- 1Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 2Otolaryngology & Head and Neck Center, Cancer Center, Department of Head and Neck Surgery, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, Zhejiang, China
- 3Zhejiang Key Laboratory of Precision Medicine Research on Head & Neck Cancer, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, Zhejiang, China
- 4Zhejiang Provincial Clinical Research Center for Head & Neck Cancer, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, Zhejiang, China
Background: Esophageal granular cell tumor (GCT) is a rare benign neurogenic tumor, however, malignant transformation has been reported. And there is no consensus on the choice of esophageal reconstruction in these patients. Therefore, the study of markers of malignant potential in GCT is of great importance in guiding the choice of clinical treatment. Case presentation: Herein, we report the case of a patient with a large cervical esophageal GCT in which a myocutaneous free flap was used to repair a large defect after the resection of a localized esophageal tumor, providing strong reliability in terms of coverage capacity, tissue resistance, and distance management from the recipient vessel. The patient’s recovery was satisfactory at 48 days postoperatively, without significant complications. To further understand the specific cellular status of esophageal granular cell tumors, we performed an in-depth analysis of the tumor and its paraneoplastic tissues in this patient using single-cell RNA sequencing (scRNA-seq), which showed that neural-like cell subpopulations were enriched in the tumor, and genes such as SOX10, S100B, NCAM1, SPP1, and STMN1 were significantly upregulated. A significant copy number variation increase was observed in the X chromosome region. Conclusions: To the best of our knowledge, the present study represents the first scRNA-seq analysis of GCT, providing valuable insights for future prediction of GCT malignancy. In addition, the present study successfully repaired a large cervical oesophageal defect using a skin flap, and these findings have great potential to guide the understanding and management of postoperative large defects in benign cervical esophageal masses, paving the way for further clinical surgical practice in this area.
1 Introduction
Granular cell tumors (GCT) are rare soft tissue tumors originating from Schwann cells and were first identified in the tongue by Abrikossoff in 1926 (1). GCT can occur in any part of the body, preferentially in the tongue, mammary glands, and proximal limbs, which are usually located in the skin, subcutaneous layer, or submucosal layer. GCT in the gastrointestinal tract are rare, accounting for only 8–10% of cases, with esophageal involvement observed in only 2% of cases, occurring primarily in the lower and middle esophagus (2–4), but only 20% and 15% of these are located in the middle and upper segments. Most GCTs are benign; however, tumors with atypical cytological features or malignant transformations have been reported (5, 6). Here, we share this case to offer a reference for clinically treating similar cases and to inspire related basic medical research.
2 Case presentation
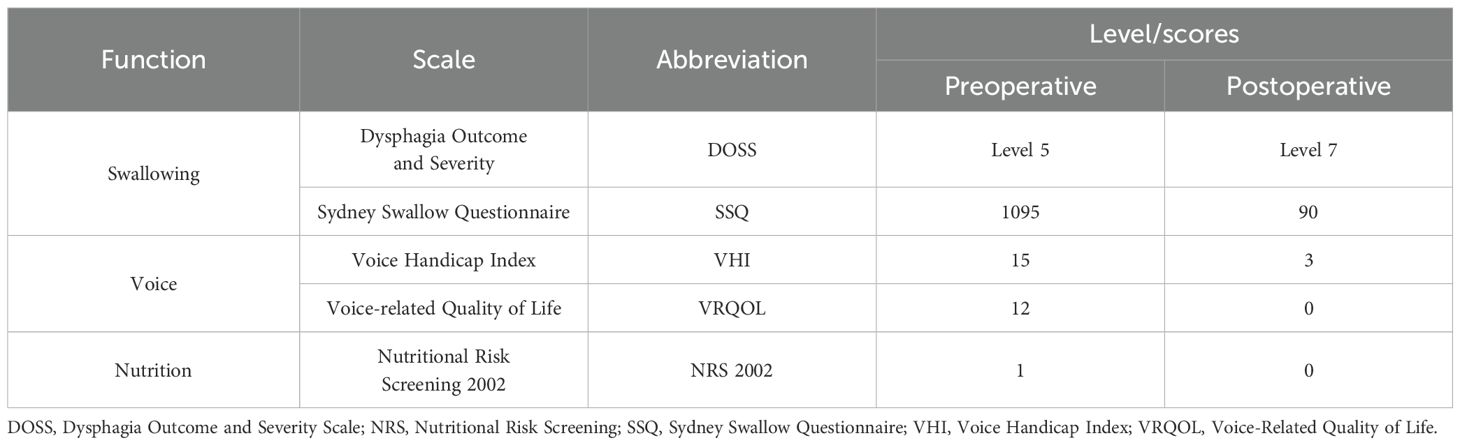
A 56-year-old female with over-year swallowing difficulty was admitted. Her height, weight, and BMI were 155 cm, 52 kg, and 23.11 kg/m², respectively. Dysphagia, voice, and nutritional status were assessed using the Sydney Swallow Questionnaire (SSQ) (7), Dysphagia Outcome and Severity Scale (DOSS) (8), Voice Handicap Index (VHI) (9), Voice-Related Quality of Life (V-RQOL) (10) and Nutritional Risk Screening 2002 (NRS 2002), preoperatively and on postoperative day 50, respectively (Table 1). All laboratory test results were normal. Preoperative and postoperative day 50 Enhancement magnetic resonance imaging (MRI), Enhanced computed tomography (CT), and endoscopic examination are shown in Figures 1A–F. A preoperative needle biopsy indicated a GCT. Immunohistochemical showed that S-100 (+), SOX10 (+), P40 (–), P63 (-), CK (Pan) (-), Vimentin (+), Ki67 (+, 3%), PAS (+).
