- 1Oncology Institute of Southern Switzerland (IOSI), Ente Ospedaliero Cantonale (EOC), Bellinzona, Switzerland
- 2Institute of Oncology Research (IOR), Bellinzona, Switzerland
- 3Department of Clinical Medicine and Surgery, University of Naples “Federico II”, Naples, Italy
- 4School of Specialization in Medical Oncology, Department of Human Pathology “G. Barresi”, University of Messina, Messina, Italy
- 5Faculty of Biomedical Sciences, Università della Svizzera Italiana, Lugano, Switzerland
- 6Istituto Cantonale di Patologia, Ente Ospedaliero Cantonale (EOC), Locarno, Switzerland
- 7Imaging Institute of Southern Switzerland, Ente Ospedaliero Cantonale, Lugano, Switzerland
- 8Urology Service, Department of Surgery, Ente Ospedaliero Cantonale, Università della Svizzera Italiana, Lugano, Switzerland
The introduction of enfortumab vedotin combined with pembrolizumab (EV-P) as a first-line treatment for advanced urothelial carcinoma (UC) has transformed the therapeutic landscape and holds great promise for improving patient outcomes. However, predictive and prognostic biomarkers for this novel regimen remain limited, and no specific subgroup has yet been identified for whom frontline EV-P could be withheld in favor of platinum-based chemotherapy. We report the first two cases of patients with BRCA-mutant metastatic UC who experienced markedly short progression-free survival with first-line EV-P but achieved more durable responses with second-line platinum-based chemotherapy. These observations raise important questions about the potential predictive role of BRCA - and more broadly, DNA damage repair - mutations in the evolving treatment paradigm of UC. Given the known sensitivity of BRCA-mutated tumors to platinum agents, frontline platinum-based chemotherapy may warrant consideration in this molecularly defined subgroup. Larger studies are needed to validate these preliminary findings and inform treatment selection.
1 Introduction
Urothelial carcinoma (UC) is the 10th most common malignancy worldwide. It is the most frequent cancer of the urinary tract, and approximately 10% of cases arise from the upper tract (UTUC) (1). Metastatic UC (mUC) is associated with an aggressive disease course and a 5-year survival rate of 5-8% (1), with metastatic UTUCs being associated with a worse prognosis (2).
The results of the III EV-302/KEYNOTE-39A trial have recently transformed the treatment landscape of mUC (3). In this study the combination of the antibody drug conjugate (ADC) enfortumab vedotin with the immune checkpoint inhibitor pembrolizumab (EV-P) as first-line treatment in patients with mUC demonstrated improved oncological outcomes compared to platinum-based chemotherapy. In particular, patients treated with EV-P had both a median progression-free survival (PFS) and a median overall survival (OS) that was almost doubled compared to patients treated with platinum-based chemotherapy (12.5 and 31.5 months vs 6.3 and 16.1 months, with an HR of 0.45 for PFS and 0.47 for OS). Patients treated with EV-P had an overall response rate (ORR) of 67.7%, with only 8.7% experiencing progressive disease as their best response.
The marked benefit in favor of EV-P was maintained regardless of the primary site of origin of disease (upper vs lower tract), PD-L1 expression (low vs high), cisplatin eligibility status (eligible vs ineligible) and site of metastasis (visceral vs lymph nodes). Based on these impressive results, international guidelines have included EV-P as the new standard of care for first-line therapy in patients with mUC who are eligible for this combination therapy (4, 5). However, predictive factors for EV-P efficacy remain largely unknown, highlighting a critical gap in knowledge.
The use of information derived from immunohistochemistry analysis or from next generation sequencing is becoming fundamental to personalize the treatment of patients with mUC (4, 5). For example, patients with mUC with FGFR alterations (FGFR2/3 mutations or FGFR3 fusions) can benefit from FGFR inhibitors, such as erdafitinib (6). In addition, there are interesting preliminary data for the use of Fam-trastuzumab deruxtecan-nxki in patients with HER-2 positive mUC as assessed by immunohistochemistry. This treatment has been included among the possible therapeutic options for HER-2 positive disease in advanced lines of therapy in the current NCCN guidelines (4) following the results of the DESTINY-PanTumor02 phase II Trial (7).
To date, we do not know whether the efficacy of EV-P as first-line therapy in patients with mUC can be influenced by specific molecular features. It is therefore possible that some subgroups of patients with certain mutations may still benefit more from a platinum-based regimen as first-line treatment than from EV-P.
DNA damage response and repair (DDR) gene mutations occur in up to 25% of UC cases, with BRCA1 and BRCA2 mutations present in approximately 3% and 4.5% of tumors, respectively (8).
Here, we present two cases of BRCA-mutant metastatic UTUC treated with first line EV-P followed by second-line platinum-based chemotherapy, who had a short response to EV-P and a longer response to second line platinum-based chemotherapy.
2 Case description
2.1 Case one
A 75-year-old Caucasian non-smoker male presented with worsening lower urinary tract symptoms during a routine urology visit. A contrast enhanced CT scan revealed left ureteral dilation with a filling defect in the pre-vesical and intramural ureter. A left distal ureteral resection with ureteral reimplantation was performed, and pathological analysis confirmed high-grade invasive urothelial carcinoma with muscle invasion (pT2) (Figure 1A). Concurrent bladder resection revealed multifocal high-grade pT1 papillary UC.
