- Department of Gastrointestinal Cancer Center, Chongqing University Cancer Hospital, Chongqing, China
Background: The prognostic importance of the pretreatment pan-immune-inflammation value (PIV) in colorectal cancer has been extensively documented, yet its role remains unclear. This study aims to conduct an updated meta-analysis to elucidate the relationship between the pretreatment PIV and long-term survival outcomes among patients diagnosed with colorectal cancer.
Methods: A systematic literature review was performed in PubMed, Embase, Web of Science and CNKI to identify eligible studies from inception to January 18, 2025. The primary endpoints evaluated were survival outcomes. Hazard ratios (HRs) along with their corresponding 95% confidence intervals (CIs) for survival outcomes were extracted. A random-effects model was utilized to synthesize the findings. All statistical analyses were conducted using R software, version 4.2.1.
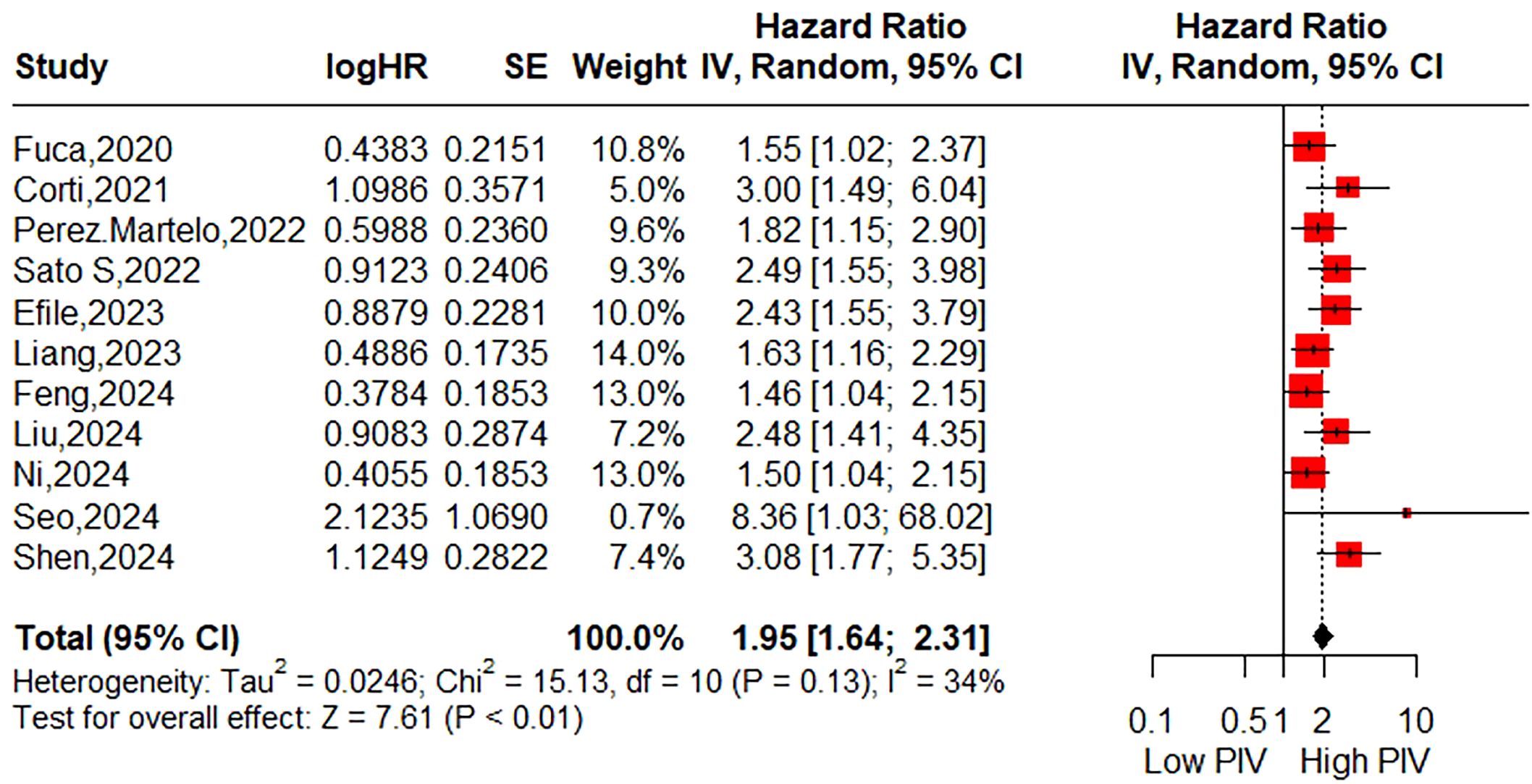
Results: Out of 81 identified studies, a total of 14 retrospective studies including 6,192 colorectal cancer patients were ultimately included. In this meta-analysis, the pooled results demonstrated that patients with higher PIV exhibited significantly poorer overall survival (11 studies, HR=1.95; 95%CI:1.64-2.31; P<0.01; I2 = 34%) and disease-free survival (10 studies, HR= 1.89; 95% CI: 1.48-2.41; P < 0.01; I2 = 66%). Furthermore, evidence pooled from two studies demonstrated that PIV may be an independent prognostic factor for cancer-specific survival (HR= 2.61; 95% CI: 1.56-4.38; P < 0.01; I2 = 0%).
Conclusion: Our study reveals that the pretreatment PIV can serve as a valuable biomarker for predicting long-term survival outcomes in patients with colorectal cancer, which may have important clinical implications for personalized treatment strategies.
1 Background
Colorectal cancer (CRC) continues to rank as the third most commonly diagnosed malignancy and the second leading cause of global cancer-related mortality (1). Despite remarkable progress in surgical techniques, chemotherapeutic regimens, radiotherapy protocols, targeted therapies, and immunotherapeutic interventions for CRC patients, clinical outcomes remain suboptimal (2). To date, the Tumor-Node-Metastasis (TNM) classification system has been universally acknowledged as the cornerstone for stratifying prognostic risks in CRC. However, extensive evidence demonstrates considerable heterogeneity in patient outcomes even within the same TNM stage, particularly in stages II and III (3). This variability underscores the limitations of relying solely on TNM staging to fully predict the prognostic outcomes. As such, there is an imperative need to identify robust biomarkers capable of refining risk stratification and pinpointing individuals at high risk of adverse prognoses.
