- 1Department of Radiology, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2Department of Radiology, The First People’s Hospital of Wenling, Wenling, Zhejiang, China
- 3Department of Radiology, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
Pancreatic extra-gastrointestinal stromal tumors (pEGISTs) are exceedingly rare, accounting for less than 5% of all extra-gastrointestinal stromal tumors (EGISTs). We report a case involving a 62-year-old woman who presented with a hypervascular pancreatic mass. Postoperative histopathological examination confirmed the diagnosis of a gastrointestinal stromal tumor (GIST). The neoplastic cells exhibited a spindle-shaped morphology without evident necrosis on histologic sections. Immunohistochemical analysis demonstrated strong, diffuse positivity for CD117, DOG-1, and CD34. Notably, the imaging characteristics of this pEGISTs displayed several distinctive features, including intralesional “island-like” enhancement, peripheral nodular enhancement, and prominent vascular “staghorn” patterns. Although histopathology remains the gold standard for definitive diagnosis of pEGISTs, correlating clinical presentation with these unique radiologic features can substantially improve diagnostic accuracy for radiologists. Additionally, we provide a comprehensive review and discussion of the current literature on the clinical and imaging hallmarks of pEGISTs.
Introduction
Gastrointestinal stromal tumors (GISTs) are mesenchymal neoplasms arising from the interstitial cells of Cajal within the gastrointestinal (GI) tract and are recognized for their potential malignant behavior (1). These tumors can develop anywhere along the GI tract, with the stomach and small intestine being the most frequently involved sites. Extra‐gastrointestinal stromal tumors (EGISTs) are defined as tumors that share identical histopathological and immunohistochemical profiles with GISTs but originate outside the GI tract, including locations such as the retroperitoneum, momentum, mesentery, or pancreas (2). EGISTs are uncommon, accounting for only 5%–10% of all GIST cases (1, 3–5). (pEGISTs are exceedingly rare; to date, only sporadic case reports have been described in both Chinese and English literature, and no systematic investigations have specifically characterized their imaging features (6). Given the lack of distinctive clinical manifestations, preoperative diagnosis is particularly challenging, and pEGISTs are often misdiagnosed as pancreatic neuroendocrine neoplasms (pNENs), solid pseudopapillary neoplasms (SPNs), mucinous cystic neoplasms (MCNs), or pancreatic pseudocysts.
In this study, we present a rare case of pEGISTs, providing a comprehensive description of its clinical presentation and imaging characteristics. We also systematically review recent Chinese and English literature on pEGISTs, with particular emphasis on studies that include computed tomography (CT) imaging. By summarizing the clinical and radiologic features of these tumors, we aim to enhance diagnostic accuracy among radiologists and inform clinical decision‐making, thereby improving therapeutic outcomes and patient prognosis.
Results
Case presentation
A 62-year-old woman underwent a routine health examination that incidentally revealed a pancreatic mass. She denied any abdominal pain or other discomfort but reported an unintentional weight loss of approximately 2.5 kg over the preceding six months. The outpatient department provisionally diagnosed her with a “pancreatic tumor,” and she was admitted for further evaluation. On physical examination, mild tenderness was elicited in the upper abdomen without rebound tenderness or guarding, and a palpable mass was noted in the epigastric region.
On admission, laboratory investigations—including complete blood count, liver and renal function tests, electrolytes, and tumor markers (CA19-9, CEA)—were all within normal limits. Her past surgical history included a cesarean delivery 28 years ago and a subtotal hysterectomy 23 years ago. Her medical history was notable for hypertension of over ten years’ duration (well controlled on oral antihypertensive medication), type 2 diabetes mellitus for approximately one year (well controlled on oral hypoglycemics), and asthma for more than ten years (currently not on any medications).
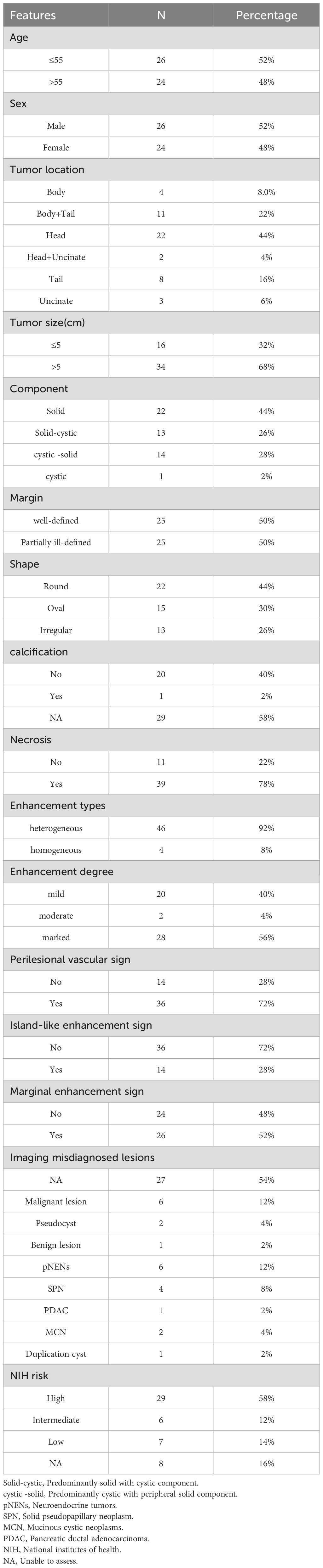
Contrast-enhanced computed tomography (CT) of the pancreas revealed a well-circumscribed, round, isoattenuating mass in the pancreatic body, measuring approximately 3.2 cm × 3.6 cm × 3.7 cm. On non-contrast imaging, the lesion’s attenuation measured 41.8 HU. Following contrast administration, the mass exhibited heterogeneous, moderate enhancement, with attenuation values of 55.9 HU in the arterial phase, 68.7 HU in the portal venous phase, and 56.9 HU in the delayed phase (Figures 1A–D; Supplementary Figure S1). Of particular note, the periphery of the lesion displayed nodular, “rim-like” enhancement (hereafter referred to as the “marginal enhancement sign”), and engorged vessels were seen at the lesion margin (the “Perilesional vascular sign,” Figures 1D, E). Figure 1 demonstrates a well-circumscribed hypervascular pancreatic mass(A-D) with marginal enhancement (D-E, red arrows) and perilesional vascular signs (E, yellow arrows), typical imaging features of pEGISTs. A nodular area of intense enhancement was observed within the lesion, referred to as the “island-like enhancement sign,” with attenuation values of 106 HU, 140 HU, and 115 HU in the arterial, portal venous, and delayed phases, respectively (Figures 2A–D). Figure 2 highlights this feature on both CT and MRI, where nodular intratumoral areas demonstrate stronger enhancement than surrounding tissue (red arrows), suggesting viable tumor components within a heterogeneous matrix. Yellow arrows indicate adjacent portal vein enhancement. Upstream, mild atrophy of the pancreatic parenchyma and slight dilation of the main pancreatic duct were observed. The splenic artery was displaced posteriorly by compression from the mass, while the surrounding fat planes remained clear, with no evidence of adjacent vascular invasion. No significantly enlarged lymph nodes were identified in the retroperitoneum. Based on these findings, a neoplastic process was favored, with the leading preoperative differential diagnoses being pancreatic neuroendocrine neoplasm (pNEN) or solid pseudopapillary neoplasm (SPN).
