- 1Division of Biliary Tract Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Department of Pathology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3West China School of Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Gallbladder neuroendocrine carcinoma (GB-NEC) is an exceptionally rare and highly aggressive malignancy, accounting for only 0.2% of gastrointestinal neuroendocrine neoplasms and 2.3% of gallbladder cancers. Due to its nonspecific clinical presentation and diagnostic challenges, most patients present with advanced disease at diagnosis, resulting in poor prognosis with median survival typically under 12 months. This study aimed to analyze clinicopathological characteristics and identify independent prognostic factors in GB-NEC patients.
Methods: We conducted a retrospective cohort study of 31 histologically confirmed GB-NEC cases treated at a tertiary referral center between 2015-2024. Comprehensive data including demographic characteristics, tumor markers, pathological features (differentiation, Ki-67 index, invasion patterns), treatment modalities (surgical approach, chemotherapy regimens), and survival outcomes were analyzed. Statistical methods included Kaplan-Meier survival analysis, log-rank tests, and multivariate Cox proportional hazards regression models.
Results: The cohort demonstrated median progression-free survival of 12 months and overall survival of 36 months. Multivariate analysis identified three independent poor prognostic factors: elevated alpha-fetoprotein (AFP) (HR 1.01, p=0.034), mixed neuroendocrine-non-neuroendocrine histology (HR 3.90, p=0.042), and delayed adjuvant chemotherapy (HR 15.62, p=0.006).
Discussion: This study establishes AFP elevation, mixed histology, and delayed chemotherapy as critical determinants of poor prognosis in GB-NEC. Our findings emphasize the importance of early diagnosis, aggressive surgical resection, and timely initiation of platinum-based adjuvant therapy.
Graphical Abstract. Graphical abstract (Created in https://BioRender.com).
1 Introduction
Neuroendocrine carcinoma (NEC) is a poorly differentiated, highly aggressive epithelial tumor with neuroendocrine differentiation features, often diagnosed at advanced stages (1). As a subtype of neuroendocrine neoplasms (NENs), NEC can occur in the digestive system, respiratory system, thyroid, and other organs. Its diagnosis relies on immunohistochemical markers such as chromogranin A (CgA) and synaptophysin (Syn) (2). According to the World Health Organization (WHO) fifth edition classification of digestive system tumors, NENs are further categorized into neuroendocrine tumors (NETs) and NECs, which exhibit distinct molecular characteristics and biological behaviors (3). Although NEC accounts for less than 1% of all malignancies (4), its aggressive nature and poor prognosis have drawn significant attention. Within the gastrointestinal tract, NEC is most commonly found in the rectum, jejunum-ileum, and pancreas (1). Gallbladder NEC (GB-NEC) is particularly rare, representing only 0.2% of gastrointestinal NEC and 2.3% of gallbladder malignancies (5, 6). While adenocarcinoma constitutes over 90% of gallbladder cancers, the clinical characteristics, treatment strategies, and prognostic factors for GB-NEC remain poorly defined (7).
The clinical manifestations of GB-NEC are nonspecific, often including right upper quadrant pain, abdominal distension and jaundice. These symptoms can be mistaken for cholelithiasis, leading to delayed diagnosis (8–10). Current research on GB-NEC is largely limited to case reports, and treatment strategies are typically extrapolated from those for gallbladder adenocarcinoma, lacking specificity. However, a recent large-scale retrospective study of 56 GB-NEC cases—the largest series reported to date in the literature—has provided valuable insights into the pathological characteristics and diagnosis of this disease. While this represents a significant advance, the authors acknowledge that clinical follow-up data remain limited, highlighting a critical area for future investigation (11). Surgery remains the primary treatment modality, with early-stage patients potentially requiring only cholecystectomy, while advanced cases may necessitate extended resection combined with platinum-based adjuvant chemotherapy (12–14). However, since GB-NEC is frequently diagnosed at advanced stages, patient prognosis is extremely poor, with a median survival of merely 8.9 months, significantly lower than that of gallbladder adenocarcinoma (15). Previous studies reported 1-year, 2-year, and 3-year survival rates as low as 20%, 10%, and 0%, respectively (16), with prognostic factors including adjuvant therapy, tumor size and TNM stage (17, 18). Currently, there are no standardized guidelines for the diagnosis and treatment of GB-NEC. To build upon the growing understanding of this disease, particularly the need for comprehensive survival and outcomes analysis, this study retrospectively analyzed clinical data from 31 GB-NEC patients treated at West China Hospital between January 2015 and December 2024, aiming to identify survival-related prognostic factors and provide evidence for clinical decision-making.
2 Method
2.1 Study cohort
Between January 2015 and December 2024, 1,207 patients underwent surgical treatment for gallbladder cancer at West China Hospital, Sichuan University. Postoperative pathological specimens were examined via histopathological evaluation (hematoxylin and eosin staining, HE) and immunohistochemistry. The antibodies used in this study included Syn, CgA, CD56, cytokeratin 7 (CK7), CK5&6, pan-cytokeratin (pan-CK), p53, retinoblastoma protein (Rb), and Ki-67 (MIB-1). According to the WHO 2022 classification of endocrine and neuroendocrine tumors, gallbladder NECs are poorly differentiated malignancies of biliary epithelial origin with neuroendocrine differentiation features. Histologically, they are classified into small cell type (small cells with hyperchromatic nuclei, scant cytoplasm, arranged in oat cell-like/nested patterns) and large cell type (large cells with prominent nucleoli, abundant cytoplasm, and organoid/trabecular structures), with a Ki-67 proliferation index ≥20%. Immunohistochemical staining confirmed neuroendocrine components in NECs via positive expression of Syn, CgA, or CD56 (at least one marker positive), while epithelial origin was verified through CK7 and pan-CK. Additionally, NECs often exhibit p53 mutations (diffuse strong positivity or null expression on immunohistochemistry) and Rb protein loss (negative Rb expression), both of which aid in NEC diagnosis. Mixed neuroendocrine-non-neuroendocrine tumors (MiNENs) require both neuroendocrine (≥30% of tumor composition) and non-neuroendocrine components (e.g., adenocarcinoma, squamous carcinoma), each meeting their respective diagnostic criteria: neuroendocrine components must fulfill NEC diagnostic requirements, while non-neuroendocrine components (adenocarcinoma or squamous carcinoma) require verification via CK7/pan-CK or CK5&6 positivity, respectively.
