- 1College of Medicine, Qatar University, Doha, Qatar
- 2Clinical Translational Science Research Group, Qatar University (QU) Health, Qatar University, Doha, Qatar
- 3Department of Mathematics and Statistics, College of Arts and Sciences, Qatar University, Doha, Qatar
Background: Pancreatic ductal adenocarcinoma (PDAC) remains one of the most aggressive malignancies, with poor outcomes despite therapeutic advancements. Immune checkpoint inhibitors (ICIs) have transformed cancer care, but their efficacy in PDAC is limited due to the tumor’s immunosuppressive microenvironment.
Methods: We systematically reviewed and meta-analyzed clinical outcomes of ICI therapy in PDAC using studies from PubMed, CINAHL, Cochrane Library, and Google Scholar, published up to February 28, 2024. Eligible studies reported objective response rate (ORR), progression-free survival (PFS), or overall survival (OS). Risk of bias was assessed using RoB 2.0 and ROBINS-I. Random-effects models estimated pooled effect sizes.
Results: Fifty-four studies (n = 2,364) were included. ORR ranged from 0% to 67%. ICI-based combinations showed a modest ORR benefit (OR = 1.10; 95% CI: 1.02–1.18) and improved OS when combined with chemotherapy (HR = 0.82; 95% CI: 0.78–0.87). However, ICIs plus radiotherapy were associated with increased mortality (HR = 1.18; 95% CI: 1.04–1.34). PFS improved in select subgroups, particularly in patients with high tumor mutational burden or mismatch repair deficiency.
Conclusion: ICIs combined with chemotherapy may modestly improve survival in PDAC. Outcomes remain heterogeneous and limited, underscoring the need for better biomarker-driven patient selection and more effective combination strategies.
1 Introduction
Pancreatic adenocarcinoma (PDAC) is among the most lethal and challenging cancers to treat, with limited improvements in survival despite decades of research and clinical advancements [see (1–3)]. Epidemiological studies, such as that by Neoptolemos et al. (4), highlight the poor prognosis associated with PDAC, with five-year survival rates remaining below 2%. This underscores the critical need for effective therapeutic strategies to address this devastating disease.
The molecular and genetic underpinnings of PDAC have been extensively studied, revealing key drivers such as mutations in KRAS, TP53, and CDKN2A [see (5, 6)]. These genetic alterations contribute to the aggressive biology of PDAC, including its dense stromal microenvironment and immunosuppressive characteristics (7, 8). The tumor microenvironment (TME), characterized by high collagen density, fibrotic stroma, and abundant immunosuppressive cells (e.g., regulatory T cells, myeloid-derived suppressor cells), further complicates treatment by promoting therapy resistance, excluding effector immune cells, and limiting drug delivery (7, 9). This “cold” immune milieu with low antigen presentation and limited T-cell infiltration is a key reason for the poor response to immune checkpoint blockade in PDAC. These factors collectively hinder the efficacy of traditional therapies, including surgery, chemotherapy, and radiation, and present a substantial challenge for immunotherapy strategies.
The emergence of immunotherapy, particularly immune checkpoint inhibitors (ICIs), has revolutionized the treatment of various cancers by harnessing the immune system to target and destroy tumor cells [see (10, 11)]. However, in pancreatic cancer, single-agent immunotherapies have generally yielded limited success. Royal et al. (12) showed that the immunosuppressive tumor microenvironment and low mutational burden are major barriers to the efficacy of ICIs in PDAC. Similarly, Quintanilha et al. (13) found that tumor mutational burden and genomic alterations play a critical role in predicting the effectiveness of ICIs. Despite these challenges, there is growing interest in combination therapies that integrate ICIs with other modalities, such as chemotherapy, radiation, targeted therapies, and immunomodulators [see (14, 15)]. These approaches aim to prime the immune system, disrupt tumor defense mechanisms, and overcome resistance to immunotherapy.
Early-phase clinical trials have shown some encouraging results, suggesting that combination therapies may enhance the efficacy of ICIs in PDAC. For example, Anderson et al. (16) demonstrated that combining ICIs with chemotherapy could improve clinical outcomes in certain patient subgroups. Similarly, O’Reilly et al. (17) reported that perioperative chemotherapy significantly enhances survival outcomes for resectable PDAC. However, conflicting outcomes persist, often influenced by variations in study designs, patient populations, and treatment regimens [see (18)]. This underscores the need for a systematic appraisal of the evidence to evaluate the effectiveness of ICIs in PDAC, clarify their role in clinical practice, and guide future research directions.
This study systematically investigates the impact of immune checkpoint inhibitors on key clinical outcomes—specifically progression-free survival (PFS), overall survival (OS), and objective response rate (ORR)—in patients with pancreatic adenocarcinoma. By synthesizing the available evidence, this review aims to provide a comprehensive understanding of the current state of immunotherapy in PDAC, identify gaps in the literature, and offer insights into optimizing treatment strategies for this challenging disease.
2 Methods
2.1 Literature search
A systematic search of PubMed, Embase, Scopus, Web of Science, and ClinicalTrials.gov was conducted from inception to February 28, 2024. The search combined MeSH and free-text terms related to “pancreatic cancer” and “immune checkpoint inhibitors” (ICIs). Full search strings used for each database are provided in Supplementary Table 1.
2.2 Study design
This systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (19). The study aimed to evaluate the clinical outcomes of immune checkpoint inhibitors (ICIs) in pancreatic adenocarcinoma (PDAC), focusing on progression-free survival (PFS), overall survival (OS), and objective response rate (ORR).
2.3 Search strategy
A comprehensive literature search was conducted across multiple databases, including PubMed, CINAHL Open Research, Cochrane Library, and Google Scholar, up to [insert date of search]. The search strategy utilized a combination of keywords and Medical Subject Headings (MeSH) terms related to immune checkpoint inhibitors, pancreatic adenocarcinoma, and clinical outcomes (see Supplementary Table 1 for the full search strings). The Rayyan tool (20) was employed to manage and screen the search results.
2.4 Inclusion and exclusion criteria
Studies were selected based on predefined eligibility criteria, modified from the PICOS framework (21):
● Population: Patients diagnosed with pancreatic adenocarcinoma (PDAC).
● Intervention: Immune checkpoint inhibitors (ICIs), either as monotherapy or in combination with other treatments (e.g., chemotherapy, radiotherapy).
● Comparison: Standard treatments (e.g., chemotherapy alone) or placebo.
● Outcomes: Progression-free survival (PFS), overall survival (OS), and objective response rate (ORR).
● Study Design: Randomized controlled trials (RCTs), phase Ib/II/III trials, retrospective studies, and observational studies.
Studies were excluded if they were reviews, meta-analyses, conference abstracts, letters, editorials, or opinion pieces. Additionally, studies involving animal models or non-human subjects were excluded.
2.5 Study selection and data extraction
The study selection process followed the PRISMA flow diagram (see Figure 1). Two independent reviewers screened titles and abstracts for eligibility, followed by full-text review of potentially relevant studies. Discrepancies were resolved through discussion or consultation with a third reviewer. Data extraction was performed using a standardized form, capturing study characteristics (e.g., author, year, study design, sample size), intervention details (e.g., type of ICI, combination therapies), and clinical outcomes (e.g., PFS, OS, ORR).
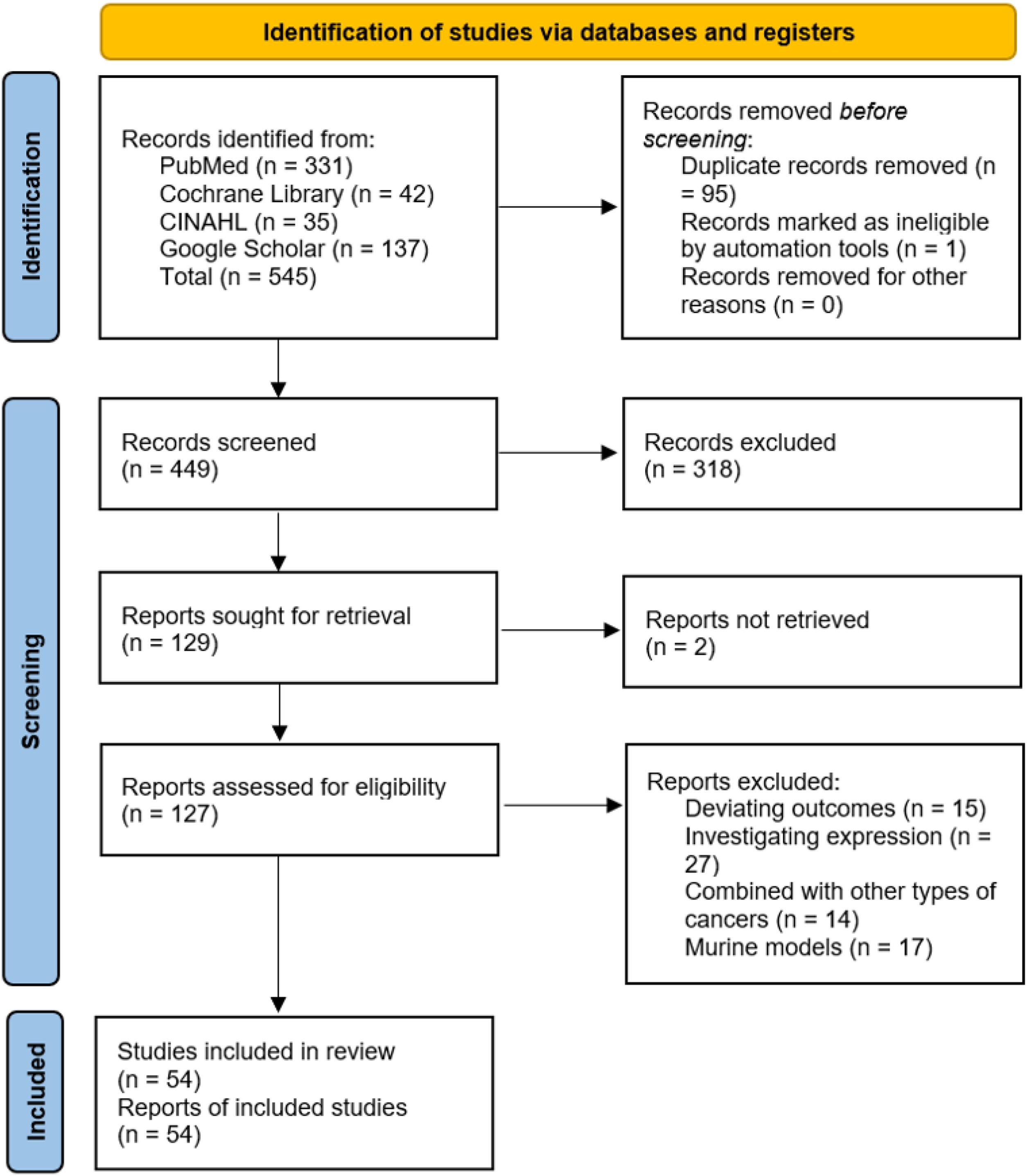
Figure 1. PRISMA flow diagram illustrating the process of study selection for inclusion in the systematic review and meta-analysis.
2.6 Quality assessment and risk of bias
Risk of bias was assessed using the Cochrane Risk of Bias 2.0 (RoB 2.0) tool for randomized controlled trials (22) and the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool for non-randomized studies (23). These tools evaluate key domains of bias, including randomization, deviations from intended interventions, missing data, and outcome measurement. The overall quality of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework (24). Assessment results were visualized using traffic-light plots and considered in the interpretation of pooled results.
2.7 Data analysis
Meta-analyses were conducted using RStudio (version 4.4.2) with the meta package Schwarzer (25). Pooled effect sizes for PFS, OS, and ORR were calculated using random-effects models to account for heterogeneity across studies. Heterogeneity was quantified using the Higgins I2 statistic, with values of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively. Forest plots were generated to visualize the pooled effect sizes and their 95% confidence intervals. Sensitivity analyses were performed using the leave-one-out method to assess the robustness of the results. Publication bias was evaluated using funnel plots and Egger’s test (26). A p-value of less than 0.05 was considered statistically significant.
