- 1Dana-Farber Cancer Institute, Boston, MA, United States
- 2University of Minnesota Medical School, Minneapolis, MN, United States
- 3Analysis Group, Inc., Montréal, QC, Canada
- 4Johnson & Johnson, Horsham, PA, United States
Background: The triplet combination of chemotherapy (docetaxel), androgen receptor pathway inhibitors (ARPI), and androgen deprivation therapy has recently become a recommended approach to treat metastatic castration-sensitive prostate cancer (mCSPC). This study aimed to compare the incremental time and cost burden of adding docetaxel to ARPI-based treatment among patients with mCSPC receiving chemotherapy-containing regimens (CCR) and non–chemotherapy-containing regimens (NCR) in the United States.
Methods: Clinical data from community urology practices linked with claims data (1/1/2016–12/31/2023) were used to select patients initiating a CCR or NCR. Outcomes, including time spent managing mCSPC (days with prostate cancer-related resource utilization or management care) and healthcare costs, were compared between balanced cohorts using weighted multivariable Poisson and linear regressions.
Results: 126 CCR and 837 NCR patients (mean age 64.7 years, 52.6% White, 14.4% Black) were followed for a mean of 6.3 (CCR) and 6.8 (NCR) months. The CCR cohort spent on average 4.1 days per-patient-per-month (PPPM) managing mCSPC vs 3.3 days PPPM in the NCR cohort (rate ratio: 1.18; 95% confidence interval [CI]; 1.03, 1.34). Mean all-cause total healthcare costs were $17,833 PPPM in the CCR cohort and $11,527 PPPM in the NCR cohort (weighted adjusted cost difference: $6,184; 95% CI: 3,515, 8,517).
Conclusions: Patients initiating a CCR experienced greater time burden managing mCSPC and higher healthcare costs than those initiating an NCR. These findings support counseling expressing these differences in burden in mCSPC treatment decision-making conversations.
1 Introduction
Prostate cancer (PC) is the second leading cause of cancer-related death among men in the United States (US) (1). Metastatic castration-sensitive prostate cancer (mCSPC), also known as metastatic hormone-sensitive prostate cancer, is an advanced stage of PC that is sensitive to hormone therapies (2). Chemotherapy (i.e., docetaxel) combined with androgen deprivation therapy (ADT) was the standard frontline treatment prior to the approval of oral androgen receptor pathway inhibitors (ARPIs), which are now widely used in combination with ADT for the treatment of mCSPC (3–5). Recently, intensifying treatment with a triple combination of chemotherapy, an ARPI (i.e., abiraterone acetate or darolutamide), and ADT has been recommended in practice guidelines as a treatment option for mCSPC (6–9).
With growing mCSPC treatment options, healthcare providers should consider various attributes of treatments that may impact patients’ outcomes and well-being (6). During cancer treatment selection, survival benefits and side effects of therapies are typically communicated with patients; however, the consideration of patients’ time and cost burden remains underacknowledged by oncologists (10, 11). Time burden of cancer treatment, also known as “time toxicity”, can be conceptualized as the time spent in coordinating care and in healthcare facility visits for receiving cancer-directed therapy, emergency care for side effects, hospitalization, and testing; the time spent during these visits also includes travel and wait times, which are often underrecognized (10, 11). Patient time burden is particularly relevant in advanced cancer settings when differences in survival benefits can be modest (or absent) between treatments, as the modest survival gain could be offset by the time lost to managing care (11, 12).
Given that chemotherapy with docetaxel has been associated with additional, potentially costly, clinical side effects in both clinical trial and real-world settings in mCSPC (13, 14), docetaxel-containing regimens may result in increased healthcare resource utilization (HRU) and costs, as well as greater time burden to manage care, relative to regimens without docetaxel. To date, however, data on the time burden and healthcare costs associated with chemotherapy-containing regimens (CCR) relative to non–chemotherapy-containing regimens (NCR) for mCSPC have been limited. This study aimed to compare the incremental HRU burden of chemotherapy intensification by assessing the number of days needed to manage PC care and healthcare costs among patients with mCSPC receiving ARPI-based treatment in the US.
2 Patients and methods
2.1 Data source
Clinical data from Precision Point Specialty Analytics (hereafter PPS) linked with administrative claims data from the Komodo Research Database (hereafter KRD) were used (01/01/2016–12/31/2023). The PPS database contains electronic medical record data collected as part of routine clinical care from >90 US community-based urology practices. The KRD contains claims data sourced from various payers and healthcare organizations covering >320 million US patients (see Supplementary Methods for more details). As all data are de-identified and Health Insurance Portability and Accountability Act compliant, approval from an institutional review board was not required.
2.2 Study design
A retrospective longitudinal analysis among propensity score–weighted cohorts of ARPI-naïve patients with mCSPC initiated on a CCR or an NCR was conducted. Patients were assigned to two mutually exclusive treatment cohorts based on the presence or absence of docetaxel intensification in the ARPI-based treatment: the CCR cohort included patients treated with the triplet regimen of docetaxel + abiraterone acetate or darolutamide + ADT, and the NCR cohort included patients treated with the doublet regimen of abiraterone acetate + ADT (as of the time of study conduct, darolutamide + ADT was not approved for treatment of mCSPC without the concurrent use of docetaxel (15)).
For the CCR cohort, the index date was the earliest of docetaxel initiation or first claim for abiraterone acetate or darolutamide on/after US Food and Drug Administration mCSPC indication approval dates for abiraterone acetate (02/07/2018 [high-risk mCSPC]) (16) or darolutamide (08/05/2022) (17). For the NCR cohort, the index date was the first claim for abiraterone acetate on/after the approval date for the high-risk mCSPC indication by the US Food and Drug Administration. The baseline period was the 6 months preceding the index date. The observation period spanned from the index date until the earliest of 12 months following the index date, discontinuation of the index ARPI or initiation of a new ARPI, initiation of docetaxel (NCR cohort only), end of continuous closed claims insurance eligibility, or end of data availability (including death).
