- 1Department of Clinical Laboratory, Peking University First Hospital, Beijing, China
- 2Institute of Reproductive and Child Health/National Health Commission Key Laboratory of Reproductive Health, School of Public Health, Peking University, Beijing, China
- 3Department of Urology, Peking University First Hospital and Institute of Urology, Beijing, China
Background and aims: Radical cystectomy (RC) remains the standard treatment for localized and regionally muscle-invasive bladder cancer (MIBC). However, only half of patients with MIBC survive more than 5 years after RC. We explored the factors associated with overall survival (OS) and constructed a prognostic nomogram for predicting 1-, 3-, and 5-year OS after RC.
Methods: The data were sourced from the Surveillance, Epidemiology, and End Results (SEER) database and Peking University First Hospital (PKUFH). Univariate and multivariate Cox regression analyses were performed using the minimum value of the Akaike information criterion to select independent prognostic factors that significantly contributed to patient survival. A prognostic nomogram was designed to predict 1-, 3-, and 5-year OS.
Results: Among the 16,949 patients with MIBC undergoing surgery, 31.15% survived for more than 5 years. The nomogram we created demonstrated satisfactory discriminative ability to predict the survival of MIBC patients with RC, with area under curve (AUC) of 0.939, 0.880 and 0.852 for 1-, 3- and 5-year OS in the testing set. Moreover, the nomogram still exhibited good performance in an externally independent dataset (1-year: AUC=0.970; 3-year: AUC=0.847; 5-year: AUC=0.790). Furthermore, decision curve analyses showed a modest net benefit for the use of the MIBC nomogram in the current cohort compared to the use of American Joint Committee on Cancer staging alone.
Conclusions: A prognostic nomogram was developed and validated to help clinicians evaluate the prognosis of postoperative MIBC patients. The future integration of additional data will likely improve model performance and accuracy for personalized prognostics.
1 Introduction
Bladder cancer is the ninth most commonly diagnosed cancer worldwide, with approximately 614,298 new cases and 220,596 deaths in 2022 (1). Of all cases, 25% are muscle invasive with significant risk of mortality. Non-muscle-invasive bladder cancer has a low lethality rate but can progress to muscle-invasive bladder cancer (MIBC) at a rate of up to 40–80% rate within 5 years (2, 3).
MIBC is characterized by high recurrence, metastasis, and poor prognosis, and is associated with a 5-year survival rate of 60–70%. For MIBC patients with distant metastasis, the 5-year survival rate is only 5% (4). This imposes a heavy economic burden on both patients and society.
Radical cystectomy (RC) remains the standard treatment for patients with MIBC (T2–4) (5, 6). However, the 5-year overall survival (OS) rate in male and female patients is 58–66% and at least 30% of patients experience local recurrence within 5 years after surgery. Most recurrences are diagnosed during the first 2 years (7–10). Thus, it is crucial to identify patients with poor prognosis or high risk of recurrence. These patients may need to be considered for adjuvant treatments or followed more closely for the early monitoring of disease progression and treatment.
At present, tumor stage and regional lymph node status are the primary prognostic variables following RC (11). In general, patients with early-stage and low-grade tumors have a better prognosis, while those with advanced-stage and high-grade tumors have a poorer prognosis. Lymph node metastasis is an important indicator of poor prognosis in bladder cancer, typically suggesting advanced disease progression. The 10-year recurrence-free survival for patients without lymph node involvement is 76% for T1-pT3a, 61% for T3b, and 45% for T4, but when lymph nodes are involved, it drops to 34%, regardless of the stage (12).
Nomograms for cancer-specific survival (CSS) following RC have been developed (13, 14) but their wider use cannot be recommended until more data are available. Shariat et al. (13) reported that the accuracy of a nomogram for the prediction of CSS that included pathologic stage, lymph node status, lymph vascular invasion, neoadjuvant chemotherapy, and adjuvant external beam radiotherapy was significantly superior to the staging based on the American Joint Committee on Cancer (AJCC). However, due to it not being applicable to patients with a non-transitional cell carcinoma histology, as well as the limited sample size and lack of a validation group, that model may lack generalizability in clinical practice. In addition, the effects of racial factors on predictive models in MIBC have not been studied.
The novelty of this work lies in the construction and validation of a nomogram that incorporates various clinical characteristics, ethnicities, and social factors for the prediction of OS, based on the Surveillance, Epidemiology, and End Results (SEER) database. In addition, we conducted external validation in a Chinese population to further confirm its clinical applicability.
