- 1Department of Hepatobiliary and Pancreatic Surgery, Shaoxing People’s Hospital, Shaoxing, China
- 2School of Medicine, Graduate School, Zhejiang University, Hangzhou, China
Background: Gallbladder neuroendocrine neoplasms (GB-NENs) are exceedingly rare in clinical practice. To date, no large-scale, multicenter prospective studies have been conducted on this disease, resulting in a lack of established diagnostic and therapeutic experience or consensus. This case series reports seven GB-NEN patients who underwent different treatment modalities with varying outcomes. By integrating our institutional experience with previous literature, we aim to provide some therapeutic recommendations for GB-NEN patients.
Methods: The clinicopathological data of seven GB-NEN patients treated at our institution between June 2013 and June 2024 were retrospectively analyzed, with a focus on their treatment courses.
Results: Seven GB-NEN patients did not exhibite specific clinical manifestations or distinctive imaging features. All patients underwent surgical intervention, including radical resection in four cases. The overall survival ranged from 3 to 55 months, with a median survival of 19 months.
Conclusion: GB-NENs are highly aggressive and associated with poor prognosis. We recommend: 1) Radical cholecystectomy as the primary treatment for resectable GB-NENs; 2) Platinum-based chemotherapy as the first-line regimen, with close monitoring for drug resistance; 3) Early assessment of chemosensitivity to guide further treatment decisions, postoperative chemotherapy combined with adjuvant therapies may improve surgical efficacy.
Introduction
Primary gallbladder neuroendocrine neoplasms (GB-NENs) represent a rare subset of neuroendocrine tumors (NETs), exhibiting a predilection for female individuals, comprising approximately 0.5% of all NETs, while gallbladder neuroendocrine carcinomas (GB-NECs) constitute 2.1% of all gallbladder malignancies based on data from the U.S. Surveillance, Epidemiology, and End Results (SEER) database (1, 2). GB-NENs are rarely encountered in clinical practice since the gallbladder is devoid of enterochromaffin cells from which the neuroendocrine neoplasia originates. Furthermore, these neoplasms often present without symptoms initially and have no specific tumor markers, resulting in frequent preoperative misdiagnosis. Histopathological analysis remains the diagnostic gold standard. No standardized clinical guidelines or consensus exist for GB-NENs; thus, management typically mirrors protocols for gallbladder cancer or other NENs. Surgical resection remains the cornerstone of treatment, with platinum-based chemotherapy regimens constituting the first-line systemic treatment. Adjuvant therapy is considered a potential survival-prolonging strategy for advanced-stage GB-NENs, which often present with distant metastases (Supplementary Figure 1) (3).
Case series presentation
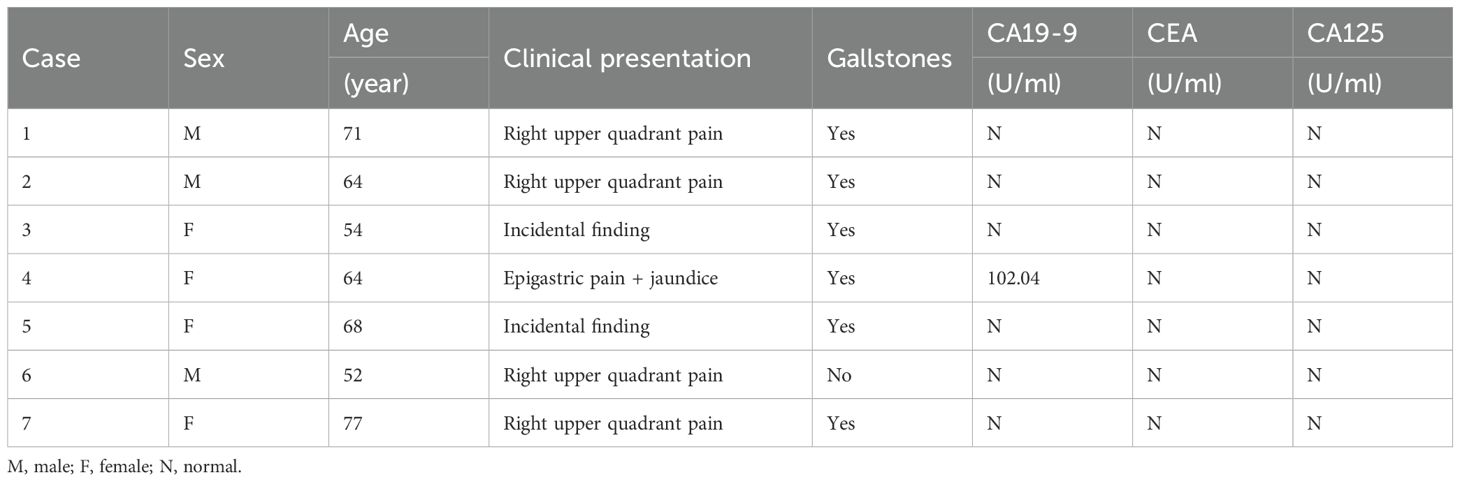
This study retrospectively analyzed clinical characteristics and treatment outcomes of seven GB-NEN cases treated at our institution, comprising three male and four female patients, with a median age of 64 years (range: 52–77). None exhibited disease-specific clinical manifestations at presentation. Four patients reported epigastric pain, two cases were detected during routine examinations, and one presented with jaundice due to bile duct involvement. Six patients had no evidence of associated hereditary syndromes (e.g., multiple endocrine neoplasia type 1) but a history of cholelithiasis complicated by cholecystitis. Except for one patient with elevated serum CA19–9 levels, all other biomarkers were within normal ranges(Table 1). Preoperative imaging uniformly revealed diffuse or focal gallbladder wall thickening. Among these, four cases were initially misdiagnosed as benign conditions (e.g., acute/chronic cholelithiasis or adenomyomatosis), whereas the remaining three exhibited space-occupying lesions, suggesting malignancy (Figure 1).
