- 1The Graduate School, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2The First Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 3Department of Ultrasound, Ningbo No. 2 Hospital, Ningbo, Zhejiang, China
- 4Department of General Surgery, Ningbo No. 2 Hospital, Ningbo, Zhejiang, China
Background: Currently, the axillary management strategy of omitting axillary lymph node dissection (ALND) in early-stage breast cancer (BC) patients with cT1-2, clinically node-negative (cN0), and sentinel lymph node biopsy (SLNB) revealing 1–2 sentinel lymph nodes (SLNs) macro-metastases remains controversial. This study aims to systematically evaluate the safety of omitting ALND in this population.
Methods: This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered with the registration number: CRD42025645388. A systematic literature search was conducted across five electronic databases (PubMed, Web of Science, Cochrane Library, Ovid Medline, and Embase) from inception through December 2024. Randomized controlled trials (RCTs) and cohort studies meeting the predefined eligibility criteria were included. Primary outcomes included disease-free survival (DFS) and overall survival (OS). The association between ALND omission and long-term outcomes was assessed using pooled hazard ratios (HRs) with 95% confidence intervals (CIs).
Results: Fifteen studies (6 RCTs, 9 cohort studies) involving 33,599 patients in the SLNB-only group and 95,711 controls receiving SLNB+ALND were analyzed. No significant differences in DFS (HR = 0.99, 95%CI:0.85-1.14, p=0.857) or OS (HR = 1.03, 95%CI: 0.92-1.14 p=0.251) were observed in both groups. Subgroup analyses by follow-up duration (5-years and 10-years), study design (RCTs and cohort studies), and region (Eastern and Western) showed no survival differences between the experimental and control groups. (all p values are greater than 0.05).
Conclusion: Omitting ALND is safe for early-stage BC patients with cT1-2, cN0, and 1–2 SLNs macro-metastases.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42025645388.
Introduction
Breast cancer (BC) is the second most common cancer globally and the most frequently diagnosed cancer in women, accounting for 25% of all female cancer cases. It ranks fourth among cancer-related deaths worldwide and is responsible for 16.7% of female cancer-related fatalities (1). Current therapeutic modalities for BC encompass radiation therapy, chemotherapy, targeted therapy, immunotherapy, and endocrine therapy, while surgical intervention remains the cornerstone of curative treatment. Within surgical oncology, axillary lymph node dissection (ALND) has historically held significant prognostic importance. It is well known that axillary lymph nodes are an important pathway for BC metastasis, making precise axillary nodal staging essential for both prognostic evaluation and therapeutic decision-making (2). ALND can remove potentially metastatic lymph nodes, provides definitive axillary staging and improve long-term prognosis, and it has long been the standard surgical procedure for BC (3). However, ALND carries substantial postoperative morbidity including lymphedema, sensory nerve injury, infection, and hemorrhage, all of which adversely impact patients’ quality of life (4). Early-stage BC is typically defined as primary tumors classified as T1-2, regional lymph node status N0-N1, and without distant metastasis (5). With the widespread application of screening technologies, the detection rate of early-stage BC has significantly increased, leading to markedly improved patient survival outcomes (6). A major clinical challenge in contemporary BC management is to achieve accurate axillary staging while minimizing surgical trauma and optimizing quality of life - specifically, by reducing unnecessary ALND.
The advent of sentinel lymph node biopsy (SLNB) in the 1990s revolutionized axillary staging, mitigating these morbidity concerns while maintaining oncological safety (7). Relevant studies demonstrated a 97.5% concordance rate between sentinel lymph nodes (SLNs) status and axillary lymph node pathology, with SLNB achieving diagnostic accuracy rates up to 97% (8). These findings support the omission of ALND in patients with negative SLNB results (9). To date, ALND remains standard care for positive SLNB cases. However, in relevant studies, it was found that among early-stage BC patients cT1-2, clinically node-negative (cN0) and SLNB positive who underwent ALND, approximately 75.7% had only one positive SLN (10). In other words, for such patients, the clinical value of ALND is somewhat limited. Subsequent randomized controlled trials (RCTs) including Z0011, SENOMAC, and AMAROS have rigorously investigated this clinical dilemma (11–13). The results showed that for early-stage BC patients who were cT1-2, cN0 and had 1–2 positive SLNs after SLNB, omitting ALND was not inferior to ALND in terms of prognosis. While existing meta-analyses have explored the safety of omitting ALND in early-stage BC patients, limitations persist. Most studies focus on populations with micro-metastases or isolated tumor cells (ITC) (for this population, the axillary node disease burden is low, indicating a favorable prognosis), constrained by insufficient sample sizes and further subgroup stratification (14, 15). Therefore, this study, through a systematic review of published RCTs and cohort studies, aims to explore the impact of omitting ALND on long-term prognosis in early-stage BC patients who are cT1-2, cN0 and 1–2 SLNs macro-metastatic after SLNB.
Methods
Search strategy
This meta-analysis, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16), was registered in the international Prospective Register of Systematic Reviews (PROSPERO) under the number CRD42025645388. A literature search was conducted across five electronic databases: PubMed, Web of Science, Cochrane Library, Ovid Medline, and Embase. The search spanned from the inception of each database to December 2024. The search strategy combined Medical Subject Headings (MeSH) terms and free-text terms related to “breast neoplasms” and “sentinel lymph node”. The search string was as follows: ((((((((((((((((breast neoplasms [mesh]) OR (breast tumor)) OR (breast cancer)) OR (cancer of breast)) OR (malignant neoplasm of breast)) OR (malignant tumor of breast)) OR (mammary cancer)) OR (mammary neoplasms, human)) OR (breast carcinoma)) OR (human mammary carcinoma)) OR (breast carcinoma in situ)) OR (carcinoma, ductal, breast)) OR (carcinoma, lobular)) OR (inflammatory breast neoplasms)) OR (triple negative breast neoplasms)) OR (inflammatory breast neoplasms)) AND ((sentinel lymph node [mesh]) OR (sentinel lymph nodes)) OR (sentinel node))).
The search terms and strategies were adjusted according to the indexing systems and requirements of each database. To avoid overlooking potential studies, a secondary manual search was conducted on the references of the included articles.
Inclusion criteria and exclusion criteria
Articles were considered for inclusion only if they fulfilled the following criteria defined by the PICOS principle: (1) patients were diagnosed with early-stage BC, with clinical staging of cT1-2, cN0, and M0; (2) all patients underwent SLNB, with pathological results demonstrating macro-metastases in 1–2 positive SLNs; (3) the experimental group underwent SLNB alone without subsequent ALND; (4) the control group underwent SLNB followed by ALND; (5) studies reported long-term outcomes, including overall survival (OS) and disease-free survival (DFS); (6) study design included RCTs and cohort studies.
The exclusion criteria were as follows: (1) data were unavailable; (2) relevant outcomes of interest were not reported; (3) full-text articles could not be obtained; (4) articles were not in English; (5) when articles with data updates were available, only the most recent and/or the most comprehensive ones were considered for inclusion.
Data extraction and quality assessment
Two independent investigators extracted data using standardized forms capturing: first author, publication year, study design, enrollment period, surgical procedures, follow-up duration, sample characteristics (SLN status, molecular subtypes, histologic grades), and survival outcomes.