2 Methods
2.1 Data source and study population
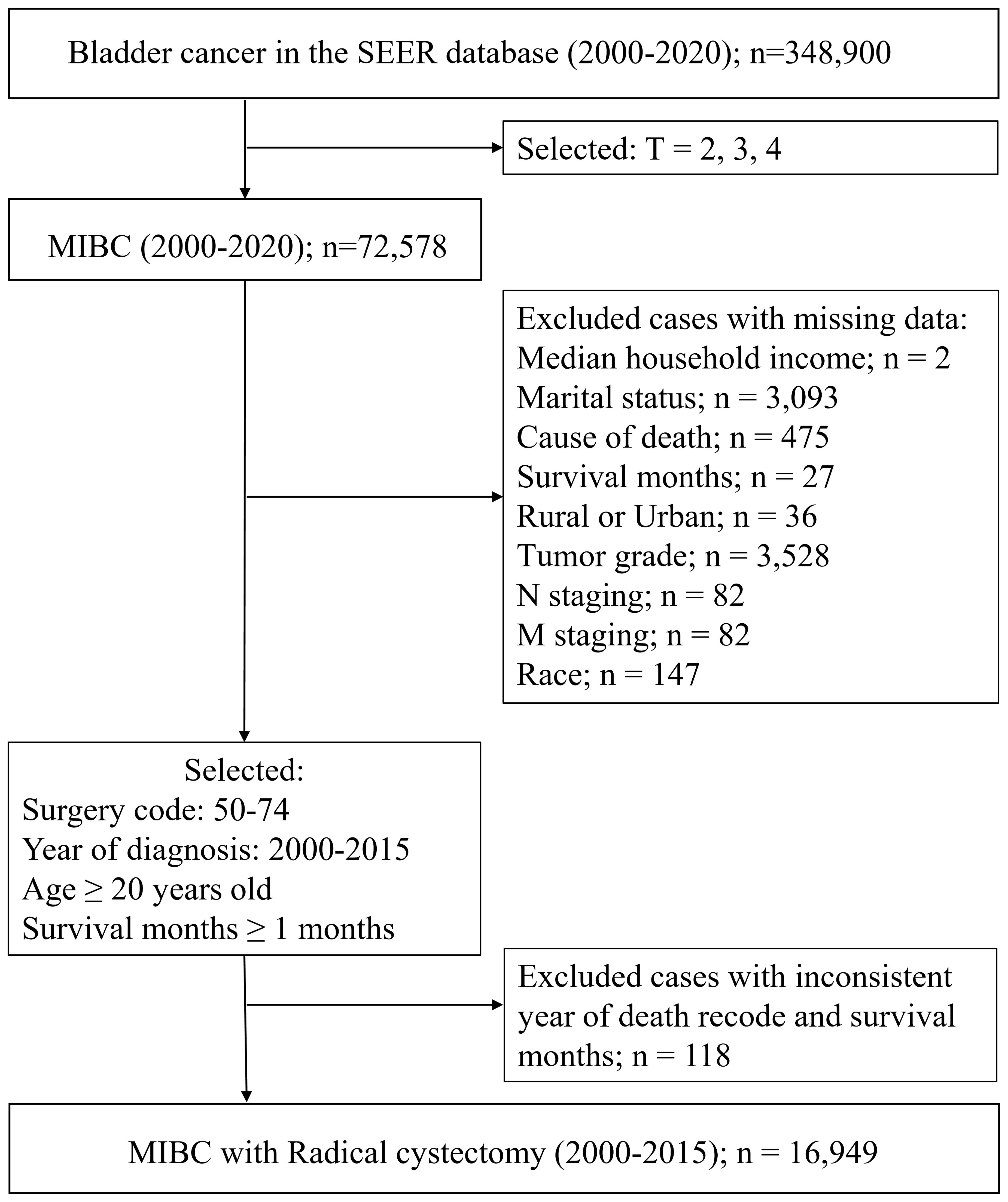
SEER Database. Data were obtained from 2000–2020 SEER research data covering 17 registries. SEER collects data on cancer diagnoses and survival for approximately 48% of the US population, and it benefits from extensive quality review (15). We selected patients aged 20 years and older who were diagnosed with MIBC and received treatment with RC between January 1, 2000, and December 31, 2015. Patients with missing survival data and those who died within 1 month after initial diagnosis were excluded. Complete data for patient demographics and tumor and treatment variables were retrieved with the use of SEER*Stat version 8.4.3 software. A flow chart for the study procedure is shown in Figure 1. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.
External validation. Only patients diagnosed with MIBC who underwent RC were included in the study. The same criteria were used to retrospectively collect information from Peking University First Hospital from January 1, 2016, to January 1, 2019, to form an external cohort. Survival status and research variables were determined by telephone or obtained from the most recent medical review by a urologist. Ethical approval was approved by Peking University First Hospital Ethics Committee.
2.2 Study variables
Variables were obtained as coded within SEER. Patient demographic variables were age (at diagnosis), sex, race, marital status, household income, and rural or urban residence. Tumor and treatment variables were tumor grade (I, well differentiated; II, moderately differentiated; III, poorly differentiated; and IV, undifferentiated), TNM staging, primary site, and histology. For treatment data, we chose surgery codes 50–74 for RC in the “RX Summ–Surg Prim Site (1998+),” while chemotherapy status was determined as the use of a “chemotherapy recode” in SEER. The endpoint of survival analysis was OS, determined from all causes of death.
2.3 Statistical analysis
After cases with insufficient clinicopathologic characteristics were excluded, the remaining eligible patients (n = 16949) were randomly divided into 1:1 training and testing sets. To determine whether there was multicollinearity among the variables, a collinearity diagnosis was performed, and the results showed that the tolerance values for the included variables were less than 1, and the variance impact factor values were all less than 10. A univariate Cox proportional-hazards regression was first performed to screen potential prognostic factors in the training set. Variables with p < 0.1 in univariate Cox regression were considered potential candidate factors for the creation of nomograms. Utilizing bootstrap methods, a series of 1,000 resampling processes undertaken via backward stepwise multivariate Cox regression analyses were conducted, as determined by the Akaike Information Criterion (AIC), to select the best combination of variables. The independent prognostic factors that significantly contributed to patient survival were selected to construct the nomogram, as well as the nomogram for 1-, 3-, and 5-year OS.
The accuracy and discrimination ability of the nomogram across three cohorts (training, testing, and external validation set) were assessed and compared in relation to the calibration curves, concordance index (C-index), and receiver operating characteristic (ROC) curves. To determine clinical practicability, a decision curve analysis (DCA) was performed. The clinicopathological characteristics for patient survival were analyzed by applying the Kaplan–Meier curve and comparing the results using the log-rank test. All analyses were performed using R software (version 4.3.3, R Foundation for Statistical Computing), with the survival package for univariate and multivariable Cox regression analyses, the rms package for the nomogram and calibration curve, the pROC package for the ROC curve, and the ggDCA package for DCA analysis. In each analysis, statistical significance was set at p < 0.05. All analyses were performed using R software (version 4.3.3, R Foundation for Statistical Computing).
3 Results
3.1 Patient characteristics
From 2000 to 2020, data from a total of 72,578 MIBC patients were drawn from the SEER database. The results indicated an increase in numbers of cases of MIBC per year, while the proportion of patients undergoing RC decreased (Supplementary Figure S1A). The survival rate of patients who had undergone RC was nearly twice that of those who do not. The 5-year survival rates for patients who underwent RC and for those who did not were 31% and 15%, respectively (Supplementary Figure S1B).