A large amount of evidence underscores the pivotal role of host inflammation and immune status in modulating the progression, treatment responsiveness, and survival trajectories of cancer patients (4, 5). Drawing upon this insight, a number of inflammation/immune-related biomarkers has emerged to forecast clinical outcomes in oncology, including the monocyte-to-lymphocyte ratio (MLR) (6), neutrophil-to-lymphocyte ratio (NLR) (7), and platelet-to-lymphocyte ratio (PLR) (8). Recently, a novel prognostic biomarker—the pan-immune-inflammation value (PIV)—has captured the attention of clinicians worldwide (9, 10). By integrating neutrophils, platelets, monocytes, and lymphocytes into a single metric, PIV has demonstrated superior prognostic accuracy compared to its simpler counterparts, such as NLR, NLR, and PLR (9). Specifically, PIV is calculated via the formula: serum neutrophil × platelet × monocyte ÷ lymphocyte, a methodology first introduced by Fuca et al. (11) in 2020 as a prognostic index for metastatic colorectal cancer patients undergoing chemotherapy combined with targeted therapy. Subsequently, the prognostic utility of PIV has been progressively investigated across various clinical settings of CRC patients (12–14). In 2022, Yang et al. (15) conducted the first meta-analysis encompassing six studies, preliminarily demonstrating the prognostic significance of PIV in CRC patients. Nevertheless, they conceded that the limited number of included studies rendered the prognostic implications of PIV in CRC somewhat ambiguous. In light of the burgeoning recent literature, we undertook an updated meta-analysis to further illuminate the correlation between pretreatment PIV and long-term oncological outcomes in CRC patients.
2 Methods
2.1 Search strategy
The present meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16). A systematic and comprehensive search for relevant studies was performed across multiple databases, including PubMed, Embase, CNKI, and Web of Science, spanning from their inception to January 18, 2025. The search strategy involved a combination of predefined keywords: (pan-immune-inflammation value) AND (((colorectal) OR (colon) OR (rectum) OR (rectal)) AND ((cancer) OR (tumor) OR (carcinoma))). No restrictions were imposed on language during the search process. Additionally, the reference lists of the included studies were thoroughly examined to identify further relevant reports. Two investigators (L-J and P-HY) independently executed the search procedure.
2.2 Study selection
