775, and was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (9).
Data source and study selection
An exhaustive systematic search was performed in databases including PubMed, Scopus, Embase, Web of Science, and Cochrane Library. A combination of Medical Subject Headings terms and free texts was utilized to identify all studies published up to the 9th of November 2024. The search queries were constructed using the refined terms: ‘Triglyceride-Glucose Index’, ‘Colorectal Neoplasms’, and ‘Colorectal Cancer’. The details of searching strategies are presented in Supplementary Table 1. No filters based on language or publication status were applied during the initial search to maximize the comprehensiveness of our retrieval.
We included studies that (i) investigated the relationship between TyG index and colorectal carcinogenesis, including both colorectal adenomas and carcinoma, (ii) provided data on odds ratios (OR), relative risks (RR), or hazard ratios (HR). The following types of studies were excluded: (i) Reviews, (ii) case reports, and (iii) preclinical studies.
The literature was consolidated and duplicate entries were eliminated using EndNote. Following this, two independent researchers conducted an initial review of study titles and abstracts. Eligible studies were then subjected to a full-text evaluation. Any discrepancies between the reviewers were arbitrated by a third investigator.
Data extraction and quality assessment
Data were collected and summarized by one investigator and reviewed by another reviewer. Any discrepancies were resolved by consensus between the reviewers. The subsequent details were collected and presented in a table: first author’s name, publication year, country of origin, study design, study population, sample size, details about the TyG index, mean age, proportion of males, along with the ORs, RRs, and HRs accompanied by their 95% confidence intervals (CI) for colorectal adenoma and/or carcinoma, when available.
The quality assessment was performed utilizing the Newcastle-Ottawa Scale (NOS) (10). Studies assessed with NOS scores between seven and nine were categorized as high-quality, while those scoring between five and six were classified as moderate-quality. Studies scoring four or below were regarded as low-quality.
Statistical analysis
All statistical analyses were conducted using R (v4.3.2). A meta-analysis was performed to compare the association between continuous increase level in TyG index and the risk of colorectal carcinogenesis, including adenomas and carcinoma, yielding a combined effect size (ES) and its 95% CI. The impact of a one-unit increase in the TyG index on colorectal carcinogenesis risk was evaluated by calculating ORs, RRs, or HRs along with their 95% CIs through meta-analysis to obtain an overall estimate. Additionally, separate meta-analyses were conducted to assess the risk of colorectal carcinogenesis for each quartile of the TyG index relative to the lowest quartile.
The choice between a fixed-effects model and a random-effects model for producing combined ES depended on the level of heterogeneity observed. Heterogeneity was evaluated using the I² statistic and the Cochrane Q test, with significance defined as an I² exceeding 50% and a Q test p-value below 0.1. Publication bias was detected using funnel plots and Egger’s test. Sensitivity analyses, using the leave-one-out method, were conducted to evaluate the influence of individual studies on the pooled effect estimates. In this meta-analysis, OR, RR, and HR were considered equivalent in their interpretation. A two-tailed P-value less than 0.05 was considered statistically significant.
Results
Study selection
The initial search retrieved 64 records. After removing duplicates, 35 studies remained for preliminary screening. After carefully reviewing the titles and abstracts, 23 studies were considered irrelevant and thus excluded. Subsequently, 12 studies were subjected to more detailed evaluation. Eventually, a total of 9 studies that fulfilled all the eligibility requirements were included in the meta-analysis (11–19). The study selection process is illustrated in Figure 1.
Characteristics of the included studies
Among the included studies, there was three cross-sectional studies (13, 14, 18), one case-control study (16), and five cohort studies (11, 12, 15, 17, 19), encompassing a total of 1,056,401 individuals. These studies were conducted between 2020 and 2024, spanning regions in East Asia (China, Japan, and South Korea) and Europe. The sample sizes of these studies varied from 1,472 to 510,471 participants. A higher proportion of males was observed among patients with colorectal neoplasms compared to the control group. Additionally, there was a trend of increasing male prevalence with higher levels of the TyG index. According to the NOS criteria for evaluating the quality of observational studies, all the included studies were of high quality, as detailed in Supplementary Table 2. The characteristics of the included studies are summarized in Table 1.
Association between continuous increase in the TyG and risk of colorectal carcinogenesis
The relationship between the continuous elevation of the TyG index and the risk of colorectal carcinogenesis was investigated in two studies, encompassing a total of 32,388 participants (14, 17). Despite its smaller sample size, Choi et al. contributed 85.6% of the overall weight because its effect estimate was more precise (OR 1.16, 95% CI 1.02–1.33) than that of Okamura et al. (HR 1.38, 95% CI 1.00–1.91). Given the absence of heterogeneity (I² = 0%), a fixed-effect model was used to calculate the pooled estimate, and a random-effects model was additionally performed as a sensitivity check showing consistent results. Overall, the meta-analysis showed that a continuous increase in the TyG index was associated with a 19% higher risk of colorectal carcinogenesis (ES, 1.19; 95%CI, 1.05-1.34; Figure 2).