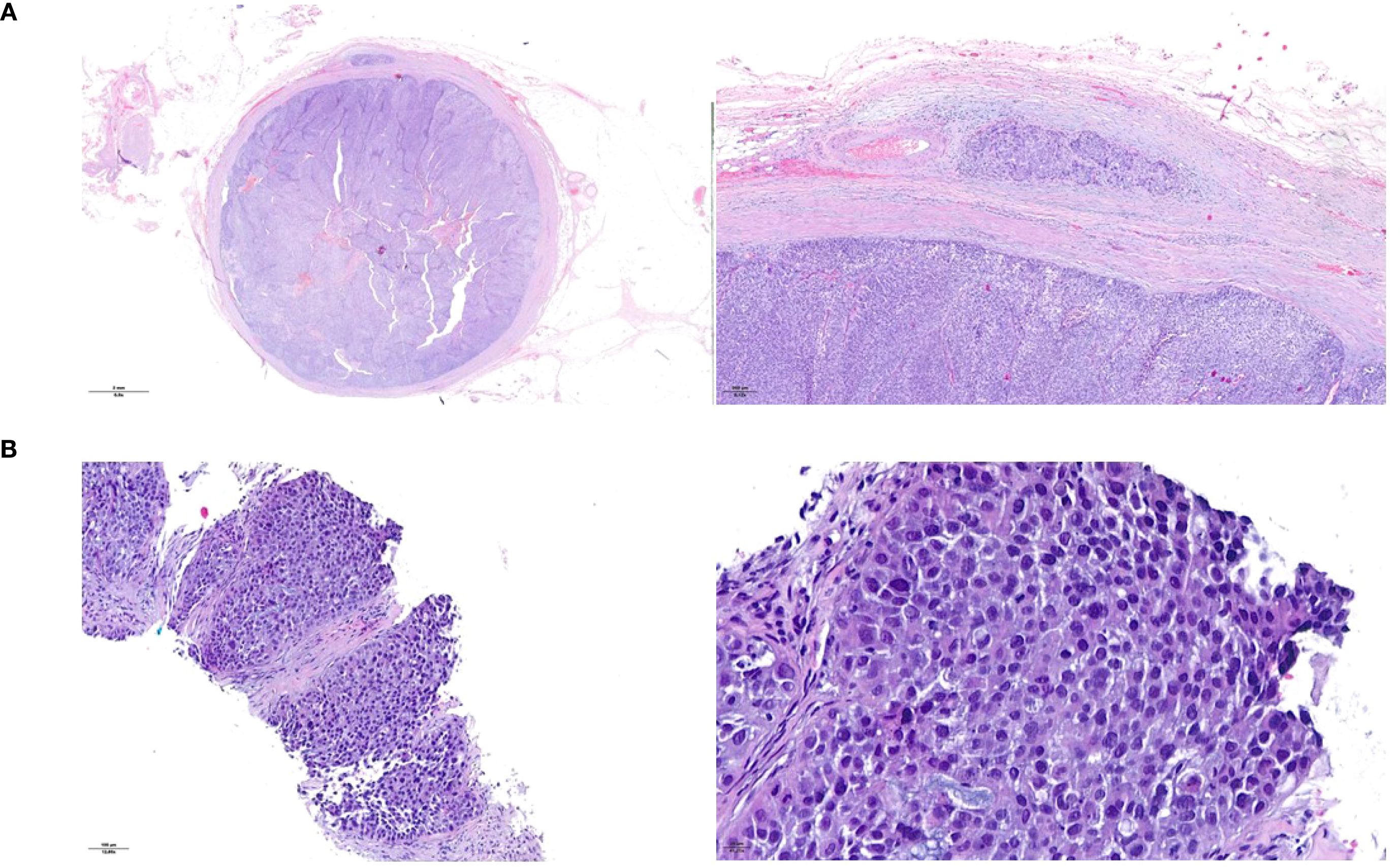
Figure 1. Histopathological presentation of the urothelial ureteral primary tumor (A) and of the peritoneal metastasis (B). Hematoxylin and eosin staining.
Staging CT of the chest and abdomen identified solid lesions in the left renal pelvis and ureter, bladder wall thickening, ascites, and peritoneal nodules. A biopsy of a peritoneal nodule revealed poorly differentiated carcinoma of urothelial origin (Figure 1B). The final diagnosis was pT2N0M1, stage IV UTUC.
Somatic next generation sequencing (NGS) evaluation of the ureteral specimen was performed by Ion Torrent (Thermo Fisher Scientific, Waltham, MA) using Oncomine Comprehensive Assay v3 (Thermo Fisher Scientific, Waltham, MA) and identified a BRCA2 p.S2887* variant, a TP53 p.P151Sfs*13 variant, and increased copy numbers of CCND1, FGF19, FGF3, PPARG, and RAF1. The tumor was classified as microsatellite-stable while tumor mutational burden analysis was not performed. The case was discussed at our molecular tumor board where the BRCA alteration found was considered pathogenic and with an elevated allele frequency (69.37%). Considering the current evidence and recommendations (9), patient was referred for germinal testing, which did not show any pathogenic variants in BRCA2 or the other examined genes.
The patient was referred to the Oncology Department and in March 2024 started first line EV-P treatment. A CT scan was performed after the administration of the third cycle of the treatment and showed a RECIST 1.1 partial response (PR), with reduction in pyelo-ureteral lesions and peritoneal nodules, as well as a complete resolution of the ascites. However, a single pre-vesical peritoneal lesion had increased in size (8mm diameter increase, from 13 to 21 mm). A follow-up CT after three additional cycles (July 2024) demonstrated progressive disease, with new peritoneal lesions, suspected left pyeloureteral junction disease relapse, and an enlarged right obturator lymph node (Figure 2A). EV-P treatment was therefore discontinued after six cycles. All treatment cycles were administered without dose reduction or delay. During the treatment, the patient developed grade 1 oral mucositis and peripheral neuropathy.
