- Department of Thoracic Surgery, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu, China
Purpose: The aim of this study was to identify the relationship between preoperative controlling nutritional status (CONUT) score and long-term and short-term outcomes in patients with esophageal cancer receiving esophagectomy.
Methods: The Web of Science, EMBASE, PubMed, and CNKI databases were searched up to 24 January 2025. Primary outcome was long-term survival such as the overall survival (OS), disease-free survival (DFS), and cancer-specific survival (CSS). Secondary outcomes included the postoperative overall complication, incision infection, anastomotic fistula, pneumonia, respiratory complication, 90-day death, cardiovascular complication, major adverse cerebrocardiovascular event (MACCE), pulmonary atelectasis, and pulmonary embolism. Hazard ratio (HR) and odds ratio (OR) with 95% confidence interval (CI) were separately combined for the primary and secondary outcomes. Subgroup analysis for the OS and DFS by neoadjuvant therapy and pathological type was further conducted.
Results: Eighteen studies with 5,495 cases were included. Pooled results manifested that elevated preoperative CONUT score predicted significantly worse OS (HR = 1.75, 95% CI: 1.30–2.37, p<0.001), DFS (HR=1.21, 95% CI: 1.13–1.30, p < 0.001), and CSS (HR = 2.60, 95% CI: 1.65–4.10, p<0.001). Subgroup analysis for the OS and DFS by the history of neoadjuvant therapy and pathological type demonstrated similar results. Furthermore, elevated CONUT score was significantly related to increased risk of overall complication (OR = 1.50, 95% CI: 1.14–1.96, p=0.004), pneumonia (OR= 1.60, 95% CI: 1.23–2.08, p<0.001), respiratory complication (OR = 1.60, 95% CI: 1.26–2.03, p<0.001), cardiovascular complication (OR = 3.660, 95% CI: 1.068–12.550, p=0.039), MACCE (OR = 1.920, 95% CI: 1.068–3.452, p=0.040), and pulmonary atelectasis (OR = 2.314, 95% CI: 1.408–3.805, p<0.001).
Conclusion: Preoperative CONUT score might serve as a prognostic indicator in surgical esophageal cancer, and patients with elevated CONUT score are suggested to experience worse long-term and short-term clinical outcomes.
Introduction
Over the past few decades, remarkable advances in cancer treatment—including surgery, chemotherapy, radiotherapy, immunotherapy, and targeted therapy—have substantially improved patient survival and quality of life (1). Nevertheless, despite these therapeutic breakthroughs, certain malignancies, such as esophageal cancer, remain associated with high incidence and mortality rates, particularly in regions such as China (2). According to the World Health Organization, the global incidence of esophageal cancer has shown an upward trend, especially among male patients (3, 4). Major risk factors include long-term smoking, alcohol consumption, poor dietary habits, and chronic esophageal conditions such as esophagitis and gastroesophageal reflux (5, 6). Surgical treatment continues to serve as the cornerstone for early-stage or localized esophageal cancer, while multimodal therapies combining surgery with chemotherapy and radiotherapy are commonly applied for locally advanced disease (5). However, because of the biological heterogeneity of esophageal cancer, treatment outcomes after surgery vary widely among patients, underscoring the importance of accurate prognostic assessment to guide postoperative management.
Postoperative prognosis prediction for esophageal cancer is a significant challenge. In the short term, factors such as surgical complications, postoperative recovery, and comorbidities may impact patient recovery. Long-term prognosis, on the other hand, is influenced by multiple factors, including tumor staging, lymph node metastasis, and the patient’s overall health status. While certain prognostic tools (such as TNM staging and tumor marker detection) are available, the existing methods still have limitations due to the heterogeneity of esophageal cancer and individual patient differences (7, 8). Consequently, identifying more accurate short-term and long-term prognostic indicators has become a key focus in current esophageal cancer research.
Controlling nutritional status (CONUT) score is a simple, cost-effective, and reliable nutritional assessment tool, derived from indicators such as serum albumin, total cholesterol levels, and peripheral blood lymphocyte count. In patients with cancer, particularly those undergoing surgery, chemotherapy, or radiotherapy, there is a high risk of malnutrition, which can impair immune function, postoperative recovery, and treatment tolerance, ultimately affecting treatment outcomes and survival rates (9, 10). Research has shown that patients with a higher CONUT score generally have poorer nutritional status, suppressed immune function, and worse cancer prognosis (11). Conversely, patients with a lower CONUT score typically have better nutritional status, faster postoperative recovery, and longer survival. The prognostic value of CONUT has been well-established in various types of cancer such as gastric and colorectal cancer (11, 12). However, it remains unclear whether CONUT can effectively predict the short-term and long-term prognosis of patients with esophageal cancer undergoing surgical treatment.
This study aimed to further identify the relationship between preoperative CONUT score and long-term and short-term outcomes in patients with esophageal cancer receiving esophagectomy.
Materials and methods
The current meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (13).