2.8 Ethical considerations
This study utilized publicly available data from published studies and did not involve direct human or animal subjects. Therefore, ethical approval was not required.
3 Results
3.1 Study selection process and characteristics
The literature search identified a total of 545 records from PubMed (n = 331), Cochrane Library (n = 42), CINAHL (n = 35), and Google Scholar (n = 137). After removing 95 duplicate records and 1 record marked as ineligible by automation tools, 449 records were screened. Of these, 318 records were excluded based on title and abstract review, leaving 129 reports sought for retrieval. Two reports were not retrieved, and 127 reports were assessed for eligibility. After excluding studies with deviating outcomes (n = 15), those investigating expression (n = 27), studies combining PDAC with other types of cancers (n = 14), and studies involving murine models (n = 17), a total of 54 studies were included in the review. The study selection process is summarized in Figure 1.
The included studies comprised 3 single-center open-label trials, 31 phase II/1b trials, and 14 multi-center randomized studies, with a total participant population of 2,364. The sample sizes of the included studies ranged from 3 to 312 participants, reflecting the heterogeneity in trial phases and study designs. The studies compared various immune checkpoint inhibitor (ICI) dosing regimens with standard chemotherapy (e.g., Paclitaxel, Gemcitabine), other ICIs (e.g., Nivolumab/Ipilimumab), and radiotherapy or other modalities such as vaccines. The data estimation point was 12 months after the targeted drug therapy, with varying follow-up periods. Detailed characteristics of the included studies are presented in Table 1.
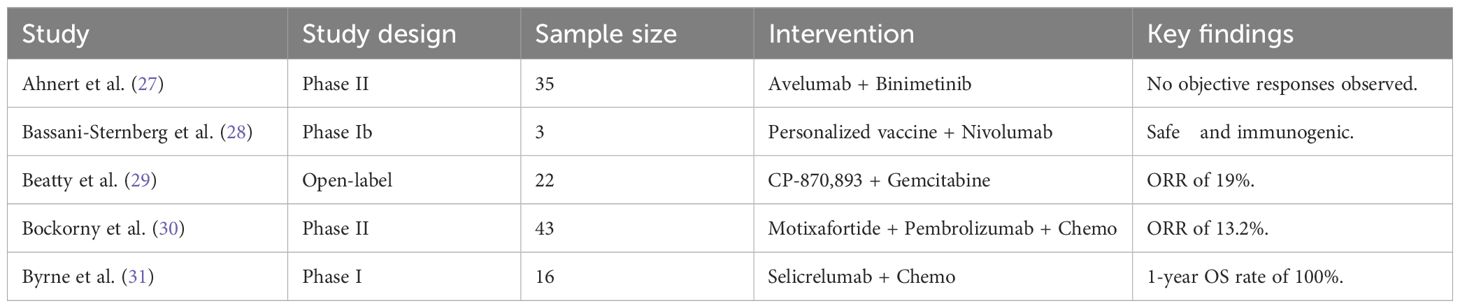
Table 1. Detailed search strategy used for the systematic review across PubMed, CINAHL, Cochrane Library, and Google Scholar.
3.2 Risk of bias assessment
The risk of bias in the included studies was assessed using the Cochrane Risk of Bias 2.0 (RoB 2.0) tool for randomized controlled trials (RCTs) and the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool for non-randomized studies. The results of the risk of bias assessment are summarized in Figures 2, 3.
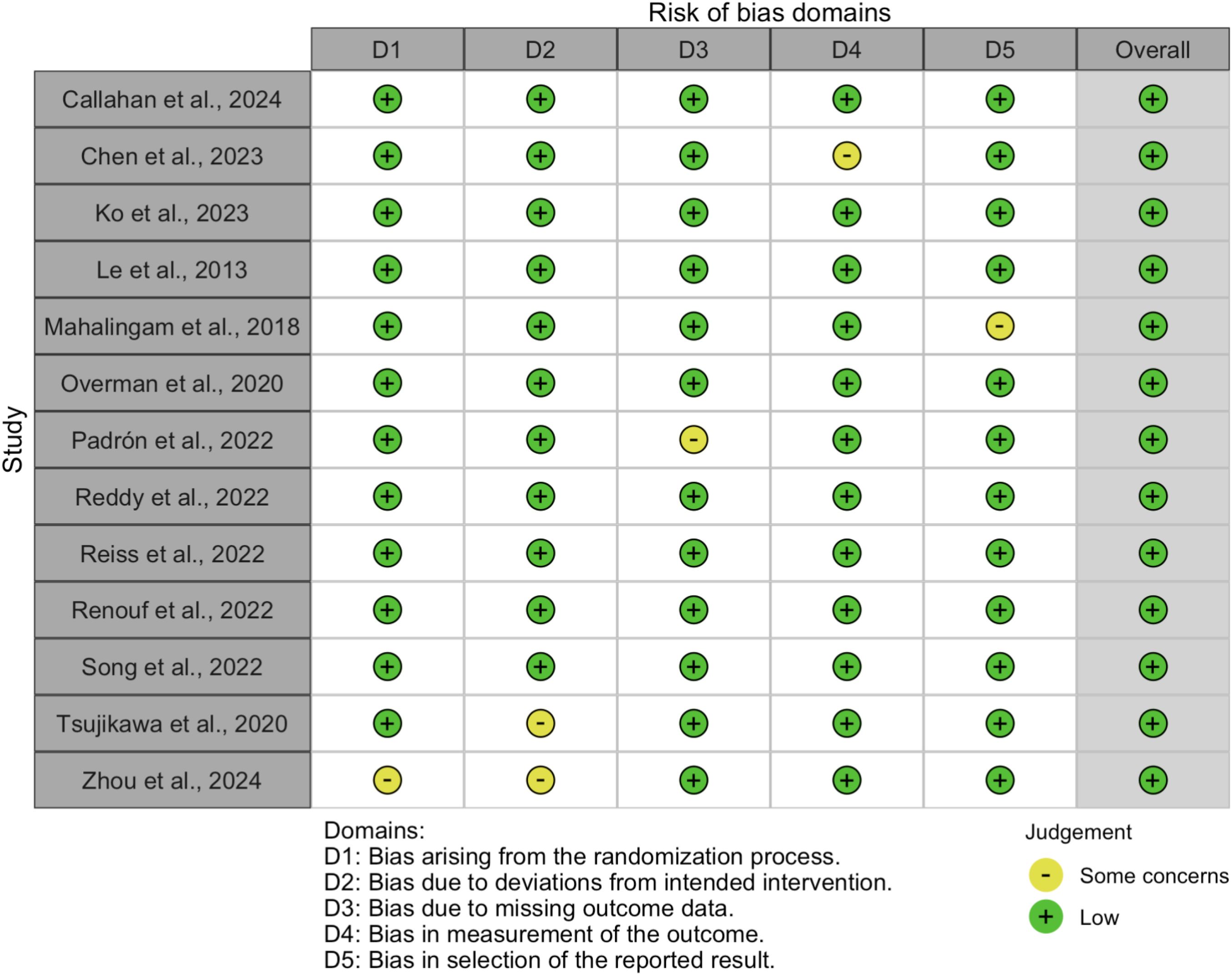
Figure 2. Summary of risk of bias across domains for randomized controlled trials, assessed using the Cochrane RoB 2.0 tool.
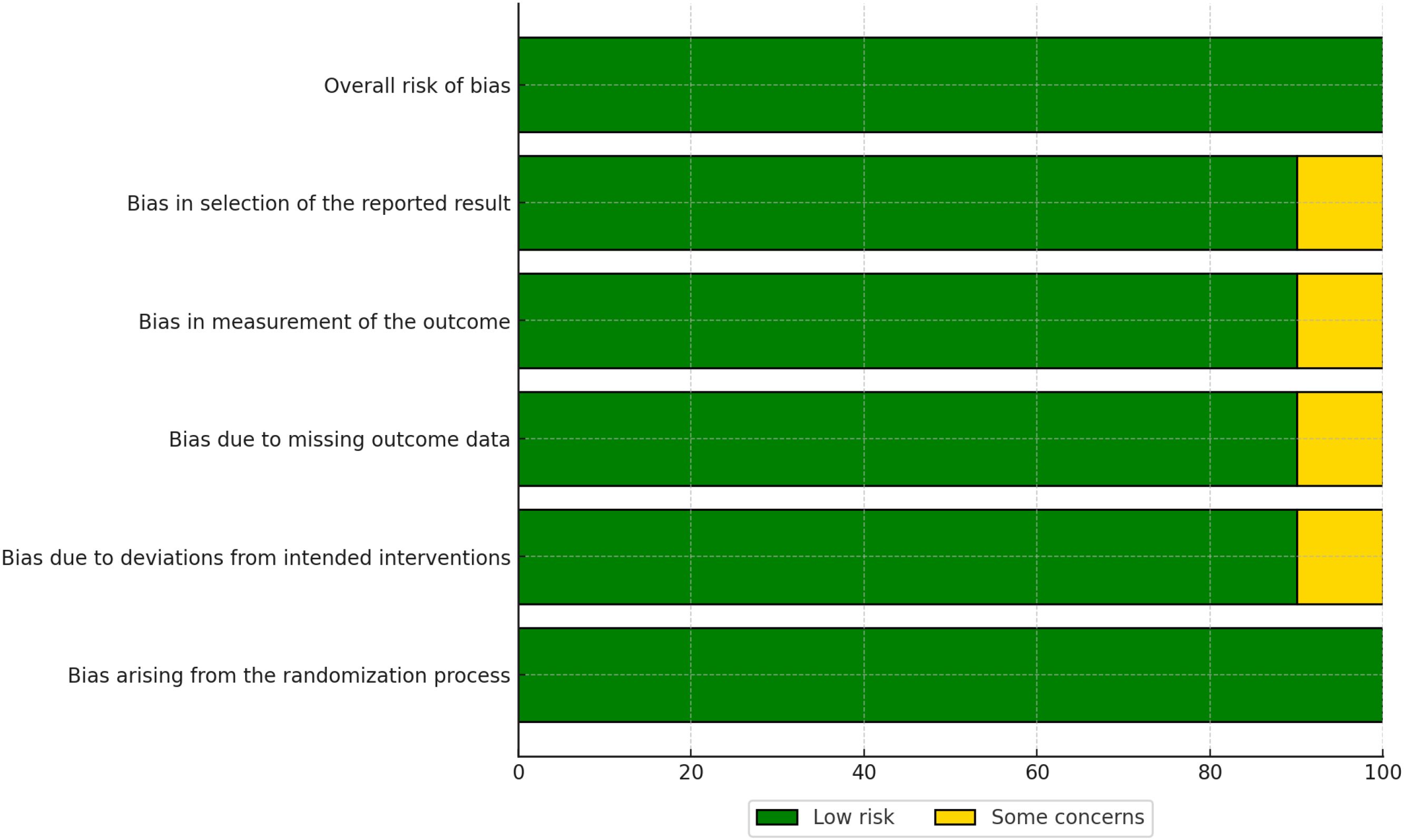
Figure 3. Traffic light plot showing domain-level risk of bias judgments for each included randomized controlled trial.
3.2.1 Randomized controlled trials
For RCTs, the RoB 2.0 tool evaluated five domains of bias:
● Bias arising from the randomization process (D1),
● Bias due to deviations from intended interventions (D2),
● Bias due to missing outcome data (D3),
● Bias in measurement of the outcome (D4), and
● Bias in selection of the reported result (D5).
The overall risk of bias for each RCT is visualized in Figure 3. Most RCTs were judged to have a low risk of bias across all domains, although some studies raised concerns in specific areas, such as deviations from intended interventions (D2) and missing outcome data (D3).
3.2.2 Non-randomized studies
For non-randomized studies, the ROBINS-I tool assessed seven domains of bias:
● Bias due to confounding (D1),
● Bias due to selection of participants (D2),
● Bias in classification of interventions (D3),
● Bias due to deviations from intended interventions (D4),
● Bias due to missing data (D5),
● Bias in measurement of outcomes (D6), and
● Bias in selection of the reported result (D7).
