- 1Department of Neurosurgery, The Jikei University School of Medicine, Daisan Hospital, Tokyo, Japan
- 2Department of Neurosurgery, The Jikei University School of Medicine, Tokyo, Japan
Objective: To develop and validate a simple 0–7 point prognostic score for patients with synchronous brain metastases (s-BM) at initial consultation and to provide an exploratory comparison with metachronous BM (m-BM).
Methods: We retrospectively analyzed 297 patients with BM (2014–2022): s-BM n=64 and m-BM n=233. The score uses five pre-treatment factors available at time-zero: age <70 y (1 point), KPS ≥70 (2 points), absence of extracranial metastases (ECM) (2 points), presence of a lung mass on imaging (1 point), and absence of a digestive-tract mass (1 point). Patients were stratified as Score A (0), B (1–4), and C (5–7). Discrimination and parsimony were assessed by Harrell’s C-index and AIC; proportional-hazards (PH) assumptions by global Schoenfeld test; time-dependent AUCs at 6/12 months were calculated; bootstrap (B = 500) provided optimism-corrected C-indices. (Synchronous was defined as ≤30 days between first ascertainment of primary tumor and BM; m-BM >30 days).
Results: Median OS appeared similar for s-BM and m-BM in this cohort (5.2 vs 6.4 months; exploratory). In s-BM, KPS ≥70 and absence of ECM were independent predictors of longer survival. The proposed score produced stepwise separation across A/B/C. It showed the highest C-index (0.690) and lowest AIC (375.57) versus RPA, GPA, SIR, and BSBM, with no PH violations (global p=0.983). AUCs were 0.779 (6 mo) and 0.795 (12 mo); bootstrap-corrected C-index ≈0.691. A coefficient-weighted variant yielded a similar C-index (0.694) but a higher AIC (381.9).
Conclusions: A five-factor, 1–2 point score usable at initial consultation provides superior discrimination and parsimony in s-BM compared with established systems. External, multicenter validation is warranted.
Introduction
Brain metastases (BM) are considered to be the “terminal stage” of cancers due to their dismal prognosis. Multimodal therapeutics including surgical resection, chemoradiation therapy, and immunotherapy have contributed to extending median overall survival (mOS) and improving the quality of life for patients with cancer involving BM.
Considering the timing of initial diagnoses of BM along with primary lesions, BM could develop after the progression of the primary lesion [defined as metachronous BM (m-BM)] or BM develop simultaneously with or before treatment for the primary lesions [defined as synchronous BM (s-BM)]. So far, several prognostic parameters, including recursive partitioning analysis (RPA), graded prognostic assessment (GPA), score index for radiosurgery (SIR), and basic score for brain metastases (BSBM), have been utilized for validation for focal radiotherapy such as stereotactic radiosurgery (SRS) (1–4). In the era of combined immunotherapy of immune checkpoint inhibitors (ICIs), molecular target agents, and radiation therapy, aggressive treatment for BM, even under the co-existence of primary lesion and BM, might provide clinical benefit. Given that the genetic profile for driver mutations of cancer-related oncogenes and immune checkpoint molecules of PD-L1 with tumor mutation burden and microsatellite instability might stratify clinical outcomes in patients with the same primary cancers, we need a predictive prognostic biomarker for reflecting the clinical prognosis of patients with BM.
The purpose of this study was to explore several parameters extracted by multivariate analysis in a retrospective cohort of patients with s-BM and m-BM, to establish a novel predictive score based on these parameters for validation of multimodal treatment including molecular targeted drugs and ICIs. This novel scoring system might provide accurate prognostic prediction judged from the initial parameters to facilitate medical collaboration with other departments and to determine an appropriate sequential therapeutic schedule. We present a descriptive, exploratory comparison of s-BM and m-BM to contextualize our cohort, recognizing that the study is not designed or powered to test equivalence. Regardless of apparent similarity in survival, clinical decision-making at initial consultation differs fundamentally in s-BM; therefore, our primary objective was to develop an s-BM–specific prognostic score based on pre-treatment information.
Materials and methods
Study design and patients
We conducted a single-center retrospective cohort study of consecutive patients with brain metastases (BM) treated at The Jikei University School of Medicine Daisan Hospital between January 2014 and December 2022. Baseline demographic and clinical variables were recorded and are reported in the Results (Table 1); no descriptive counts are repeated in Methods.
Definitions
“Synchronous” BM (s-BM) was defined as an absolute interval ≤30 days between the first ascertainment of the primary tumor and the first ascertainment of BM, regardless of order; “metachronous” (m-BM) as >30 days. Ascertainment dates were the earliest imaging or histopathology confirmations. (We did not use “after start of primary therapy” as a criterion to avoid conflict with the time-window definition).
Pretreatment variables and imaging surrogates
Five time-zero variables were prespecified: age, Karnofsky Performance Status (KPS), extracranial metastases (ECM), lung mass, and digestive-tract mass. Clinically interpretable cutoffs were set a priori (age <70 y; KPS ≥70). Because the score is intended for use at initial consultation, when histology may be unavailable, we used imaging-based surrogates for primary site when necessary: “lung mass” (discrete intrathoracic mass on chest radiograph/CT judged by the treating team to represent a presumptive lung primary) and “digestive mass” (discrete mass in the esophagus, stomach, intestine, pancreas, or hepatobiliary system on CT/US). These definitions used the earliest imaging before brain-directed therapy and were not retroactively altered by later pathology to avoid look-ahead/incorporation bias.
Score construction
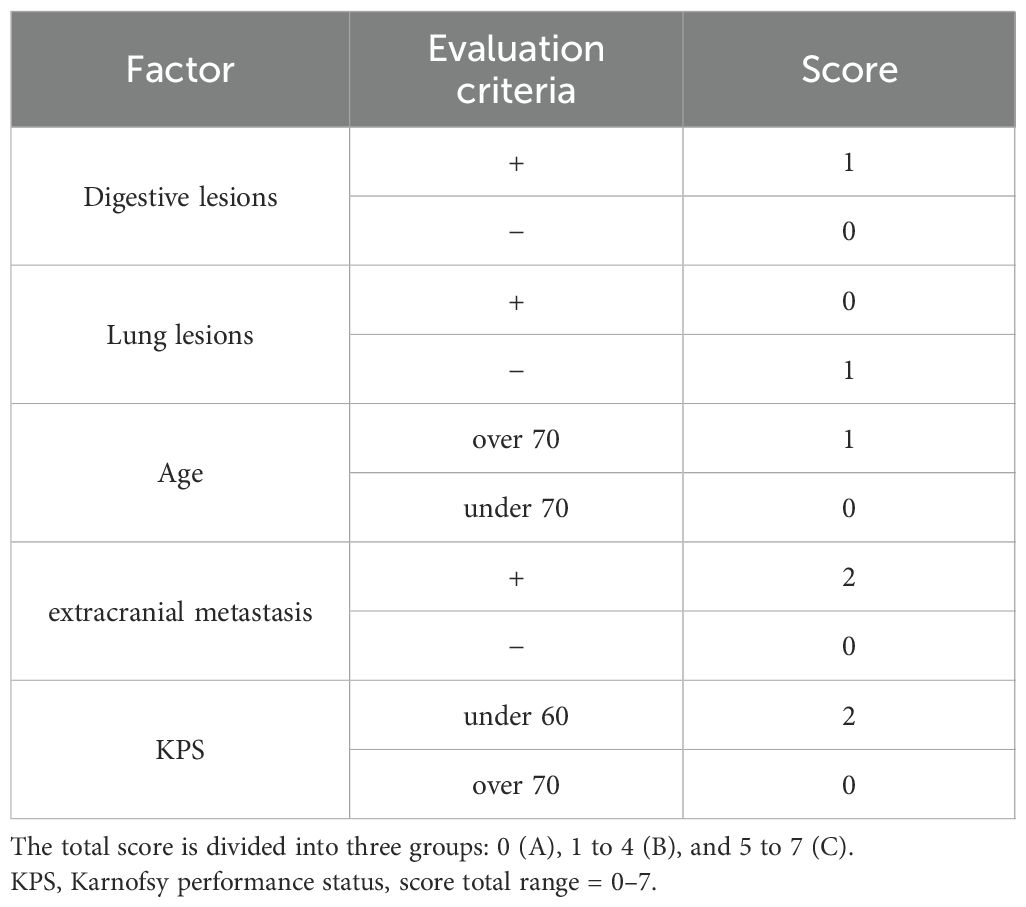
Variables significant in univariable Cox received 1 point; those retaining significance in a multivariable Cox model received 2 points, yielding a 0–7 total. As sensitivity analyses, we (i) derived a coefficient-weighted score from the multivariable β estimates and (ii) fitted a reduced score including only multivariable-significant factors; both were compared with the simple scheme using C-index and AIC (Supplement).