Literature search
The Web of Science, EMBASE, PubMed, and CNKI databases were searched from database inception up to 24 January 2025 with the following terms: controlling nutritional status core, CONUT, esophageal, esophagus, tumor, cancer, neoplasm, and carcinoma. Detailed search strategy in the PubMed was as follows: (controlling nutritional status core OR CONUT) AND (esophageal OR esophagus) AND (tumor OR cancer OR neoplasm OR carcinoma) (Supplementary File 1). MeSH terms and free texts were applied. Moreover, all references in included studies were also reviewed.
Inclusion criteria
Studies that met the following criteria were included: (1) patients were diagnosed with primary esophageal cancer and receiving the surgical therapy; (2) the CONUT score was evaluated before the surgery based on the cholesterol level, serum albumin level, and total lymphocyte level according to the previous published formula (14); (3) the association of preoperative CONUT score with at least one of the clinical outcomes was explored; (4) articles were published in English or Chinese; and (5) full texts were available.
Exclusion criteria
Studies that met the following criteria were excluded: (1) letters, editorials, case reports, reviews, or animal trials; and (2) duplicate or overlapping data.
Data collection
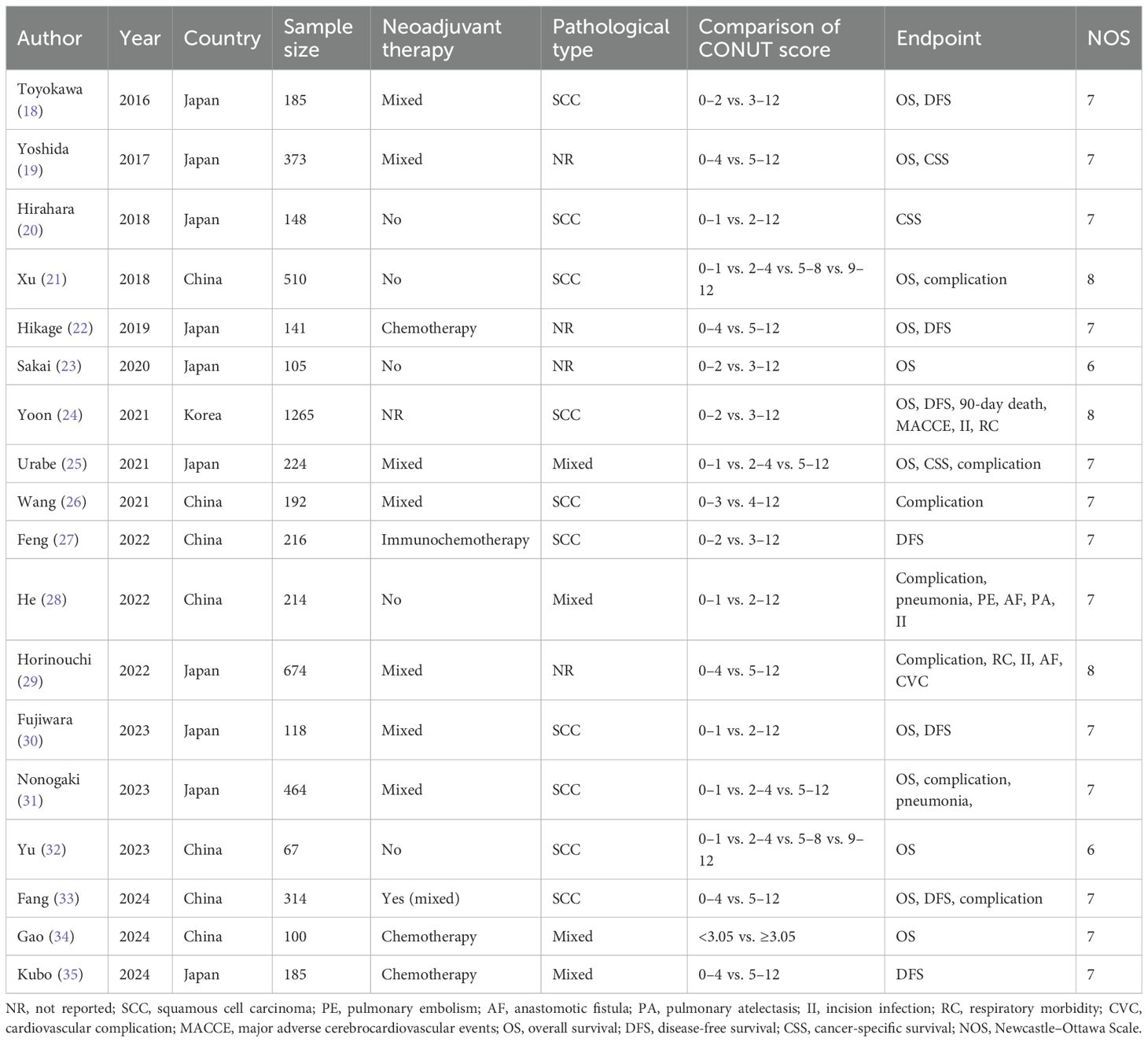
The following information was collected: the name of the first author, publication year, country, sample size, history of neoadjuvant therapy, pathological type, comparison of CONUT score, endpoint, hazard ratio (HR), odds ratio (OR), and 95% confidence interval (CI).
