- 1Department of Pediatric Cardiology, Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain
- 2Cardiovascular Research Group iCare4Kids, Institut de Recerca Sant Joan de Déu, Esplugues de Llobregat, Spain
- 3Data and Digital Strategy Department, Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain
- 4Department of Pediatric Oncology, Barcelona Pediatric Cancer Center, Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain
- 5Department of Neonatology, Barcelona Pediatric Cancer Center, Hospital Sant Joan de Déu, Esplugues de Llobregat, Spain
- 6Departament of Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA, United States
Background and objectives: Advances in cancer therapies have significantly improved survival rates in children. However, treatment-related toxicities remain common. This study aims to evaluate the incidence and characteristics of cardiotoxicity in a pediatric cancer cohort.
Methods: This prospective study included pediatric patients who received chemotherapy between September 2020 and March 2023. Patients were categorized into five groups according to treatment phase: baseline, early treatment, late treatment, end-of-treatment, and relapse. Cardiovascular evaluation included anthropometric assessment, laboratory biomarkers, electrocardiogram (ECG) and functional echocardiography. Patients were stratified for cardiotoxicity according to pediatric and adult clinical practice guidelines.
Results: 265 patients were included (mean age 9.95 ± 5.26 years). The incidence of ventricular dysfunction was 2.3%. A decline in LVEF > 10% from baseline was observed in 16.5% of patients. Abnormal global longitudinal strain (GLS) values were found in 34.7%; significant ECG changes in 16.2%, and elevated Troponin I levels in 7.1%. Based on echocardiographic and laboratory findings, patients undergoing treatment showed greater cardiac involvement compared with those in other groups.
Conclusions: Although the overall incidence of overt ventricular dysfunction was low, the use of ECG and GLS enhanced the sensitivity for detecting of subclinical cardiac impairment in pediatric patients receiving chemotherapy.
Introduction
Cancer is the leading cause of disease-related mortality in the pediatric age group (1). Thanks to advances in the diagnosis and treatment of childhood cancer, more patients are now surviving to adulthood. However, this improved survival has also brought to light the substantial burden of cardiovascular disease, with increased morbidity and mortality attributed to cardiotoxicity (2, 3).
Childhood cancer survivors (CCS) face a significantly elevated risk of cardiovascular disease as a late effect of cancer therapy. According to data from the Childhood Cancer Survivor Study (CCSS), the risk of developing cardiovascular disease is 5 to 15 times higher in CCS compared to the general population, depending on the specific malignancy and treatment exposure. Moreover, the risk of heart failure is up to 8 times greater in CCS than in their healthy siblings. The spectrum of cardiotoxicity-related complications is broad and includes cancer therapy-related cardiac dysfunction (CTRCD), arrhythmias, valvular disease, and pericardial involvement. Among these, CTRCD is one of the most common and clinically significant. Despite its potential reversibility with early detection and intervention, it often remains undiagnosed in its subclinical stages (4–10).
Cardiotoxicity can manifest in the short, medium, or long term (11). Its etiology and pathogenesis are multifactorial, influenced by both the underlying disease and the treatment received. Moreover, patient-specific risk factors, including individual predisposition and lifestyles, also modulate the development of cardiotoxicity (12).
Currently, the evaluation of cardiovascular function is mostly based on echocardiography and the analysis of serum biomarkers such as Troponin or the N-terminal pro b-type natriuretic peptide (NT-proBNP) (10). Myocardial function is easily assessed using echocardiography. Particularly useful for the quantification of the myocardial function are the left ventricular ejection fraction (LVEF) and the Global Longitudinal Strain (GLS) (13, 14) an echocardiographic measure of myocardial deformation that is more sensitive than LVEF in the detection of subclinical dysfunction or asymptomatic CTRCD (10, 13–15)3. Current guidelines establish a cardiotoxicity risk stratification by measuring of LVEF and GLS, and assessing their decline from baseline.
Follow-up recommendations for pediatric cancer survivors have been recently published by the American Society of Echocardiography (16). According to these guidelines, an LVEF greater than 55% is considered within normal limits, whereas an LVEF below 50% and/or a GLS above –16% (less negative) is classified as abnormal.
This study aimed to perform a comprehensive cardiovascular evaluation in children with cancer at different stages of their disease from diagnosis to the end of treatment.
Materials and methods
Study design and population
A descriptive, single-center, cross-sectional study was conducted at a tertiary, referral, pediatric cancer center in Spain. The study enrolled all consecutive children (< 18 years old) with a diagnosis of cancer who underwent cardiovascular assessment at the cardio-oncology unit between September 2020 and March 2023. Only children with a diagnosis of onco-hematological disease requiring chemotherapy were included in the study.
Patients were included at different disease stages and assessed at a single point in time without follow-up. Thus, five different cohorts were defined according to their stage of treatment (Figure 1): i) Baseline: before the initiation of chemotherapy; ii) Early treatment: 3 months after initiation of treatment; iii) Late treatment: 6 months after the initiation of treatment; iv) End-of-treatment: 2 months after the end of treatment; v) Relapse: assessment at the time of relapse before the initiation of new onco-hematological treatment.