Comparator systems
For external benchmarks, we implemented the original descriptions of RPA (1), GPA (2), SIR (3), and BSBM (4), using their published category definitions (canonical sources cited).
Outcome
The primary endpoint was overall survival (OS) from initial consultation to death; patients alive at last contact were censored.
Statistical analysis
Kaplan–Meier curves were compared with two-sided log-rank tests. Prognostic effects were estimated using Cox proportional-hazards models. Proportional-hazards (PH) assumptions were assessed by the Grambsch–Therneau test on Schoenfeld residuals (cox.zph) for global and variable-specific tests; diagnostic plots were inspected. To compare systems beyond hypothesis testing, we reported Harrell’s C-index (discrimination), Akaike information criterion (AIC) (parsimony), and time-dependent AUCs at 6 and 12 months (timeROC); for categorical systems (RPA, GPA, SIR, BSBM), discrimination was evaluated via the Cox linear predictor. Bootstrap internal validation (B = 500 resamples) provided optimism-corrected C-indices. Missing data were handled by complete-case analysis per model. All tests were two-sided with α = 0.05. Analyses were performed in R and EZR (based on R) (5).
Results
Comparison of mOS between s-BM and m-BM
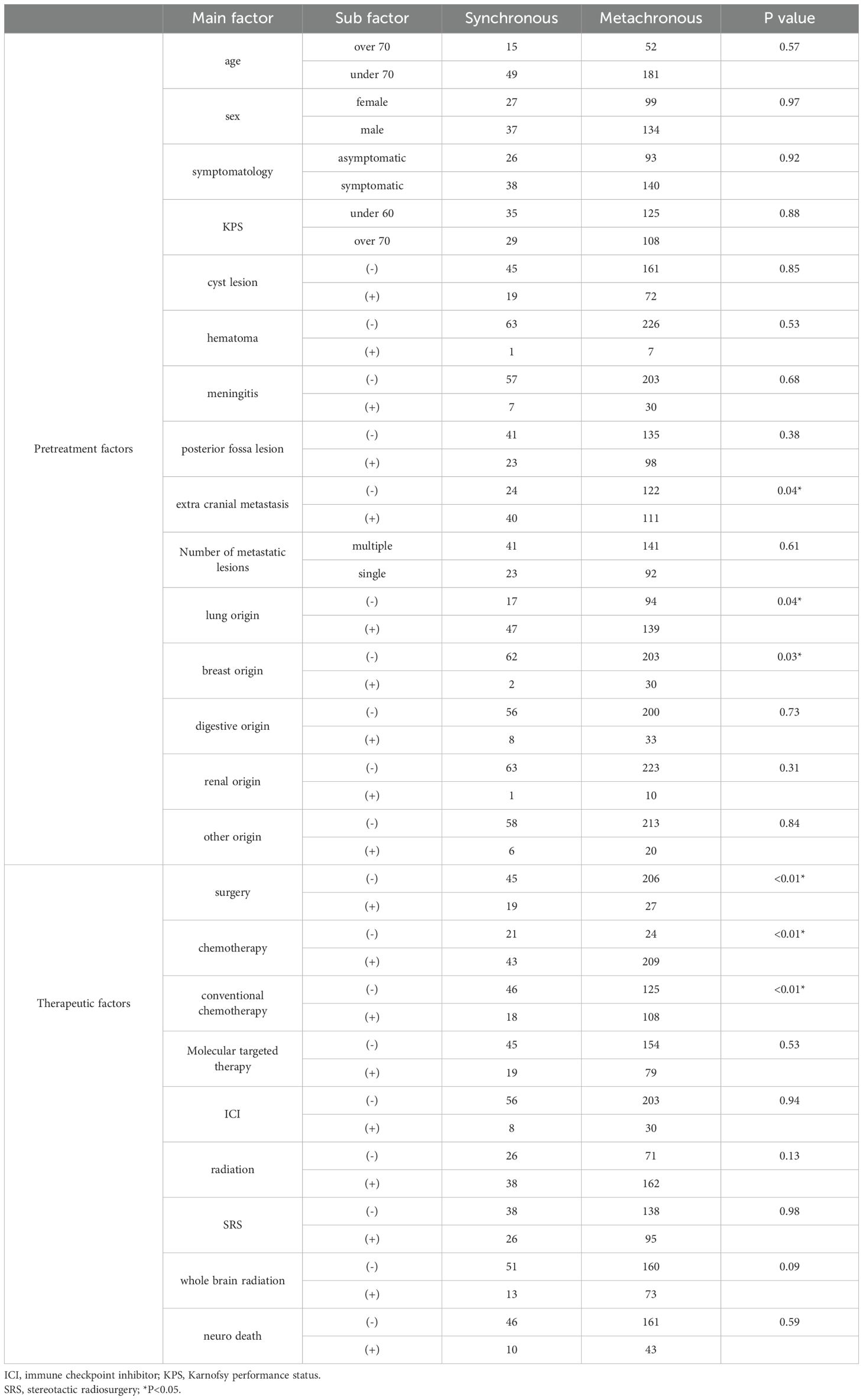
Median overall survival (mOS) was 5.2 months (95% CI, 4.1–7.3) for s-BM and 6.4 months (95% CI, 3.5–8.0) for m-BM. We did not detect a statistically significant difference between groups (Figure 1); this two-group comparison is exploratory and not powered for equivalence/non-inferiority. Our primary analyses focus on developing and validating the s-BM prognostic score. As pretreatment background, the s-BM group had more extracranial metastasis, more primary lung cancer, and fewer breast cancer cases, and in terms of treatment, surgery was more frequent and chemotherapy—especially conventional regimens—was less frequently administered in s-BM. Cohort characteristics.