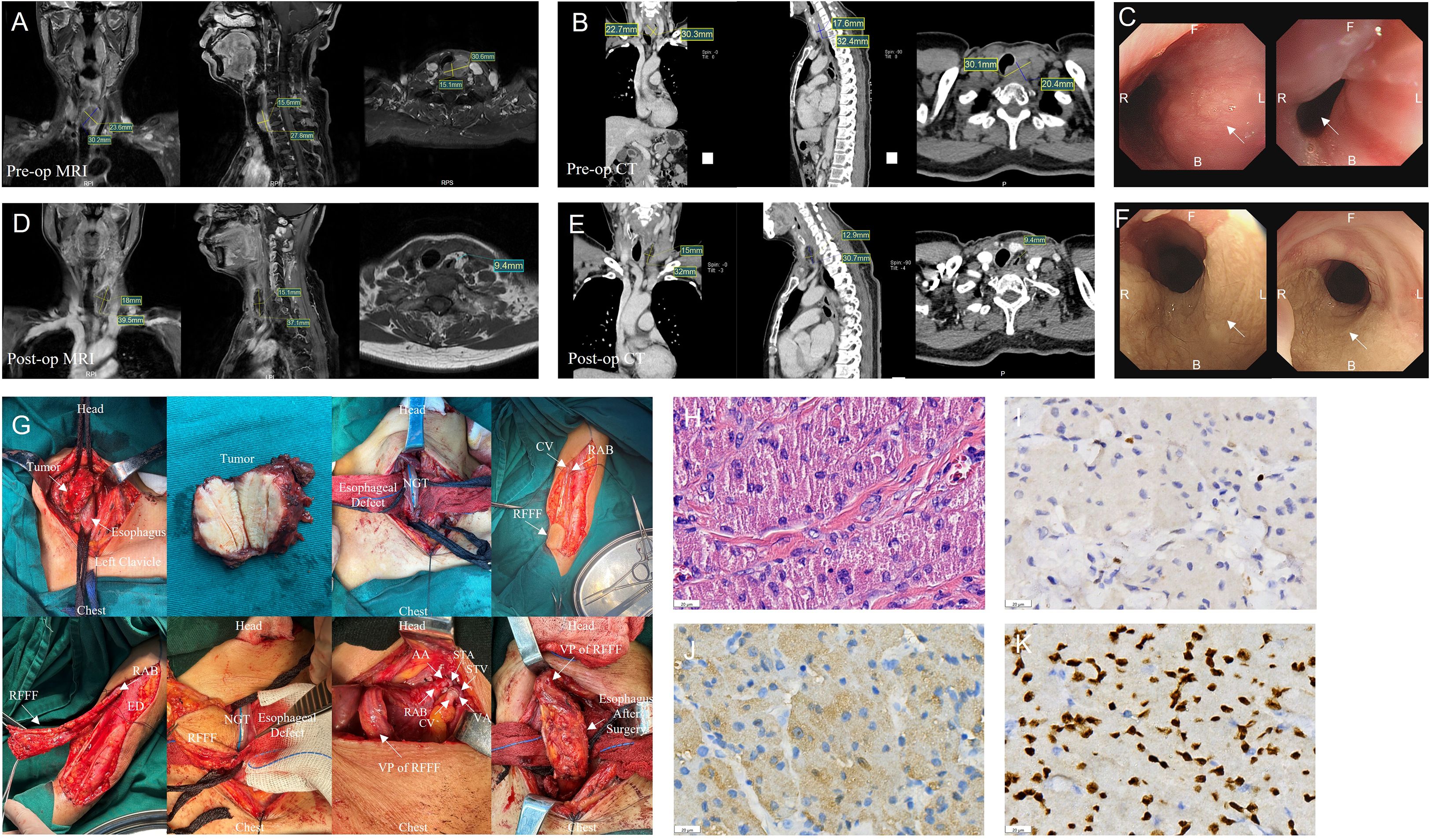
Figure 1. Information for patient. (A-C) Preoperative (pre-op) enhancement magnetic resonance imaging (MRI), enhanced computed tomography (CT) and Endoscopic examination results. (D-F) Postoperative (post-op) enhancement magnetic resonance imaging (MRI), enhanced computed tomography (CT) and Endoscopic examination results. (G) Surgery images. (H) Numerous eosinophilic granules visible within the cytoplasm under high magnification. Hematoxylin eosin staining; scale bar, 20 µm. (I) Tumor cells express Ki67 with about 3% in the hotspot. (J). Positive expression of S100 in tumor cells. (K) Positive expression of SOX10 in tumor cells. (AA, Arterial anastomosis; B, back; CV, cephalic vein; ED, Extensor digitorum; F, Front; NGT, Nasogastric tube; RAB, radial arteriovenous bundle; RFFF, radial forearm free flap; STA, superior thyroid artery; STV, superior thyroid vein; VA, venous anastomosis; VP, Vascular pedicle).
After a multi-disciplinary discussion, a decision was made to perform partial cervical esophagectomy, radial forearm free flap (RFFF) grafting. The intraoperative observation is shown in Figure 1G. Routine histopathological examination of the esophageal tumor (Figures 1H–K) revealed a 3.2 × 3 × 1.3 cm GCT invading the esophageal subserosa without vascular or perineural invasion. Margins were negative, and no neoplasm invasion was observed. Immunohistochemistry results: S100(+), SOX10(+), P40(-), P63(-), CK(Pan) (-), Vimentin (+), Ki67(+), hot spot area about 3%, P53(+, varying intensity), PAS (+). The patient was discharged on day 20 after surgery with no complications like stenosis or fistula during follow-up.