For RCTs, methodological quality was evaluated using the Cochrane Risk of Bias Tool 1.0 (RoB 1.0) (17). This instrument assesses potential biases across multiple domains, including randomization sequence generation, allocation concealment, blinding of participants/investigators, blinding of outcome assessment, completeness of outcome data, and selective reporting. Risk of bias was categorized as “high”, “low”, or “unclear”. For cohort studies, methodological rigor was assessed via the Newcastle-Ottawa Scale (NOS), which evaluates three domains: selection bias, comparability, and outcome measurement. A scoring system was applied to each domain, with studies achieving NOS scores≥6 classified as high-quality. Discrepancies between reviewers were resolved through consensus discussion during the data extraction and quality appraisal processes.
Statistical analysis
Meta-analyses were conducted using Stata 12.0 and Review Manager 5.3. The association between omission of ALND and long-term prognosis was evaluated by calculating pooled hazard ratios (HRs) with 95% confidence intervals (CIs). Effect estimates were interpreted as favoring the experimental group when HR<1 and the control group when HR>1. Heterogeneity was assessed using Cochran’s Q statistic (χ² test) and I² quantification, with I² values>50% indicating substantial heterogeneity and ≤ 50% indicating low heterogeneity (18). Subgroup analyses stratified by follow-up times, study design, and geographic region were performed to investigate potential sources of heterogeneity.
Given anticipated heterogeneity due to variations in population characteristics, ethnicities, treatment protocols, and BC subtypes, a random-effects model was uniformly applied to enhance result reliability. Sensitivity analyses were conducted by sequentially excluding each study to evaluate result stability. Potential publication bias was examined using Begg’s test (19). All analyses employed two-tailed tests, with statistical significance defined at p<0.05.
Results
Study selection
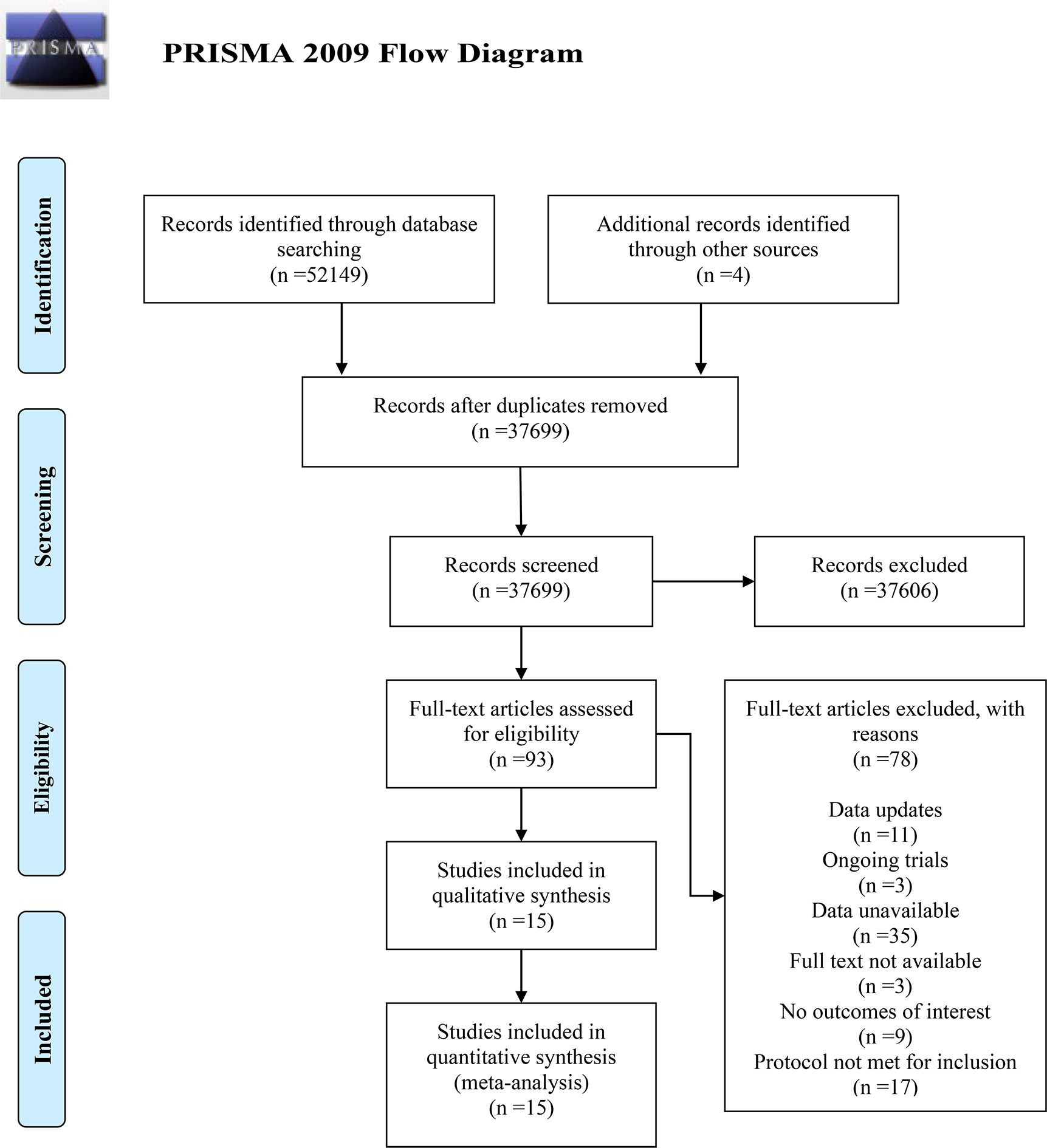
A total of 52,153 terms were identified through the initial search of five electronic databases and additional secondary manual indexing. After duplicates were removed using Note Express 4.0 software, 37,699 terms remained. By reading the titles and abstracts, 37,606 terms were excluded. The full texts of the remaining 93 articles were reviewed, and 78 were excluded for the following reasons: updated data (n=11); unavailable data (n=35); full text unobtainable (n=3); lack of interesting outcomes (n=9); methodological ineligibility (n=17); and ongoing studies (n=3). Ultimately, 15 articles were included in the meta-analysis. The detailed process of inclusion and exclusion is shown in the PRISMA flow diagram (Figure 1).