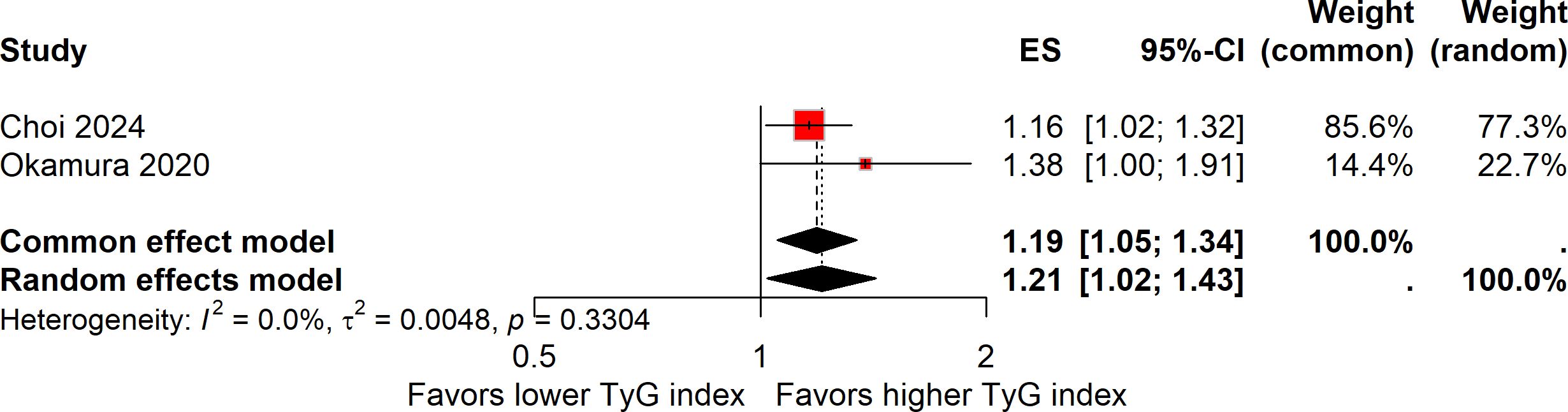
Figure 2. Forest plot showing the association between a continuous increase in the TyG index and the risk of colorectal carcinogenesis. Both common-effect (fixed-effect) and random-effects models were applied as a sensitivity analysis despite the absence of heterogeneity (I² = 0%). The weight of each study reflects precision rather than sample size.
Impact of a one-unit increment in the TyG index on colorectal carcinogenesis risk
Five studies explored the association between a one-unit increment in the TyG index and the risk of colorectal carcinogenesis (12, 13, 16, 18, 19). The meta-analysis revealed that a one-unit increase in the TyG index was linked to a 24% higher risk of colorectal carcinogenesis (ES, 1.24; 95%CI, 1.15-1.32; Figure 3). As with the previous analysis, no heterogeneity was detected across these studies (I² = 0%). The funnel plot with Egger’s test suggested no significant publication bias (Figure 4A). The sensitivity analysis conducted via the leave-one-out method indicated that the pooled result was reliable (Figure 5A).
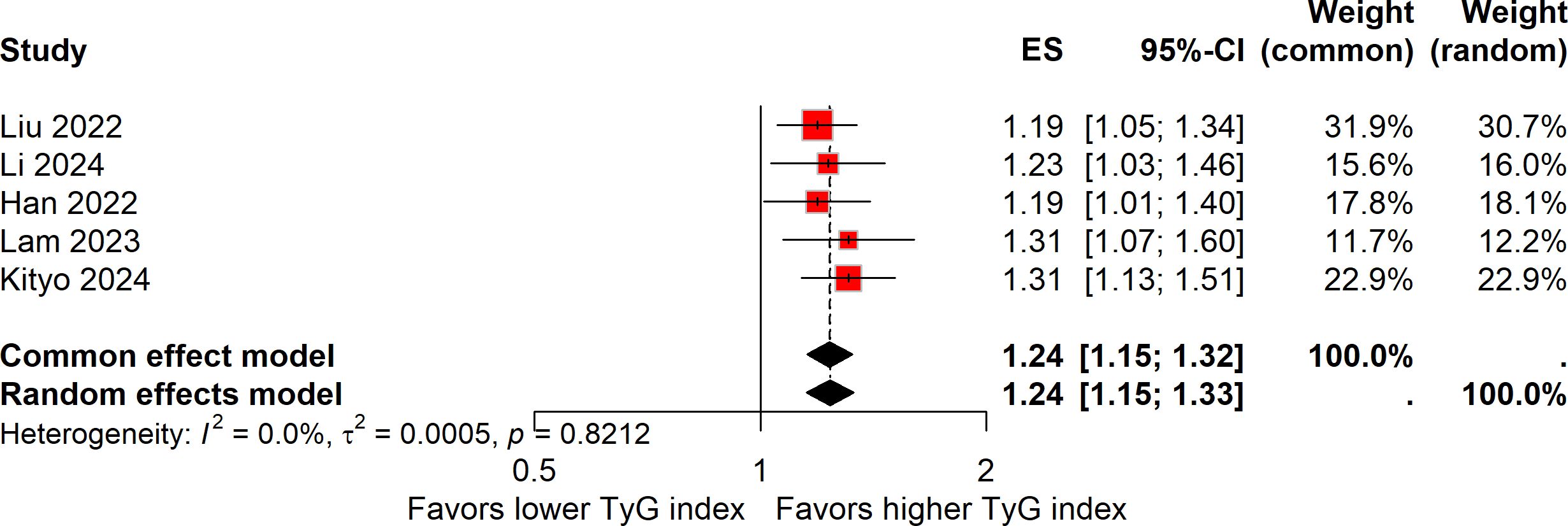
Figure 3. Forest plot of the association between one-unit increase in the TyG index and colorectal carcinogenesis.