The overall risk of bias for non-randomized studies is presented in Figure 4. While many studies were judged to have a moderate risk of bias, some exhibited significant concerns, particularly in the domains of confounding (D1) and selection of participants (D2).
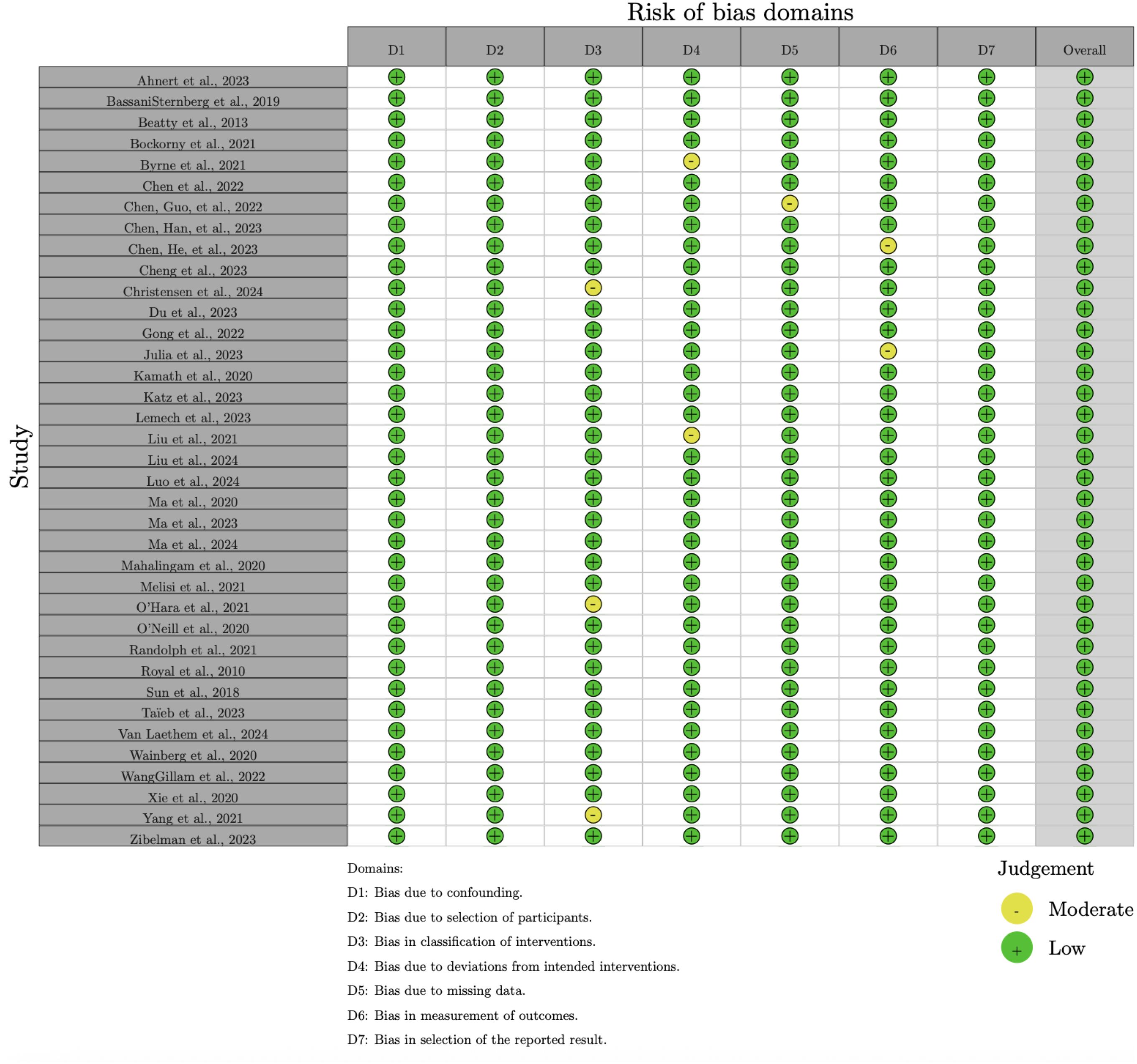
Figure 4. Summary of risk of bias across domains for non-randomized studies, assessed using the ROBINSI tool.
The risk of bias assessment revealed that the majority of RCTs had a low risk of bias, whereas non-randomized studies more frequently had a moderate risk of bias, particularly in domains such as confounding and selection of participants. No studies were rated as having a high overall risk of bias. These findings highlight the importance of considering study design when interpreting the results of this meta-analysis. Detailed risk of bias assessments for individual studies are provided in Figures 4, 5.
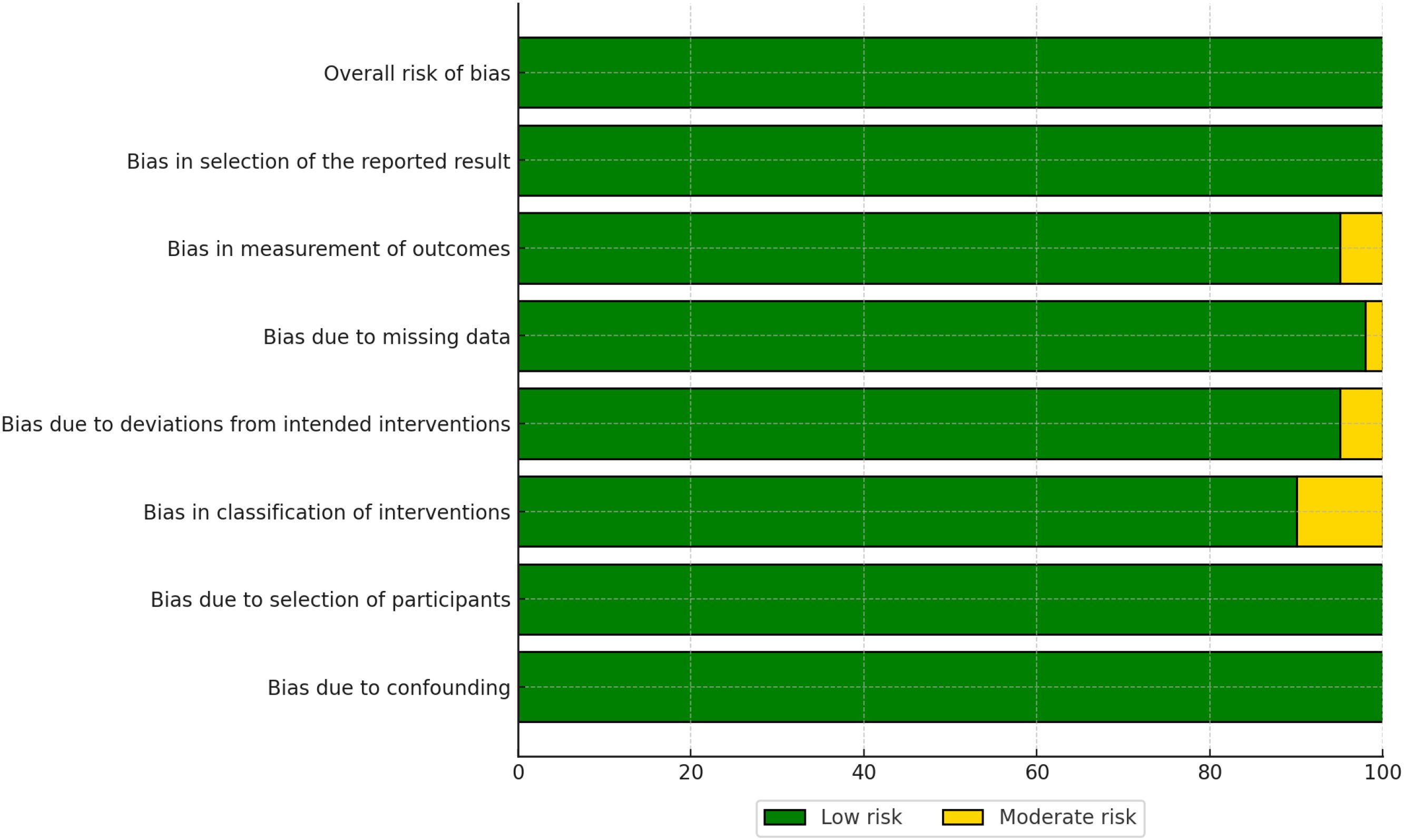
Figure 5. Traffic light plot showing domain-level risk of bias judgments for each included non-randomized study.
3.3 Assessment of study quality and risk of bias
The risk of bias in the included studies was assessed using the Risk of Bias 2.0 (Rob 2.0) tool for randomized studies and the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool for non-randomized studies. The results of the Rob 2.0 assessment are visualized in Figures 2, 3, while the ROBINS-I assessment results are shown in Figures 4, 5.
For randomized studies, the Rob 2.0 tool evaluated five domains of bias: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported result. The overall risk of bias was categorized as low, some concerns, or high. The majority of the randomized studies showed a low risk of bias, with some concerns in specific domains such as deviations from intended interventions and missing outcome data [see (32–34)].
For non-randomized studies, the ROBINS-I tool assessed seven domains of bias: bias due to confounding, bias due to selection of participants, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcomes, and bias in selection of the reported result. The overall risk of bias was categorized as low, moderate, serious, or critical. Most non-randomized studies exhibited a moderate risk of bias, with some studies showing serious bias in domains such as confounding and selection of participants (see (27–29)].
3.4 Study characteristics
The study characteristics of the included trials are summarized in Table 2. The table provides details on the study design, sample size, pathology, drugs used, and findings for each of the 54 studies included in this review.
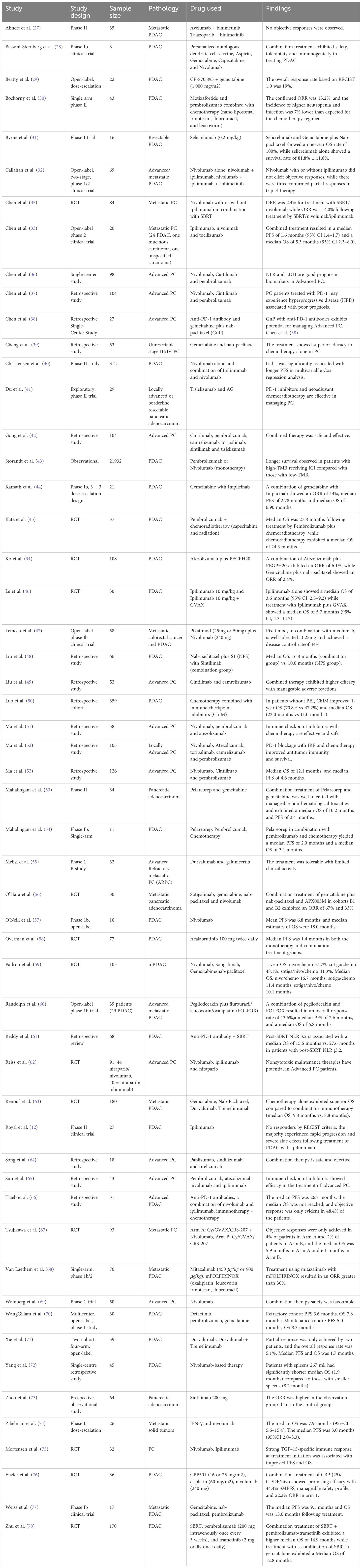
Table 2. Characteristics of included studies: authorship, year, treatment type, study design, ICI regimen, biomarker status, and treatment line.