2.3 Patient selection criteria
Adult patients were included if they i) had ≥1 paid pharmacy claim (in KRD) or dispensation (in PPS) for abiraterone acetate or darolutamide (first paid pharmacy claim or dispensation was defined as the ARPI initiation date); ii) had ≥1 medical or paid pharmacy claim or procedure for ADT observed ± 180 days around the ARPI initiation date; iii) (CCR cohort only) had ≥1 medical or paid pharmacy claim or procedure for docetaxel observed 90 days prior to or 180 days after the ARPI initiation date; iv) had mCSPC status on the index date, with <9 months between the date of metastasis and the index date (see Supplementary Methods for assessment details); and v) had ≥6 months of claims activity prior to (and including) the index date during a period of continuous closed claims insurance eligibility.
Patients were excluded if they had prior use of advanced PC-related medications (i.e., radiopharmaceutical therapy, chemotherapy [except docetaxel for the CCR cohort], apalutamide, enzalutamide, immunotherapy, or poly ADP-ribose polymerase inhibitors) any time prior to or on the index date; or if they became castration-resistant during the time between ARPI initiation and docetaxel initiation.
2.4 Study outcomes
Time spent managing mCSPC, defined as the number of days with PC-related HRU or PC management care, was assessed during the observation period. HRU categories included inpatient admission and days, emergency room (ER) visits, outpatient visits, and other services (e.g., dental or vision care, durable medical equipment services). PC management care included imaging, biopsy, chemotherapy management (i.e., iron/red blood cell transfusion, treatment with erythropoiesis-stimulating agents or granulocyte colony-stimulating factor [G-CSF]), prostate-specific antigen assessment, and genetic testing). When multiple HRU services for one patient on the same day were observed, these services only contributed one day. For inpatient admission, the number of days was calculated from the admission and discharge dates. In instances where the date of the claim differed from the date the service was received (e.g., laboratory tests or imaging), the date on which the service was received was used.
All-cause and PC-related HRU and healthcare costs were also assessed during the observation period. Total healthcare costs were the sum of medical costs (i.e., inpatient, ER, outpatient, and other services costs) and pharmacy costs. Separately, costs related to chemotherapy management (as defined above) in any setting were also assessed. Costs were inflated to 2023 US dollars using the medical care component of the Consumer Price Index.
All outcomes are reported per-patient-per-month (PPPM).
2.5 Statistical analyses