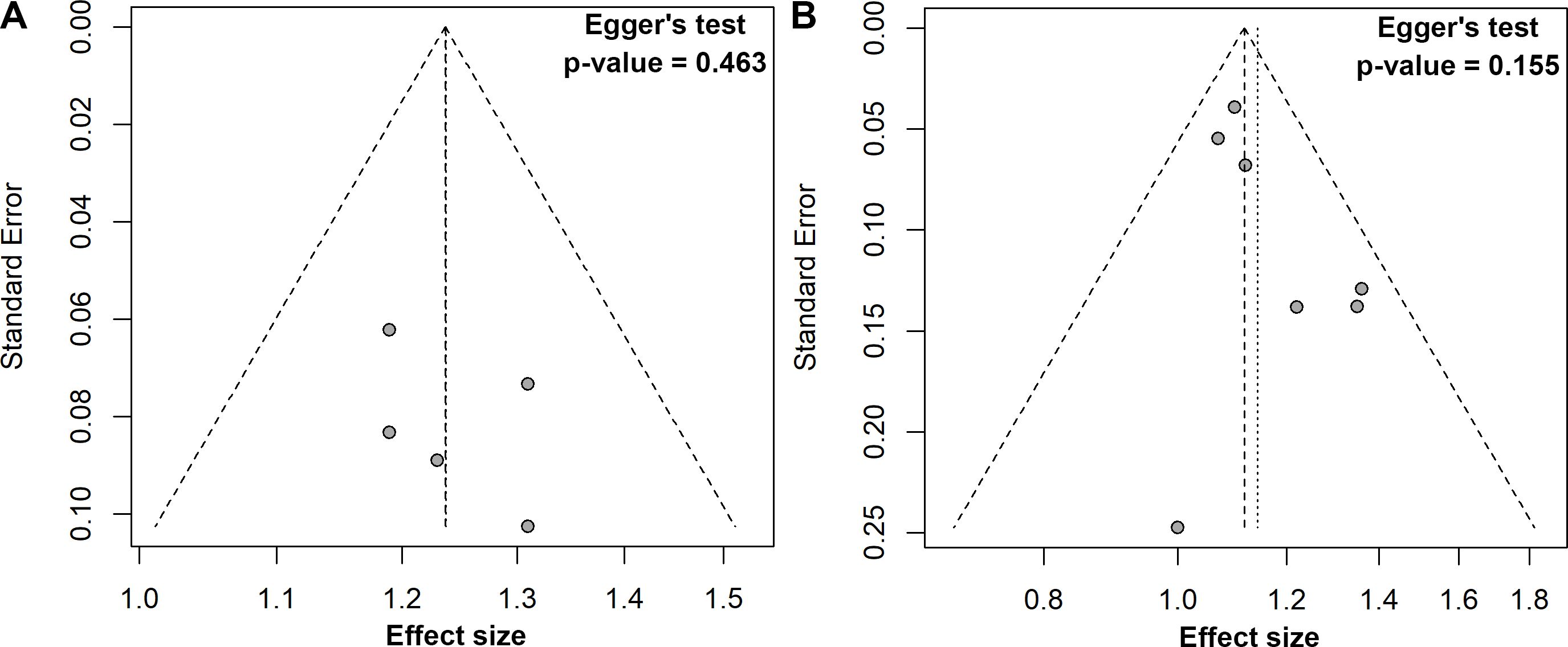
Figure 4. Funnel plots with Egger’s regression tests for publication bias: (A) one unit increase in the TyG index, (B) quartile 3 vs. quartile 1.
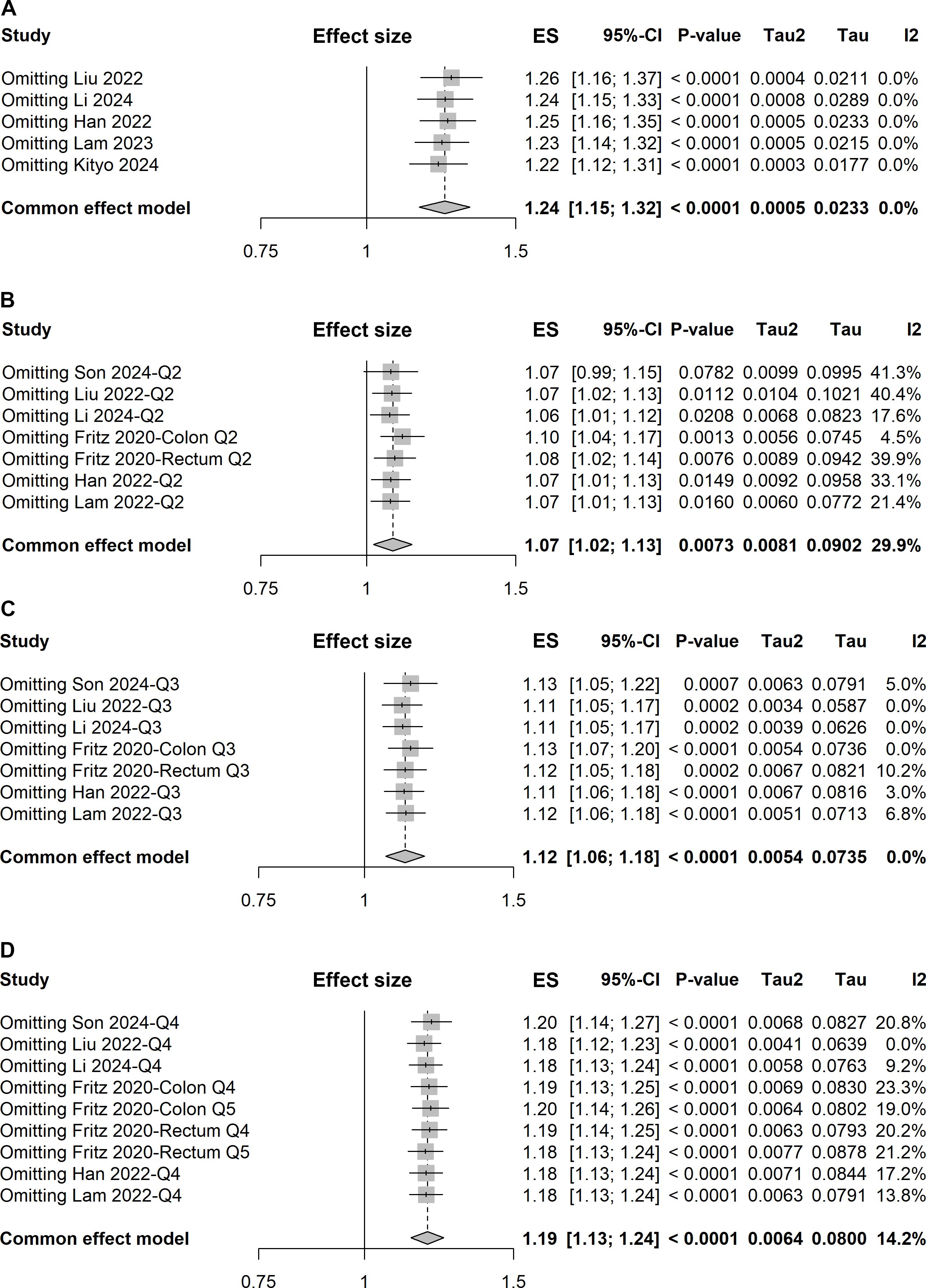
Figure 5. Forest plots of sensitivity analyses using the leave-one-out approach for pooled results: (A) one-unit increase in the TyG index, (B) quartile 2 vs. quartile 1, (C) quartile 3 vs. quartile 1, and (D) quartile 4 vs. quartile 1.
Assessment of colorectal carcinogenesis risk across TyG index quartiles
Six studies evaluated the risk of colorectal carcinogenesis across quartiles of the TyG index (11–13, 16, 18, 19), while one study used quintiles (15). To facilitate a uniform comparison, we extracted ESs for the second quartile versus the first quartile and the third quartile versus the first quartile from all seven studies. For the comparison of the fourth quartile versus the first quartile, we included ESs from the fourth quartile of the six studies with quartile data and the fifth quartile from the study with quintile data. This method allowed us to integrate the additional data from the quintile study for the highest TyG category while maintaining a consistent framework for the other quartile comparisons.
Given a relatively low level of heterogeneity (I² = 29.9%) was observed across the studies, the pooled ES with 95% CI was determined using the fixed-effect model. The meta-analysis demonstrated that individuals in the second quartile of the TyG index had a 7% increased risk of colorectal carcinogenesis compared to the reference quartile (quartile 1) (ES, 1.07; 95% CI, 1.02-1.13; Figure 6A). However, the sensitivity analysis revealed that the pooled result was significantly influenced by the study conducted by Son et al. (Figure 5B).
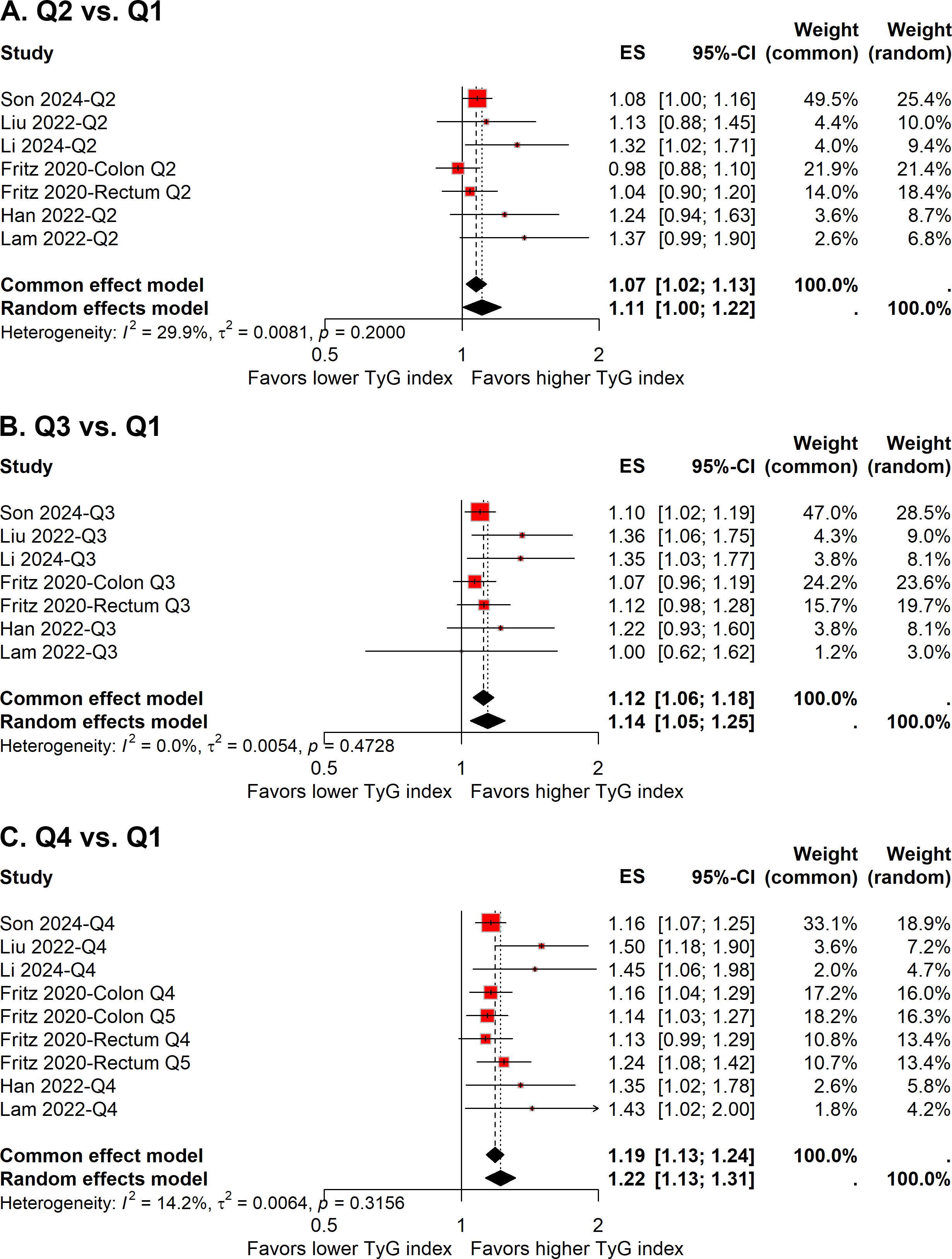
Figure 6. Forest plot of the association between quartile 2 vs. quartile 1 (A), quartile 3 vs. quartile 1 (B), and quartile 4 vs. quartile 1 (C) of TyG index and colorectal carcinogenesis.