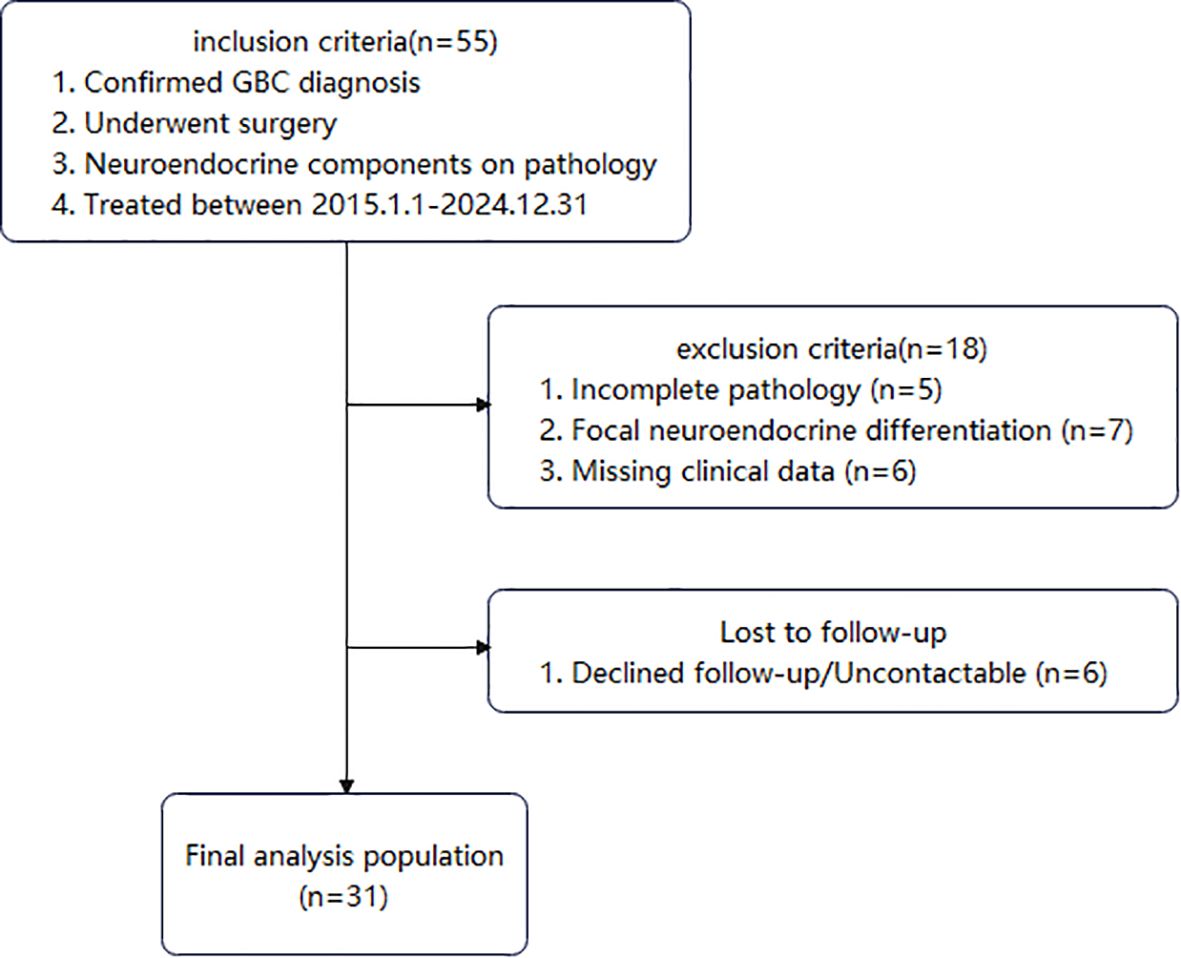
Among these patients, 55 had pathological findings indicating neuroendocrine components. Patients with incomplete pathology, focal neuroendocrine differentiation, or missing clinical data were excluded. Ultimately, 31 patients diagnosed with GB-NEC were included (Figure 1). The study protocol was approved by the hospital’s Institutional Review Board.
2.2 Characteristics
Medical records of all 31 GB-NEC patients were retrospectively reviewed, including age, sex, BMI, symptoms, comorbidities, HBsAg status, alkaline phosphatase (ALP), lactate dehydrogenase (LDH), tumor markers (AFP, CEA, CA19-9), and Child-Pugh liver function classification. Pathological features included tumor diameter, liver invasion, liver metastasis, histological type, histological component, lymph node metastasis, lymphovascular invasion (LVI), perineural invasion (PNI), and Ki-67 index. Treatment-related characteristics encompassed surgical approach, neoadjuvant therapy, adjuvant chemotherapy, and radiotherapy. Tumor staging followed the 8th edition American Joint Committee on Cancer (AJCC) staging system.
2.3 Follow-up
Postoperatively, patients underwent blood tests and abdominal ultrasound every 3 months in the first year, then every 6 months thereafter. Suspected recurrence or distant metastasis was further evaluated via CT/MRI or PET/CT. Overall survival (OS) was defined as the time from treatment initiation to death, while progression-free survival (PFS) was defined as the time from treatment initiation to first confirmed recurrence. Follow-up ended at patient death or the last follow-up date (April 1, 2025).