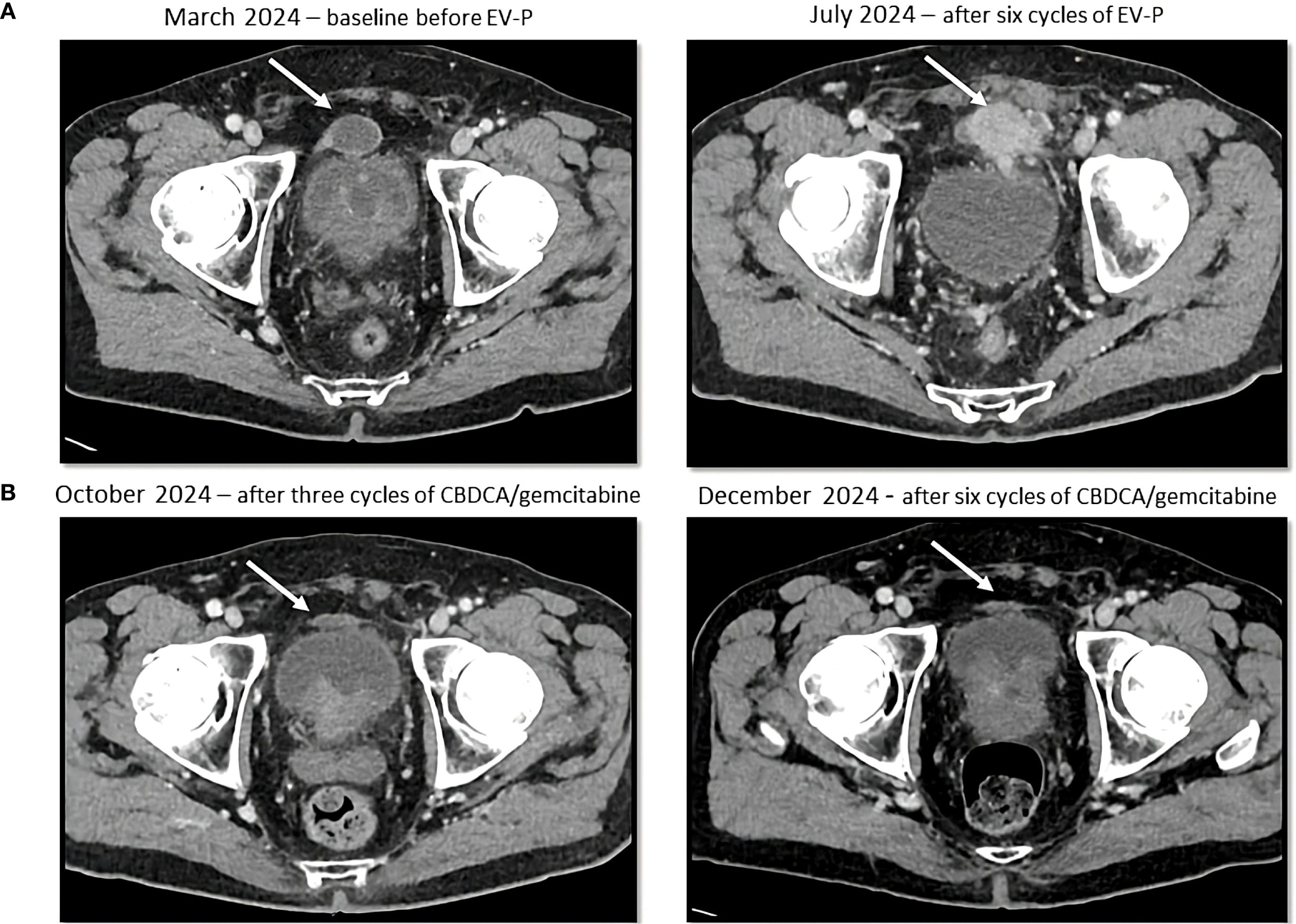
Figure 2. Sequential CT scans showing disease progression during treatment with enfortumab vedotin and pembrolizumab (A) and partial response with platinum-based chemotherapy (B). Cystic lesion (white arrow) above the bladder, that increased in density and size during enfortumab vedotin and pembrolizumab treatment and then decreased in size after platinum-based therapy administration.
Second-line carboplatin AUC 5 day 1 plus gemcitabine 1000 mg/m² day 1 and day 8 every 3 weeks was subsequently initiated in August 2024. Carboplatin was chosen because of a mildly reduced glomerular filtration rate of 50 mL/min/1.73 m2.
A CT scan after three cycles (October 8, 2024) showed a PR by RECIST 1.1 with a significant reduction in tumor burden with a reduction in size of numerous peritoneal lesions (the biggest being the aforementioned prevescical lesion that decreased from 46 mm to 31 mm) and of the right obturator lymphadenopathy (with a short axis from 10 mm to 6 mm). The case was subsequently discussed at our multidisciplinary board which, due to the good treatment response, suggested continuing treatment with carboplatin and gemcitabine for up to six total cycles. Subsequent cycles were administered at a reduced dose (25% dose reduction for both drugs), due to grade 2 anemia and grade 1 peripheral neuropathy. The CT scan performed after six cycles (December 18, 2024) confirmed an even deeper response, with complete response of some peritoneal nodules (Figure 2B). A follow-up CT scan in March 2025 further demonstrated the stability of the achieved response.
