- 1Department of Medical Oncology, Kindai University Faculty of Medicine, Osaka-sayama, Osaka, Japan
- 2Department of Pathology, Kindai University Faculty of Medicine, Osaka-sayama, Osaka, Japan
- 3Department of Genome Biology, Kindai University Faculty of Medicine, Osaka-sayama, Osaka, Japan
- 4Department of Immunology, Kindai University Faculty of Medicine, Osaka-sayama, Osaka, Japan
Transformation to small cell lung cancer (SCLC) is a resistance mechanism in epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) after EGFR-tyrosine kinase inhibitor (EGFR-TKI) treatment. The efficacy of immune checkpoint inhibitor (ICI) in transformed SCLC remains to be elucidated. The present case report highlights a patient whose tumor underwent transformation to SCLC after developing resistance to an EGFR-TKI treatment. The patient subsequently achieved long-term remission lasting more than 5 years through treatment with an anti-PD-1 antibody nivolumab. Generally, the efficacy of ICI is inferior in EGFR-mutated NSCLC compared to those with EGFR wild-type NSCLC. However, some cases that have transformed to SCLC may be sensitive to ICI treatment. Further investigation is necessary to determine the efficacy of ICI in cases that have undergone transformation to SCLC.
1 Introduction
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are the standard of care for patients with EGFR-mutated non-small cell lung cancer (NSCLC), and most patients achieve tumor shrinkage (1, 2). However, resistance to EGFR-TKIs eventually develops in all tumors. The mechanisms of resistance vary, including secondary EGFR mutations, amplification of MET, and transformation to small cell lung cancer (SCLC) (1). Approximately 3–10% of tumors that acquired resistance to EGFR-TKI transform to SCLC (3). The treatment of such cases is generally a combination of platinum and etoposide, which is the standard of care for patients with extensive stage SCLC (3).
Immune checkpoint inhibitors (ICIs), including PD-1/PD-L1 inhibitors, represent an established standard of care for NSCLC (4). However, the efficacy of ICI therapy is limited in patients with EGFR-mutated NSCLC, specifically, the response rate in a phase II trial of nivolumab monotherapy in patients after resistance to EGFR-TKI therapy was 9.6% (5). And the median progression-free survival with nivolumab was also 1.7 months (95% CI 1.3−2.3 months), which was worse than standard platinum combination chemotherapy (stratified log-rank test P = 0.001; stratified Cox 313 PH model HR of 1.92, with a 95% CI of 1.27-2.90) (5). The role of ICIs as a treatment following the development of resistance to EGFR-TKI therapy remains to be delineated (6). Conversely, in extensive SCLC, the combination of ICI and chemotherapy significantly prolonged overall survival when compared to chemotherapy alone (7, 8). However, patients with SCLC that has transformed from EGFR-mutated NSCLC constitute a relatively small population, and the efficacy of ICIs in such cases remains to be fully elucidated.
In this report, we describe a case of SCLC that transformed from EGFR-mutated NSCLC after EGFR-TKI treatment and achieved a long-term response with subsequent anti-PD-1 monotherapy.
2 Case presentation
A 76-year-old woman presented to our clinic with the chief complaint of bloody sputum in November 2016. The patient had smoked 20 cigarettes per day for the past 25 years and had a medical history of left upper lobectomy for lung adenocarcinoma at another hospital in 2007. Chest and abdominal computed tomography (CT) images showed a metastatic lung tumor in the left pulmonary hilar region, multiple mediastinal lymph node metastases, and a rib metastasis (Figure 1). A bronchoscopic lung biopsy was performed for pathologic diagnosis. The final diagnosis was stage IVA (c-T3N2M1b) lung adenocarcinoma harboring an EGFR exon 19 deletion (Figure 2).