3 Analytical results of single-cell RNA-seq
In this study, we used single-cell RNA-sequencing (scRNA-seq) to analyze cell clusters in tumor and paraneoplastic tissues. Following data quality control, 12,953 high-quality cells were obtained and analyzed using the UMAP clustering algorithm. We annotated 14 cell clusters, including neural cell-like cells (marker genes: NGFR, NAV2, GAD2, and PLP1), endothelial cells (marker genes: PLVAP and VWF), smooth muscle cells (marker genes: ACTA2 and PLVAP), and fibroblasts (marker genes: TAGLN and COL1A2) (Figures 2A–B). Significant differences were observed in cell composition between the tumor and paraneoplastic tissues (Figure 2C). Paraneoplastic tissues were mainly epithelial, while tumor tissues were more diverse, containing endothelial cells (16.7%), smooth muscle cells (12.6%), fibroblasts (19.5%), macrophages (3.4%), B cells (11.6%), NK cells (20.1%), and T cells (9.3%). Neural cell-like clusters (6.4%) were enriched in tumor tissues (Figure 2D) and further analyzed the subpopulations of these neural cell-like clusters. Differentially expressed genes (DEGs) analysis identified key genes (SOX10, SOX4, S100B, NCAM1, SPP1, NGFR, IGFBP5, STMN1, RUNX2, and TGFBI) (Figure 2E), with expression levels compared across clusters (Figures 2F–I). Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses highlighted pathways like cell leading edge, cell–substrate junction, oxidative phosphorylation, and axon development in neural cell-like clusters (Figure 2J). Copy number variation (CNV) analysis revealed higher CNV levels in neural cell-like clusters, particularly in the X chromosome region (Figures 2K–L).