In the comparison of the third quartile, no heterogeneity was present (I² = 0%), and the fixed-effect model was used to estimate the pooled ES. The meta-analysis suggested that individuals in the third quartile of the TyG index had a 12% higher risk of colorectal carcinogenesis than the reference quartile (ES, 1.12; 95% CI, 1.06-1.18; Figure 6B). The funnel plot with Egger’s test suggested no significant publication bias (Figure 4B), and the sensitivity analysis indicated that the pooled result was robust (Figure 5C).
For the fourth quartile, the meta-analysis indicated a 19% increased risk of colorectal carcinogenesis relative to the reference quartile (ES, 1.19; 95% CI, 1.13-1.24; Figure 6C). A low level of heterogeneity (I² = 14.2%) was also noted across these studies. Sensitivity analysis confirmed the robustness of the pooled result (Figure 5D).
Discussion
CRC remains one of the most prevalent and deadly cancers worldwide, with an increasing burden on healthcare systems and societies (1, 2). It is well-established that lifestyle and metabolic factors play a critical role in the pathogenesis of CRC (20, 21). One of the major contributors to CRC development is IR, a condition often associated with metabolic syndrome, obesity, and diabetes (5). However, measuring IR directly is complex and costly, limiting its use in large-scale epidemiological studies. In this context, the TyG index, a surrogate marker for IR, has garnered attention due to its simplicity and ease of use. The TyG index offers several advantages, including its accessibility, simplicity, and validity. Firstly, TyG index can be easily calculated using readily available clinical data, making it a cost-effective and convenient tool for large-scale population studies and routine clinical practice. Moreover, it requires only two readily available blood tests, fasting triglycerides, and fasting glucose, avoiding the need for more complex and expensive methods like the hyperinsulinemic-euglycemic clamp technique. Additionally, it has been shown to correlate well with other established measures of IR, such as the HOMA-IR (22).