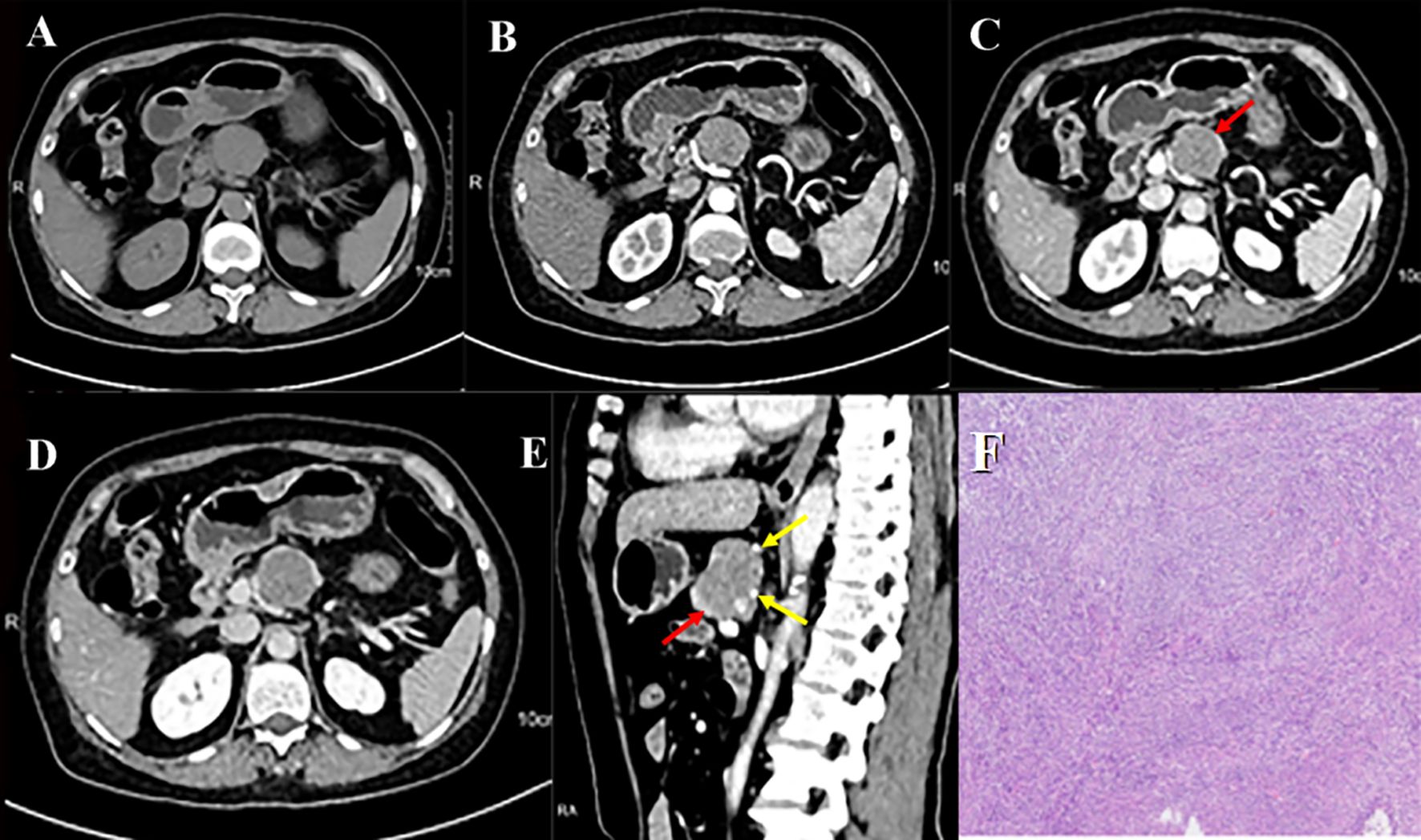
Figure 1. pEGISTs, pancreatic CT scan shows multiple-phase enhancement with marginal enhancement and Perilesional vascular sign. Axial unenhanced (A) reveals an isodense mass in the body of the pancreas; arterial (B), portal venous (C), and equilibrium (D) phases show moderate enhancement with marginal enhancement signs (red arrow); sagittal enhanced (E) shows both marginal enhancement (red arrow) and Perilesional vascular sign (yellow arrow). (F) Histological examination of pathological biopsy showed spindle cell tumor in the pancreatic body. The tumor was mainly composed of spindle cells. Original magnification: ×100.