We analyzed 297 patients overall (s-BM n=64; m-BM n=233). Key baseline features are summarized in Table 1, including age, sex, primary tumor sites, KPS, extracranial metastases, lesion characteristics, and initial treatments. Values are presented as median (IQR) or n (%) (Table 1).
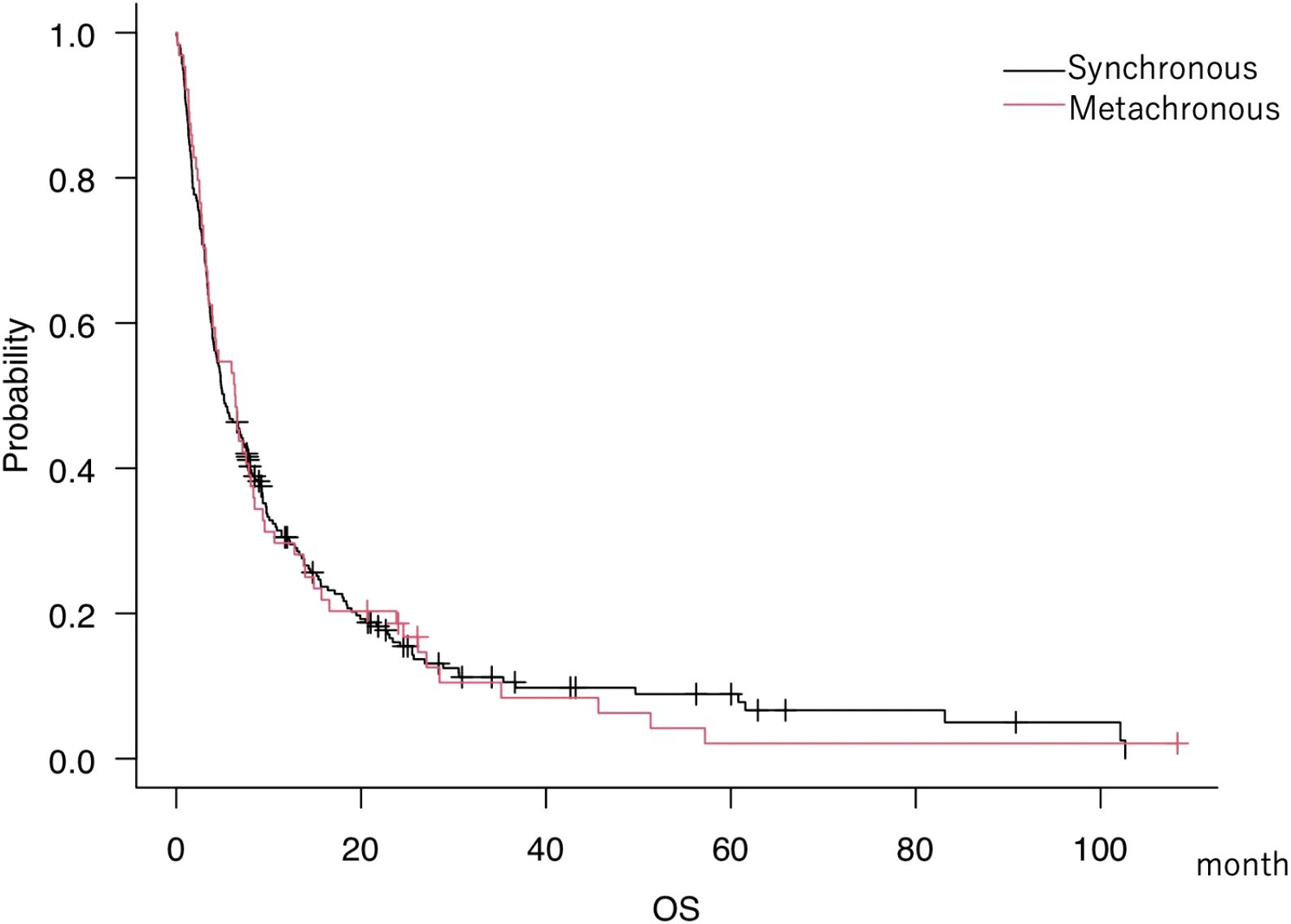
Figure 1. Kaplan–Meier overall survival (OS) curves for synchronous brain metastases (s-BM; n=64) and metachronous BM (m-BM; n=233) at initial consultation. OS was measured from initial consultation to death; tick marks indicate censoring. Medians with 95% CIs are shown (s-BM 5.2 months [4.1–7.3]; m-BM 6.4 months [3.5–8.0]). This two-group comparison is exploratory and not powered for equivalence/non-inferiority. Differences were assessed with a two-sided log-rank test; an unadjusted hazard ratio (Cox model) is reported in the Supplement for context. Abbreviations: OS, overall survival; s-BM, synchronous brain metastases; m-BM, metachronous brain metastases.
Pretreatment factors associated with survival in s-BM
In s-BM, univariable analyses identified age <70 years, KPS ≥70, absence of extracranial metastasis (ECM), presence of primary lung cancer, and absence of primary digestive cancer as factors associated with prolonged mOS (Table 2). In multivariable analysis including these five factors, KPS ≥70 and absence of ECM remained independently associated with longer mOS (Table 3).
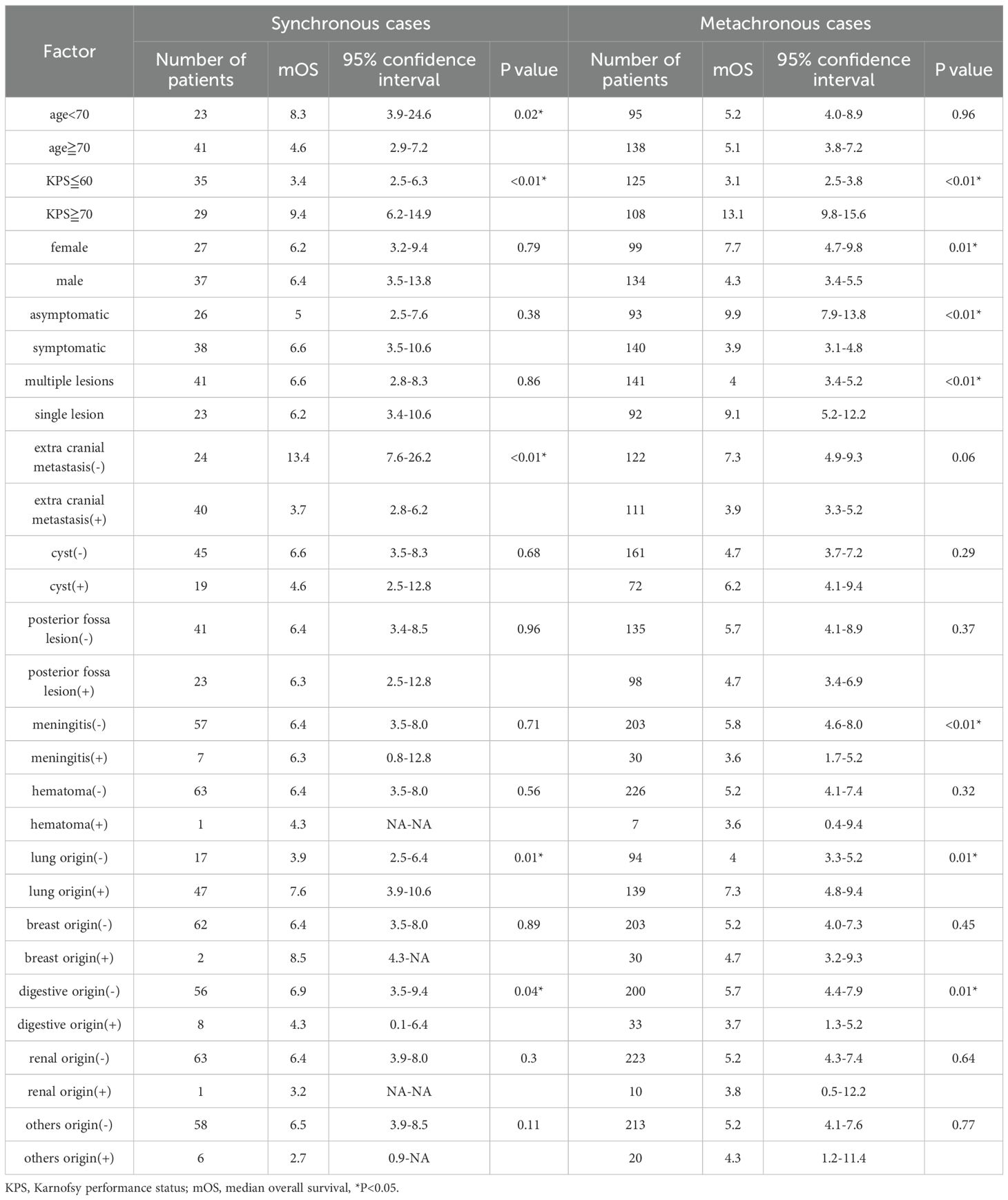
Table 2. Univariate analysis of mean survival time in patients where synchronous and metachronous brain metastasi.
