Abstract
Background:
Alpha-fetoprotein (AFP) is an established biomarker for liver cancer, but its role in gastric cancer (GC) remains unclear. This study evaluated AFP’s prognostic value in GC and developed a survival prediction model incorporating AFP and other clinical factors.
Methods:
We analyzed 766 GC patients from Changzhou Traditional Chinese Medicine Hospital, categorizing them as AFP-positive (>20 ng/mL) or AFP-negative (≤20 ng/mL). Kaplan-Meier and Cox regression analyses assessed the association between AFP levels and overall survival (OS). A nomogram based on identified prognostic factors was created and evaluated using ROC curves, calibration curves, and decision curve analysis (DCA).
Results:
Among 766 gastric cancer (GC) patients, 3.3% (n=25) exhibited elevated AFP levels (>20 ng/mL). The AFP-positive group demonstrated significantly more aggressive clinicopathological features, including larger tumor size (p < 0.05), deeper invasion (higher T-stage), increased lymph node metastasis (higher N-stage), and higher rates of distant metastasis (p = 0.035). Survival analysis revealed markedly worse outcomes for AFP-positive patients (Log-rank P < 0.001), with a 68% higher mortality risk (unadjusted HR =1.68, 95% CI: 1.27–2.23). Multivariate Cox regression confirmed AFP positivity as an independent prognostic factor (adjusted HR = 1.8, 95% CI: 1.03–3.14, p =0.04), alongside T4-stage, N3-stage, and distant metastasis. A prognostic nomogram integrating AFP levels and TNM staging achieved superior predictive accuracy (AUCs: 0.80–0.84) compared to TNM staging alone (AUCs: 0.70–0.74) across 1-, 3-, and 5-year survival. Calibration and decision curve analyses further validated the model’s clinical utility, supporting its role in risk stratification and treatment planning.
Conclusions:
AFP is a significant independent prognostic factor in gastric cancer, and its inclusion in a multivariate model enhances survival prediction. The prognostic nomogram developed in this study offers clinicians a valuable tool for predicting patient outcomes and guiding treatment decisions. Further validation and prospective studies are necessary to confirm the model’s clinical applicability.
Introduction
Gastric cancer constitutes a major global health challenge, particularly within Asia, where its incidence and mortality rates are considerably elevated (1). As reported by the World Health Organization (WHO), gastric cancer ranks as the fifth most common cancer worldwide, maintaining a consistently high mortality rate (2). Despite notable advancements in surgical techniques, radiotherapy, chemotherapy, and targeted therapies, the early manifestations of gastric cancer often remain insidious (3). This frequently results in diagnoses at advanced stages, at which point treatment efficacy is significantly diminished. The elevated mortality rate and associated treatment complexities render gastric cancer a pressing public health concern, necessitating urgent action. Therefore, the implementation of early screening procedures, timely diagnoses, and effective prognostic evaluations is essential for enhancing patient survival rates (4).
Alpha-fetoprotein (AFP) is identified as a glycoprotein predominantly secreted by the fetal liver and gastrointestinal tract, with concentrations typically remaining low in adults (5). Elevated AFP levels are frequently associated with hepatocellular carcinoma, specific germ cell tumors, and gastric hepatoid adenocarcinoma (GHA), among other malignancies (6). In recent years, AFP’s potential diagnostic and prognostic utility in gastric cancer has garnered increasing attention, particularly within subtypes such as gastric hepatoid adenocarcinoma (GHA), where elevated levels are significantly correlated with tumor development, progression, and prognosis. Elevated AFP levels may not only correlate with tumor occurrence but could also reflect underlying tumor behavior, encompassing factors such as invasiveness, metastatic potential, and treatment response. Consequently, AFP is regarded as a significant biomarker in gastric cancer research (7).
While numerous studies underscore the potential of AFP in the context of gastric cancer, existing literature predominantly focuses on its correlation with specific clinical and pathological features. Research indicates that elevated AFP is closely associated with advanced tumor stage, increased tumor size, lymph node involvement, and distant metastasis. Patients with AFP-positive status frequently present with larger tumors, higher rates of lymph node metastasis, and more frequent liver metastasis, all of which are commonly linked to a poorer prognosis. However, systematic studies specifically addressing AFP-positive gastric cancer patients are sparse, with many characterized by limitations such as small sample sizes and patient heterogeneity (8). As a result, despite preliminary research efforts, the absence of comprehensive prognostic models constrains the effective clinical application of AFP as a biomarker in gastric cancer.
This study seeks to retrospectively analyze the clinical and pathological characteristics of AFP-positive gastric cancer patients and investigate the relationship between AFP levels and clinical outcomes. By integrating AFP with other critical clinical and pathological factors, this research aims to develop a prognostic model using multifactorial analysis, with the objective of providing a more precise reference for prognostic evaluation and individualized treatment in clinical practice.
Materials and methods
Study population
A retrospective analysis was conducted on 1,100 gastric cancer patients treated at Changzhou Traditional Chinese Medicine Hospital, an affiliate of Nanjing University of Chinese Medicine. Of these, 766 patients meeting the inclusion criteria were selected for comprehensive analysis. D2 radical gastrectomy was performed on all patients, except for 18 individuals with gastric cancer liver metastasis who underwent simultaneous hepatogastric lesion resection. The inclusion criteria were as follows (1): a confirmed diagnosis of gastric adenocarcinoma (2), comprehensive clinical and pathological data, and (3) available preoperative AFP measurement results. Patients with underlying liver diseases (e.g., cirrhosis, hepatic fibrosis, or hepatocellular carcinoma) or other conditions known to elevate AFP levels were excluded, as these conditions could bias the AFP’s role as a gastric cancer biomarker. The protocol of this study was approved by the institutional ethical board of Changzhou Traditional Chinese Medicine Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
AFP measurement and grouping
Preoperative serum AFP levels were determined using a chemiluminescent immunoassay, with a normal reference range of 0-20 ng/mL. Patients were classified into two groups based on serum AFP level (8) (1): AFP-positive (serum AFP >20 ng/mL) and (2) AFP-negative (serum AFP ≤ 20 ng/mL). This threshold was selected in accordance with established clinical practices for determining AFP positivity in gastric cancer.
Data collection and follow-up
Clinical and pathological data were retrospectively obtained from medical records. The collected data encompassed demographic information (age, sex), tumor characteristics (size), and laboratory results (AFP, CEA, and CA19-9 levels). Pathological staging information, including T-stage (tumor depth), N-stage (lymph node involvement), and M-stage (distant metastasis), was cataloged. Follow-up procedures included outpatient visits, telephone calls, emails, and letters. The final follow-up was conducted in December 2019. Survival time was defined as the interval from surgery to either the last follow-up date or the date of death, whichever occurred first. Patients who were lost to follow-up were not included in the survival analysis.