The inclusion criteria were as follows: (1) Studies investigating the association between the pretreatment PIV and survival outcomes in patients with CRC, including overall survival (OS), recurrence-free survival (RFS), disease-free survival (DFS), progression-free survival (PFS) and cancer-specific survival (CSS); (2) Hazard ratios (HRs) along with their 95% confidence intervals (CIs) were either directly reported or could be calculated based on the original survival curves; (3) The specific cut-off value of the PIV was clearly defined. The exclusion criteria were as follows: (1) Studies that failed to provide distinct data for CRC patients; (2) Case reports, reviews, conference abstracts, and correspondence; (3) Overlapping datasets.
2.3 Data extraction and quality assessment
Two independent reviewers (L-J and P-HY) performed data extraction and conducted cross-verification of all results. The extracted data encompassed essential information, including the first author’s name, publication year, study period, country, study design, blood sampling time, whether diseases affecting biomarker testing were excluded, sample size, cut-off value determination method, cut-off value of the PIV, and clinicopathological characteristics such as age, sex, primary treatment, tumor stage, tumor location, survival outcomes, and follow-up duration. The quality of the included studies was rigorously evaluated using the Newcastle-Ottawa Scale (NOS) (17), which consists of eight predefined items. Each study was assigned a final score ranging from 0 to 9 based on a comprehensive assessment; scores of 7–9 were considered indicative of high-quality research.
2.4 Statistical analysis
In this study, since RFS, PFS, and DFS share similar endpoints, they were collectively analyzed as a single outcome measure (DFS), consistent with previous literature (18, 19). The HRs along with their corresponding 95% CIs were used as the effect size for these survival outcomes. Statistical heterogeneity among the included studies was assessed using the I²statistic, and I²≥ 50% was considered indicative of significant heterogeneity. A random-effects model was employed to synthesize HRs during the meta-analysis due to the substantial clinical heterogeneity across studies. Subgroup analysis was conducted to evaluate the robustness of the pooled results. Additionally, sensitivity analysis was performed to explore the source of heterogeneity in the pooled results when significant heterogeneity was present. Begg’s funnel plot was utilized to assess potential publication bias. For pooled outcomes exhibiting significant publication bias, the trim-and-fill method was further applied. A two-tailed P value less than 0.05 was deemed statistically significant. All statistical analyses were conducted using R software, version 4.2.1.