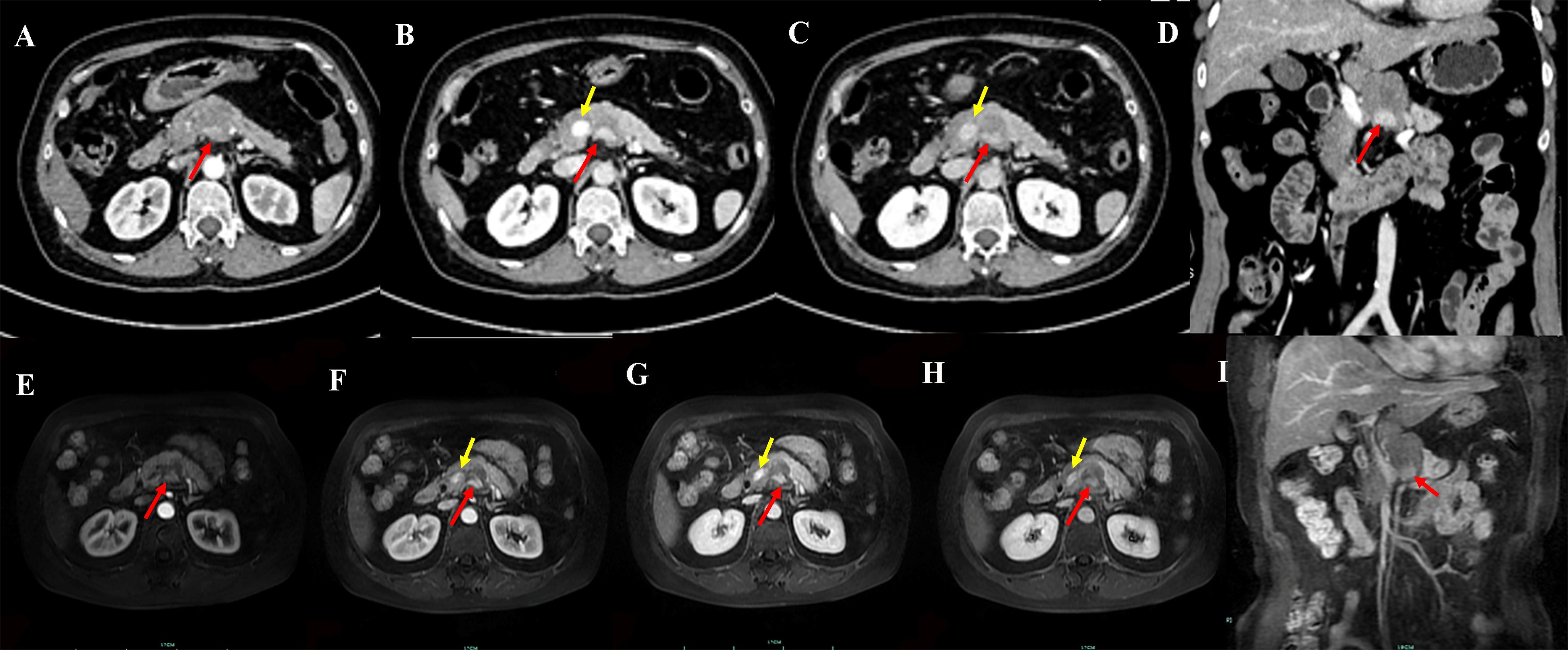
Figure 2. Pancreatic CT multi-phase enhancement in axial (A-C) and coronal (D) views shows a nodular area with more prominent enhancement, referred to as ‘island-like enhancement’ (red arrow), and enhanced portal vein (yellow arrow). Pancreatic MR multi-phase enhancement in axial (E-H) and coronal (I) views shows ‘island-like enhancement’ (red arrow) and enhanced portal vein (yellow arrow).
Magnetic resonance imaging (MRI) of the pancreas further characterized the lesion. On T1-weighted imaging (T1WI), the mass was hypointense, while on T2-weighted imaging (T2WI) it appeared uniformly mildly hyperintense, with well-defined margins. Diffusion-weighted imaging (DWI) demonstrated marked hyperintensity, and the corresponding apparent diffusion coefficient (ADC) map showed substantially reduced signal, indicating restricted diffusion (Figures 3A–D). Following multiphase contrast enhancement, the lesion exhibited heterogeneous moderate enhancement (Figures 3E–H), with nodular foci of more intense enhancement observed within the mass (Figures 2E–I). Figure 3 illustrates the MRI features of the pancreatic lesion.
Figure 3. MRI axial unenhanced (A) fat-suppressed T1WI shows the lesion (red arrow) as low signal, and fat-suppressed T2WI shows high signal; (C) DWI shows high signal, (D) ADC mapping shows low signal; axial multi-phase enhancement (E-H) shows the lesion with mild enhancement, marginal enhancement signs, and faint island-like enhancement signs.
Under general anesthesia, the patient underwent a laparoscopic distal pancreatectomy with splenectomy and peripancreatic neurolysis. Gross examination of the resected specimen and adjoining splenic tissue was followed by histopathologic analysis, which revealed a spindle-cell neoplasm composed of densely packed cells exhibiting mild atypia(Figure 1F).Immunohistochemistry showed the following profile: CK-PAN (–), EMA (–), CD34 (+), S100 (focally +), Desmin (–), Ki-67 (3%+), STAT6 (–), β-catenin (–), α-SMA (focally +), MDM2 (scant +), SS18-SSX (–), NKX2.2 (–), CD99 (focally +), pan-TRK (weak +), DOG1 (+), CD117 (+), SOX10 (–), H3 K27me3 (focally +), GLI-1 (–), WT1 (scant +), BCOR (–), SDHB (positive expression), Synaptophysin (–), Chromogranin A (–), INSM1 (–), Calretinin (–), GATA3 (occasional +), CDK4 (focally +), CD21 (–), CD23 (–), and CD35 (–). Molecular genetic testing for KIT gene exons 9, 11, 12, 13, 14, 17, 18, and PDGFRA gene exons 12, 14, and 18 showed no detectable mutations. Taken together, the histologic, immunophenotypic, and molecular findings supported a diagnosis of a borderline or low‐malignant‐potential spindle‐cell neoplasm, consistent with a wild‐type gastrointestinal stromal tumor (wild-type GIST).