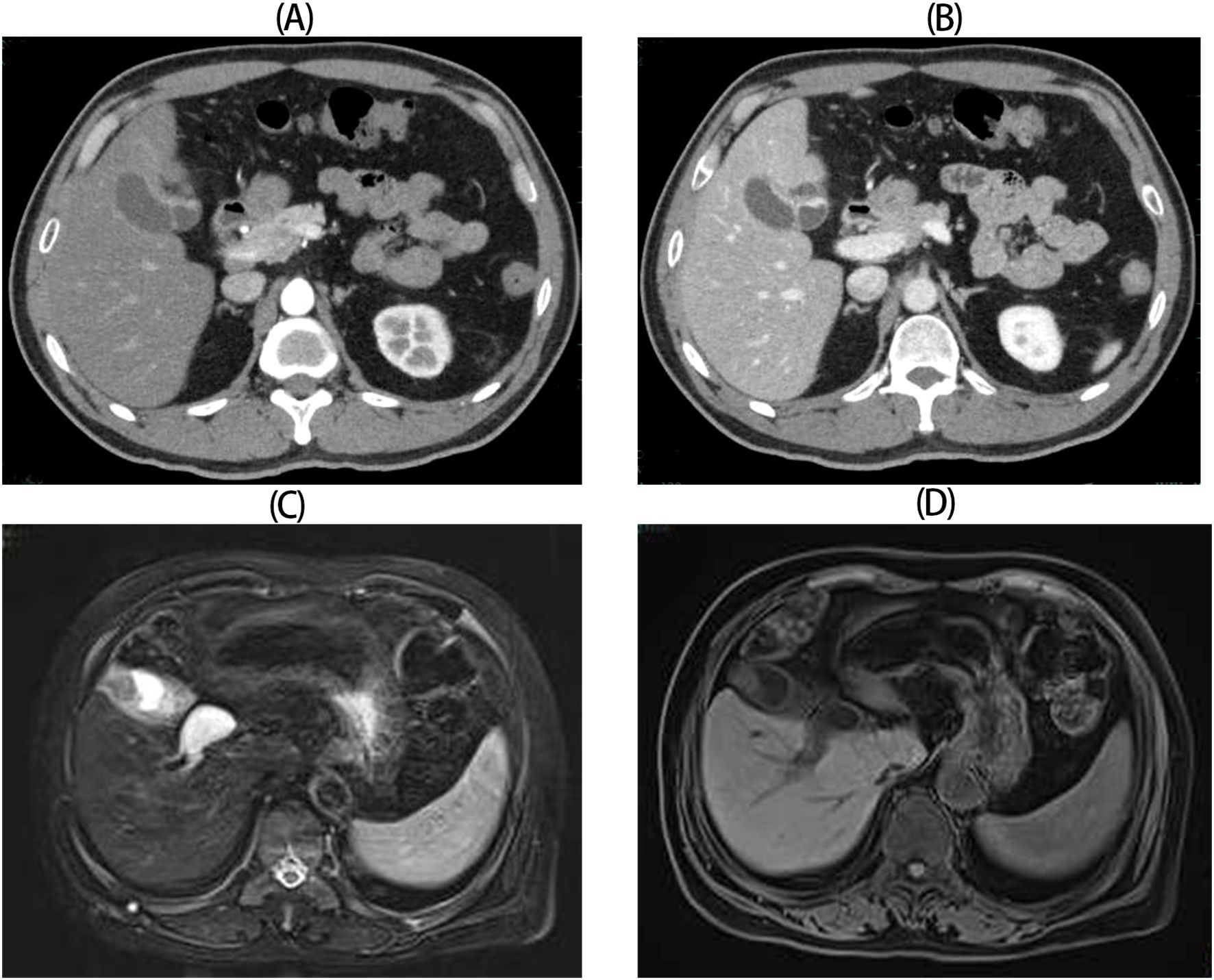
Figure 1. Radiologic images for GB-NENs. (A, B) Case 6: Irregular gallbladder wall thickening with arterial and venous phase enhancement (CT). (C, D) Case 5: An irregular, patchy soft-tissue signal at the gallbladder fundus (MRCP).