Statistical analysis
Descriptive statistics were employed to summarize the baseline characteristics of the study population. Continuous variables were expressed as means with standard deviations (SD), whereas categorical variables were presented as frequencies and percentages. Differences between the AFP-positive and AFP-negative groups were analyzed using statistical tests, such as Student’s t-test for continuous variables and chi-squared tests for categorical variables. Kaplan-Meier survival analysis was employed to evaluate overall survival (OS) between the two groups. Survival curves were generated, and differences were assessed using the log-rank test. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated to quantify the mortality risk associated with AFP positivity. Univariate and multivariate Cox proportional hazards regression analyses were utilized to identify significant prognostic factors. Key variables consisted of AFP levels, T-stage, N-stage, tumor size, and other relevant clinical parameters. Predictors identified as significant in univariate analysis were subsequently included in a multivariate Cox regression model to determine independent prognostic factors.
Prognostic model construction
A nomogram was constructed based on findings from the multivariate Cox regression analysis. It incorporated significant independent prognostic factors, including AFP levels, T-stage, N-stage, and tumor size, to project individual patient survival probabilities at various time points. To ensure the robustness and generalizability of the model, the study population was randomly divided into a modeling cohort and a validation cohort in a ratio of 7:3. The modeling cohort was utilized for constructing the nomogram, while the validation cohort was used to evaluate its performance. This nomogram functions as an efficient tool for clinicians to estimate patient outcomes and facilitate clinical decision-making.
Model evaluation
The nomogram’s performance was assessed using receiver operating characteristic (ROC) curves, with the area under the curve (AUC) providing a measure of its discriminative ability. Calibration curves were utilized to compare predicted survival probabilities with observed outcomes, thus evaluating the nomogram’s predictive accuracy. Decision curve analysis (DCA) provided insights into the clinical utility of the nomogram by comparing the potential benefits of its application versus treating all or no patients.
Statistical analyses were conducted using R software (version 4.2). A p-value of <0.05 was considered statistically significant for all analyses.
Results
Patient characteristics
The study cohort comprised 766 gastric cancer patients, among whom 25 (3.3%) were identified as AFP-positive (serum AFP > 20 ng/mL). The mean patient age was 62.9 ± 11.6 years, predominantly male (60.8%). In Table 1, baseline characteristics of both AFP-positive and AFP-negative groups are detailed. Significantly higher occurrences of advanced tumor stages, including larger tumor size, greater invasion (higher T-stage), and more frequent lymph node involvement (higher N-stage), were observed in the AFP-positive group compared to the AFP-negative group (all p < 0.05). Additionally, liver and distant metastases were more common in the AFP-positive group (p = 0.263 and p = 0.035, respectively). To further validate the consistency of AFP’s prognostic value, we performed stratified analyses by sex (male/female), age (≤65/>65 years), TNM stage (I-II/III-IV), and liver metastasis status (yes/no). While some subgroups did not reach statistical significance due to limited sample size (all interaction P-values >0.05), The forest plot (Supplementary Figure S1) demonstrated good homogeneity across subgroups, supporting the stability of AFP’s prognostic value regardless of patient characteristics.
Table 1
| Variables | Total (n = 766) | AFP Negative (n = 741) | AFP Positive (n = 25) | P |
|---|---|---|---|---|
| Age (years) | 62.9 ± 11.6 | 62.8 ± 11.7 | 65.5 ± 11.0 | 0.268 |
| Tumor Size (cm) | 4.80 ± 2.35 | 4.75 ± 2.30 | 5.90 ± 2.70 | 0.032* |
| CA19-9 (U/mL) | 83.84 ± 418.39 | 79.86 ± 411.95 | 201.83 ± 576.39 | 0.152 |
| CEA (ng/mL) | 5.86 ± 16.39 | 5.51 ± 15.52 | 16.10 ± 32.07 | 0.113 |
| Number of lymph node | 21.66 ± 9.80 | 21.75 ± 9.84 | 19.00 ± 8.21 | 0.168 |
| Lymph node (mm) | 5.61 ± 7.16 | 5.56 ± 7.20 | 7.08 ± 5.80 | 0.297 |
| Survival Time (days) | 1395.74 ± 925.38 | 1414.51 ± 923.66 | 839.24 ± 810.06 | 0.002* |
| Sex, n (%) | ||||
| Female | 300 (39.2) | 290 (39.1) | 10 (40.0) | 0.918 |
| Male | 466 (60.8) | 451 (60.9) | 15 (60.0) | |
| T, n (%) | 0.024* | |||
| 1 | 127 (16.57) | 127 (17.14) | 0 (0.00) | |
| 2 | 92 (12.01) | 91 (12.28) | 1 (4.00) | |
| 3 | 197 (25.71) | 188 (25.37) | 9 (36.00) | |
| 4 | 350 (45.69) | 335 (45.21) | 15 (60.00) | |
| N, n (%) | 0.047* | |||
| 0 | 249 (32.51) | 246 (33.20) | 3 (12.00) | |
| 1 | 138 (18.02) | 132 (17.81) | 6 (24.00) | |
| 2 | 135 (17.62) | 132 (17.81) | 3 (12.00) | |
| 3 | 244 (31.85) | 231 (31.17) | 13 (52.00) | |
| M, n (%) | 0.035* | |||
| 0 | 743 (97.00) | 721 (97.30) | 22 (88.00) | |
| 1 | 23 (3.00) | 20 (2.70) | 3 (12.00) | |
| Histology, n (%) | ||||
| AD | 500 (65.3) | 486 (65.6) | 14 (56.0) | 0.263 |
| MAD | 110 (14.4) | 106 (14.3) | 4 (16.0) | |
| SRCC | 80 (10.4) | 77 (10.4) | 3 (12.0) | |
| Other | 76 (9.9) | 72 (9.7) | 4 (16.0) | |
| Liver Metastases, n (%) | ||||
| Absent | 748 (97.65) | 726 (97.98) | 22 (88.00) | 0.263 |
| Present | 18 (2.35) | 15 (2.02) | 3 (12.00) | |
Analysis of the sociodemographic characteristics of the research object.
t, t-test; χ², Chi-square test; -, Fisher exact; *P<0.05.
SD, standard deviation.