The patient remains in follow-up with no evidence of disease progression, currently eleven months post-second-line treatment initiation, and seven months from the last cycle of platinum-based chemotherapy.
2.2 Case two
A 69-year-old Caucasian female smoker presented with pelvic pain and macrohematuria. A CT scan revealed a suspicious tissue mass in the distal third of the left ureter, with ureteral dilation but no other suspicious urinary tract lesions or lymphadenopathies (Figure 3). She subsequently underwent robot-assisted left nephroureterectomy with bladder cuff excision and pelvic and hilar loco-regional lymphadenectomy. Histological analysis confirmed high-grade urothelial carcinoma with infiltration of the muscularis propria, focal angioinvasion, and negative resection margins.
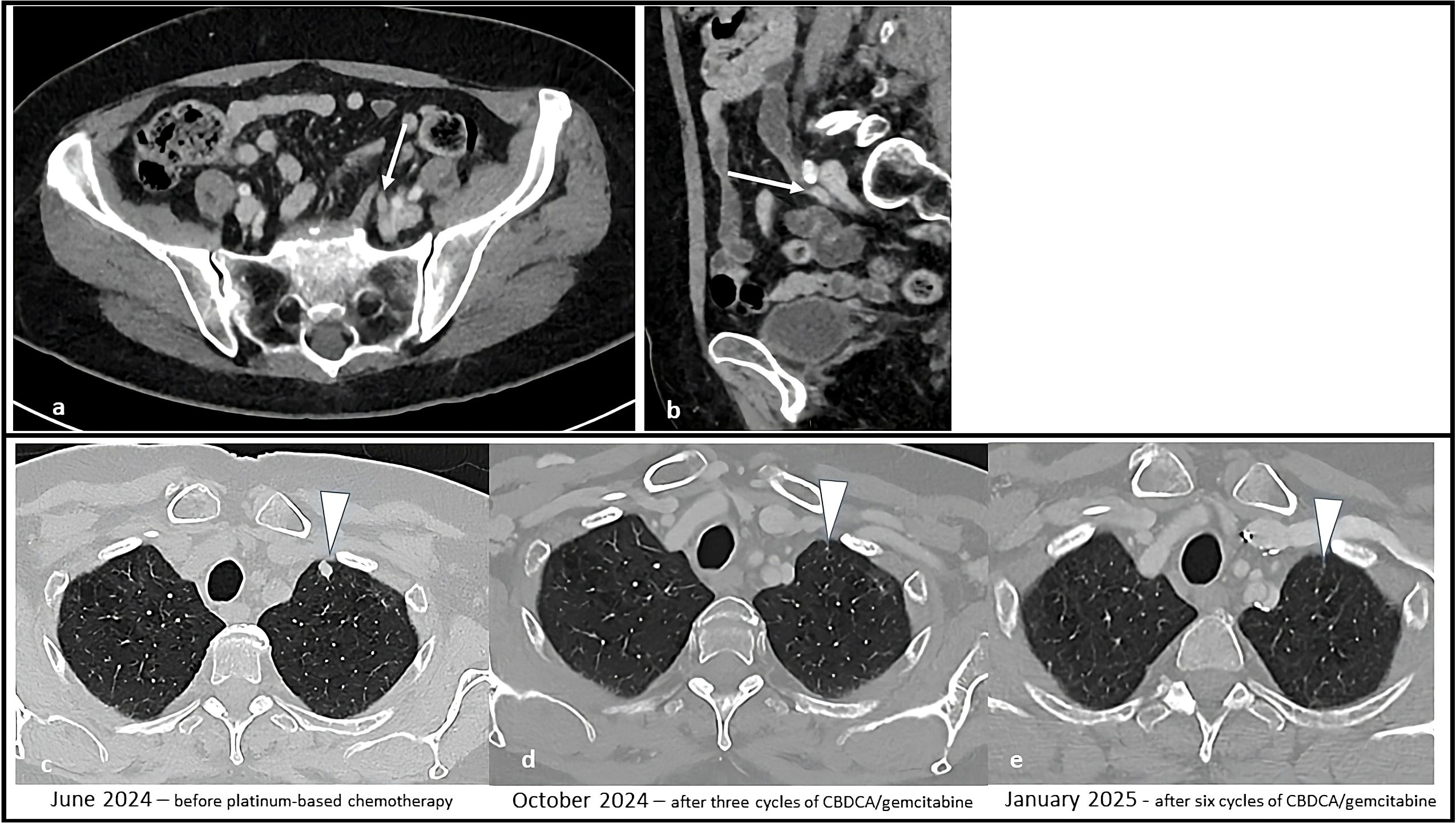
Figure 3. Sequential CT scans showing disease progression during treatment with enfortumab vedotin plus pembrolizumab and subsequent partial response with platinum-based chemotherapy. Solid tissue within the left ureter causing hydronephrosis (white arrow) in the axial (a) and sagittal (b) CT images. Afterwards, the appearance of solid lung nodules was noted [white arrowhead in (c)], that progressively responded to therapy (d) and disappeared (e).
The final pathological staging was pT2, L/V1, Pn0, G3, R0. However, carcinomatous lymphangitis was also noted in the adipose tissue adjacent to the left common iliac lymph nodes (Figure 4A).
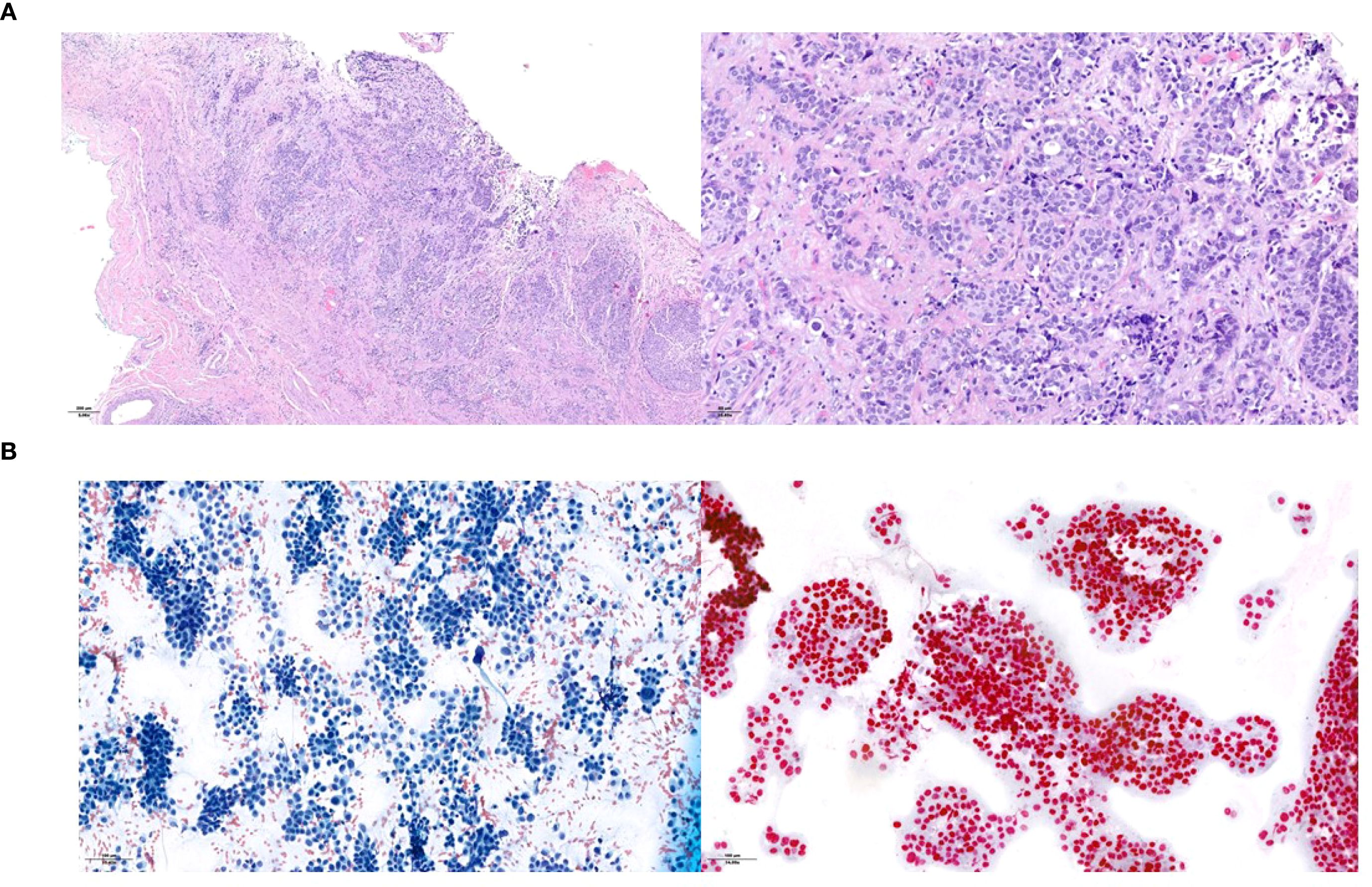
Figure 4. Histopathological presentation of the urothelial ureteral primary tumor (A) and of the supraclavicular lymph node metastasis (B). Hematoxylin and eosin staining in the primary tumor and assessment of neoplastic cellularity and GATA-3 expression in the lymph node.