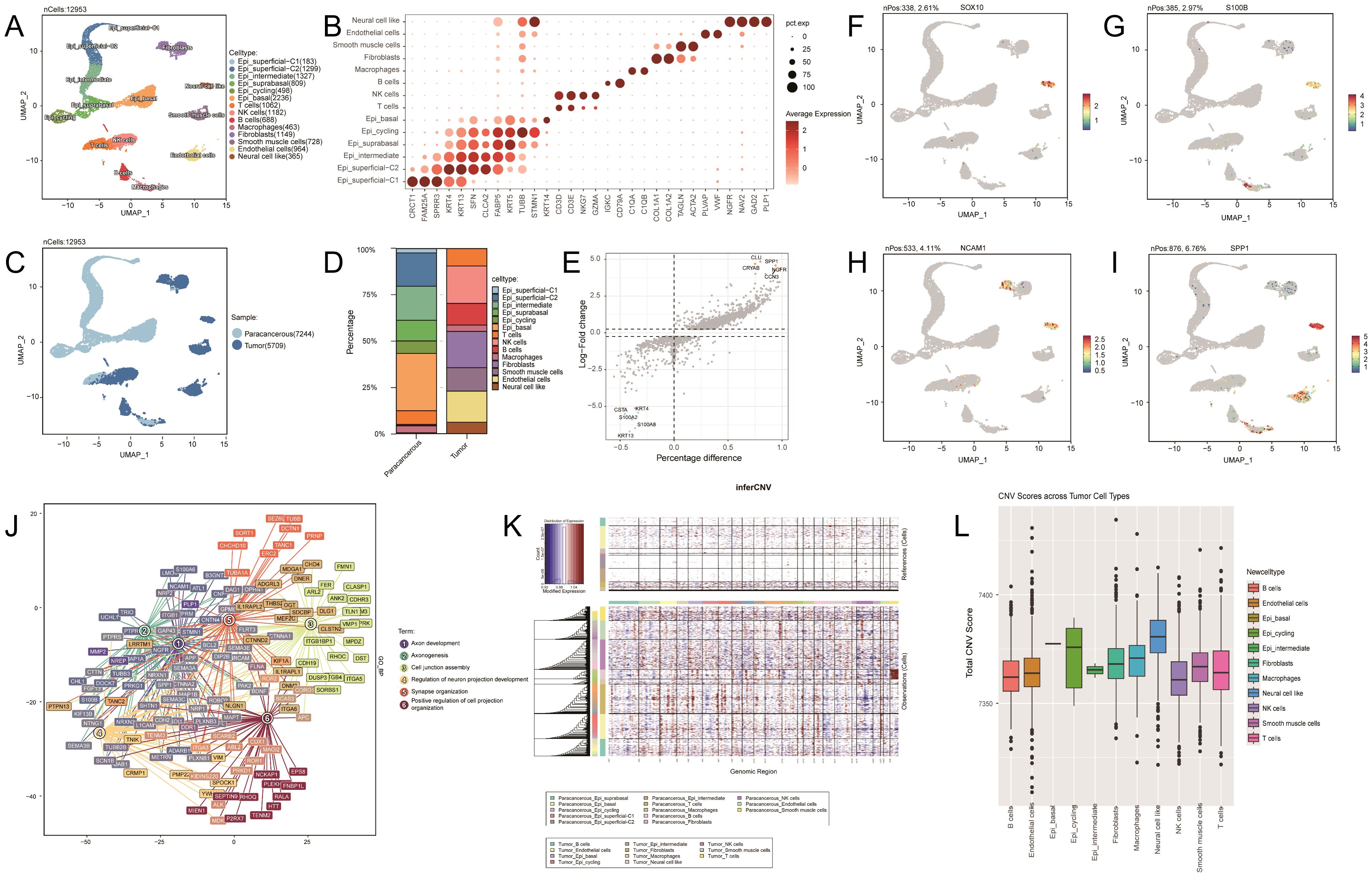
Figure 2. (A) Uniform Manifold Approximation and Projection (UMAP) representation of 14 identified cell types. N=12,953 cells. (B) Dot plot of the gene expression level of marker genes across cells. (C) UMAP representation showing two sample types, paracancerous and tumor. (D) Bar plot showing the cell type composition in paraneoplastic and tumor samples. (E) Volcano plot showing differential gene expression analysis for the neural cell like cluster. (F–I): UMAP plots showing the expression levels of SOX10, S100B, NCAM1, and SPP1 across different cell clusters. (J) Gene Ontology analysis of biological processes for the neural cell-like cluster. (K) Heatmap showing large-scale copy number variations (CNVs) profile of each tumor cell cluster. (L) Box plot showing the CNV scores across different tumor cell types.