3 Results
3.1 Study characteristics
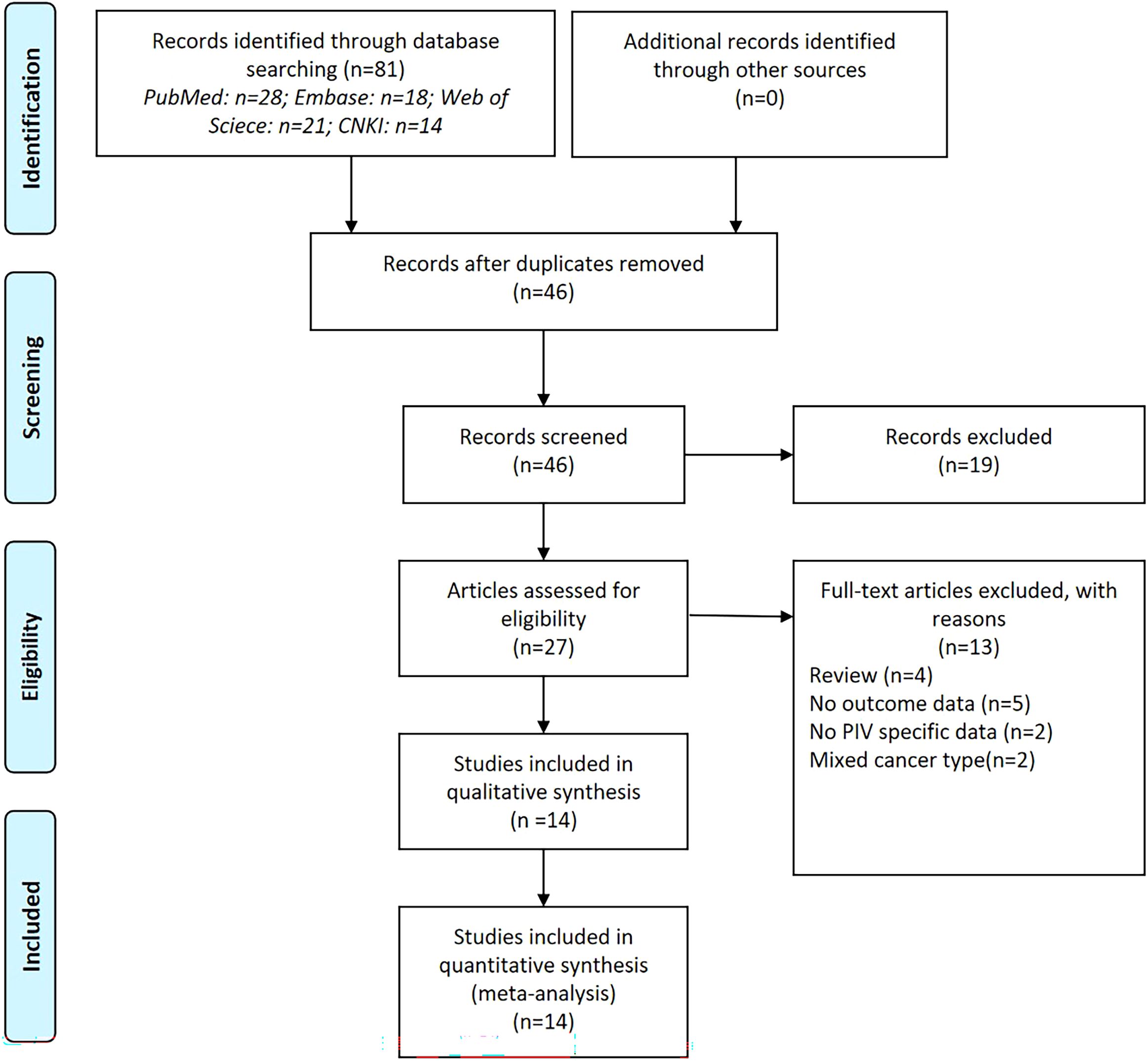
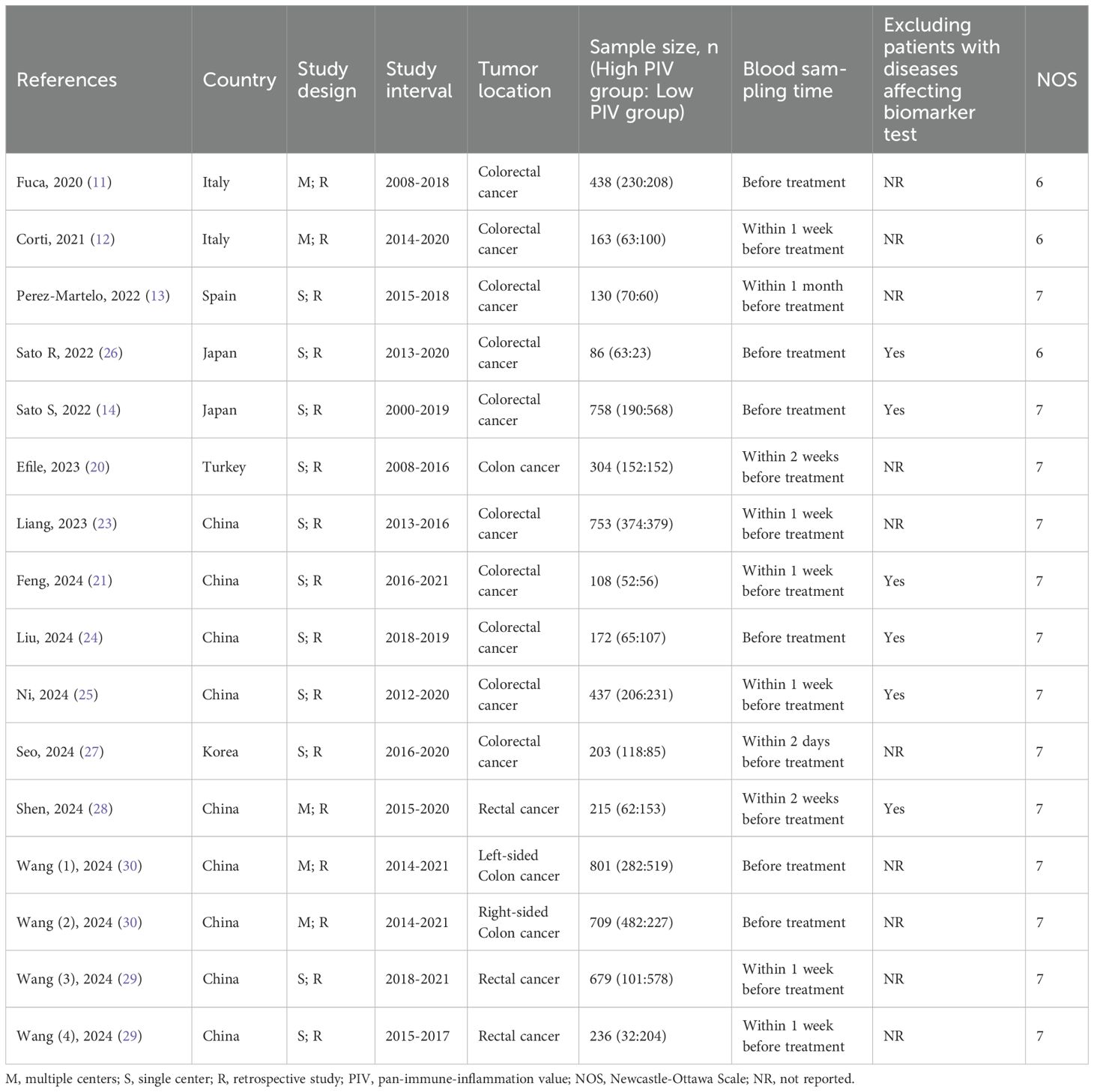
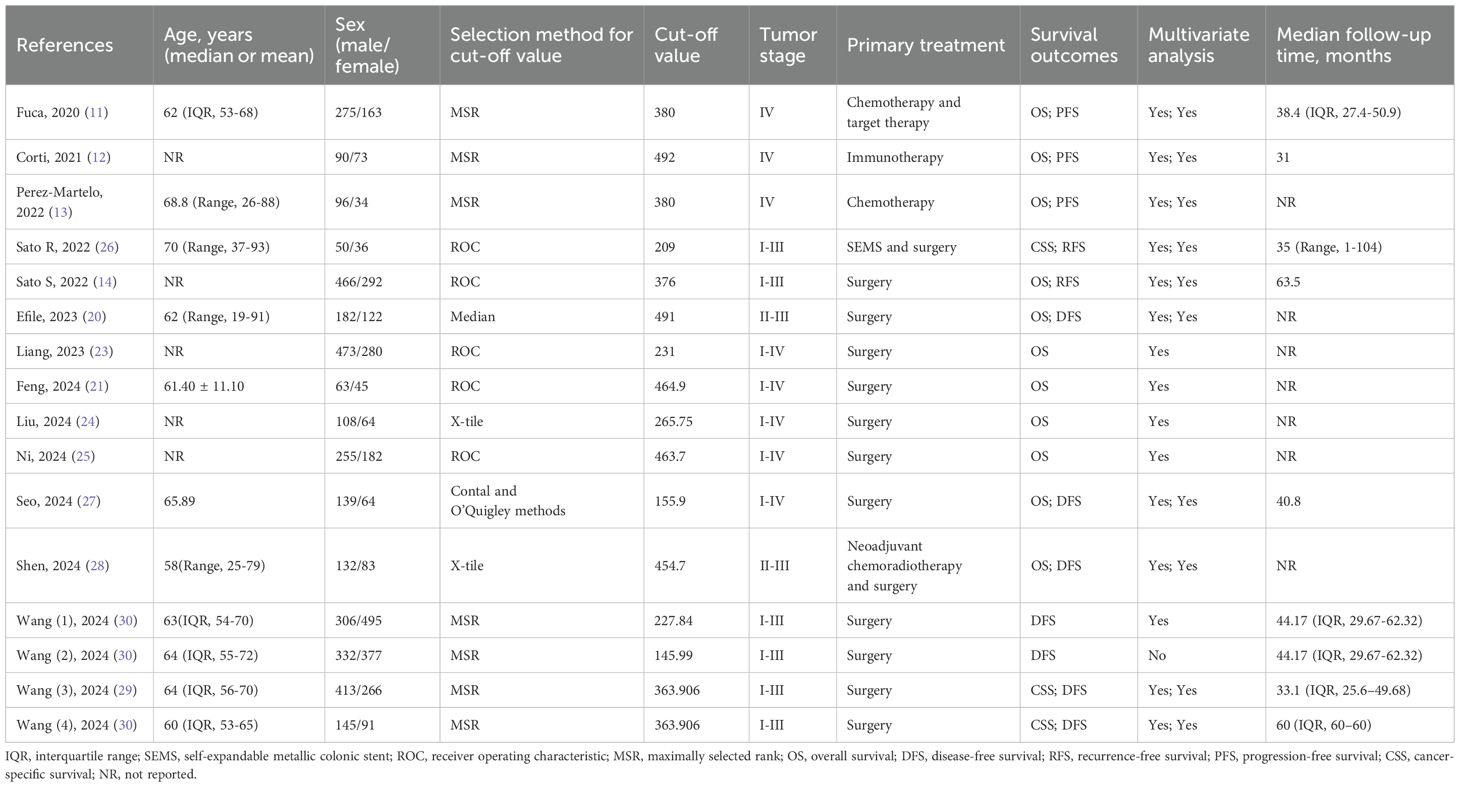
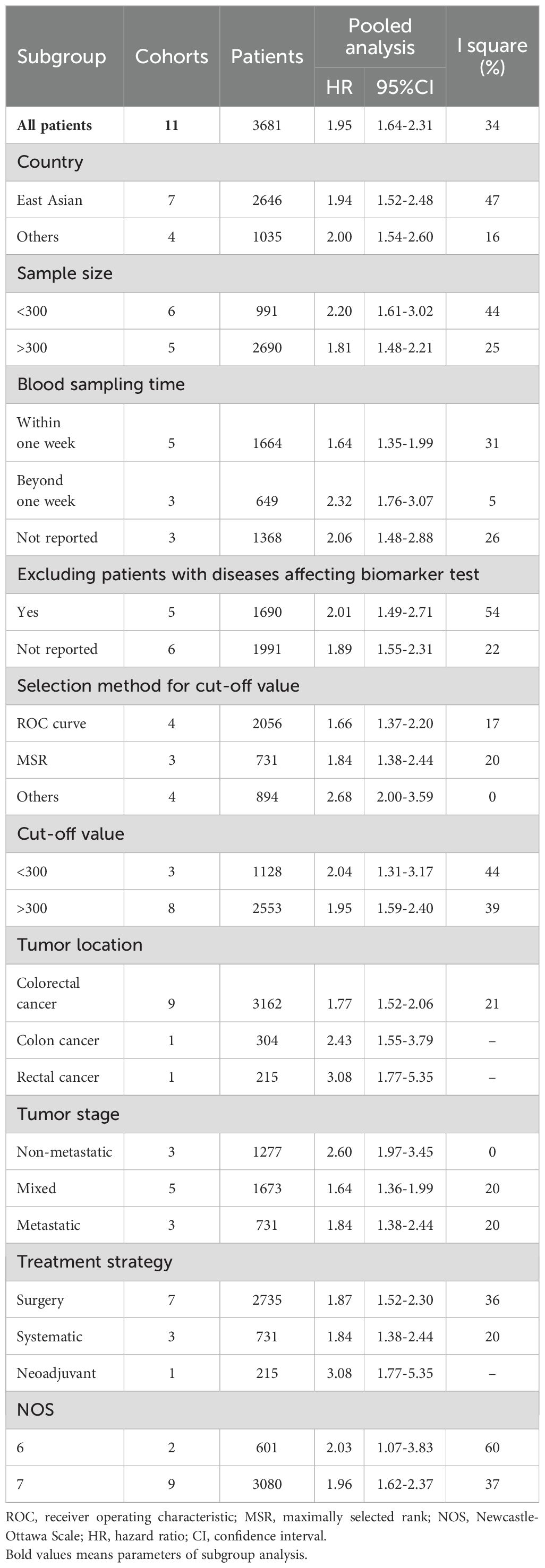
The database search resulted in a total of 81 records, as illustrated in Figure 1. Following a rigorous assessment of titles, abstracts, and full texts, 14 studies (11–14, 20–30) were ultimately selected for inclusion in this analysis. Tables 1 and 2 presented detailed summaries of the basic characteristics and survival outcomes of these studies, respectively. In summary, this meta-analysis included a total of 6,192 patients from six countries: China, Japan, Italy, Korea, Turkey, and Spain. The publication years spanned from 2020 to 2024, with sample sizes ranging from 86 to 801 participants. Among the included studies, 10 studies focused on colorectal cancer, 2 studies on colon cancer, and 2 studies on rectal cancer. Concerning primary treatment modalities, 10 studies involved surgical interventions, 3 studies involved systemic treatments, and 1 study focused on neoadjuvant therapy. Regarding survival endpoints, 11 studies evaluated OS, 5 studies assessed DFS, 3 studies examined PFS, 2 studies analyzed RFS, and 2 studies evaluated CSS. Notably, all studies exhibited high quality, with NOS scores ranging from 6 to 7 (as shown in Table 1, Supplementary Table S1).
3.2 Relationship between the pretreatment PIV and OS
The association between the pretreatment PIV and OS was evaluated in 11 studies involving 3,681 patients. The pooled HR was 1.95 (95% CI: 1.64-2.31; P < 0.01), indicating a significant correlation between higher PIV and poorer OS in CRC patients (Figure 2). Additionally, subgroup analyses were performed to assess the robustness of the pooled result across various factors, including country (East Asian vs. Others), sample size (<300 vs. >300), sampling time (Within one week vs. Beyond one week vs. Not reported), exclusion of patients with diseases affecting biomarker testing (Yes vs. Not reported), selection method for cut-off value (ROC curve vs. MSR vs. Others), cut-off value (<300 vs. >300), tumor location (Colorectal cancer vs. Colon cancer vs. Rectal cancer), tumor stage (Non-metastatic vs. Mixed vs. Metastatic), treatment strategy (Surgery vs. Neoadjuvant vs. Systemic therapy), and NOS score (6 vs. 7). As shown in Table 3 and Supplementary Figure S1, all subgroup analyses consistently revealed that patients with higher PIV had significantly reduced OS compared to those with lower PIV.