Seven patients underwent surgical intervention. Three patients received radical cholecystectomy for gallbladder cancer based on preoperative diagnosis: Case 6 transitioned from etoposide-cisplatin (EP) to irinotecan-cisplatin (IP) due to treatment intolerance, supplemented with anlotinib. After six chemotherapy cycles developed bone marrow suppression, treatment was changed to penpulimab combined with anlotinib. The patient ultimately died of tumor recurrence 40 months postoperatively. Case 7 received only one EP cycle before refusing further chemotherapy. Liver metastases were detected at 2 months postoperation, with death occurring at 10 months. Case 5 exhibited unexpected 55-month disease-free survival (DFS) without adjuvant treatment. One patient received pancreaticoduodenectomy for distal bile duct involvement: Case 4 completed six gemcitabine-oxaliplatin (GMOX) cycles without subsequent adjuvant therapy and liver metastases emerged at 12 months, subsequent treatments(transarterial chemoembolization, EP chemotherapy, and lenvatinib plus camrelizumab) all proved ineffective, with death at 23 months postoperation. Two patients underwent simple cholecystectomy: Case 3 developed hepatic and distant metastases at 7 months, dying at 15 months. Case 2 was lost to follow-up. Case 1 with preexisting hepatic metastases underwent palliative resection without additional treatment (3-month survival) (Table 2).
Postoperative histopathological examination confirmed the diagnosis of GB-NENs in all cases, with predominant tumor localization in the gallbladder body(diameter range: 0.5–3 cm; invasion depth: 0.2–2 cm). Immunohistochemical profiling confirmed universal Ki-67 positivity, with proliferation indices >20% in six cases. According to the World Health Organization (WHO) 2019 classification, NETs are graded based on Ki-67 proliferation index as follows: G1 (<3%), G2 (3-20%), and G3 NEC (>20%) (4), so most patients were already diagnosed with NEC at presentation. The majority of cases represented large-cell neuroendocrine carcinoma(LCNECs), with only one exception being small-cell neuroendocrine carcinoma(SCNECs). By definition, a neoplasm can be qualified as mixed neuroendocrine-non-neuroendocrine neoplasms(MiNEN) when a neuroendocrine or a non-neuroendocrine component is morphologically and immunohistochemically recognizable and constitutes ≥ 30% of the tumor burden (5). Pathological analysis revealed adenocarcinoma components (7-10% of tumor) in five cases, which did not meet the diagnostic threshold for MiNEN. Uniform immunoreactivity was observed for three established neuroendocrine markers: chromogranin A (CgA), synaptophysin (Syn), and CD56, aligning with existing literature (4) (Table 2, Figure 2).
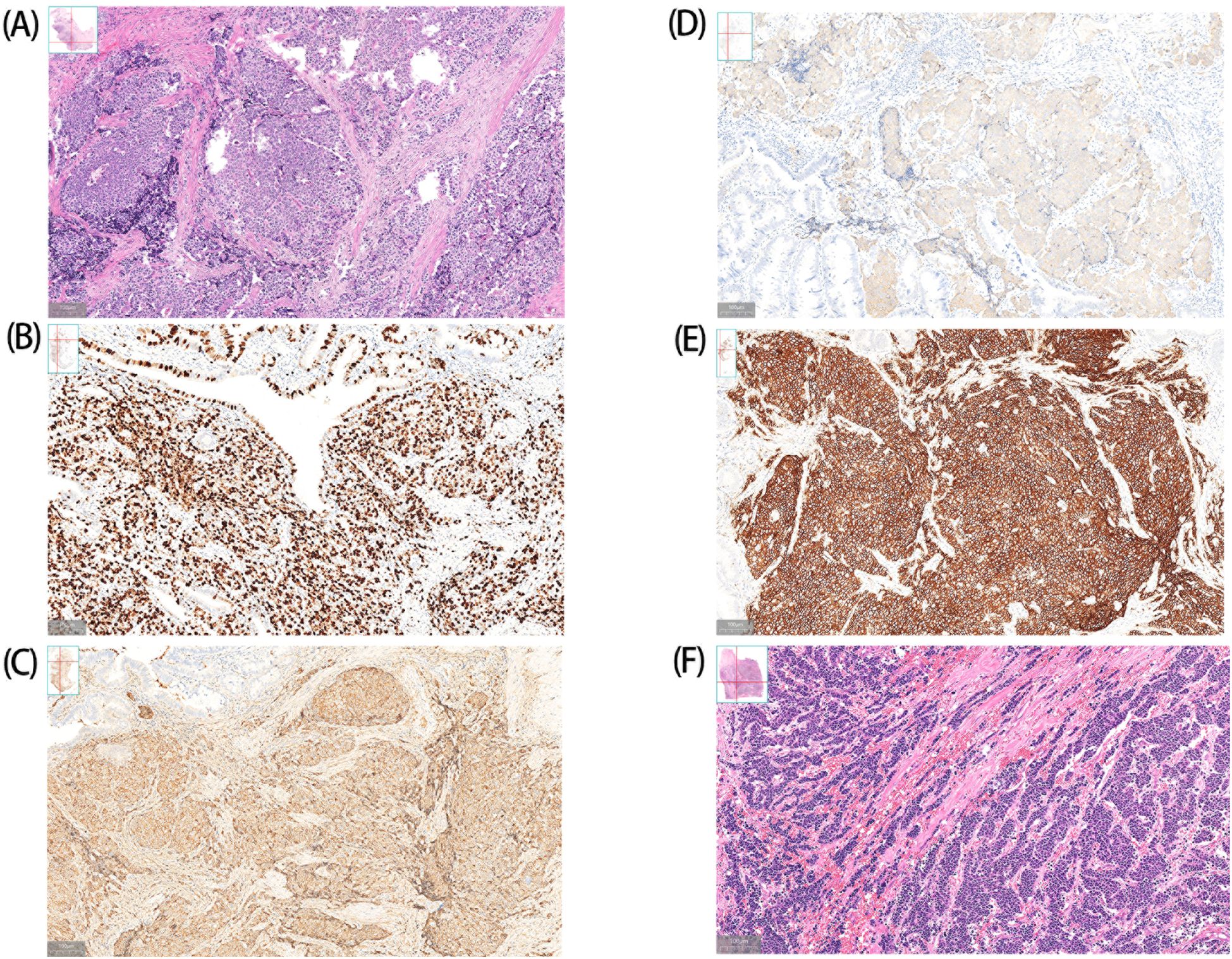
Figure 2. Histopathologic findings of GB-NENs. A (Case 6) and F (Case 1) show LCNECs and SCNECs, respectively. Case 6: Immunohistochemical analysis demonstrates positive staining for CgA (C), Syn (D) and CD56 (E), with a Ki-67 proliferation index of approximately 70% (B).