Review of literature
A literature search identified 76 published articles on pancreatic EGISTs (pEGISTs). Of these, 48 studies (encompassing 50 patients) included CT imaging data and were thus selected for detailed analysis. We extracted and aggregated clinical and imaging characteristics for all 50 patients (Supplementary Table S1; Table 1). The median age at diagnosis was 55 years (range: 30–74 years), with a nearly equal sex distribution (24 females [52%] and 26 males [48%]). The most common tumor location was the pancreatic head (22 cases, 44%), followed by the pancreatic body–tail region (11 cases, 22%). Tumor size ranged from 2.0 cm to 35.0 cm in maximum diameter, with a mean size of 10.2 cm.
On CT imaging, 27 lesions (54%) appeared predominantly cystic‐solid (14 cystic‐predominant; 13 solid‐predominant), 22 lesions (44%) were purely solid, and only one lesion (2%) was purely cystic. Among the 16 tumors measuring ≤ 5 cm in diameter, 12 (75%) manifested as solid masses, three (19%) exhibited solid‐predominant cystic‐solid change, and one (6%) was predominantly cystic. In contrast, of the 34 tumors > 5 cm, cystic or cystic‐solid morphology predominated (24 cases, 70.6%). Tumor margins were clear in most cases; however, lesions with borderline or overtly malignant potential occasionally demonstrated ill‐defined borders. Morphologically, tumors were round in 22 cases (44%), oval in 15 cases (30%), and irregular in 13 cases (26%). Necrosis was a frequent finding (39 cases, 78%), whereas calcification was rare (1 case, 2%). On contrast-enhanced CT, 46 lesions (92%) exhibited heterogeneous enhancement. Enhancement intensity was most commonly described as marked or mild, with moderate enhancement being less frequent. Notably, some lesions demonstrated characteristic imaging signs, including “island-like enhancement,” “marginal enhancement,” and a “Perilesional vascular sign.”
Among all reported cases in our review, none were correctly diagnosed preoperatively. Of 23 cases with documented preoperative misdiagnoses, six (26%) were incorrectly classified as malignant lesions of undetermined origin, six (26%) as pNENs, four (18%) as SPNs, two (9%) as MCNs, two (9%) as pseudocysts, one (4%) as pancreatic adenocarcinoma, one (4%) as a duplication cyst, and one (4%) as a benign cystic lesion.
Discussion
GISTs represent the most common mesenchymal neoplasms of the gastrointestinal tract, arising from interstitial cells of Cajal or their precursors. Since GISTs were first delineated as a distinct pathological entity in 1983, they have become a focal point of research in gastrointestinal oncology. Their characteristic histological morphology and immunohistochemical profile—most notably strong expression of CD117 (KIT) and DOG1—provide a clear diagnostic framework (1). However, tumors with morphologic and immunophenotypic features indistinguishable from gastrointestinal GISTs but occurring outside the gastrointestinal tract, without anatomical continuity to the bowel wall or serosa, are classified as EGISTs. EGISTs account for only 5–10% of all GISTs, reflecting their relative rarity (2). The majority of EGISTs originate in mesenteric, omental, retroperitoneal, abdominal wall, hepatic, or pancreatic locations, with pEGISTs comprising less than 5% of cases (1–5). Because the pancreas is an uncommon site for EGISTs, pEGISTs are exceedingly rare; most published data derive from isolated case reports or small retrospective series, and a unified understanding of their clinical and radiologic features remains lacking.
The cellular origin of pEGISTs are not yet fully elucidated, although the prevailing hypothesis posits that they derive from ectopic interstitial cells of Cajal or undifferentiated mesenchymal precursors located in the peri-pancreatic or retroperitoneal region, which subsequently undergo stromal differentiation. Similar to gastrointestinal-type GISTs, pEGISTs lack specific clinical manifestations: early lesions are often asymptomatic, and many tumors are discovered incidentally during routine health examinations or imaging studies. Symptomatic patients may present with abdominal pain, discomfort, weight loss, distension, or an abdominal mass (6–54). Our present findings corroborate these clinical features (Supplementary Table S1). In our cohort of 50 patients, the median age was 55 years; 24 (48%) were female and 26 (52%) were male. The head of the pancreas was the most frequently involved site (22/50, 44%), followed by the body and tail (11/50, 22%). This distribution is consistent with earlier reports, which predominantly describe pEGISTs in middle-aged and elderly patients, with predilection for the pancreatic head and tail (55), and the gender distribution aligns with findings by Gupta and colleagues (55). Tumor sizes ranged from 2.0 to 35.0 cm, with a mean maximum diameter of 10.2 cm—again in agreement with prior literature, which attributes large tumor volumes at diagnosis to the insidious growth of pEGISTs and the absence of early, specific symptoms. Consequently, most patients have sizeable lesions at presentation that often compress or infiltrate adjacent structures (15). Consistent with our series and the reviewed literature, serum tumor markers such as CA19–9 and CEA were uniformly negative, indicating minimal utility of traditional serum markers in diagnosing pEGISTs (7–25).