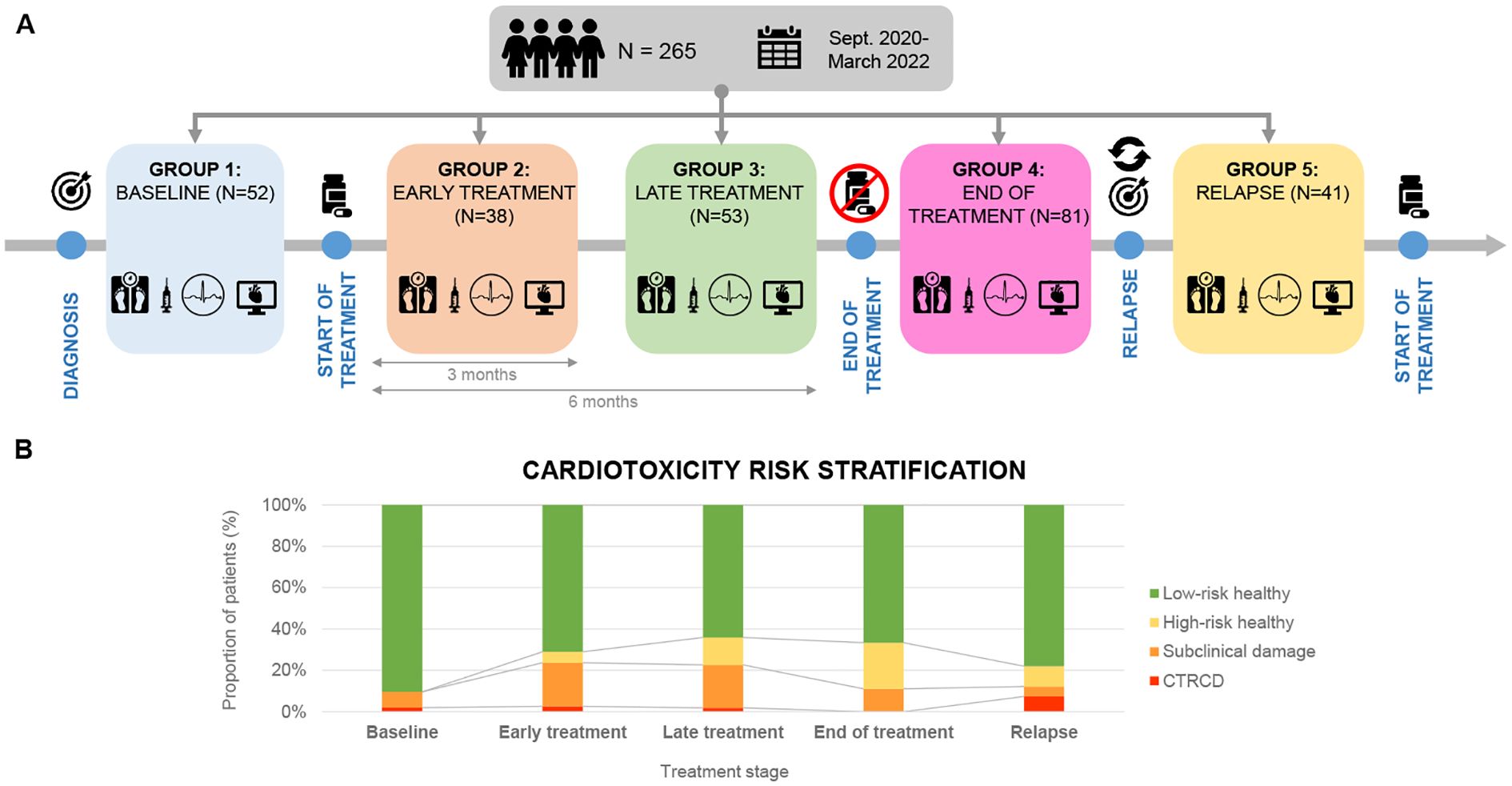
Figure 1. (A) Cross-sectional study with a sample of 265 patients recruited from September 2020 to March 2022. Patients were divided into 5 groups according to chemotherapeutic treatment phase. (B) Cardiotoxicity risk classification in each treatment phase (Ventricular dysfunction due to cardiotoxicity- Subclinical damage- High risk healthy- Low risk healthy).
Study variables
A complete medical history and physical examination were performed, including the determination of body mass index (BMI) and blood pressure (BP) with respective Z score values. Overweight was defined as BMI ≥ 2 standard deviations (SD) and underweight as BMI < 2 SD. Systolic hypertension was defined as systolic blood pressure (SBP) ≥ 2 SD and diastolic hypertension as diastolic blood pressure (DBP) ≥2 SD. Data regarding chemo- and radiotherapy were collected. Patients receiving anthracycline doses greater than >249 mg/m2 and/or chest radiotherapy over15Gy were further classified as High-Risk.
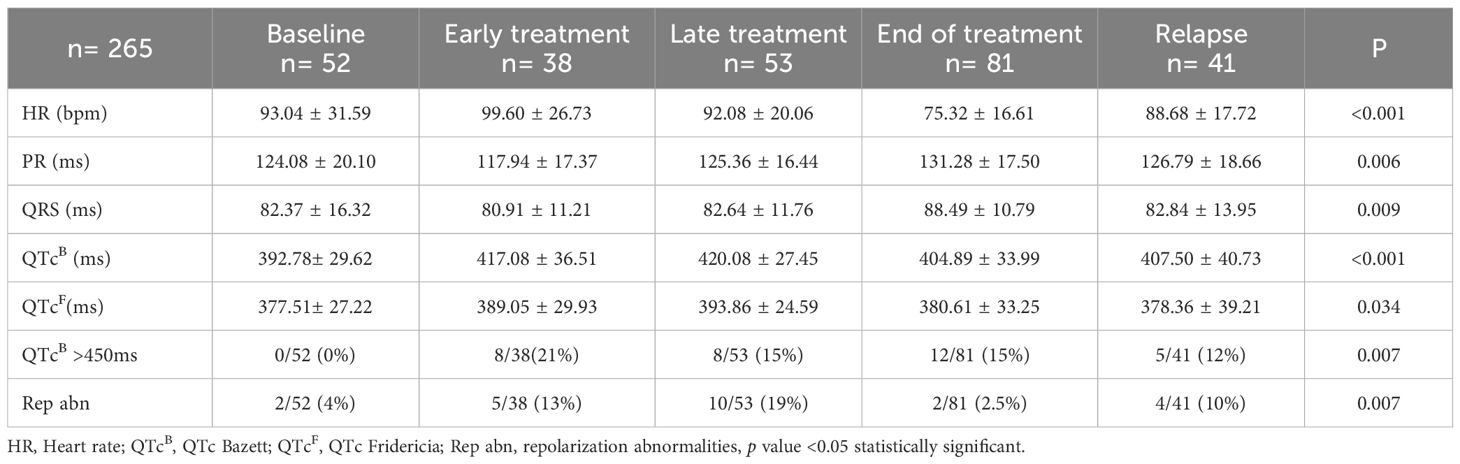
In addition, the following complementary tests were performed: 12-lead electrocardiogram (ECG), a functional echocardiography and, serum biomarkers. ECG included the measurement of the following parameters: heart rate (HR), PR interval (ms), QRS complex (ms), and QT interval corrected according to the Fridericia and Bazett (B) formula. Long QT was defined as a QTc (B) ≥ 450 ms. Repolarization disturbance was defined as flattening or inversion of the T wave in the left anterior precordial leads (II, III, aVF, V5, V6) (Figure 2).