3.3 Relationship between the pretreatment PIV and DFS
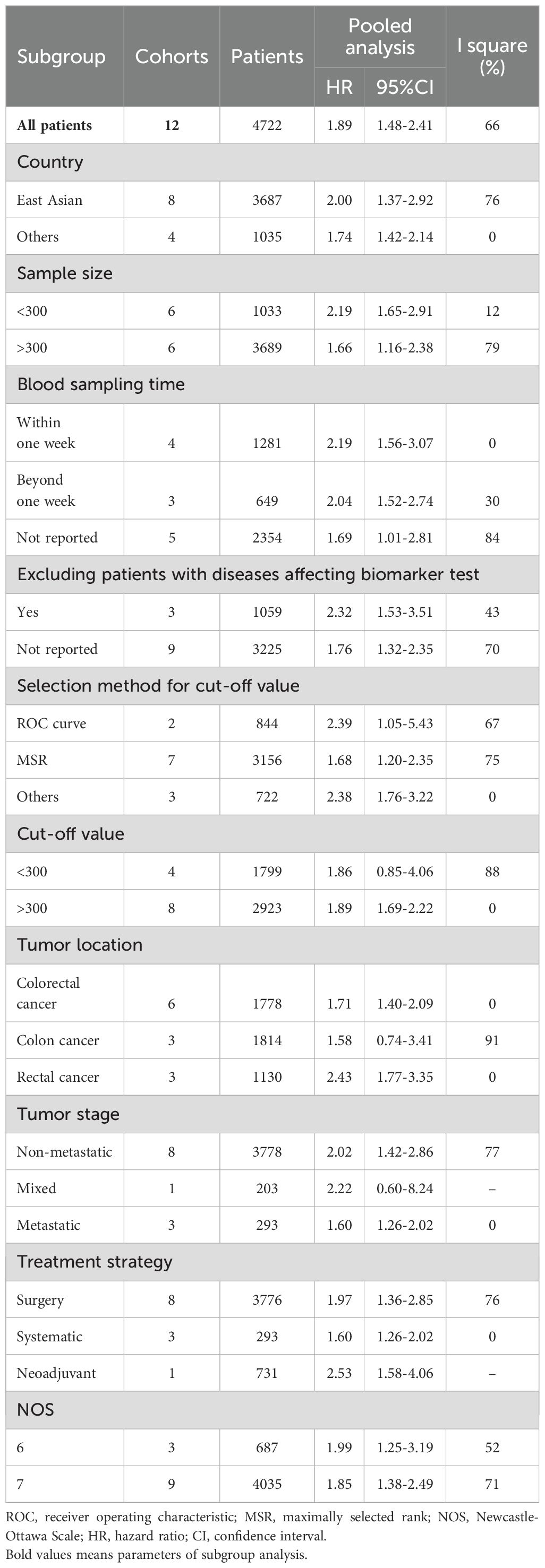
A total of ten studies (12 cohorts) involving 4,722 patients reported on DFS. The pooled HR was HR=1.89 (95%CI: 1.48-2.41; P<0.01; I2 = 66%), indicating a significant association between higher PIV and poorer DFS (Figure 3). As shown in Table 4 and Supplementary Figure S2, subgroup analyses based on the aforementioned factors revealed that patients in the higher PIV group experienced worse DFS across most subpopulations, with exceptions including subgroups with a cut-off value < 300 (HR = 1.86; 95% CI: 0.85–4.06), colon cancer (HR = 1.58; 95% CI: 0.74–3.41), and mixed tumor stages (HR = 2.22; 95% CI: 0.60–8.24). Sensitivity analysis demonstrated that the pooled DFS remained statistically significant when omitting each study at a time, and its heterogeneity was substantially reduced (I² = 3%) upon exclusion of the second cohort reported by Wang et al. (30). (Supplementary Figure S3).
![Forest plot from a meta-analysis, showing hazard ratios for different studies. Each study is represented by a square and horizontal line indicating the hazard ratio and 95% confidence interval, respectively. Pooled estimate is shown as a diamond at 1.89 with a range of [1.48; 2.41]. Heterogeneity measures: Tau² = 0.1124, Chi² = 32.22, I² = 66%. The overall effect Z-score is 5.12 with P < 0.01.](https://www.frontiersin.org/files/Articles/1599075/fonc-15-1599075-HTML/image_m/fonc-15-1599075-g003.jpg)
Figure 3. Forest plot accessing the relationship between the pretreatment PIV and disease-free survival.
3.4 Relationship between the pretreatment PIV and CSS
The relationship between the pretreatment PIV and CSS was assessed in two studies (3 cohorts) involving 1,001 patients. The pooled HR was 2.61 (95% CI: 1.56-4.38; P < 0.01; I2 = 0%), suggesting a possible association between higher PIV and relatively poorer CSS (Figure 4). Due to the limited number of included studies, subgroup analyses were not conducted.
![Forest plot showing hazard ratios with 95% confidence intervals for three studies: Sato R, 2022; Wang (3), 2024; and Wang (4), 2024. The combined effect size is 2.61 [1.56, 4.38]. Weight percentages are 21.6%, 34.9%, and 43.5%, respectively. Heterogeneity measures show Tau-squared is zero, Chi-squared is 0.36 with two degrees of freedom, and I-squared is zero percent. Test for overall effect yields Z = 3.63 with P < 0.01. Red squares represent individual studies with varying sizes, and a diamond shape indicates the overall effect size.](https://www.frontiersin.org/files/Articles/1599075/fonc-15-1599075-HTML/image_m/fonc-15-1599075-g004.jpg)
Figure 4. Forest plot accessing the relationship between the pretreatment PIV and cancer-specific survival.
3.5 Publication bias
The Begg’s funnel plots are illustrated in Figure 5. The results of the Begg’s test indicated a significant publication bias concerning OS (P=0.0063). However, the trim-and-fill analysis revealed that the pooled result remained robust after accounting for four additional hypothetical unpublished studies (HR=1.74; 95% CI: 1.45-2.09; P<0.01; I2 = 50.8%). For DFS and CSS, the funnel plots exhibited bilateral symmetry, with P values of 0.1926, and 0.2963, respectively, as determined by the Begg’s test.