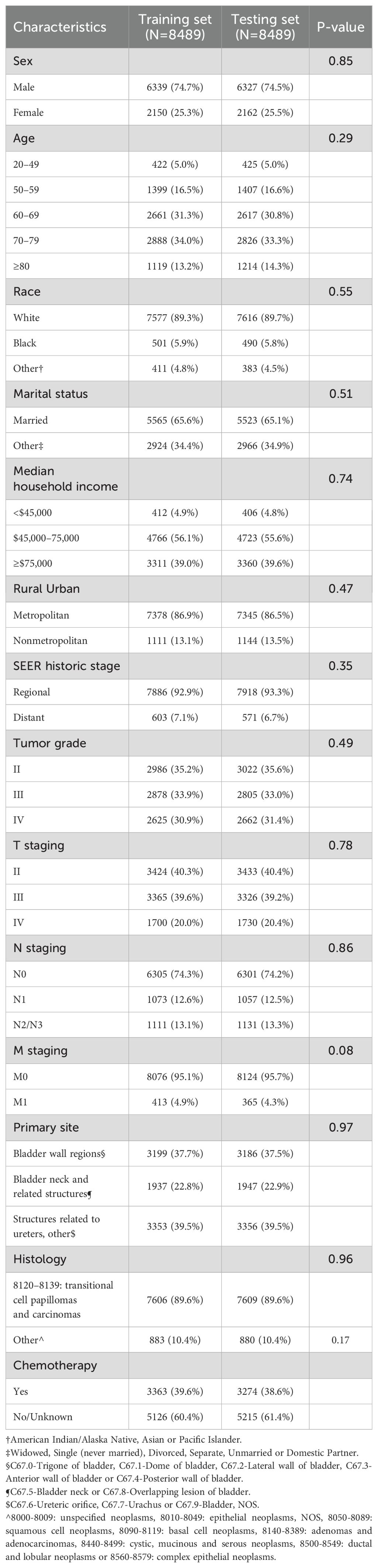
After removing cases that lacked adequate clinicopathological characteristics, the remaining eligible patients (n = 16,949) were randomly divided into training (8,489 patients) and testing (8,489 patients) sets. The baseline characteristics of each cohort are listed in Table 1. In addition, 175 patients from the Chinese cohort who met the eligibility criteria were included in this study (Supplementary Table S1).
Among the MIBC patients in the training set, the majority were male, accounting for about 74.7%. The age distribution ranged from 20 to over 80 years, with 34% of the patients between 70 and 79 years old. White people formed the vast majority of patients (89.3%), followed by Black people (5.9%) and other races, including American Indian, Alaska Native, and Asian or Pacific Islander (4.8%). In terms of marital status, most were married (65.6%). Tumor characteristics, including tumor grade, TNM staging, primary site, and histology showed an even distribution between the two groups without significant deviation. Regarding chemotherapy, about 39.6% of the patients chose to undergo treatment, while around 60.4% did not receive chemotherapy or had no record of receiving it.
3.2 Risk factors associated with OS
The detailed results of the univariate Cox regression analysis of the training set are presented in Table 2. Sex, age, race, marital status, tumor grade, TNM staging, primary site, histology, and chemotherapy were all significantly related to OS. In a subsequent multivariate Cox regression, all of these significant factors were first incorporated into a Cox regression model. To select independent prognostic factors that significantly contributed to patient survival and that could be included in the nomogram, we conducted 1,000 bootstrap resampling processes of the AIC for variable selection. Ultimately, the key factors for predicting OS were determined, including sex, age, race, marital status, tumor grade, TNM staging, histology, and chemotherapy (Table 2).
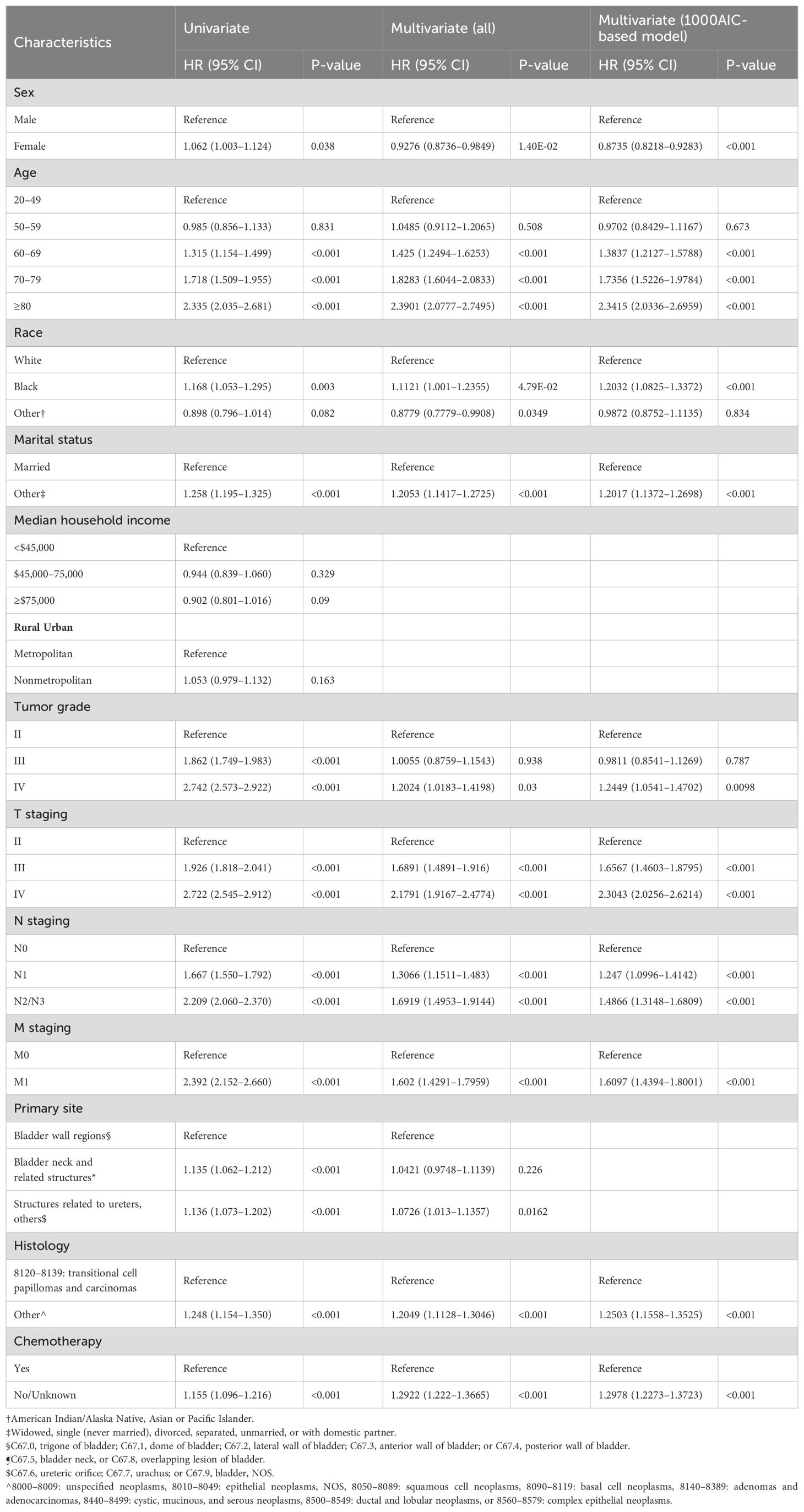
Table 2. Results of univariate and multivariate Cox analyses for OS of MIBC patients with RC in the training set.