Primary outcome was long-term survival including the overall survival (OS), disease-free survival (DFS), and cancer-specific survival (CSS). Secondary outcomes were postoperative complications including the overall complication, incision infection, anastomotic fistula, pneumonia, respiratory complication, 90-day death, cardiovascular complication, major adverse cerebrocardiovascular event (MACCE), pulmonary atelectasis, and pulmonary embolism.
Methodological quality assessment
The quality of included studies was assessed by the Newcastle–Ottawa Scale (NOS) score tool and studies with a NOS score >5 were defined as high-quality studies (15).
Two authors independently performed the literature search, study selection, data extraction, and methodological quality assessment. The inter-rater reliability between the two reviewers was assessed, and any discrepancies were resolved through discussion and consensus with a third investigator.
Statistical analysis
Statistical analyses were performed by STATA (version 15.0) software. Heterogeneity between included studies was assessed by I2 statistics. If significant heterogeneity was detected (I2 > 50%), the random-effects model was applied; otherwise, the fixed-effects model was applied. HRs and 95% CIs were combined to evaluate the association between CONUT score and survival. ORs and 95% CIs were combined to evaluate the predictive role of postoperative complications. Sensitivity analysis was conducted to detect the sources of heterogeneity and assess the stability of the overall results. Meanwhile, Begg’s funnel plot and Egger’s test were conducted to detect publication bias (16, 17).
Results
Literature search and selection
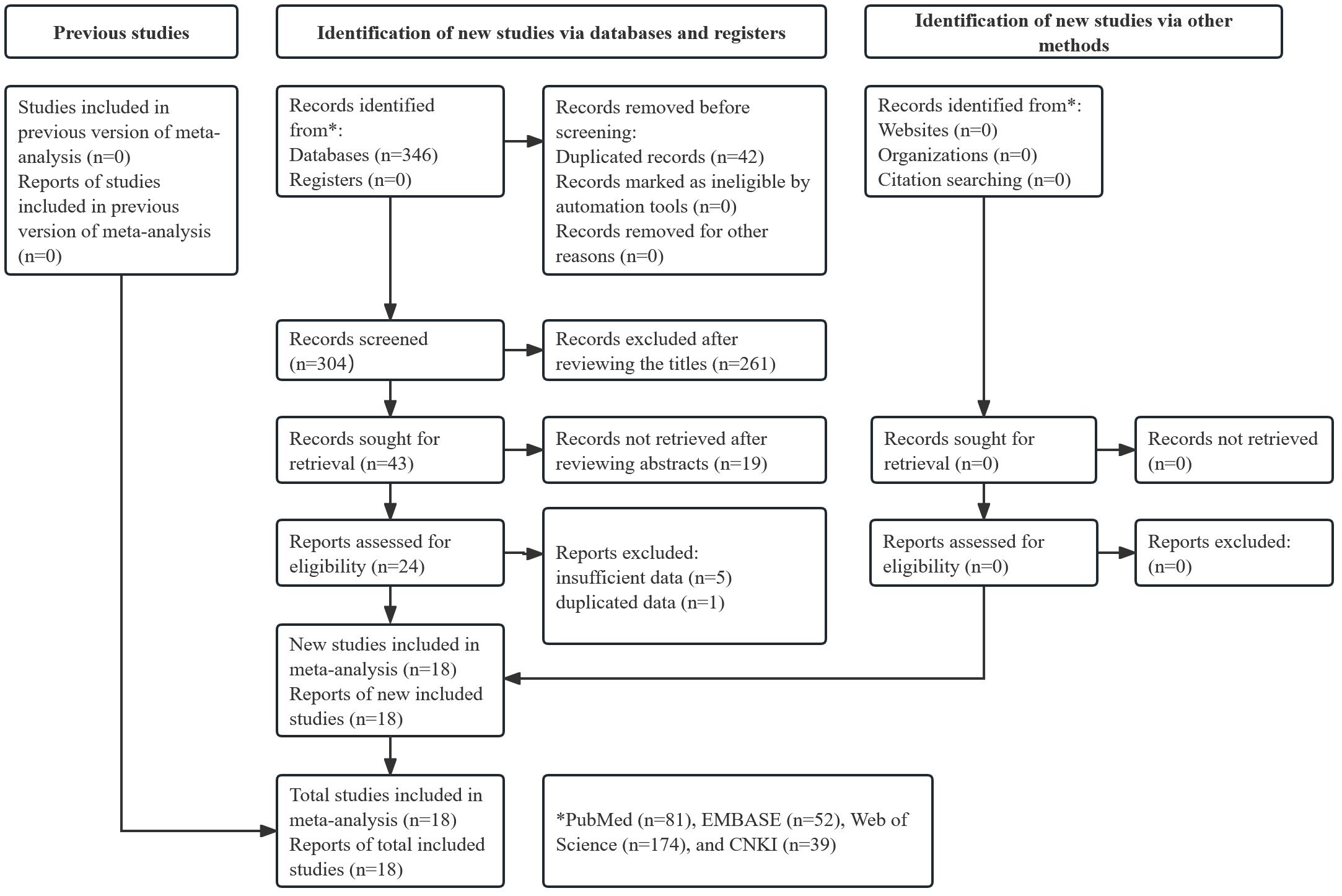
Figure 1 presents the literature search and selection process. Initially, 346 records were searched from databases and 42 duplicated records were removed. After reviewing titles, abstracts, and full texts, 286 publications were excluded. A total of 18 studies were included in this meta-analysis (18–35).