Overlap weighting, based on the propensity score, was used to account for differences in baseline characteristics between the CCR and NCR cohorts (Supplementary Methods) (18, 19). Balancing of baseline characteristics between both the non-weighted and weighted cohorts was assessed using standardized differences (<10% was considered well-balanced) (20).
The time spent managing mCSPC and HRU outcomes were compared between the weighted cohorts using weighted Poisson regression models with an offset to account for different follow-up times to calculate rate ratios (RRs). Cost outcomes were compared between the weighted cohorts using weighted ordinary least squares regression models. Non-parametric bootstrapping procedure with 500 replications was used to calculate 95% confidence intervals (CIs) and p-values for all models. All weighted regression models were further adjusted for baseline characteristics that remained imbalanced after weighting (Supplementary Methods).
3 Results
3.1 Baseline characteristics
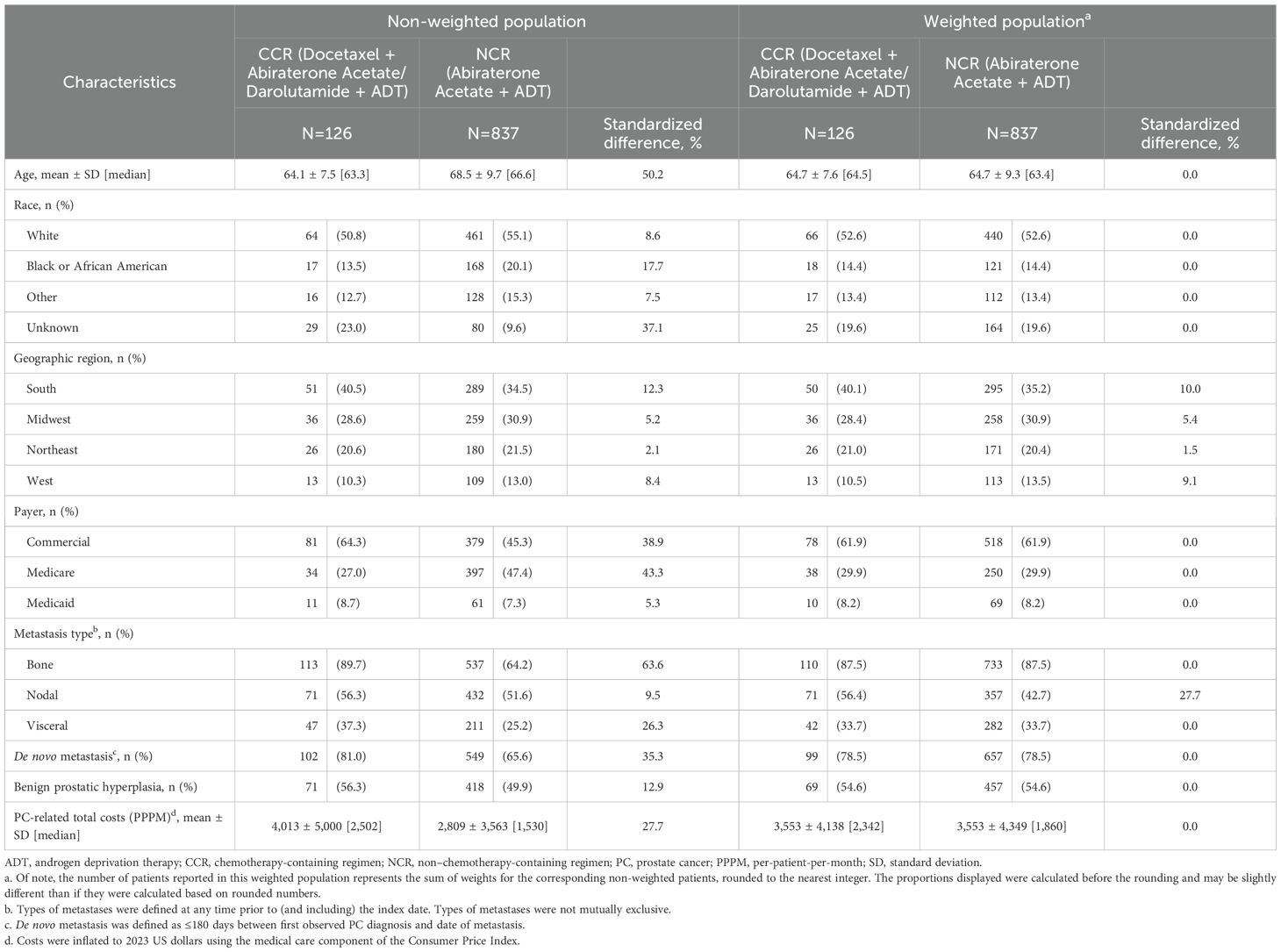
Overall, 126 patients were included in the CCR cohort and 837 in the NCR cohort (Figure 1). Baseline patient characteristics were generally well-balanced between the weighted cohorts, with standardized differences <10% (Table 1). In both the weighted CCR and NCR cohorts, mean age was 64.7 years, 52.6% were White patients, and 14.4% were Black or African American patients. Over 60% (61.9%) of patients were covered by commercial insurance, whereas 29.9% and 8.2% were Medicare and Medicaid beneficiaries, respectively. During baseline, most patients had bone metastasis (87.5% in both cohorts), about half had nodal metastasis (56.4% in CCR cohort; 42.7% in NCR cohort), and one-third had visceral metastasis (33.7% in both cohorts). Nearly 80% (78.5%) of patients in both cohorts had de novo metastasis and 54.6% had benign prostatic hyperplasia.
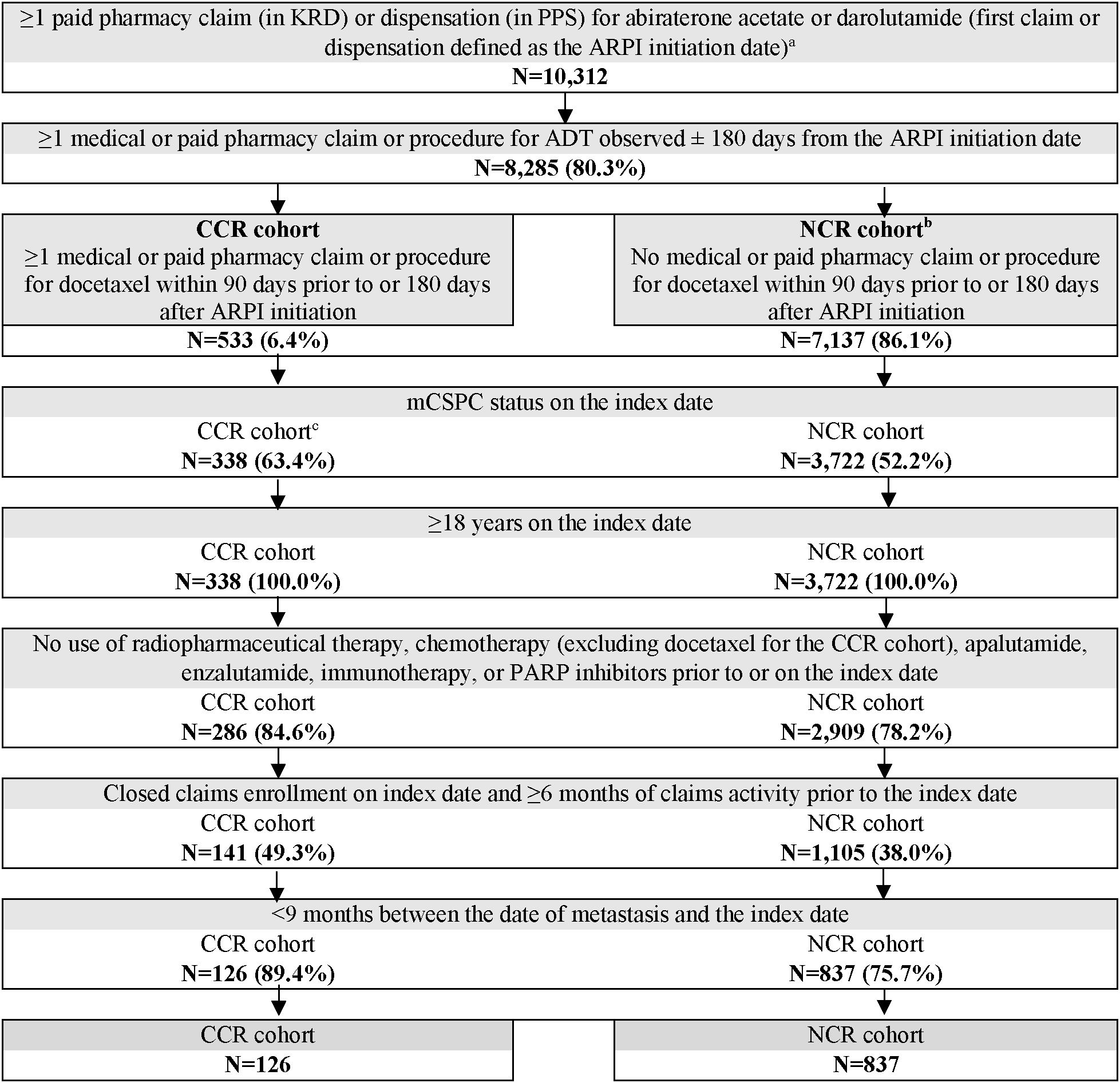
Figure 1. Patient selection flowchart. ADT, androgen deprivation therapy; ARPI, androgen receptor pathway inhibitor; CCR, chemotherapy-containing regimen; KRD, Komodo Research Database; mCSPC, metastatic castration-sensitive prostate cancer; NCR, non–chemotherapy-containing regimen; PARP, poly ADP-ribose polymerase; PPS, Precision Point Specialty; US, United States. a. ARPI initiation date on or after US Food and Drug Administration approval for mCSPC (i.e., 02/07/2018 for abiraterone acetate [high-risk mCSPC], 08/05/2022 for darolutamide). b. Only ARPI-naïve patients who initiated abiraterone acetate + ADT were included in the NCR cohort given as of the time of study conduct, darolutamide + ADT was not approved for treatment of mCSPC without the concurrent use of docetaxel. c. Patients were excluded if they became castration-resistant during the time between ARPI initiation and docetaxel initiation.