The Kaplan-Meier survival curves for OS are displayed in Supplementary Figure S2. Female, age > 60 years, unmarried status, high tumor grade and TNM staging, diagnosis of non-transitional cell carcinoma, and absence of chemotherapy were associated with poor survival prognosis.
Interestingly, in a Cox regression analysis, we found an interaction between age and sex. Between the ages of 20 and 60 years, males demonstrated a higher survival probability than females, suggesting a protective effect of sex on survival in this period. However, this pattern reversed over time, and after 80 years old, the probability of survival for females exceeded that of males. Nonetheless, overall, being of the male sex remains a protective factor. This reveals the dynamic changes in the effect of sex on survival time with age (Supplementary Figure S3).
3.3 Nomogram construction and validation
We included the above factors as potential predictors for the 1-, 3-, and 5-year OS in the training set to present a nomogram graph as shown in Figure 2. The 1-, 3-, and 5-year calibration curves for the training and testing sets did not show large fluctuations, indicating that the predicted results were basically consistent with the actual results in the two cohorts, and the model had good accuracy (Supplementary Figure S4). The C-index of the nomogram used to estimate OS in the training set was 0.671 (95% confidence interval [CI] 0.663–0.679). The AUC values of the 1-, 3-, and 5-year OS were 0.941, 0.883, and 0.857 in the training set. For the 1-, 3-, and 5-year OS of the testing set, the nomogram demonstrated favorable discrimination, with AUC values of 0.939, 0.880, and 0.852, respectively. The C-index of the nomogram used to predict OS was 0.672 (95% CI 0.664–0.680), indicating that the prediction model had good discrimination (Figure 3).
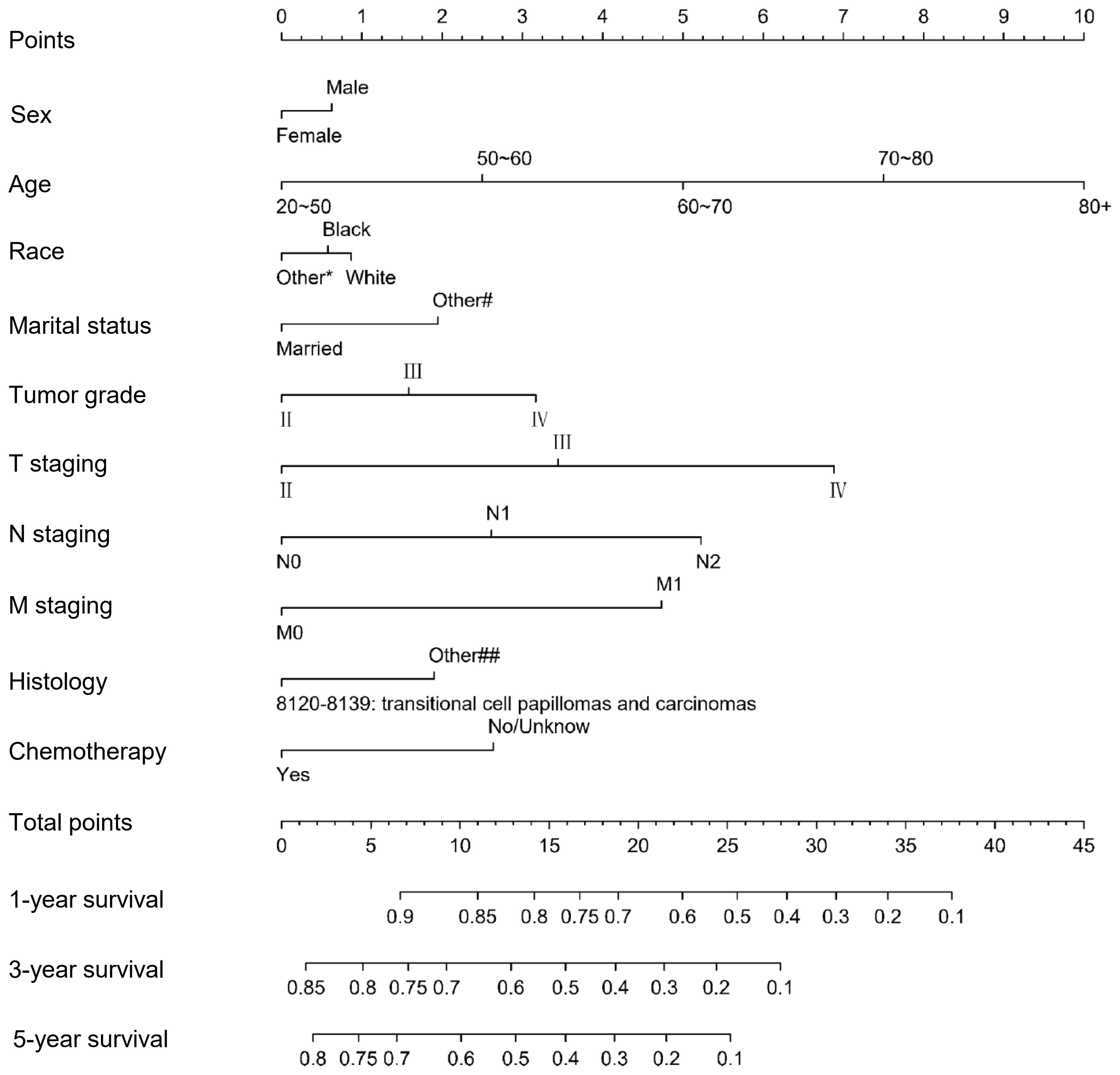
Figure 2. Nomogram for predicting 1-, 3-, and 5-year overall survival for MIBC patients with RC in the training set.