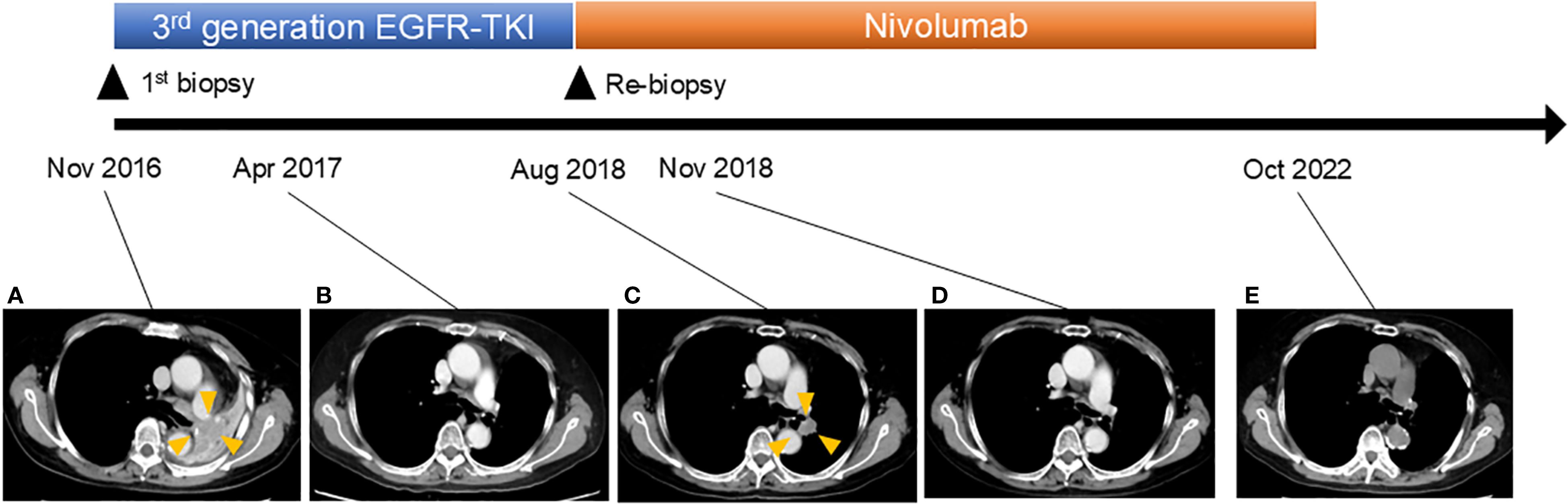
Figure 1. Clinical course of the patient. Computed tomography scan revealed the following: (A) Recurrence of lung cancer in the left pulmonary hilar region 9 years after surgery. (B) Disappearance of tumor after osimertinib treatment. (C) Regrowth of tumor. Osimertinib treatment was stopped and nivolumab treatment was initiated. (D) Disappearance of tumor after nivolumab treatment. (E) Continued tumor disappearance under nivolumab treatment.
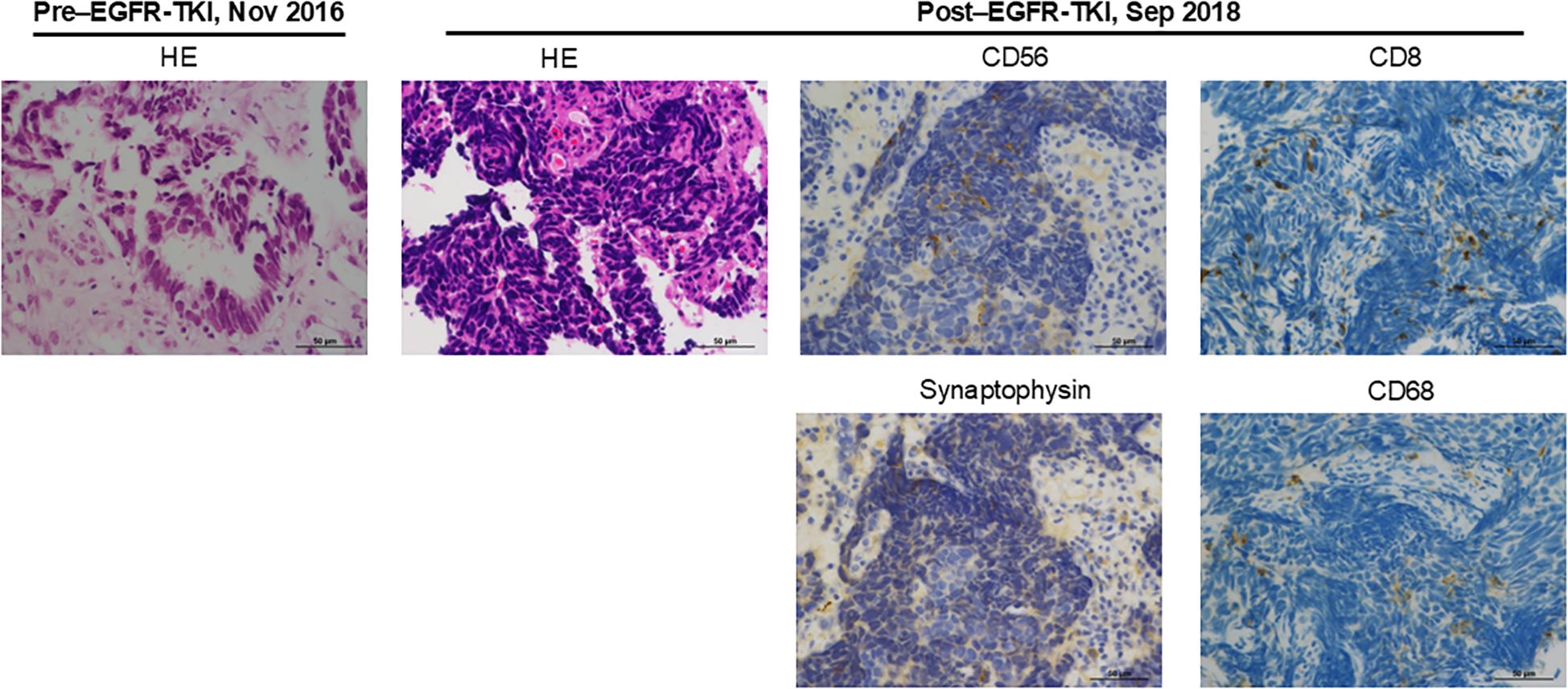
Figure 2. Pathologic examination of tumor obtained before EGFR-TKI treatment and after developing resistance to EGFR-TKI. The images show hematoxylin and eosin (HE) or immunohistochemical staining of CD56, synaptophysin, CD8, and CD68.
In December 2016, the patient was initiated on a third-generation EGFR-TKI, resulting in complete response to the treatment (Figure 1). However, in August 2018, EGFR-TKI was discontinued due to the recurrence of the tumor in the left lung (Figure 1). In order to obtain a pathological diagnosis of this exacerbated pulmonary tumor, a bronchoscopic re-biopsy was performed. The pathology results indicated a transformation to SCLC, with partly positive expression of CD56 and synaptophysin (Figure 2). Polymerase chain reaction testing showed that the EGFR exon 19 deletion remained positive, without secondary EGFR mutations detected. Additionally, programmed death-ligand 1 (PD-L1) tumor proportion score was negative (less than 1%).
In September 2018, the patient was enrolled in a clinical trial and was initiated on monotherapy with the anti-PD1 antibody nivolumab, 3 mg/kg, on day 1 every 2 weeks. Notably, 3 months later, in November 2018, CT imaging revealed left pulmonary tumor shrinkage. In May 2019, grade 3 adrenocorticotropic hormone deficiency was observed as an adverse event related to nivolumab treatment. However, nivolumab treatment was continued with corticosteroid replacement therapy. Subsequently, nivolumab treatment was continued until October 2022, when treatment was discontinued due to patient relocation. As of the end of 2023, there was no evidence of tumor progression.
3 Discussion