Basic characteristics of included studies
Among the 18 included studies, 5,495 patients were involved. Most studies were from China or Japan and focused on patients with squamous cell carcinoma (SCC). The sample size ranged from 67 to 1,265. The NOS scores of all included studies were all higher than 5, with detailed information in Supplementary Table 1. Other specific data are shown in Table 1.
Association between preoperative CONUT score and primary outcomes
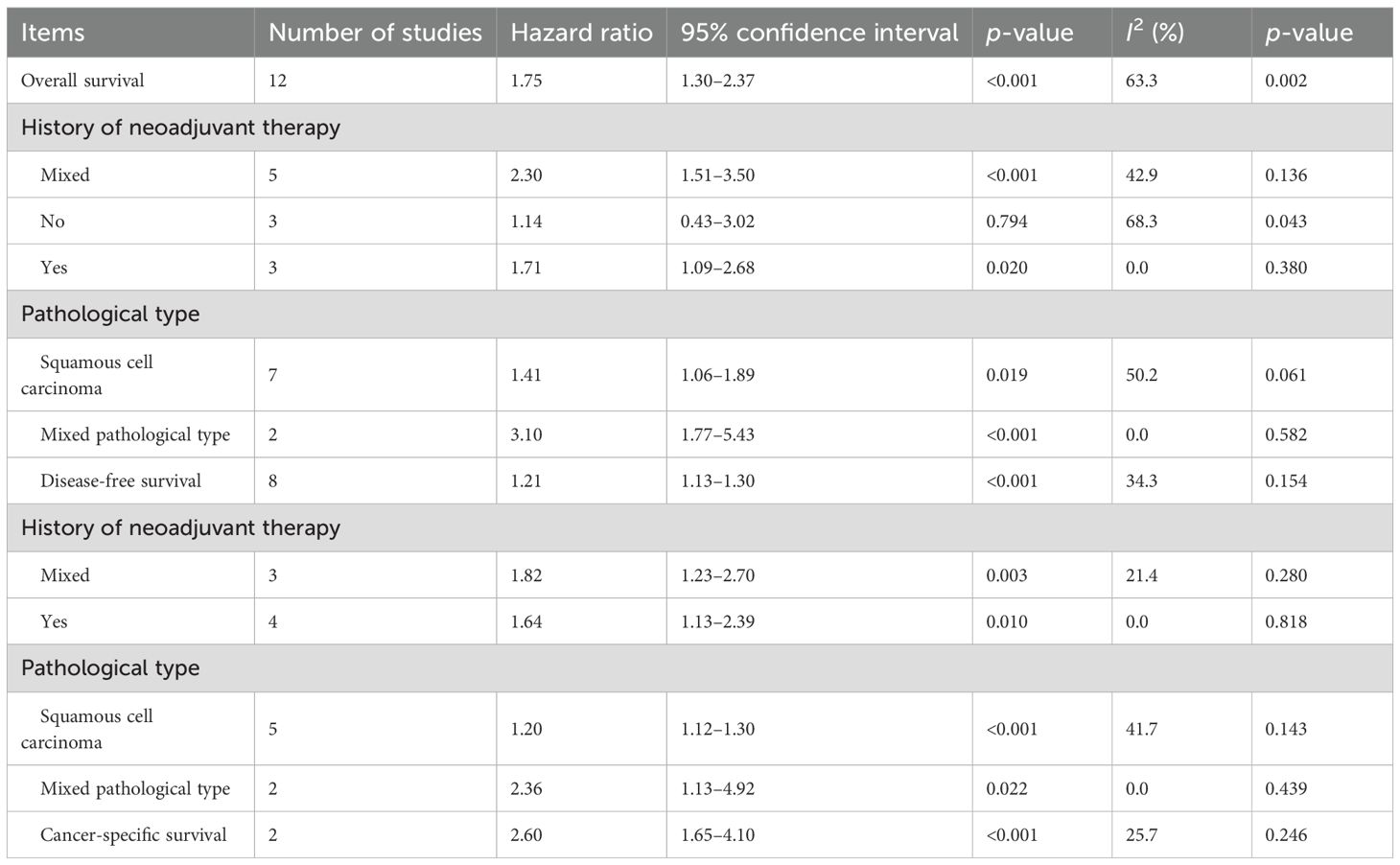
Fifteen studies explored the predictive role of preoperative CONUT score for long-term survival among surgical patients with esophageal cancer (18–25, 27, 30–35). Pooled results demonstrated that elevated preoperative CONUT score was related to decreased OS (HR = 1.75, 95% CI: 1.30–2.37, p<0.001; I2 = 63.3%, p=0.002) (Figure 2). Subgroup analysis based on the history of neoadjuvant therapy (mixed: HR = 2.30, 95% CI: 1.51–3.50, p<0.001; I2=42.9%, p=0.136; no: HR = 1.14, 95% CI: 0.43–3.02, p=0.794; I2 = 68.3%, p=0.043; yes: HR = 1.71, 95% CI: 1.09–2.68, p=0.020; I2 = 0.0%, p=0.380) (Supplementary Figure 1A) manifested similar results, although the association between CONUT score and OS among patients without history of neoadjuvant therapy did not reach the statistical difference. Subgroup analysis by the pathological type (SCC: HR = 1.41, 95% CI: 1.06–1.89, p= 0.019; I2 = 50.2%, p=0.061; mixed type: HR = 3.10, 95% CI: 1.77–5.43, p<0.001; I2 = 0.0%, p=0.582) (Supplementary Figure 1B) also indicated the association between CONUT score and OS (Table 2).