2.4 Data analysis
Statistical analyses were performed using R software (version 4.2.3). Continuous variables were expressed as mean ± standard deviation (SD) or median (range). Kaplan-Meier curves were generated using GraphPad Prism 9.5 to assess OS and PFS differences in the overall population and subgroups. To ensure sufficient events per variable (EPV) for survival analysis, BMI, N stage, and TNM stage were dichotomized based on clinical relevance and sample distribution. The log-rank test compared survival differences across patient, tumor, and treatment-related characteristics. Univariate and multivariate Cox proportional hazards regression models identified independent risk factors. To avoid information loss in this exploratory study, restricted cubic splines (RCS) were used to test nonlinear relationships, retaining continuous variables. For multivariate analysis, candidate variables were first selected from univariate analyses with P<0.05. Continuous variables were evaluated for multicollinearity using Pearson correlation analysis (with correlation coefficients >0.7 considered clinically significant), while categorical variable associations were examined through χ2 tests. All selected variables subsequently underwent variance inflation factor (VIF) assessment, where VIF values ≥5 indicated problematic collinearity requiring exclusion. This systematic approach ensured the final multivariate model incorporated only statistically independent predictors while maintaining clinical relevance and minimizing overfitting. P<0.05 was considered statistically significant.
3 Result
3.1 Clinical, pathological characteristics and surgical methods
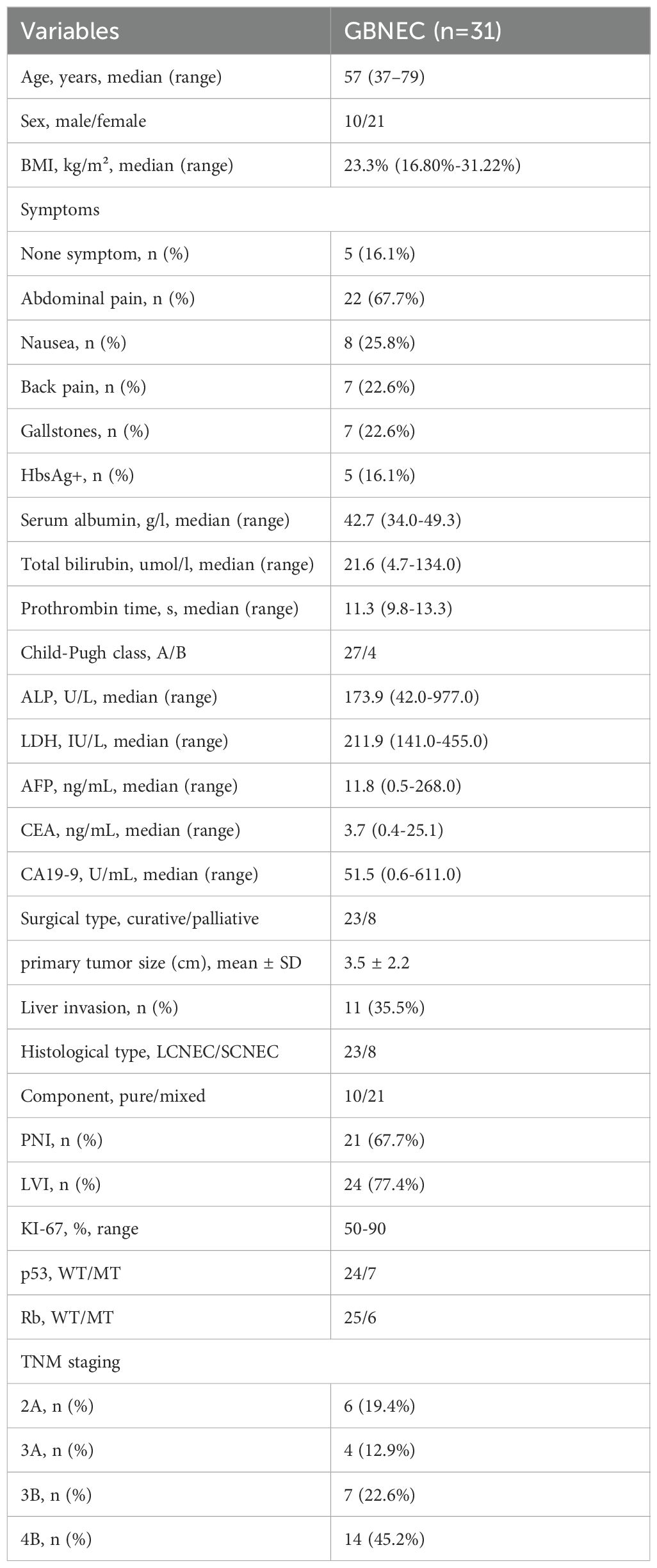
Complete clinical and pathological data were obtained for all 31 GB-NEC patients, with a median follow-up of 31 months (Table 1). The cohort included 21 (67.7%) females and 10 (32.3%) males, aged 37–79 years. The most common symptom was abdominal pain (22, 67.7%), followed by nausea (8, 25.8%) and back pain (7, 22.6%). Five (16.1%) patients were asymptomatic, diagnosed incidentally during routine examinations. Seven (22.6%) had a history of gallstones, and five (16.1%) were HBsAg-positive.
Regarding treatment, 23 (74.2%) patients received radical resection with histologically confirmed R0 margins, while eight (25.8%) received palliative surgery. Among radical resection patients, 11 underwent cholecystectomy with wedge liver resection, and 12 underwent extended resection (at least gallbladder plus liver segments 4b/5). Palliative procedures included cholecystectomy alone (5 patients), cholecystectomy with hepaticojejunostomy (2 patients), and cholecystectomy with liver radiofrequency ablation (1 patient). All patients underwent lymph node dissection for pathological staging.
Pathological and immunohistochemical findings showed a mean tumor diameter of 3.5 cm, with liver invasion in 11 (35.5%), PNI in 21 (67.7%), and LVI in 24 (77.4%) cases. Tumor components were classified as large cell neuroendocrine carcinoma (LCNEC) (23, 74.2%) or small cell neuroendocrine carcinoma (SCNEC, 8, 25.8%). Since MiNEN requires each component to constitute ≥30% of the tumor (1), “atypical mixed NEC” cases in this study were classified based on coexistence with other tumor types: 10 (32.3%) had pure NEC, while 21 (67.7%) had mixed tumors. According to AJCC 8th edition staging, six (19.4%) patients had stage IIA disease, four (12.9%) stage IIIA, seven (22.6%) stage IIIB, and 14 (45.2%) stage IVB. Among 20 patients receiving chemotherapy, 13 received platinum-based regimens.