Somatic NGS evaluation of the ureteral specimen was performed by Ion Torrent (Thermo Fisher Scientific, Waltham, MA) using Oncomine Comprehensive Assay v3 (Thermo Fisher Scientific, Waltham, MA) and identified an isolated BRCA2 p.N2930Ifs*7 mutation with a 25.5% allele frequency. The case was discussed at our molecular board where the BRCA alteration found was considered pathogenic. Germline genetic testing did not reveal any pathogenic variants (specifically in BRCA2) but identified three variants of uncertain significance (VUS) in CHEK2, BUB1B, and MUTYH, which are currently considered to be of no clinical relevance. The tumor was classified as microsatellite-stable while tumor mutational burden analysis was not performed.
A follow-up FDG-PET scan performed nine months post-surgery revealed new suspicious lymphadenopathies in the left supraclavicular, mediastinal, and retroperitoneal regions, as well as metabolically active lesions in the right scapula and upper right lung lobe. Fine-needle aspiration of the left supraclavicular lymph node confirmed metastatic urothelial carcinoma (Figure 4B).
Metastatic disease progression was therefore established, the patient was referred to the Oncology Department and initiated first-line treatment with EV-P in January 2024. After four cycles she developed an immune-related colitis with grade 3 diarrhea. The patient also underwent a colonoscopy which revealed collagenous and apoptotic colitis consistent with immunotherapy toxicity. Pembrolizumab was therefore discontinued. The colitis completely resolved following corticosteroid treatment. EV monotherapy was continued from cycle five onward without dose reductions.
A CT scan performed after four cycles (March 26, 2024) showed stable disease per RECIST 1.1, with minimal reduction in the left supraclavicular. However, a follow-up CT scan five months after treatment initiation (June 2024, after 8 cycles of EV and 4 cycles of P) revealed multiple new suspicious millimetric pulmonary nodules. A confirmatory CT imaging performed one month later confirmed disease progression (Figure 3).
EV treatment was discontinued and second-line chemotherapy with carboplatin AUC 5 plus gemcitabine 1000 mg/m² was initiated in August 2024. Carboplatin was chosen because of a mildly reduced glomerular filtration rate of 45 mL/min/1.73 m2. From the third cycle onwards, both drugs were administered at a 25% reduced dosage because of emergent grade 3 thrombocytopenia and neutropenia. A CT scan after three cycles (October 31, 2024) showed a partial response according to RECIST 1.1., with both numerical and dimensional reduction of the pulmonary nodules. The case was subsequently discussed at our multidisciplinary board which, due to the good response to treatment, suggested continuing treatment with carboplatin plus gemcitabine for up to six cycles. After the completion of six chemotherapy cycles (January 2025) the patient repeated a CT staging which showed a further reduction in lung nodules (Figure 3). A follow-up CT scan performed in March 2025 showed no sign of disease progression.
The patient remains on active follow-up with no signs of progression, currently seven months after the last chemotherapy cycle.
3 Discussion and conclusions
We report two cases of patients with mUC and BRCA 2 mutations who exhibited a short progression free survival (PFS) of 4 and 6 months to first-line EV-P therapy. In contrast they both achieved a deep and prolonged response with second-line platinum-based chemotherapy. Since international guidelines recommend the use of targeted therapies (e.g., erdafitinib in patients with mUC with FGFR alterations) as a possible second-line therapy (4, 5), at our institution, comprehensive next-generation sequencing (NGS) is routinely performed at the time of diagnosis in all patients with metastatic urothelial carcinoma, as part of standard clinical care, to determine in advance whether we can use a targeted therapy as second-line therapy. Since the results of these analyses are not always rapid, we prefer to test our patients at the diagnosis of metastatic disease in order to have these results readily available in case of disease progression. To date, between September 2021 and December 2024, a total of 23 patients were treated with EV-containing regimens at our Institute. Among these, 11 patients received EV-P as first-line treatment for mUC, starting from December 2023. Of these 11 patients, only 2 harbored pathogenic BRCA mutations, corresponding to the two cases described in our study. In the remaining 12 patients who received EV monotherapy, no BRCA alterations were identified (3).
Current guidelines recommend EV-P as the first-line standard of care for all eligible patients, regardless of tumor location or molecular profile (4, 5). However, given its recent introduction in clinical practice, predictive factors for response and survival outcomes with EV-P are largely unknown.
Our two patients had a much shorter PFS than the median reported in the EV-302 study (4 and 6 months vs 12.5 months) (3). The comparatively poor outcome on EV-P in our patients with BRCA2-mutated tumors raises questions about the potential negative predictive role of BRCA2 mutations in mUC. In the EV-302 study, no analyses of the efficacy of EV-P based on the results of molecular analyses (e.g. pathogenic BRCA mutations) were reported. Therefore, to date, we have no data on the efficacy of EV-P versus platin-based therapy in patients with pathogenic BRCA alterations. Based on the history of our two patients one could hypothesize that patients with pathogenic BRCA mutation may respond better to platin-based therapy than to EV-P. And there may be some biological rationale for that.
There is a strong rationale for the observed sensitivity of BRCA-mutant mUC to platinum-based therapy. BRCA mutations are well established as predictive markers of platinum sensitivity in other malignancies (10), and further data suggest that mUC patients with BRCA mutations achieve better outcomes with first-line platinum-based chemotherapy (11) and in the perioperative setting (12).
One of the possible, yet speculative, explanations for the short response to EV-P in patients with BRCA alterations may lie on its mechanism of action. After the antibody binds to Nectin-4, the cytotoxic payload, monomethyl auristatin E (MMAE), is released. MMAE does not directly induce DNA damage; instead, it disrupts microtubule polymerization, impairing chromosomal assembly and segregation during mitosis, ultimately leading to mitotic arrest and cell death through mitotic catastrophe (13). We could hypothesize that this mechanism of action is inherently less effective in BRCA-mutated tumors, where genomic instability is a key driver of cancer progression. In such contexts, agents that cause direct DNA damage, such as platinum-based compounds, may achieve more durable disease control due to the tumor’s underlying inability to repair double-strand DNA breaks efficiently.