Study characteristics
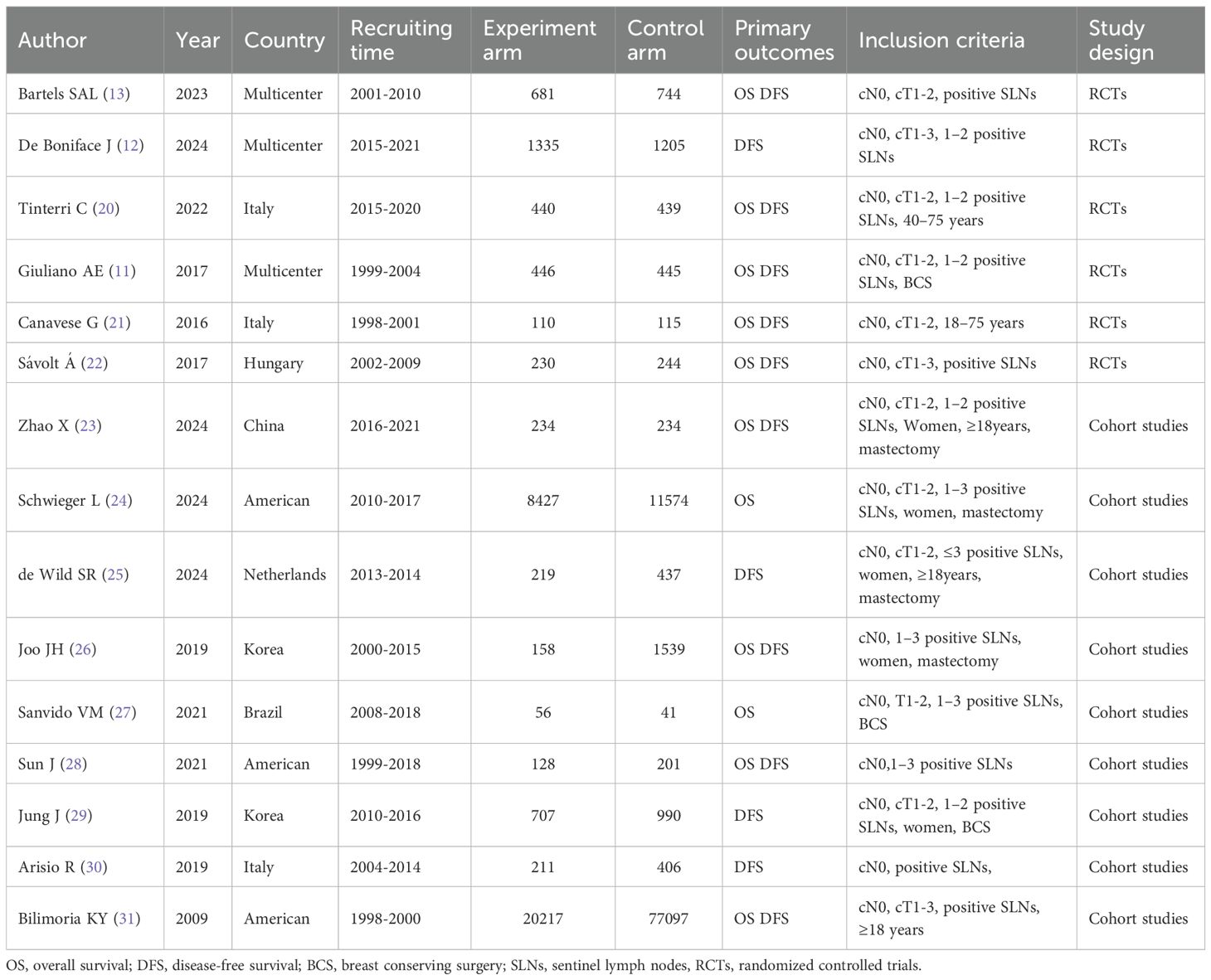
The 15 included articles, comprising 6 RCTs (11–13, 20–22)and 9 cohort studies (23–31), published between 2009 and 2024, were mostly conducted in Western countries. The recruitment period ranged from 1998 to 2021, with more than 33,000 participants in the experimental group and more than 95,000 in the control group. The recruited patients generally suffered from early-stage BC and were characterized by cT1-2, cN0, M0, and had 1–2 positive SLNs after SLNB. Surgical procedures included breast-conserving surgery and mastectomy. Specific information is presented in Table 1. Further details regarding the median follow-up time of the included studies, the median age of patients at recruitment, the distribution of the number of positive SLNs, and the distribution of histological grading are summarized in Supplementary Tables 1–1, 1-2.
Methodological quality of included studies
The RoB 1.0 was used to evaluate the quality of RCTs, revealing a high risk of bias due to the nature of the interventions (mainly because blinding was not performed). The risk of bias assessment for RCTs is detailed in Supplementary Figures 1, 2. The NOS as used to evaluate cohort studies, with all studies rated as moderate to high quality, scoring at least 7 out of 9 points. Specific scores are shown in Supplementary Table 2.
Long-term prognosis
DFS
A total of 13 studies reported DFS, with more than 25,000 participants in the experimental group and more than 84,000 in the control group. The pooled results showed no significant difference in DFS between the two groups (HR = 0.99, 95%CI:0.85-1.14, p=0.857, I²=20.5%) as presented in Figure 2.
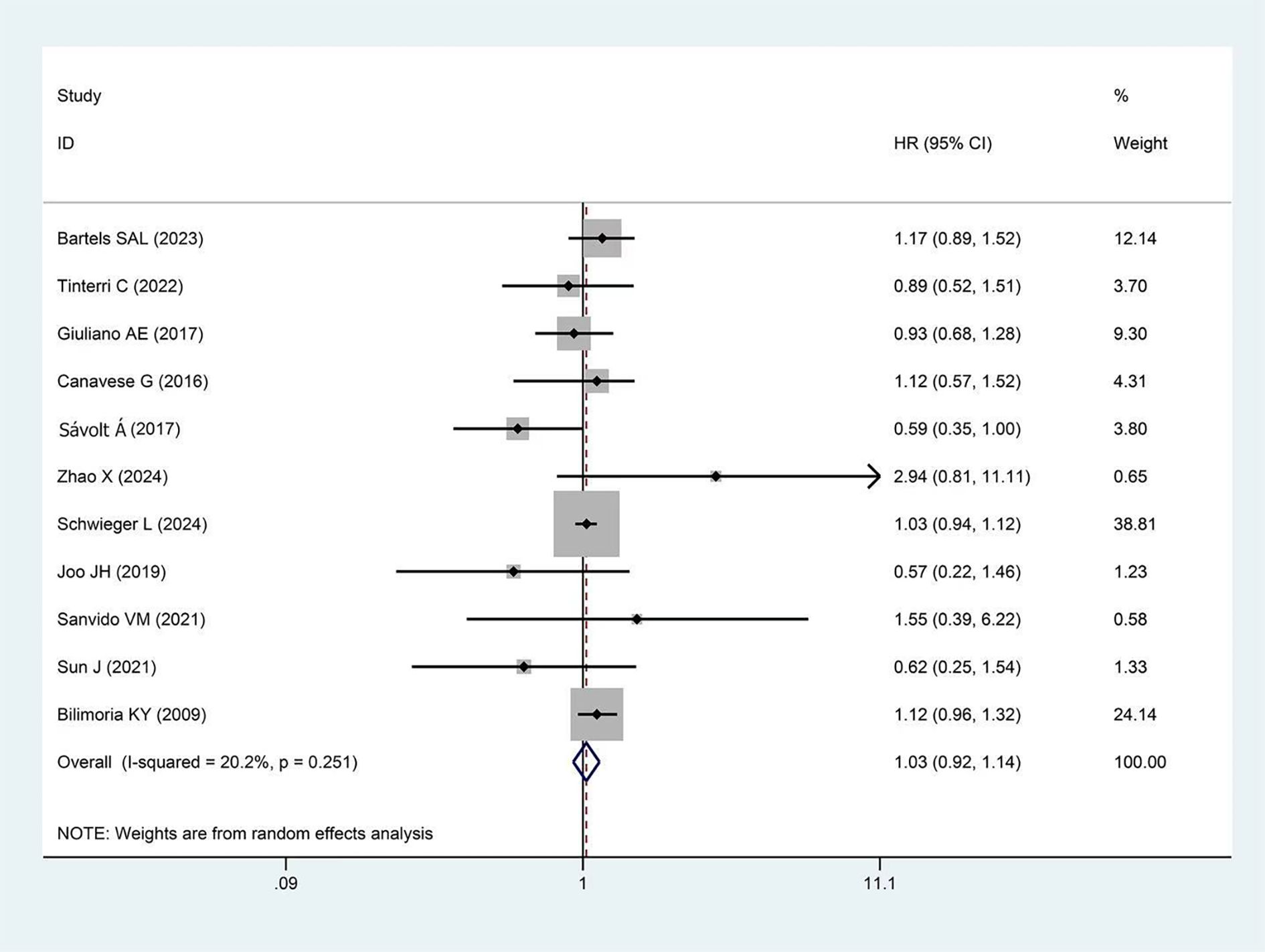
Figure 2. Forest plot showing the effect of omitting ALND on DFS in early-stage BC cT1-2, cN0, and 1–2 SLNs macro-metastases (p=0.857).