Discussion
The preoperative diagnosis of GB-NENs remains a significant clinical challenge, with definitive confirmation typically achieved only through postoperative pathological examination of cholecystectomy specimens. Due to sampling limitations, percutaneous biopsy is not recommended. An overview of the 2022 WHO Classification of Neuroendocrine Neoplasms stated that CgA, Syn, and insulinoma-associated protein 1 (INSM1) represent general neuroendocrine markers and recommended the simultaneous use of Syn and INSM1, as this combination can reliably identify the neuroendocrine component (both NET and NEC) in nearly all cases. Notably, CgA staining may be negative or only focally positive in NEC components (6). Case 6 immunohistochemistry results were consistent with the above findings, showing strong Syn positivity but weaker CgA staining. Radiological findings provide supportive but non-definitive diagnostic value. A well-defined margin and intact overlying mucosa help differentiate GB-NENs from carcinomas (7). This distinguishing pattern is similarly observed when comparing gastric NENs to adenocarcinomas (8). Suspicious radiological characteristics warranting consideration of GB-NENs include GB-replacing masses, extensive growth patterns or bulky lymph node metastases at initial presentation (7, 9). In differential diagnosis, dual-energy CT plays a pivotal role in distinguishing between benign and malignant gallbladder pathologies (10). Quantitative parameters (iodine concentration and spectral curve characteristics) demonstrate significant differences between NENs and adenocarcinomas, thereby enhancing preoperative diagnostic accuracy. For non-emergency cases, gallium-68 or FDG-labeled PET imaging offers superior diagnostic precision. While histopathology remains the diagnostic gold standard, further investigation of GB-NEN-specific pathological signatures is warranted. Novel histopathological correlates, such as well-defined margins (neuroendocrine cells reside in subepithelial lamina propria which help to preserve overlying mucosa) (7), may enhance imaging interpretation and preoperative diagnostic accuracy. This integrated diagnostic approach, combining advanced pathological characterization with refined imaging criteria, represents a promising direction for improving GB-NEN diagnosis prior to surgical intervention.
NENs are thought to arise from neuroendocrine cells distributed systemically, but since the normal gallbladder lacks enterochromaffin cells from which the neuroendocrine neoplasia originates, the cellular origin of GB-NENs remains controversial, with two predominant hypotheses currently proposed. The first suggests GB-NENs may originate from gastric/intestinal metaplasia of gallbladder mucosal epithelium induced by chronic inflammation (11, 12), while the second posits that GB-NENs could derive from transdifferentiation of gallbladder adenocarcinoma (4). Emerging research increasingly favors the transdifferentiation hypothesis: 1) Existing literature demonstrates that the majority of reported GB-NEN cases are associated with gallstone-related chronic inflammation (4), a finding that aligns with our observed incidence of 85.71%, which is also a well-established risk factor for gallbladder cancer (12, 13). Chirag et al. have further delineated the inflammatory mechanisms driving gallbladder carcinogenesis (14). Both GB-NETs and carcinomas exhibit gastric/intestinal metaplasia and CgA-positive cells (11), suggesting a potential common precursor lesion. 2) Persistent histological alterations frequently drive the acquisition of molecular changes. A recent study unveiled the mutation landscape of 15 cases of GB-NENs by using whole-exome sequencing (WES) technology and found that TP53 showed the highest mutation frequency (73%, 11/15) (15). Intriguingly, TP53 mutations are also the earliest and most prevalent genomic event in gallbladder cancer (14, 16), implicating overlapping oncogenic pathways. 3) Emerging insights into GB-MiNENs, coupled with molecular evidence demonstrating intimate associations between neuroendocrine and non-neuroendocrine components in intestinal counterparts and strongly supporting a monoclonal origin asthe most frequent genetic event (17), which lend credence to the hypothesis of shared cellular origins and subsequent lineage divergence in GB-NENs (18). Elucidating GB-NET origins could inform risk mitigation strategies and refine mechanistic understanding of tumor evolution to guide targeted therapies, though further investigation is needed to fully resolve the cellular and molecular mechanisms underlying these processes.