Our hypothesis remains speculative, as there are currently no further data to support its mechanism in patients with bladder cancer or in those receiving MMAE-based ADCs for other malignancies. In other cancer types treated with microtubule-targeting agents, the evidence is inconsistent: BRCA2-mutant tumors in some contexts exhibit limited sensitivity or inherent resistance (14–17), while in others, BRCA2 alterations appear to enhance sensitivity to these therapies (18).
Furthermore, some data suggest that alterations in DDR genes may be associated with improved outcomes in urothelial cancer patients treated with PD-1/PD-L1 inhibitors (19), complicating the interpretation of our findings. Preclinical (20) and clinical (21) data further suggest that DDR alterations could represent a biologically heterogeneous group of UCs, with individual mutations potentially linked to variable treatment sensitivities. As a result, their predictive value may be limited when assessed collectively rather than as distinct genomic entities.
When considering potential molecular predictors of response to EV-P, Nectin-4 expression assessed by immunohistochemistry was not a predictive factor of response to the EV-P combination (22). Further data on clinical and molecular predictors of EV-P response remain scarce, and genomic determinants of treatment efficacy have not yet been explored.
Predictive factors for EV monotherapy have been more extensively studied, given its earlier incorporation into clinical practice following positive outcomes in patients pretreated with platinum-based chemotherapy and immune checkpoint inhibitors in the EV-101 (23) and EV-201 (24) trials, with subsequent accelerated FDA approval in 2021 (25). For instance, the multicenter UNITE cohort analysis (26) have found that TSC1 alterations correlated with improved ORR, CDKN2A/2B alterations predicted shorter PFS, and high tumor mutational burden was associated with better OS following EV treatment. However, only 20 (11.8%) of the patients in this cohort had DDR mutations, including BRCA2, and they were not significantly associated with response to EV monotherapy or patient survival outcomes.
To sum up, while the EV-P combination represents a new, highly effective, life-prolonging standard-of-care treatment for treatment-naïve mUC patients, the identification of new clinical and genetic factors that predict treatment response, as highlighted in our report, will enable better prognostic and predictive stratification, potentially paving the way for personalized management strategies. Notably, platinum-based chemotherapy (combined with nivolumab or avelumab maintenance) remains a viable first-line treatment option for patients that are ineligible for EV-P or where it is unavailable. It can also serve as a second-line therapy after progression on EV-P in eligible patients although at the moment we have no evidence on what is the standard second line therapy in patients with urothelial carcinoma progressing after EV-P (27, 28). Both of our patients were treated with carboplatin-based chemotherapy as a second-line treatment because they were both cisplatin-unfit due to a reduced glomerular filtration rate. The positive results obtained with carboplatin-based chemotherapy as second line treatment in our two patients are encouraging, since many patients are cisplatin-unfit.
Finally, it remains uncertain whether clinical or genomic factors, such as the presence of BRCA alteration, could help identify subgroups of patients who would derive greater survival benefit from frontline platinum-based therapy even in settings where EV-P is accessible (29).Exploring this question in large prospective trials is needed. A comparative analysis of outcomes between BRCA-altered and non-HRR-altered patients treated with first and second-line platinum-based regimens could clarify whether BRCA-mutant tumors are more responsive to DNA-damaging agents or, conversely, inherently less responsive to EV-P. These two hypotheses are not mutually exclusive and warrant systematic investigation. A prospective collection of genomic and treatment response data would be an important step toward addressing this question and better defining the optimal treatment approach for patients with BRCA-mutated mUC.
Beyond the metastatic setting, the identification of predictive biomarkers for EV-P and other UC treatment regimens may have important implications in other contexts, such as the evolving perioperative landscape (30). Ongoing studies are evaluating EV-based combinations with immunotherapy in earlier disease stages (31–33), and even biomarker-driven, risk-adaptive strategies such as bladder preservation trials are being explored (34). Ultimately, the role of BRCA and other genomic alterations as predictive factors in these contexts remains a critical area for future research.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comitato etico cantonale c/o Ufficio di sanità Via Orico 5 6501 Bellinzona. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GS: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. CC: Writing – original draft, Writing – review & editing. GP: Writing – review & editing. MPe: Writing – review & editing. MPu: Writing – original draft, Writing – review & editing. LT: Writing – review & editing. RP: Writing – review & editing. UV: Writing – review & editing. IC: Writing – review & editing. JB: Data curation, Visualization, Writing – review & editing. MF: Writing – review & editing. RG: Writing – review & editing. SR: Visualization, Writing – review & editing. AG: Writing – review & editing. FM: Writing – review & editing. NF: Writing – review & editing. SG: Writing – review & editing. FT: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
SG received personal honoraria for participation in advisory boards for Sanofi, Orion, Roche, Amgen, MSD; other honoraria from RSI Televisione Svizzera Italiana; invited speaker for ESMO, Swiss group for Clinical Cancer Research SAKK, Swiss Academy of Multidisciplinary oncology SAMO, Orikata academy research group, China Anti-Cancer Association Genitourinary Oncology Committee CACA-GU; Speaker’s bureau for Janssen Cilag; travel grant from ProteoMEdiX; institutional honoraria for advisory boards for Bayer, Janssen Cilag, Roche, AAA International including Indipendent Data Monitoring Committee and IDMC and Steering Committee member for Amgen, Menarini Silicon Biosystems, Astellas Pharma, Tolero Pharmaceuticals, MSD, Pfizer, Telixpharma, BMS and Orion; patent royalties and other intellectual property for a research method for biomarker WO2009138392. UV received a grant from Fond’Action; received institutional personal honoraria from Pfizer, Astellas, Janssen, Merck, MSD, BMS, Ipsen, Healthbook, Novartis AAA, Bayer, Grasso consulting, and Vaccentis; received private personal honoraria from Healthbook, SAMO, Inselspital Bern, Kantonsspital Chur, Kantonsspital Baden, and Ipsen; and received a travel grant from Astra Zeneca and Janssen. FT: payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Silvio Grasso Consulting Speaker “Opinioni a confronto in oncologia” 11.2022/09.2024; institution; SAKK: Speaker “SAKK Ticino meeting” 04.2022 institution; Speaker “GU Cancer Forum” 03.2024 institution; Speaker “GU Cancer Forum” 04.2025 institution; Merck Speaker “Swiss Educational Events Bladder Cancer from MIBC to Metastasis An Integrated Approach to Personalized Treatment Optimization”; institution; Novartis Interview about the role about lutetium-PSMA in prostate cancer; institution. Support for attending meetings and/or travel: Bayer Travel support EAU 2022 and Travel support ASCO GU 2025; Participation on a Data Safety Monitoring Board or Advisory Board: Bayer Ad board 11.2024; institution. IC declares institutional funding for clinical trials as PI from AstraZeneca, Merck Sharp & Dhome, Vivesto, Tolremo, Orion, Bayer, lncyte, consultancy/advisor role from AstraZeneca, GlaxoSmithKline, Merck Sharp & Dhome, AbbVie, Biontech, lncyte, Beigene outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1648230/full#supplementary-material
References
1. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Hu X, Miao J, Qian L, Zhang D, and Wei H. The predictors and surgical outcomes of different distant metastases patterns in upper tract urothelial carcinoma: A SEER-based study. Front Surg. (2022) 9:1045831. doi: 10.3389/fsurg.2022.1045831
3. Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. New Engl J Med. (2024) 390:875–88. doi: 10.1056/NEJMoa2312117
4. Flaig TW, Spiess PE, Abern M, Agarwal N, Bangs R, Buyyounouski MK, et al. Bladder cancer, version 3.2024. J Natl Compr Cancer Netw. (2024) 22:216–25. doi: 10.6004/jnccn.2024.0024
5. European Association of Urology Guidelines. Muscle-invasive and Metastatic Bladder Cancer. European Association of Urology (2024). Available online at: https://uroweb.org/guidelines/muscle-invasive-and-metastatic-bladder-cancer (Accessed June 10, 2025).