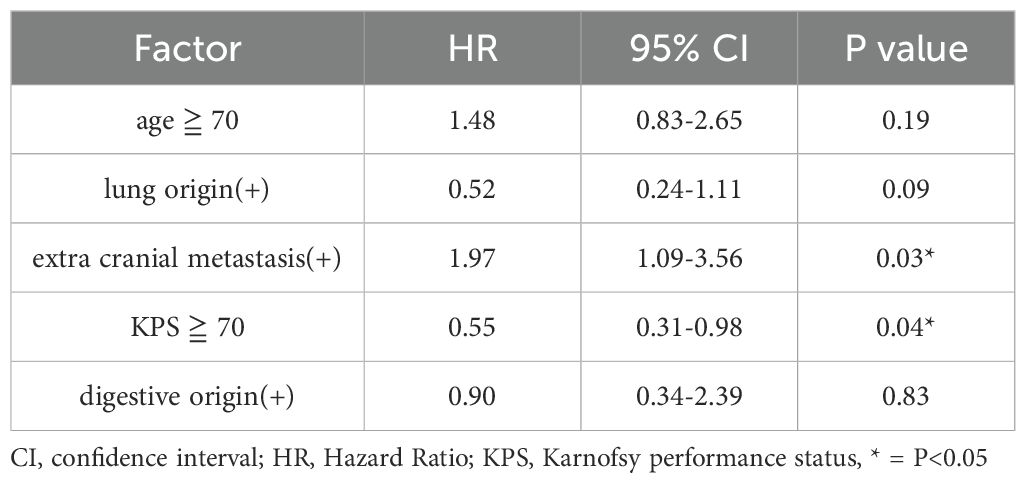
Table 3. Multivariate analysis of median survival times for patients with synchronous and metachronous brain metastases.
Verification of the proposed prognostic score in s-BM
Based on the above findings, we constructed a five-item score (age <70, KPS ≥70, no ECM, lung mass present, no digestive mass). Patients were stratified into three categories: Score A (0 points; n=6), Score B (1–4 points; n=31), and Score C (5–7 points; n=27) (Table 4).
mOS for Score A, Score B, and Score C was 57.2 months (95% CI, 8.3–NA), 7.6 months (95% CI, 4.2–12.8), and 3.2 months (95% CI, 2.1–4.6), respectively (Figure 2A), showing a clear stepwise gradient across categories.
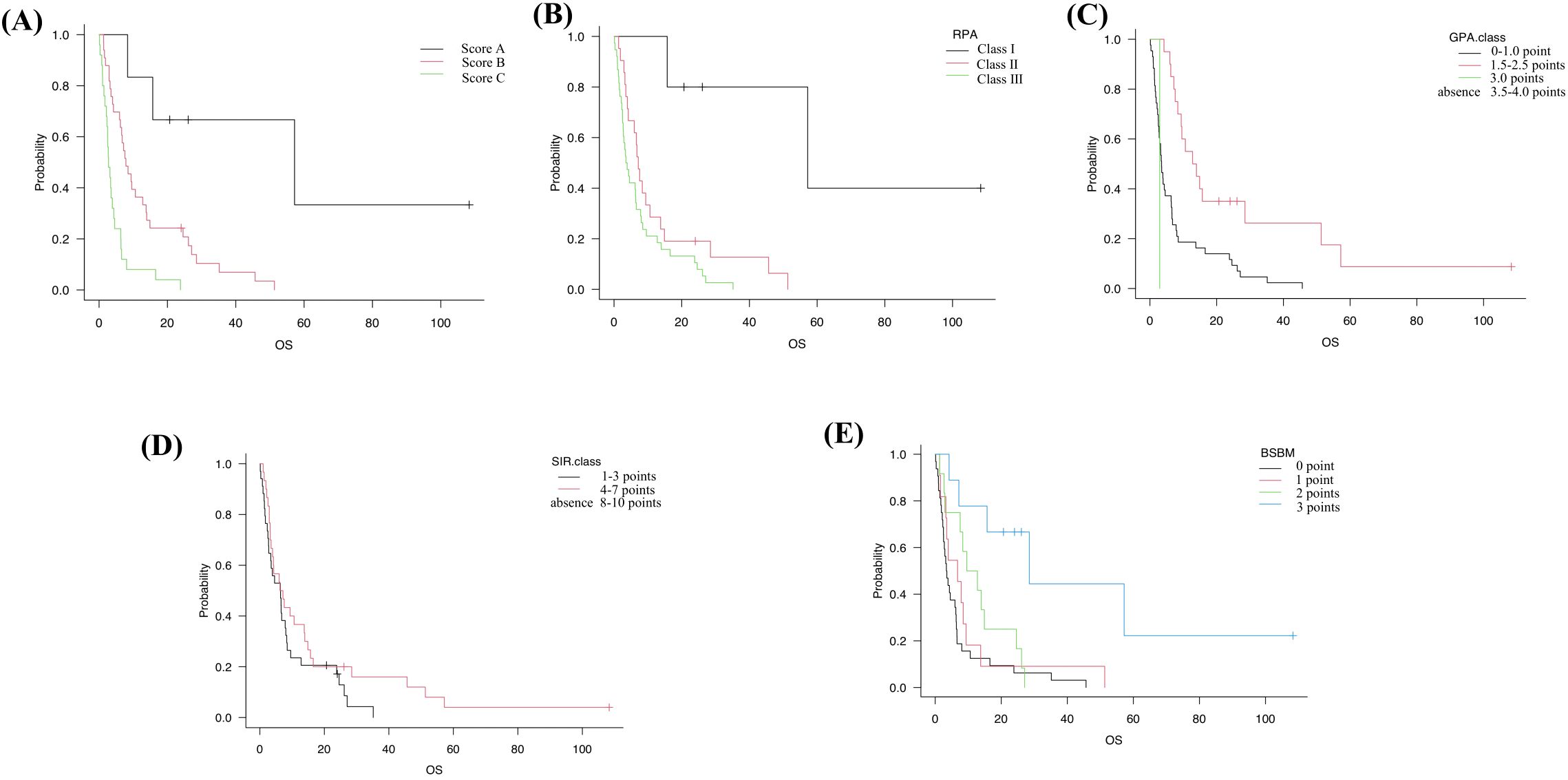
Figure 2. Kaplan–Meier OS in the s-BM cohort stratified by five prognostic systems. (A) Our score: Score A (0 points; n=6), Score B (1–4 points; n=31), Score C (5–7 points; n=27). (B) RPA (Recursive Partitioning Analysis): Class I (n=5), Class II (n=21), Class III (n=38). (C) GPA (Graded Prognostic Assessment): 0–1.0 (n=43), 1.5–2.5 (n=20), 3.0 (n=1). (D) SIR (Score Index for Radiosurgery): 1–3 (n=34), 4–7 (n=30). (E) BSBM (Basic Score for Brain Metastases): 0 (n=32), 1 (n=11), 2 (n=12), 3 (n=9). Tick marks indicate censoring; medians (with NE where appropriate) are reported in the text. Group differences were evaluated with two-sided log-rank tests across strata (trend interpretation for ordered categories); no multiplicity adjustment was applied, and pairwise p-values are not emphasized. These panels are provided for a clinical context; model-based performance comparisons are summarized in Supplementary Tables S1, S2.