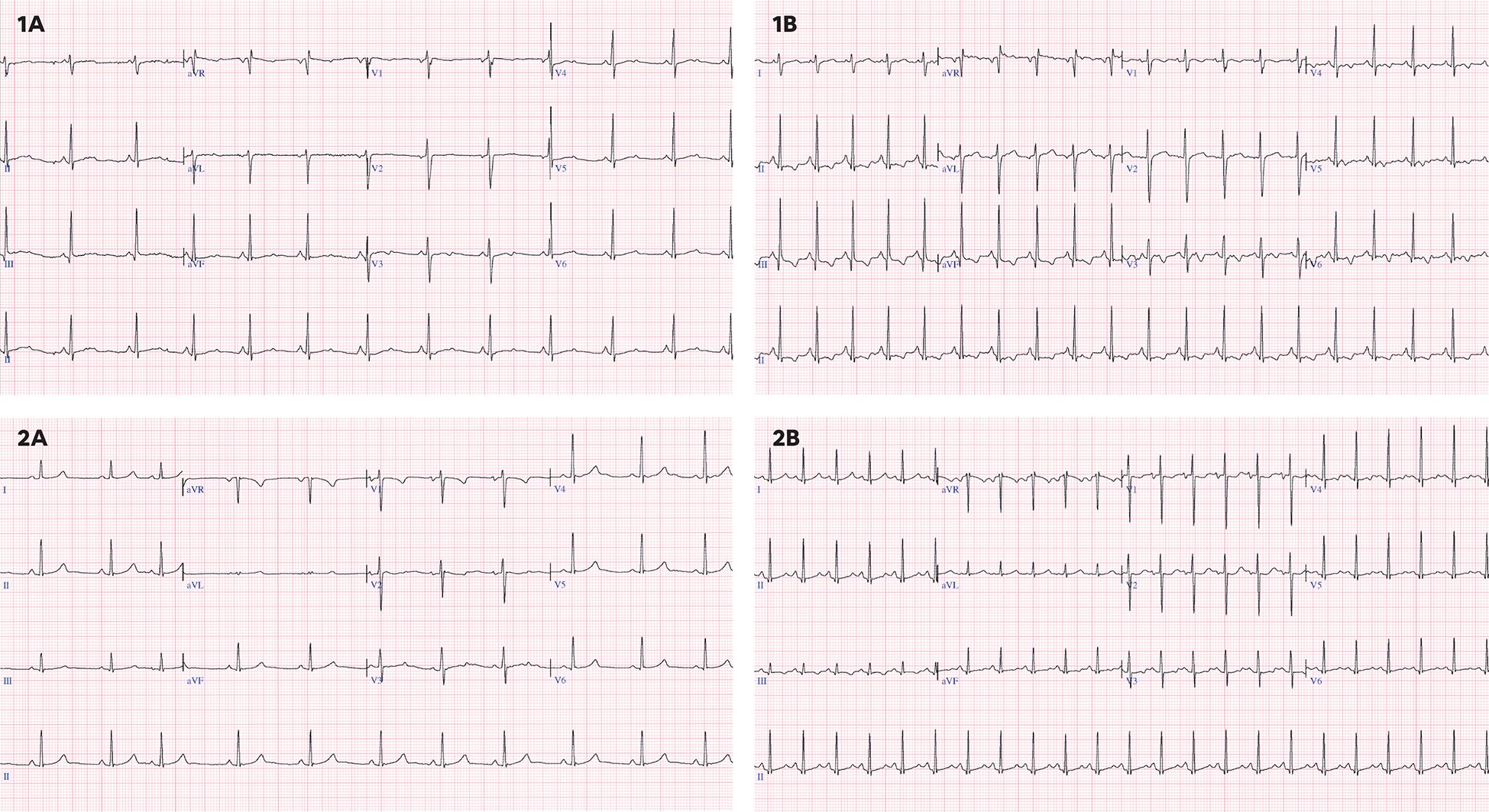
Figure 2. (1A) 16 years old, baseline ECG with sinus rhythm at 73 bpm, QTc(B) 441 ms, QTc(F) 427 ms without repolarization disturbance. (1B) 16 years old, ECG under chemotherapy with sinus rhythm at 118 bpm, QTc(B) 448 ms, QTc(F) 400 ms with repolarization alteration and negative T waves in II, III, aVF, V5,V6. (2A) 12 years, baseline ECG with sinus rhythm at 66 bpm, QTc(B) 377 ms, QTc(F) 371 ms, without repolarization alteration. (2B) 12 years, ECG under chemotherapy with sinus rhythm at 136 bpm, with QT segment lengthening and asymmetric T-wave rise with QTc(B) 481 ms, QTc(F) 420 ms, without repolarization changes.
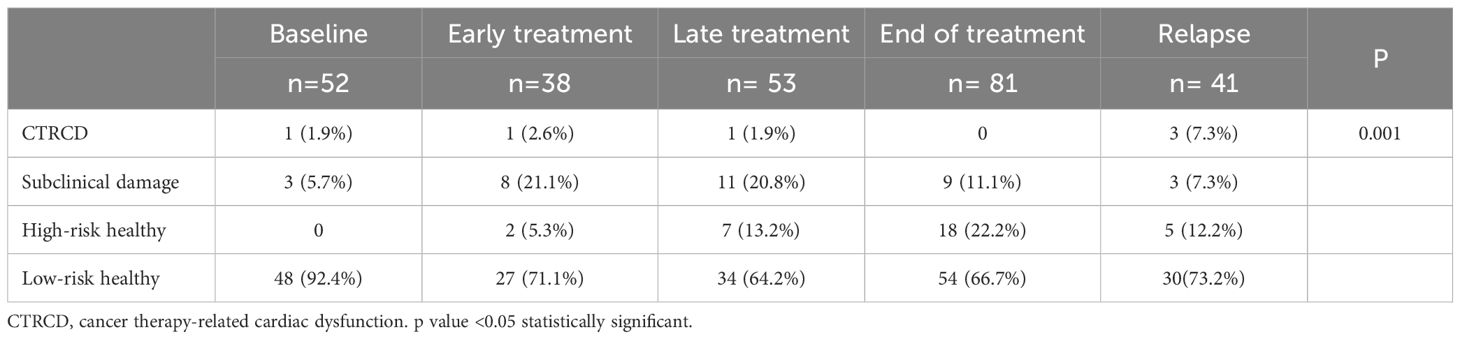
All patients underwent a comprehensive echocardiographic study including both morphological and, functional assessment. Images were acquired and further analyzed following the institutional echocardiographic study protocol. Speckle Tracking Echocardiography (STE) method was used to measure Global Longitudinal Strain (GLS) in all study patients (Supplementary Material 1). In addition, all patients had a baseline echocardiography performed prior to the initiation of treatment, with LVEF measured at that time. This baseline value was used to determine whether a >10% drop in LVEF had occurred. Troponin I and NT-proBNP levels were measured in all study patients. Both biomarkers were analyzed using a chemiluminescent microparticle immunoassay (CMIA) on the Alinity analyzer platform (Abbott Laboratories, Abbott Park, IL, USA). Using the aforementioned Cardioongology guidelines, with the caveat of not having baseline measurements of the cardiac function, four different groups of cardiovascular function were defined for this study (16). (Figure 1): Group A (CTRCD): LVEF <55%, Group B (subclinical damage): LVEF >55% and/or positive Troponin I, Group C (high-risk healthy): LVEF >55% and negative Troponin I, Group D (low-risk healthy): LVEF >55% and negative Troponin I.