Survival analysis based on AFP levels
For the purposes of survival analysis, patients were segregated into AFP-positive and AFP-negative groups. Kaplan-Meier survival curves demonstrated a significant difference in OS between the two groups. Those in the AFP-positive group had significantly poorer outcomes (Log-rank P < 0.001). The hazard ratio (HR) for the AFP-positive cohort, compared to the AFP-negative cohort, was calculated to be 1.68 (95% confidence interval (CI: 1.27–2.23), suggesting that elevated AFP levels correlate with a 68% increased mortality risk (Figure 1).
Figure 1
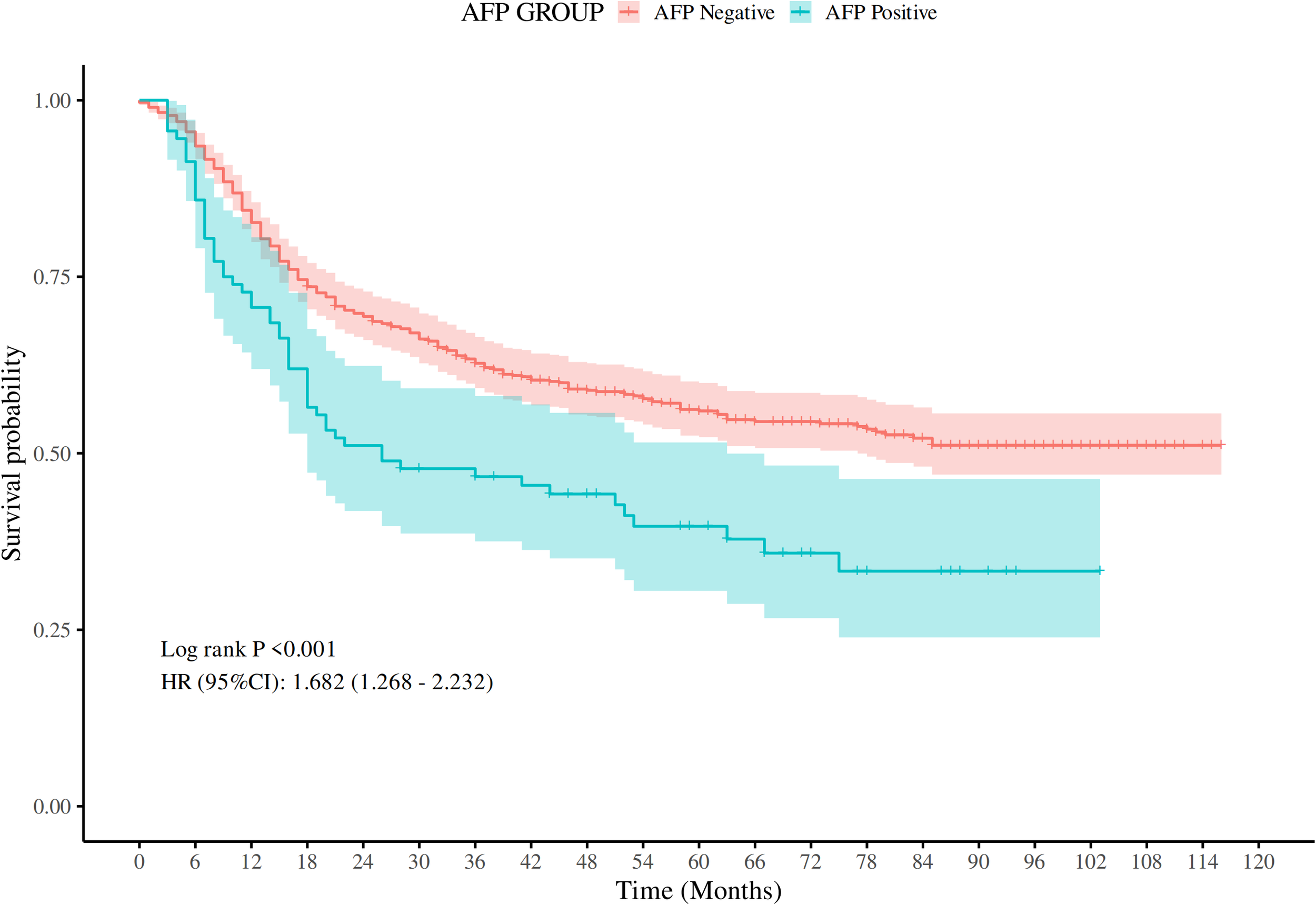
Comparison of OS rates between AFP-positive GC and AFP-negative GC (P < 0.001).
Univariate and multivariate Cox regression analysis
Univariate Cox regression analysis indicated that several clinical and pathological factors held significant associations with OS, such as AFP levels, T-stage, N-stage, tumor size, and distant metastasis occurrence. Notably, elevated AFP levels within the AFP-positive group correlated with a significantly poorer prognosis (HR = 2.36, 95% CI: 1.37–4.05, p < 0.001). Multivariate Cox regression analysis, accounting for potential confounders, confirmed AFP positivity as an independent prognostic factor (adjusted HR = 1.8, 95% CI: 1.03–3.14, p =0.04), alongside T4-stage, N3-stage, and distant metastasis (Table 2). Prior to modeling, multicollinearity was assessed through generalized variance inflation factors (GVIF), with all adjusted GVIF^(1/(2*Df)) values below 1.68 (T-stage:1.06; N-stage:1.22; metastasis:1.02), confirming variable independence (Supplementary Table S1). This unadjusted association was further validated in multivariable Cox analysis, where AFP positivity remained significant after adjusting for T/N/M-stage(HR = 1.8, 95% CI: 1.03–3.14, p = 0.04), along with T4-stage (HR = 3.31, 95% CI: 1.79–6.12, p < 0.01), N3-stage (HR = 2.35, 95% CI: 1.49–3.71, p < 0.01), and distant metastasis (HR = 1.85, 95% CI: 1.08–3.16, p =0.03).