Comparison with established grading systems
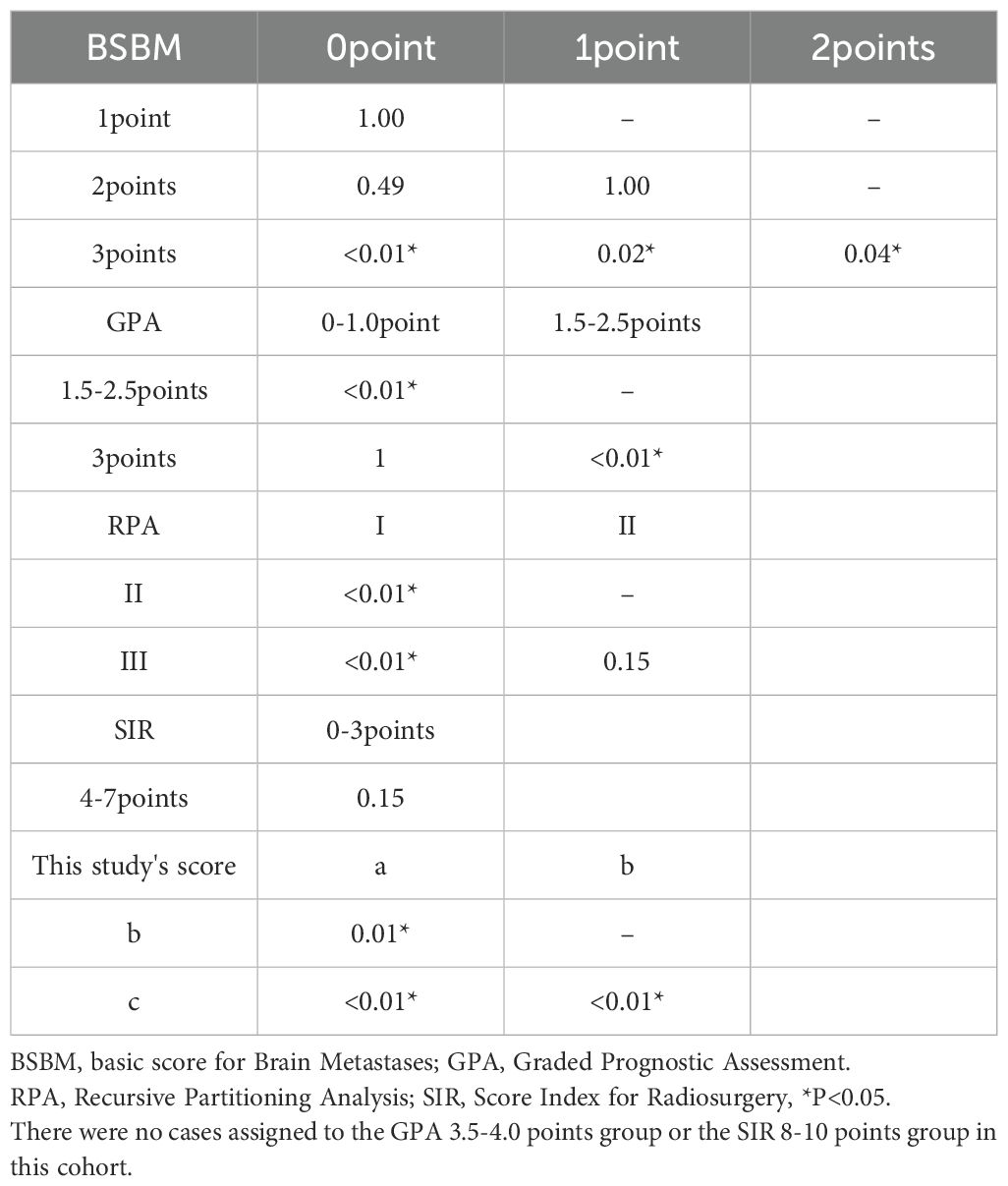
For external context, the cohort was also classified by four established systems. RPA: Class I (n=5), Class II (n=21), Class III (n=38); GPA: 0–1.0 (n=43), 1.5–2.5 (n=20), 3.0 (n=1), 3.5–4.0 (n=0); SIR: 1–3 (n=34), 4–7 (n=30), 8–10 (n=0); BSBM: 0 (n=32), 1 (n=11), 2 (n=12), 3 (n=9).
Corresponding mOS values were as follows: RPA Class I, II, III: 57.2, 7.2, and 3.7 months (95% CI, 15.7–NA; 3.9–10.6; 2.5–6.4) (Figure 2B). GPA 0–1.0, 1.5–2.5, 3.0: 3.5, 13.4, and 2.9 months (95% CI, 2.5–6.3; 7.6–28.5; NA–NA) (Figure 2C). SIR 1–3 and 4–7: 6.4 and 6.7 months (95% CI, 2.7–8.0; 3.5–13.9) (Figure 2D). BSBM 0, 1, 2, 3: 3.4, 6.8, 11.2, and 28.5 months (95% CI, 2.3–6.2; 2.5–6.7; 2.7–24.6; 4.2–NA) (Figure 2E, Table 5). Among all systems, only our proposed score separated all categories distinctly across the cohort.