Statistical analysis
Statistical analysis of the data was performed with SPSS 28.0 for Windows. Normality was tested according to the Kolmogorov-Smirnov and Shapiro-Wilk criteria. Differences between groups were analyzed for statistical significance using one-way ANOVA followed by post-hoc Tukey test for all pairwise comparisons. To perform hypothesis testing on qualitative categorical variables, the χ2 test was used. In all analyses, a p-value <0.05 was considered statistically significant.
Ethical aspects
Informed consent was obtained from patients/legal guardians before their inclusion in the study. The project developed according to the Declaration of Helsinki in its latest revision of 2013 and under the guidelines of the Law 14/2007 on Biomedical Research. Furthermore, the study was approved by the Sant Joan de Déu Foundation Research Ethics Committee and the processing, communication, and transferring of personal data of all participants complies with current legislation (European Regulation EU2016/679 and Organic Law3/2018 of 5 December on the Protection of Personal Data): PIC-227-19; PIC-88-24.
Results
Clinical and laboratory variables
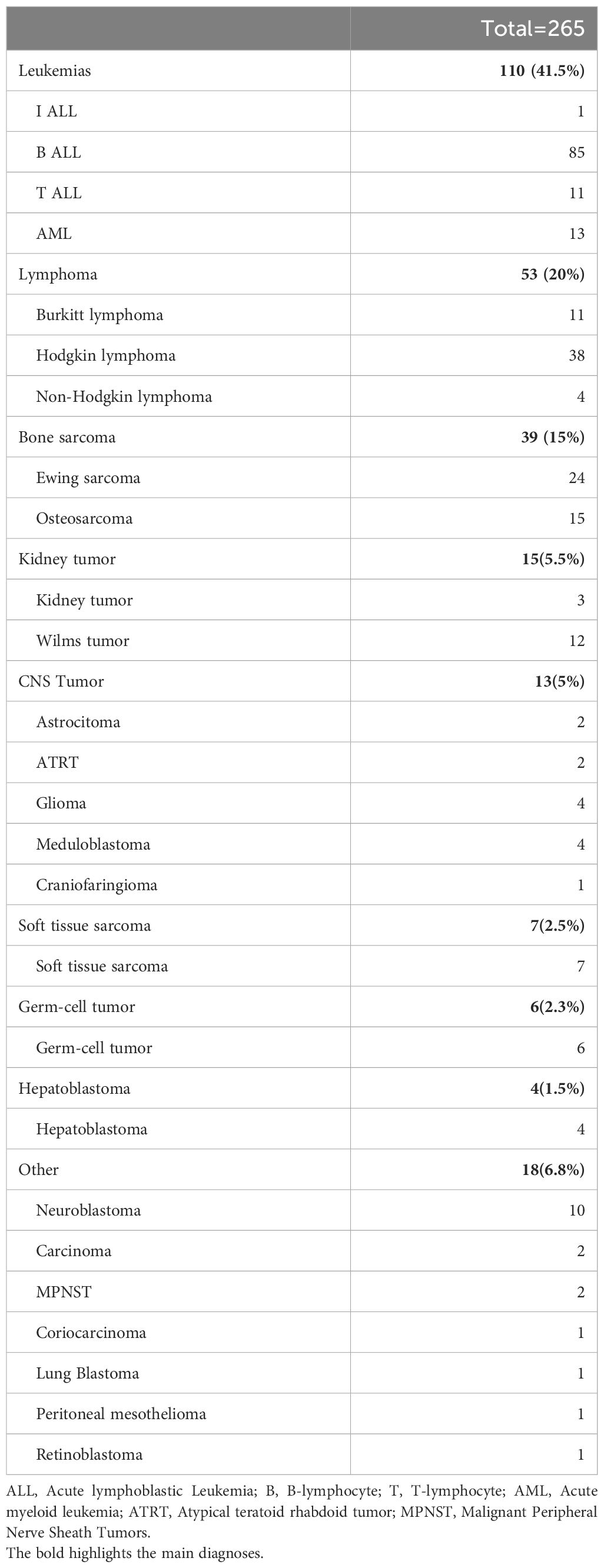
Two hundred sixty-five patients were analyzed, of whom 156 (58.8%) were male. Their mean age at enrollment was 9.95 ± 5.26 years. There were no significant differences in BMI across groups, with 4.5% (12/265) of patients classified as overweight and 4.9% (13/265) as underweight. The overall incidence of systolic hypertension was 17/265 (6.41%).
The most common diagnoses included: leukemia: 110 (41.5%), lymphoma: 53(20%), bone sarcomas: 39 (15%), kidney tumors 15 (5.5%) and CNS tumors: 13 (5%) (Table 1). Only 5/265 (1.9%) patients received cardiovascular medications such as ACE inhibitors or beta-blockers, and 4/265 (1.5%) dexrazoxane for cardioprotection. Patients were allocated to the five different groups as follows: baseline, 52/265 (19.6%); early treatment, 38/265 (14.3%); late treatment, 53/265 (20%); end-of-treatment, 81/265 (30.6%); relapse, 41/265 (15.5%).
The cumulative anthracyclines doses and radiotherapy exposure at the end-of-treatment of each of the study groups is detailed in Table 2.