The present meta-analysis synthesizes evidence from nine observational studies involving over a million participants, revealing a significant association between the TyG index and the risk of colorectal carcinogenesis. Specifically, a continuous increase in the TyG index was associated with an elevated risk of colorectal carcinogenesis, with an ES of 1.19 (95% CI, 1.05-1.34). Moreover, a one-unit increase in the TyG index corresponded to a 24% higher risk of colorectal carcinogenesis (ES, 1.24; 95% CI, 1.15-1.32). These findings underscore the potential utility of the TyG index as a surrogate marker for predicting colorectal carcinogenesis risk. Additionally, the association between TyG index with colorectal carcinogenesis risk was also evident when comparing different quartiles of the index. Participants in the second, third, and highest quartiles exhibited significantly higher risks of colorectal carcinogenesis compared to those in the lowest quartile, with ESs of 1.07 (95% CI, 1.02-1.13), 1.12 (95% CI, 1.06-1.18), and 1.19 (95% CI, 1.13-1.24), respectively. This dose-response relationship suggests that the TyG index may serve as a valuable tool for stratifying individuals based on their risk of colorectal carcinogenesis, thereby informing personalized screening and prevention strategies.
The results of this meta-analysis align with earlier studies that have suggested a role for IR and metabolic dysfunction in colorectal carcinogenesis. This meta-analysis extends these findings by demonstrating that the TyG index, a more accessible and cost-effective alternative to HOMA-IR, can also be used to predict colorectal carcinogenesis risk. Moreover, this meta-analysis adds value to the growing body of evidence linking metabolic disorders, particularly IR, to colorectal carcinogenesis. Studies have demonstrated that insulin and glucose metabolism abnormalities contribute to CRC progression through insulin signaling pathways (23, 24). Additionally, the association between the TyG index and colorectal carcinogenesis risk observed in this meta-analysis is consistent with studies that have found a link between metabolic syndrome and increased colorectal carcinogenesis incidence (25). Studies found that individuals with metabolic syndrome had a higher risk of developing CRC (26, 27).