3.2 Time spent managing mCSPC and HRU
Patients were observed for a mean of 6.3 months (median 6.0 months) in the CCR cohort and 6.8 months (median 6.7 months) in the NCR cohort. For the CCR cohort, a mean of 4.0 docetaxel infusions were observed per patient, with a mean of 22 days between successive infusions. During the observation period, the CCR cohort spent a mean of 4.1 days PPPM (median 3.6 days PPPM) managing mCSPC, compared with 3.3 days PPPM (median 2.4 days PPPM) in the NCR cohort (RR: 1.18, 95% CI: 1.03, 1.34; p=0.016; Figure 2).
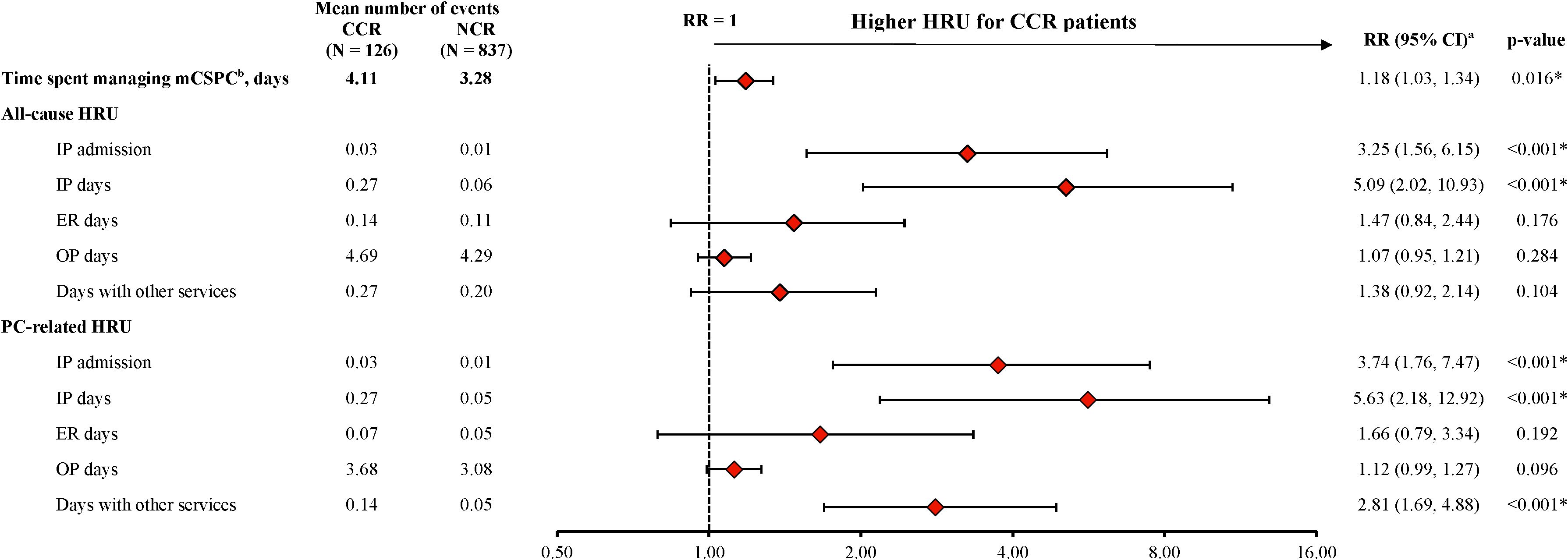
Figure 2. HRU among patients receiving CCR or NCR, PPPM. CCR, chemotherapy-containing regimen; CI, confidence interval; ER, emergency room; HRU, healthcare resource utilization; IP, inpatient; mCSPC, metastatic castration-sensitive prostate cancer; NCR, non–chemotherapy-containing regimen; OP, outpatient; PC, prostate cancer; PPPM, per-patient-per-month; RR, rate ratio. * p-value <0.05. a. The weighted model was adjusted for the following baseline variables: all-cause pharmacy costs, categorical age (≤70, 71-80, ≥81), time between metastasis and index date, baseline time spent managing mCSPC, erectile dysfunction, nodal metastasis and Quan-Charlson comorbidity index score. b. The time spent managing mCSPC is reported from the patients’ perspective, by days with a PC-related HRU or PC management care HRU outcome. When multiple HRU services for one patient on the same day were observed, these services only contributed one day. For inpatient stays, the number of days was calculated from the admission and discharge dates. In instances where the date of the claim differed from the date the service was received (e.g., laboratory tests or imaging), the date in which the service was received was used. All days were summed per person, and time spent managing mCSPC was reported PPPM.