3.2 Postoperative recurrence
During follow-up, tumor recurrence occurred in 22 patients (71.0%; Figure 2a). Median PFS was 12 months, with 6-, 12-, and 24-month cumulative recurrence rates of 45.4% (95% CI: 24.6-60.4%), 59.9% (95% CI: 37.6-74.3%), and 67.2% (95% CI: 44.6-80.6%), respectively. Survival analysis revealed significant associations between recurrence and M stage (P = 0.006, Figure 2b), TNM stage (P = 0.013, Figure 2c), liver metastasis (P = 0.027, Figure 2d), histological component (P = 0.024, Figure 2e), PNI (P = 0.005, Figure 2f), LVI (P = 0.010, Figure 2g), and adjuvant chemotherapy (P<0.001, Figure 2h). Multivariate Cox regression identified elevated AFP (HR: 1.01, 95% CI: 1.003-1.024, P = 0.013), mixed histology (HR: 3.90, 95% CI: 1.048-14.552, P = 0.042), and delayed chemotherapy (HR: 18.22, 95% CI: 3.561-93.211, P = 0.042) as independent prognostic factors for recurrence (Table 2, Supplementary Table S1).
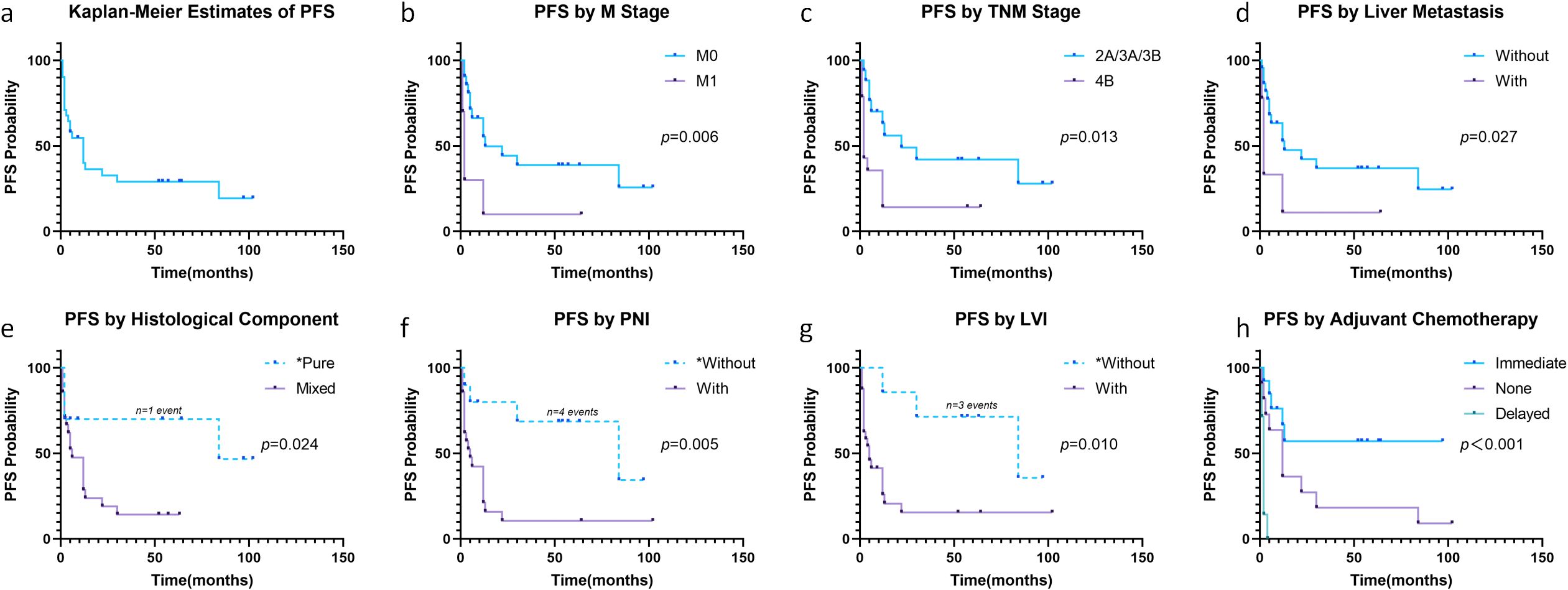
Figure 2. Progression-free survival in all patients (a). Progression-free Survival stratified by M stage (b), TNM stage (c), liver metastasis (d), histological component (e), PNI (f), LVI (g) and adjuvant chemotherapy (h). *Caution in interpretation due to <5 events in these subgroups.