Table 2
| Factor | Category | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | ||
| Age (years) | 1.02 | 1.01–1.03 | 0.01* | 1.01 | 0.99–1.02 | 0.08 | |
| Sex | Female | 1.00 | – | ||||
| Male | 1.04 | 0.99–1.09 | 0.16 | – | |||
| AFP Custom (ng/mL) | AFP- | 1.00 | 1.00 | ||||
| AFP+ | 2.36 | 1.37–4.05 | <0.001* | 1.80 | 1.03–3.14 | 0.04* | |
| T Stage | T1 | 1.00 | 1.00 | ||||
| T2 | 1.32 | 0.56–3.08 | 0.53 | 1.01 | 0.43–2.38 | 0.98 | |
| T3 | 3.82 | 2.11–6.91 | <0.01* | 2.55 | 1.38–4.74 | <0.001* | |
| T4 | 6.91 | 3.92–12.19 | <0.01* | 3.31 | 1.79–6.12 | <0.01* | |
| N Stage | N0 | 1.00 | 1.00 | ||||
| N1 | 1.58 | 0.94–2.64 | 0.08 | 1.21 | 0.71–2.05 | 0.48 | |
| N2 | 3.17 | 2.12–4.73 | <0.01* | 1.91 | 1.24–2.93 | <0.001* | |
| N3 | 5.35 | 3.72–7.69 | <0.01* | 2.35 | 1.49–3.71 | <0.01* | |
| M Stage | M0 | 1.00 | – | ||||
| M1 | 3.50 | 2.10–5.85 | <0.01* | 1.85 | 1.08–3.16 | 0.03* | |
| Histology | AD | 1.00 | – | ||||
| MAD | 0.95 | 0.85–1.05 | 0.37 | – | |||
| SRCC | 0.95 | 0.71–1.26 | 0.72 | – | |||
| Other | 0.88 | 0.73–1.03 | 0.13 | – | |||
| Liver Metastases | Absent | 1.00 | – | ||||
| Present | 2.45 | 1.42–4.22 | <0.001* | – | |||
| Tumor Size (cm) | 1.15 | 1.08–1.22 | <0.01* | 1.07 | 0.99–1.15 | 0.08 | |
| CA19-9 (U/mL) | 1.01 | 1.01–1.01 | <0.01* | – | |||
| CEA (ng/mL) | 1.01 | 1.01–1.01 | 0.04* | – | |||
| Number of lymph node | 0.99 | 0.98–1.01 | 0.29 | – | |||
| Lymph node (mm) | 1.05 | 1.04–1.06 | <0.01* | 1.02 | 1.01–1.04 | 0.01* | |
COX regression analysis of risk factors for DM in training cohort.
Multivariable HR adjusted for T/N/M-stage, tumor size. *P<0.05.
Prognostic model construction and evaluation
Given the significant prognostic factors identified through multivariate analysis, a nomogram was crafted to predict 1-year, 3-year, and 5-year OS probabilities for AFP-positive gastric cancer patients (Figure 2). Incorporating AFP level, T-stage, N-stage, and distant metastasis, the nomogram demonstrated superior discriminatory power compared to the conventional TNM staging system. Specifically, the area under the curve (AUC) for 1-year survival in the training cohort was 0.80 (95% CI: 0.73–0.87) (Figure 3A)versus 0.71 (0.63–0.78) (Figure 3B) for TNM staging, and 0.84 (0.74–0.94) (Figure 3A) versus 0.74 (0.63–0.86) (Figure 3B) in the validation cohort. Similarly, for 3-year survival, the nomogram achieved AUCs of 0.80 (0.75–0.86) and 0.84 (0.76–0.93) in the training and validation cohorts(Figure 3C), respectively, outperforming TNM staging (training: 0.72 [0.67–0.77]; validation: 0.71 [0.63–0.79]) (Figure 3D). At 5 years, the nomogram maintained robust performance with AUCs of 0.78 (0.73–0.83) and 0.83 (0.75–0.90) (Figure 3E), compared to 0.70 (0.64–0.76) and 0.74 (0.65–0.82) for TNM staging (Figure 3F). Calibration curves showed excellent agreement between predicted and observed survival probabilities (Figures 4A-C), while DCA further highlighted the nomogram’s clinical utility. Across threshold probabilities, the nomogram provided significantly higher net benefits than both TNM staging and the “treat-all” or “treat-none” strategies, underscoring its value in guiding personalized treatment decisions (Figure 5).
Figure 2
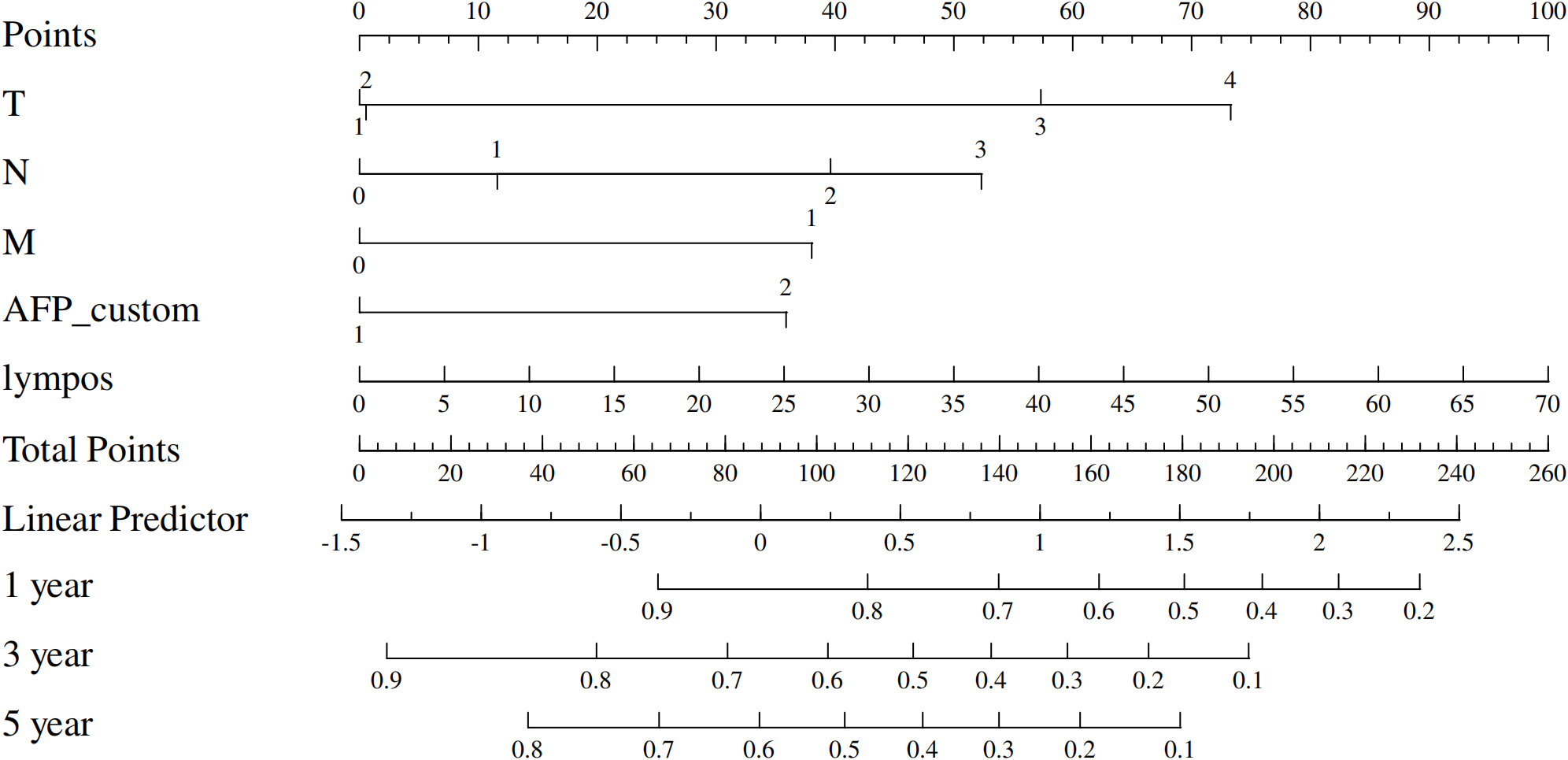
Prognostic nomogram for predicting 1-, 3-, and 5-year overall survival in gastric cancer patients.