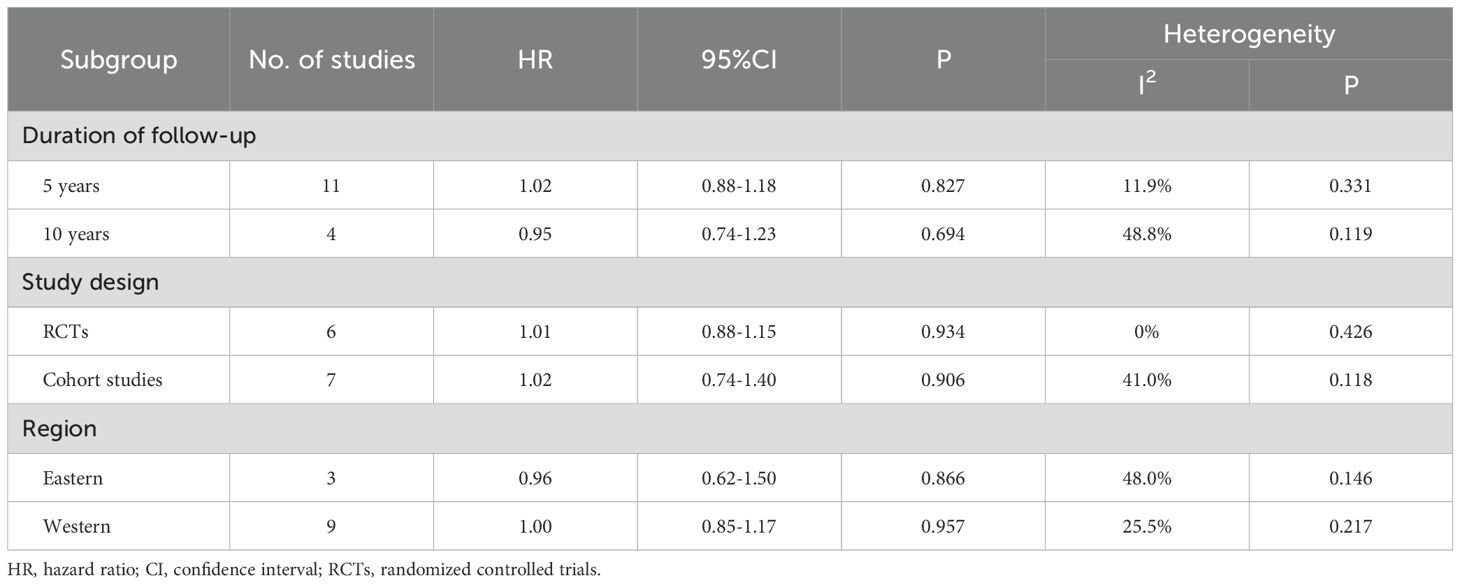
Subgroup analysis results are shown in Table 2. Studies were divided into 5-year and 10-year subgroups based on follow-up duration. No statistical difference in DFS was observed in either the 5-year subgroup (HR = 1.02, p=0.827) or the 10-year subgroup (HR = 0.95, p=0.694) between the experimental and control groups. When stratified by study design, no significant difference in DFS was observed between the experimental and control groups in the RCTs subgroup (HR = 1.01, p=0.934) or the cohort studies subgroup (HR = 1.02, p=0.906). In terms of geographical region, studies were divided into Eastern and Western subgroups. Neither the Eastern subgroup (HR = 0.96, p=0.866) nor the Western subgroup (HR = 1.00, p=0.957) showed a statistical difference in DFS between the experimental and control groups. Sensitivity analysis demonstrated the stability of the pooled DFS results (Supplementary Figure 3). Moreover, Begg’s test indicated no significant publication bias (p=0.951, Supplementary Figure 4).
OS
A total of 11 studies reported OS, with more than 31,000 participants in the experimental group and more than 92,000 in the control group. The pooled results showed no significant difference in OS between the two groups (HR = 1.03, 95%CI: 0.92-1.14 p=0.251, I²=20.2%) as presented in Figure 3.
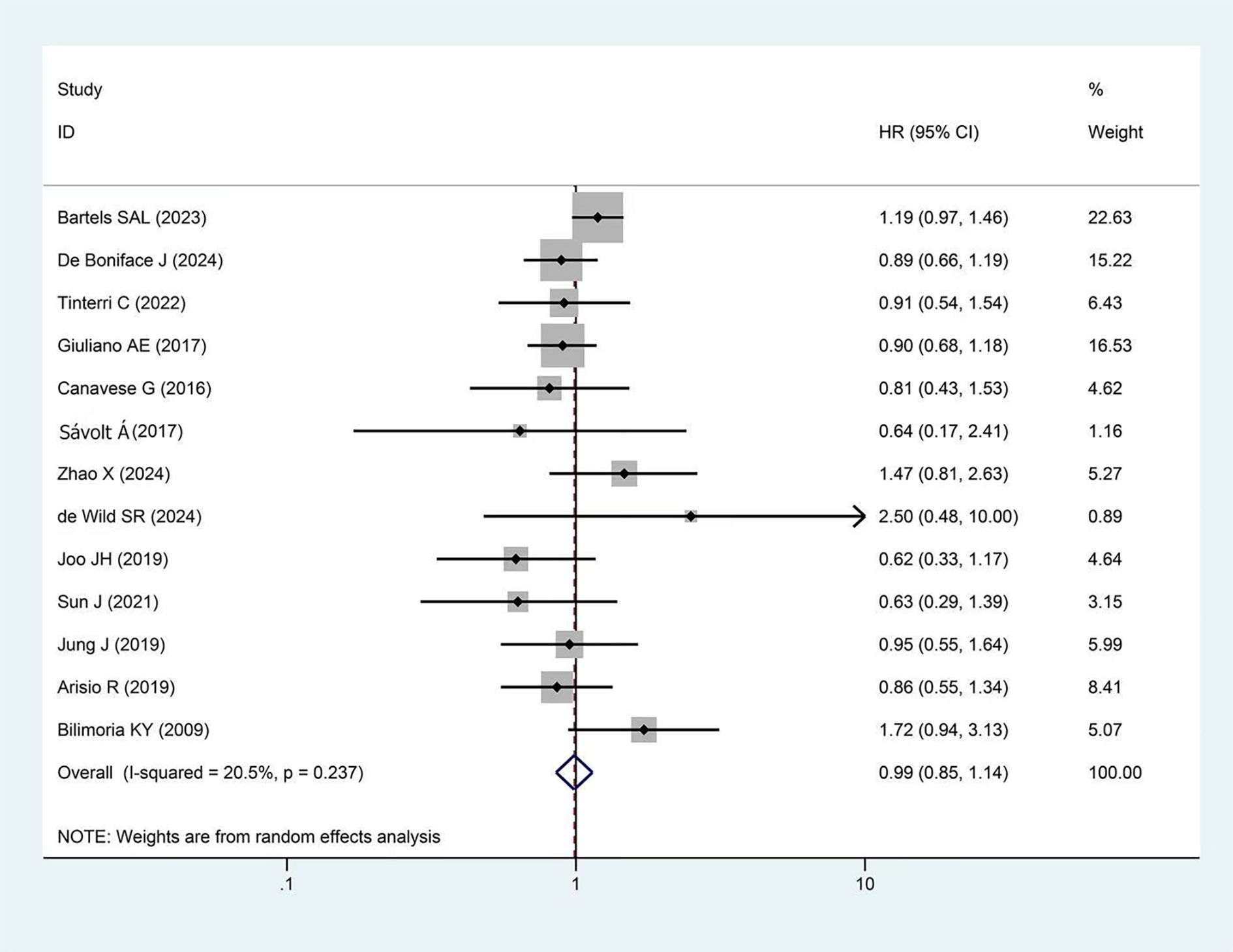
Figure 3. Forest plot showing the effect of omitting ALND on OS in early-stage BC cT1-2, cN0, and 1–2 SLNs macro-metastases (p=0.606).