Model-based performance in s-BM (C-index, AIC, PH)
To complement Kaplan–Meier comparisons, we fitted univariable Cox models for each system in s-BM. Our score achieved the highest discrimination (Harrell’s C-index 0.690) and the lowest AIC (375.57) compared with RPA (C = 0.631/AIC=378.65), GPA (0.666/386.13), SIR (0.629/388.63), and BSBM (0.671/379.67). Global Schoenfeld tests indicated no PH violations for any model (our score p=0.983). (Supplementary Table S1) Global Schoenfeld tests did not indicate PH violations for our score (p = 0.983) or for the comparator models (all non-significant), supporting the validity of the Cox analyses. We summarized performance in Table 6 (Harrell’s C-index, AIC, and 6/12-month AUCs). A compact one-panel plot (Figure 3) shows C-index point estimates across systems for visual comparison. In sensitivity analyses, the β-weighted variant achieved similar discrimination but a higher AIC, and the multivariable-only score did not outperform the 5-factor scheme; thus, we retained the pragmatic 1–2 point score for the main analyses (Table 6; Supplementary Tables S1, S2).
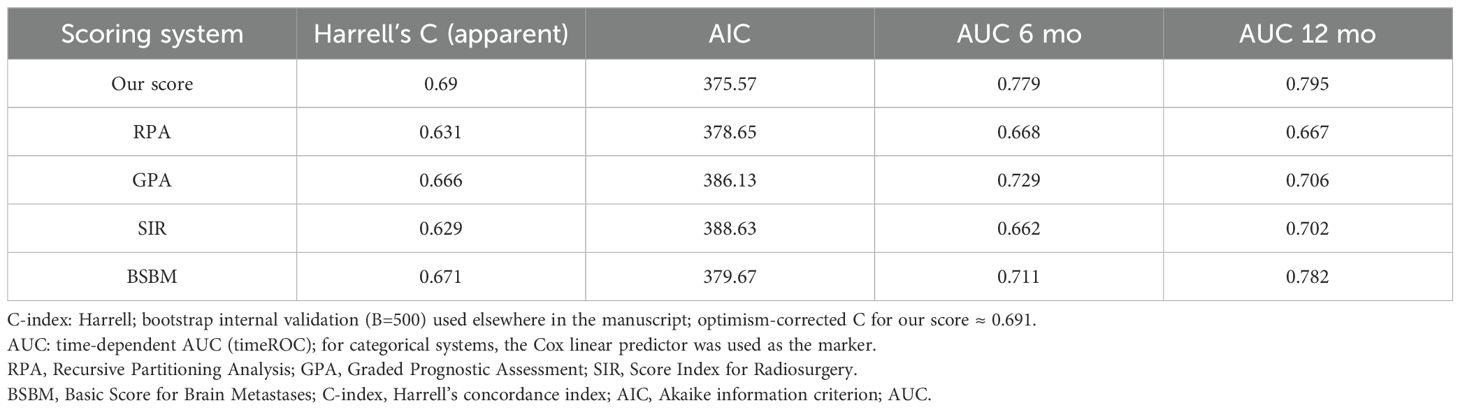
Table 6. Comparative prognostic performance of five scoring systems in the s-BM cohort—Harrell’s C-index, AIC, and time-dependent AUCs at 6 and 12 months.
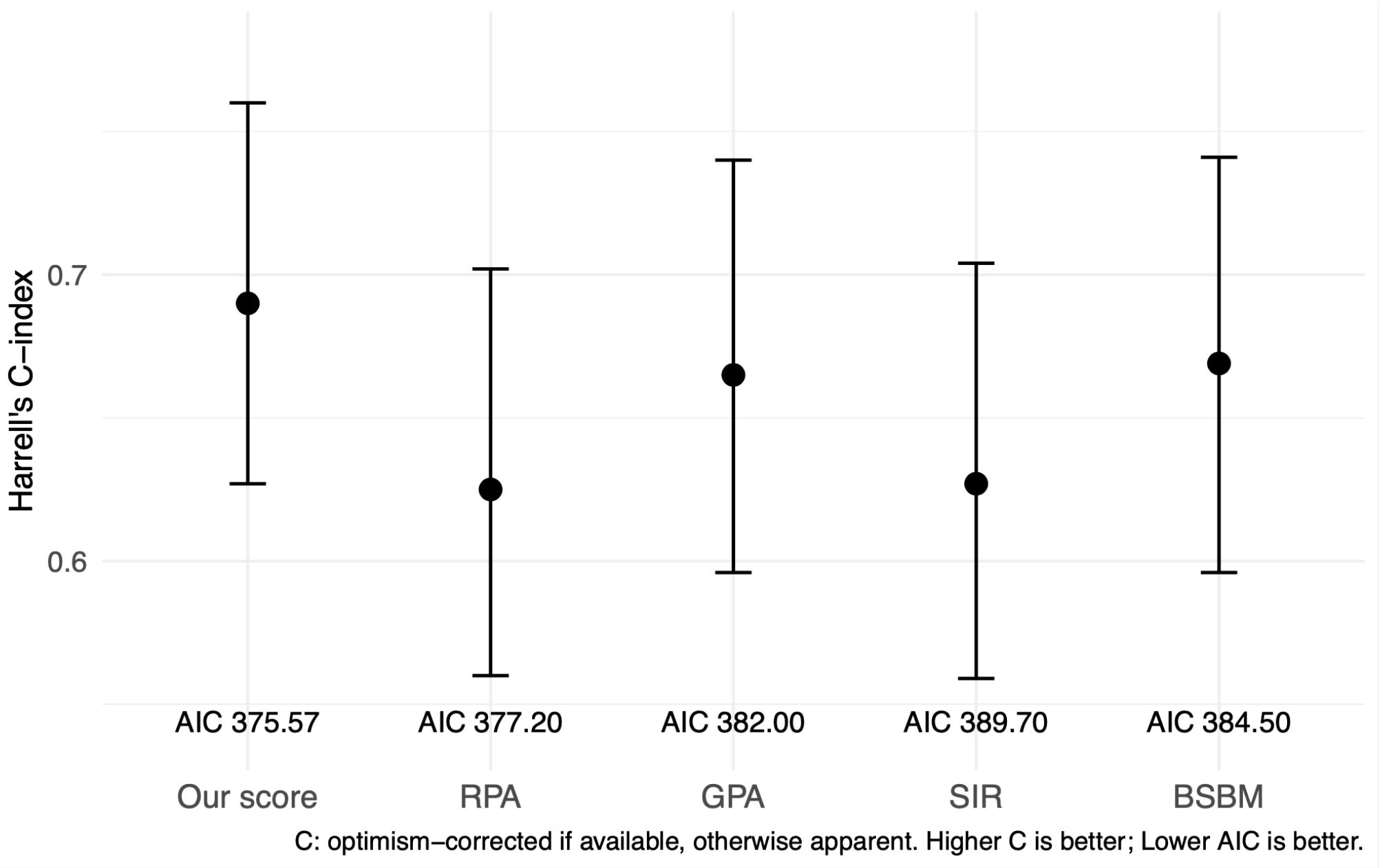
Figure 3. Harrell’s C-index with 95% confidence intervals for each prognostic scoring system in the s-BM cohort. AIC values are displayed below the labels. Higher C indicates better discrimination; lower AIC indicates better parsimony. Comparisons across systems are descriptive; no hypothesis testing was performed between C-indices. C-index, Harrell’s concordance index; AIC, Akaike information criterion; RPA, Recursive Partitioning Analysis; GPA, Graded Prognostic Assessment; SIR, Score Index for Radiosurgery; BSBM, Basic Score for Brain Metastases.