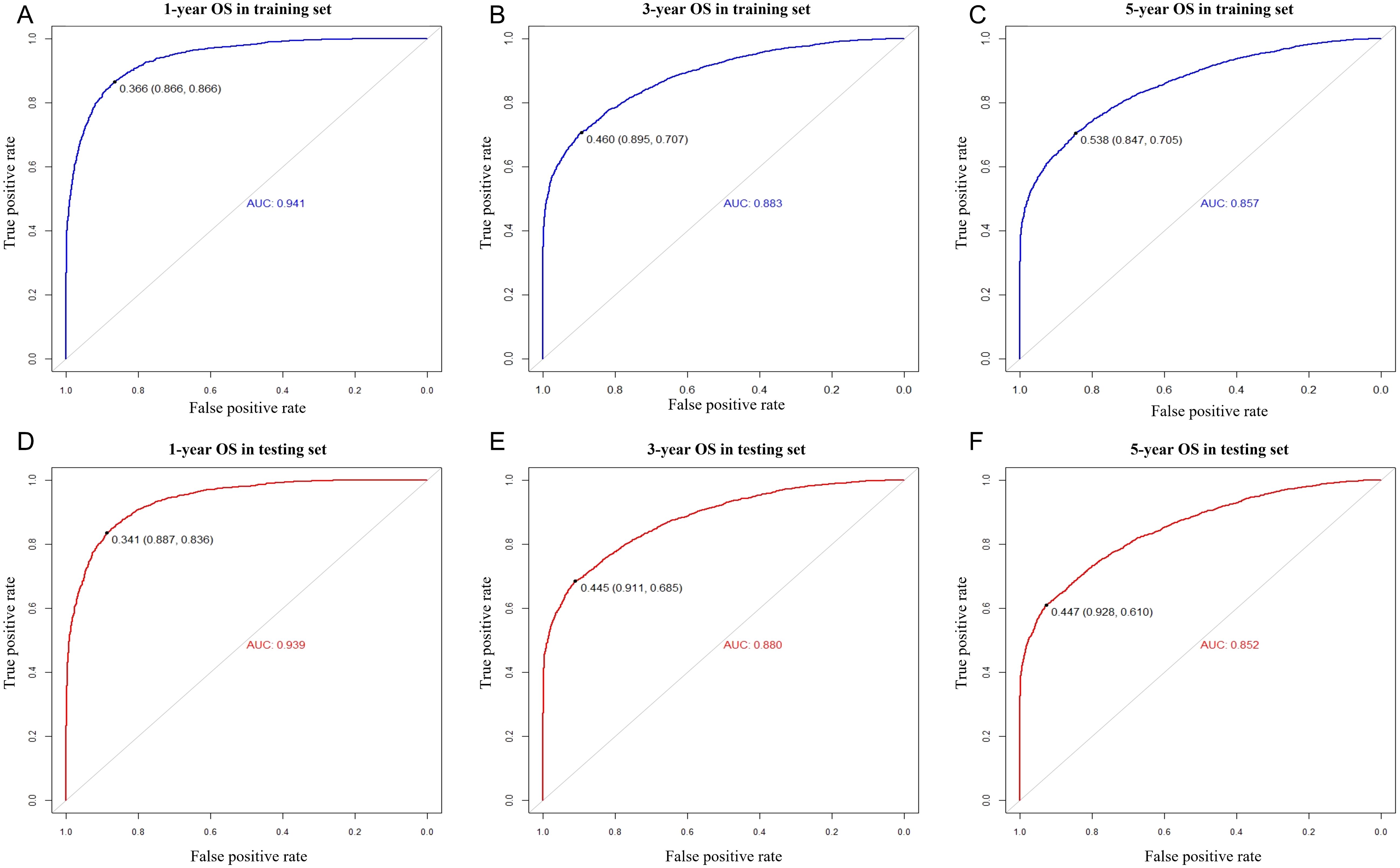
Figure 3. ROC curves for predicting 1-, 3-, and 5-year overall survival for MIBC patients with RC in the training set (A–C) and the testing set (D–F).
After resolving the accuracy of the model, the testing set was used to perform DCA to make the nomogram clinically practical. The nomogram showed had high clinical potential for the prediction of OS, with a wide and practical threshold probability range through 1-, 3-, and 5-year OS. Moreover, it outperformed the AJCC TNM staging system because more clinical net benefits were obtained within a wider threshold probability range using it (Supplementary Figure S5), meaning that our nomogram can aid clinical decision-making and predict outcomes for patients.
Finally, we tested the nomogram in a Chinese cohort (Peking University First Hospital, 2016–2019) and found that it maintained good discrimination and consistency in the Asian population. Specifically, the C-index reached 0.631 (95% CI 0.533–0.729), and the AUC values for 1-, 3-, and 5-year OS were 0.970, 0.847, and 0.790. These indicators suggest that the nomogram has relatively good predictive power, which makes it suitable for clinical practice in Asian populations (Figure 4).