6. Loriot Y, Matsubara N, Park SH, Huddart RA, Burgess EF, Houede N, et al. Erdafitinib or chemotherapy in advanced or metastatic urothelial carcinoma. New Engl J Med. (2023) 389:1961–71. doi: 10.1056/NEJMoa2308849
7. Meric-Bernstam F, Makker V, Oaknin A, Oh D-Y, Banerjee S, González-Martín A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-panTumor02 phase II trial. J Clin Oncol. (2024) 42:47–58. doi: 10.1200/JCO.23.02005
8. Heeke AL, Pishvaian MJ, Lynce F, Xiu J, Brody JR, Chen W-J, et al. Prevalence of homologous recombination–related gene mutations across multiple cancer types. JCO Precis Oncol. (2018) 2:1–13. doi: 10.1200/PO.17.00286
9. Kuzbari Z, Bandlamudi C, Loveday C, Garrett A, Mehine M, George A, et al. Germline-focused analysis of tumor-detected variants in 49,264 cancer patients: ESMO Precision Medicine Working Group recommendations. Ann Oncol. (2023) 34:215–27. doi: 10.1016/j.annonc.2022.12.003
10. Jia X, Wang K, Xu L, Li N, Zhao Z, and Li M. A systematic review and meta-analysis of BRCA1/2 mutation for predicting the effect of platinum-based chemotherapy in triple-negative breast cancer. Breast. (2022) 66:31–9. doi: 10.1016/j.breast.2022.08.012
11. Teo MY, Bambury RM, Zabor EC, Jordan E, Al-Ahmadie H, Boyd ME, et al. DNA damage response and repair gene alterations are associated with improved survival in patients with platinum-treated advanced urothelial carcinoma. Clin Cancer Res. (2017) 23:3610–8. doi: 10.1158/1078-0432.CCR-16-2520
12. Plimack ER, Dunbrack RL, Brennan TA, Andrake MD, Zhou Y, Serebriiskii IG, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol. (2015) 68:959–67. doi: 10.1016/j.eururo.2015.07.009
13. Best RL, LaPointe NE, Azarenko O, Miller H, Genualdi C, Chih S, et al. Microtubule and tubulin binding and regulation of microtubule dynamics by the antibody drug conjugate (ADC) payload, monomethyl auristatin E (MMAE): Mechanistic insights into MMAE ADC peripheral neuropathy. Toxicol Appl Pharmacol. (2021) 421:115534. doi: 10.1016/j.taap.2021.115534
14. Pellegrino B, Cavanna L, Boggiani D, Zamagni C, Frassoldati A, Schirone A, et al. Phase II study of eribulin in combination with gemcitabine for the treatment of patients with locally advanced or metastatic triple negative breast cancer (ERIGE trial). Clinical and pharmacogenetic results on behalf of the Gruppo Oncologico Italiano di Ricerca Clinica (GOIRC). ESMO Open. (2021) 6:100019. doi: 10.1016/j.esmoop.2020.100019
15. Olmos D, Lorente D, Jambrina A, Tello-Velasco D, Ovejero-Sánchez M, Gonzalez-Ginel I, et al. BRCA1/2 and homologous recombination repair alterations in high- and low-volume metastatic hormone-sensitive prostate cancer: prevalence and impact on outcomes. Ann Oncol. (2025) 36(10):1190–202. doi: 10.1016/j.annonc.2025.05.534
16. Nientiedt C, Heller M, Endris V, Volckmar A-L, Zschäbitz S, Tapia-Laliena MA, et al. Mutations in BRCA2 and taxane resistance in prostate cancer. Sci Rep. (2017) 7:4574. doi: 10.1038/s41598-017-04897-x
17. Wysocki PJ, Korski K, Lamperska K, Zaluski J, and Mackiewicz A. Primary resistance to docetaxel-based chemotherapy in metastatic breast cancer patients correlates with a high frequency of BRCA1 mutations. J Clin Oncol. (2008) 26:1079–9. doi: 10.1200/jco.2008.26.15_suppl.1079
18. Tan DSP, Yap TA, Hutka M, Roxburgh P, Ang J, Banerjee S, et al. Implications of BRCA1 and BRCA2 mutations for the efficacy of paclitaxel monotherapy in advanced ovarian cancer. Eur J Cancer. (2013) 49:1246–53. doi: 10.1016/j.ejca.2012.11.016
19. Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol. (2018) 36:1685–94. doi: 10.1200/JCO.2017.75.7740
20. Shanmugam S P, Zhou Y, Stelter I, Hanlon T, Bekele RT, Bellmunt J, et al. Impact of DNA repair deficiency on sensitivity to antibody-drug conjugate (ADC) payloads in bladder cancer. Bladder Cancer. (2025) 11. doi: 10.1177/23523735251317865
21. Iyer G, Choi W, Luo B, Carvalho F, Hanlon T, Reis H, et al. Relationship among DNA damage response gene alterations, molecular subtypes, and survival outcomes in patients with metastatic bladder cancer treated on CALGB 90601. JCO Precis Oncol. (2025) 9:e2400938. doi: 10.1200/PO-24-00938
22. Powles TB, van der Heijden MS, Gupta S, Perez Valderrama B, Galsky MD, Bedke J, et al. 1966MO EV-302: Exploratory analysis of nectin-4 expression and response to 1L enfortumab vedotin (EV) + pembrolizumab (P) in previously untreated locally advanced or metastatic urothelial cancer (la/mUC). Ann Oncol. (2024) 35:S1137–8. doi: 10.1016/j.annonc.2024.08.2051