The CCR cohort experienced a greater number of all-cause inpatient admissions (RR: 3.25, 95% CI: 1.56, 6.15; p<0.001) and inpatient days (RR: 5.09, 95% CI: 2.02, 10.93; p<0.001) compared with the NCR cohort during the observation period (Figure 2). Similar results were seen for PC-related inpatient admissions (RR: 3.74; 95% CI: 1.76, 7.47; p<0.001) and inpatient days (RR: 5.63; 95% CI: 2.18, 12.92; p<0.001). In addition, the number of PC-related days with other services was also greater in the CCR cohort than in the NCR cohort (RR: 2.81, 95% CI: 1.69, 4.88; p<0.001; Figure 2). The number of days spent on all-cause and PC-related ER visits trended higher in the CCR cohort relative to the NCR cohort, although the differences were not statistically significant (RR for all-cause ER days: 1.47, 95% CI: 0.84, 2.44; p=0.176; PC-related ER days: 1.66, 95% CI: 0.79, 3.34; p=0.192; Figure 2).
3.3 Economic burden
During the observation period, the CCR cohort incurred significantly higher healthcare costs compared with the NCR cohort (Figure 3A, Supplementary Table 1). The mean all-cause total healthcare costs were $17,833 PPPM in the CCR cohort and $11,527 PPPM in the NCR cohort (cost difference: $6,184; 95% CI: 3,515, 8,517; p<0.001). Medical costs constituted nearly 40% of the all-cause total costs and were $6,592 PPPM in the CCR cohort and $4,240 PPPM in the NCR cohort (cost difference: $2,060; 95% CI: 865, 3,369; p<0.001). All-cause pharmacy costs were $11,290 PPPM in the CCR cohort and $7,288 PPPM in the NCR cohort (cost difference: $4,124; 95% CI: 1,914, 6,168; p<0.001).
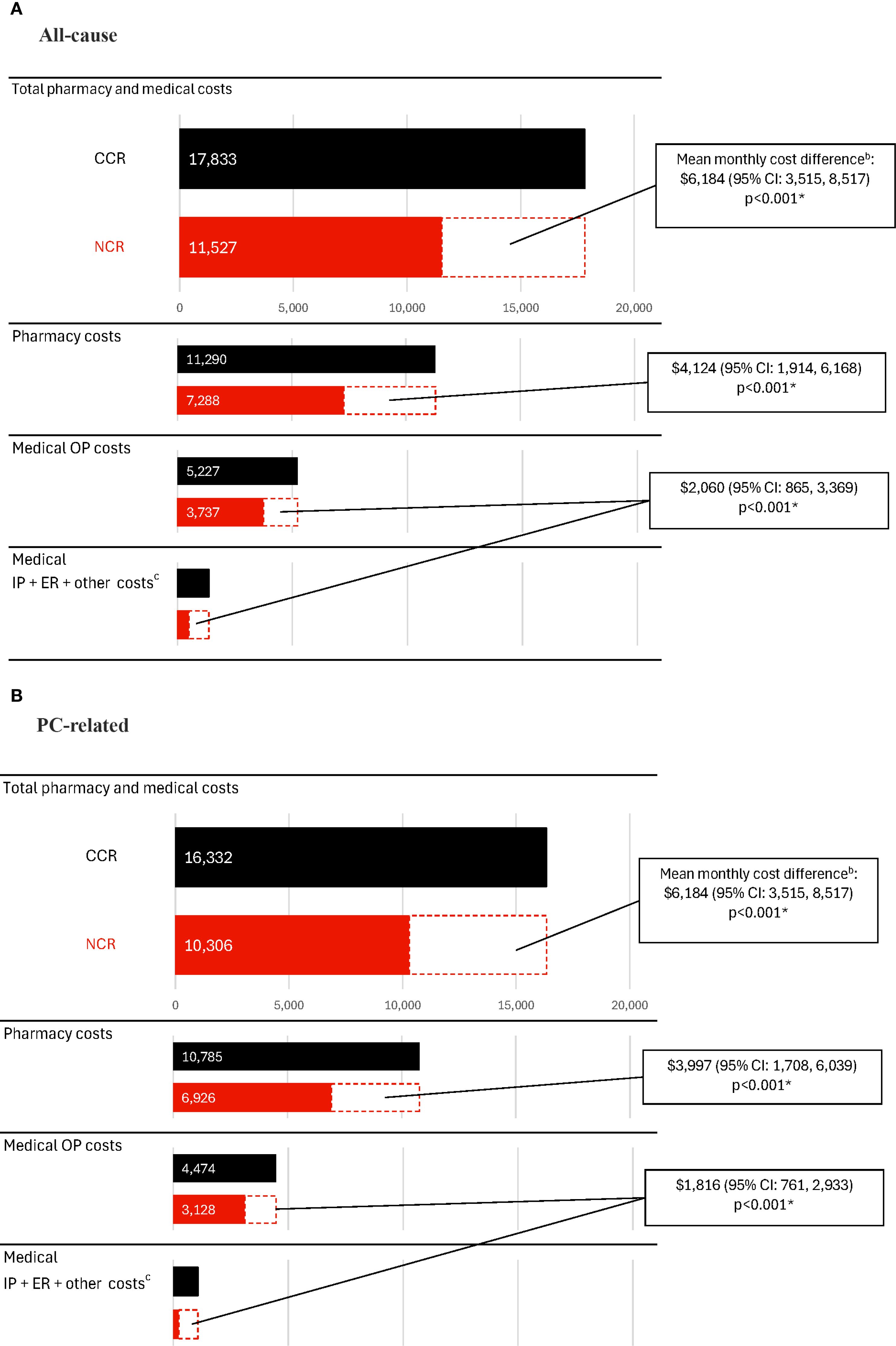
Figure 3. Healthcare costs among patients receiving CCR or NCR, PPPMa. (A) All-cause costs. (B) PC-related costs. CCR, chemotherapy-containing regimen; CI, confidence interval; ER, emergency room; IP, inpatient; mCSPC, metastatic castration-sensitive prostate cancer; NCR, non–chemotherapy-containing regimen; OP, outpatient; PC, prostate cancer; PPPM, per-patient-per-month; US, United States. * p-value <0.05. a. Costs were inflated to 2023 US dollars using the medical care component of the Consumer Price Index and were reported PPPM. b. The weighted model was adjusted for the following baseline variables: all-cause pharmacy costs, categorical age (≤70, 71-80, ≥81), time between metastasis and index date, baseline time spent managing mCSPC, erectile dysfunction, nodal metastasis and Quan-Charlson comorbidity index score. c. Other costs included durable medical equipment, dental, and vision care costs.