Figure 3
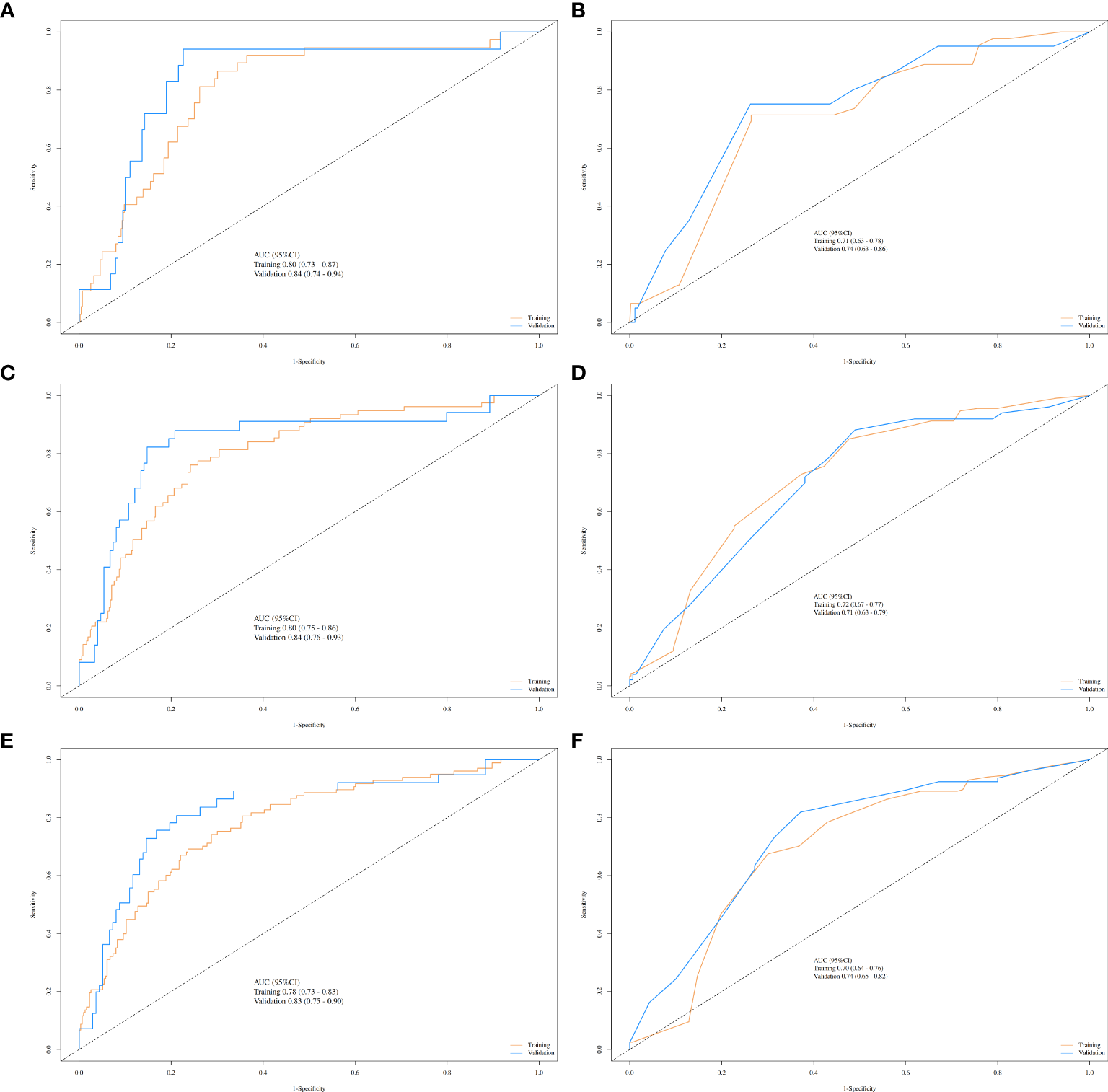
ROC curves comparing the nomogram and TNM staging system for predicting survival in gastric cancer. Nomogram performance (yellow lines: training cohort; blue lines: validation cohort) for 1-year (A), 3-year (C), and 5-year (E) survival. TNM staging performance (yellow lines: training cohort; blue lines: validation cohort) for 1-year (B), 3-year (D), and 5-year (F) survival.