23. Rosenberg JE, O’Donnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol. (2019) 37:2592–600. doi: 10.1200/JCO.19.01140
24. Yu EY, Petrylak DP, O’Donnell PH, Lee J-L, van der Heijden MS, Loriot Y, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): a multicenter, single-arm, phase 2 trial. Lancet Oncol. (2021) 22:872–82. doi: 10.1016/S1470-2045(21)00094-2
25. Chang E, Weinstock C, Zhang L, Charlab R, Dorff SE, Gong Y, et al. FDA approval summary: enfortumab vedotin for locally advanced or metastatic urothelial carcinoma. Clin Cancer Res. (2021) 27:922–7. doi: 10.1158/1078-0432.CCR-20-2275
26. Jindal T, Jiang C, Alhalabi O, Nizam A, Nguyen C, Talukder R, et al. Genomic biomarkers associated with enfortumab vedotin outcomes for patients with advanced urothelial carcinoma: analysis of UNITE study data. Eur Urol Oncol. (2024) 8(2):258–62. doi: 10.1016/j.euo.2024.12.006
27. Powles T, Bellmunt J, Comperat E, De Santis M, Huddart R, Loriot Y, et al. ESMO Clinical Practice Guideline interim update on first-line therapy in advanced urothelial carcinoma. Ann Oncol. (2024) 35:485–90. doi: 10.1016/j.annonc.2024.03.001
28. Vogl UM, Testi I, and De Santis M. Front-line platinum continues to have a role in advanced bladder cancer. Eur Urol Focus. (2024) 10:217–8. doi: 10.1016/j.euf.2024.03.005
29. Klümper N and Eckstein M. Biomarkers of response to anti-NECTIN4 antibody-drug conjugate enfortumab vedotin in urothelial cancer. Eur Urol Focus. (2024) 10:224–6. doi: 10.1016/j.euf.2024.04.001
30. Vogl U, Salfi G, Pecoraro G, Pedrani M, Pereira Mestre R, Puglisi M, et al. Current advances in the perioperative treatment of muscle-invasive bladder cancer. Healthbook TIMES Oncol Hematol. (2025) 24(2):54–63. doi: 10.36000/HBT.OH.2025.24.184
31. Necchi A, Bedke J, Galsky MD, Shore ND, Plimack ER, Xylinas E, et al. Phase 3 KEYNOTE-905/EV-303: Perioperative pembrolizumab (pembro) or pembro + enfortumab vedotin (EV) for muscle-invasive bladder cancer (MIBC). J Clin Oncol. (2023) 41:TPS585–5. doi: 10.1200/JCO.2023.41.6_suppl.TPS585
32. Powles T, Drakaki A, Teoh JY-C, Grande E, Fontes-Sousa M, Porta C, et al. A phase 3, randomized, open-label, multicenter, global study of the efficacy and safety of durvalumab (D) + tremelimumab (T) + enfortumab vedotin (EV) or D + EV for neoadjuvant treatment in cisplatin-ineligible muscle-invasive bladder cancer (MIBC) (VOLGA). J Clin Oncol. (2022) 40:TPS579–9. doi: 10.1200/JCO.2022.40.6_suppl.TPS579
33. Hoimes CJ, Bedke J, Loriot Y, Nishiyama H, Fang X, Kataria RS, et al. KEYNOTE-B15/EV-304: Randomized phase 3 study of perioperative enfortumab vedotin plus pembrolizumab versus chemotherapy in cisplatin-eligible patients with muscle-invasive bladder cancer (MIBC). J Clin Oncol. (2021) 39:TPS4587–TPS4587. doi: 10.1200/JCO.2021.39.15_suppl.TPS4587
Keywords: metastatic urothelial carcinoma, enfortumab vedotin, pembrolizumab, EV-302, BRCA, genomic biomarkers
Citation: Salfi G, Clerici CMA, Pecoraro G, Pedrani M, Puglisi M, Tortola L, Pereira Mestre R, Vogl U, Colombo I, Barizzi J, Frattini M, Graffeo R, Rizzo S, Gallina A, Monni F, Fossati N, Gillessen S and Turco F (2025) Case Report: Could genetic factors influence the outcomes of first-line enfortumab vedotin plus pembrolizumab therapy in patients with metastatic urothelial carcinoma? Two cases of patients harbouring a BRCA mutation. Front. Oncol. 15:1648230. doi: 10.3389/fonc.2025.1648230
Received: 16 June 2025; Accepted: 19 September 2025;
Published: 03 October 2025.
Edited by:
Steven Kregel, Loyola University Chicago, United StatesReviewed by:
Kent William Mouw, Dana–Farber Cancer Institute, United StatesMichal Sternschuss, Davidoff Cancer Treatment and Research Center, Israel
Copyright © 2025 Salfi, Clerici, Pecoraro, Pedrani, Puglisi, Tortola, Pereira Mestre, Vogl, Colombo, Barizzi, Frattini, Graffeo, Rizzo, Gallina, Monni, Fossati, Gillessen and Turco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabio Turco, ZmFiaW8udHVyY29AZW9jLmNo
 Giuseppe Salfi
Giuseppe Salfi Chiara Maria Agrippina Clerici1
Chiara Maria Agrippina Clerici1 Martino Pedrani
Martino Pedrani Marialuisa Puglisi
Marialuisa Puglisi Ricardo Pereira Mestre
Ricardo Pereira Mestre Ursula Vogl
Ursula Vogl Ilaria Colombo
Ilaria Colombo Jessica Barizzi
Jessica Barizzi Milo Frattini
Milo Frattini Stefania Rizzo
Stefania Rizzo Fabio Turco
Fabio Turco