Similar trends in cost differences between the CCR and NCR cohorts were observed for PC-related costs (difference for PC-related total healthcare costs: $5,813, 95% CI: 3,135, 8,125; PC-related medical costs: $1,816, 95% CI: 761, 2,933; PC-related pharmacy costs: $3,997, 95% CI: 1,708, 6,039; all p<0.001; Figure 3B; Supplementary Table 1).
In the CCR cohort, chemotherapy management costs were $1,225 PPPM, driven largely by G-CSF use ($920 PPPM).
4 Discussion
In this retrospective analysis, during the first 12 months following ARPI-based treatment initiation, patients with chemotherapy intensification experienced 18% more days lost to mCSPC management, and incurred significantly greater healthcare costs, than those not treated with chemotherapy. For patients receiving a CCR, incremental costs of chemotherapy amounted to over $6,000 per month overall, and over $2,000 per month for medical costs, relative to those receiving an NCR.
To the best of our knowledge, this study is the first to quantify time burden associated with chemotherapy intensification in the context of ARPI-based treatment in mCSPC. Although time burden of cancer-related care, including chemotherapy, has been reported, few studies were specific to metastatic PC (21–23). One recent study in the metastatic castration-resistant PC setting found that chemotherapy was associated with more days spent with healthcare contact (e.g., for infusion, imaging, hospitalization, ER visits) compared with supportive care during the first quarter of the year prior to patients’ death, and such association was not observed with ARPIs (24). The results of that study highlight the time burden associated with chemotherapy for metastatic PC treatment, albeit in a different setting from the current study.
Time burden is most relevant for patients with advanced disease such as metastatic PC when life expectancy becomes limited (11). Indeed, research indicates that the consideration of both time and survival attributes could impact patients’ cancer treatment decisions (25). While clinical trials have demonstrated survival benefits of the triplet combination of docetaxel, ARPI (specifically abiraterone acetate and darolutamide), and ADT over docetaxel and ADT doublet regimens in patients with mCSPC (8, 9), a systematic review and meta-analysis of mCSPC trials found that the triplet combination of docetaxel, ARPI, and ADT, compared with the doublet combination without docetaxel, was not associated with improved overall survival (26). Real-world studies on the effectiveness of triplet therapy in mCSPC are emerging and have largely shown that the therapy is associated with high response rates and tolerable adverse event profiles (27–30), but survival data have been limited due to short and variable follow-up times and thus potential survival benefits over doublet regimens remain to be elucidated. Importantly, the current study provides insights on the additional time commitment required to manage chemotherapy and attend healthcare service visits among patients receiving a CCR relative to those receiving an NCR, highlighting the substantial incremental burden associated with chemotherapy intensification in mCSPC and the importance of informed discussions that include tradeoffs between increased time burden and potential survival benefit during treatment decision-making.
This study also found that patients with mCSPC incurred considerable incremental healthcare costs from chemotherapy intensification when receiving triplet versus doublet regimen. While the economic burden associated with mCSPC in relation to HRU and disease progression has been previously reported (31, 32), the cost findings in the current study shed light on the healthcare costs specifically attributable to the addition of docetaxel to ARPI-based treatment. In addition to greater pharmacy costs partially owing to the additional chemotherapy-related agents used in the triplet regimen such as G-CSF products, a sizable portion of the incremental total costs in patients receiving CCR was accounted for by additional medical costs, mainly the costs for outpatient visits and inpatient admissions. The significant increase in incremental healthcare costs observed in this study may be in part due to the management of side effects associated with chemotherapy (13, 14).
Notably, patients with chemotherapy intensification had more than three times the number of inpatient admissions and five times the number of inpatient days each month compared with those not treated with chemotherapy. These substantially increased rates in acute care may be in part due to unmanaged disease or treatment side effects requiring hospitalizations among patients receiving CCR (14). As studies have suggested that patients with metastatic PC generally prefer treatments that delay chemotherapy and require fewer additional hospital visits (33), the reduced inpatient admission rates and days associated with NCR therapies may help mitigate the stress and burden imposed on patients, as well as on the healthcare system (31).
Together, the substantial time and economic burden associated with chemotherapy intensification in ARPI-based treatment illustrated in this study underscore the importance of counseling expressing these differences in burden in decision-making conversations when selecting treatment for mCSPC. Strategies to incorporate the consideration of time burden in research and routine mCSPC care, such as the development and implementation of standardized metrics, are warranted to improve care quality (11, 34, 35).
The study findings should be considered with the following limitations. First, miscoding or misclassification in the clinical record or through the administrative claims may introduce selection and information biases despite efforts to balance the study populations. Second, overlap weighting and adjusted regression analyses could only adjust for measured covariates, and residual confounding may be present. Third, the cost component of the results may not be generalizable to healthcare systems outside of the US. Additionally, larger studies with more recent patient cohorts and longer follow-up are required to continue monitoring and evaluating the findings of this study for broader applicability.