The low incidence of GB-NENs has limited current literature predominantly to case reports and small case series, no large-scale, multicenter prospective studies have been conducted. Consequently, prognostic predictors remain poorly characterized, and no internationally standardized management strategies exist. Given that all NENs originate from neuroendocrine cells and emerging evidence suggesting GB-NENs may share a common origin with gallbladder cancer, we propose that treatment strategies for GB-NECs may be partially informed by protocols for gallbladder cancer and NENs at other sites. Furthermore, the widespread adoption of WES has enabled the identification of novel driver mutations and pathways potentially involved in GB-NENs pathogenesis, revealing promising therapeutic targets and informing the application of existing targeted therapies:
1 Radical cholecystectomy recommended for surgically eligible GB-NENs regardless of grade or stage
There is no consensus on the optimal treatment for GB-NENs (4). Surgical resection remains the primary therapeutic approach, with options including simple cholecystectomy, radical cholecystectomy, and palliative cholecystectomy. Radical cholecystectomy is the standard surgical procedure for gallbladder cancer and is also the mainstay of treatment for GB-NENs. Shekhar et al. conducted a retrospective analysis of data from the SEER database (1973–2016) encompassing all GB-NEN patients and found that those who underwent surgery had significantly better survival outcomes (mean survival: 111.0 ± 8.3 vs. 8.3 ± 1.2 months, P < 0.01) (19). In our case series, four patients underwent radical surgery and achieved relatively favorable survival. A systematic review of surgical management for T1-stage gallbladder cancer indicated that simple cholecystectomy is sufficient for T1a lesions, with no evidence supporting superior outcomes for T1b patients receiving radical cholecystectomy (20). Nevertheless, we recommend radical resection with regional lymphadenectomy when clinically feasible, as GB-NENs demonstrate markedly higher aggressiveness and poorer prognosis compared to conventional gallbladder cancers. A retrospective study have revealed that lymph node metastasis (N2) occurs significantly more frequently in GB-NEC than in gallbladder cancer (70.0% vs. 34.0%; P < 0.05) (2). Furthermore, EMS of GB-NEC identified ZFHX3 as the second most frequently mutated gene (15). Given that ZFHX3 mutations in endometrial tumors are associated with higher tumor grade and increased lymphovascular space invasion (21), it raises the question of whether such mutations also contribute to elevated lymphatic metastasis risk in GB-NENs.
2 Platinum-based first-line chemotherapy optional, with adjuvant therapies for potential prognostic improvement
2.1 Role of chemotherapy
Current evidence regarding adjuvant chemotherapy for GB-NENs remains inconclusive. Some studies report improved median overall survival (OS) and DFS with postoperative chemotherapy (11, 22). For instance, Case 6 in our series, initially presenting with N1 lymph node metastasis, achieved 40 months of survival following multimodal therapy, including EP, IP with anlotinib, and pembrolizumab-anlotinib regimens, surpassing the reported median survival of 23.2 months (19). However, a decade-long multicenter study by Wang et al. found no statistically significant prognostic benefit from adjuvant chemotherapy (4). Notably, Case 5 in our cohort remained recurrence-free for 55 months after radical cholecystectomy without chemotherapy. We posit that chemotherapeutic responsiveness typically manifests early; discontinuation should be considered for non-responders to avoid severe adverse events, such as hepatorenal toxicity or leukopenia (3, 23).
2 Chemotherapy regimens
The rarity of GB-NENs precludes large-scale prospective or retrospective studies, leaving no standardized chemotherapy protocol. Platinum-etoposide combinations, extrapolated from small-cell lung cancer treatment paradigms and supported by small retrospective studies demonstrating chemosensitivity in NENs (objective response rate: 40-70%) (23–25), have become the consensus first-line regimen. Updated European Neuroendocrine Tumor Society Guidelines(ENETS) guidelines recommend carboplatin or irinotecan as potential substitutes for cisplatin and etoposide, respectively (26). A retrospective analysis by Hiroo et al. indicate comparable progression-free survival (PFS) (95% CI: 3.1–7.0 vs. 3.5–6.3, P = 0.781) and OS (95% CI: 11.2–14.6 vs. 8.9–17.4, P = 0.593) between EP and carboplatin-etoposide regimens (27), though an international survey by Lamarca et al. revealed significant intercenter heterogeneity in platinum-etoposide protocols, including variations in dosing, administration routes, and treatment cycles (28). Alternative gemcitabine-based regimens, typically used for biliary tract cancers, may also be considered. In our Case 4, where the tumor involved the gallbladder neck and common bile duct, the patient achieved 1-year PFS following pancreaticoduodenectomy and six cycles of GEMOX without additional adjuvant therapy. Notably, GB-NENs may exhibit heightened chemoresistance potential. RB1 mutations, frequent in GB-NECs but absent in carcinomas, correlate with chemoresistance (15). We recommend platinum-based regimens as first-line, with gemcitabine alternatives for non-responders or intolerant patients, while monitoring for resistance.