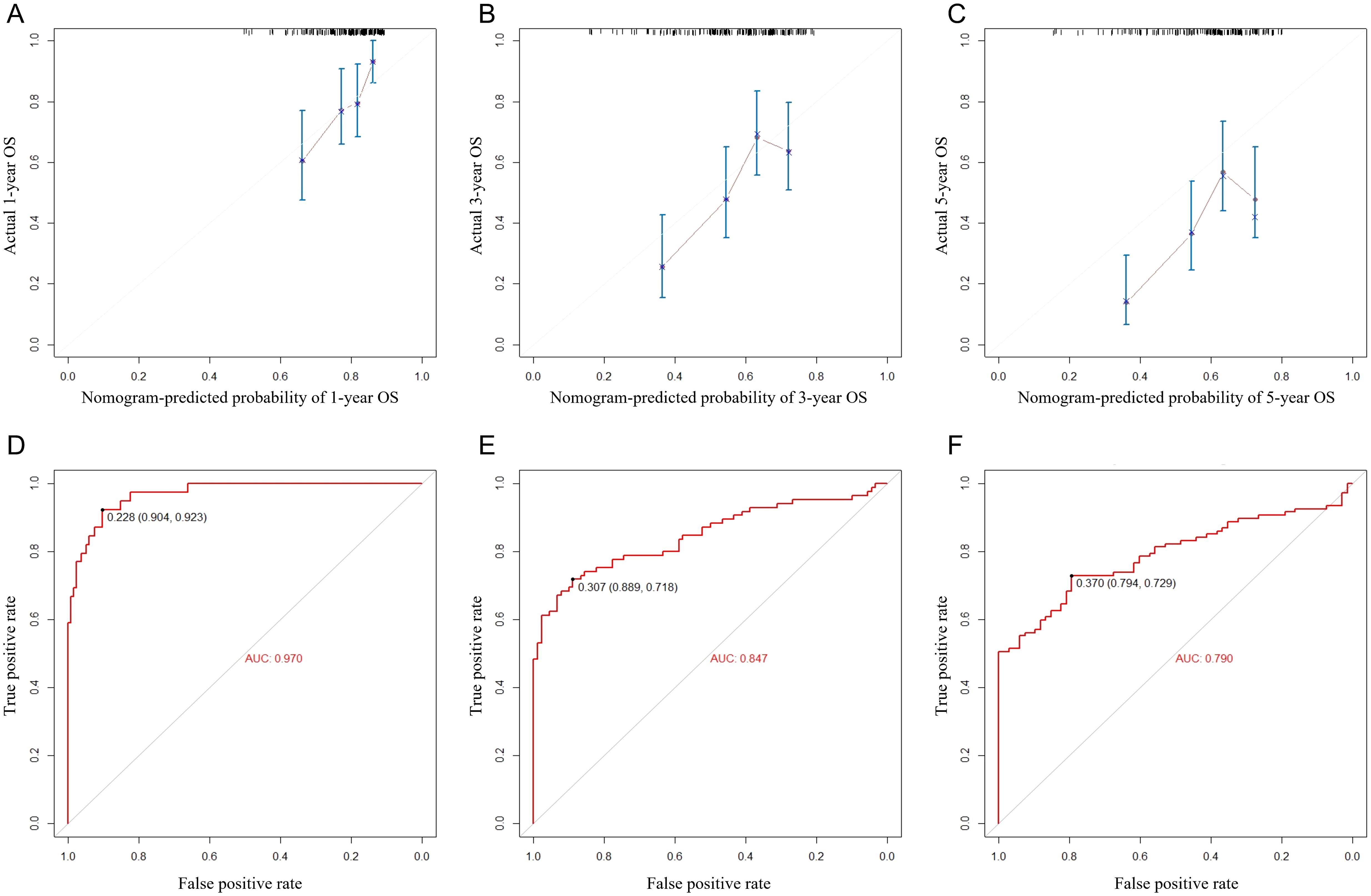
Figure 4. Calibration curves (A–C) and ROC curves (D–F) for predicting 1-, 3-, and 5-year overall survival for MIBC patients with RC in the external validation set.
4 Discussion
RC with pelvic lymphadenectomy is the most effective and most widely used definitive local treatment for invasive bladder cancer. In this study, we selected 72,578 MIBC patients from the SEER database; the results showed an increasing incidence of MIBC per year, with the highest incidence in patients aged 70–79 years. As found in previous studies, 50% of MIBC patients who underwent RC had improved survival rates (16, 17). However, postoperative morbidity remains high, partly because of postoperative complications (such as ureteric reflux and the deterioration of renal function, chronic/recurrent pyelonephritis, and metabolic disorders) or recurrent bladder cancer (18, 19). The reported OS rates after RC in elderly patients (≥70 year) range between 36% and 66% at 3 years and between 8% and 54% at 5 years (18). Therefore, it is imperative to investigate the prognostic factors that affect the survival of patients undergoing surgery to provide an estimate of the risk of disease progression in MIBC patients undergoing RC.
In recent years, several researchers have contributed to the development of postoperative predictive models for bladder cancer. A study based on data from more than 9,000 bladder cancer patients from 12 centers worldwide constructed a database that contained patient characteristics and pathological details. They developed an international bladder cancer nomogram that predicted recurrence risk for bladder cancer after RC. Although the accuracy of that study nomogram is significantly better than commonly used standards, it does not contain treatment information. It simply provides a way to assess the risk of disease recurrence in an individual patient after RC assuming no adjuvant therapy is received (20). Based on demographic, pathological, and treatment information on 640 patients, Maria et al. (21) developed a unique real-world tool for the prediction of bladder cancer death in patients with MIBC who had received NAC before RC. However, it remains unclear whether other treatment regimens and demographic characteristics, such as marital status, income, and race, can be used to predict postoperative survival.
In this study, 16,496 MIBC patients who received RC treatment were selected from the SEER database, who were randomized to the training and testing sets at a 1:1 ratio. Based on multivariate Cox regression analysis, sex, age, race, marital status, tumor grade, TNM staging, histology, and chemotherapy were identified as independent prognostic predictors of OS. On the basis of these factors, we developed a nomogram to predict the OS of MIBC patients undergoing RC for 1, 3, and 5 years and further evaluated its performance. The findings suggest that older age, being male, and of the Black people race are epidemiological features associated with poor MIBC prognosis. Bladder cancer was found to more commonly affect older individuals, with >78% of cases occurring in ages > 60 years. Several studies with large sample sizes have demonstrated a significant relationship between age at RC and perioperative complication rate (18, 22, 23). Other factors, such as a long history of smoking, underlying health problems, and late diagnosis and treatment limitations, may also contribute to lower OS in older patients. Males are more commonly affected by bladder cancer, with a male-to-female ratio of approximately 3:1 in the training and testing sets. Several hypotheses have been proposed for this, including differences in tobacco use and exposure to specific compounds in the workplace, hormonal factors, and the influence of sex chromosomes (24–26). Interestingly, in this study, we noted an interaction between age and sex, indicating that as age increases, the difference in 5-year OS rates between males and females become less significant. Further exploration and validation are required to corroborate these findings. In addition, we found that unmarried patients, including widowed patients, are at significantly greater risk for poor prognosis and mortality than married patients. This may be due to the fact that married patients are less likely to develop metastatic disease and are more likely to receive definitive therapy (27). In line with previous studies, we found that OS was better in people with low-grade, early-stage tumors than in those with high-grade, advanced-stage tumors (11, 20, 28). About 90% of cases of bladder cancer are transitional cell papillomas and carcinomas (TCCs) (3), and 10% are squamous cell neoplasms and other histology subtypes. In this study, we found that having TCC was an advantage for patients undergoing RC. Several studies have shown that SCC accounts for <3% of all newly diagnosed cases of bladder cancer in the United States, and is more aggressive and lethal than urothelial cancer (29, 30). In addition, our study confirms that chemotherapy for MIBC is associated with improved OS (21). Furthermore, the results of C-index and AUC analyses demonstrated strong concordance with our nomogram. Indeed, the DCA results validate its clinical utility. Therefore, the variables used to construct the prognostic model in this study are statistically reliable.