Time-dependent AUCs at 6 and 12 months
Time-dependent AUCs favored our score at both landmarks: 0.779 (6 months) and 0.795 (12 months), versus RPA 0.668/0.667, GPA 0.729/0.706, SIR 0.662/0.702, and BSBM 0.711/0.782 (categorical scores evaluated using Cox linear predictors). (Supplementary Table S1).
Internal validation and sensitivity analysis
Bootstrap internal validation (B = 500) yielded an optimism-corrected C-index of approximately 0.691 for our score, closely matching the apparent estimate, suggesting minimal optimism. Comparator systems had corrected C-indices of ~0.625–0.671.
In a sensitivity analysis, a coefficient-weighted variant of our score (multivariable Cox linear predictor) showed C-index 0.694 and AIC 381.9—comparable discrimination but worse parsimony than the simple scheme. An integer approximation of the weighted model is provided in the Supplement. (Supplementary Tables S1, S2).
Discussion
Previously, it has been considered that the prognosis of BM was dependent on tumor size in terms of sensitivity to radiation therapy, which was supposed to play a pivotal role in the treatment of BM.
However, prognostic stratification for clinical outcomes following the introduction of molecular targeted drugs and ICIs has become more evident than prognostication after radiation therapy, therefore the credibility of traditional prognosis grading has become questionable.
The concept of chemo-immunotherapy differs significantly from radiation therapy; specifically, its effectiveness is determined by sensitivity rather than tumor size.
Furthermore, intracranial irradiation is a double-edged treatment that is beneficial due to its cytotoxic effect from radiation therapy, but it also induces cerebral edema and radiation necrosis. Although steroids can help alleviate cerebral edema, they also suppress the immune system.
Instead, VEGF inhibitors like bevacizumab have been introduced. They provide beneficial dual effects by reducing cerebral edema and enhancing tumor immunity. Therefore, with the advancement of these multimodalities for BM, the traditional grading system must be reevaluated for accurate prognostication.
So far, the GPA scoring system for BM has been revised, incorporating genetic backgrounds over time (2, 6–8). Accurate prognostic predictions made during the initial consultation, as analyzed in this study, are essential for further treatment.
In this cohort, overall survival appeared similar between s-BM and m-BM; however, this two-group comparison is exploratory and not powered for equivalence/non-inferiority. Historically, s-BM has been reported to fare worse (9, 10). Differences in case-mix and treatment exposure likely counterbalanced (e.g., s-BM: more lung, fewer breast, more surgery, and less conventional chemotherapy; m-BM: exclusion of early failures and intracranial therapy prioritized once primary treatment is established) (11), and contemporary systemic therapies may further attenuate historical gaps (12). Accordingly, we emphasize the development of an s-BM–specific, pre-treatment score usable at the initial consultation.
In the background analysis of groups of s-BM and m-BM of this cohort, there were more lung cancers in groups of s-BM and more breast cancers in groups of m-BM, which is consistent with previous reports, including the characteristic that breast cancer often metastasizes to other organs after a while. The higher number of surgical interventions in the groups of s-BM is presumably due to the high need for surgical treatment to improve symptomatic cases and the need for tissue confirmation. Potthoff et al. stated that there was no difference in prognosis after surgical intervention between s-BM and m-BM, thus surgical treatment should be preferentially considered (13).
Previous reports have shown that prognosis was dependent on primary cancer, especially digestive cancer, being particularly associated with a poor prognosis (14). Interestingly, similar results were observed in s-BM in this cohort.
There are several reports summarizing the treatment outcomes of the group of s-BM by primary cancer type, and it has been reported that age, tissue type, systemic treatment, and surgical treatment are involved as prognostic factors in BM from breast cancer and lung cancer (15–18).
Our score demonstrated the highest discrimination (C-index 0.690) and the lowest AIC (375.57) compared with RPA, GPA, SIR, and BSBM, with no PH violations; time-dependent AUCs at 6/12 months were concordant, and bootstrap-corrected C-indices were nearly identical to apparent estimates—suggesting limited optimism. A coefficient-weighted variant achieved similar discrimination but a higher AIC, supporting the pragmatic 1–2 point scheme for time-zero use. Variable selection based solely on multivariable significance can be sample-size dependent and unstable in correlated predictors, risking the omission of clinically salient time-zero information. In our data, a β-weighted score and a multivariable-only score offered no material performance gain over the simple scheme, while reducing usability; hence the final model prioritizes parsimony and interpretability with internal bootstrap validation.
When considering the treatment outcomes of BM, the influence of racial differences could not be ignored because of the wide variety of primary tumors. The proportion of melanoma is higher in cohorts that include Caucasians, while the proportion of gastrointestinal and thyroid cancers tends to be higher in cohorts that include Asians (19, 20). Among these, the results of this cohort are an analysis of a single institution, but they are likely to reflect the treatment outcomes of patients with BM in Japan.
The score we proposed here is intended to predict the prognosis at the time of initial consultation in cases where both conditions are discovered synchronously. As mentioned above, this score is capable of more sensitive classification than other scores, but to assess the usefulness of this score, it is necessary to take into account the unique characteristics of BM treatment. Naturally, unlike other intracranial BM, treatment must be carried out in coordination with the treatment of the primary lesion. Unlike the group of m-BM, in which metastasis was discovered after the diagnosis was confirmed and treatment had progressed, in the group of s-BM, collaboration with other departments is particularly necessary to determine whether tissue confirmation should be performed at the primary or metastatic lesion, and how the treatment should be scheduled. We also formulate treatment schedules in consultation with the department in charge of the primary tumor, and it is important to be able to make highly accurate prognostic predictions at this time. Based on this, a common understanding can be reached as to whether aggressive treatment should be recommended or palliative treatment should be considered, and we hope that our proposal scoring system will be utilized appropriately in the future to promote collaboration.
Limitation
This study was conducted at a single center and retrospectively analyzed. Although bootstrap internal validation (B = 500) suggested minimal optimism, the score has not yet undergone external validation. All modeling and performance estimates were derived from the same institution and period, so generalizability across centers, imaging protocols, disease mix, and systemic therapy patterns remains uncertain. Given the modest size of the s-BM cohort (n=64; ~60 events) and the use of the same cohort for both model development and validation, a non-negligible risk of overfitting remains. We prespecified the five predictors and their cut-offs and avoided data-driven stepwise selection to mitigate this risk, and the optimism-corrected C-index (0.691) was close to the apparent estimate; nevertheless, residual optimism cannot be excluded. A limitation of this time-zero design is possible misclassification of the primary site when using imaging surrogates; such an error is likely non-differential and would attenuate associations. External, multicenter validation with standardized imaging adjudication and, where available, pathology cross-check is warranted. In line with reporting guidance for prediction models, the present work represents model development with internal validation; external, multicenter—preferably prospective—validation and potential recalibration are required before broad implementation. We plan such validation in an independent, multicenter s-BM cohort.