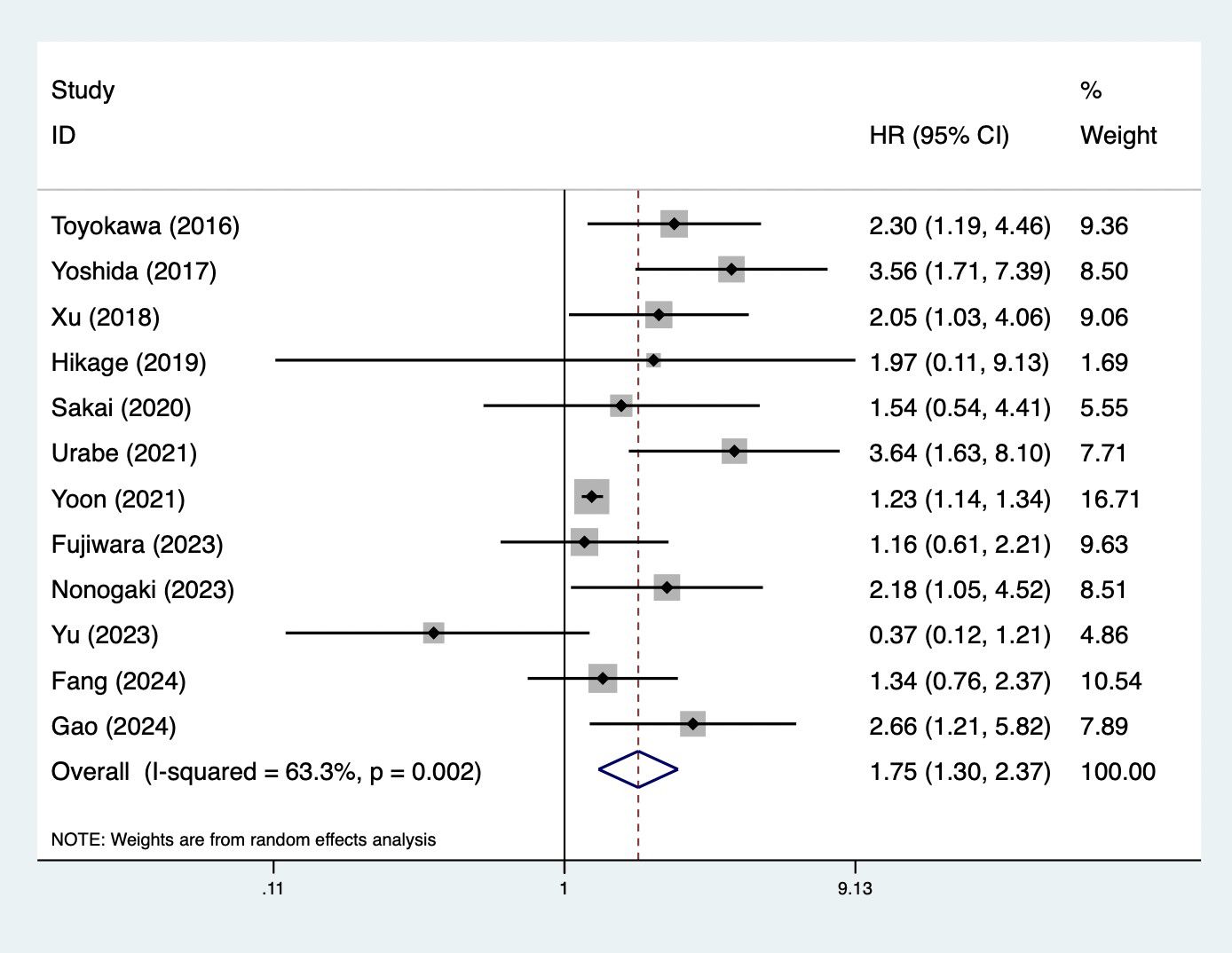
Figure 2. Association of preoperative controlling nutritional status score with overall survival among surgical patients with esophageal cancer.