Figure 4
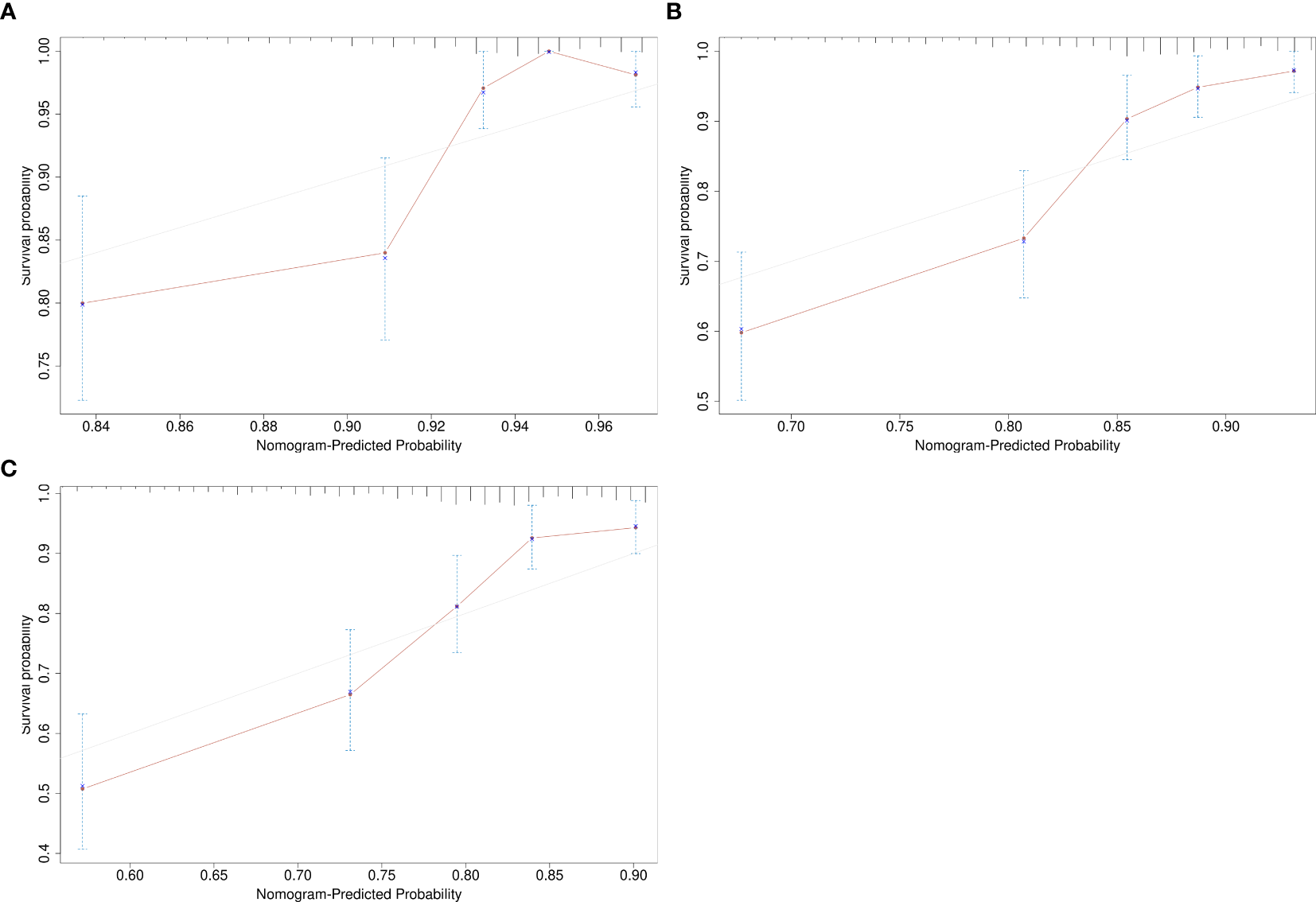
Calibration curves assessing the agreement between nomogram-predicted and observed survival probabilities. (A) 1-year survival; (B) 3-year survival; (C) 5-year survival.
Figure 5
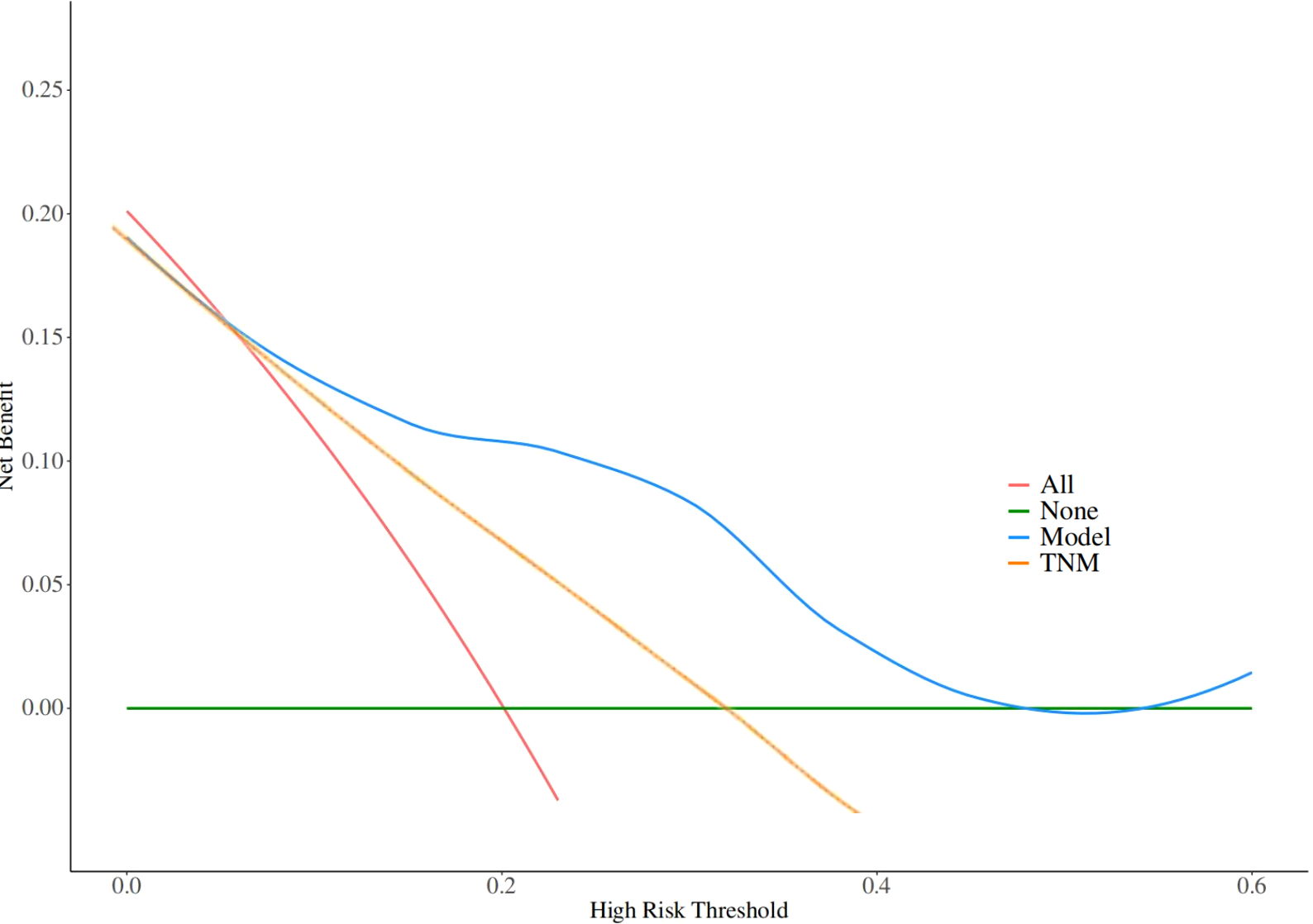
Decision curve analysis (DCA) evaluating the clinical utility of the nomogram across threshold probabilities. The nomogram (blue line) demonstrates significantly higher net benefit compared to the TNM staging system (yellow line) and the 'treat-all' (red lines) or 'treat-none' strategies (green lines).