2.3 Other adjuvant therapies
A single-arm, open-label phase II trial conducted by Chen et al. demonstrated a 30% increase in objective response rate with GEMOX plus camrelizumab in biliary tract cancer,while prolonging both OS and PFS (29), prompting inquiry into immunotherapy combinations for GB-NENs. Peptide receptor radionuclide therapy (PRRT), well-established for G1/G2 gastroenteropancreatic NENs, now shows efficacy in somatostatin receptor imaging (SRI)-positive G3 NENs (30), suggesting potential applicability to GB-NECs. Genomic characterization of GB-NECs has revealed clinically actionable alterations. EMS has identified ALK mutations (TKI-sensitive) and other targets (MYC, ZFHX3, Wnt pathway) (15). While immune-targeted therapies currently serve as late-line options with limited efficacy, their integration with chemotherapy may enhance outcomes, particularly in consolidating surgical results and advancing therapeutic discovery.
NENs show different characteristics depending on their location. While pancreatic and appendiceal NETs are often well-differentiated (G1/G2), GB-NENs are typically diagnosed as NET G3/NEC (4, 31). Analysis of median OS data from the SEER database by Arvind et al. demonstrated significant survival disparities: patients with rectal (24.6 years), appendiceal (>30 years), lung (5.5 years) and pancreatic (3.6 years) NETs live significantly longer than those with GB-NENs (23.2 months) (19, 32). This difference may reflect the distinct age distribution patterns between NETs at different anatomical sites, with GB-NETs demonstrating significantly later onset (median age at diagnosis: 63 years) compared to appendiceal NETs (median age: 34 years) (4, 31) or NETs at other anatomical sites (33). Notably, although gallbladder cancer and GB-NENs share the same organ origin and may arise from common precursor lesions, their divergent biological evolution results in significantly different outcomes. GB-NEN patients exhibit substantially worse survival compared (3-year overall survival rate: 31.1% vs 63.8%, P<0.01) (34). These differences likely reflect that the differentiation status and pathological characteristics of NENs may reflect distinct tumor origins and molecular mechanisms of pathogenesis, which critically influence disease prognosis. Consequently, while treatment strategies for non-gallbladder NENs or gallbladder cancer may provide valuable references, therapeutic approaches for GB-NENs require specific modifications and innovations based on clinical response patterns. This adaptive approach will facilitate the development of novel, more effective treatment strategies tailored to the unique biology of GB-NENs. Moreover, emerging evidence supports circulating cytokines as dynamic biomarkers for assessing treatment response in NEN patients, facilitating adaptive therapeutic interventions. Concurrently, systemic inflammation markers (neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, PD-1/PD-L1) show prognostic utility, advancing precision management strategies for GB-NENs (35).
3 Conclusion
Our case series examines GB-NENs patients receiving different treatments, sharing our center’s clinical experience while comparing management strategies with gallbladder cancer and NENs from other sites. Certainly, this study still has several important limitations: the extended timeframe introduces variability due to evolving diagnostic and surgical techniques, and as a single-center retrospective analysis with small sample size, our findings require validation through future multicenter prospective studies. These results should be viewed as preliminary experience rather than definitive evidence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Shaoxing People’s Hospital Academic Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
JZ: Conceptualization, Data curation, Investigation, Software, Writing – original draft. JL: Conceptualization, Investigation, Software, Writing – original draft. YD: Investigation, Writing – original draft. BL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Medical Science and Technology Project of Zhejiang Province under grant no.2025KY1663.
Acknowledgments
We would like to thank all of our colleagues in the medical and nursing team of the hepatobiliary and pancreatic surgery.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1606850/full#supplementary-material
Abbreviations
GB-NENs, Gallbladder neuroendocrine neoplasms; NET, neuroendocrine tumors; SEER, Surveillance, Epidemiology, and End Results; EP, etoposide-cisplatin; IP, irinotecan-cisplatin; GMOX, gemcitabine-oxaliplatin; DFS, disease-free survival; NEC, neuroendocrine carcinoma; WHO, World Health Organization; CgA, chromogranin A; Syn, synaptophysin; WES, whole-exome sequencing; GB-MiNENs, gallbladder mixed neuroendocrine-non-neuroendocrine neoplasms; OS, overall survival; ENETS, European Neuroendocrine Tumor Society Guidelines; PFS, progression-free survival; PRRT, Peptide receptor radionuclide therapy.
References
1. Cen D, Liu H, Wan Z, Lin Z, Wang Y, Xu J, et al. Clinicopathological features and survival for gallbladder NEN: a population-based study. Endocr Connect. (2019) 8:1273–81. doi: 10.1530/EC-19-0124
2. Chen C, Wang L, Liu X, Zhang G, Zhao Y, and Geng Z. Gallbladder neuroendocrine carcinoma: report of 10 cases and comparision of clinicopathologic features with gallbladder adenocarcinoma. Int J Clin Exp Pathol. (2015) 8:8218–26.