Furthermore, preoperative CONUT score was associated with DFS (HR = 1.21, 95% CI: 1.13–1.30, p<0.001; I2 = 34.3%, p=0.154) (Figure 3). Meanwhile, subgroup analysis stratified by the history of neoadjuvant therapy (mixed: HR = 1.82, 95% CI: 1.23–2.70, p=0.003; I2 = 21.4%, p=0.260; yes: HR = 1.64, 95% CI: 1.13–2.39, p=0.010; I2 = 0.0%, p=0.818) (Supplementary Figure 1C) and pathological type (SCC: HR = 1.20, 95% CI: 1.12–1.30, p<0.001; I2 = 41.7%, p=0.143; mixed type: HR = 2.36, 95% CI: 1.13–4.92, p=0.022; I2 = 0.0%, p=0.439) (Supplementary Figure 1D) indicated consistent results (Table 2).
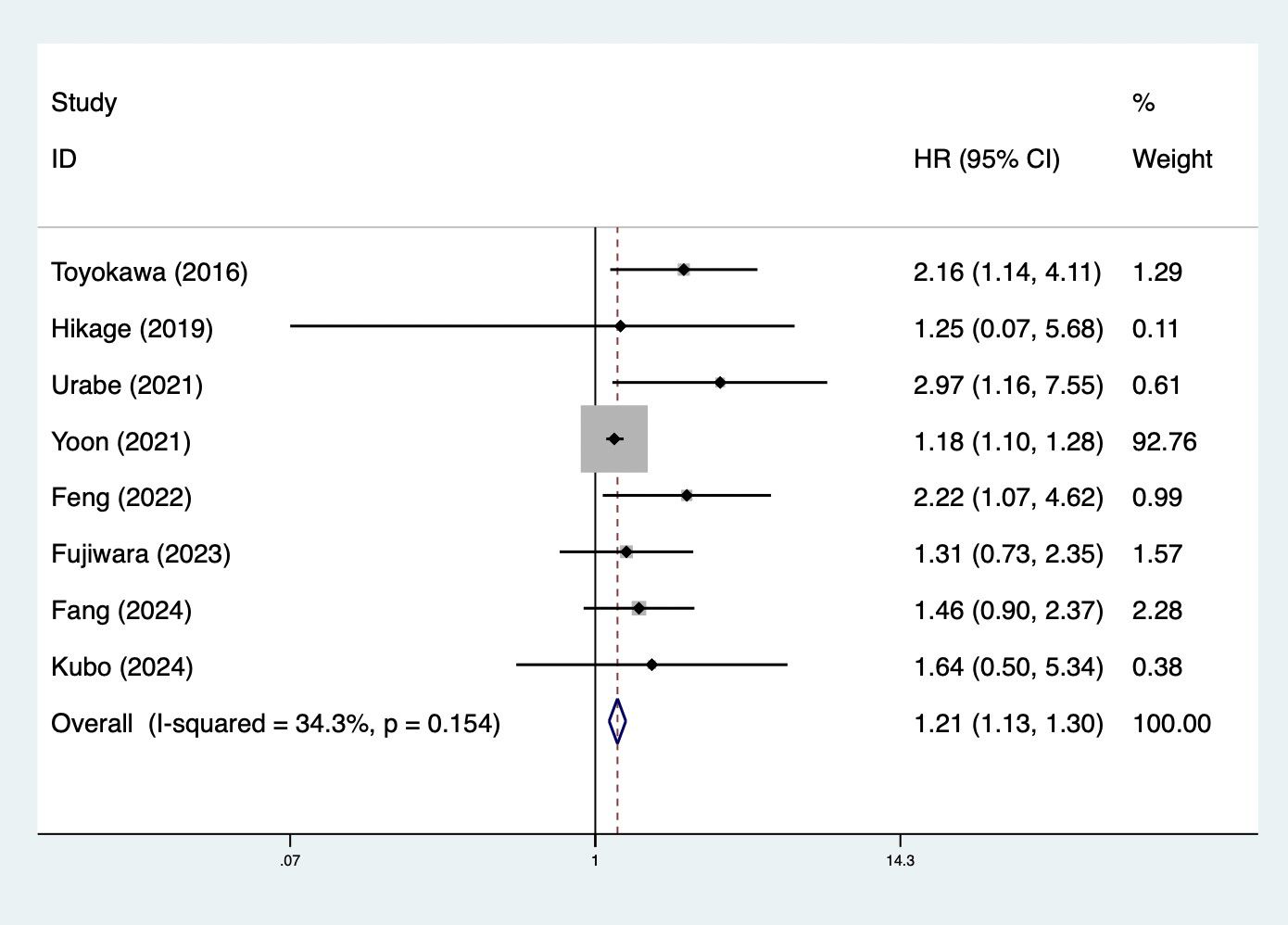
Figure 3. Association of preoperative controlling nutritional status score with disease-free survival among surgical patients with esophageal cancer.
Furthermore, elevated preoperative CONUT score predicted worse CSS (HR = 2.60, 95% CI: 1.65–4.10, p<0.001; I2 = 25.7%, p=0.246) (Table 2).