Our study also had several limitations. First, the external validation cohort consisted of single-center data, with certain characteristics, such as race and marital status, being relatively uniform. Therefore, it is essential to perform further validation through prospective multicenter clinical trials. Second, the accuracy of the model should be further improved; more risk factors related to OS could be included in the future, such as preoperative laboratory results and tumor markers other than PD1/PDL1, which may also be significant predictors and prognostic indicators for MIBC disease progression and treatment resistance. Finally, the SEER database also lacks information on specific treatment plans, such as adjuvant therapy and neoadjuvant therapy. Further analysis is needed to evaluate their impact on patient prognosis.
5 Conclusions
In summary, a nomogram was constructed and validated from diverse groups of MIBC patients. Compared to the conventional staging system, our nomogram offers increased accuracy, good clinical utility, and more precise prognosis for 1-, 3-, and 5-year OS. It provides an accurate risk assessment for postoperative MIBC patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Peking University First Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HMH: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. HH: Conceptualization, Data curation, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing. JD: Data curation, Formal Analysis, Validation, Visualization, Writing – review & editing. LP: Data curation, Formal Analysis, Writing – review & editing. QM: Data curation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing. HL: Conceptualization, Funding acquisition, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing. LJ: Formal Analysis, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the Scientific Research Seed Fund of PKUFH (2022SF47 to HHM). National High Level Hospital Clinical Research Funding (Research Achievement Transformation Project of PKUFH) (2024CX13 to HL). National High Level Hospital Clinical Research Funding (Interdepartmental Clinical Research Project of PKUFH) (2023IR44 to HH).
Acknowledgments
We thank all staff of the SEER database for their contribution in data collection, maintenance, distribution and so on. Also we would like to thank all the developers of the R programming package for selflessly sharing their code.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1597107/full#supplementary-material
Supplementary Figure 1 | Incidence of MIBC in the SEER database (A) and survival rates between MIBC with and without RC (B).
Supplementary Figure 2 | The clinicopathologic characteristics on patients’ overall survival of MIBC patients with RC in training set and the results suggested that sex(A), age(B), race(C), marital status(D), tumor grade(E), T staging(F), N staging(G), M staging(H), primary site(I), histology(G) and chemotherapy(K) was significance impact patients’ prognostic.
Supplementary Figure 3 | Differences in 5-year survival between males and females across different age groups aged 20–50 years (A), 50–60 years (B), 60–70 years (C), 70–80 years (D), 80+ years (E), and all ages (F).
Supplementary Figure 4 | Calibration curves for predicting 1-, 3-, and 5-year overall survival for MIBC patients with RC in the training set (A–C) and the testing set (D–F).
Supplementary Figure 5 | Decision curve analysis between the nomogram and the AJCC staging system for 1-, 3-, and 5-year overall survival of MIBC patients with RC in the testing set.
Abbreviations
RC, Radical cystectomy; MIBC, muscle-invasive bladder cancer; OS, overall survival; SEER database, the Surveillance, Epidemiology, and End Results database; PKUFH, Peking University First Hospital; NMIBC, non-muscle-invasive bladder cancer; CSS, cancer-specific survival; AJCC, the American Joint Committee on Cancer; AIC, Akaike Information Criterion; C-index, concordance index; ROC curves, receiver operating characteristic curves; DCA, decision curve analysis; TCC, transitional cell papillomas and carcinomas.
References
1. Ferlay J EM, Lam F, Laversanne M, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today (version 1). Lyon, France: International Agency for Research on Cancer (2024). Available at: https://gco.iarc.who.int/today (Accessed April 9, 2025).