4 Discussion
The clinical presentation of esophageal granular cell tumors is nonspecific, and they are mostly observed incidentally during endoscopic examinations. Esophageal granulosa cell tumors (EGCT) often appear as submucosal protrusive lesions or sessile polyps on endoscopy and can be easily misdiagnosed as polyps or esophageal smooth muscle tumors. Significant esophageal granular cell tumors may result in esophageal stricture, retrosternal discomfort, or dysphagia.
The treatment of esophageal cell tumors should encompass a thorough evaluation of factors such as size, location, depth of invasion, and the individual patient’s clinical circumstances. Some researchers state that patients who are asymptomatic or are mildly symptomatic with tumors measuring <1 cm can be managed through regular monitoring. Conversely, other scholars advocate primary tumor resection as the preferred treatment for GCT, emphasizing the importance of endoscopic ultrasonography for pretreatment assessment. For tumors limited to the mucosal layer or with minimal submucosal infiltration and a size of <2 cm, Endoscopic Mucosal Resection or Endoscopic Submucosal Dissection is recommended. In cases of extensive submucosal infiltration, invasion of the lamina propria, or tumors >2 cm, complete surgical resection is recommended. Long-term postoperative follow-up is essential to monitor tumor recurrence and potential complications.
Free-flap repair of esophageal defects following surgery for giant GCT in the cervical esophagus is uncommon. In 2006, a single case involving the use of a forearm radial free flap for the reconstruction of a 5-cm defect in the cervical esophagus was reported (11). The patient underwent a one-year follow-up period, during which they exhibited normal diet intake, absence of dysphagia, normal speech, and a regular lifestyle. There is no consensus on the method of esophageal reconstruction following partial esophagectomy for benign esophageal lesions such as GCT. Currently, the methods used to reconstruct the esophagus include gastric pull-up (GPU), colonic interposition (CI), jejunal flap (JF), and myocutaneous or fasciocutaneous free flaps (MC/FCFFs). GPUs are widely used for esophageal reconstruction because of their rich blood supply and the advantage of requiring only a single anastomosis. However, reflux esophagitis, mechanical obstruction, dumping syndrome, and aspiration pneumonia are common postoperative complications associated with GPU (12, 13). Some surgeons prefer to use CI for esophageal reconstruction, which has fewer complications associated with gastric reflux than gastric pull-ups and is mostly used for esophageal reconstruction after resection of tumors in the upper third of the esophagus (13). Overall complication rates for CI range from 40 to 60%, with most mortality rates <10% (14). Conversely, studies have indicated that JF outperforms CI in various aspects, including the graft necrosis rate, anastomotic leakage incidence, length of hospital stay, and postoperative weight loss (15, 16). However, a limitation of JF is its limited ability to address extensive esophageal defects owing to its limited vascular mesenteric arcades (15, 16). The aforementioned esophageal reconstruction methods necessitate transabdominal surgery, which is unsuitable for esophageal reconstruction after local resection of benign cervical esophageal tumors, posing the risks of intestinal obstruction and delayed transoral feeding in the postoperative phase.
The advantages of MC/FCFFs for esophageal reconstruction include no requirement for transabdominal surgery, a low incidence of ischemic graft necrosis, and no catheter redundancy. However, the drawbacks are a high rate of anastomotic leakage and short food transport time (13). One study suggested that multilayer closure could reduce the incidence of fistulae (17). In previous clinical practice, MC/FCFFs were selected only when esophageal reconstruction via gastrointestinal catheterization failed. MC/FCFFs are often used as an option for the secondary repair of esophageal defects because secondary repair is often accompanied by the risks of enlarged tissue damage and poor recipient vascularization. Sturdy pedicle flaps are a safer alternative with a lower incidence of flap necrosis, fistulae, and strictures (18). The preferred application of MC/FCFF to repair esophageal defects after local esophageal resection has occasionally been reported. In 2015, Mohammad et al. reported a case of primary early squamous cell carcinoma of the cervical esophagus reconstructed by local resection with a radial forearm free flap (19). The patient maintained good esophageal function for almost 4 years after resection without any signs of recurrence. In 2024, a study reported six cases of cervical esophageal defect reconstruction using pedicle flaps without flap necrosis, esophageal fistula, hematoma, or wound dehiscence (20). Although MC/FCFFs for the repair of localized esophageal defects have demonstrated very reliable efficacy, their clinical application in esophageal reconstructive surgery has been limited because of obscuring of the tracheal structure, the high variability of vascular anatomy, and the highly demanding surgeon’s microvascular anastomosis technique.