Association between preoperative CONUT score and secondary outcomes
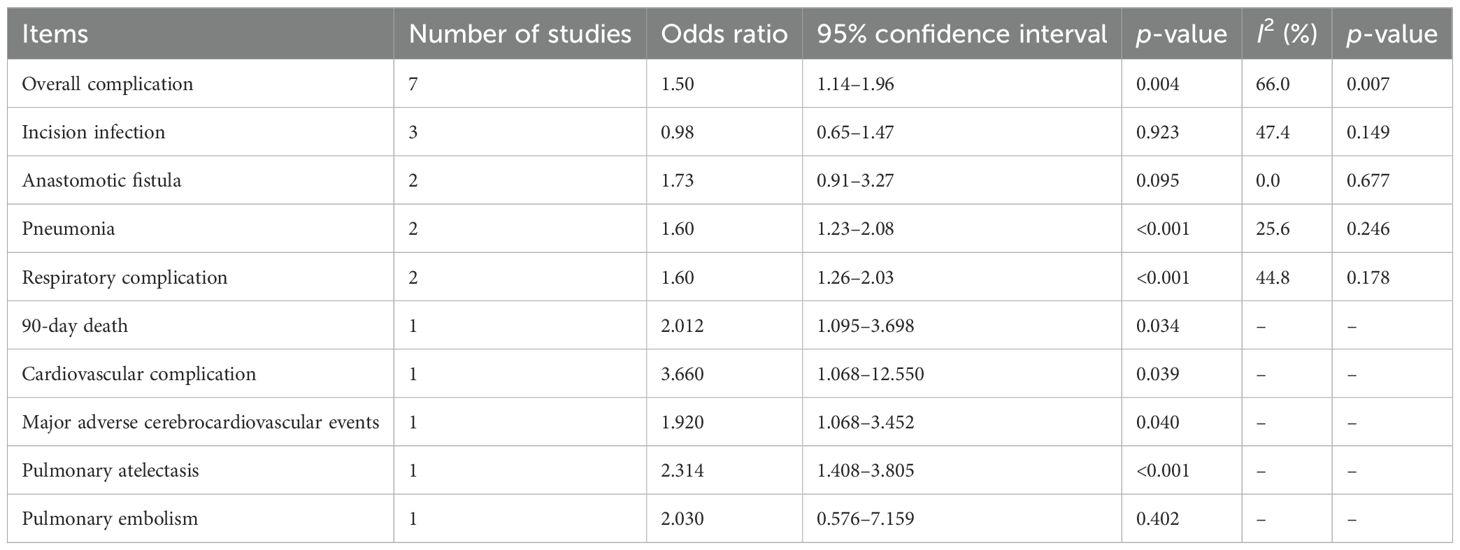
Eight studies explored the relationship between the CONUT score and the risk of postoperative complications (21, 24–26, 28, 29, 31, 33). Overall, pooled results demonstrated that elevated preoperative CONUT score predicted increased risk of postoperative complications (OR = 1.50, 95% CI: 1.14–1.96, p=0.004; I2 = 66.0%, p =0.007) (Figure 4). For specific complications, elevated CONUT score was significantly related to increased risk of pneumonia (OR = 1.60, 95% CI: 1.23–2.08, p <0.001; I2 = 25.6%, p=0.246) (Supplementary Figure 2A), respiratory complication (OR = 1.60, 95% CI: 1.26–2.03, p<0.001; I2 = 44.8%, p=0.178) (Supplementary Figure 2B), cardiovascular complication (OR = 3.660, 95% CI: 1.068–12.550, p=0.039), MACCE (OR = 1.920, 95% CI: 1.068–3.452, p=0.040), and pulmonary atelectasis (OR = 2.314, 95% CI: 1.408–3.805, p<0.001) (Table 3).
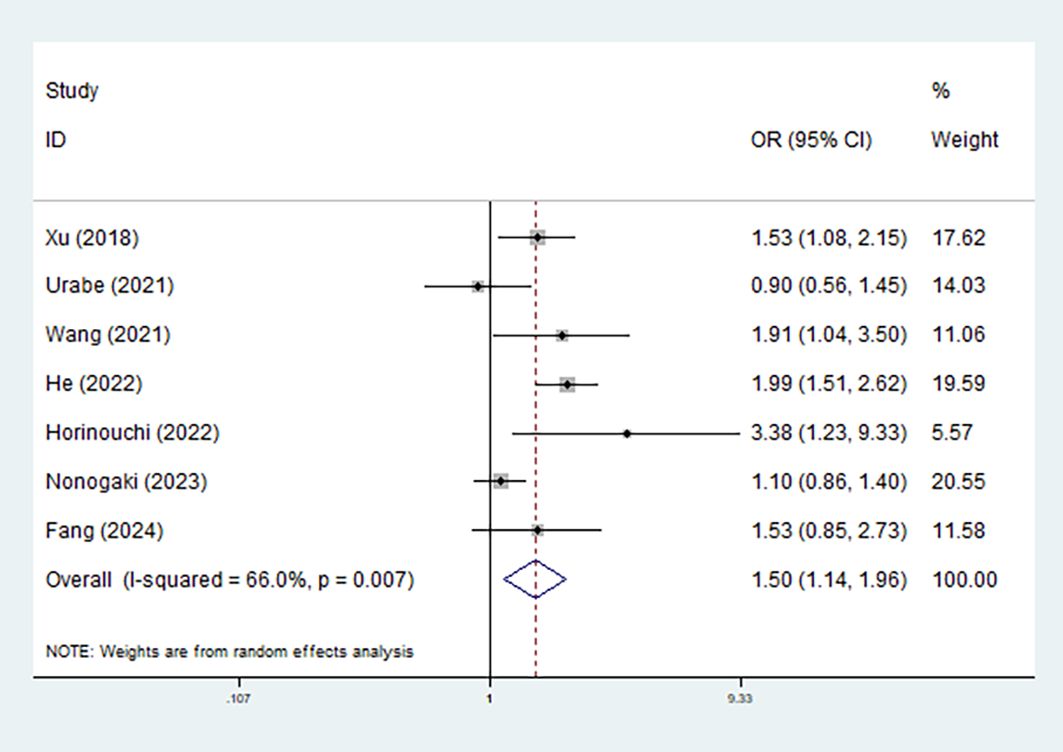
Figure 4. Association of preoperative controlling nutritional status score with postoperative overall complications among surgical patients with esophageal cancer.