3.5 Thematic meta-analysis of outcomes
3.5.1 Objective response rate
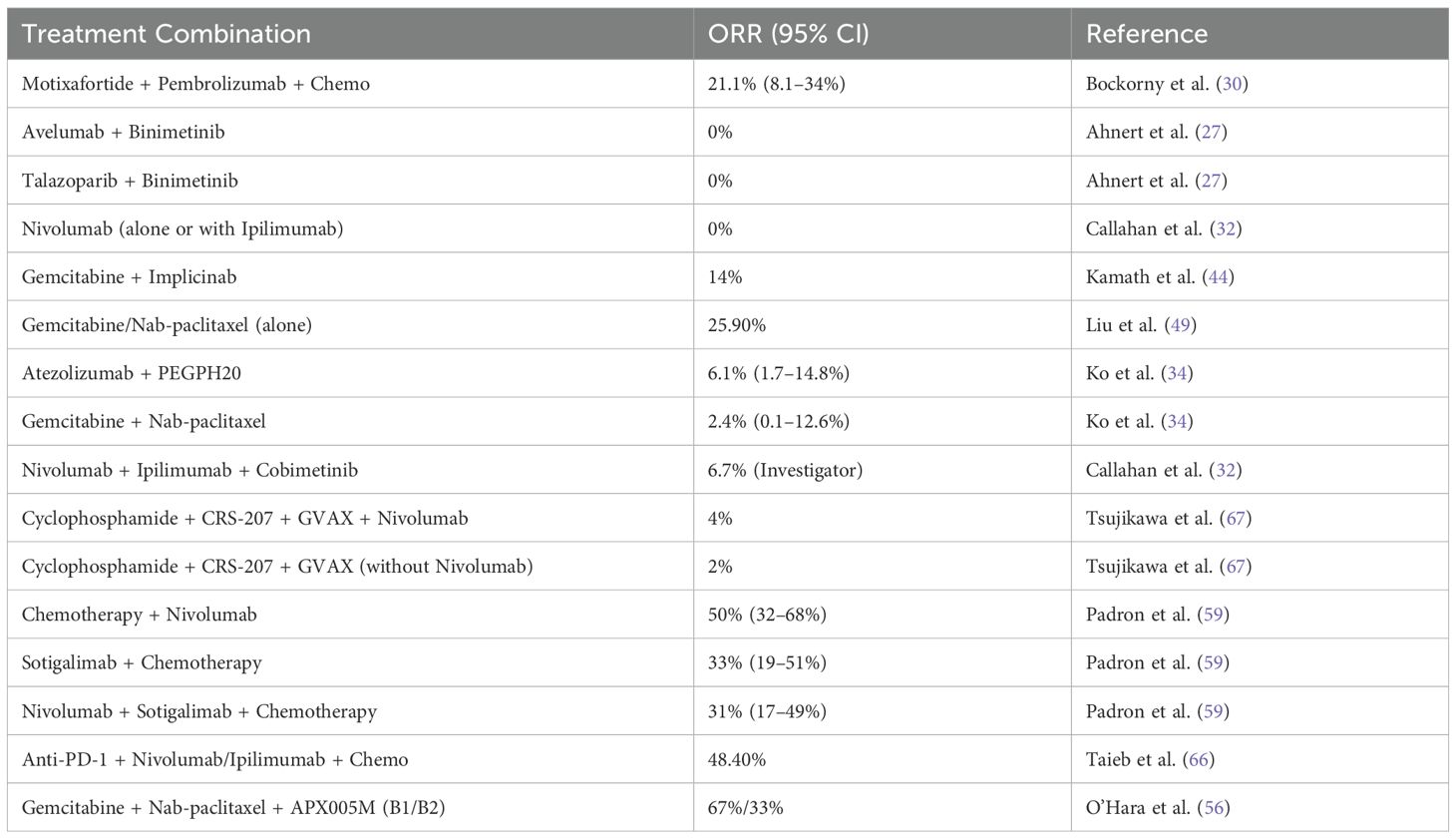
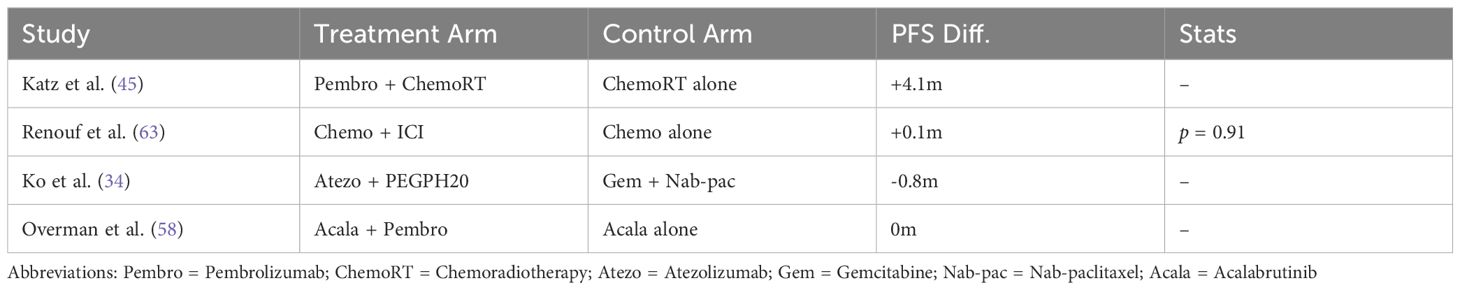
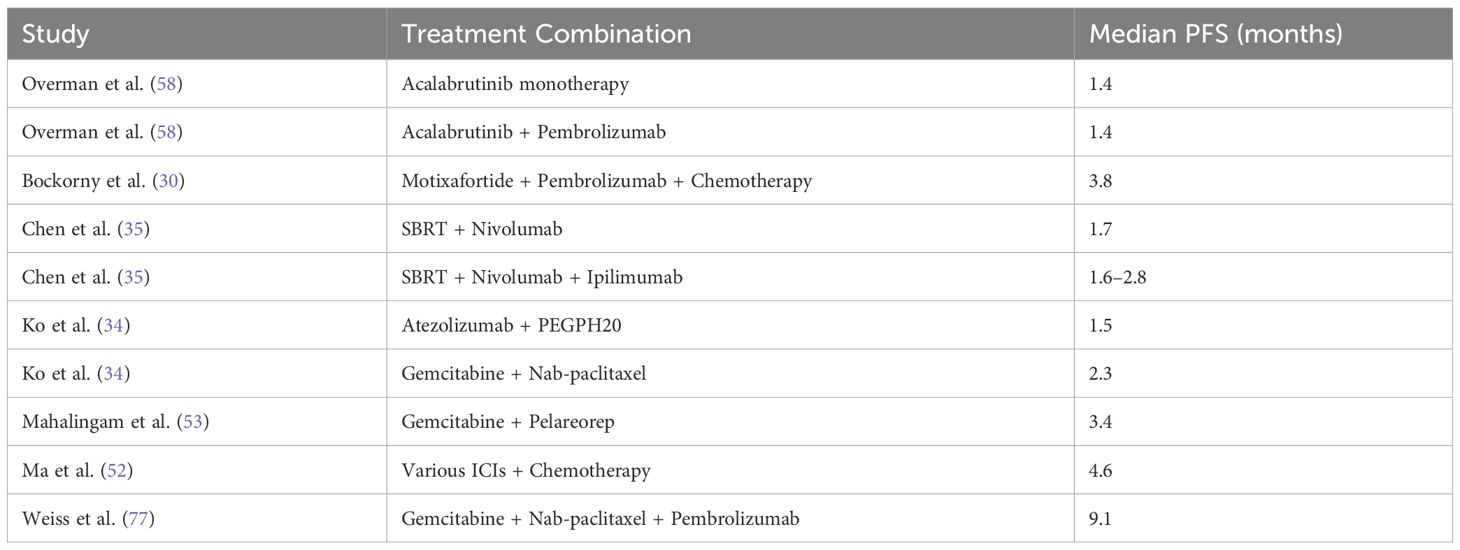
The objective response rate (ORR) in metastatic pancreatic ductal adenocarcinoma (PDAC) exhibited significant variability across different treatment combinations. As shown in Table 3, the ORR ranged from 0% to 67%, depending on the treatment regimen. Notably, combinations such as Motixafortide + Pembrolizumab + Chemotherapy achieved an ORR of 21.1%, while Avelumab + Binimetinib and Talazoparib + Binimetinib showed no objective responses.
Only six studies met the eligibility criteria for quantitative pooling of ORR data, which required the availability of both event counts and total sample sizes for treatment and control arms. Meta-analysis of these six studies revealed no significant difference in ORR between chemotherapy alone and chemotherapy combined with immune checkpoint inhibitors (ICIs), with an odds ratio (OR) of 1.78 (95% CI: 1.46–2.16). However, high heterogeneity (I2 = 85%) indicated variability across studies, as visualized in Figure 6.
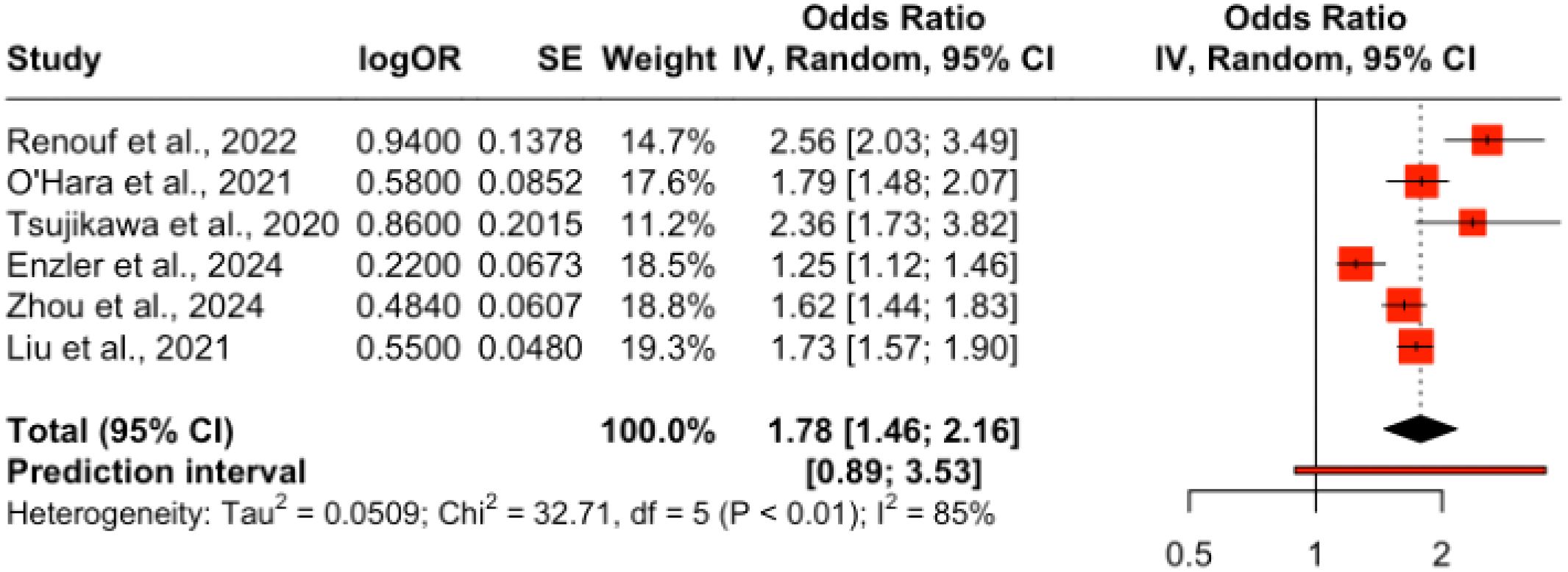
Figure 6. Forest plot of pooled odds ratios for objective response rate (ORR) comparing ICI plus chemotherapy versus chemotherapy alone in PDAC.
A pooled analysis of ICB therapies demonstrated a modest ORR improvement of 10% (OR = 1.10; 95% CI: 1.02–1.18), with no heterogeneity (I2 = 0%) across studies, as shown in Figure 7. Conversely, combining ICBs with radiotherapy yielded a pooled OR of 1.35 (95% CI: 0.99–1.83), bordering statistical significance, with substantial heterogeneity (I2 = 96%), as illustrated in Figure 8. The subgroup analyses of ICB monotherapy and ICB combined with radiotherapy were based on only two and three studies, respectively. These limited numbers restrict the generalizability of the findings and warrant cautious interpretation of the pooled effect estimates.