3. Imai H, Saijo K, Kawamura Y, Kodera S, Komine K, Iwasaki T, et al. Comparison of Efficacy and Safety between Carboplatin-Etoposide and Cisplatin-Etoposide Combination Therapy in Patients with Advanced Neuroendocrine Carcinoma: A Retrospective Study. Oncology. (2024) 102:359–67. doi: 10.1159/000534747
4. Wang Y, Huang B, Fu Q, Wang J, Ye M, Hu M, et al. Comprehensive Clinical Analysis of Gallbladder Neuroendocrine Neoplasms: A Large-Volume Multicenter Study During One Decade. Ann Surg Oncol. (2022) 29:7619–30. doi: 10.1245/s10434-022-12107-w
5. Sciarra A, Missiaglia E, Trimech M, Melloul E, Brouland JP, Sempoux C, et al. Gallbladder Mixed Neuroendocrine-Non-neuroendocrine Neoplasm (MiNEN) Arising in Intracholecystic Papillary Neoplasm: Clinicopathologic and Molecular Analysis of a Case and Review of the Literature. Endocr Pathol. (2020) 31:84–93. doi: 10.1007/s12022-020-09605-6
6. Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. (2022) 33:115–54. doi: 10.1007/s12022-022-09708-2
7. Bae JS, Kim SH, Yoo J, Kim H, and Han JK. Differential and prognostic MRI features of gallbladder neuroendocrine tumors and adenocarcinomas. Eur Radiol. (2020) 30:2890–901. doi: 10.1007/s00330-019-06588-9
8. Kim SH, Kim SH, Kim MA, Shin CI, Han JK, and Choi BI. CT differentiation of poorly-differentiated gastric neuroendocrine tumours from well-differentiated neuroendocrine tumours and gastric adenocarcinomas. Eur Radiol. (2015) 25:1946–57. doi: 10.1007/s00330-015-3600-z
9. Kim TH, Kim SH, Lee KB, and Han JK. Outcome and CT differentiation of gallbladder neuroendocrine tumours from adenocarcinomas. Eur Radiol. (2017) 27:507–17. doi: 10.1007/s00330-016-4394-3
10. Ratanaprasatporn L, Uyeda JW, Wortman JR, Richardson I, and Sodickson AD. Multimodality Imaging, including Dual-Energy CT, in the Evaluation of Gallbladder Disease. Radiographics. (2018) 38:75–89. doi: 10.1148/rg.2018170076
11. Eltawil KM, Gustafsson BI, Kidd M, and Modlin IM. Neuroendocrine tumors of the gallbladder: an evaluation and reassessment of management strategy. J Clin Gastroenterol. (2010) 44:687–95. doi: 10.1097/MCG.0b013e3181d7a6d4
12. Zanchetta M, Adani GL, Micheletti G, Poto GE, Piccioni SA, Carbone L, et al. Perforated Calculous Cholecystitis and Incidental Squamous Cell Carcinoma of the Gallbladder-A Complex Relationship with a Difficult Management in the Acute Setting. Med (Kaunas). (2025) 61:452. doi: 10.3390/medicina61030452
13. Roa JC, García P, Kapoor VK, Maithel SK, Javle M, and Koshiol J. Gallbladder cancer. Nat Rev Dis Primers. (2022) 8:69. doi: 10.1038/s41572-022-00398-y
14. Nepal C, Zhu B, O’Rourke CJ, Bhatt DK, Lee D, Song L, et al. Integrative molecular characterisation of gallbladder cancer reveals micro-environment-associated subtypes. J Hepatol. (2021) 74:1132–44. doi: 10.1016/j.jhep.2020.11.033
15. Liu F, Li Y, Ying D, Qiu S, He Y, Li M, et al. Whole-exome mutational landscape of neuroendocrine carcinomas of the gallbladder. Signal Transduct Target Ther. (2021) 6:55. doi: 10.1038/s41392-020-00412-3
16. Moreno M, Pimentel F, Gazdar AF, Wistuba II, and Miquel JF. TP53 abnormalities are frequent and early events in the sequential pathogenesis of gallbladder cancer. Ann Hepatol. (2005) 4:192–9. doi: 10.1016/S1665-2681(19)32065-4
17. Furlan D, Cerutti R, Genasetti A, Pelosi G, Uccella S, La Rosa S, et al. Microallelotyping defines the monoclonal or the polyclonal origin of mixed and collision endocrine-exocrine tumors of the gut. Lab Invest. (2003) 83:963–71. doi: 10.1097/01.lab.0000079006.91414.be
18. de Bitter TJJ, Kroeze LI, de Reuver PR, van Vliet S, Vink-Börger E, von Rhein D, et al. Unraveling Neuroendocrine Gallbladder Cancer: Comprehensive Clinicopathologic and Molecular Characterization. JCO Precis Oncol. (2021) 5:PO.20.00487. doi: 10.1200/PO.20.00487
19. Gogna S, Samson D, Gachabayov M, Rojas A, Felsenreich DM, Koo D, et al. Neuroendocrine neoplasms of the gallbladder: early detection and surgery is key to improved outcome. Langenbecks Arch Surg. (2022) 407:197–206. doi: 10.1007/s00423-021-02256-z