Free flap esophageal reconstruction, a more complex surgery requiring intensive postoperative monitoring, offers superior reliability in terms of coverage capacity, tissue resistance, and distance management from the recipient vessel. We recommend that thin flaps be considered when reconstructing cervical esophageal defects after local resection of benign tumors. Surgeons should consider their expertise and adopt a customized, multidisciplinary approach to manage such cases.
To date, to our knowledge, we have found no published data on single-cell analysis of EGCT. To further understand the specific cellular status of esophageal granular cell tumors, we analyzed the differences in cell clusters between the tumor and paraneoplastic tissues of patients via scRNA-seq. The results revealed significant cellular heterogeneity in EGCT, characterized by a diversity of cell types, particularly enriched with the neural cell-like clusters (Figure 2A). Our findings are consistent with those of previous studies on the characteristics of its pathological origin, further supporting its neurogenic pathological features (1). By comparing the expression levels of DEGs, we observed that SOX10, S100B, and NCAM1 (CD68) were significantly upregulated in the neural cell-like cluster (Supplementary Figure 1F–H), which is consistent with the findings of previous studies (1, 21). Our research further delineated the specific cell types that exhibited positive immune responses to these markers in GCT tissues and validated their neurogenic pathological features at single-cell resolution. Furthermore, SPP1, NGFR, IGFBP5, STMN1, RUNX2, and TGFBI were highly expressed in the neural cell-like cluster (Figure 2I; Supplementary Figures 1A–F). These genes are involved in the activation of signaling pathways related to tumor growth, metastasis, and immune evasion. Notably, SPP1 is overexpressed in various cancers, promoting malignant progression and correlating with poor prognosis by enhancing cell survival, proliferation, and angiogenesis (22, 23). In multiple cancers, including non-small cell lung cancer, STMN1 is abnormally expressed at high levels and promotes tumor cell proliferation by influencing microtubule stability and regulating phosphorylation levels (24–26). RUNX2 plays a critical role in tumor progression and bone metastasis (27, 28), whereas TGFBI may contribute to immune evasion in the tumor immune microenvironment (29). Therefore, the high expression of genes identified in the neural cell-like cluster of the patient’s tumor tissue may indicate that this cluster plays an important role in the progression and malignancy of EGCT. Furthermore, CNV analysis revealed a significant increase in CNVs in the X-chromosome region of the neural cell-like cluster. This finding is consistent with that of previous research indicating a higher incidence of EGCT in females (30). The increase in CNVs on the X chromosome may represent a potential mechanism for the higher incidence of this tumor in females, and the results of this study suggest that sex differences play a significant role in the occurrence and progression of this tumor. This article emphasizes two aspects: the selection of flaps for esophageal defect repair and scRNA-seq technology. Both are aimed at providing clinical guidance for EGCT patients, with the ultimate goal of enhancing their diagnostic and therapeutic outcomes.
In summary, we present a rare case of a giant EGCT in the cervical segment that was analyzed for the first time using scRNA-seq. Assessment of the malignant potential of a tumor is the key to its management. Clinicians should consider the clinical presentation, choice of treatment options, and prognostic follow-up of esophageal GCT. We strongly recommend the early radical surgical resection of esophageal tumors with potentially malignant features. The MCFFs is an effective and reliable technique for reconstructing esophageal function post-surgery for esophageal tumors. Our patient had significantly reduced dysphagia, good nutritional status, normal phonation, and no postoperative complications such as fistulas or gastrointestinal stenosis. However, long-term follow-up and further statistical comparisons with other modalities for the reconstruction of esophageal function are needed to repair esophageal defects with MCFFs.
5 Conclusion
We strongly recommend the early radical surgical resection of esophageal tumors with potentially malignant features, and the MCFF is an effective and reliable technique for reconstructing esophageal function post-surgery for esophageal tumors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Zhejiang Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. KC: Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. YD: Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing. JX: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Scientific Research Fund of the National Health Commission, Zhejiang Provincial Health Major Science and Technology Plan Project (No. WKJ-ZJ-2415), and the Basal Research Fund of Hangzhou Medical College (No. KYZD2023012).