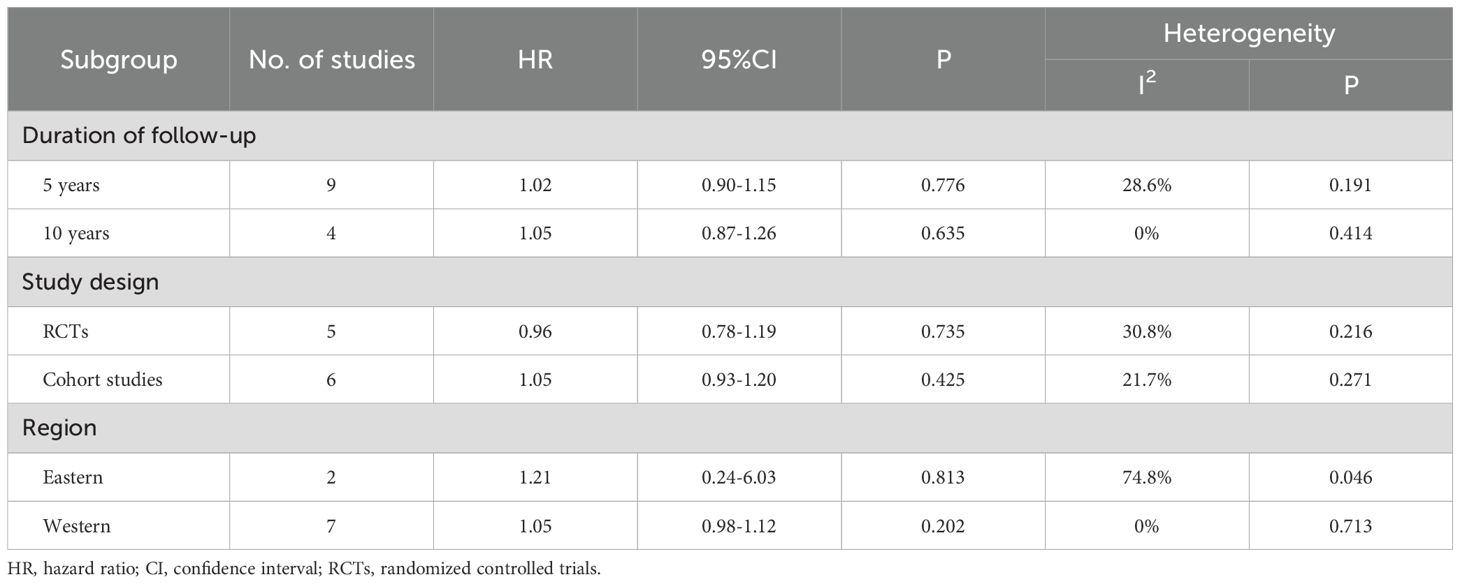
Subgroup analysis results are shown in Table 3. Based on follow-up duration, studies were divided into 5-year (HR = 1.02, p=0.776) and 10-year subgroups (HR = 1.05, p=0.635). In terms of study design, studies were divided into RCTs (HR = 0.96, p=0.735) and cohort study subgroups (HR = 1.05, p=0.425). Studies were also divided into Western (HR = 1.05, p=0.202) and Eastern subgroups (HR = 1.21, p=0.813). In the subgroup analyses mentioned above, no significant statistical differences were observed in OS between the experimental and control groups. Sensitivity analysis demonstrated the stability of the pooled OS results. (Supplementary Figure 5). Additionally, Begg’s test showed no significant publication bias (p=0.640, Supplementary Figure 6).
Discussion
In early-stage BC, lymphatic dissemination represents the predominant metastatic pathway, with approximately 33% of patients presenting with regional lymph node involvement at initial diagnosis (32). The axillary lymph nodes, due to their anatomical location and lymphatic drainage characteristics, are the primary target organs for metastasis. Traditional ALND was once the standard procedure for blocking metastasis and assessing axillary status. However, its complications significantly affect patients’ postoperative quality of life. With the development of SLNB technology, characterized by ≥98% accuracy in axillary staging and a lower risk of complications, it has become the preferred method for axillary staging (33). For patients with negative SLNs, SLNB has become the standard treatment when combined with comprehensive systemic therapy. For patients with positive SLNs, ALND is still required.
Lymph node metastasis can be classified into three types based on the diameter of tumor cells in the lymph nodes: ITC (≤0.2 mm), micro-metastasis (>0.2 mm and ≤2 mm), and macro-metastasis (>2 mm) (34). Currently, patients with positive SLNs routinely undergo ALND, but recent studies have revealed potential for optimizing this strategy. In cN0 patients and positive SLNs after SLNB, data show that in about 60% of cases, only positive SLNs is found during ALND, with no other lymph node metastasis (35). Further research by Sonia Martinez Alcaide’s team found that among patients with macro-metastasis in SLNs, 66.7% had only SLN involvement after ALND; 7.9% had one non-sentinel lymph node (NSLN) involved; 6.3% had two NSLNs involved; and 19% had three or more involved NSLNs (36). In patients with micro-metastasis, a lower axillary metastatic burden was observed: 83.9% had only SLN involvement, 12.9% had one NSLN involved, and 3.2% had two NSLNs involved. This finding was confirmed by the long-term follow-up results of the IBCSG 23–01 study (37). Specifically, in early-stage BC patients who were cT1-2, cN0, and had micro-metastasis (including ITC) in SLNB, there were no statistically significant differences in OS, DFS, or regional control between patients who received ALND and those who underwent SLNB alone. The latest European Society for Medical Oncology (ESMO) guidelines (38) and multiple meta-analyses (14, 39) also suggest that for patients with the least axillary metastatic burden, SLNB alone is sufficient.