At the time this patient was treated with nivolumab, the efficacy of ICI in EGFR-mutated NSCLC was not clear, and nivolumab treatment was administered as a clinical trial. Currently, the efficacy of ICIs in EGFR-mutated NSCLC is generally considered limited. However, the efficacy of ICIs remains to be fully evaluated in the relatively small subpopulation of patients whose tumor has transformed to SCLC after developing resistance to EGFR-TKIs (9). The present case with a SCLC transformation showed a long-term response to anti-PD-1 despite the presence of a positive EGFR mutation. In other retrospective observation studies, there is a trend toward a better prognosis in patients with EGFR-mutated and transformed SCLC treated with ICI and chemotherapy compared to chemotherapy alone (10, 11). Specifically, the median overall survival was 10 months for patients treated with chemotherapy alone versus 13 months for patients treated with chemotherapy plus ICI in patients whose tumor transformed after developing resistance to EGFR-TKI therapy (Hazard ratio 0.75, 0.36−1.56) (10). Similarly, in patients with EGFR-mutated NSCLC that had transformed to SCLC, Zhang CY et al. reported that the median overall survival of patients who received immunotherapy was significantly longer than that of patients who did not receive immunotherapy (20.2 m versus 7.9 m, P < 0.01) (11). In contrast to these observations, other groups reported no long-term efficacy of ICI alone in their cohorts including cases of EGFR-mutated and transformed SCLC (12, 13). Specifically, Fujimoto D et al. reported only one case of response among 15 patients with EGFR-mutated NSCLC whose tumor has transformed to small cell lung cancer after developing resistance to EGFR-TKIs (12). Marcoux N et al. also reported no response among 17 patients with EGFR-mutated NSCLC who received ICI alone after SCLC-transformation (13). Collectively, the response rate to ICI alone may not necessarily be high in EGFR-mutated NSCLC, even with small cell transformation. However, it is noteworthy that ICI treatment may result in long-term survival in subpopulation with SCLC transformation, as evidenced in the present case report. Therefore, patient selection by biomarkers may be desirable for ICI treatment in patients with EGFR-mutated NSCLC with SCLC transformation.