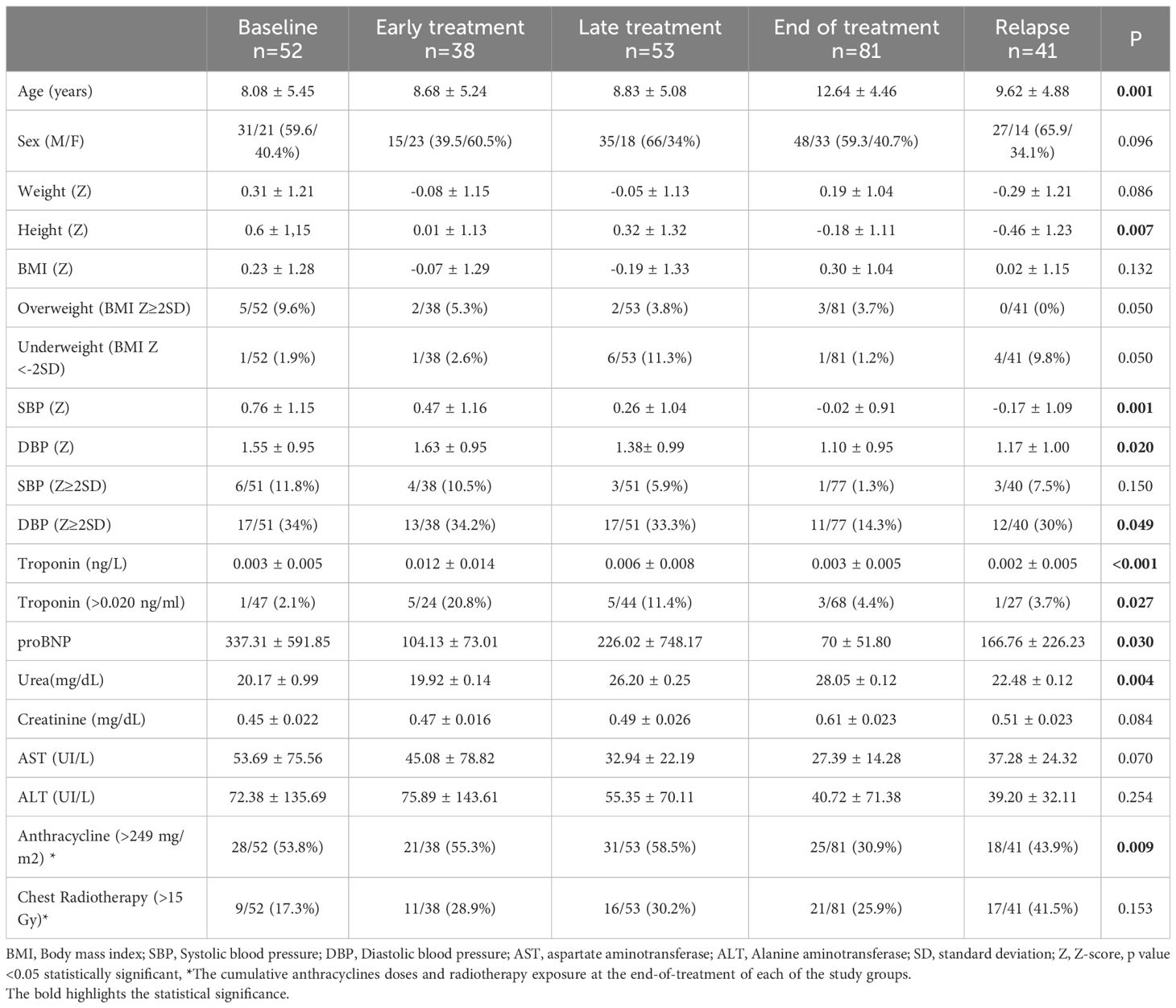
Table 2. Analysis of anthropometric variables in the different groups according to the treatment phase.
Serum biomarkers
Troponin I: Elevated levels were observed in 7.1% of patients, with the highest values in the early treatment group (0.012 ± 0.014 ng/ml; p < 0.001). The percentage of patients with high troponin levels was significantly higher in the early and late groups compared to the end-of-treatment group (20.8 vs 11.4 vs 4.4%; p=0.027).
NT-proBNP: The highest values were found in the baseline group, while the lowest levels seen in those of the end-of-treatment group (337.31 ± 591.85 vs 70 ± 51.80 ng/L; p=0.030).
Electrocardiographic variables
Electrocardiographic abnormalities were identified in 16.2% of patients. Prolonged QTc (QTc > 450 ms) was observed in 12.4% (33/265) of patients (Table 3). Compared to the baseline group, a significantly higher proportion of patients in the early and late treatment groups had prolonged QTc (0% vs. 21.1% vs. 15.1%; p = 0.007). Patients in the early and late groups had significantly longer QTc intervals than those in the baseline group, regardless of whether Bazett’s (417.08 ± 36.51; 420.08 ± 27.45 vs. 392.78 ± 29.62 ms; p = 0.001) or Fridericia’s correction was applied (389.05 ± 29.93; 393.86 ± 24.59 vs. 377.51 ± 27.22 ms; p = 0.034). Repolarization abnormalities were present in 8.7% (23/265) of patients, defined by flat or negative T waves in left precordial leads. Compared to baseline, both early and late treatment groups had a significantly higher proportion of patients with repolarization abnormalities (3.8% vs. 13.2% vs. 18.9%; p = 0.007). Although statistically significant differences were found in PR interval and QRS duration between groups, all values remained within normal limits.33/265 (12.4%).
Echocardiographic variables
Left ventricular systolic function
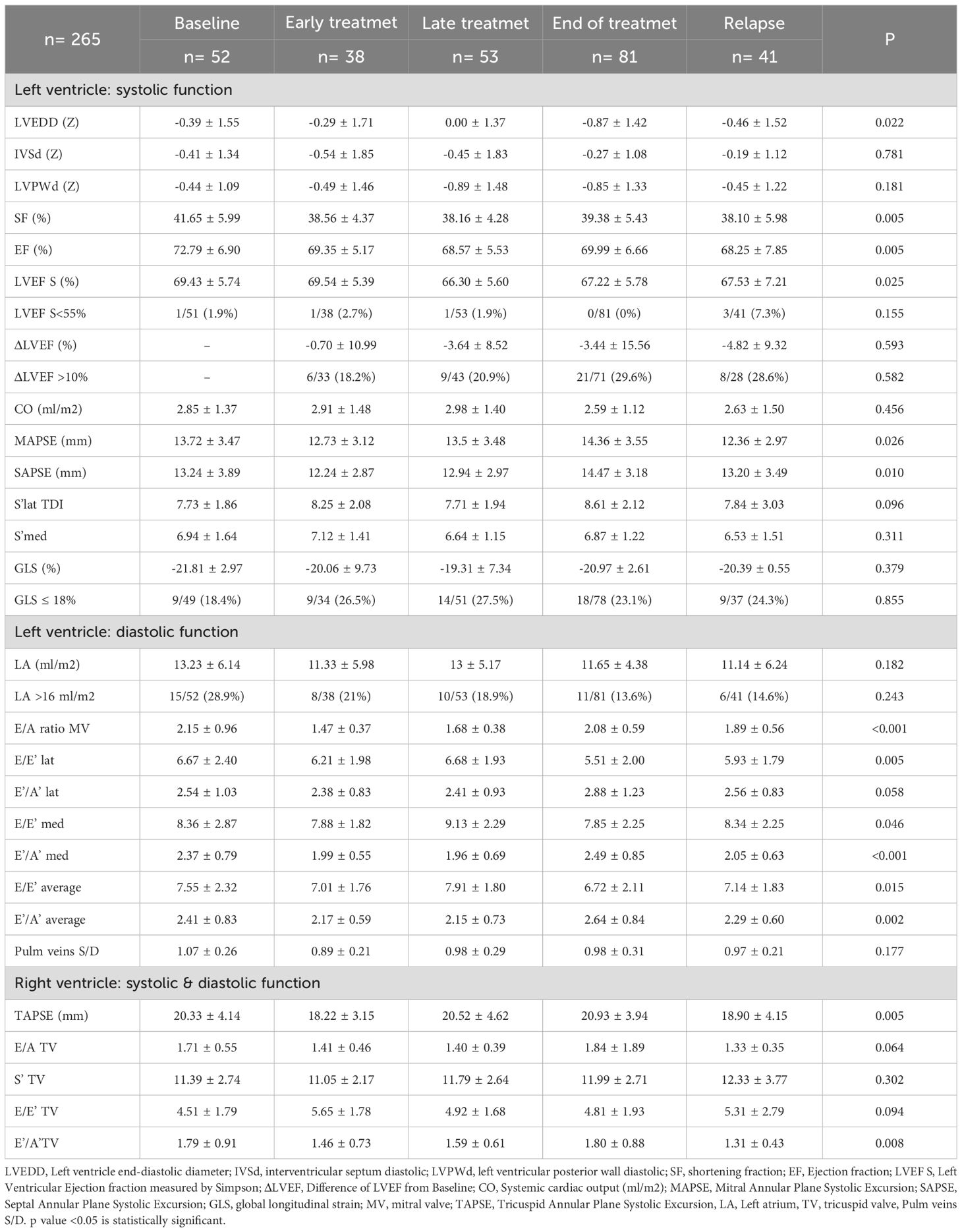
The overall incidence of CTRCD was 2.3%. Patients in late treatment group had a lower LVEF compared to the baseline group (66.30 ± 5.60 vs 69.43 ± 5.74%; p=0.025); 16.5% presented a >10% drop in LVEF from baseline, but no significant differences were found between groups (Table 4). In 34.7% of cases, GLS was ≤18%. Although the early and late groups had a higher proportion of patients with reduced GLS than the baseline group, these differences were not statistically significant. LV dilatation was observed in 6.8%, with the largest dimensions found in the baseline and late groups compared to the end-of-treatment group (Z-score: 0.39 ± 1.55 vs. 0.00 ± 1.37 vs. -0.87 ± 1.42; p = 0.022).