Discussion
While alpha-fetoprotein (AFP) is a well-recognized biomarker for liver cancer (9), its role in gastric cancer (GC) remains less explored. This study provides substantial evidence supporting the prognostic value of AFP in gastric cancer, particularly for patients who are AFP-positive (10). Our findings reveal a correlation between AFP-positive gastric cancer (AFPGC) and more aggressive disease characteristics, coupled with significantly worse OS. Specifically, AFP-positive patients displayed a hazard ratio (HR) of 1.68 (95% CI: 1.27–2.23), indicating a 68% increase in mortality risk compared to AFP-negative patients. These results concur with existing literature (11), which have demonstrated an association between AFP positivity and advanced tumor stages, alongside poor prognosis in various malignancies.
A central finding of our study is the link between AFP positivity and advanced clinical as well as pathological features, including larger tumor size, deeper invasion (T-stage), more frequent lymph node metastasis (N-stage), and presence of distant metastasis. Such attributes suggest that AFP-positive GC epitomizes a more aggressive disease variant. In line with previous reports, we observed a notable association between AFP-positive GC and liver metastasis; 12.0% of AFP-positive patients exhibited liver metastasis, a figure lower than the 33%-72% reported elsewhere (12). This variation might result from our inclusion criteria, focusing solely on surgically treated patients, thereby excluding more advanced, untreated cases. Nonetheless, our findings still support the notion that AFP-positive gastric cancer harbors a propensity for liver metastasis, contributing to its poorer prognosis.
Elevated AFP levels in GC may reflect both the tumor’s biological behavior and its interaction with the liver. Typically, AFP is produced in the fetal liver and yolk sac, with levels remaining low in adults (13). Reactivation of AFP expression can occur in certain tumors, especially those with hepatocellular traits (6). A potential biological mechanism underlying elevated AFP levels in gastric cancer involves hepatic differentiation or hepatocellular-like differentiation of gastric tumor cells (10). The stomach and liver originate from the primitive foregut during embryogenesis, suggesting that some gastric tumors may acquire hepatocellular features, culminating in AFP production (14). This process is more likely in gastric cancers manifesting liver metastasis, as tumor cells may acquire hepatic features during liver invasion (15). In our study, AFP-positive patients exhibited significantly larger tumor sizes (5.90 ± 2.70 cm vs. 4.75 ± 2.30 cm, P = 0.032), supporting the hypothesis that AFP-positive tumors tend to be more aggressive (16). Larger tumors have a higher propensity to metastasize, particularly to the liver, consistent with the higher rate of liver metastasis observed in AFP-positive patients (17). Additionally, increased AFP production may reflect hepatic differentiation presence in tumor cells, potentially enhancing their metastatic potential (18). This mechanism proposes that AFP might not only serve as a prognostic biomarker but also offer insights into the underlying biology of gastric cancer (6). Biological aggressiveness of alpha-fetoprotein (AFP)-positive gastric cancer The prognostic implications of elevated AFP levels are considerable (19). In our study, AFP-positive gastric cancer patients demonstrated significantly lower survival rates at 1, 3, and 5 years compared to AFP-negative patients. The 5-year survival rates for AFP-positive and AFP-negative patients were 28% and 51%, respectively, underscoring the strong association between AFP positivity and poor long-term outcomes (10). This finding implies that AFP could serve not only as a prognostic biomarker but also as a potential therapeutic target. Future research should explore whether targeting pathways related to AFP production or hepatic differentiation in GC offers novel therapeutic avenues (15).
Our study demonstrates AFP’s strong prognostic value in gastric cancer, particularly regarding metastatic propensity. This observation aligns with emerging understanding of AFP-producing tumors’ unique biology. The staining intensity of CXCR4 and SDF-1 in cancer cells was significantly higher in intestinal-type than in diffuse-type gastric cancer. Furthermore, intestinal-type cancer cells that permeated the vascular or lymphatic channels as well as liver and lymph node metastases showed strong CXCR4 and SDF-1 staining (20). Additionally, AFP acts as a co-chaperone of heat shock protein 90 (HSP90), stabilizing oncoproteins c-MYC and c-MET, thereby facilitating tumor progression in liver and gastric cancers (21). The molecular mechanism underlying AFP’s role in GC involves activation of the Wnt signaling pathway, leading to increased cell growth and aggression (22).
Our study also devised a nomogram to predict survival in AFP-positive gastric cancer patients, incorporating AFP levels along with T-stage, N-stage, and distant metastasis presence. The nomogram demonstrated robust discriminatory ability, evidenced by AUC values of 0.80 for 1-year survival in the training cohort and 0.84 in the validation cohort. Calibration curves further validated the model, confirming its accuracy in predicting actual patient outcomes. DCA also indicated that the nomogram offered a net benefit across various threshold probabilities, thus making it a valuable tool for clinical decision-making (23). This nomogram could assist clinicians in predicting individual patient survival probabilities and facilitate more informed treatment decisions, particularly for those with AFP-positive gastric cancer (24, 25).
Despite its contributions, this study has limitations. The retrospective design may carry inherent biases, including issues with data completeness and selection bias. Although the study involved a large cohort, its single-center nature limits the external validity of the findings. Future multicenter studies are necessary to validate these results and assess the nomogram’s generalizability. Additionally, AFP testing variability across laboratories may affect result reproducibility. Standardization of AFP measurement techniques is crucial for broader clinical application.