Radiologic imaging is central to preoperative assessment of pEGISTs and can yield important diagnostic clues. Modalities include abdominal ultrasound, contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasound–guided fine-needle aspiration (EUS-FNA), and positron emission tomography–CT (PET-CT), with CT and MRI being paramount. However, previous publications have predominantly described CT features, with fewer reports of MRI characteristics; thus, a comprehensive summary of imaging findings is still needed. In our study, the MRI characteristics of the index case paralleled CT findings: on unenhanced sequences, the lesion exhibited low signal intensity on T1-weighted imaging (T1WI), high signal intensity on T2-weighted imaging (T2WI), and diffusion restriction on diffusion-weighted imaging. We systematically reviewed and summarized CT imaging features from 50 pEGISTs lesions. Although pEGISTs most frequently occur in the pancreatic head and body/tail regions, our illustrative case arose in the pancreatic body, a less common location. We stratified lesions by maximum diameter into ≤5 cm and >5 cm subgroups and analyzed their solid versus cystic composition. Among patients with lesions ≤5 cm (n = 16), 75% (12/16) were predominantly solid, 19% (3/16) were predominantly solid with patchy cystic change, and only 6% (1/16) were predominantly cystic. In contrast, patients with lesions >5 cm (n = 34) exhibited a higher proportion of cystic or mixed cystic-solid masses—70.6% (24/34). pEGISTs are hypervascular neoplasms, and their enhancement patterns on contrast-enhanced CT have the following salient features: (1) heterogeneous enhancement: 92% (46/50) in our series demonstrated heterogeneous enhancement, whereas 8% (4/50)—including one entirely cystic lesion—exhibited homogeneous enhancement. Marked heterogeneity is especially diagnostically suggestive. (2) Marginal enhancement sign and Perilesional vascular sign: 52% (26/50) showed Marginal enhancement sign. The “Perilesional vascular sign,” previously described in the literature (7–13), was observed in 72% of cases in our review, and we herein formally designate it as the Perilesional vascular sign. Combined Marginal enhancement sign and Perilesional vascular sign occurred in 40% of cases. Marginal enhancement sign on contrast-enhanced CT or MRI is characterized by pronounced enhancement of the tumor periphery with lack of central enhancement. This is typically due to central necrosis or cystic degeneration in GISTs resulting from rapid growth, leading to insufficient blood supply and minimal or absent enhancement centrally; in contrast, the proliferative, active tumor regions are often located at the periphery, where vascularity is rich and contrast permeability is high, producing marked rim enhancement. In addition, some GISTs develop a pseudocapsule with an abundant vascular network, which also manifests as a ring-like enhancement. The Perilesional vascular sign on contrast-enhanced CT or MRI appears as enhancing vessels at the tumor margin (whether dilated or not). This arises because pancreatic GISTs often receive blood supply from the peripancreatic arterial network or mesenteric collateral vessels; tumor growth can stimulate angiogenesis in adjacent vessels or cause dilation, congestion, or remodeling of nearby vessels, resulting in the Perilesional vascular sign on imaging (3). Nodule-in-nodule (or “island-like”) enhancement: this feature was less common; our index case exhibited classic “island-like” enhancement (Figures 2A–D), and similar findings have been sporadically reported (11, 15, 16, 18, 20, 21). Island-like enhancement is seen as scattered nodular enhancing areas within the solid portion of the lesion, resembling islands. This feature stems from intratumoral heterogeneity in GISTs—with variable cellular density, blood supply, and degrees of necrosis—where the “island” regions likely represent focal residual high-density active tumor tissue. To our knowledge, this sign has not been previously named, and we propose “island-like enhancement” as a novel, diagnostically valuable feature specific to pancreatic GIST.
The preoperative misdiagnosis rate of pEGISTs is extremely high, given that its imaging appearances overlap with various other pancreatic lesions. According to literature review, small solid pancreatic GIST (especially ≤5cm) should be distinguished from pNENs first, followed by SPNs in the differential diagnosis (56). pNENs are the second most common solid tumors after pancreatic ductal adenocarcinoma (PDAC) (57, 58) and often exhibit hypervascularity. Additionally, FDG-PET is valuable for evaluating metastatic spread and assessing the biological behavior of GISTs. Although 68Ga-DOTATATE PET is more specific for neuroendocrine tumors, it is occasionally utilized to differentiate GISTs from pNENs due to their overlapping hypervascular imaging characteristics (59, 60).Neither pNENs nor SPNs demonstrates the Perilesional vascular sign or island-like enhancement. Furthermore, SPNs typically occur in young women, have a well-defined capsule, exhibit progressive enhancement that does not exceed the degree of adjacent pancreatic parenchyma, and show no perilesional vascularity. For lesions >5 cm, the predominant presentation is cystic-solid masses with hyperenhancing solid components; differentiation from other mesenchymal tumors of the pancreas—especially hypervascular sarcomas such as leiomyosarcoma—is required. Although pEGISTs imaging characteristics (e.g., Perilesional vascular sign and island-like enhancement) may aid in distinguishing them from other pancreatic sarcomas, further study is needed to validate their diagnostic utility. It is important to emphasize that, regardless of a preoperative misdiagnosis, surgical resection remains the first-line treatment for pEGISTs and is unlikely to be delayed once imaging suggests a resectable hypervascular pancreatic mass. When pEGISTs exhibit multiloculated cystic-solid or predominantly cystic features, differentiation from MCNs and pancreatic pseudocysts becomes essential. Malignant-transformed MCNs often demonstrate elevated tumor markers (e.g., CA19-9), “eggshell” calcifications, mild enhancement of the solid components, and possible lymph node metastases; they do not exhibit perilesional vascularity. Pancreatic pseudocysts are rarely located within the pancreatic parenchyma per se and are typically associated with a history of pancreatitis. Consequently, a multimodality imaging approach is recommended for accurate preoperative diagnosis of pEGISTs, with careful consideration of the differential list.
From a pathological standpoint, CD117 and DOG1 are critical diagnostic markers for GISTs and EGISTs, with overwhelmingly high positivity rates. Recent studies indicate that combined testing for CD117 and DOG1 further enhances diagnostic sensitivity, especially in CD117-negative or atypical cases, where DOG1 serves as a valuable adjunct. It is crucial to note that pEGISTs characteristically have friable stroma; preoperative puncture biopsy, such as EUS-FNA or percutaneous core biopsy, may carry a risk of tumor seeding into the peritoneal cavity. Therefore, in cases with imaging findings highly suggestive of pEGISTs and definitive surgical intent, routine preoperative biopsy is not recommended.
Moreover, imaging plays an indispensable role in guiding treatment decisions. Contrast-enhanced CT can accurately assess tumor involvement of adjacent vascular structures and determine resectability, serving as a key component of preoperative risk stratification. MRI offers superior soft-tissue contrast and is advantageous in detecting hepatic metastases. PET-CT may be indicated to evaluate for distant metastatic disease and to inform postoperative adjuvant therapy and surveillance protocols.