While our study did not directly explore the biological mechanisms underlying the observed association, several plausible pathways may explain the link between a higher TyG index and colorectal carcinogenesis. The TyG index integrates fasting triglyceride and glucose levels, thereby capturing the combined metabolic burden of hyperglycemia and dyslipidemia—two key components of insulin resistance. Elevated fasting glucose can lead to chronic hyperglycemia, which promotes oxidative stress and the formation of advanced glycation end products (AGEs). These AGEs activate inflammatory signaling cascades such as the NF-κB and STAT3 pathways, resulting in increased production of pro-inflammatory cytokines (e.g., interleukin-6 and tumor necrosis factor-alpha) that induce DNA damage, inhibit apoptosis, and promote tumor progression (28). Hyperglycemia also augments insulin and insulin-like growth factor (IGF-1) signaling, activating the PI3K/AKT/mTOR pathway, which enhances cellular proliferation, survival, and angiogenesis while suppressing antitumor immune surveillance (29, 30). In parallel, elevated fasting triglycerides reflect impaired lipid metabolism and are associated with increased circulating free fatty acids, lipotoxicity, and oxidative stress (31–33). Excess triglyceride-derived lipid intermediates can disrupt membrane lipid composition, activate the PKC and JNK pathways, and promote chronic inflammation, creating a tumor-permissive microenvironment (31–34). Dysregulated lipid and bile acid metabolism may further influence the gut microbiota, leading to dysbiosis that generates carcinogenic metabolites, disrupts epithelial barrier integrity, and enhances mucosal inflammation (35–37). Together, these component-specific processes illustrate that the TyG index is more than a surrogate marker of insulin resistance—it integrates the oncogenic consequences of both hyperglycemia and hypertriglyceridemia. This dual metabolic stress may synergistically drive epithelial proliferation, inflammation, and genomic instability, thereby contributing to colorectal tumorigenesis (4, 5).
The findings of this meta-analysis hold important implications for clinical practice and public health. The TyG index offers a simple, cost-effective, and non-invasive method for assessing IR, making it particularly suitable for large-scale epidemiological studies and routine clinical settings. Incorporating the TyG index into CRC screening protocols enables healthcare providers to identify high-risk individuals who could potentially benefit from targeted interventions, such as lifestyle modifications and pharmacological treatments to improve insulin sensitivity. Clinicians could then prioritize these individuals for more intensive screening procedures, such as colonoscopy or fecal occult blood testing, potentially leading to earlier detection of CRC and enhanced patient outcomes. Moreover, the TyG index could serve as a biomarker for monitoring the effectiveness of preventive strategies. Longitudinal studies tracking changes in the TyG index over time can help evaluate the impact of interventions on reducing CRC risk. For instance, interventions that lower the TyG index, such as weight loss programs, exercise regimens, and dietary adjustments, may be associated with decreased incidence of CRC. Additionally, public health initiatives should prioritize metabolic health education, emphasizing the TyG index as a modifiable CRC risk factor. In resource-limited settings, where advanced screening modalities are scarce, TyG-based risk algorithms could optimize resource allocation by triaging high-risk populations for priority screening.
Several limitations of this meta-analysis should be acknowledged. First, all included studies were observational in nature, precluding causal inference. Although most studies adjusted for a broad range of confounders, residual and unmeasured confounding (e.g., dietary patterns, physical activity, medication use, metabolic comorbidities, and CRC screening behaviors) may persist. Reverse causation is also plausible, as preclinical or early-stage colorectal neoplasms may influence glucose and lipid metabolism through systemic inflammation and metabolic dysregulation, thereby elevating the TyG index (38). Second, exposure assessment was heterogeneous: the TyG index was generally measured at a single baseline time point, which may not represent long-term metabolic status, and variation in fasting status, assay methodology, and study-specific cutoffs could contribute to between-study heterogeneity. Moreover, “colorectal carcinogenesis” encompassed both adenoma and carcinoma, representing different biological stages and detection probabilities; separate analyses for these entities were not feasible due to limited available data. Third, despite the lack of significant publication bias on funnel plots and Egger’s tests, small-study effects cannot be entirely excluded. Fourth, the included studies were primarily conducted in East Asian and European populations, which may limit generalizability to other ethnic groups. Fifth, the cut-off values of the TyG index varied across studies, and optimal thresholds for predicting CRC risk have not been standardized. Additionally, in our quartile-based analysis, we incorporated a study that reported data in quintiles by equating its highest quintile with the highest quartile of other studies (15). While this was a necessary and pragmatic approach to include all available data, it represents a methodological simplification that should be considered when interpreting the results. Moreover, the conclusion regarding a continuous increase in the TyG index and CRC risk is based on only two studies; while the result is statistically significant, it requires validation in a larger number of cohorts.