5 Conclusions
In this real-world study, patients initiating an intensified ARPI-based treatment with docetaxel experienced greater time burden managing mCSPC and higher healthcare costs, including medical costs, than those initiating treatment without docetaxel. Treatment decision-making for mCSPC should include informed discussions considering time and cost burden of chemotherapy when included in treatment with an ARPI and ADT.
Data availability statement
The datasets presented in this article are not readily available because the data that support the findings of this study are from PPS Analytics and Komodo Health Solutions, and restrictions apply to the availability of these data, which were used under license for this study. Requests to access the datasets should be directed to https://portal.ppsanalytics.com/ or https://www.komodohealth.com/.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
DSL: Writing – original draft, Writing – review & editing, Methodology, Formal analysis, Conceptualization. AG: Methodology, Writing – original draft, Conceptualization, Writing – review & editing, Formal analysis. CR: Conceptualization, Methodology, Investigation, Data curation, Writing – review & editing, Writing – original draft, Formal analysis. SB: Formal analysis, Writing – review & editing, Writing – original draft, Methodology, Conceptualization. JK: Writing – original draft, Investigation, Writing – review & editing, Data curation, Methodology, Formal analysis, Conceptualization. LD: Writing – review & editing, Formal analysis, Writing – original draft, Data curation, Investigation, Methodology, Conceptualization. GW: Writing – original draft, Writing – review & editing, Formal analysis, Investigation, Conceptualization, Data curation, Methodology. DP: Conceptualization, Writing – original draft, Methodology, Writing – review & editing, Formal analysis, Data curation, Investigation. IK: Methodology, Writing – review & editing, Formal analysis, Writing – original draft, Conceptualization. AKM: Formal analysis, Writing – original draft, Writing – review & editing, Methodology, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article.
Acknowledgments
Medical writing assistance was provided by professional medical writer, Flora Chik, PhD, MWC, an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson, which funded the development and conduct of this study.
Conflict of interest
CR, JK, LD, GW, and DP are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Johnson & Johnson. SB and IK are employees and stockholders of Johnson & Johnson.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was funded by Johnson & Johnson. The study sponsor was involved in several aspects of the research, including the study design, interpretation of data, and writing of the manuscript.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1650378/full#supplementary-material
References
1. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Desai K, McManus JM, and Sharifi N. Hormonal therapy for prostate cancer. Endocr Rev. (2021) 42:354–73. doi: 10.1210/endrev/bnab002
3. Meagher MF, Salmasi A, and Stewart TF. Treatment landscape for metastatic castrate-sensitive prostate cancer: A review. Res Rep Urol. (2023) 15:509–17. doi: 10.2147/RRU.S398129
4. James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. (2016) 387:1163–77. doi: 10.1016/S0140-6736(15)01037-5
5. Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. (2015) 373:737–46. doi: 10.1056/NEJMoa1503747
6. Mittal A, Sridhar SS, Ong M, and Jiang DM. Triplet therapy in metastatic castrate sensitive prostate cancer (mCSPC)-A potential new standard of care. Curr Oncol. (2023) 30:4365–78. doi: 10.3390/curroncol30040332
7. Lowrance W, Dreicer R, Jarrard DF, Scarpato KR, Kim SK, Kirkby E, et al. Updates to advanced prostate cancer: AUA/SUO guideline (2023). J Urol. (2023) 209:1082–90. doi: 10.1097/JU.0000000000003452
8. Fizazi K, Foulon S, Carles J, Roubaud G, McDermott R, Flechon A, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 x 2 factorial design. Lancet. (2022) 399:1695–707. doi: 10.1016/S0140-6736(22)00367-1
9. Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. (2022) 386:1132–42. doi: 10.1056/NEJMoa2119115
11. Gupta A, Eisenhauer EA, and Booth CM. The time toxicity of cancer treatment. J Clin Oncol. (2022) 40:1611–5. doi: 10.1200/JCO.21.02810
12. Gupta A, O’Callaghan CJ, Zhu L, Jonker DJ, Wong RPW, Colwell B, et al. Evaluating the time toxicity of cancer treatment in the CCTG CO.17 trial. JCO Oncol Pract. (2023) 19:e859–e66. doi: 10.1200/OP.22.00737
13. Wang L, Li C, Zhao Z, Li X, Tang C, Guan Z, et al. Comparison of doublet and triplet therapies for metastatic hormone-sensitive prostate cancer: A systematic review and network meta-analysis. Front Oncol. (2023) 13:1104242. doi: 10.3389/fonc.2023.1104242
14. Rulach RJ, McKay S, Neilson S, White L, Wallace J, Carruthers R, et al. Real-world uptake, safety profile and outcomes of docetaxel in newly diagnosed metastatic prostate cancer. BJU Int. (2018) 121:268–74. doi: 10.1111/bju.14025
15. NUBEQA® (darolutamide) prescribing information. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc. (2023).
16. National Cancer Institute. Abiraterone approved for earlier use in men with metastatic prostate cancer (2018). Available online at: https://www.cancer.gov/news-events/cancer-currents-blog/2018/abiraterone-fda-prostate-hormone-sensitive (Accessed December 4, 2024).
17. U.S. Food and Drug Administration. FDA approves darolutamide tablets for metastatic hormone-sensitive prostate cancer (2022). Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-darolutamide-tablets-metastatic-hormone-sensitive-prostate-cancer (Accessed December 4, 2024).