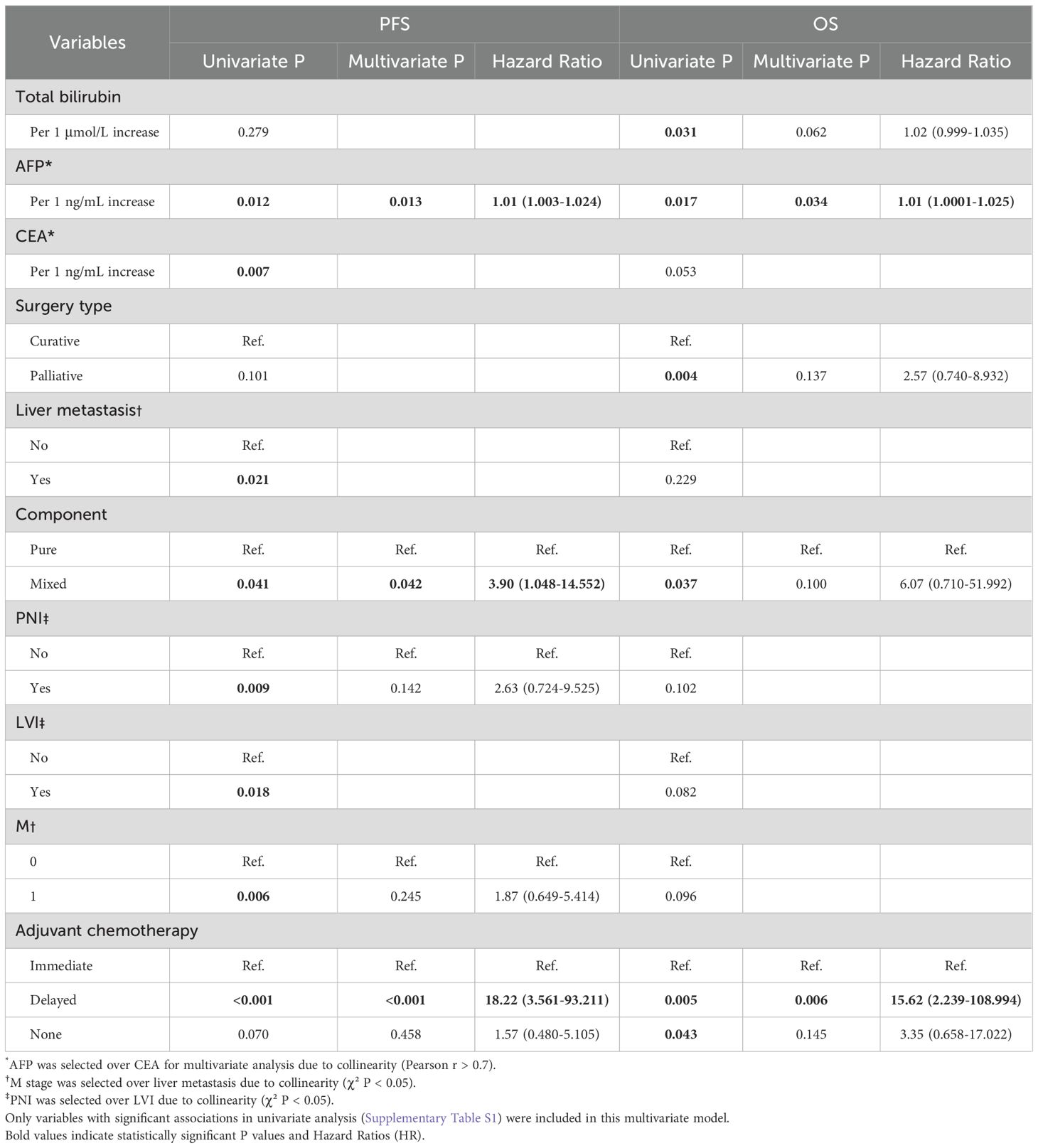
Table 2. Multivariate Cox regression analysis of progression-free survival and overall survival in all patients.
3.3 Survival outcomes
Fifteen (48.4%) patients died during follow-up (Figure 3a). Median OS was 36 months, with 6-, 12-, and 24-month cumulative mortality rates of 22.6% (95% CI: 6.4-36.0%), 34.9% (95% CI: 14.4-50.6%), and 48.0% (95% CI: 24.4-64.2%), respectively. Survival analysis showed OS was associated with surgical type (P = 0.002, Figure 3b), histological component (P = 0.013, Figure 3c), and adjuvant chemotherapy (P = 0.006, Figure 3d). Multivariate Cox regression confirmed elevated AFP (HR: 1.01, 95% CI: 1.000-1.028, P = 0.034) and delayed chemotherapy (HR: 15.62, 95% CI: 2.239-108.994, P = 0.006) as independent prognostic factors for OS (Table 2, Supplementary Table S1).
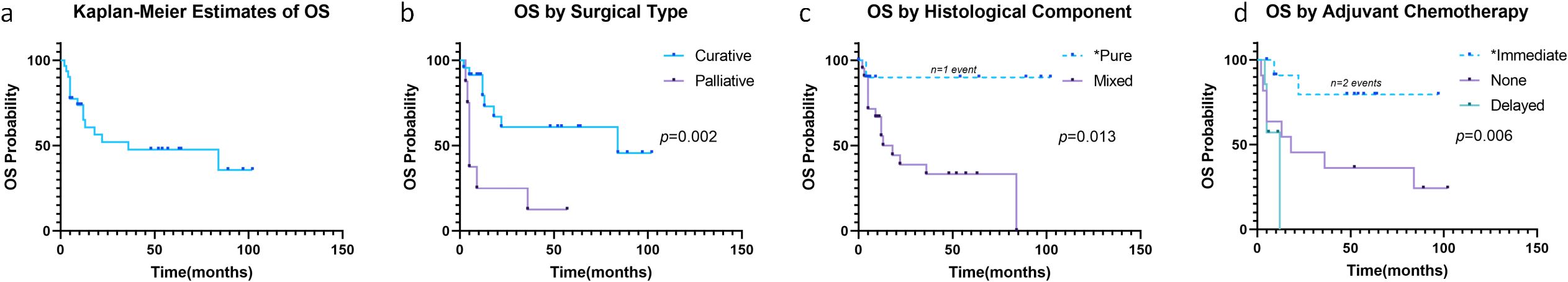
Figure 3. Overall survival in all patients (a). Overall Survival stratified by surgical type (b), histological component (c) and adjuvant chemotherapy (d). *Caution in interpretation due to <5 events in these subgroups.
4 Discussion
This retrospective study analyzed 31 GB-NEC patients, revealing a 71.0% recurrence rate (median PFS: 12 months) and 48.4% mortality rate (median OS: 36 months). Univariate analysis identified AFP, M stage, TNM stage, liver metastasis, surgical type, tumor composition, PNI, LVI, and adjuvant chemotherapy as significant prognostic factors. Multivariate analysis further established elevated AFP, mixed histology, and delayed chemotherapy as independent risk factors for recurrence, while AFP and delayed chemotherapy also independently impacted OS.