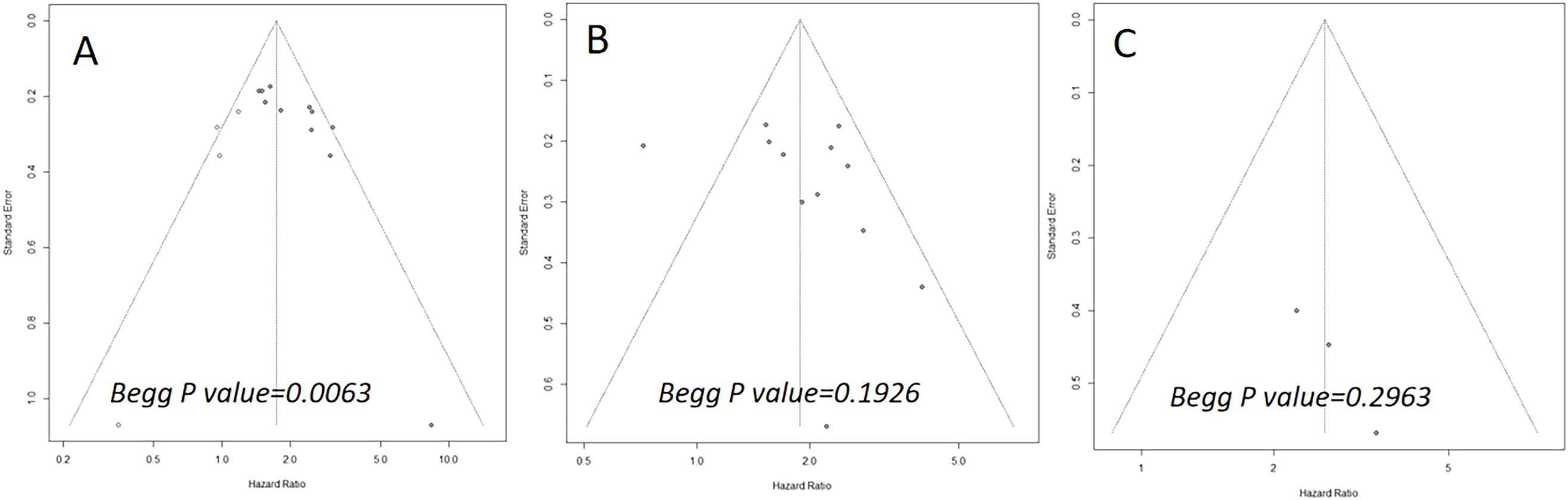
Figure 5. Begg’s funnel plots assessing publication bias between the pretreatment PIV and overall survival (A), disease-free survival (B), and cancer-specific survival (C).
4 Discussion
Cancer-related inflammation is pervasive among patients with malignant diseases and has been firmly established as a driving force in cancer progression and metastasis (31). Traditionally, the host’s inflammatory state could be reflected by blood-based biomarkers to some extent, including neutrophil count, platelet count, and lymphocyte count (32). Moreover, extensive evidence from numerous studies underscores the predictive power of their ratios for evaluating both short-term and long-term patient outcomes, particularly within the context of oncology (8). Notably, these markers possess intrinsic advantages—being non-invasive, objective, and economically viable—thereby offering great promise for broad clinical implementation (2).
In recent years, a novel biomarker known as the PIV—a composite indicator encompassing serum neutrophils, platelets, monocytes, and lymphocytes—has garnered significant attention from clinicians (11). This is largely due to its remarkable prognostic potential reported in various malignancies. In ovarian cancer, Liao et al. (33) demonstrated that patients in the high PIV group exhibited poorer OS and PFS compared to those in the low PIV group. In breast cancer, Li et al. (34) revealed that the pretreatment PIV was a useful predictive indicator for pathological complete response and long-term survival in patients undergoing neoadjuvant chemotherapy. Furthermore, a recent meta-analysis by Kuang et al. (35) confirmed that elevated PIV levels are associated with reduced OS and PFS in cancer patients receiving immune checkpoint inhibitors. In CRC, although a previous meta-analysis by Yang et al. (15) in 2022 showed the significant efficacy of PIV in predicting long-term survival, this study incorporated only 6 studies with 1,879 patients, which may limit the clarity and generalizability of its conclusions. Therefore, further investigation into the prognostic value of PIV in CRC patients remains essential.
By synthesizing data from 14 studies encompassing a total of 6,192 CRC patients, our meta-analysis demonstrated that patients in the high PIV group exhibited a 1.95-fold increased risk of poor OS. Furthermore, subgroup analyses conducted based on eligible factors reinforced the prognostic significance of PIV across patients with diverse clinical characteristics. Although significant publication bias was detected, the trim-and-fill method consistently corroborated the robustness of the pooled results. Meanwhile, this meta-analysis revealed that patients in the high PIV group faced a 1.89-fold increased risk of poor DFS, with no substantial publication bias observed. Subgroup analyses indicated that pretreatment PIV held significant prognostic value in most subsets, except for subgroups defined by a cut-off value < 300, colon cancer, and mixed tumor stages. These inconsistent findings in certain subgroups should be interpreted with caution due to the limited number of cohorts included in these analyses. Sensitivity analysis further identified that these inconsistencies primarily stemmed from the second cohort reported by Wang et al. (30), which suggested limited prognostic utility of PIV for DFS in patients with right-sided colon cancer (HR = 0.72; 95% CI: 0.48–1.06). Upon excluding this cohort, the heterogeneity (I²) markedly decreased from 66% to 3%. Additionally, two studies initially explored the association between PIV and CSS, yielding statistically significant outcomes. Compared to prior meta-analysis, the primary strength of the current study lies in its inclusion of a more heterogeneous population with varied clinical features, thereby enhancing the generalizability of PIV’s prognostic value.
The potential mechanism by which the PIV can effectively predict prognosis in CRC patients can be explained through the following aspects. First, neutrophils, which are the most common innate immune cells, have been documented to facilitate tumor invasion and metastasis through the secretion of vascular endothelial growth factor A (VEGFA), matrix metalloproteinases (MMPs), and other chemokines such as interleukin-6 (IL-6) and transforming growth factor-beta (TGF-β) (36). Additionally, elevated levels of neutrophils can impair T cell activation by releasing substantial amounts of nitric oxide, arginase, and reactive oxygen species, thereby suppressing the body’s cytotoxic effect on cancer cells (37). Second, monocytes, particularly those that differentiate into tumor-associated macrophages (TAMs), can induce apoptosis in antitumor T cells (38). Furthermore, TAM density has been shown to influence tumor tissue angiogenesis by promoting the production and secretion of pro-angiogenic factors (39). Third, platelets have been reported to induce epithelial-mesenchymal transition (EMT) and angiogenesis via the secretion of TGF-β, VEGF, and fibroblast growth factor (FGF) (40). Moreover, platelets can recruit neutrophils and monocytes, thus facilitating the distant metastasis of tumor cells (32). Finally, lymphocytes serve as the primary effector cells of the immune system, coordinating immune responses against tumor cells (41). Tumor-infiltrating lymphocytes secrete a variety of cytokines, such as IFN-γ and TNF-α, which not only inhibit tumor growth but also promote tumor cell apoptosis (42). Specifically, CD8+ T cells can directly induce tumor cell death through the release of perforin and granzyme (43). Consequently, a reduction in lymphocyte count impairs the body’s capacity to effectively suppress tumor progression.