18. Li F, Thomas LE, and Li F. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol. (2019) 188:250–7. doi: 10.1093/aje/kwy201
19. Thomas LE, Li F, and Pencina MJ. Overlap weighting: A propensity score method that mimics attributes of a randomized clinical trial. JAMA. (2020) 323:2417–8. doi: 10.1001/jama.2020.7819
20. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28:3083–107. doi: 10.1002/sim.3697
21. Bange EM, Doucette A, Gabriel PE, Porterfield F, Harrigan JJ, Wang R, et al. Opportunity costs of receiving palliative chemotherapy for metastatic pancreatic ductal adenocarcinoma. JCO Oncol Pract. (2020) 16:e678–e87. doi: 10.1200/JOP.19.00328
22. Lim SA, Hao SB, Boyd BA, Mitsakos A, Irish W, Burke AM, et al. Opportunity costs of surgical resection and perioperative chemotherapy for locoregional pancreatic adenocarcinoma. JCO Oncol Pract. (2022) 18:302–9. doi: 10.1200/OP.21.00311
23. Yabroff KR, Davis WW, Lamont EB, Fahey A, Topor M, Brown ML, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. (2007) 99:14–23. doi: 10.1093/jnci/djk001
24. Sentana-Lledo D, Barnett A, Einstein DJ, Gupta A, and Morgans AK. Days spent with healthcare contact by patients with metastatic castrate resistant prostate cancer in the last year of life. Oncologist. (2025) 30:oyaf046. doi: 10.1093/oncolo/oyaf046
25. Gupta A, Brundage MD, Galica J, Karim S, Koven R, Ng TL, et al. Patients’ considerations of time toxicity when assessing cancer treatments with marginal benefit. Oncologist. (2024) 29:978–85. doi: 10.1093/oncolo/oyae187
26. Riaz IB, Naqvi SAA, He H, Asghar N, Siddiqi R, Liu H, et al. First-line systemic treatment options for metastatic castration-sensitive prostate cancer: A living systematic review and network meta-analysis. JAMA Oncol. (2023) 9:635–45. doi: 10.1001/jamaoncol.2022.7762
27. Kafka M, Giannini G, Artamonova N, Neuwirt H, Ofner H, Kramer G, et al. Real-world evidence of triplet therapy in metastatic hormone-sensitive prostate cancer: An Austrian multicenter study. Clin Genitourin Cancer. (2024) 22:458–66.e1. doi: 10.1016/j.clgc.2023.12.018
28. Mondal D, Roy S, Bhattacharjee A, Roy R, Meyur S, and Biswas B. Triplet therapy in metastatic hormone-sensitive prostate cancer: A real-world Indian multicentre study. J Clin Oncol. (2024) 42:e17086–e. doi: 10.1200/JCO.2024.42.16_suppl.e17086
29. Urabe F, Imai Y, Goto Y, Tashiro K, Hashimoto M, Yoshihara K, et al. Real-world evidence of triplet therapy efficacy in patients with metastatic castration-sensitive prostate cancer: a Japanese multicenter study. Jpn J Clin Oncol. (2024) 54:1208–13. doi: 10.1093/jjco/hyae098
30. Kwon WA, Song YS, and Lee MK. Strategic advances in combination therapy for metastatic castration-sensitive prostate cancer: Current insights and future perspectives. Cancers (Basel). (2024) 16:3187. doi: 10.3390/cancers16183187
31. Kaye DR, Khilfeh I, Muser E, Morrison L, Kinkead F, Lefebvre P, et al. Characterizing the real-world economic burden of metastatic castration-sensitive prostate cancer in the United States. J Med Econ. (2024) 27:381–91. doi: 10.1080/13696998.2024.2323901
32. Kaye DR, Khilfeh I, Muser E, Morrison L, Kinkead F, Lefebvre P, et al. Real-world economic burden associated with disease progression from metastatic castration-sensitive to castration-resistant prostate cancer on treatment in the United States. J Manag Care Spec Pharm. (2024) 30:684–97. doi: 10.18553/jmcp.2024.30.7.684
33. Connor MJ, Genie MG, Burns D, Bass EJ, Gonzalez M, Sarwar N, et al. A systematic review of patients’ values, preferences, and expectations for the treatment of metastatic prostate cancer. Eur Urol Open Sci. (2022) 36:9–18. doi: 10.1016/j.euros.2021.10.003
34. Parsons HM, Gupta A, Jewett P, and Vogel RI. The intersecting time, administrative and financial burdens of a cancer diagnosis. J Natl Cancer Inst. (2025) 117:595–600. doi: 10.1093/jnci/djae252
Keywords: androgen receptor antagonists, chemotherapy, hormone receptor agonists, mCSPC, prostate neoplasms, time burden, triplet therapy
Citation: Sentana-Lledo D, Gupta A, Rossi C, Burbage S, Korsiak J, Diaz L, Wong G, Pilon D, Khilfeh I and Morgans AK (2025) Time and cost burden associated with docetaxel in patients with metastatic castration-sensitive prostate cancer initiating an androgen receptor pathway inhibitor-based regimen. Front. Oncol. 15:1650378. doi: 10.3389/fonc.2025.1650378
Received: 19 June 2025; Accepted: 29 August 2025;
Published: 26 September 2025.
Edited by:
Feifei Sun, Shandong University, ChinaReviewed by:
Bernhard Ralla, Charité University Medicine Berlin, GermanyKaran Jatwani, George Washington University Hospital, United States
Copyright © 2025 Sentana-Lledo, Gupta, Rossi, Burbage, Korsiak, Diaz, Wong, Pilon, Khilfeh and Morgans. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Sentana-Lledo, ZGFuaWVsX3NlbnRhbmFsbGVkb0BkZmNpLmhhcnZhcmQuZWR1
 Daniel Sentana-Lledo
Daniel Sentana-Lledo Arjun Gupta
Arjun Gupta Carmine Rossi
Carmine Rossi Sabree Burbage4
Sabree Burbage4