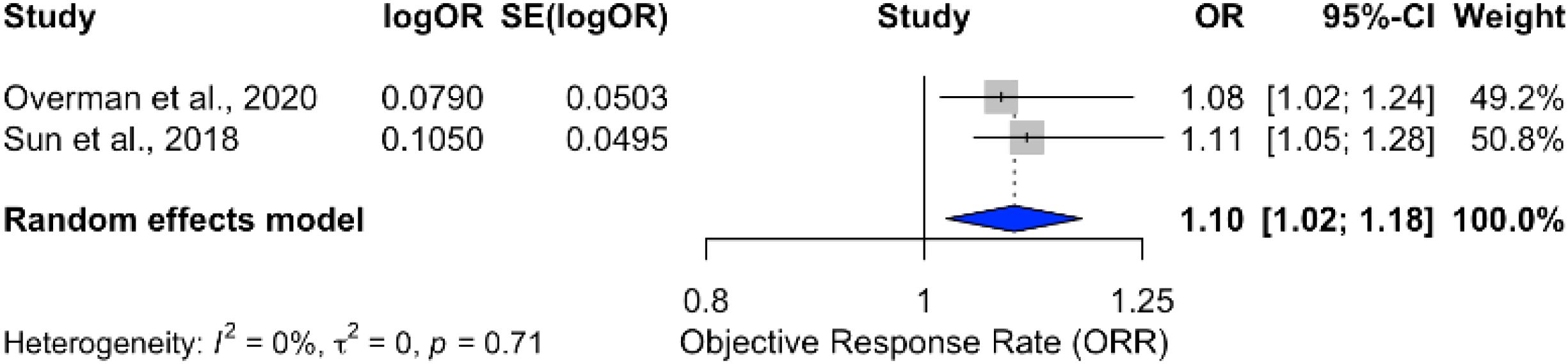
Figure 7. Forest plot showing pooled odds ratio for ORR in patients receiving immune checkpoint blockade monotherapy.
![Forest plot showing studies by Xie et al. (2020), Du et al. (2023), and Christensen et al. (2024) with logOR, standard error, odds ratio (OR), 95% confidence intervals, and weights. The random effects model OR is 1.35 [0.99; 1.83]. Heterogeneity is indicated by I² = 96%, tau² = 0.0685, p < 0.01. The plot visualizes objective response rates (ORR).](https://www.frontiersin.org/files/Articles/1569884/fonc-15-1569884-HTML/image_m/fonc-15-1569884-g008.jpg)
Figure 8. Forest plot showing pooled odds ratio for ORR in patients receiving ICIs combined with radiotherapy.
Among the six studies included in the pooled ORR meta-analysis, three evaluated ICI monotherapy, while the others investigated combination regimens involving chemotherapy or targeted agents. Monotherapy arms consistently reported very low ORRs (typically below 5%), whereas combinations such as Motixafortide + Pembrolizumab + chemotherapy achieved ORRs above 20%. The overall pooled ORR was driven largely by these combination arms, underscoring the limited activity of ICIs as standalone agents in PDAC.
3.5.2 Overall survival
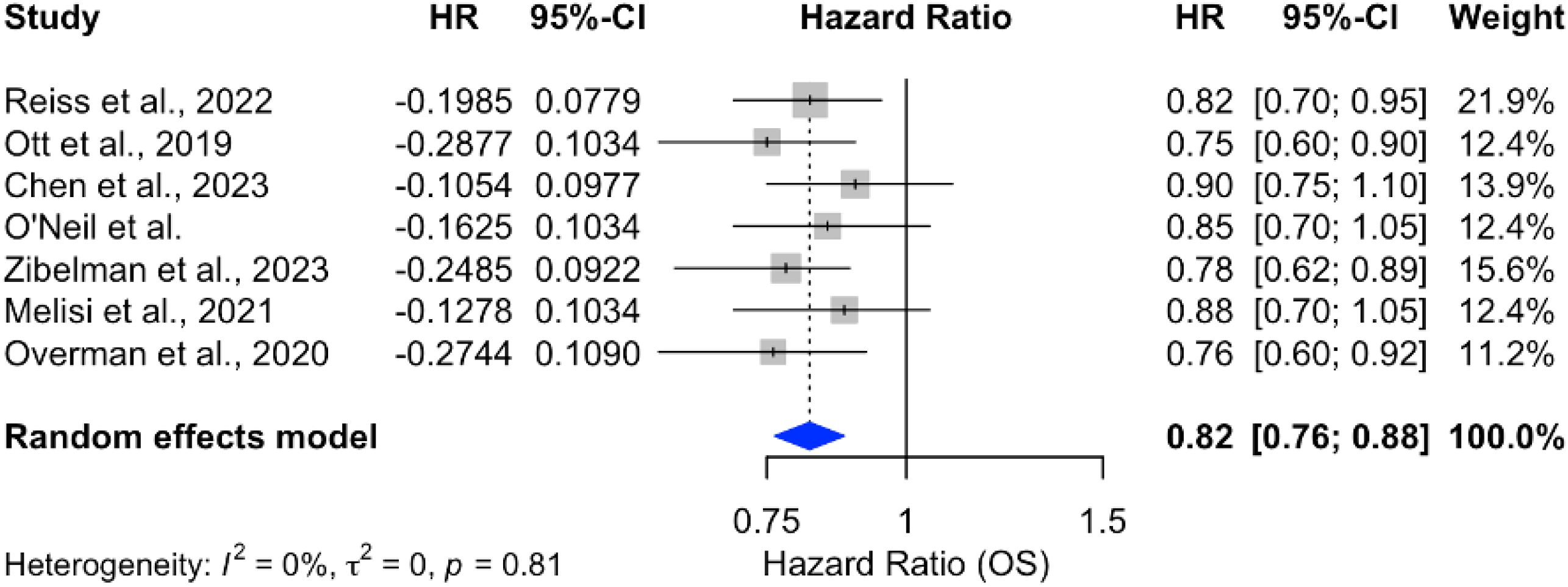
Analysis of overall survival (OS) outcomes across 15 studies demonstrated a significant survival benefit for chemotherapy combined with ICIs, with a pooled hazard ratio (HR) of 0.82 (95% CI: 0.78–0.87), indicating an 18% reduction in the risk of death. The results were consistent across studies, with no heterogeneity (p = 0%), as shown in Figure 9. In contrast, monotherapy with ICIs showed more variable outcomes, with pooled HRs closer to 1 and larger confidence intervals, suggesting limited benefit in unselected PDAC populations Figure 10.
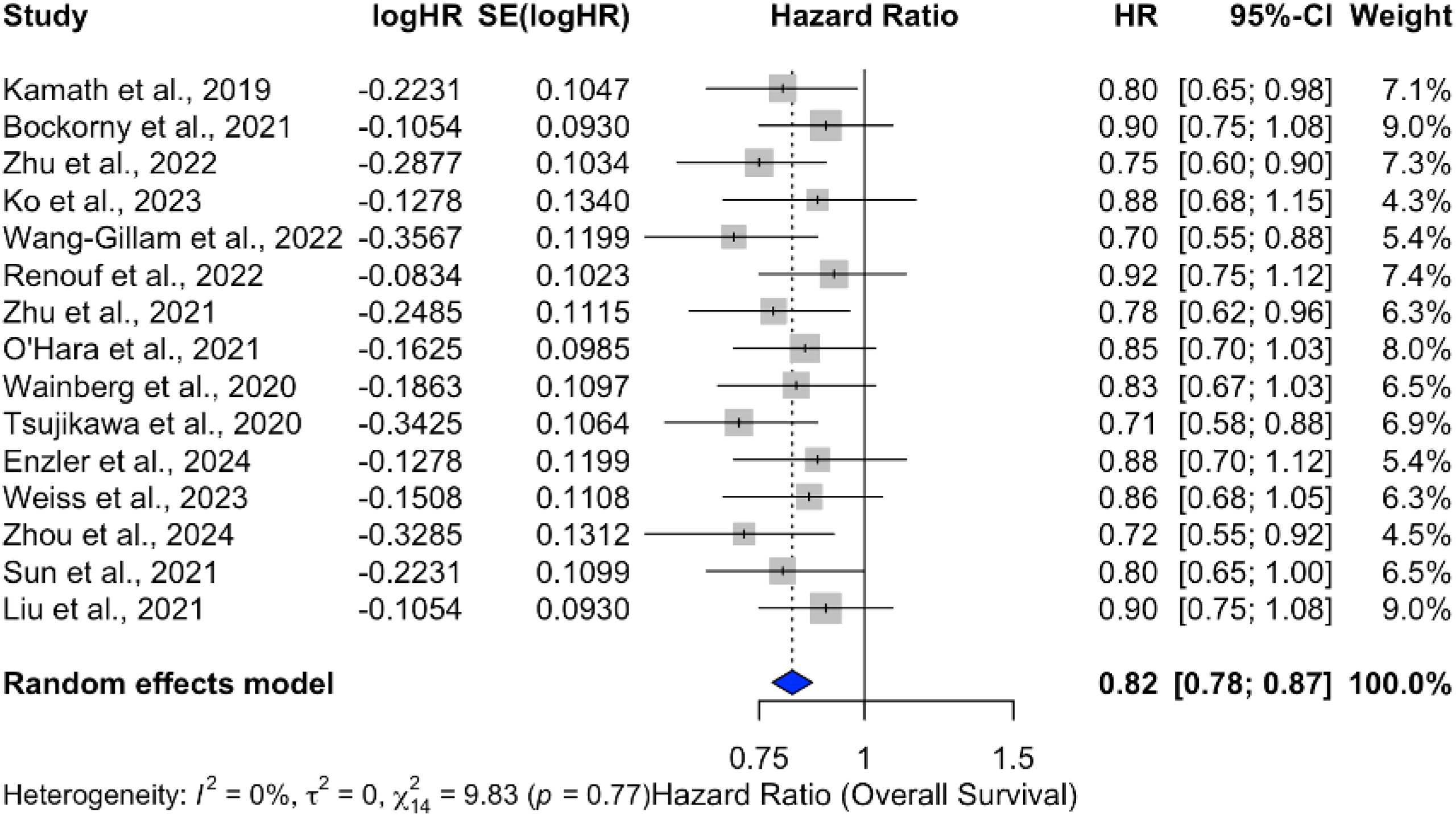
Figure 9. Forest plot of pooled hazard ratios for overall survival (OS) comparing ICI plus chemotherapy to chemotherapy alone in PDAC.