In summary, pEGISTs are rare but clinically significant entity whose early identification depends on a comprehensive analysis of epidemiologic, radiologic, and pathologic features. Although its imaging characteristics lack absolute specificity, the presence of a well-circumscribed hypervascular pancreatic mass demonstrating marked heterogeneous enhancement, Marginal enhancement sign, Perilesional vascular sign, and/or island-like enhancement should prompt strong consideration of pEGISTs. Definitive diagnosis, however, ultimately relies on postoperative histopathology and immunohistochemistry. Future efforts should focus on larger, multicenter studies to refine the diagnostic pathway, develop standardized preoperative models for identification, and optimize management strategies to improve clinical outcomes and patient prognosis. Importantly, to minimize the risk of needle-track seeding and peritoneal dissemination, preoperative puncture biopsy is discouraged in cases where imaging suggests a resectable pEGISTs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
JX: Investigation, Methodology, Writing – original draft. MG: Formal analysis, Methodology, Writing – original draft. YW: Data curation, Investigation, Methodology, Writing – original draft. XH: Investigation, Methodology, Supervision, Writing – original draft. JD: Investigation, Software, Writing – original draft. XZ: Data curation, Investigation, Methodology, Writing – original draft. XC: Investigation, Resources, Writing – original draft. RY: Resources, Visualization, Writing – review & editing. JX: Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Scientific Research Fund of Wenling Science and Technology Bureau (2022S00119).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1638850/full#supplementary-material
Supplementary Figure 1 | Contrast enhancement curve of the pancreatic lesion.
References
1. Ibrahim A and Montgomery EA. Gastrointestinal stromal tumors: variants and some pitfalls that they create. Adv Anat Pathol. (2024) 31:354–63. doi: 10.1097/PAP.0000000000000463
2. Costa E Silva T, Jorge Alves H, Vasconcelos M, Moreira AP, and Sousa Picado B. Extragastrointestinal stromal tumor in the peritoneum: A case report. Cureus. (2024) 16:e61411. doi: 10.7759/cureus.61411
3. Martinez R, Lezcano M, Randhawa J, Aijaz S, Al Mazouni Y, Altamimi A, et al. Extragastrointestinal stromal tumor mimicking appendicitis: A case study. Am J Case Rep. (2025) 26:e944665. doi: 10.12659/AJCR.944665
4. Papamattheou E, Katsaros I, Papadakos SP, Lianos E, and Kontis E. Rectovaginal extra-gastrointestinal stromal tumors (EGISTs): A systematic review of the literature and a pooled survival analysis. Cancers (Basel). (2025) 17:1382. doi: 10.3390/cancers17081382
5. Chahar OS, Br L, Raina S, Singh KD, Tripathi S, Singh O, et al. A rare case of extragastrointestinal stromal tumor of vocal cord - case report. Indian J Otolaryngol Head Neck Surg. (2024) 76:3625–8. doi: 10.1007/s12070-024-04652-5
6. Ali MS, Cheleng A, Behera P, and Sahoo MR. A gastrointestinal stromal tumor (GIST) and a pseudocyst of the pancreas: A peculiar case of both co-existing in the same patient. Cureus. (2024) 16:e61642. doi: 10.7759/cureus.61642
7. Gupta V, Chopde A, Chaudhari V, Bal M, Shrikhande SV, and Bhandare MS. Primary pancreatic GIST - A-single centre case series and systematic review of literature. J Gastrointest Cancer. (2024) 55:572–83. doi: 10.1007/s12029-024-01024-8
8. Xie Q, Cai P, and Liu C. Gastrointestinal stromal tumor four cases outside the pancreas and the literature review. J Chin Liver Surg. (2021) 11:858–9. doi: 10.3760/cma.J.c.n113884-20210507-00157
9. Sharma S, Shetty V, and Ali IM. Gastrointestinal stromal tumor (GIST) masquerading as a pancreatic pseudocyst: A rare case report. Cureus. (2024) 16:e66491. doi: 10.7759/cureus.66491
10. Yin X, Yang H, Zhang B, and Yin Y. A rare simultaneous coexistence of pancreatic gastrointestinal stromal tumor and esophageal schwannoma: a case report and review of literature. Front Oncol. (2024) 14:1428910. doi: 10.3389/fonc.2024.1428910
11. Song T, Hong Q, and Wu Y. Pancreatic extragastrointestinal stromal tumor: A case report. Cureus. (2024) 16:e54514. doi: 10.7759/cureus.54514
12. Marbun VMG, Jamtani I, Krisnuhoni E, and Panigoro SS. Rare double primary Malignancies: A pancreatic gastrointestinal stromal tumor mimicking as a metastatic lesion of myoepithelial carcinoma of parotid gland. Case Rep Med. (2023) 2023:8274226. doi: 10.1155/2023/8274226
13. Beji H, Bouassida M, Mroua B, Belfkih H, M’ farrej MK, and Touinsi H. Extra-gastrointestinal stromal tumor of the pancreas: a case report. Int I Surg Case Rep. (2022) 98:107581. doi: 10.1016/j.iijscr
14. Chen X and Meng X. Pancreatic stromal tumors: case report and literature review. J Hepatobil Pancreat Surg. (2021) 34:299–302. doi: 10.11952/j.issn.1007-1954.2022.05.009
15. Zhao R, Gong X, and Zhao H. A case report of pancreatic stromal tumor. J Pract Radiol. (2021) 38:1907–8. doi: 10.3969/j.issn.1002-1671.2022.11.045