Left ventricular diastolic function
In all groups, the E/A ratio was >1. The lowest values were seen in early group and the highest in the baseline group (1.47 ± 0.37 vs 2.15 ± 0.96 vs; p < 0.001). At the end-of-treatment, the E/E’ ratio (both lateral and medial) was significantly lower than in the late treatment group (E/E’ lateral: 5.51 ± 2.00 vs. 6.68 ± 1.93; p = 0.005; E/E’ medial: 7.85 ± 2.25 vs. 9.13 ± 2.29; p = 0.046), although all values remained within normal ranges. Left atrial dilatation was uncommon, with only one case detected with an indexed volume > 34 ml/m². The baseline group had a higher proportion of patients with left atrial dilatation than the end-of-treatment group (28.9% vs. 13.6%), although this was not statistically significant.
Right ventricular systolic function
Patients in the early group had lower TAPSE values compared to those in the end-of-treatment group (18.22 ± 3.15 vs 20.93 ± 3.94 mm; p=0.008).
No significant valvar insufficiencies, signs of pulmonary hypertension or pericardial effusion were observed during the echocardiographic assessments.
Risk stratification - incidence of cardiotoxicity
There were statistically significant differences in the distribution of cardiovascular risk categories across treatment groups. A higher proportion of patients in the treatment groups had subclinical damage or high cardiovascular risk compared to the baseline group (33.3% vs. 5.7%; p = 0.001) (Table 5). When patients were classified solely by LVEF, no significant differences were observed among groups.
Discussion
Our analysis of a large cohort of pediatric oncology patients demonstrates that cardiovascular risk stratification evolves throughout chemotherapy treatment, with a greater proportion of patients presenting subclinical myocardial damage or high cardiovascular risk during therapy. The overall incidence of CTRCD was 2.3%, but 16.5% experienced a ≥10% decrease in LVEF from their baseline, and 34.7% had abnormal GLS values, indicating subclinical dysfunction. ECG abnormalities were observed in 16.2% of patients, primarily QT prolongation and repolarization disturbances, and 7.1% had elevated troponin levels. Interestingly, patients in the early and late treatment groups showed greater cardiac involvement, as evidenced by echocardiographic and biomarker alterations. These findings reinforce the need for continuous cardiac surveillance throughout treatment to detect early cardiotoxicity and prevent irreversible damage.
The incidence of CTRCD related to cardiotoxicity in adulthood is widely reported in the scientific literature. Cardinale et al., described an incidence of cardiotoxicity of 9% in their studied sample (14). However, information in children is scarce and there is great variability depending on the population studied and the diagnostic methods used. Bu-Lock et al. (17), analyzed 125 pediatric patients and reported an incidence of CTRCD of 5% with 19.2% of patients experiencing a significant fall in LVEF. Similarly, Agha et al. (18), in a study including 40 patients, described 5% of CTRCD with 40% of the patients experiencing decrease in LVEF. However, Kocabas et al. (19), was not able to identify CTRCD in 72 patients. In our study, despite only 2.3% of patient having CTRCD, 16.5% had subclinical impairment of the myocardial function.
Traditionally, LV systolic function has been assessed using LVEF and FS, though these measures often fail to detect subtle myocardial changes, as chemotherapy-induced damage tends to be regional and asymmetric (20). More sensitive approaches, such as tissue Doppler imaging (TDI), have been suggested as potential alternatives, particularly for tracking medial S’ velocity declines during chemotherapy (17–19, 21). Although the late treatment group in our study had worse TDI values than the other groups, these differences were not statistically significant.
Systematic measurement of GLS also allows early identification of systolic function abnormalities and has been correlated with the development of long-term cardiotoxicity (22). Thavendiranathan et al., demonstrated that the early fall in GLS was more sensitive than LVEF analysis (21). In this study, a 10-15% decrease in GLS was considered the most useful parameter for predicting long-term cardiovascular disease. However, the SUCCOUR study also questions the usefulness of this parameter, finding no significant difference in patient outcomes when function assessment at follow-up was based on GLS rather than LVEF (23). In our study, GLS was altered in 34.7% of patients, significantly increasing the sensitivity for the detection of cardiovascular risk groups compared to traditional function assessment parameters. However, without longitudinal follow-up, it remains unclear how these early GLS alterations translate into long-term cardiac dysfunction in children surviving from cancer.
Diastolic dysfunction has been explored as an early marker of cardiotoxicity in several studies. To date, there is no scientific evidence to clarify the diagnostic and/or prognostic value of its assessment. Furthermore, in the pediatric age group, changes in diastolic function seem to occur later and have great variability according to age, making their use difficult (17, 24).
Right ventricular function assessment in pediatric oncology patients presents similar challenges, as classical indices such as TAPSE and RV fractional area change have limited sensitivity in detecting subtle RV dysfunction (25). In this study, no significant alterations in RV function or pulmonary hypertension were detected, suggesting that RV involvement may be less prominent in early chemotherapy exposure.