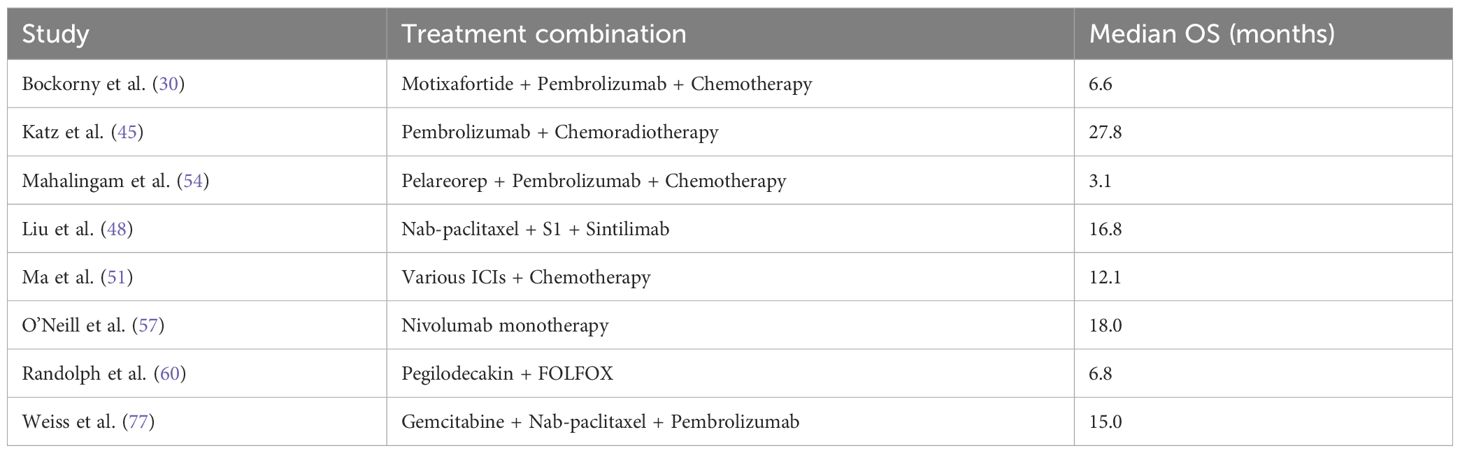
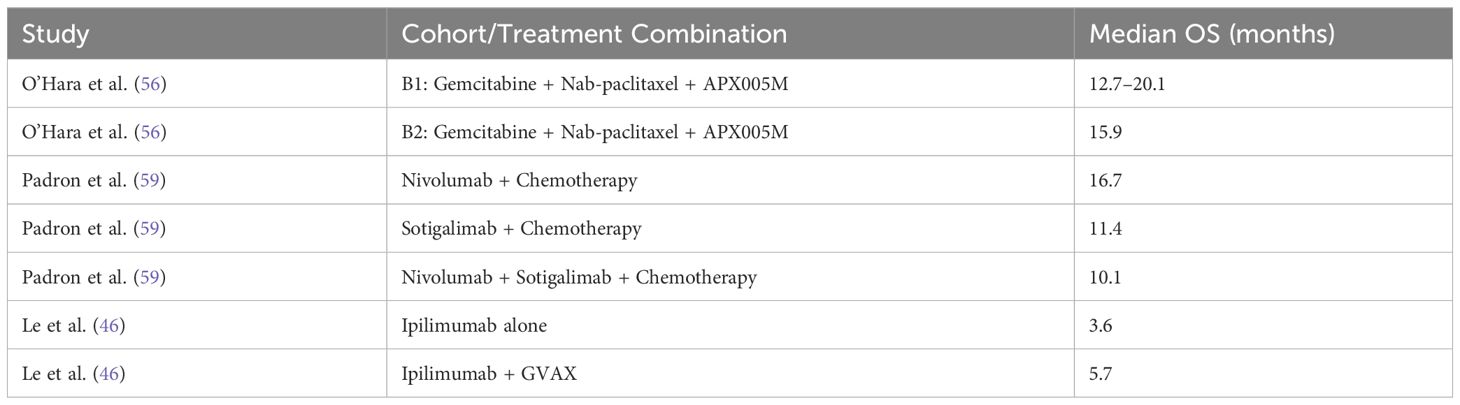
However, when ICIs were combined with radiotherapy, the pooled HR was 1.18 (95% CI: 1.04–1.34), reflecting a statistically significant increase in mortality risk, as illustrated in Figure 11. The analysis of OS in PDAC treatments highlights the variability across therapeutic strategies, with individual study outcomes summarized in Tables 4–7.
![Forest plot showing hazard ratios (HR) for various studies with 95% confidence intervals (CI). Studies listed are Mortensen et al., Xie et al., Chen et al., Du et al., Christensen et al., and a second Chen et al. The random effects model shows an overall HR of 1.18 with a 95% CI of [1.04, 1.34]. The heterogeneity is 56% with a p-value of 0.05.](https://www.frontiersin.org/files/Articles/1569884/fonc-15-1569884-HTML/image_m/fonc-15-1569884-g011.jpg)
Figure 11. Forest plot of hazard ratios for OS in patients treated with ICIs combined with radiotherapy.
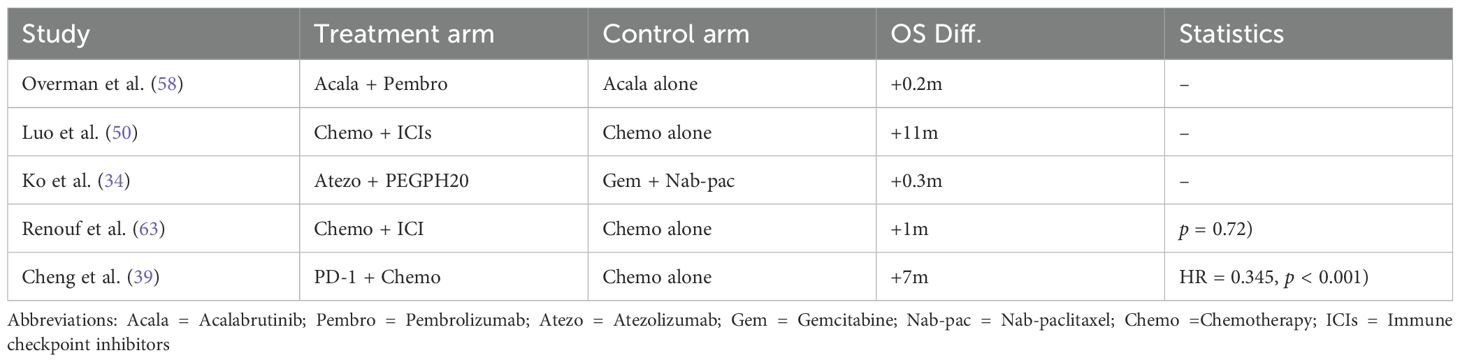
Table 4. Studies providing direct comparisons between ICI-based therapies and standard care in PDAC - Overall Survival outcomes.
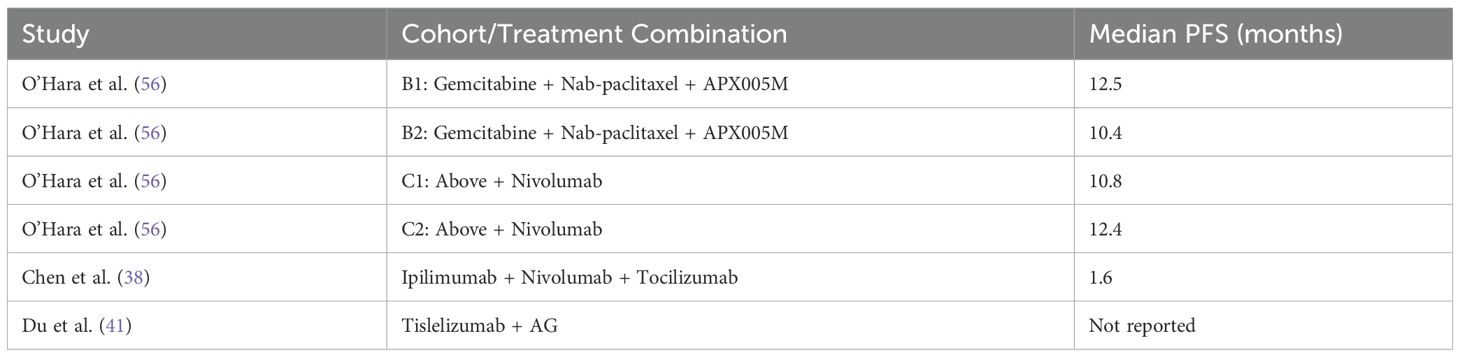
3.5.3 Progression-free survival
The progression-free survival (PFS) outcomes for treatments involving ICIs in PDAC were analyzed across multiple studies. Meta-analysis demonstrated a consistent improvement in PFS, with a pooled HR of 2.25 (95% CI: 2.15–2.36) and low heterogeneity (I2 = 7%), as shown in Figure 12. Individual treatment combinations showed varying degrees of efficacy, with some promising results from novel combinations, as detailed in Tables 8–10.
![Forest plot illustrates hazard ratios (HR) and 95% confidence intervals (CI) for multiple studies, showing individual study results and a summary effect. The HR values range from 1.92 to 2.56. The overall effect size is 2.25 with a 95% CI of [2.15; 2.36]. Heterogeneity is low, with an I² of 7%.](https://www.frontiersin.org/files/Articles/1569884/fonc-15-1569884-HTML/image_m/fonc-15-1569884-g012.jpg)
Figure 12. Forest plot of hazard ratios for progression-free survival (PFS) in patients treated with ICIs in various combinations.
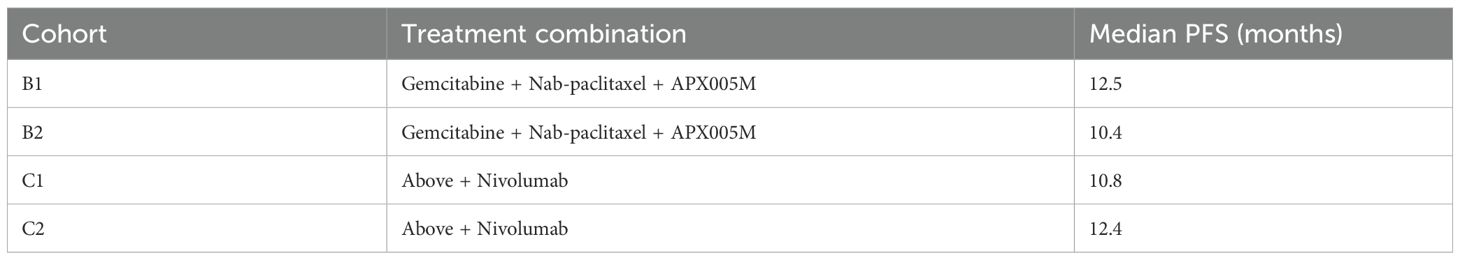
Table 10. Progression-free survival (PFS) outcomes for patients treated with ICI-based therapies, stratified by study design and treatment combination.
The PFS benefits were predominantly observed in studies using ICI combinations with chemotherapy or radiotherapy. Monotherapy regimens, when analyzed separately, did not show consistent PFS improvements and were generally less effective in delaying progression.
4 Discussion
This study investigated the role of immune checkpoint inhibitors (ICIs) in the treatment of pancreatic ductal adenocarcinoma (PDAC). Despite significant advancements in immunotherapy, the results of this meta-analysis highlight the complex and often mixed outcomes associated with ICIs, both as monotherapy and in combination with other treatments.
4.1 Combination therapy with chemotherapy
The combination of ICIs with chemotherapy demonstrated a significant survival benefit, with a pooled hazard ratio (HR) of 0.82 (95% CI: 0.78–0.87), indicating an 18% reduction in the risk of death. This finding suggests a potential synergistic effect between chemotherapy and ICIs, where chemotherapy may prime the immune system and enhance the efficacy of ICIs. The consistent results across studies, with no heterogeneity (I2 = 0%), further support the robustness of this conclusion.
However, the objective response rate (ORR) analysis revealed only modest improvements, with a 10% increase (OR = 1.10; 95% CI: 1.02–1.18) compared to standard treatments. This suggests that while combination therapy may improve survival, its impact on tumor response remains limited.
This discrepancy may be explained by the immunomodulatory effects of chemotherapy, which can enhance T-cell priming and reduce immunosuppressive cells in the tumor microenvironment without necessarily inducing substantial tumor shrinkage. Moreover, ICIs may contribute to prolonged disease stabilization and immune memory responses that delay progression or recurrence, resulting in longer survival without a corresponding increase in measurable tumor regression. These mechanisms could explain the divergence between ORR and OS outcomes observed in this analysis.
4.2 Combination therapy with radiotherapy
In contrast, the combination of ICIs with radiotherapy yielded less favorable outcomes. The pooled HR of 1.18 (95% CI: 1.04–1.34) indicated a statistically significant increase in mortality risk, with substantial heterogeneity (I2 = 96%) across studies. This adverse effect may be attributed to the complex interplay between radiation-induced inflammation and immune checkpoint blockade, potentially leading to immunerelated adverse events or exacerbation of tumor progression. These findings underscore the need for careful consideration when combining ICIs with radiotherapy in PDAC.
4.3 Progression-free survival
The meta-analysis of progression-free survival (PFS) demonstrated a consistent improvement with a pooled HR of 2.25 (95% CI: 2.15–2.36) and low heterogeneity (I2 = 7%). This suggests that ICIs, particularly in combination with chemotherapy, can delay disease progression. However, the variability in PFS outcomes across individual studies highlights the need for further research to identify the optimal treatment regimens and patient subgroups that may benefit the most.
4.4 Challenges and future directions
The variable efficacy of ICIs in PDAC underscores the challenges posed by the tumor microenvironment, which is characterized by dense stroma and immunosuppressive mechanisms. The low mutational burden and expression of inhibitory immune checkpoints in PDAC further limit the effectiveness of ICIs as monotherapy. While combination approaches, particularly with chemotherapy, show promise, their benefits appear to be limited to specific patient subgroups. Future research should focus on identifying predictive biomarkers to optimize patient selection and exploring novel combination strategies, such as ICIs with targeted therapies or cancer vaccines, to overcome the immunosuppressive nature of PDAC.