16. Ene D, Florescu LM, Ene R, Popescu B, and Gheonea IA. An extremely uncommon case of pancreatic extragastrointestinal stromal tumor in a 53-year-old female patient. Rom J Morphol Embryol. (2021) 62:569–73. doi: 10.47162/RJME.62.2.24
17. Yang F, Jin C, and Fu D-L. Diagnosis and treatment and prognosis of pancreatic interstitial tumor. J Liver Gallbladder Surg J. (2011) 7:558–61. doi: 10.3760/cma.J
18. Duan Y, Yang Z-X, and Ye Y-L. Pancreas gastrointestinal stromal tumor in 4 cases report and literature analysis. Chin J Pract Surg. (2019) 33:844–849853. doi: 10.19538/j.carolcarrollJPS
19. Dhillon J. Mesenchymal tumors of the pancreas. Monogr Clin Cytol. (2020) 26:122–128. doi: 10.1159/00045573
20. Rasool Z, Mushtag S, Samoon N, Malik S, Gul S, Khan M, et al. Pancreatic extra gastrointestinal stromaltumor: a case report and review of the literature. J Med Sci Clin Res. (2018) 6:846–54. doi: 10.18535/jmscr/v6i5.133
21. Yol S, Polat E, Duman M, Uzun O, Yasar NF, Peker KD, et al. Pancreatic extragastrointestinal stromal tumorinvading the duodenum. Turk J Surg. (2018) 34:231–3. doi: 10.5152/turkjsurg
22. Yeo SJ, Cho CM, Kwon HI, Cho SH, Kim GC, Seo AN, et al. An extragastrointestinal stromal tumor originating from thepancreas. Case Rep Gastroenterol. (2018) 12:671–8. doi: 10.1159/000494553
23. Xu W-B and Liu X-J. Imaging research & Medical application. CT diagnosis of pancreatic stromal tumor: a case report and literature review. (2018) 2:168–9. doi: 10.3969/j.issn.2096-3807.2018.19.115
24. Chi M and Wan YM. A case of giant pancreatic stromal tumor with multiple metastases to liver and abdominal cavity. Pancreat Dis. (2018) 18:83–4. doi: 10.3760/cma.J.iSSN.1674-1935.2018.02.004
25. Abderrahmen D, Waad F, Mohamed B, Fathia H, Ohamed A, and Ali B. Pancreatic gastrointestinal stromal tumour: a case reportand review of the literature. J Cancer Ther. (2017) 8:954–61. doi: 10.4236/jct.2017.811085
26. Kwon HJ. Extra-gastrointestinal stromal tumor of the pancreas:report of a case. Ann Hepatobil Pancreat Surg. (2017) 21:23742. doi: 10.14701/ahbps.2017.21.4.237
27. Ren S, Chen X, Wang J, Zhao R, Song L, Wang Z, et al. Differentiation of duodenal gastrointestinal stromal tumors from hypervascular pancreatic neuroendocrine tumors in the pancreatic head using contrast-enhanced computed tomography. Abdom Radiol (NY). (2019) 44:867–76. doi: 10.1007/s00261-018-1803-x
28. Ren S, Qian LC, Cao YY, Daniels MJ, Song LN, Wang ZQ, et al. Computed tomography-based radiomics diagnostic approach for differential diagnosis between early- and late-stage pancreatic ductal adenocarcinoma. World J Gastrointest Oncol. (2024) 16:1256–67. doi: 10.4251/wjgo.v16.i4.1256
29. Ren S, Qian L, Daniels MJ, Duan S, Chen R, and Wang Z. Evaluation of contrast-enhanced computed tomography for the differential diagnosis of hypovascular pancreatic neuroendocrine tumors from chronic mass-forming pancreatitis. Eur J Radiol. (2020) 133:109360. doi: 10.1016/j.ejrad.2020.109360
30. Narushima K, Shuto K, Okazumi S, Ohira G, Mori M, Hayano K, et al. Malignant diagnosis and prognostic analysis of 89 GIST patients using preoperative FDG-PET. Sci Rep. (2023) 13:2266. doi: 10.1038/s41598-023-29038-5
31. Saleh M, Bhosale PR, Yano M, Itani M, Elsayes AK, Morani AC, et al. New frontiers in imaging including radiomics updates for pancreatic neuroendocrine neoplasms. Abdom Radiol (NY). (2022) 47:3078–100. doi: 10.1007/s00261-020-02833-8
32. Wu B, Fei J, and Song Z. Complexity of the diagnosis and treatment of pancreatic interstitial tumor. Chin Digest Surg. (2017) 10:1081–3. doi: 10.3760/cma.J.iSSN.1673-9752.2017.10.018
33. Liu L, Zhu Y, Wang D, Yang C, Zhang QL, Li X, et al. Coexisting and possible primary extra-gastrointestinal stromal tumorsof the pancreas and liver: a single case report. Oncol Lett. (2016) 11:3303–7. doi: 10.3892/o1.2016.4420
34. Elgeidie A, El-Magd EA, EI-Maaty SRA, and El-Hawary AK. Pancre-atic gastrointestinal stromal tumor: a case report. Int J Surg Case Rep. (2016) 29:67–70. doi: 10.1016/j.ijscr.2016.08.019
35. Xu H and Ren K. A case of pancreatic stromal tumor. Radiol Pract. (2016) 31:97. doi: 10.13609/j.cnki.1000-0313.2016.01.024
36. Aziret M, Cetinkiinar S, Aktas E, Irkörüicüi O, Bali I, and Erdem H. Pancreatic gastrointestinal stromal tumor after upper gastrointestinal hemorrhage and performance of whipple procedure: a casereport and literature review. Am J Case Rep. (2015) 16:509–13. doi: 10.12659/AJCR.893803
37. Xiao P, Chen CF, and Huang WN. A case report of pancreatic Malignant stromal tumor. Radiol Pract. (2015) 9:969–70. doi: 10.13609/j.cnki.1000-0313.2015.09.022
38. Lu X, Changyong E, and Yao X. Pancreas stromal tumor with liver metastasis in 1 case report and literature review. Chin J Pract Surg. (2015) 4):422–5. doi: 10.7504/CJPSISSN1005-2208.2015.04.21
39. Stanek M, Pedziwiatr M, Mattok M, and Budzyúski A. Laparo-scopic removal of gastrointestinal stromal tumors of uncinateprocess of pancreas. Wideochir Inne Tech Maloinwazyine. (2015) 10:311–5. doi: 10.5114/wiitm