Acknowledgments
We wish to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1580121/full#supplementary-material
Abbreviations
AA, Arterial anastomosis; B, Back; CI, Colonic interposition; CT, Computed tomography; CV, Cephalic vein; CNV, Copy number variation; DEGs, Differentially expressed genes; DOSS, Dysphagia Outcome and Severity Scale; ED, Extensor digitorum; F, Front; GCT, Granulosa cell tumor; GPU, Gastric pull-up; NGT, nasogastric tube; JF, Jejunal flap; MC/FCFF, Myocutaneous or fasciocutaneous free flaps; MRI, Magnetic resonance imaging; NRS 2002, Nutritional Risk Screening 2002; RAB, Radial arteriovenous bundle; RFFF, Radial forearm free flap; scRNA-seq, Single-cell RNA-sequencing; SSQ, Sydney Swallow Questionnaire; STA, Superior thyroid artery; STV, Superior thyroid vein; VA, Venous anastomosis; VHI, Voice Handicap Index; VP, Vascular pedicle; V-RQOL, Voice-Related Quality of Life
References
1. Abrikossoff A and Myome Uber. Virchows Archiv fur Pathologische Anatomie und Physiologie und fur Klinische Medizin. (1926) 260:215–33. doi: 10.1007/BF02078314
2. Lack EE, Worsham RGF, Callihan MD, Crawford BE, Klappenbach S, Rowden G, et al. Granular cell tumor: A clinicopathologic study of 110 patients. J Surg Oncol. (1980) 13:301–16. doi: 10.1002/jso.2930130405
3. Johnston MJ and Helwig EB. Granular cell tumors of the gastrointestinal tract and perianal region A study of 74 cases. Digest Dis Sci. (1981) 26:807–16. doi: 10.1007/bf01309613
4. An S, Jang J, Min K, Kim M, Park H, Park YS, et al. Granular cell tumor of the gastrointestinal tract: histologic and immunohistochemical analysis of 98 cases. Hum Pathol. (2015) 46:813–9. doi: 10.1016/j.humpath.2015.02.005
5. Piecuch J, Wiewiora M, and Latos W. Surgical treatment of a rare case of granular cell tumour of the cervical oesophagus. Videosurgery Miniinv. (2013) 8(2):166–9. doi: 10.5114/wiitm.2011.32819
6. Loo CKC, Santos LD, and Killingsworth MC. Malignant oesophageal granular cell tumour: a case report. Pathology. (2004) 36:506–8. doi: 10.1080/00313020412331282744
7. Audag N, Toussaint M, Prigent H, and Reychler G. Interpretation of Sydney Swallow Questionnaire results using the oropharyngeal dysphagia risk matrix. Neurogastroenterol Motility. (2024) 36(11):e14916. doi: 10.1111/nmo.14916
8. O’Neil KH, Purdy M, Falk J, and Gallo L. The dysphagia outcome and severity scale. Dysphagia. (1999) 14:139–45. doi: 10.1007/PL00009595
9. Ribeiro VV, Batista DDJ, Silveira WL, Barbosa I, Casmerides MCB, Dornelas R, et al. Reliability, measurement error, and responsiveness of the voice handicap index: A systematic review and meta-analysis. J Voice. (2024). 18:S0892-1997(24)00169-3. doi: 10.1016/j.jvoice.2024.05.017
10. Narasimhan SV, Puttegowda K, and Sahana K. Adaptation and validation of the voice-related quality of life measure into kannada. J Voice. (2022) 39(2):568.e1–568.e6. doi: 10.1016/j.jvoice.2022.09.011
11. Marin VP, Yu P, and Weber RS. Isolated cervical esophageal reconstruction for rare esophageal tumors. Head Neck. (2006) 28:856–60. doi: 10.1002/hed.20442
12. Cheng B, Chang S, Mao Z, Li M, Huang J, Wang Z, et al. Surgical treatment of giant esophageal leiomyoma. World J gastroenterology: WJG. (2005) 11:4258–60. doi: 10.3748/wjg.v11.i27.4258
13. Merritt RE. Conduit selection for reconstruction after esophagectomy for esophageal cancer. Surg Oncol Clin N Am. (2024) 33:549–56. doi: 10.1016/j.soc.2024.01.001
14. Sanchez MV, Alicuben ET, Luketich JD, and Sarkaria IS. Colon interposition for esophageal cancer. Thorac Surg Clin. (2022) 32:511–27. doi: 10.1016/j.thorsurg.2022.07.006
15. Doki Y, Okada K, Miyata H, Yamasaki M, Fujiwara Y, Takiguchi S, et al. Long-term and short-term evaluation of esophageal reconstruction using the colon or the jejunum in esophageal cancer patients after gastrectomy. Dis Esophagus. (2008) 21:132–8. doi: 10.1111/j.1442-2050.2007.00738.x