Conclusion
In conclusion, AFP represents a significant prognostic marker in gastric cancer, and its inclusion in multivariate models enhances survival prediction. The nomogram developed in this study offers clinicians a valuable tool for predicting patient outcomes and facilitating informed treatment decisions.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Changzhou Traditional Chinese Medicine hospital Ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FZ: Writing – original draft, Conceptualization, Methodology. YT: Validation, Investigation, Writing – review & editing. WM: Visualization, Formal analysis, Writing – review & editing. ZT: Writing – review & editing, Supervision, Conceptualization. TL: Writing – original draft, Project administration, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1598337/full#supplementary-material
References
1
Zhou Q Tao F Qiu L Chen H Bao H Wu X et al . Somatic alteration characteristics of early-onset gastric cancer. J Oncol. (2022) 2022:1498053. doi: 10.1155/2022/1498053
2
Ferlay J Colombet M Soerjomataram I Mathers C Parkin DM Pineros M et al . Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. (2019) 144:1941–53. doi: 10.1002/ijc.v144.8
3
Smyth EC Nilsson M Grabsch HI van Grieken NC Lordick F . Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
4
Waddingham W Nieuwenburg SAV Carlson S Rodriguez-Justo M Spaander M Kuipers EJ et al . Recent advances in the detection and management of early gastric cancer and its precursors. Frontline Gastroenterol. (2021) 12:322–31. doi: 10.1136/flgastro-2018-101089
5
Wang Y Zhang S Ding B Tang Z Ji Y Yu Y et al . Development and validation of an individualized nomogram for gastric cancer patients treated with perioperative chemotherapy followed by radical surgery. Transl Gastroenterol Hepatol. (2024) 9:39. doi: 10.21037/tgh-23-75
6
Wepsic HT Kirkpatrick A . Alpha-fetoprotein and its relevance to human disease. Gastroenterology. (1979) 77:787–96. doi: 10.1002/prca.201800062
7
Zhouwei Z Bijuan C Jiami Y Jingxian Z Yi Z Mingyao S et al . Elevated serum alpha-fetoprotein is a significant prognostic factor for patients with gastric cancer: results based on a large-scale retrospective study. . Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.901061
8
Wang Y Shen L Jiao X Zhang X . Predictive and prognostic value of serum AFP level and its dynamic changes in advanced gastric cancer patients with elevated serum AFP. World J Gastroenterology. (2018) 24:266–73. doi: 10.3748/wjg.v24.i2.266
9
Lin H Hsieh Y Fang W Huang K Li A . Clinical manifestations in patients with alpha-fetoprotein–producing gastric cancer. Curr Oncology. (2014) 21:e394. doi: 10.3747/co.21.1768
10
Wang D Li C Xu Y-C Xing Y Qu L Guo Y et al . Clinicopathological characteristics and prognosis of alpha-fetoprotein positive gastric cancer in Chinese patients. Int J Clin Exp pathology. (2015) 8 6:6345–55.
11
Sauzay C Petit A Bourgeois A Barbare J Chauffert B Galmiche A et al . Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma. Clinica chimica acta; Int J Clin Chem. (2016) 463:39–44. doi: 10.1016/j.cca.2016.10.006
12
Trevisani F Garuti F Neri A . Alpha-fetoprotein for diagnosis, prognosis, and transplant selection. Semin Liver Disease. (2019) 39:163–77. doi: 10.1055/s-0039-1677768
13
He R Yang Q Dong X Wang Y Zhang W Shen L et al . Clinicopathologic and prognostic characteristics of alpha-fetoprotein-producing gastric cancer. Oncotarget. (2017) 8:23817–30. doi: 10.18632/oncotarget.15909
14
Kono K Amemiya H Sekikawa T Iizuka H Takahashi A Fujii H et al . Clinicopathologic features of gastric cancers producing alpha-fetoprotein. Dig Surg. (2002) 19:359–65. doi: 10.1159/000065838
15
Ishigami S Natsugoe S Nakashima H Tokuda K Nakajo A Okumura H et al . Biological aggressiveness of alpha-fetoprotein (AFP)-positive gastric cancer. Hepatogastroenterology. (2006) 53:338–41.
16
Shoji H Shuhei K Daisuke I Takeshi K Kazuma O Atsushi S et al . Liver metastasis is the only independent prognostic factor in AFP-producing gastric cancer. World J Gastroenterol. (2013) 19(36):6055–6061. doi: 10.3748/wjg.v19.i36.6055
17
Chen H Egan JO Chiu JF . Regulation and activities of alpha-fetoprotein. Crit Rev Eukaryot Gene Expr. (1997) 7:11–41. doi: 10.1615/CritRevEukarGeneExpr.v7.i1-2.20
18
Tatli AM Urakci Z Kalender ME Arslan H Tastekin D Kaplan MA . Alpha-fetoprotein (AFP) elevation gastric adenocarcinoma and importance of AFP change in tumor response evaluation. Asian Pac J Cancer Prev. (2015) 16:2003–7. doi: 10.7314/APJCP.2015.16.5.2003
19
Ooi A Nakanishi I Sakamoto N Tsukada Y Takahashi Y Minamoto T et al . Alpha-fetoprotein (AFP)-producing gastric carcinoma. Is it hepatoid differentiation? Cancer. (1990) 65:1741–7. doi: 10.1002/1097-0142(19900415)65:8<1741::AID-CNCR2820650814>3.0.CO;2-3
20
Iwasa S Yanagawa T Fan J Katoh R . Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric cancer is associated with lymph node and liver metastasis. . Anticancer Res. (2009) 29 11:4751–8.
21
Lin Z Pan R Wu L Zhu F Fang Q Kwok HF et al . AFP-HSP90 mediated MYC/MET activation promotes tumor progression in hepatocellular carcinoma and gastric cancers. Cancer Cell Int. (2024) 24:283. doi: 10.1186/s12935-024-03455-6
22
Chen D Lin X Zhang C An G Li Z Dong B et al . Activated Wnt signaling promotes growth and progression of AFP-producing gastric cancer in preclinical models. Cancer Manag Res. (2019) 11:1349–62. doi: 10.2147/CMAR.S187219
23
Da X Juan Z Zhijun H Zhongchuan L . Case report: Significant response of alpha-fetoprotein-producing gastric cancer from combined chemotherapy and immunotherapy. Front Immunol. (2024) 15:1448875. doi: 10.3389/fimmu.2024.1448875
24
Yu C Zhang Y . Development and validation of prognostic nomogram for young patients with gastric cancer. Ann Transl Med. (2019) 7:641. doi: 10.21037/atm.2019.10.77
25
Adachi Y Tsuchihashi J Shiraishi N Yasuda K Etoh T Kitano S . AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. (2003) 65:95–101. doi: 10.1159/000072332
Summary
Keywords
alpha-fetoprotein, gastric cancer, prognosis, metastasis, nomogram
Citation
Zuo F, Tan Y, Mao W, Tang Z and Luo T (2025) Clinical prognosis evaluation of alpha-fetoprotein-positive gastric cancer: comprehensive analysis and development of a novel nomogram for survival prediction. Front. Oncol. 15:1598337. doi: 10.3389/fonc.2025.1598337
Received
23 March 2025
Accepted
05 May 2025
Published
23 May 2025
Volume
15 - 2025
Edited by
Matteo De Pastena, University of Verona, Italy
Reviewed by
Lei Wang, The First Affiliated Hospital of Zhengzhou University, China
Alice Cattelani, University of Verona, Italy
Updates
Copyright
© 2025 Zuo, Tan, Mao, Tang and Luo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianping Luo, robalance@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.