The number of pathologically SLNs in ALND demonstrated a significant positive correlation with NSLN positivity rates (40, 41). Among patients with 1–3 positive SLNs, the NSLN positivity rate was 37.9%; however, this rate increased dramatically to 83.3% when ≥4 SLNs were involved. It is worth noting, even in patients with 1–2 macro-metastases in SLNs, the overall axillary metastatic burden remains low, prompting exploration of whether ALND can be safely omitted for such patients. The 5-year follow-up results of the SENOMAC trial demonstrated that omitting ALND in patients with macro-metastases was non-inferior to ALND in terms of survival outcomes (12), a finding that aligns with the conclusions of this meta-analysis. No significant differences in DFS and OS were observed between the patients underwent ALND and those receiving SLNB alone. This may be due to the following reasons: 1. specific patients: as previously mentioned, the axillary burden in BC patients is low, with 60% of patients having tumor cells involving only SLNs (35), which is removed through SLNB. 2. Postoperative radiotherapy: most patients received postoperative radiotherapy, which effectively clears potential occult lesions in breast tissue, chest wall, or regional lymph nodes, reducing the risk of local recurrence (42). Therefore, even if there are residual positive lymph nodes after SLNB, postoperative regional lymph node radiotherapy can provide effective salvage. By the way studies have also indicated that for women with 1–3 positive lymph nodes, regional lymph node radiotherapy reduces the absolute risk of 15-year BC mortality by approximately 2-3% (43). 3. Postoperative adjuvant systemic therapy: at least 90% of patients received adjuvant systemic therapy, including chemotherapy, as well as targeted, immunotherapy, and endocrine therapy based on the molecular subtypes of BC. Current evidence suggests that BC is a disease with systemic tendencies, and even in early-stage BC, there may be microscopic metastases (44, 45). Adjuvant systemic therapy is crucial for improving patient prognosis. Studies have shown a 50% reduction in the 10-year recurrence rate and a 20% decrease in the 20-year risk of breast cancer mortality, significantly improving survival rates (46). Additionally, personalized treatment strategies are available based on different BC subtypes. For human epidermal growth factor receptor 2-positive (HER2-positive) BC patients, targeted therapy is available. Relevant studies have shown that compared to the observation group, patients receiving one year of trastuzumab treatment had a 12-year OS rate increased from 73% to 79% (47). For hormone receptor-positive (HormR-positive) BC, endocrine therapy can be used. Tamoxifen can reduce the risk of recurrence by about 50% and the risk of death by about 28% in estrogen receptor-positive (ER-positive) patients (48). For the most aggressive triple-negative breast cancer (TNBC), immunotherapy can be used. For instance, pembrolizumab combined with chemotherapy as adjuvant therapy has significantly improved OS. In the KEYNOTE-522 trial, with a median follow-up of 75.1 months, the 5-year OS rate was 86.6% in the pembrolizumab-chemotherapy group compared to 81.7% in the placebo-chemotherapy group, and the 5-year event-free survival rates were 81.2% and 72.2%, respectively (49). Postoperative adjuvant systemic therapy effectively targets systemic microscopic metastases, reduces recurrence risk, and improves survival rates, providing personalized treatment strategies for patients with different molecular subtypes and significantly enhancing BC survival rates.
BC subtypes demonstrate distinct recurrence timelines (32, 50). Luminal A subtype BC generally carries a low risk of recurrence, but some patients may experience delayed metastasis over 10 years post-diagnosis. Luminal B subtype BC typically exhibits a higher risk of recurrence within the first 5 years following diagnosis. HER2-positive BC often presents with the highest risk of recurrence within the first 3–5 years post-diagnosis. TNBC demonstrates the most aggressive early recurrence pattern, usually peaking within the first 3 years after diagnosis. Additionally, research indicates that the risk of BC recurrence can persist for 10 to 32 years (51). Therefore, for strategies that omitting ALND, long-term recurrence risk assessment and management must be considered. This study divided the included articles into 5-year and 10-year subgroups. Fortunately, no significant differences were observed in OS and DFS between the experimental group and the control group in either the 5-year subgroup or the 10-year subgroup. Studies were categorized into RCTs and cohort studies subgroups, with no statistically significant differences in clinical outcomes observed between these two groups. This study also examined the differences between Eastern and Western patients, including age at onset, subtype distribution, and genetic factors (52, 53). In Western countries, BC typically occurs at a later age, predominantly after 60 years old, whereas in Eastern regions, the peak incidence age is approximately 50 years old. Regarding subtype distribution: the most prevalent molecular subtype globally is HormR-positive/HER2-negative. However, in Eastern regions, the incidence of TNBC and HER2-positive BC is relatively higher. Concerning genetic factors: family history accounts for a larger proportion of BC cases in Western countries, with certain hereditary syndromes such as breast cancer susceptibility gene (BRCA) related breast and ovarian cancer being more common. In contrast, while family history remains an important risk factor in Eastern regions, its contribution to overall BC cases is relatively lower, and the types and characteristics of genetic syndromes differ from those observed in Western populations. Considering these differences, this study divided the included articles into Eastern and Western subgroups, and the results showed no significant differences. All results indicate that for early-stage BC, cT1-2, cN0, and with 1–2 macro-metastases after SLNB, omitting ALND may be safe.
The advantages of this study are: (1) this is the most comprehensive systematic meta-analysis to date for SLNs macro-metastases, proving the safety of omitting ALND. (2) Subgroup analyses were set based on follow-up time, study type, and region. (3) High-quality RCTs and well-designed cohort studies were included, with high credibility and a large sample size.
The disadvantages are: (1) subgroup analyses based on molecular subtypes are missing, and the potential impact of different molecular subtypes on axillary treatment strategies has not been clarified. (2) The maximum follow-up duration in this study was 10 years; however, certain subtypes (e.g., Luminal A) may require extended follow-up beyond this period. The absence of data beyond 10 years limits the assessment of long-term safety, thereby restricting the generalizability and clinical applicability of the findings. (3) The number of included Eastern studies is limited, and the study conclusions cannot yet be directly applied globally. (4) Heterogeneity in clinical practice patterns across different regions and time periods-particularly in surgical techniques and adjuvant treatment protocols may influence patient prognosis, thereby restricting the generalizability of our findings to a global population. (5) The lack of blinding in the included RCTs and regional differences in imaging and pathology standards for assessing recurrence may introduce subjectivity into DFS evaluation, potentially biasing the results. The findings of this study still require validation through larger sample sizes and multicenter randomized controlled trials.
This study shows that omitting ALND is safe for specific BC patients. In the future, axillary surgery for BC may be further downscaled. Emerging large-scale RCTs like INSEMA (54) and SOUND (55) have initially confirmed the feasibility of omitting SLNB in certain patients. These advancements promise to enhance post-operative quality of life for BC patients.
Conclusion
This study demonstrates that for early-stage BC, cT1-2, cN0, and 1–2 SLN macro-metastases, omitting ALND is safe. This conclusion remains robust across varying follow-up periods, differing evidence hierarchies, and diverse geographic regions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YC: Writing – original draft, Validation, Formal Analysis, Project administration, Visualization, Data curation, Conceptualization. XZ: Data curation, Formal Analysis, Writing – original draft. QW: Validation, Writing – original draft, Data curation, Visualization. SG: Project administration, Formal Analysis, Writing – original draft. LW: Software, Resources, Writing – original draft. MZ: Writing – original draft, Resources, Software. LG: Funding acquisition, Writing – review & editing, Conceptualization. CS: Funding acquisition, Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the Zhu Xiu Shan Talent Project of Ningbo No. 2 Hospital (Project Number: 2023HMYQ09), supported by Zhejiang Medical Science and Technology Research Foundation, China (Grant No. 2021KY1021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1620034/full#supplementary-material
Supplementary Figure 1 | Risk of bias assessment of included RCTs studies.
Supplementary Figure 2 | Summary of risk of bias across RCTs studies.
Supplementary Figure 3 | Sensitivity analysis of DFS.
Supplementary Figure 4 | Evaluation of publication bias in DFS studies(p=0.951).
Supplementary Figure 5 | Sensitivity analysis of OS.
Supplementary Figure 6 | Evaluation of publication bias in OS studies(p=0.640).