16. Hung P, Chen H, Tu Y, and Kao Y. A comparison of different types of esophageal reconstructions: A systematic review and network meta-analysis. J Clin Med. (2022) 11:5025. doi: 10.3390/jcm11175025
17. Tan NC, Lin PY, Kuo PJ, Tsai YT, Chen YC, Nguyen KT, et al. An objective comparison regarding rate of fistula and stricture among anterolateral thigh, radial forearm, and jejunal free tissue transfers in circumferential pharyngo-esophageal reconstruction. Microsurg. (2015) 35:345–9. doi: 10.1002/micr.22359
18. Ramella V, Ferrari A, Novati FC, Arnež ZM, Marchi G, Rodda A, et al. Secondary microsurgical reconstruction of the cervical esophagus: safer flaps and practical tips in a challenging situation. J Clin Med. (2024) 13:2726. doi: 10.3390/jcm13092726
19. Ali Mohammad FHM, Go PM, Ghanem TM, Stachler RM, and Hammoud ZM. Long-term survival after local resection of cervical esophageal cancer. Ann Thorac surgery. (2015) 99:2202–3. doi: 10.1016/j.athoracsur.2014.08.050
20. Agarwal A, Philips R, Fiorella M, Amin DR, Krein H, and Heffelfinger R. Complications and functional outcomes after esophageal reconstruction with an intact larynx. Laryngoscope. (2024) 134:1227–33. doi: 10.1002/lary.31055
21. Malik F, Bernieh A, and Saad AG. Esophageal granular cell tumor in children: A clinicopathologic study of 11 cases and review of the literature. Am J Clin Pathol. (2023) 160:106–12. doi: 10.1093/ajcp/aqad025
22. Wei J, Chen Z, Hu M, He Z, Jiang D, Long J, et al. Characterizing intercellular communication of pan-cancer reveals SPP1+ Tumor-associated macrophage expanded in hypoxia and promoting cancer Malignancy through single-cell RNA-seq data. Front Cell Dev Biol. (2021) 2021:9. doi: 10.3389/fcell.2021.749210
23. Lu D, Yeh W, Huang S, Tang C, Lin H, and Chou S. Osteopontin increases heme oxygenase–1 expression and subsequently induces cell migration and invasion in glioma cells. Neuro-Oncology. (2012) 14:1367–78. doi: 10.1093/neuonc/nos262
24. Rubin CI and Atweh GF. The role of stathmin in the regulation of the cell cycle. J Cell Biochem. (2004) 93:242–50. doi: 10.1002/jcb.20187
25. Iancu-Rubin C and Atweh GF. p27Kip1 and stathmin share the stage for the first time. Trends Cell Biol. (2005) 15:346–8. doi: 10.1016/j.tcb.2005.05.008
26. Zeng L, Lyu X, Yuan J, Chen Y, Wen H, Zhang L, et al. STMN1 promotes tumor metastasis in non-small cell lung cancer through microtubule-dependent and nonmicrotubule-dependent pathways. Int J Biol Sci. (2024) 20:1509–27. doi: 10.7150/ijbs.84738
27. Yin X, Teng X, Ma T, Yang T, Zhang J, Huo M, et al. RUNX2 recruits the NuRD(MTA1)/CRL4B complex to promote breast cancer progression and bone metastasis. Cell Death Differentiation. (2022) 29:2203–17. doi: 10.1038/s41418-022-01010-2
29. Lecker LSM, Berlato C, Maniati E, Delaine-Smith R, Pearce OMT, Heath O, et al. TGFBI production by macrophages contributes to an immunosuppressive microenvironment in ovarian cancer. Cancer Res. (2021) 81:5706–19. doi: 10.1158/0008-5472.CAN-21-0536
Keywords: granular cell tumor, single-cell RNA sequencing, myocutaneous free flap, esophageal tumor, esophageal reconstruction
Citation: Shao C, Chen K, Duan Y and Xu J (2025) Case Report: A large granular cell tumor of the cervical esophagus with single cell RNA sequencing analysis. Front. Oncol. 15:1580121. doi: 10.3389/fonc.2025.1580121
Received: 20 February 2025; Accepted: 15 July 2025;
Published: 02 September 2025.
Edited by:
Yuquan Chen, Monash University, AustraliaReviewed by:
Ötrs Péter Horváth, Pécs University, HungaryMeiqi Miao, Kunshan Traditional Chinese Medicine Hospital, China
Copyright © 2025 Shao, Chen, Duan and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiajie Xu, eHVqaWFqaWVAaG1jLmVkdS5jbg==; Yanting Duan, ZHl0MTk4ODA4MThAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Chengying Shao1,2†
Chengying Shao1,2† Jiajie Xu
Jiajie Xu