The 2010 WHO classification of digestive system tumors categorized well-differentiated NETs as G1/G2 and poorly differentiated NETs (G3) as NECs, with mixed adenoneuroendocrine carcinomas (MANECs) representing mixed tumors (19). The 2022 WHO classification distinguished G3 NETs from NECs and introduced mixed neuroendocrine-non-neuroendocrine neoplasms (MiNENs) for tumors containing ≥30% of both components (20). Studies confirm NETs and NECs exhibit distinct survival patterns, necessitating separate consideration (21, 22). Among NECs, colorectal cases show the best survival, while gallbladder/biliary NECs have the worst (23). Preoperative diagnosis of GB-NEC remains challenging, often requiring postoperative pathology (5), and its rarity and poor prognosis complicate management. The management of rare and aggressive neoplasms like GB-NEC is best guided by multidisciplinary teams leveraging established international guidelines. While specific guidelines for GB-NEC are limited, frameworks from the European Neuroendocrine Tumor Society (ENETS) and the National Comprehensive Cancer Network (NCCN) for poorly differentiated neuroendocrine carcinomas of other sites provide valuable direction, emphasizing the critical importance of radical surgery and platinum-based chemotherapy (23, 24). Our findings strongly align with these principles.
Previous studies report varying survival outcomes for GB-NEC: Chen et al. (16) reported a median OS of 3 months, Jiang et al. (17) 16.8 months, and our study 36 months—likely due to higher rates of radical resection and chemotherapy in our cohort. Another study of 34 GB-NEC patients reported 1-, 3-, and 5-year OS rates of 64%, 35%, and 19%, respectively (18), aligning with our findings. While the recent large-scale study of 56 GB-NEC cases has provided valuable pathological insights (11), comprehensive survival data remain limited due to the reported follow-up constraints. High recurrence rates in GB-NEC (6) were also observed.
This study newly associates elevated AFP as a factor associated with poor prognosis in GB-NEC, potentially due to its association with liver metastasis. While most MiNENs share proliferative indices and genomic alterations with NECs/adenocarcinomas (25), our inclusion of all GB-NECs regardless of neuroendocrine component percentage revealed worse recurrence in mixed tumors—a novel finding suggesting increased aggressiveness in mixed cases. Thus, atypical MiNENs with <30% of either component may require refined classification.
Radical resection, particularly R0 resection, is paramount for GB-NEC (26, 27). Although radical surgery prolonged survival in our study (nonsignificant in multivariate analysis), we strongly recommend early radical resection for resectable tumors. The role of adjuvant therapy remains debated: some studies report no survival benefit (26, 28), while others demonstrate significant OS improvement (17, 18, 29). In our cohort, 13/20 chemotherapy patients received platinum-based regimens, with timely chemotherapy significantly improving outcomes versus delayed treatment. Thus, early adjuvant chemotherapy is strongly recommended. This observation is consistent with current clinical understanding of NEC management. For poorly-differentiated neuroendocrine carcinomas like GB-NEC, platinum-based chemotherapy forms the cornerstone of systemic treatment. While no randomized trials have specifically addressed G3 extra-pulmonary NENs, platinum-etoposide combinations remain the regimen of choice based on retrospective evidence, with carboplatin often preferred over cisplatin due to comparable efficacy and better tolerance (30). Beyond first-line treatment, the role of second-line chemotherapy remains limited and requires careful consideration of patient performance status and potential risks versus benefits. Case reports highlight successes with platinum-based regimens (10, 14, 31–36), though some patients still experience rapid progression (37, 38), underscoring the need for multimodal therapy. Multidisciplinary team (MDT) management is essential, particularly for mixed tumors requiring component-specific chemotherapy. Although neoadjuvant chemotherapy (NACT) showed no survival benefit here (limited cases), prior studies suggest tumor downsizing via NACT facilitates resection (39). Biotherapy and adjuvant chemoradiation may also hold promise (40); these modalities should be considered in MDT discussions, warranting further exploration of personalized, multimodal approaches for GB-NEC.
This study has several limitations. Its single-center, retrospective nature and small sample size limit the statistical power for some subgroup analyses. The preoperative diagnosis of GB-NEC was challenging, and insufficient data on biomarkers like neuron-specific enolase (NSE) and CgA precluded their prognostic evaluation. Furthermore, as over half of our patients were still alive at the time of analysis, our current survival data require further maturation. Therefore, we plan to extend the follow-up period for this cohort and continuously enroll newly diagnosed patients in the future. This ongoing effort will be crucial for obtaining more robust survival statistics, increasing the sample size, and ultimately validating our findings with greater accuracy. Moreover, prospective, multi-institutional collaborations will be essential to independently validate our findings, refine risk stratification, and explore the efficacy of novel therapeutic agents for this challenging disease.
5 Conclusion
In conclusion, GB-NEC is a highly aggressive malignancy with dismal prognosis. Elevated AFP, mixed histology, and delayed chemotherapy are key prognostic factors for poor outcomes. Preoperative diagnosis remains difficult; thus, heightened clinical awareness, R0 resection combined with early platinum-based chemotherapy, and multimodal therapy are crucial. Multicenter studies incorporating pathological and immunohistochemical profiling are needed to optimize individualized treatment strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Biomedical Ethics Review Committee of West China Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because (1) the research involved no more than minimal risk to participants (2); the waiver would not adversely affect subjects’ rights and welfare (3); the study could not practicably be carried out without the waiver; and (4) all data were anonymized prior to analysis.