While echocardiographic parameters provide valuable insights, ECG abnormalities have also been reported in pediatric patients undergoing chemotherapy. Previous studies have described conduction disturbances, repolarization abnormalities, and QT prolongation in up to 25% of patients (18, 26). In this study, 16.2% of patients had ECG alterations, most commonly QT prolongation and repolarization disturbances, findings that were particularly pronounced in patients undergoing active chemotherapy. These results suggest that ECG monitoring could be useful for tracking transient electrophysiological changes during treatment, though the long-term significance of these findings remains to be determined.
Biomarkers such as troponin and NT-proBNP remain the most widely used markers for detecting chemotherapy-induced myocardial injury, though interest is growing in newer biomarkers such as microRNAs, and proteomics (27). Troponins, particularly troponin I and troponin T, are the gold standard for detecting myocardial necrosis. In this study, troponin elevations were found in 7.1% of patients, with the highest values observed in those currently undergoing treatment, reinforcing its role as a potential early indicator of myocardial damage (28). NT-proBNP, a well-established marker in heart failure, has also been associated with chemotherapy-induced cardiotoxicity, with levels above 100 ng/L linked to an increased risk of cardiac events (29, 30). In pediatric patients, elevated NT-proBNP levels have been correlated with CTRCD compared to healthy controls (31). Interestingly, in our study, the highest values of NT-proBNP were found in the baseline group, while the lowest levels found in those of the end-of-treatment group suggesting than other factors, such as, inflammation, fluid management, might play a role.
The International Late Effects of Childhood Cancer Guideline Harmonization Group has developed evidence-based recommendations for long-term cardiovascular monitoring in childhood cancer survivors (32). Risk stratification is primarily based on cumulative anthracycline exposure and chest radiotherapy, which determine follow-up intervals. Interestingly, when applying adult guideline criteria, a higher percentage of patients in the early (23.7%) and late (26.4%) treatment groups were classified as having subclinical myocardial dysfunction or high cardiovascular risk. These same groups also had the highest prevalence of ECG abnormalities, reinforcing the need for continuous cardiovascular monitoring throughout treatment. The findings from this study suggest that pediatric oncology patients should undergo dynamic cardiovascular risk stratification at each stage of treatment, allowing for early interventions that may help mitigate long-term cardiovascular complications.
Conclusion
Cardiovascular function in children with cancer changes dynamically throughout chemotherapy, with significant alterations occurring during active treatment phases. Frequent cardiovascular assessments are essential for early detection of myocardial dysfunction, allowing for timely interventions to prevent irreversible cardiac damage. GLS and ECG abnormalities appear to improve sensitivity in detecting subclinical cardiotoxicity, though long-term studies are needed to confirm their prognostic value in children. Pediatric oncology patients require individualized, stage-specific cardiovascular risk stratification to optimize long-term cardiac outcomes. Future studies with larger cohorts and longitudinal follow-up are necessary to refine screening protocols and risk stratification models in pediatric cardio-oncology.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Sant Joan de Déu Foundation Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
EA: Methodology, Data curation, Validation, Project administration, Conceptualization, Supervision, Formal Analysis, Writing – original draft, Investigation, Software, Writing – review & editing, Resources, Funding acquisition, Visualization. PG: Writing – review & editing, Formal Analysis, Visualization, Methodology, Data curation, Resources, Validation, Investigation, Software, Project administration, Supervision, Funding acquisition, Writing – original draft, Conceptualization. MG: Validation, Writing – review & editing. CR: Writing – original draft, Data curation. MC: Methodology, Writing – review & editing, Supervision, Validation. PR: Data curation, Writing – review & editing. AM: Conceptualization, Writing – review & editing, Supervision. JS: Funding acquisition, Project administration, Validation, Formal Analysis, Supervision, Methodology, Writing – review & editing, Conceptualization, Investigation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The present work has been partially funded by the Spanish Society of Cardiology: Clinical Research Projects in Cardiology of the Spanish Society of Cardiology (SEC/FEC-INV-CLIN20/010). Grant TED2021-132025B-C44 funded by MICIU/AEI/10.13039/501100011033 and by the European Union Next Generation EU/PRTR. PGC acknowledges support from the grant#RYC2023-043724-I by MCIU/AEI/10.13039/501100011033 and from FSE+.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1623081/full#supplementary-material
Abbreviations
BMI, body mass index; BP, Blood pressure; CTRCD, cancer therapy-related cardiac dysfunction; GLS, Global Longitudinal Strain; LVEF, left ventricular ejection fraction.; LVDI, left ventricular diastolic diameter.; MAPSE, Mitral Annular Plane Mitral Systolic Excursion; SAPSE, Septal Annular Septal Plane Systolic Excursion; SD, standard deviation; TAPSE, Tricuspid Annular Plane Systolic Excursion; TDI, Tissue Doppler.
References
1. Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, et al. Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5—a population-based study. Lancet Oncol. (2014) 15:35–47. doi: 10.1016/S1470-2045(13)70548-5
2. Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the childhood cancer survivor study. JNCI J Natl Cancer Inst. (2008) 100:1368–79. doi: 10.1093/jnci/djn310
3. Fidler MM, Reulen RC, Henson K, Kelly J, Cutter D, Levitt GA, et al. Population-based long-term cardiac-specific mortality among 34–489 five-year survivors of childhood cancer in Great Britain. Circulation. (2017) 135:951–63. doi: 10.1161/CIRCULATIONAHA.116.024811
4. Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. (2009) 339:b4606–6. doi: 10.1136/bmj.b4606
5. Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American heart association. Circulation. (2013) 128:1927–95. doi: 10.1161/CIR.0b013e3182a88099
6. Hooning MJ, Botma A, Aleman BMP, Baaijens MHA, Bartelink H, Klijn JGM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. JNCI J Natl Cancer Inst. (2007) 99:365–75. doi: 10.1093/jnci/djk064