3. Lopez-Beltran A, Cookson MS, Guercio BJ, and Cheng L. Advances in diagnosis and treatment of bladder cancer. BMJ (Clinical Res ed.). (2024) 384:e076743. doi: 10.1136/bmj-2023-076743
4. Xie B, Lin J, Chen X, Zhou X, Zhang Y, Fan M, et al. CircXRN2 suppresses tumor progression driven by histone lactylation through activating the Hippo pathway in human bladder cancer. Mol Cancer. (2023) 22:151. doi: 10.1186/s12943-023-01856-1
5. Compérat E, Amin MB, Cathomas R, Choudhury A, De Santis M, Kamat A, et al. Current best practice for bladder cancer: a narrative review of diagnostics and treatments. Lancet. (2022) 400:1712–21. doi: 10.1016/S0140-6736(22)01188-6
6. Alfred Witjes J, Max Bruins H, Carrión A, Cathomas R, Compérat E, Efstathiou JA, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2023 guidelines. Eur Urol. (2024) 85:17–31. doi: 10.1016/j.eururo.2023.08.016
7. Stenzl A, Cowan NC, De Santis M, Kuczyk MA, Merseburger AS, Ribal MJ, et al. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol. (2011) 59:1009–18. doi: 10.1016/j.eururo.2011.03.023
8. Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. (2001) 19:666–75. doi: 10.1200/JCO.2001.19.3.666
9. Madersbacher S, Hochreiter W, Burkhard F, Thalmann GN, Danuser H, Markwalder R, et al. Radical cystectomy for bladder cancer today–a homogeneous series without neoadjuvant therapy. J Clin Oncol. (2003) 21:690–6. doi: 10.1200/JCO.2003.05.101
10. Hautmann RE, Volkmer BG, Schumacher MC, Gschwend JE, and Studer UE. Long-term results of standard procedures in urology: the ileal neobladder. World J Urol. (2006) 24:305–14. doi: 10.1007/s00345-006-0105-z
11. Stein JP, Lieskovsky G, Cote R, Groshen S, Feng A-C, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. (2023) 41:3772–81. doi: 10.1200/JCO.22.02762
12. Ma J and Black PC. Current perioperative therapy for muscle invasive bladder cancer. Hematology/oncology Clinics North America. (2021) 35:495–511. doi: 10.1016/j.hoc.2021.02.002
13. Shariat SF, Karakiewicz PI, Palapattu GS, Amiel GE, Lotan Y, Rogers CG, et al. Nomograms provide improved accuracy for predicting survival after radical cystectomy. Clin Cancer Res. (2006) 12:6663–76. doi: 10.1158/1078-0432.CCR-06-0372
14. Zaak D, Burger M, Otto W, Bastian PJ, Denzinger S, Stief CG, et al. Predicting individual outcomes after radical cystectomy: an external validation of current nomograms. BJU Int. (2010) 106:342–8. doi: 10.1111/j.1464-410X.2009.09138.x
15. Adamo MP, Boten JA, Coyle LM, Cronin KA, Lam CJK, Negoita S, et al. Validation of prostate-specific antigen laboratory values recorded in Surveillance, Epidemiology, and End Results registries. Cancer. (2017) 123:697–703. doi: 10.1002/cncr.30401
16. Cookson MS, Chang SS, Wells N, Parekh DJ, and Smith JA. Complications of radical cystectomy for nonmuscle invasive disease: comparison with muscle invasive disease. J Urol. (2003) 169:101–4. doi: 10.1016/S0022-5347(05)64045-1
17. Meyer J-P, Blick C, Arumainayagam N, Hurley K, Gillatt D, Persad R, et al. A three-centre experience of orthotopic neobladder reconstruction after radical cystectomy: revisiting the initial experience, and results in 104 patients. BJU Int. (2009) 103:680–3. doi: 10.1111/j.1464-410X.2008.08204.x
18. Froehner M, Brausi MA, Herr HW, Muto G, and Studer UE. Complications following radical cystectomy for bladder cancer in the elderly. Eur Urol. (2009) 56:443–54. doi: 10.1016/j.eururo.2009.05.008
19. Lawrentschuk N, Colombo R, Hakenberg OW, Lerner SP, Månsson W, Sagalowsky A, et al. Prevention and management of complications following radical cystectomy for bladder cancer. Eur Urol. (2010) 57(6):983–1001. doi: 10.1016/j.eururo.2010.02.024
20. Bochner BH, Kattan MW, and Vora KC. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol. (2006) 24:3967–72. doi: 10.1200/JCO.2005.05.3884
21. Mir MC, Marchioni M, Zargar H, Zargar-Shoshtari K, Fairey AS, Mertens LS, et al. Nomogram predicting bladder cancer-specific mortality after neoadjuvant chemotherapy and radical cystectomy for muscle-invasive bladder cancer: results of an international consortium. Eur Urol Focus. (2021) 7:1347–54. doi: 10.1016/j.euf.2020.07.002
22. lting LS, Pettaway C, Bekele BN, Grossman HB, Cooksley C, Avritscher EBC, et al. Correlation between annual volume of cystectomy, professional staffing, and outcomes: a statewide, population-based study. Cancer. (2005) 104:975–84. doi: 10.1002/cncr.v104:5
23. Konety BR, Dhawan V, Allareddy V, and Joslyn SA. Impact of hospital and surgeon volume on in-hospital mortality from radical cystectomy: data from the health care utilization project. J Urol. (2005) 173:1695–700. doi: 10.1097/01.ju.0000154638.61621.03
24. Theodorescu D, Li Z, and Li X. Sex differences in bladder cancer: emerging data and call to action. Nat Rev Urol. (2022) 19:447–9. doi: 10.1038/s41585-022-00591-4
25. Teoh JY-C, Huang J, Ko WY-K, Lok V, Choi P, Ng C-F, et al. Global trends of bladder cancer incidence and mortality, and their associations with tobacco use and gross domestic product per capita. Eur Urol. (2020) 78:893–906. doi: 10.1016/j.eururo.2020.09.006
26. Nickerson ML, Dancik GM, Im KM, Edwards MG, Turan S, Brown J, et al. Concurrent alterations in TERT, KDM6A, and the BRCA pathway in bladder cancer. Clin Cancer Res. (2014) 20:4935–48. doi: 10.1158/1078-0432.CCR-14-0330
27. Aizer AA, Chen M-H, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. J Clin Oncol. (2013) 31:3869–76. doi: 10.1200/JCO.2013.49.6489
28. Li Y, Chen T, Fu B, Luo Y, and Chen L. Survival nomogram for high-grade bladder cancer patients after surgery based on the SEER database and external validation cohort. Front In Oncol. (2023) 13:1164401. doi: 10.3389/fonc.2023.1164401
29. Scosyrev E, Yao J, and Messing E. Urothelial carcinoma versus squamous cell carcinoma of bladder: is survival different with stage adjustment? Urology. (2009) 73:822–7. doi: 10.1016/j.urology.2008.11.042
Keywords: muscle-invasive bladder cancer, radical cystectomy, nomogram, risk factors, overall survival
Citation: Huang H, Hao H, Du J, Pang L, Ma Q, Li H and Jin L (2025) A nomogram for predicting overall survival in patients with muscle-invasive bladder cancer undergoing radical cystectomy: a retrospective cohort study. Front. Oncol. 15:1597107. doi: 10.3389/fonc.2025.1597107
Received: 20 March 2025; Accepted: 29 May 2025;
Published: 19 June 2025.
Edited by:
Arun Azad, Peter MacCallum Cancer Centre, AustraliaCopyright © 2025 Huang, Hao, Du, Pang, Ma, Li and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Jin, amlubGVpQGJqbXUuZWR1LmNu; Haixia Li, YmR5eWxoeEAxMjYuY29t; Qian Ma, bWFxaWFuNTdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Haiming Huang
Haiming Huang Han Hao3†
Han Hao3† Qian Ma
Qian Ma Haixia Li
Haixia Li Lei Jin
Lei Jin