Author contributions
YL: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing. GW: Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. SS: Data curation, Investigation, Writing – original draft, Writing – review & editing. ZY: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing. DJ: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1686515/full#supplementary-material
References
1. Assarzadegan N and Montgomery E. What is new in the 2019 world health organization (WHO) classification of tumors of the digestive system: review of selected updates on neuroendocrine neoplasms, appendiceal tumors, and molecular testing. Arch Pathol Lab Med. (2021) 145:664–77. doi: 10.5858/arpa.2019-0665-RA
2. Inzani F and Rindi G. Introduction to neuroendocrine neoplasms of the digestive system: definition and classification. Pathologica. (2021) 113:1–4. doi: 10.32074/1591-951X-227
3. World Health Organization. WHO classification of tumours . Available online at: https://publications.iarc.who.int/Book-And-Report-Series/Who-Classification-Of-Tumours/Endocrine-And-Neuroendocrine-Tumours-2025 (Accessed April 14, 2025).
4. Rothenstein J, Cleary SP, Pond GR, Dale D, Gallinger S, Moore MJ, et al. Neuroendocrine tumors of the gastrointestinal tract: a decade of experience at the Princess Margaret Hospital. Am J Clin Oncol. (2008) 31:64–70. doi: 10.1097/COC.0b013e31807a2f49
5. Liu W, Chen W, Chen J, Hong T, Li B, Qu Q, et al. Neuroendocrine carcinoma of gallbladder: a case series and literature review. Eur J Med Res. (2019) 24:8. doi: 10.1186/s40001-019-0363-z
6. Vidal Panduro DA, Zegarra Buitron E, Cochella Tizon OJ, and Morales Luna DA. Neuroendocrine carcinoma of the gallbladder. Cureus. (2022) 14:e27022. doi: 10.7759/cureus.27022
7. Pirenne S, Manzano-Núñez F, Loriot A, Cordi S, Desmet L, Aydin S, et al. Spatial transcriptomics profiling of gallbladder adenocarcinoma: a detailed two-case study of progression from precursor lesions to cancer. BMC cancer. (2024) 24:1025. doi: 10.1186/s12885-024-12770-0
8. Chablou M, Mabrouk Y, Maamar K, Jabi R, and Bouziane M. Neuroendocrine carcinoma of the gallbladder concomitant with adenocarcinoma of the sigmoid colon: A rare case report. Ann Med Surg. (2012) 66:102359. 2021. doi: 10.1016/j.amsu.2021.102359
9. Buscemi S, Orlando E, Damiano G, Portelli F, Palumbo VD, Valentino A, et al. Pure" large cell neuroendocrine carcinoma of the gallbladder. Report of a case and review of the literature. Int J Surg (London England). (2016) 28 Suppl 1:S128–32. doi: 10.1016/j.ijsu.2015.12.045
10. Ikezawa K, Takada R, Fukutake N, Otsuka T, Nagata S, and Ohkawa K. Gallbladder neuroendocrine carcinoma: An important differential diagnosis of gallbladder adenocarcinoma. JGH open: an Open Access J Gastroenterol hepatology. (2021) 5:717–9. doi: 10.1002/jgh3.12556
11. Pal PR, Paul P, Patne SCU, Chowdhury Z, Dhal I, and Haiyat S. Neuroendocrine carcinoma of the gall bladder: A clinicopathological report of 56 Cases from a tertiary care cancer center in North India. J Cancer Res Ther. (2025) 21:1052–8. doi: 10.4103/jcrt.jcrt_1366_24
12. Chu H, Shi Y, Liu J, Huang D, Zhang J, and Dou C. Update in clinical management for gallbladder neuroendocrine carcinoma. Medicine. (2021) 100:e25449. doi: 10.1097/MD.0000000000025449
13. Liao Y, Cao W, Li Z, Xu X, Zhang Y, Liu Z, et al. Gallbladder neuroendocrine carcinoma: A report of two cases and literature review. Oncol letters. (2023) 25:229. doi: 10.3892/ol.2023.13815
14. Yao X, Wu K, Lu B, and Lin F. Neuroendocrine carcinoma of the gallbladder: A case report and literature review. Medicine. (2024) 103:e39147. doi: 10.1097/MD.0000000000039147
15. Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol. (2008) 98:485–9. doi: 10.1002/jso.21141
16. Chen C, Wang L, Liu X, Zhang G, Zhao Y, and Geng Z. Gallbladder neuroendocrine carcinoma: report of 10 cases and comparision of clinicopathologic features with gallbladder adenocarcinoma. Int J Clin Exp pathology. (2015) 8:8218–26.