Glossary
ALND: axillary lymph node dissection
BC: breast cancer
BRCA: breast cancer susceptibility gene
CI: confidence interval
cN0: clinically node-negative
DFS: disease-free survival
ER: estrogen receptor
ESMO: European Society for Medical Oncology
HER2: human epidermal growth factor receptor 2
HR: hazard ratio
ITC: isolated tumor cells
HormR: hormone receptor
MeSH: Medical Subject Headings
NOS: Newcastle-Ottawa Scale
NSLN: non-sentinel lymph node
OS: overall survival
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
PROSPERO: International Prospective Register of Systematic Reviews
RCT: randomized controlled trial
RoB1.0: Cochrane risk of bias tool 1.0
SLN: sentinel lymph node
SLNB: sentinel lymph node biopsy
TNBC: triple-negative breast cancer
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Bi Z and Wang Y. Advances in regional nodal management of early-stage breast cancer. Chin J Cancer Res = Chung-kuo Yen Cheng Yen Chiu. (2024) 36:215–25. doi: 10.21147/j.issn.1000-9604.2024.02.08
3. Magnoni F, Galimberti V, Corso G, Intra M, Sacchini V, and Veronesi P. Axillary surgery in breast cancer: An updated historical perspective. Semin Oncol. (2020) 47:341–52. doi: 10.1053/j.seminoncol.2020.09.001
4. Appelgren M, Sackey H, Wengström Y, Johansson K, Ahlgren J, Andersson Y, et al. Patient-reported outcomes one year after positive sentinel lymph node biopsy with or without axillary lymph node dissection in the randomized SENOMAC trial. Breast. (2022) 63:16–23. doi: 10.1016/j.breast.2022.02.013
5. Park KU, Somerfield MR, Anne N, Brackstone M, Conlin AK, Couto HL, et al. Sentinel lymph node biopsy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. (2025) 43:1720–41. doi: 10.1200/JCO-25-00099
6. Duffy SW, Tabár L, Yen AMF, Dean PB, Smith RA, Jonsson H, et al. Beneficial effect of consecutive screening mammography examinations on mortality from breast cancer: A prospective study. Radiology. (2021) 299:541–7. doi: 10.1148/radiol.2021203935
7. Hersh EH and King TA. De-escalating axillary surgery in early-stage breast cancer. Breast. (2022) 62:S43–9. doi: 10.1016/j.breast.2021.11.018
8. Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. (1997) 349:4. doi: 10.1016/S0140-6736(97)01004-0
9. Si J, Guo R, Pan H, Lu X, Guo Z, Han C, et al. Axillary lymph node dissection can be omitted in breast cancer patients with mastectomy and false-negative frozen section in sentinel lymph node biopsy. Front Oncol. (2022) 12:869864. doi: 10.3389/fonc.2022.869864
10. Carlo JT, Grant MD, Knox SM, Jones RC, Hamilton CS, Livingston SA, et al. Survival analysis following sentinel lymph node biopsy: A validation trial demonstrating its accuracy in staging early breast cancer. Bayl Univ Med Cent Proc. (2005) 18:103–7. doi: 10.1080/08998280.2005.11928044
11. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (alliance) randomized clinical trial. JAMA. (2017) 318:918. doi: 10.1001/jama.2017.11470
12. De Boniface J, Filtenborg Tvedskov T, Rydén L, Szulkin R, Reimer T, Kühn T, et al. Omitting axillary dissection in breast cancer with sentinel-node metastases. N Engl J Med. (2024) 390:1163–75. doi: 10.1056/NEJMoa2313487
13. Bartels SAL, Donker M, Poncet C, Sauvé N, Straver ME, Van De Velde CJH, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-year results of the randomized controlled EORTC 10981–22023 AMAROS trial. J Clin Oncol. (2023) 41:2159–65. doi: 10.1200/JCO.22.01565
14. Peristeri DV and Harissis HV. Axillary lymph node dissection vs sentinel biopsy only among women with early-stage breast cancer and sentinel node metastasis: A systematic review and meta-analysis. Breast J. (2021) 27:158–64. doi: 10.1111/tbj.14140
15. Petousis S, Christidis P, Margioula-Siarkou C, Liberis A, Vavoulidis E, Margioula-Siarkou G, et al. Axillary lymph node dissection vs. sentinel node biopsy for early-stage clinically node-negative breast cancer: A systematic review and meta-analysis. Arch Gynecol Obstet. (2022) 306:1221–34. doi: 10.1007/s00404-022-06458-8
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (clin Res Ed). (2021) 372:n71. doi: 10.1136/bmj.n71
17. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (clin Res Ed). (2011) 343:d5928. doi: 10.1136/bmj.d5928
18. Melsen WG, Bootsma MCJ, Rovers MM, and Bonten MJM. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. (2014) 20:123–9. doi: 10.1111/1469-0691.12494
19. Lin L, Chu H, Murad MH, Hong C, Qu Z, Cole SR, et al. Empirical comparison of publication bias tests in meta-analysis. J Gen Intern Med. (2018) 33:1260–7. doi: 10.1007/s11606-018-4425-7
20. Tinterri C, Gentile D, Gatzemeier W, Sagona A, Barbieri E, Testori A, et al. Preservation of axillary lymph nodes compared with complete dissection in T1–2 breast cancer patients presenting one or two metastatic sentinel lymph nodes: The SINODAR-ONE multicenter randomized clinical trial. Ann Surg Oncol. (2022) 29:5732–44. doi: 10.1245/s10434-022-11866-w
21. Canavese G, Bruzzi P, Catturich A, Tomei D, Carli F, Garrone E, et al. Sentinel lymph node biopsy versus axillary dissection in node-negative early-stage breast cancer: 15-year follow-up update of a randomized clinical trial. Ann Surg Oncol. (2016) 23:2494–500. doi: 10.1245/s10434-016-5177-4
22. Sávolt Á, Péley G, Polgár C, Udvarhelyi N, Rubovszky G, Kovács E, et al. Eight-year follow up result of the OTOASOR trial: The optimal treatment of the axilla – surgery or radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer. Eur J Surg Oncol (ejso). (2017) 43:672–9. doi: 10.1016/j.ejso.2016.12.011
23. Zhao X, Yang L, Cao C, and Song Z. The prognostic analysis of further axillary dissection in breast cancer with 1–2 positive sentinel lymph nodes undergoing mastectomy. Front Oncol. (2024) 14:1406981. doi: 10.3389/fonc.2024.1406981
24. Schwieger L, Postlewait LM, Subhedar PD, Geng F, Liu Y, Gillespie T, et al. Patterns of completion axillary dissection for patients with cT1-2N0 breast cancer undergoing total mastectomy with positive sentinel lymph nodes. J Surg Oncol. (2024) 129:468–80. doi: 10.1002/jso.27503
25. De Wild SR, Van Roozendaal LM, De Wilt JHW, Van Dalen T, Van Der Hage JA, Van Duijnhoven FH, et al. De-escalation of axillary treatment in the event of a positive sentinel lymph node biopsy in cT1–2 N0 breast cancer treated with mastectomy: Nationwide registry study (BOOG 2013-07). Br J Surg. (2024) 111:znae077. doi: 10.1093/bjs/znae077
26. Joo JH, Kim SS, Son BH, Ahn SD, Jung JH, Choi EK, et al. Axillary lymph node dissection does not improve post-mastectomy overall or disease-free survival among breast cancer patients with 1–3 positive nodes. Cancer Res Treat. (2019) 51:1011–21. doi: 10.4143/crt.2018.438
27. Sanvido VM, Elias S, Facina G, Bromberg SE, and Nazário ACP. Survival and recurrence with or without axillary dissection in patients with invasive breast cancer and sentinel node metastasis. Sci Rep. (2021) 11:19893. doi: 10.1038/s41598-021-99359-w