In general, tumor mutation burden (TMB) is relatively high in SCLC, partly due to heavy smoking. However, despite a smoking history, the TMB value is low in the present patient. While an association between TMB and the effect of ICI has been reported, the therapeutic effect of ICI in the present case is difficult to explain from TMB (14). The expression level of PD-L1 is also associated with the therapeutic effect of ICI in NSCLC and other types of cancer, but was negative in this case (15). Furthermore, an association between the infiltration of inflammatory cells into the tumor area and the therapeutic effect of ICI has been reported (16). We observed an infiltration of CD8- or CD68-positive immune cells in the current SCLC-transformed tumor (Figure 2). Additionally, for assessing the infiltration levels of multiple immune cells to estimate the tumor microenvironment, CIBERSORTx was performed using transcriptome data obtained from EGFR-mutated NSCLC tumor developing resistance to EGFR-TKIs (Figure 3, Supplementary Material and Methods) (17). Among 35 tumors obtained from patients with EGFR-mutated NSCLC treated with EGFR-TKIs, the present tumor showed relatively increased inflammatory cells, including exhausted CD4 and CD8 positive cells and M1-like macrophages, compared to other tumors (Figure 3). Consistent with the present case, Haratani K et al. reported that in patients with EGFR-mutated NSCLC, nivolumab responders had significantly higher CD8+ tumor-infiltrating lymphocyte (TIL) density than non-responders (18). Moreover, according to a retrospective analysis of extensive-stage SCLC, patients with high TIL demonstrated significantly superior progression-free survival compared to those with low TIL (19). Overall, ICI therapy may be beneficial in patients with EGFR-mutated NSCLC that has transformed into SCLC accompanied by CD8-positive TIL infiltration.

Figure 3. Tumor inflammatory cell profiles from 35 patients with EGFR-mutated NSCLC that has developed resistance to EGFR-TKIs. Inflammatory cell types were determined by CIBERSORTx analysis. Red dots indicate the present case, which has transformed to small cell lung cancer.
As a limitation, this is only a single case report of sustained response to ICI treatment, and further studies are needed to determine which patients would benefit most from ICI treatment after SCLC transformation based on clinical or genetic background. The combination of ICI and chemotherapy may also need continued investigation given its success in extensive-stage SCLC. Specifically, limited response rates to ICI monotherapy have been reported in extensive-stage SCLC, ranging from 2.3% for monotherapy with the anti-PD-L1 antibody atezolizumab to 9.5% for monotherapy with the anti-PD-L1 antibody durvalumab (20, 21). However, the combination of ICI and chemotherapy (i.e., platinum and etoposide) demonstrated a significant improvement in overall survival compared to chemotherapy alone in extensive-stage SCLC (7, 8).
4 Conclusion
ICI might be a treatment option in cases with EGFR-mutated and transformed SCLC. Further case series are required to evaluate the relationship between the tumor immune environment or the expression profile of immune checkpoint molecules and therapeutic response of ICI treatment.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by The Institutional Review Boards of Kindai Hospital, Kishiwada Hospital, and Izumi Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YK: Resources, Writing – original draft, Investigation. KY: Formal analysis, Resources, Conceptualization, Writing – review & editing, Investigation. JT: Resources, Writing – review & editing. OM: Investigation, Writing – review & editing. KS: Formal analysis, Writing – review & editing. KK: Writing – review & editing, Supervision. KN: Writing – review & editing, Supervision. HH: Resources, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The authors thank the patient and her family for cooperation in this report. We also thank Hiroki Goto, Eri Otsuka, Hiroaki Okida, Masanori Funabashi, Yuuri Hashimoto, Kenji Hirotani, Yasuki Kamai, Takashi Kagari for the supplementary data analysis.