17. Jiang M and Zhang Y. Clinical features and outcomes analysis of Gallbladder neuroendocrine carcinoma. J Cancer Res Ther. (2023) 19:910–6. doi: 10.4103/jcrt.jcrt_1959_21
18. Lee SM and Sung CO. Neuroendocrine carcinomas of the gallbladder: A clinicopathologic and immunohistochemical analysis of 34 resected cases. Am J Surg Pathol. (2020) 44:1308–21. doi: 10.1097/PAS.0000000000001536
19. Meoni G, Antonuzzo L, Messerini L, Giommoni E, Muto A, Petreni P, et al. Gallbladder neuroendocrine neoplasm: a case report and critical evaluation of WHO classification. Endocr J. (2014) 61:989–94. doi: 10.1507/endocrj.EJ14-0191
20. Couvelard A, Cazes A, and Cros J. Updates in histopathological classification and tissue biomarkers of digestive neuroendocrine neoplasms: What the clinician should know. Best Pract Res Clin Endocrinol Metab. (2023) 37:101795. doi: 10.1016/j.beem.2023.101795
21. Dhruv S, Atodaria KP, Mukherjee I, Kankaria A, and Shah UK. Large cell neuroendocrine carcinoma of the gallbladder: where survival is a rare entity - case report and review of the literature. Case Rep Gastroenterol. (2023) 17:333–8. doi: 10.1159/000534520
22. Zhang YR, Hu GC, Fan MK, Yao HL, Jiang C, Shi HY, et al. Nomograms for predicting survival outcomes in patients with neuroendocrine neoplasms of the gallbladder undergoing primary tumor resection: A population-based study. Curr Oncol. (2023) 30:2889–99. doi: 10.3390/curroncol30030221
23. Sorbye H, Grande E, Pavel M, Tesselaar M, Fazio N, Reed NS, et al. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for digestive neuroendocrine carcinoma. J neuroendocrinology. (2023) 35:e13249. doi: 10.1111/jne.13249
24. Shah MH, Goldner WS, Benson AB, Bergsland E, Blaszkowsky LS, Brock P, et al. Neuroendocrine and adrenal tumors, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:839–68. doi: 10.6004/jnccn.2021.0032
25. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. (2020) 76:182–8. doi: 10.1111/his.13975
26. Wang W, Yang CX, Yu XZ, Zhang SL, Wang J, and Wang J. Clinicopathological characteristics and prognostic factors of patients with primary gallbladder neuroendocrine carcinomas. J Dig Dis. (2022) 23:166–73. doi: 10.1111/1751-2980.13088
27. Zhang Z, Guo T, Huang X, Xie P, Wang L, and Yu Y. Age-specific clinicopathological characteristics and prognostic analysis of neuroendocrine carcinomas of the gallbladder. Cancer Med. (2022) 11:641–53. doi: 10.1002/cam4.4463
28. Wang Y, Huang B, Fu Q, Wang J, Ye M, Hu M, et al. Comprehensive clinical analysis of gallbladder neuroendocrine neoplasms: A large-volume multicenter study during one decade. Ann Surg Oncol. (2022) 29:7619–30. doi: 10.1245/s10434-022-12107-w
29. Chu H, Zhang C, Shi Y, Wu W, Hu Z, Zhang J, et al. Gallbladder neuroendocrine carcinoma: A single center experience. Medicine. (2020) 99:e21912. doi: 10.1097/MD.0000000000021912
30. Espinosa-Olarte P, La Salvia A, Riesco-Martinez MC, Anton-Pascual B, and Garcia-Carbonero R. Chemotherapy in NEN: still has a role? Rev Endocr Metab Disord. (2021) 22:595–614. doi: 10.1007/s11154-021-09638-0
31. Kamei K, Shindoh J, Kiya Y, Matsumoto I, Hashimoto M, and Takeyama Y. Conversion surgery after extensive chemotherapy for stage IV mixed adenoneuroendocrine carcinoma (MANEC) of the gallbladder: clinical implications from the patterns of response and recurrence. Clin J Gastroenterol. (2020) 13:240–6. doi: 10.1007/s12328-019-01053-y
32. Rennie AT and Halbreich SL. Rare case of gallbladder neuroendocrine carcinoma. Cureus. (2022) 14:e28531. doi: 10.7759/cureus.28531
33. Takeda Y, Kobayashi N, Kessoku T, Okubo N, Suzuki A, Tokuhisa M, et al. Case reports: chemoradiotherapy for locally advanced neuroendocrine carcinoma of the gallbladder. Clin J Gastroenterol. (2022) 15:803–8. doi: 10.1007/s12328-022-01645-1
34. Shaikh S, Abouzead L, Leone C, and Talishinskiy T. A rare occurrence of a poorly differentiated large cell neuroendocrine carcinoma of the gallbladder: A case report and review of the literature. Int J Surg Case Rep. (2023) 109:108476. doi: 10.1016/j.ijscr.2023.108476
35. Li H, Qiao J, Kou X, Wu C, Liu H, and Qiu J. Complete remission of gallbladder neuroendocrine carcinoma with liver metastasis by tislelizumab plus chemotherapy: a case report. Front Oncol. (2024) 14:1346290. doi: 10.3389/fonc.2024.1346290
36. Oe S, Nakamura K, Shinohara N, Uchihara D, Miyagawa K, Tsukamoto T, et al. Multimodal treatment approach for mixed neuroendocrine-non-neuroendocrine neoplasms of the gallbladder: A case report of a patient with a favorable survival outcome. Intern Med. (2025) 64(16):2433–38. doi: 10.2169/internalmedicine.4996-24
37. Dülger UC, Erdem Ş, Cünük ES, and Altıntoprak F. A rare case of small cell neuroendocrine carcinoma of gallbladder origin. J Surg Case Rep. (2024) 2024:rjae386. doi: 10.1093/jscr/rjae386
38. Wang H, Li Q, Han S, and Tian H. Rare case report: primary small-cell neuroendocrine carcinoma of the gallbladder. Front Oncol. (2025) 15:1524974. doi: 10.3389/fonc.2025.1524974
39. Kanetkar AV, Patkar S, Khobragade KH, Ostwal V, Ramaswamy A, and Goel M. Neuroendocrine carcinoma of gallbladder: A step beyond palliative therapy, experience of 25 cases. J gastrointestinal cancer. (2019) 50:298–303. doi: 10.1007/s12029-018-0070-y
Keywords: gallbladder neuroendocrine carcinoma, platinum-based chemotherapy, survival analysis, prognostic factors, retrospective study
Citation: Luo Y, Zhou Y, Wang G, Saad SA, Jiang D and You Z (2025) Clinical management and prognostic determinants of gallbladder neuroendocrine carcinoma: a single-institutional analysis of 31 cases. Front. Oncol. 15:1686515. doi: 10.3389/fonc.2025.1686515
Received: 15 August 2025; Accepted: 29 October 2025;
Published: 17 November 2025.
Edited by:
Evan Vosburgh, United States Department of Veterans Affairs, United StatesReviewed by:
Anna La Salvia, National Institute of Health (ISS), ItalyYanpeng Tian, Hunan University of Chinese Medicine, China
Copyright © 2025 Luo, Zhou, Wang, Saad, Jiang and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Jiang, ZGFuamlhbmdAc2N1LmVkdS5jbg==; Zhen You, eW91emhlbkB3Y2hzY3UuY24=
†These authors have contributed equally to this work and share first authorship
 Yuting Luo1†
Yuting Luo1† Saud Ahmad Saad
Saud Ahmad Saad Zhen You
Zhen You