Future research should aim to address these methodological limitations through individual participant data meta-analyses that allow harmonized definitions of TyG exposure, consistent covariate adjustment, and evaluation of repeated TyG trajectories over time. Purpose-built prospective cohorts with standardized fasting protocols, adjudicated outcomes, and detailed colonoscopy data would further strengthen temporal inference. Dose–response analyses using restricted cubic splines could delineate whether the association is linear or threshold-dependent, while meta-regression or subgroup analyses by sex, age, BMI, diabetes status, and region may help explain residual heterogeneity. Complementary approaches such as Mendelian randomization could also provide stronger evidence for causality by assessing whether genetically predicted triglyceride–glucose profiles are associated with CRC risk independent of confounding and reverse causation. Furthermore, mechanistic and translational studies are warranted to clarify the biological underpinnings of this association. Experimental work should explore how TyG-related insulin resistance promotes colorectal tumorigenesis through insulin/IGF-1 signaling, PI3K–AKT–mTOR activation, chronic inflammation, and microbiome or bile acid alterations. Interventional studies evaluating TyG-lowering strategies—such as lifestyle modification, weight control, or pharmacologic agents including GLP-1 receptor agonists—should examine colorectal endpoints to determine whether the TyG index represents a modifiable causal determinant or a surrogate marker of metabolic risk.
Conclusion
This meta-analysis demonstrates a significant positive association between the TyG index and the risk of colorectal carcinogenesis. The findings suggest that the TyG index could serve as a valuable surrogate marker for predicting colorectal carcinogenesis risk, offering a practical, accessible tool for identifying individuals at elevated risk. These results have important implications for CRC screening and prevention, and future research should aim to further validate the role of the TyG index in clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
LW: Conceptualization, Investigation, Writing – original draft, Data curation, Formal analysis, Methodology. YuL: Data curation, Formal analysis, Writing – original draft, Software, Visualization. YoL: Data curation, Formal analysis, Visualization, Writing – original draft, Methodology. JS: Visualization, Writing – original draft, Conceptualization, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Acknowledgments
The authors expressed their gratitude to the databases that supplied the essential data for this investigation.
Conflict of interest
The authors declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was used in the creation of this manuscript. Improve the quality of the Manuscript
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1569824/full#supplementary-material
References
1. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics, 2025. CA Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
2. Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. (2023) 72:338–44. doi: 10.1136/gutjnl-2022-327736
3. Jasperson KW, Tuohy TM, Neklason DW, and Burt RW. Hereditary and familial colon cancer. Gastroenterology. (2010) 138:2044–58. doi: 10.1053/j.gastro.2010.01.054
4. Lee S-H, Park S-Y, and Choi C. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. (2021) 46:15–37. doi: 10.4093/dmj.2021.0280
5. Yoon Y, Keum N, Zhang X, Cho E, and Giovannucci E. Hyperinsulinemia, insulin resistance and colorectal adenomas: A meta-analysis. Metabolism. (2015) 64:1324–33. doi: 10.1016/j.metabol.2015.06.013
6. DeFronzo RA, Tobin JD, and Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. (1979) 237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214
7. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, and Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) 28:412–9. doi: 10.1007/BF00280883
8. Park HM, Lee HS, Lee Y-J, and Lee J-H. The triglyceride–glucose index is a more powerful surrogate marker for predicting the prevalence and incidence of type 2 diabetes mellitus than the homeostatic model assessment of insulin resistance. Diabetes Res Clin Pract. (2021) 180:109042. doi: 10.1016/j.diabres.2021.109042
9. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
10. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
11. Son M, Moon SY, Koh M, Kang Y, and Lee JY. Association between surrogate markers of insulin resistance and the incidence of colorectal cancer in korea: A nationwide population-based study. J Clin Med. (2024) 13:1628. doi: 10.3390/jcm13061628
12. Liu T, Zhang Q, Wang Y, Ma X, Zhang Q, Song M, et al. Association between the TyG index and TG/HDL-C ratio as insulin resistance markers and the risk of colorectal cancer. BMC Cancer. (2022) 22:1007. doi: 10.1186/s12885-022-10100-w
13. Li J, Chen J, Liu H, Yan S, Wang Y, Xing M, et al. Association of the triglyceride-glucose index with the occurrence and recurrence of colorectal adenomas: a retrospective study from China. BMC Public Health. (2024) 24:579. doi: 10.1186/s12889-024-18076-x
14. Choi CH, Moon SY, and Lee JY. The relationship between surrogate markers of insulin resistance and occurrence of colorectal adenoma in individuals under 50 years old: A single-center retrospective cross-sectional study. J Pers Med. (2024) 14:971. doi: 10.3390/jpm14090971