Finally, systemic therapy has evolved rapidly in recent years (e.g., immune checkpoint inhibitors and targeted agents). Our development cohort spans both the pre-immunotherapy era and its early adoption. However, the score was based on regimen-agnostic pretreatment factors; its performance may differ in a setting dominated by immunotherapy. Given the limited number of patients treated exclusively with immunotherapy in our s-BM cohort, we did not perform a restricted analysis for this subgroup. This limitation will be addressed in future external, multicenter validation. Treatment-era heterogeneity remains essential, and we plan to pursue an immunotherapy-only subanalysis in a contemporary, multicenter cohort.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical Committee of The Jikei University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YY: Writing – original draft, Investigation, Data curation, Conceptualization. GS: Data curation, Project administration, Writing – review & editing. TM: Project administration, Data curation, Investigation, Writing – review & editing. YT: Data curation, Writing – review & editing, Project administration, Formal Analysis. NK: Resources, Visualization, Data curation, Writing – review & editing, Supervision, Investigation. TI: Resources, Visualization, Writing – review & editing, Supervision. YA: Writing – review & editing, Visualization, Investigation, Supervision. YM: Supervision, Writing – review & editing, Visualization. TT: Visualization, Validation, Writing – review & editing, Conceptualization, Writing – original draft, Supervision.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1617366/full#supplementary-material
Abbreviations
ANOVA, analysis of variance; BBB, blood-brain barrier; Bev, Bevacizumab; BM, Brain metastases; BSBM, basic score for Brain Metastases; CT, computed tomography; GPA, Graded Prognostic Assessment; ICI, immune checkpoint inhibitor; KPS, Karnofsky performance scale; m-BM, metachronous brain metastases; mOS, median overall survival; NA, Not Available; NE (not estimable); NCCN, National Comprehensive Cancer Network; OS, overall survival; PFS, progression-free survival; RCC, renal cell carcinoma; ROC, receiver operating characteristic; RPA, Recursive Partitioning Analysis; s-BM, synchronous brain metastases; SD, standard deviation; SIR, Score Index for Radiosurgery; SRS, stereotactic radiosurgery; SRT, stereotactic radiation therapy; VEGF, vascular endothelial growth factor.
References
1. Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. (1997) 37:745–51. doi: 10.1016/s0360-3016(96)00619-0
2. Sperduto PW, Berkey B, Gaspar LE, Mehta M, and Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. (2008) 70:510–4. doi: 10.1016/j.ijrobp.2007.06.074
3. Weltman E, Salvajoli JV, Brandt RA, de Morais Hanriot R, Prisco FE, Cruz JC, et al. Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys. (2000) 46:1155–61. doi: 10.1016/s0360-3016(99)00549-0
4. Lorenzoni J, Devriendt D, Massager N, David P, Ruiz S, Vanderlinden B, et al. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys. (2004) 60:218–24. doi: 10.1016/j.ijrobp.2004.02.017
5. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transp. (2013) 48:452–8. doi: 10.1038/bmt.2012.244
6. Ribeiro LM, Bomtempo FF, Rocha RB, Telles JPM, Neto EB, and Figueiredo EG. Development and adaptations of the Graded Prognostic Assessment (GPA) scale: a systematic review. Clin Exp Metast. (2023) 40:445–63. doi: 10.1007/s10585-023-10237-3
7. Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. (2010) 77:655–61. doi: 10.1016/j.ijrobp.2009.08.025
8. Li J, Jing W, Zhai X, Jia W, Zhu H, and Yu J. Estimating survival in patients with non-small-cell lung cancer and brain metastases: A verification of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). Onco Targets Ther. (2021) 14:1623–31. doi: 10.2147/ott.S288928
9. Thomas AJ, Rock JP, Johnson CC, Weiss L, Jacobsen G, and Rosenblum ML. Survival of patients with synchronous brain metastases: an epidemiological study in southeastern Michigan. J Neurosurg. (2000) 93:927–31. doi: 10.3171/jns.2000.93.6.0927
10. Tonse R, Rubens M, Appel H, Tom MC, Hall MD, Odia Y, et al. Systematic review and meta-analysis of PD-L1 expression discordance between primary tumor and lung cancer brain metastasis. Neurooncol Adv. (2021) 3:vdab166. doi: 10.1093/noajnl/vdab166
11. Tonse R, Rubens M, Appel H, Tom MC, Hall MD, Odia Y, et al. Systematic review and meta-analysis of lung cancer brain metastasis and primary tumor receptor expression discordance. Discov Oncol. (2021) 12:48. doi: 10.1007/s12672-021-00445-2
12. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. (2012) 366:925–31. doi: 10.1056/NEJMoa1112824
13. Potthoff AL, Heimann M, Lehmann F, Ilic I, Paech D, Borger V, et al. Survival after resection of brain metastasis: impact of synchronous versus metachronous metastatic disease. J Neurooncol. (2023) 161:539–45. doi: 10.1007/s11060-023-04242-5
14. Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, et al. Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol. (2020) 38:3773–84. doi: 10.1200/jco.20.01255
15. Che W, Wang Y, Wang X, and Lyu J. Association between age and the presence and mortality of breast cancer synchronous brain metastases in the United States: A neglected SEER analysis. Front Public Health. (2022) 10:1000415. doi: 10.3389/fpubh.2022.1000415
16. Ho VK, Gijtenbeek JM, Brandsma D, Beerepoot LV, Sonke GS, and van der Heiden-van der Loo M. Survival of breast cancer patients with synchronous or metachronous central nervous system metastases. Eur J Can. (2015) 51:2508–16. doi: 10.1016/j.ejca.2015.07.040
17. Zhou G, Zhang Z, Yu P, Geng R, Wang G, Ma W, et al. Predictive value of clinical characteristics on risk and prognosis of synchronous brain metastases in small-cell lung cancer patients: A population-based study. Cancer Med. (2023) 12:1195–203. doi: 10.1002/cam4.4978
18. Shen H, Deng G, Chen Q, and Qian J. The incidence, risk factors and predictive nomograms for early death of lung cancer with synchronous brain metastasis: a retrospective study in the SEER database. BMC Can. (2021) 21:825. doi: 10.1186/s12885-021-08490-4
19. Gomez D, Feng JJ, Cheok S, Shah I, Dicharry H, Cote DJ, et al. Incidence of brain metastasis according to patient race and primary cancer origin: a systematic review. J Neurooncol. (2024) 169:457–67. doi: 10.1007/s11060-024-04748-6
Keywords: brain metastases (BM), synchronous brain metastases, prognostic factors (PF), prognostic scoring system, survival analysis
Citation: Yamamoto Y, Saito G, Maetani T, Terasawa Y, Kato N, Ishii T, Akasaki Y, Murayama Y and Tanaka T (2025) Validation of a prognostic scoring system for brain metastases with synchronous detection of primary cancers at initial consultation. Front. Oncol. 15:1617366. doi: 10.3389/fonc.2025.1617366
Received: 24 April 2025; Accepted: 27 October 2025;
Published: 17 November 2025.
Edited by:
Nima Aghdam, New York University, United StatesReviewed by:
David Wasilewski, Charité University Medicine Berlin, GermanyPablo Flores-Paco, Hospital Universitario Reina Sofia, Spain
Copyright © 2025 Yamamoto, Saito, Maetani, Terasawa, Kato, Ishii, Akasaki, Murayama and Tanaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshihide Tanaka, dHRhbmFrYUBqaWtlaS5hYy5qcA==
 Yohei Yamamoto
Yohei Yamamoto Gentoku Saito1
Gentoku Saito1 Tomona Maetani
Tomona Maetani Yasuharu Akasaki
Yasuharu Akasaki Toshihide Tanaka
Toshihide Tanaka