40. Tian YT, Liu H, Shi SS, Xie YB, Xu Q, Zhang JW, et al. Malignant extra-gastrointestinal stromaltumor of the pancreas: report of two cases and review of the lit-erature. World J Gastroenterol. (2014) 20:863–8. doi: 10.3748/wjg.v20.i3.863
41. Akbulut S, Yavuz R, Otan E, and Hatipoglu S. Pancreatic extragastro-intestinal stromal tumor: a case report and comprehensive literaturereview. World J Gastrointest Surg. (2014) 6:175–82. doi: 10.4240/wigs.v6.i9.175
42. Beltrame V, Gruppo M, Pastorelli D, Pizzi S, Merigliano S, and Sperti C. Extra-gastrointestinal stromal tumor of the pancreas:case report and review of the literature. World J Surg Oncol. (2014) 12:105. doi: 10.1186/1477-7819-12-105
43. Ding H-J, Jiang X-H, and Xu D-K. Pancreatic tail primary gastrointestinal outer gliomas in 1 case and the literature review. J Clin Exp Pathol. (2014) 4:453–454455. doi: 10.13315 / j.carol carroll nki cjcep
44. Serin KR, Keskin M, Gulluoglu M, and Emre A. Atypical localisa-tion of a gastrointestinal stromal tumor: a case report of pancreasgastrointestinal stromal tumor. Ulusal Cer Derg. (2013) 29:42–4. doi: 10.5152/UCD.2013.11
45. Soufi M, Bouziane M, Massrouri R, and Chad B. Pancreatic GIST withpancreas divisum: a new entity. Int J Surg Case Rep. (2013) 4:68–71. doi: 10.1016/j.ijscr.2012.09.007
46. Wegge J, Bartholomew DM, Burke LH, and Miller LA. Pancreatic extra-gastrointestinal stromal tumour masquerading as a bleeding duodenalmass. BMI Case Rep. (2012) 2012:bcr2012007040. doi: 10.1136/bcr-2012-007040
47. Kim HH, Koh YS, Park EK, Seoung JS, Hur YH, Kim HJ, et al. Primary extragastrointestinal stromal tumor arisingin the pancreas: report of a case. Surg Today. (2012) 42:386–90. doi: 10.1007/s00595-011-0080-x
48. Cecka F, Jon B, Ferko A, $ubrt Z, Nikolov DH, and Tyěová V. Long term survival of a patient after resection of a gastrointestinalstromal tumor arising from the pancreas. Hepatobil Pancreat Dis Int. (2011) 10:330–2. doi: 10.1016/S1499-3872(11)60056-8
49. Wang G, Quan Z, and Ren J. Pancreatic extra-gastrointestinal stromal tumor: a case report. Chin J Pract Surg. (2012) 32(9):790–1. doi: 1005-2208(2012)09-0790-02
50. Meng L, Fang SH, and Jin M. An unusual case of pancreatic andgastric neoplasms (2010b): Malignant GISTs originating from thepancreas and stomach. Eur Radiol. (2011) 21:663–5. doi: 10.1007/s00330-010-1893-5
51. Wang S, Chen Y, and Yang D. A case report of extra-gastrointestinal stromal tumor of the pancreas. J Hepatobil Pancreat Surg. (2011) 36:508–11. doi: 10.11952/j.issn.1007-1954.2024.08.014
52. Zhu L, Li K, and Zou L. Pancreatic stromal tumor: a case report and literature review. Chin J Med Comput Imaging. (2011) 17:89–91. doi: 10.3969/j.issn.1006-5741.2011.01.019
53. Padhi S, Sarangi R, and Mallick S. Pancreatic extragastrointestinal stromal tumors, interstitial Cajal like cells, and telocytes. JOP. (2013) 14(1):1–14. doi: 10.6092/1590-8577/1293
54. Lee W, Li X, Lee S, and Chandan VS. Primary pancreatic gastrointestinal stromal tumor. Hepatobiliary Pancreat Dis Int. (2021) 20(4):391–3. doi: 10.1016/j.hbpd.2020.08.005
55. Liu Z, Tian Y, Xu G, Liu S, Guo M, Lian X, et al. Pancreatic gastrointestinal stromal tumor: clinicopathologic features and prognosis. J Clin Gastroenterol. (2017) 51(9):850–6. doi: 10.1097/MCG.0000000000000719
56. Feng W, Bao Y, and Maoyun. Pancreas stromal tumor case. [J] Ordinary Surg. (2010) 25(4):325. doi: 10.3760/cma.J.iSSN.1007-631-x.2010.04.019
57. Zackria R and Jayaraman V. The gastrointestinal stromal tumor (GIST) of a pancreatic cyst. Cureus. (2022) 14(6):e26197. doi: 10.7759/cureus.26197
58. Daum O, Klecka J, Ferda, Treska V, Vanecek T, Sima R, et al. Gastrointestinal stromal tumor of thepancreas: case report with documentation of KIT gene mutation. Virchows Arch. (2005) 446:470–2. doi: 10.1007/s00428-004-1200-4.PMID:15756592
59. Hirata H, Ishiguro N, Ito K, Suga A, Yasuura N, Egami H, et al. Gastrointestinal: A case of pancreatic gastrointestinal stromal tumor diagnosed by endoscopic ultrasound fine needle biopsy. J Gastroenterol Hepatol. (2023) 38(10):1680. doi: 10.1111/jgh.16213
Keywords: pancreas, extragastrointestinal stromal tumors, literature review, case report, imaging features
Citation: Xu J-X, Gu M-Y, Wang Y-H, Huang X-S, Duan J-Q, Zheng X-Z, Chen X-X, Yu R-S and Xiang J-Y (2025) Imaging features of pancreatic extragastrointestinal stromal tumors: a case report and literature review. Front. Oncol. 15:1638850. doi: 10.3389/fonc.2025.1638850
Received: 31 May 2025; Accepted: 09 July 2025;
Published: 29 July 2025.
Edited by:
Shuai Ren, Affiliated Hospital of Nanjing University of Chinese Medicine, ChinaReviewed by:
Giorgia Porrello, University of Palermo, ItalyZongyu Xie, The First Affiliated Hospital of Bengbu Medical College, China
T. G. Balachandar, Apollo Main Hospitals, India
Copyright © 2025 Xu, Gu, Wang, Huang, Duan, Zheng, Chen, Yu and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ri-Sheng Yu, cmlzaGVuZy15dUB6anUuZWR1LmNu; Jun-Yi Xiang, eGp5eHpiZHl4QDE2My5jb20=
 Jian-Xia Xu
Jian-Xia Xu Meng-Yin Gu1
Meng-Yin Gu1 Yi-Hua Wang
Yi-Hua Wang Xiao-Shan Huang
Xiao-Shan Huang Xiao-Zhong Zheng
Xiao-Zhong Zheng Jun-Yi Xiang
Jun-Yi Xiang