Conflict of interest
KY has received honoraria from Chugai Pharmaceutical Co., Ltd., AstraZeneca K.K., MSD K.K., Amgen Inc., and Daiichi Sankyo Co., Ltd. HH has received grants or contracts from IQVIA Services JAPAN K.K., Eisai Co., Ltd., Syneos Health Clinical K.K., EP-CRSU Co., Ltd., EPS Corporation., Shionogi & Co., Ltd., Nippon Kayaku Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., GlaxoSmithKline K.K., MSD K.K., Sanofi K.K., Amgen Inc., Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Bristol Myers Squibb Company, PRA Health Sciences Inc., CMIC CO., Ltd., Astellas Pharma Inc., Pfizer R&D Japan G.K., Ascent Development Services, Labcorp Development Japan K.K., Eisai Inc., Kobayashi Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Pfizer Japan Inc., AstraZeneca K.K., AbbVie Inc., Daiichi Sankyo Co., Ltd., A2 Healthcare Corp., Novartis Pharma K.K., Eli Lilly Japan K.K., Merck Biopharma Co., Ltd, Medpace Japan K.K., Kyowa Kirin Co., Ltd., Japanese Gastric Cancer Association, Thoracic Oncology Research Group, Clinical Research Support Center Kyushu, West Japan Oncology Group, Japan Clinical Cancer Research Organization, Comprehensive Support Project for Oncological Research of Breast Cancer, EPS International Co., Ltd., Mebix, Inc., Ono Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Covance Japan Inc., Japan Clinical Research Operations and Medical Research Support; payment or honoraria from Ono Pharmaceutical Co., Ltd., Merck Biopharma Co., Ltd., Daiichi Sankyo Co., Ltd., 3H Clinical Trial Inc., AstraZeneca K.K., Novartis Pharma K.K., Chugai Pharmaceutical Co., Ltd., Bristol Myers Squibb Company, Eli Lilly Japan K.K., Amgen Inc., MSD K.K., Sysmex Corporation, Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Janssen Pharmaceutical K.K. and Guardant Health Japan Corp.; and has participated on a data safety monitoring board or advisory board of Bristol Myers Squibb Company, AbbVie Inc., Chugai Pharmaceutical Co., Ltd., Novocure K.K., AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Janssen Pharmaceutical K.K.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1651248/full#supplementary-material
References
1. Zhou X, Zeng L, Huang Z, Ruan Z, Yan H, Zou C, et al. Strategies beyond 3rd EGFR-TKI acquired resistance: opportunities and challenges. Cancer Med. (2025) 14:e70921. doi: 10.1002/cam4.70921
2. Singh S, Sadhukhan S, and Sonawane A. 20 years since the approval of first EGFR-TKI, gefitinib: Insight and foresight. Biochim Biophys Acta Rev Cancer. (2023) 1878:188967. doi: 10.1016/j.bbcan.2023.188967
3. Zhang H, Gao H, Gao P, Liu H, and Chen J. Molecular mechanisms and therapeutic strategies for small−cell lung cancer transformation after TKI therapy in EGFR−mutated lung adenocarcinoma (Review). Mol Clin Oncol. (2025) 23:62. doi: 10.3892/mco.2025.2857
4. Raskova Kafkova L, Mierzwicka JM, Chakraborty P, Jakubec P, Fischer O, Skarda J, et al. NSCLC: from tumorigenesis, immune checkpoint misuse to current and future targeted therapy. Front Immunol. (2024) 15:1342086. doi: 10.3389/fimmu.2024.1342086
5. Hayashi H, Sugawara S, Fukuda Y, Fujimoto D, Miura S, Ota K, et al. A randomized phase II study comparing nivolumab with carboplatin-pemetrexed for EGFR-mutated NSCLC with resistance to EGFR tyrosine kinase inhibitors (WJOG8515L). Clin Cancer Res. (2022) 28:893–902. doi: 10.1158/1078-0432.CCR-21-3194
6. Zhu P, Li Z, Sun Y, Liu T, and Yin R. Persist or resist: Immune checkpoint inhibitors in EGFR-mutated NSCLC. Cancer Sci. (2025) 116:581–91. doi: 10.1111/cas.16428
7. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
8. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. (2019) 394:1929–39. doi: 10.1016/S0140-6736(19)32222-6