15. Fritz J, Bjørge T, Nagel G, Manjer J, Engeland A, Häggström C, et al. The triglyceride-glucose index as a measure of insulin resistance and risk of obesity-related cancers. Int J Epidemiol. (2020) 49:193–204. doi: 10.1093/ije/dyz053
16. Han M, Wang H, Yang S, Zhu S, Zhao G, Shi H, et al. Triglyceride glucose index and Atherogenic index of plasma for predicting colorectal neoplasms in patients without cardiovascular diseases. Front Oncol. (2022) 12:1031259. doi: 10.3389/fonc.2022.1031259
17. Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, and Fukui M. Triglyceride-glucose index (TyG index) is a predictor of incident colorectal cancer: A population-based longitudinal study. BMC Endocr Disord. (2020) 20:113. doi: 10.1186/s12902-020-00581-w
18. Lam TY, Zhu Z, Tang RS, Wong SH, Lui RN, Ng SS, et al. TRIGLYCERIDE–GLUCOSE INDEX (TYG INDEX) IS ASSOCIATED WITH HIGHER RISK OF COLORECTAL ADENOMA IN ASYMPTOMATIC INDIVIDUALS. Gastroenterology. (2023) 164:S–321. doi: 10.1016/S0016-5085(23)01725-0
19. Kityo A and Lee SA. Triglyceride-glucose index, modifiable lifestyle, and risk of colorectal cancer: A prospective analysis of the korean genome and epidemiology study. J Epidemiol Glob Health. (2024) 14:1249–56. doi: 10.1007/s44197-024-00282-w
20. Huxley R, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr C, and Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: A quantitative overview of the epidemiological evidence. Int J Cancer. (2009) 125:171–80. doi: 10.1002/ijc.24343
21. Yu J, Feng Q, Kim J, and Zhu Y. Combined effect of healthy lifestyle factors and risks of colorectal adenoma, colorectal cancer, and colorectal cancer mortality: systematic review and meta-analysis. Front Oncol. (2022) 12:827019. doi: 10.3389/fonc.2022.827019
22. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1210/jc.2010-0288
23. Kasprzak A. Insulin-like growth factor 1 (IGF-1) signaling in glucose metabolism in colorectal cancer. Int J Mol Sci. (2021) 22:6434. doi: 10.3390/ijms22126434
24. Hopkins B, Goncalves M, and Cantley L. Insulin–PI3K signalling: an evolutionarily insulated metabolic driver of cancer. Nat Rev Endocrinol. (2020) 16:276–83. doi: 10.1038/s41574-020-0329-9
25. Ahmed R, Schmitz K, Anderson K, Rosamond W, and Folsom A. The metabolic syndrome and risk of incident colorectal cancer. Cancer. (2006) 107:28–36. doi: 10.1002/cncr.21950
26. Choi Y, Lee D, Han K, Shin C, and Kim N. Abdominal obesity, glucose intolerance and decreased high-density lipoprotein cholesterol as components of the metabolic syndrome are associated with the development of colorectal cancer. Eur J Epidemiol. (2018) 33:1077–85. doi: 10.1007/s10654-018-0440-6
27. Chen H, Zheng X, Zong X, Li Z, Li N, Hur J, et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut. (2020) 70:1147–54. doi: 10.1136/gutjnl-2020-321661
28. Chang S-C and Yang W. Hyperglycemia, tumorigenesis, and chronic inflammation. Crit Rev Oncol Hematol. (2016) 108:146–53. doi: 10.1016/j.critrevonc.2016.11.003
29. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. (2008) 8:915–28. doi: 10.1038/nrc2536
30. Lau M-T and Leung P. The PI3K/Akt/mTOR signaling pathway mediates insulin-like growth factor 1-induced E-cadherin down-regulation and cell proliferation in ovarian cancer cells. Cancer Lett. (2012) 326:191–8. doi: 10.1016/j.canlet.2012.08.016
31. Corn KC, Windham MA, and Rafat M. Lipids in the tumor microenvironment: From cancer progression to treatment. Prog Lipid Res. (2020) 80:101055. doi: 10.1016/j.plipres.2020.101055
32. Lee-Rueckert M, Canyelles M, Tondo M, Rotllan N, Kovanen PT, Llorente-Cortes V, et al. Obesity-induced changes in cancer cells and their microenvironment: Mechanisms and therapeutic perspectives to manage dysregulated lipid metabolism. Semin Cancer Biol. (2023) 93:36–51. doi: 10.1016/j.semcancer.2023.05.002
33. Li S, Yuan H, Li L, Li Q, Lin P, and Li K. Oxidative stress and reprogramming of lipid metabolism in cancers. Antioxidants (Basel). (2025) 14:201. doi: 10.3390/antiox14020201
34. Chen K, Guo J, Zhang T, Gu J, Li H, and Wang J. The role of dyslipidemia in colitis-associated colorectal cancer. J Oncol. (2021) 2021:6640384. doi: 10.1155/2021/6640384
35. Brennan C and Garrett W. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol. (2016) 70:395–411. doi: 10.1146/annurev-micro-102215-095513
36. Chen J, Pitmon E, and Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol. (2017) 32:43–53. doi: 10.1016/j.smim.2017.09.006
37. Genua F, Raghunathan V, Jenab M, Gallagher W, and Hughes D. The role of gut barrier dysfunction and microbiome dysbiosis in colorectal cancer development. Front Oncol. (2021) 11:626349. doi: 10.3389/fonc.2021.626349
Keywords: triglyceride-glucose index, colorectal carcinogenesis, surrogate marker, meta-analysis, risk factor
Citation: Wang L, Lin Y, Liao Y and Shen J (2025) Association between triglyceride-glucose index and risk of colorectal carcinogenesis: a meta-analysis of observational studies. Front. Oncol. 15:1569824. doi: 10.3389/fonc.2025.1569824
Received: 17 June 2025; Accepted: 27 November 2025; Revised: 05 November 2025;
Published: 17 December 2025.
Edited by:
Ramesh Pothuraju, Rajiv Gandhi Centre for Biotechnology, IndiaReviewed by:
Xianlei Cai, Zhejiang University, ChinaTuo Han, the Second Affiliated Hospital of Xi’an Jiaotong University, China
Copyright © 2025 Wang, Lin, Liao and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhai Shen, c2hlbmpoX3BoYXJtQDEyNi5jb20=; Lichao Wang, Mzk3MDExNDM0QHFxLmNvbQ==
 Lichao Wang
Lichao Wang Yuxuan Lin
Yuxuan Lin Yonghe Liao
Yonghe Liao Jinhai Shen
Jinhai Shen