7. Armstrong GT, Joshi VM, Ness KK, Marwick TH, Zhang N, Srivastava D, et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer. J Am Coll Cardiol. (2015) 65:2511–22. doi: 10.1016/j.jacc.2015.04.013
8. Bansal N, Amdani SM, Hutchins KK, and Lipshultz SE. Cardiovascular disease in survivors of childhood cancer. Curr Opin Pediatr. (2018) 30:628–38. doi: 10.1097/MOP.0000000000000675
9. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy. J Am Coll Cardiol. (2010) 55:213–20. doi: 10.1016/j.jacc.2009.03.095
10. López-Fernández T, Martín García A, Santaballa Beltrán A, Montero Luis Á, García Sanz R, Mazón Ramos P, et al. Cardio-onco-hematology in clinical practice. Position paper and recommendations. Rev Esp Cardiol Engl Ed. (2017) 70:474–86. doi: 10.1016/j.rec.2016.12.041
11. Harake D, Franco VI, Henkel JM, Miller TL, and Lipshultz SE. Cardiotoxicity in childhood cancer survivors: strategies for prevention and management. Future Cardiol. (2012) 8:647–70. doi: 10.2217/fca.12.44
12. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail. (2017) 19:9–42. doi: 10.1002/ejhf.654
13. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert Consensus for Multimodality Imaging Evaluation of Adult Patients during and after Cancer Therapy: A Report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2014) 27:911–39. doi: 10.1016/j.echo.2014.07.012
14. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. (2015) 131:1981–8. doi: 10.1161/CIRCULATIONAHA.114.013777
15. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. (2022) 43:4229–361. doi: 10.1093/eurheartj/ehac244
16. Mertens L, Singh G, Armenian S, Chen MH, Dorfman AL, Garg R, et al. Multimodality imaging for cardiac surveillance of cancer treatment in children: recommendations from the American society of echocardiography. J Am Soc Echocardiogr. (2023) 36:1227–53. doi: 10.1016/j.echo.2023.09.009
17. Bu’Lock FA, Mott MG, Oakhill A, and Martin RP. Early identification of anthracycline cardiomyopathy: possibilities and implications. Arch Dis Child. (1996) 75:416–22. doi: 10.1136/adc.75.5.416
18. Agha H, Shalaby L, Attia W, Abdelmohsen G, Aziz OA, and Rahman MYA. Early ventricular dysfunction after anthracycline chemotherapy in children. Pediatr Cardiol. (2016) 37:537–44. doi: 10.1007/s00246-015-1311-5
19. Kocabaş A, Kardelen F, Ertuğ H, Aldemir-Kocabaş B, Tosun Ö, Yeşilipek A, et al. Assessment of early-onset chronic progressive anthracycline cardiotoxicity in children: different response patterns of right and left ventricles. Pediatr Cardiol. (2014) 35:82–8. doi: 10.1007/s00246-013-0745-x
20. Armenian SH. Improving screening practices in childhood cancer survivors at risk for treatment-related heart failure. J Clin Oncol. (2014) 32:3923–5. doi: 10.1200/JCO.2014.58.5562
21. Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, and Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy. J Am Coll Cardiol. (2014) 63:2751–68. doi: 10.1016/j.jacc.2014.01.073
22. Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese society of echocardiography. Eur J Echocardiogr. (2011) 12:167–205. doi: 10.1093/ejechocard/jer021
23. Negishi T, Thavendiranathan P, Penicka M, Lemieux J, Murbraech K, Miyazaki S, et al. Cardioprotection using strain-guided management of potentially cardiotoxic cancer therapy. JACC Cardiovasc Imaging. (2023) 16:269–78. doi: 10.1016/j.jcmg.2022.10.010
24. Ganame J, Claus P, Uyttebroeck A, Renard M, D’hooge J, Bijnens B, et al. Myocardial dysfunction late after low-dose anthracycline treatment in asymptomatic pediatric patients. J Am Soc Echocardiogr. (2007) 20:1351–8. doi: 10.1016/j.echo.2007.04.007
25. Keramida K and Farmakis D. Right ventricular involvement in cancer therapy–related cardiotoxicity: the emerging role of strain echocardiography. Heart Fail Rev. (2021) 26:1189–93. doi: 10.1007/s10741-020-09938-8
26. Gupta M, Thaler HT, and Steinherz L. Presence of prolonged dispersion of qt intervals in late survivors of childhood anthracycline therapy. Pediatr Hematol Oncol. (2002) 19:533–42. doi: 10.1080/08880010290097387
27. Ananthan K and Lyon AR. The role of biomarkers in cardio-oncology. J Cardiovasc Transl Res. (2020) 13:431–50. doi: 10.1007/s12265-020-10042-3
28. Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. (2004) 109:2749–54. doi: 10.1161/01.CIR.0000130926.51766.CC
29. De Iuliis F, Salerno G, Taglieri L, De Biase L, Lanza R, Cardelli P, et al. Serum biomarkers evaluation to predict chemotherapy-induced cardiotoxicity in breast cancer patients. Tumor Biol. (2016) 37:3379–87. doi: 10.1007/s13277-015-4183-7
30. Zardavas D, Suter TM, Van Veldhuisen DJ, Steinseifer J, Noe J, Lauer S, et al. Role of troponins I and T and N -terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early-stage human epidermal growth factor receptor 2–positive breast cancer receiving trastuzumab: A herceptin adjuvant study cardiac marker substudy. J Clin Oncol. (2017) 35:878–84. doi: 10.1200/JCO.2015.65.7916
31. Hayakawa H, Komada Y, Hirayama M, Hori H, Ito M, and Sakurai M. Plasma levels of natriuretic peptides in relation to doxorubicin-induced cardiotoxicity and cardiac function in children with cancer. Med Pediatr Oncol. (2001) 37:4–9. doi: 10.1002/mpo.1155
32. Ehrhardt MJ, Leerink JM, Mulder RL, Mavinkurve-Groothuis A, Kok W, Nohria A, et al. Systematic review and updated recommendations for cardiomyopathy surveillance for survivors of childhood, adolescent, and young adult cancer from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. (2023) 24:e108–20. doi: 10.1016/S1470-2045(23)00012-8
Keywords: cardiotoxicity, pediatric cancer, chemotherapy, ventricular dysfunction, echocardiography
Citation: Aurensanz-Clemente E, Garcia-Canadilla P, Gorostegui M, Rivera C, Clara Escobar-Díaz M, Randanne P, Morales La Madrid A and Sanchez-de-Toledo J (2025) Uncovering subclinical cardiotoxicity across chemotherapy phases in pediatric oncology. Front. Oncol. 15:1623081. doi: 10.3389/fonc.2025.1623081
Received: 06 May 2025; Accepted: 29 August 2025;
Published: 25 September 2025.
Edited by:
Anna Borowiec, Maria Sklodowska-Curie National Research Institute of Oncology, PolandReviewed by:
Asmaa Abdel Sameea Mahmoud, University of Menoufia, EgyptHager Allam, Benha University Hospital, Egypt
Diana Lazar, Children’s Emergency Clinical Hospital Cluj-Napoca, Romania
Copyright © 2025 Aurensanz-Clemente, Garcia-Canadilla, Gorostegui, Rivera, Clara Escobar-Díaz, Randanne, Morales La Madrid and Sanchez-de-Toledo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Esther Aurensanz-Clemente, ZXN0aGVyLmF1cmVuc2FuekBzamQuZXM=
 Esther Aurensanz-Clemente
Esther Aurensanz-Clemente Patricia Garcia-Canadilla
Patricia Garcia-Canadilla Maite Gorostegui
Maite Gorostegui Cristina Rivera
Cristina Rivera María Clara Escobar-Díaz
María Clara Escobar-Díaz Paula Randanne5
Paula Randanne5 Andres Morales La Madrid
Andres Morales La Madrid Joan Sanchez-de-Toledo
Joan Sanchez-de-Toledo