20. Lee SE, Jang JY, Lim CS, Kang MJ, and Kim SW. Systematic review on the surgical treatment for T1 gallbladder cancer. World J Gastroenterol. (2011) 17:174–80. doi: 10.3748/wjg.v17.i2.174
21. Walker CJ, Miranda MA, O’Hern MJ, McElroy JP, Coombes KR, Bundschuh R, et al. Patterns of CTCF and ZFHX3 Mutation and Associated Outcomes in Endometrial Cancer. J Natl Cancer Inst. (2015) 107:djv249. doi: 10.1093/jnci/djv249
22. Adachi T, Haraguchi M, Irie J, Yoshimoto T, Uehara R, Ito S, et al. Gallbladder small cell carcinoma: a case report and literature review. Surg Case Rep. (2016) 2:71. doi: 10.1186/s40792-016-0200-3
23. Mitry E and Rougier P. The treatment of undifferentiated neuroendocrine tumors. Crit Rev Oncol Hematol. (2001) 37:47–51. doi: 10.1016/s1040-8428(00)00073-1
24. Fjällskog ML, Granberg DP, Welin SL, Eriksson C, Oberg KE, Janson ET, et al. Treatment with cisplatin and etoposide in patients with neuroendocrine tumors. Cancer. (2001) 92:1101–7. doi: 10.1002/1097-0142(20010901)92:5<1101::aid-cncr1426>3.0.co;2-v
25. Mitry E, Baudin E, Ducreux M, Sabourin JC, Rufié P, Aparicio T, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. (1999) 81:1351–5. doi: 10.1038/sj.bjc.6690325
26. Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology. (2016) 103:186–94. doi: 10.1159/000443172
27. Imai H, Saijo K, Kawamura Y, Kodera S, Komine K, Iwasaki T, et al. Comparison of Efficacy and Safety between Carboplatin-Etoposide and Cisplatin-Etoposide Combination Therapy in Patients with Advanced Neuroendocrine Carcinoma: A Retrospective Study. Oncology. (2024) 102:359–67. doi: 10.1159/000534747
28. Lamarca A, Frizziero M, Barriuso J, McNamara MG, Hubner RA, Valle JW, et al. Urgent need for consensus: international survey of clinical practice exploring use of platinum-etoposide chemotherapy for advanced extra-pulmonary high grade neuroendocrine carcinoma (EP-G3-NEC). Clin Transl Oncol. (2019) 21(7):950–3. doi: 10.1007/s12094-018-1996-z
29. Chen X, Wu X, Wu H, Gu Y, Shao Y, Shao Q, et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial. J Immunother Cancer. (2020) 8:e001240. doi: 10.1136/jitc-2020-001240
30. Sorbye H, Kong G, and Grozinsky-Glasberg S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr Relat Cancer. (2020) 27:R67–77. doi: 10.1530/ERC-19-0400
31. Holmager P, Willemoe GL, Nielsen K, Grøndahl V, Klose M, Andreassen M, et al. Neuroendocrine neoplasms of the appendix: Characterization of 335 patients referred to the Copenhagen NET Center of Excellence. Eur J Surg Oncol. (2021) 47:1357–63. doi: 10.1016/j.ejso.2021.02.005
32. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. (2017) 3:1335–42. doi: 10.1001/jamaoncol.2017.0589
33. Nuñez-Valdovinos B, Carmona-Bayonas A, Jimenez-Fonseca P, Fonseca P, Capdevila J, Castaño-Pascual Á, et al. Neuroendocrine Tumor Heterogeneity Adds Uncertainty to the World Health Organization 2010 Classification: Real-World Data from the Spanish Tumor Registry (R-GETNE). Oncologist. (2018) 23:422–32. doi: 10.1634/theoncologist.2017-0364
34. Yan S, Wang Y, Chen X, Zhang Y, Huang Z, Zhao J, et al. Clinical Analysis of 15 Cases of Gallbladder Neuroendocrine Carcinoma and Comparison with Gallbladder Adenocarcinoma Using a Propensity Score Matching. Cancer Manag Res. (2020) 12:1437–46. doi: 10.2147/CMAR.S227501
Keywords: neuroendocrine neoplasms, neuroendocrine carcinoma, gallbladder, surgery, adjuvant therapy
Citation: Zhang J, Li J, Dong Y and Lu B (2025) Case Report: Diagnosing and treating gallbladder neuroendocrine neoplasms through comparative analysis: a case series and literature review. Front. Oncol. 15:1606850. doi: 10.3389/fonc.2025.1606850
Received: 06 April 2025; Accepted: 26 May 2025;
Published: 16 June 2025.
Edited by:
Jiang Chen, Zhejiang University, ChinaReviewed by:
Natale Calomino, University of Siena, ItalyAnna La Salvia, National Institute of Health (ISS), Italy
Copyright © 2025 Zhang, Li, Dong and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baochun Lu, c3hsdWJhb2NodW5AMTYzLmNvbQ==
 Jiayao Zhang
Jiayao Zhang Jiandong Li1
Jiandong Li1