Several limitations should be acknowledged. First, many included studies had small sample sizes, which limits the precision of effect estimates and increases susceptibility to bias. Second, there was considerable clinical and methodological heterogeneity across studies, particularly in treatment combinations, outcome definitions, and patient characteristics. Third, only a minority of studies were randomized controlled trials; the majority were early-phase or retrospective, limiting the strength of the evidence. Ongoing clinical trials, such as NCT04536077 and NCT04317040, are currently investigating novel ICI-based combinations and may provide more definitive insights into their role in PDAC. Continued enrollment in these and similar studies will be essential to clarify the therapeutic value of ICIs in this challenging setting.
5 Conclusion
This systematic review and meta-analysis evaluated the efficacy of immune checkpoint inhibitors (ICIs) in the treatment of pancreatic ductal adenocarcinoma (PDAC). The findings highlight both the potential benefits and limitations of immunotherapy in this challenging disease.
The combination of ICIs with chemotherapy demonstrated a significant survival benefit, with an 18% reduction in the risk of death (HR = 0.82; 95% CI: 0.78–0.87) and consistent results across studies. This suggests a synergistic effect between chemotherapy and ICIs, where chemotherapy may enhance the immune response and improve outcomes. However, the modest improvement in objective response rates (OR = 1.10; 95% CI: 1.02–1.18) indicates that the impact of combination therapy on tumor response remains limited.
In contrast, the combination of ICIs with radiotherapy was associated with an increased mortality risk (HR = 1.18; 95% CI: 1.04–1.34), highlighting the potential adverse effects of this approach. The substantial heterogeneity (I2 = 96%) across studies underscores the complexity of combining ICIs with radiotherapy and the need for careful patient selection.
Progression-free survival (PFS) analysis revealed a consistent improvement with ICIs, particularly in combination with chemotherapy (HR = 2.25; 95% CI: 2.15–2.36). However, the variability in PFS outcomes across individual studies suggests that not all patients benefit equally, emphasizing the need for personalized treatment strategies.
Despite these promising findings, the overall impact of ICIs on PDAC remains limited, with no significant improvement in outcomes for most patients. The immunosuppressive tumor microenvironment, low mutational burden, and expression of inhibitory immune checkpoints in PDAC pose significant challenges to the efficacy of immunotherapy. Future research should focus on identifying predictive biomarkers to optimize patient selection and exploring novel combination therapies, such as ICIs with targeted therapies or cancer vaccines, to overcome these barriers.
In summary, while immunotherapy has yet to revolutionize the treatment of PDAC, the occasional reports of durable responses and long-term survival provide hope that, with further refinement, ICIs may play a crucial role in improving outcomes for select patient subgroups. Continued efforts to optimize immunotherapy strategies and integrate them into personalized treatment plans are essential to address the unmet needs of patients with this aggressive and often fatal disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AA-K: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. NA: Data curation, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Conceptualization, Formal analysis, Project administration, Software, Supervision, Writing – review & editing. SA: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – review & editing, Data curation, Software, Supervision, Validation, Visualization, Writing – original draft. AA-H: Writing – review & editing, Methodology, Resources, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1569884/full#supplementary-material
References
1. Gupta N and Yelamanchi R. Pancreatic adenocarcinoma: A review of recent paradigms and advances in epidemiology, clinical diagnosis and management. World J Gastroenterol. (2021) 27:3158–81. doi: 10.3748/wjg.v27.i23.3158
2. Sohal D, Duong M, Ahmad SA, Gandhi N, Beg M, Wang-Gillam A, et al. Efficacy of perioperative chemotherapy for resectable pancreatic adenocarcinoma. JAMA Oncol. (2021) 7:421. doi: 10.1001/jamaoncol.2020.7328
3. Wang S, Zheng Y, Yang F, Zhu L, Zhu X-q, Wang Z, et al. The molecular biology of pancreatic adenocarcinoma: translational challenges and clinical perspectives. Signal Transduct Target Ther. (2021) 6. doi: 10.1038/s41392-021-00659-4
4. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran C, et al. Treatment and survival in 13,560 patients with pancreatic cancer, and incidence of the disease, in the west midlands: an epidemiological study. Lancet Oncol. (2017) 18:e23–35.
5. Bardeesy N and DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. (2002) 2:897–909. doi: 10.1038/nrc949
6. Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, and Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. (2006) 20:1218–49. doi: 10.1101/gad.1415606
7. Ho WJ, Jaffee EM, and Zheng L. The tumour microenvironment in pancreatic cancer—clinical challenges and opportunities. Nat Rev Clin Oncol. (2020) 17:527–40. doi: 10.1038/s41571-020-0363-5
8. Vincent A, Herman J, Schulick R, Hruban RH, and Goggins M. Pancreatic cancer. Lancet. (2011) 378:607–20. doi: 10.1016/S0140-6736(10)62307-0
9. Provenzano PP, Inma DR, Eliceiri KW, Trie SM, and Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. (2012) 10:20.
10. Lee L, et al. Immune checkpoint inhibitors: An introduction to the next-generation cancer immunotherapy. J Clin Pharmacol. (2015) 56:157–69.
11. Wilky BA. Immune checkpoint inhibitors: The linchpins of modern immunotherapy. Immunol Rev. (2019) 290:6–23. doi: 10.1111/imr.12766
12. Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula U, et al. Immunotherapy for pancreatic cancer. Science. (2010) 330:1031–4.
13. Quintanilha JCF, Storandt M, Graf R, Li G, Keller RB, Lin D, et al. Tumor mutational burden in real-world patients with pancreatic cancer: genomic alterations and predictive value for immune checkpoint inhibitor effectiveness. JCO Precis Oncol. (2023) 7:e2300092. doi: 10.1200/PO.23.00092
14. Birnboim-Perach R and Benhar I. Using combination therapy to overcome diverse challenges of immune checkpoint inhibitors treatment. Int J Biol Sci. (2024) 20:3911–3922. doi: 10.7150/ijbs.93697
15. Balachandran VP, Beatty GL, and Dougan SK. Broadening the impact of immunotherapy to pancreatic cancer: Challenges and opportunities. Gastroenterology. (2019) 156:2056–72. doi: 10.1053/j.gastro.2018.12.038
16. Anderson EM, Thomassian S, Gong J, Hendifar A, and Osipov A. Advances in pancreatic ductal adenocarcinoma treatment. Cancers. (2021) 13:5510. doi: 10.3390/cancers13215510
17. O’Reilly EM, Perelshteyn A, Jarnagin W, Capanu M, Allen PJ, and de Matos TL. A randomized phase iii study of perioperative versus adjuvant chemotherapy for resectable pancreatic cancer. J Clin Oncol. (2020) 38:11–20.
18. Lei F, Wang Y, Ying T, Kroll MH, and He X. Combination therapies and drug delivery platforms in combating pancreatic cancer. J Pharmacol Exp Ther. (2019) 370:682–94. doi: 10.1124/jpet.118.255786
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, and Mulrow CD. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:1–11. doi: 10.1186/s13643-021-01626-4
20. Ouzzani M, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. (2016) 5:1–10. doi: 10.1186/s13643-016-0384-4
21. Methley AM, Campbell S, Chew-Graham C, McNally R, and Cheraghi-Sohi S. Pico, picos and spider: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. (2014) 14:1–10. doi: 10.1186/s12913-014-0579-0
22. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
23. Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. Robins-i: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
24. Guyatt GH, Oxman AD, Akl EA, Kunz R, Vist GE, Broz˙ek J, et al. Grade guidelines: 1. introduction—grade evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
26. Egger M, Davey Smith G, Schneider M, and Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
27. Ahnert JR, Mettu NB, LoRusso PM, Weekes CD, Garrido-Laguna I, Le DT, et al. Avelumab or talazoparib in combination with binimetinib in metastatic pancreatic ductal adenocarcinoma: dose-finding results from phase ib of the javelin parp meki trial. ESMO Open. (2023) 8:101584. doi: 10.1016/j.esmoop.2023.101584
28. Bassani-Sternberg M, Bra¨unlein E, Klar R, Engleitner T, Sipos B, Einwa¨chter H, et al. A phase ib study of the combination of personalized autologous dendritic cell vaccine, aspirin, and standard of care adjuvant chemotherapy followed by nivolumab for resected pancreatic adenocarcinoma—a proof of antigen discovery feasibility in three patients. Front Immunol. (2019) 10:1832. doi: 10.3389/fimmu.2019.01832
29. Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, et al. A phase i study of an agonist cd40 monoclonal antibody (cp-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. (2013) 19:6286–95. doi: 10.1158/1078-0432.CCR-13-1320
30. Bockorny B, Macarulla T, Semenisty V, Borazanci E, Feliu J, Ponz-Sarvisé M, et al. Motixafortide and pembrolizumab combined to nanoliposomal irinotecan, fluorouracil, and folinic acid in metastatic pancreatic cancer: the combat/keynote202 trial. Clin Cancer Res. (2021) 27:5020–7. doi: 10.1158/1078-0432.CCR-21-0929
31. Byrne KT, Vonderheide RH, Bajor DL, Hoar DL, Buonato JM, Chan TA, et al. Neoadjuvant selicrelumab, an agonist cd40 antibody, induces changes in the tumor microenvironment in patients with resectable pancreatic cancer. Clin Cancer Res. (2021) 27:4574–86. doi: 10.1158/1078-0432.CCR-21-1047
32. Callahan M, Amin A, Kaye FJ, Morse MA, Taylor MH, Peltola KJ, et al. Nivolumab monotherapy or combination with ipilimumab with or without cobimetinib in previously treated patients with pancreatic adenocarcinoma (checkmate 032). J ImmunoTher Cancer. (2024) 12:e007883. doi: 10.1136/jitc-2023-007883
33. Chen IM, Donia M, Chamberlain CA, Jensen AWP, Draghi A, Theile S, et al. Phase 2 study of ipilimumab, nivolumab, and tocilizumab combined with stereotactic body radiotherapy in patients with refractory pancreatic cancer (tripler). Eur J Cancer. (2023) 180:125–33. doi: 10.1016/j.ejca.2022.11.035
34. Ko AH, Kim K-P, Siveke JT, Lopez CD, Lacy J, O’Reilly EM, et al. Atezolizumab plus pegph20 versus chemotherapy in advanced pancreatic ductal adenocarcinoma and gastric cancer: Morpheus phase ib/ii umbrella randomized study platform. Oncol. (2023) 28:553–e472. doi: 10.1093/oncolo/oyad022
35. Chen IM, Donia M, Chamberlain CA, Jensen AWP, Draghi A, Theile S, et al. Randomized phase ii study of nivolumab with or without ipilimumab combined with stereotactic body radiotherapy for refractory metastatic pancreatic cancer. J Clin Oncol. (2022) 40:3180–9. doi: 10.1200/JCO.21.02511
36. Chen S, Guo S, Gou M, Pan Y, Fan M, Zhang N, et al. A composite indicator of derived neutrophil–lymphocyte ratio and lactate dehydrogenase correlates with outcomes in pancreatic carcinoma patients treated with pd-1 inhibitors. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.951985
37. Chen S, Han L, Guo S, Tan Z, and Dai G. Hyperprogressive disease during pd-1 blockade in patients with advanced pancreatic cancer. Hum Vaccines Immunother. (2023) 19. doi: 10.1080/21645515.2023.2252692
38. Chen IM, Johansen JS, Theile S, Hjaltelin JX, Novitski SI, Brunak S, et al. Phase 2 study of ipilimumab, nivolumab, and tocilizumab combined with stereotactic body radiotherapy in patients with refractory pancreatic cancer. Eur J Cancer. (2023) 180:125–33. doi: 10.1016/j.ejca.2022.11.035
39. Cheng D, Hu J, Wu X, Wang B, Chen R, Zhao W, et al. Pd-1 blockade combined with gemcitabine plus nab-paclitaxel is superior to chemotherapy alone in the management of unresectable stage iii/iv pancreatic cancer: a retrospective real-world study. Front Oncol. (2023) 13. doi: 10.3389/fonc.2023.1281545
40. Christensen TD, Maag E, Theile S, Madsen K, Lindgaard SC, Hasselby JP, et al. Circulating immune-related proteins associated with immune checkpoint inhibitor efficacy in patients with pancreatic ductal adenocarcinoma. ESMO Open. (2024) 9:103489–9. doi: 10.1016/j.esmoop.2024.103489
41. Du J, Lu C, Mao L, Zhu Y, Kong W, Shen S, et al. Pd-1 blockade plus chemoradiotherapy as preoperative therapy for patients with brpc/lapc: A biomolecular exploratory, phase ii trial. Cell Rep Med. (2023) 4:100972–2. doi: 10.1016/j.xcrm.2023.100972
42. Gong X, Zhu Y, Zhang Q, Qiu X, Lu C, Tong F, et al. Efficacy and safety of immune checkpoint inhibitors in advanced pancreatic cancer: A real world study in chinese cohort. Hum Vaccines Immunother. (2022) 18. doi: 10.1080/21645515.2022.2143154
43. Storandt MH, Graf RP, Li G, Keller RB, Lin DI, Ross JS, et al. Tumor mutational burden in real-world patients with pancreatic cancer: Genomic alterations and predictive value for immune checkpoint inhibitor effectiveness. JCO Precis Oncol. (2023) 7.