9. Qian X, Guo X, Li T, Hu W, Zhang L, Wu C, et al. Efficacy of immune checkpoint inhibitors in EGFR-Mutant NSCLC patients with EGFR-TKI resistance: A systematic review and meta-analysis. Front Pharmacol. (2022) 13:926890. doi: 10.3389/fphar.2022.926890
10. Saalfeld FC, Möller J, Christopoulos P, Wenzel C, Rasokat A, Wang XA, et al. Small cell transformation in EGFR-mutated non-small cell lung cancer: DLL3 expression and efficacy of immune checkpoint inhibitors or tyrosine kinase inhibitors combined with chemotherapy. Eur J Cancer. (2024) 213:115065. doi: 10.1016/j.ejca.2024.115065
11. Zhang CY, Sun H, Su JW, Chen YQ, Zhang SL, Zheng MY, et al. A potential treatment option for transformed small-cell lung cancer on PD-L1 inhibitor-based combination therapy improved survival. Lung Cancer. (2023) 175:68–78. doi: 10.1016/j.lungcan.2022.11.016
12. Fujimoto D, Akamatsu H, Morimoto T, Wakuda K, Sato Y, Kawa Y, et al. Histologic transformation of epidermal growth factor receptor-mutated lung cancer. Eur J Cancer. (2022) 166:41–50. doi: 10.1016/j.ejca.2022.02.006
13. Marcoux N, Gettinger SN, O’Kane G, Arbour KC, Neal JW, Husain H, et al. EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J Clin Oncol. (2019) 37:278–85. doi: 10.1200/JCO.18.01585
14. Galvano A, Gristina V, Malapelle U, Pisapia P, Pepe F, Barraco N, et al. The prognostic impact of tumor mutational burden (TMB) in the first-line management of advanced non-oncogene addicted non-small-cell lung cancer (NSCLC): a systematic review and meta-analysis of randomized controlled trials. ESMO Open. (2021) 6:100124. doi: 10.1016/j.esmoop.2021.100124
15. Liu W, Huo G, and Chen P. Clinical benefit of pembrolizumab in treatment of first line non-small cell lung cancer: a systematic review and meta-analysis of clinical characteristics. BMC Cancer. (2023) 23:458. doi: 10.1186/s12885-023-10959-3
16. Rother C, John T, and Wong A. Biomarkers for immunotherapy resistance in non-small cell lung cancer. Front Oncol. (2024) 14:1489977. doi: 10.3389/fonc.2024.1489977
17. Guan M, Jiao Y, and Zhou L. Immune infiltration analysis with the CIBERSORT method in lung cancer. Dis Markers. (2022) 2022:3186427. doi: 10.1155/2022/3186427
18. Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol. (2017) 28:1532–9. doi: 10.1093/annonc/mdx183
19. Shirasawa M, Yoshida T, Shiraishi K, Takigami A, Takayanagi D, Imabayashi T, et al. Identification of inflamed-phenotype of small cell lung cancer leading to the efficacy of anti-PD-L1 antibody and chemotherapy. Lung Cancer. (2023) 179:107183. doi: 10.1016/j.lungcan.2023.107183
20. Pujol JL, Greillier L, Audigier-Valette C, Moro-Sibilot D, Uwer L, Hureaux J, et al. A randomized non-comparative phase II study of anti-programmed cell death-ligand 1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: results from the IFCT-1603 trial. J Thorac Oncol. (2019) 14:903–13. doi: 10.1016/j.jtho.2019.01.008
Keywords: EGFR, EGFR-TKI, non-small cell lung cancer, small cell lung cancer transformation, case report
Citation: Kawanaka Y, Yonesaka K, Tanizaki J, Maenishi O, Sakai K, Kakimi K, Nishio K and Hayashi H (2025) Case Report: A small cell lung cancer transformed from an EGFR-mutated Adenocarcinoma demonstrated a long-term remission to anti PD-1 antibody. Front. Oncol. 15:1651248. doi: 10.3389/fonc.2025.1651248
Received: 21 June 2025; Accepted: 28 August 2025;
Published: 10 September 2025.
Edited by:
Natsuo Tomita, Nagoya City University, JapanReviewed by:
Hesong Wang, Fourth Hospital of Hebei Medical University, ChinaFranz Rödel, Goethe University Frankfurt, Germany
Copyright © 2025 Kawanaka, Yonesaka, Tanizaki, Maenishi, Sakai, Kakimi, Nishio and Hayashi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kimio Yonesaka, eW9uZXNha2FAbWVkLmtpbmRhaS5hYy5qcA==
 Yusuke Kawanaka1
Yusuke Kawanaka1 Kimio Yonesaka
Kimio Yonesaka Kazuko Sakai
Kazuko Sakai Hidetoshi Hayashi
Hidetoshi Hayashi