The present meta-analysis is subject to several notable limitations that warrant careful consideration. Firstly, all studies incorporated into this analysis were retrospective in nature, inherently carrying the risk of selection bias. This underscores the critical need for future prospective studies to establish a causal direction. Secondly, the majority of the studies were from East Asia, introducing a potential regional bias and highlighting the imperative for greater global diversity in future research endeavors. Thirdly, the study by Wang et al. (30) demonstrated that the pretreatment PIV does not exhibit prognostic value for DFS in right-sided colon cancer. And similar findings were observed in their study for other inflammatory markers, such as the NLR, PLR and systemic immune-inflammation index. However, the underlying biological mechanisms remain unclear. We contend that further clinical and basic research is warranted to elucidate the role of PIV in right-sided colon cancer. Lastly, although we preliminarily investigated the significant relationship between the pre-treatment PIV and CSS in colorectal cancer patients, our finding was derived solely from a meta-analysis of two studies. Consequently, additional studies are warranted to validate this issue.
5 Conclusions
Our research reveals that the pretreatment PIV could function as a remarkably insightful prognostic biomarker for individuals diagnosed with colorectal cancer, as individuals in the high PIV group demonstrate significantly poorer long-term survival outcomes. By harnessing this promising indicator, clinicians may effectively categorize patients and design more precise, individualized therapeutic approaches. However, further rigorous investigation is indispensable to substantiate the reliability and applicability of this biomarker in colorectal cancer prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
JL: Data curation, Writing – original draft, Methodology, Conceptualization, Visualization, Investigation, Formal analysis, Software. HP: Data curation, Formal analysis, Conceptualization, Writing – review & editing, Software. HS: Project administration, Validation, Writing – review & editing, Resources, Supervision, Funding acquisition. XL: Project administration, Software, Writing – review & editing, Supervision, Conceptualization, Investigation, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Project supported by Wu Jieping Medical Foundation research fund (No. 320.6750.2023-19-35).
Acknowledgments
We sincerely thank Professor Zhou Li from Chongqing University Cancer Hospital for her polishing of our manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1599075/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Liu H, Zhu D, Jiang D, Pang H, and Yang X. Prognostic value of the pretreatment Naples prognostic score in patients with colorectal cancer: a systematic review and meta-analysis. Front Oncol. (2025) 14. doi: 10.3389/fonc.2024.1498854
3. Sugimoto A, Fukuoka T, Shibutani M, Kasashima H, Kitayama K, Ohira M, et al. Prognostic significance of the Naples prognostic score in colorectal cancer patients undergoing curative resection: a propensity score matching analysis. BMC Gastroenterol. (2023) 23:88. doi: 10.1186/s12876-023-02722-6
4. Pang HY, Chen XF, Yan MH, Chen LH, Chen ZX, Zhang SR, et al. Clinical significance of the advanced lung cancer inflammation index in gastrointestinal cancer patients: a systematic review and meta-analysis. Front Oncol. (2023) 13:1021672. doi: 10.3389/fonc.2023.1021672
5. Petrillo A, Laterza MM, Tirino G, Pompella L, Ventriglia J, Pappalardo A, et al. Systemic-inflammation-based score can predict prognosis in metastatic gastric cancer patients before first-line chemotherapy. Future Oncol. (2018) 14:2493–505. doi: 10.2217/fon-2018-0167
6. Wu Q, Hu T, Zheng E, Deng X, and Wang Z. Prognostic role of the lymphocyte-to-monocyte ratio in colorectal cancer: An up-to-date meta-analysis. Med (Baltimore). (2017) 96:e7051. doi: 10.1097/MD.0000000000007051
7. Orditura M, Galizia G, Diana A, Saccone C, Cobellis L, Ventriglia J, et al. Neutrophil to lymphocyte ratio (NLR) for prediction of distant metastasis-free survival (DMFS) in early breast cancer: a propensity score-matched analysis. ESMO Open. (2016) 1:e000038. doi: 10.1136/esmoopen-2016-000038
8. Offi C, Romano RM, Cangiano A, Candela G, and Docimo G. Clinical significance of neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, platelet-to-lymphocyte ratio and prognostic nutritional index in low-risk differentiated thyroid carcinoma. Acta Otorhinolaryngol Ital. (2021) 41:31–8. doi: 10.14639/0392-100X-N1089
9. Hai-Jing Y, Shan R, and Jie-Qiong X. Prognostic significance of the pretreatment pan-immune-inflammation value in cancer patients: an updated meta-analysis of 30 studies. Front Nutr. (2023) 10:1259929. doi: 10.3389/fnut.2023.1259929
10. Yu D, Liu J, Meng C, Liu B, and Liao J. Pan-immune-inflammation value as a novel prognostic biomarker for digestive system cancers: a meta-analysis. World J Surg Oncol. (2024) 22:306. doi: 10.1186/s12957-024-03595-z
11. Fucà G, Guarini V, Antoniotti C, Morano F, Moretto R, Corallo S, et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer. (2020) 123:403–9. doi: 10.1038/s41416-020-0894-7
12. Corti F, Lonardi S, Intini R, Salati M, Fenocchio E, Belli C, et al. The Pan-Immune-Inflammation Value in microsatellite instability-high metastatic colorectal cancer patients treated with immune checkpoint inhibitors. Eur J Cancer. (2021) 150:155–67. doi: 10.1016/j.ejca.2021.03.043
13. Pérez-Martelo M, González-García A, Vidal-Ínsua Y, Blanco-Freire C, Brozos-Vázquez EM, Abdulkader-Nallib I, et al. Clinical significance of baseline Pan-Immune-Inflammation Value and its dynamics in metastatic colorectal cancer patients under first-line chemotherapy. Sci Rep. (2022) 12:6893. doi: 10.1038/s41598-022-10884-8
14. Sato S, Shimizu T, Ishizuka M, Suda K, Shibuya N, Hachiya H, et al. The preoperative pan-immune-inflammation value is a novel prognostic predictor for with stage I-III colorectal cancer patients undergoing surgery. Surg Today. (2022) 52:1160–9. doi: 10.1007/s00595-021-02448-6
15. Yang XC, Liu H, Liu DC, Tong C, Liang XW, and Chen RH. Prognostic value of pan-immune-inflammation value in colorectal cancer patients: A systematic review and meta-analysis. Front Oncol. (2022) 12:1036890. doi: 10.3389/fonc.2022.1036890
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
17. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