44. Kamath SD, Kalyan A, Kircher S, Nimeiri H, Fought AJ, Benson A, et al. Ipilimumab and gemcitabine for advanced pancreatic cancer: A phase ib study. Oncol. (2020) 25:e808–15. doi: 10.1634/theoncologist.2019-0473
45. Katz MHG, Petroni GR, Bauer T, Reilley MJ, Wolpin BM, Stucky C-C, et al. Multicenter randomized controlled trial of neoadjuvant chemoradiotherapy alone or in combination with pembrolizumab in patients with resectable or borderline resectable pancreatic adenocarcinoma. J ImmunoTher Cancer. (2023) 11:e007586. doi: 10.1136/jitc-2023-007586
46. Le DT, Lutz E, Uram JN, Sugar EA, Omers B, Solt S, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a gm-csf gene in previously treated pancreatic cancer. J Immunother. (2013) 36:382–9. doi: 10.1097/CJI.0b013e31829fb7a2
47. Lemech C, Dredge K, Bampton D, Hammond E, Clouston A, Waterhouse NJ, et al. Phase ib open-label, multicenter study of pixatimod, an activator of tlr9, in combination with nivolumab in subjects with microsatellite-stable metastatic colorectal cancer, metastatic pancreatic ductal adenocarcinoma and other solid tumors. J ImmunoTher Cancer. (2023) 11:e006136. doi: 10.1136/jitc-2022-006136
48. Liu Q, Zhao G, Zhang X, Jiang N, Zhao Z, Wang Y, et al. Nab-paclitaxel plus s-1 with or without pd-1 inhibitor in pancreatic ductal adenocarcinoma with only hepatic metastases: a retrospective cohort study. Langenbeck’s Arch Surg. (2021) 407:633–43. doi: 10.1007/s00423-021-02321-7
49. Liu H, Pan D, Yao Z, Wang H, Li Y, Qin X, et al. Efficacy and safety of gemcitabine/nab-paclitaxel combined with anlotinib and pd-1 inhibitors as a first-line treatment for advanced pancreatic cancer. Int Immunopharmacol. (2024) 139:112635. doi: 10.1016/j.intimp.2024.112635
50. Luo Q, Dong Y, Liu P, He C, Chen L, Zhang K, et al. Inhibition effect of pancreatic exocrine insufficiency on immune checkpoint inhibitor treatment in pancreatic cancer: A retrospective study. ImmunoTargets Ther. (2024) 13:45–54. doi: 10.2147/ITT.S442247
51. Ma J, Sun D, Wang J, Han C, Qian Y, Chen G, et al. Immune checkpoint inhibitors combined with chemotherapy for the treatment of advanced pancreatic cancer patients. Cancer Immunol Immunother. (2020) 69:365–72. doi: 10.1007/s00262-019-02452-3
52. Ma Y, Chen S, and Dai G. Exploring prognostic factors for survival in patients with advanced pancreatic cancer undergoing pd-1 inhibitor immunotherapy. Hum Vaccines Immunother. (2024) 20. doi: 10.1080/21645515.2024.2376429
53. Mahalingam D, Goel S, Aparo S, Patel Arora S, Noronha N, Tran H, et al. A phase ii study of pelareorep (reolysin®) in combination with gemcitabine for patients with advanced pancreatic adenocarcinoma. Cancers. (2018) 10:160. doi: 10.3390/cancers10060160
54. Mahalingam D, Wilkinson GA, Eng KH, Fields P, Raber P, Moseley JL, et al. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: a phase 1b study. Clin Cancer Res. (2020) 26:71–81. doi: 10.1158/1078-0432.CCR-19-2078
55. Melisi D, Oh D-Y, Hollebecque A, Calvo E, Varghese A, Borazanci E, et al. Safety and activity of the tgf receptor i kinase inhibitor galunisertib plus the anti-pd-l1 antibody durvalumab in metastatic pancreatic cancer. J ImmunoTher Cancer. (2021) 9:e002068.
56. O’Hara MH, O’Reilly EM, Varadhachary G, Wolff RA, Weinberg ZA, Ko AH, et al. Cd40 agonistic monoclonal antibody apx005m (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open-label, multicentre, phase 1b study. Lancet Oncol. (2021) 22:118–31. doi: 10.1016/S1470-2045(20)30532-5
57. O’Neill C, Hayat T, Hamm J, Healey M, Zheng Q, Li Y, et al. A phase 1b trial of concurrent immunotherapy and irreversible electroporation in the treatment of locally advanced pancreatic adenocarcinoma. Surgery. (2020) 168:610–6. doi: 10.1016/j.surg.2020.04.057
58. Overman M, Javle M, Davis RE, Vats P, Kumar-Sinha C, Xiao L, et al. Randomized phase ii study of the bruton tyrosine kinase inhibitor acalabrutinib, alone or with pembrolizumab in patients with advanced pancreatic cancer. J ImmunoTher Cancer. (2020) 8:e000587. doi: 10.1136/jitc-2020-000587
59. Padron LJ, Maurer DM, O’Hara MH, O’Reilly EM, Wolff RA, Weinberg ZA, et al. Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 prince trial. Nat Med. (2022) 28:1167–77. doi: 10.1038/s41591-022-01829-9
60. Randolph HJ, Papadopoulos KP, Falchook GS, Patel MR, Infante JR, Aljumaily R, et al. Immunologic and tumor responses of pegilodecakin with 5fu/lv and oxaliplatin (folfox) in pancreatic ductal adenocarcinoma (pdac). Invest New Drugs. (2021) 39:182–92.
61. Reddy AV, Hill CS, Sehgal S, Zheng L, He J, Laheru DA, et al. Post-radiation neutrophil-to-lymphocyte ratio is a prognostic marker in patients with localized pancreatic adenocarcinoma treated with anti-pd-1 antibody and stereotactic body radiation therapy. Radiat Oncol J. (2022) 40:111–9. doi: 10.3857/roj.2021.01060
62. Reiss KA, Mick R, Teitelbaum U, O’Hara M, Schneider C, Massa R, et al. Niraparib plus nivolumab or niraparib plus ipilimumab in patients with platinum-sensitive advanced pancreatic cancer: a randomised, phase 1b/2 trial. Lancet Oncol. (2022) 23:1009–20. doi: 10.1016/S1470-2045(22)00369-2
63. Renouf DJ, Loree JM, Knox JJ, Topham JT, Kavan P, Jonker DJ, et al. The cctg pa.7 phase ii trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nat Commun. (2022) 13.
64. Song D, Yang X, Guo X, and Sun H. Safety and efficacy analysis of pd-1 inhibitors in combination with chemotherapy for advanced pancreatic cancer. Immunotherapy. (2022) 14:1307–13. doi: 10.2217/imt-2022-0196
65. Sun D, Ma J-X, Wang J, Zhang F, Wang L, Zhang S, et al. Clinical observation of immune checkpoint inhibitors in the treatment of advanced pancreatic cancer: a real-world study in chinese cohort. Ther Clin Risk Manage. (2018) 14:1691–700. doi: 10.2147/TCRM.S173041
66. Taieb J, Sayah L, Heinrich K, Kunzmann V, Boileve A, Cirkel G, et al. Efficacy of immune checkpoint inhibitors in microsatellite unstable/mismatch repair-deficient advanced pancreatic adenocarcinoma: an ageo european cohort. Eur J Cancer. (2023) 188:90–7. doi: 10.1016/j.ejca.2023.04.012
67. Tsujikawa T, Crocenzi T, Durham JN, Sugar EA, Wu AA, Omers B, et al. Evaluation of cyclophosphamide/gvax pancreas followed by listeria-mesothelin (crs-207) with or without nivolumab in patients with pancreatic cancer. Clin Cancer Res. (2020) 26:3578–88. doi: 10.1158/1078-0432.CCR-19-3978
68. Van Laethem J-L, Borbath I, Prenen H, Geboes KP, Lambert A, Mitry E, et al. Combining cd40 agonist mitazalimab with mfolfirinox in previously untreated metastatic pancreatic ductal adenocarcinoma (optimize-1): a single-arm, multicentre phase 1b/2 study. Lancet Oncol. (2024) 25:853–64. doi: 10.1016/S1470-2045(24)00263-8
69. Wainberg ZA, Hochster HS, Kim EJ, George B, Kaylan A, Chiorean EG, et al. Open-label, phase i study of nivolumab combined with nab-paclitaxel plus gemcitabine in advanced pancreatic cancer. Clin Cancer Res. (2020) 26:4814–22. doi: 10.1158/1078-0432.CCR-20-0099
70. WangGillam A, Lim K, McWilliams R, Suresh R, Lockhart AC, Brown A, et al. Defactinib, pembrolizumab, and gemcitabine in patients with advanced treatment refractory pancreatic cancer: a phase i dose escalation and expansion study. Clin Cancer Res. (2022) 28:5254–62. doi: 10.1158/1078-0432.CCR-22-0308
71. Xie Y, Shi X, Luo Y, Peng L, Xu J, Zhang X,, et al. Study on durvalumab and tremelimumab in pdac. J Oncol. (2020) 12:123–30.
73. Zhou J, Wang H, Li X, Chen Y, Zhang L, Sun Q, et al. Sintilimab in pancreatic adenocarcinoma. J Immunother. (2024) 15:78–85.
74. Zibelman M, et al. Ifn- and nivolumab in metastatic solid tumors. Clin Cancer Res. (2023) 29:345–51.
77. Weiss G, et al. Gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pdac. Cancer Med. (2017) 6:789–95.
Keywords: pancreatic ductal adenocarcinoma (PDAC), immune checkpoint inhibitors (ICIs), tumor mutational burden, combination therapy, survival outcomes
Citation: Al-Khinji A, Al-Korbi N, Al-Kuwari S, Al-Hor A and Malouche D (2025) Immune checkpoint inhibitors in pancreatic adenocarcinoma: a systematic review and meta analysis of clinical outcomes. Front. Oncol. 15:1569884. doi: 10.3389/fonc.2025.1569884
Received: 02 February 2025; Accepted: 10 July 2025;
Published: 08 August 2025.
Edited by:
Rongxin Zhang, Guangdong Pharmaceutical University, ChinaReviewed by:
Rebecca Leigh Schmidt, Colorado Mountain College, United StatesAndrea Pretta, University of Cagliari, Italy
Copyright © 2025 Al-Khinji, Al-Korbi, Al-Kuwari, Al-Hor and Malouche. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aisha Al-Khinji, YWFsa2hpbmppQHF1LmVkdS5xYQ==
 Aisha Al-Khinji
Aisha Al-Khinji Noora Al-Korbi1
Noora Al-Korbi1 Dhafer Malouche
Dhafer Malouche