28. Sun J, Mathias BJ, Laronga C, Sun W, Zhou JM, Fulp WJ, et al. Impact of axillary dissection among patients with sentinel node-positive breast cancer undergoing mastectomy. J Natl Compr Cancer Netw : JNCCN. (2021) 19:40–7. doi: 10.6004/jnccn.2020.7597
29. Jung J, Han W, Lee ES, Jung SY, Han JH, Noh DY, et al. Retrospectively validating the results of the ACOSOG Z0011 trial in a large asian Z0011-eligible cohort. Breast Cancer Res Treat. (2019) 175:203–15. doi: 10.1007/s10549-019-05157-4
30. Arisio R, Borella F, Porpiglia M, Durando A, Bellino R, Bau MG, et al. Axillary dissection no axillary dissection in breast cancer patients with positive sentinel lymph node: A single institution experience. In Vivo. (2019) 33:1941–7. doi: 10.21873/invivo.11689
31. Bilimoria KY, Bentrem DJ, Hansen NM, Bethke KP, Rademaker AW, Ko CY, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. JCO. (2009) 27:2946–53. doi: 10.1200/JCO.2008.19.5750
32. Lapcik P, Pospisilova A, Janacova L, Grell P, Fabian P, and Bouchal P. How different are the molecular mechanisms of nodal and distant metastasis in luminal a breast cancer? Cancers. (2020) 12:2638. doi: 10.3390/cancers12092638
33. Rocco N, Velotti N, Pontillo M, Vitiello A, Berardi G, Accurso A, et al. New techniques versus standard mapping for sentinel lymph node biopsy in breast cancer: A systematic review and meta-analysis. Updates Surg. (2023) 75:1699–710. doi: 10.1007/s13304-023-01560-1
34. Sawaki M, Shien T, and Iwata H. TNM classification of Malignant tumors (breast cancer study group). Jpn J Clin Oncol. (2019) 49:228–31. doi: 10.1093/jjco/hyy182
35. Kelley MC, Hansen N, and McMasters KM. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Am J Surg. (2004) 188:49–61. doi: 10.1016/j.amjsurg.2003.10.028
36. Alcaide SM, Diana CAF, Herrero JC, Vegue LB, Perez AV, Arce ES, et al. Can axillary lymphadenectomy be avoided in breast cancer with positive sentinel lymph node biopsy? Predictors of non-sentinel lymph node metastasis. Arch Gynecol Obstet. (2022) 306:2123–31. doi: 10.1007/s00404-022-06556-7
37. Galimberti V, Cole BF, Viale G, Veronesi P, Vicini E, Intra M, et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. (2018) 19:1385–93. doi: 10.1016/S1470-2045(18)30380-2
38. Loibl S, André F, Bachelot T, Barrios CH, Bergh J, Burstein HJ, et al. Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. (2024) 35:159–82. doi: 10.1016/j.annonc.2023.11.016
39. Fan YJ, Li JC, Zhu DM, Zhu HL, Zhao Y, Zhu XB, et al. Efficacy and safety comparison between axillary lymph node dissection with no axillary surgery in patients with sentinel node-positive breast cancer: A systematic review and meta-analysis. BMC Surg. (2023) 23:209. doi: 10.1186/s12893-023-02101-8
40. Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, et al. The sentinel node in breast cancer — a multicenter validation study. N Engl J Med. (1998) 339:941–6. doi: 10.1056/NEJM199810013391401
41. Chen W, Hoffmann AD, Liu H, and Liu X. Organotropism: New insights into molecular mechanisms of breast cancer metastasis. NPJ Precis Oncol. (2018) 2:4. doi: 10.1038/s41698-018-0047-0
42. Shah C, Al-Hilli Z, and Vicini F. Advances in breast cancer radiotherapy: Implications for current and future practice. JCO Oncol Pract. (2021) 17:697–706. doi: 10.1200/OP.21.00635
43. Taylor C, Dodwell D, McGale P, Hills RK, Berry R, Bradley R, et al. Radiotherapy to regional nodes in early breast cancer: An individual patient data meta-analysis of 14–324 women in 16 trials. Lancet. (2023) 402:1991–2003. doi: 10.1016/S0140-6736(23)01082-6
44. Park M, Kim D, Ko S, Kim A, Mo K, and Yoon H. Breast cancer metastasis: Mechanisms and therapeutic implications. Int J Mol Sci. (2022) 23:6806. doi: 10.3390/ijms23126806
45. Alamoodi M, Patani N, Mokbel K, Wazir U, and Mokbel K. Reevaluating axillary lymph node dissection in total mastectomy for low axillary burden breast cancer: Insights from a meta-analysis including the SINODAR-ONE trial. Cancers. (2024) 16:742. doi: 10.3390/cancers16040742
46. Early Breast Cancer Trialists’ Collaborative Group (Ebctcg). Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100–000 women in 123 randomised trials. Lancet. (2012) 379:432–44. doi: 10.1016/S0140-6736(11)61625-5
47. Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin adjuvant (HERA) trial. Lancet (lond Engl). (2017) 389:1195–205. doi: 10.1016/S0140-6736(16)32616-2
48. Muss HB. Role of adjuvant endocrine therapy in early-stage breast cancer. Semin Oncol. (2001) 28:313–21. doi: 10.1016/S0093-7754(01)90125-3
49. Schmid P, McArthur H, Denkert C, Harbeck N, Untch M, Foukakis T, et al. Overall survival with pembrolizumab in early-stage triple-negative breast cancer. N Engl J Med. (2024) 391:1981–91. doi: 10.1056/NEJMoa2409932
50. Xu T, Zhang H, Yang BB, Qadir J, Yuan H, and Ye T. Tumor-infiltrating immune cells state-implications for various breast cancer subtypes. Front Immunol. (2025) 16:1550003. doi: 10.3389/fimmu.2025.1550003
51. Fillon M. Breast cancer recurrence risk can remain for 10 to 32 years. CA Cancer J Clin. (2022) 72:197–9. doi: 10.3322/caac.21724
52. Yu AYL, Thomas SM, DiLalla GD, Greenup RA, Hwang ES, Hyslop T, et al. Disease characteristics and mortality among asian women with breast cancer. Cancer. (2022) 128:1024–37. doi: 10.1002/cncr.34015
53. Msph ANG, Sung H, Newman LA, Freedman RA, Smith RA, Ma JS, et al. Breast cancer statistics 2024. CA Cancer J Clin. (2024) 74:477–95. doi: 10.3322/caac.21863
54. Reimer T, Stachs A, Veselinovic K, Kühn T, Heil J, Polata S, et al. Axillary surgery in breast cancer — primary results of the INSEMA trial. N Engl J Med. (2025) 392:1051–64. doi: 10.1056/NEJMoa2412063
55. Gentilini OD, Botteri E, Sangalli C, Galimberti V, Porpiglia M, Agresti R, et al. Sentinel lymph node biopsy vs no axillary surgery in patients with small breast cancer and negative results on ultrasonography of axillary lymph nodes: The SOUND randomized clinical trial. JAMA Oncol. (2023) 9:1557. doi: 10.1001/jamaoncol.2023.3759
Keywords: omitting, early-stage breast cancer, sentinel lymph node biopsy, axillary lymph node dissection, macro-metastases, meta-analysis
Citation: Chen Y, Zhang X, Wu Q, Gao S, Wang L, Zeng M, Gu L and Sheng C (2025) Safety analysis of omitting axillary lymph node dissection in early-stage breast cancer with 1–2 sentinel lymph nodes macro-metastases: a meta-analysis. Front. Oncol. 15:1620034. doi: 10.3389/fonc.2025.1620034
Received: 29 April 2025; Accepted: 09 September 2025;
Published: 25 September 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Kristina Bojanic, Josip Juraj Strossmayer University of Osijek, CroatiaTing Ye, Southwest Medical University, China
Copyright © 2025 Chen, Zhang, Wu, Gao, Wang, Zeng, Gu and Sheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changrui Sheng, U2hlbmdjcjE2ODlAMTYzLmNvbQ==; Lihu Gu, Z3VsaWh1eXVhbnpoaUAxMjYuY29t
 Yu Chen1
Yu Chen1 Lihu Gu
Lihu Gu Changrui Sheng
Changrui Sheng