18. Pang HY, Yan MH, Chen LH, Chen XF, Chen ZX, Zhang SR, et al. Detection of asymptomatic recurrence following curative surgery improves survival in patients with gastric cancer: A systematic review and meta-analysis. Front Oncol. (2022) 12:1011683. doi: 10.3389/fonc.2022.1011683
19. Sun KX, Xu RQ, Rong H, Pang HY, and Xiang TX. Prognostic significance of the Gustave Roussy immune (GRIm) score in cancer patients: a meta-analysis. Ann Med. (2023) 55:2236640. doi: 10.1080/07853890.2023.2236640
20. Efil SC, Guner G, Guven DC, Celikten B, Celebiyev E, Taban H, et al. Prognostic and predictive value of tumor infiltrating lymphocytes in combination with systemic inflammatory markers in colon cancer. Clin Res Hepatol Gastroenterol. (2023) 47:102171. doi: 10.1016/j.clinre.2023.102171
21. Feng Y and Liu Y. The correlation between preoperative pan-immune-inflammation value and prognosis in patients with colorectal cancer. J Diagn Anal. (2024) 29:1708–11. doi: 10.3969/j.issn.1009-7147.2024.09.035
22. Li K, Zeng X, Zhang Z, Wang K, Pan Y, Wu Z, et al. Pan−immune−inflammatory values predict survival in patients after radical surgery for non−metastatic colorectal cancer: A retrospective study. Oncol Lett. (2025) 29:197. doi: 10.3892/ol.2025.14943
23. Liang L, Guo X, Ye W, and Liu Y. KRAS gene mutation associated with grade of tumor budding and peripheral immunoinflammatory indices in patients with colorectal cancer. Int J Gen Med. (2024) 17:4769–80. doi: 10.2147/IJGM.S487525
24. Liu Q, Wang H, Chen Q, Luo R, and Luo C. Nomogram incorporating preoperative pan-immune-inflammation value and monocyte to high-density lipoprotein ratio for survival prediction in patients with colorectal cancer: a retrospective study. BMC Cancer. (2024) 24:740. doi: 10.1186/s12885-024-12509-x
25. Ni Z, Zhu Z, Zhou S, and Feng X. Relationship between pan-immune-inflammation value and postoperative infectiouscomplications and prognosis in patients with colorectal cancer. Modern Oncol. (2024) 31:0087 –92. doi: 10.3969/i.issn.1672-4992.2024.01.015
26. Sato R, Oikawa M, Kakita T, Okada T, Abe T, Tsuchiya H, et al. A decreased preoperative platelet-to-lymphocyte ratio, systemic immune-inflammation index, and pan-immune-inflammation value are associated with the poorer survival of patients with a stent inserted as a bridge to curative surgery for obstructive colorectal cancer. Surg Today. (2023) 53:409–19. doi: 10.1007/s00595-022-02575-8
27. Seo YJ, Kim KE, Jeong WK, Baek SK, and Bae SU. Effect of preoperative pan-immune-inflammation value on clinical and oncologic outcomes after colorectal cancer surgery: a retrospective study. Ann Surg Treat Res. (2024) 106:169–77. doi: 10.4174/astr.2024.106.3.169
28. Shen P, Xu Y, Zhu J, Qian D, Yang B, Mao Y, et al. Predictive and prognostic value of preoperative pan-immune-inflammation value in patients with locally advanced rectal cancer. Biomol BioMed. (2024) 25(5):1000–8. doi: 10.17305/bb.2024.10658
29. Wang Q, Zhong W, Xiao Y, Lin G, Lu J, Xu L, et al. Pan-immune-inflammation value predicts immunotherapy response and reflects local antitumor immune response in rectal cancer. Cancer Sci. (2025) 116:350–66. doi: 10.1111/cas.16400
30. Wang QY, Zhong WT, Xiao Y, Lin GL, Lu JY, Xu L, et al. Pan-immune-inflammation value as a prognostic biomarker for colon cancer and its variation by primary tumor location. World J Gastroenterol. (2024) 30:3823–36. doi: 10.3748/wjg.v30.i33.3823
31. Singh R, Mishra MK, and Aggarwal H. Inflammation, immunity, and cancer. Mediators Inflamm. (2017) 2017:6027305. doi: 10.1155/2017/6027305
32. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
33. Liao W, Li J, Feng W, Kong W, Shen Y, Chen Z, et al. Pan-immune-inflammation value: a new prognostic index in epithelial ovarian cancer. BMC Cancer. (2024) 24:1052. doi: 10.1186/s12885-024-12809-2
34. Li F, Wang Y, Dou H, Chen X, Wang J, and Xiao M. Association of immune inflammatory biomarkers with pathological complete response and clinical prognosis in young breast cancer patients undergoing neoadjuvant chemotherapy. Front Oncol. (2024) 14:1349021. doi: 10.3389/fonc.2024.1349021
35. Kuang T, Qiu Z, Wang K, Zhang L, Dong K, and Wang W. Pan-immune inflammation value as a prognostic biomarker for cancer patients treated with immune checkpoint inhibitors. Front Immunol. (2024) 15:1326083. doi: 10.3389/fimmu.2024.1326083
36. Ocana A, Nieto-Jiménez C, Pandiella A, and Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. (2017) 16:137. doi: 10.1186/s12943-017-0707-7
37. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, and Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. (2020) 20:485–503. doi: 10.1038/s41568-020-0281-y
38. Mantovani A, Schioppa T, Porta C, Allavena P, and Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. (2006) 25:315–22. doi: 10.1007/s10555-006-9001-7
39. Huang C, Li Z, Li N, Li Y, Chang A, Zhao T, et al. Interleukin 35 expression correlates with microvessel density in pancreatic ductal adenocarcinoma, recruits monocytes, and promotes growth and angiogenesis of xenograft tumors in mice. Gastroenterology. (2018) 154:675–88. doi: 10.1053/j.gastro.2017.09.039
40. Guven DC, Sahin TK, Erul E, Kilickap S, Gambichler T, and Aksoy S. The association between the pan-immune-inflammation value and cancer prognosis: A systematic review and meta-analysis. Cancers (Basel). (2022) 14:2675. doi: 10.3390/cancers14112675
41. Yang M, Lin SQ, Liu XY, Tang M, Hu CL, Wang ZW, et al. Association between C-reactive protein-albumin-lymphocyte (CALLY) index and overall survival in patients with colorectal cancer: From the investigation on nutrition status and clinical outcome of common cancers study. Front Immunol. (2023) 14:1131496. doi: 10.3389/fimmu.2023.1131496
42. Hou Y, Li X, Yang Y, Shi H, Wang S, and Gao M. Serum cytokines and neutrophil-to-lymphocyte ratio as predictive biomarkers of benefit from PD-1 inhibitors in gastric cancer. Front Immunol. (2023) 14:1274431. doi: 10.3389/fimmu.2023.1274431
Keywords: colorectal cancer, pan-immune-inflammation value, overall survival, disease-free survival, cancer-specific survival, meta-analysis
Citation: Li J, Pang H, Sun H and Liu X (2025) Prognostic significance of the pretreatment pan-immune-inflammation value in colorectal cancer patients: an updated meta-analysis. Front. Oncol. 15:1599075. doi: 10.3389/fonc.2025.1599075
Received: 27 March 2025; Accepted: 07 July 2025;
Published: 24 July 2025.
Edited by:
Zhen Dong, Southwest University, ChinaCopyright © 2025 Li, Pang, Sun and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Sun, c3VuaGFvNjhAc2luYS5jb20=; Xiaoyu Liu, bGl1eHkwNTE0OEAxNjMuY29t
 Jing Li
Jing Li Huayang Pang
Huayang Pang Hao Sun
Hao Sun