Sensitivity analysis and publication bias
Sensitivity analysis for the OS was conducted (Figure 5), which manifested that our results were stable and none of the included studies caused an obvious impact on the overall findings.
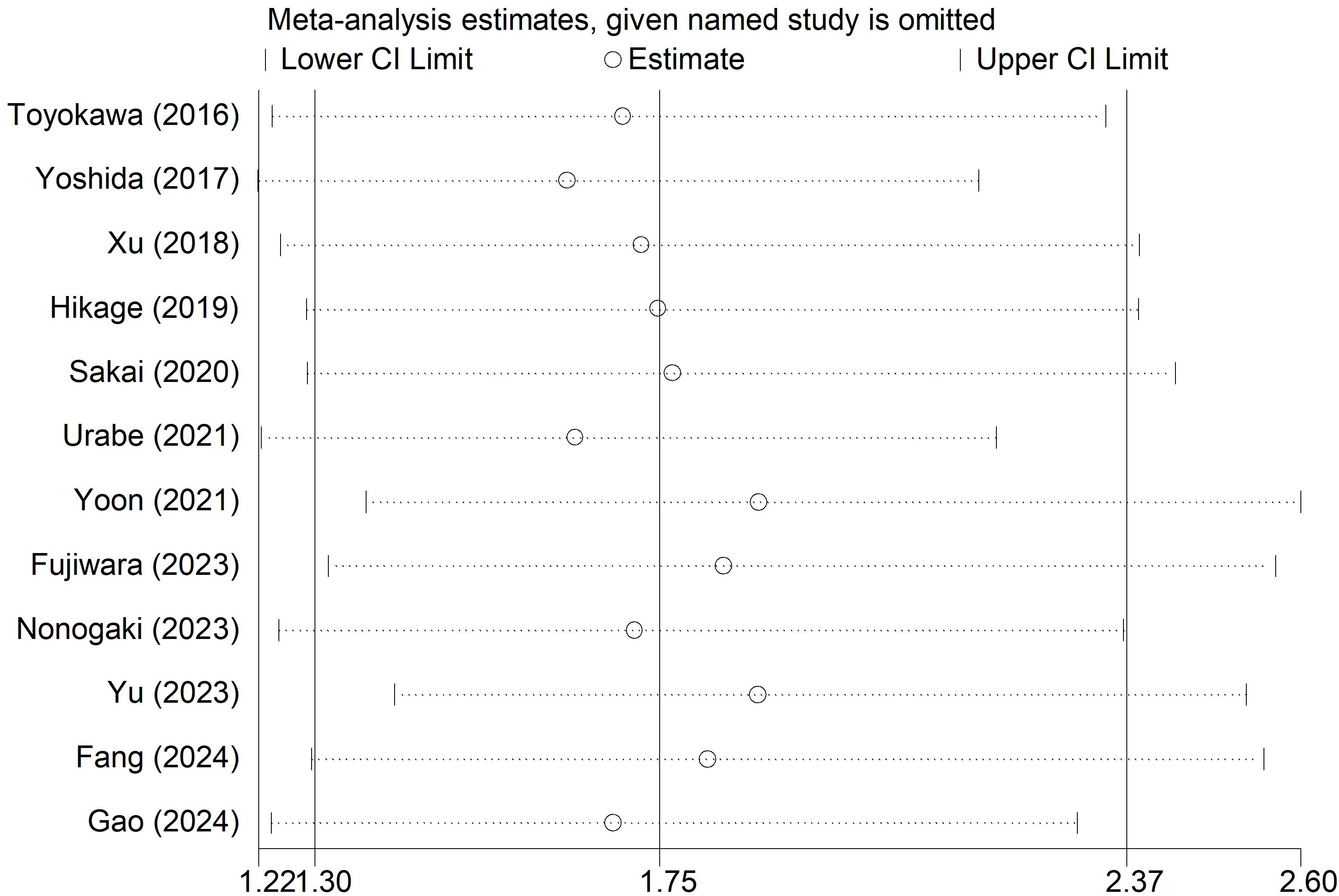
Figure 5. Sensitivity analysis for the association of preoperative controlling nutritional status score with overall survival among surgical patients with esophageal cancer.
Based on Begg’s funnel plot (Figure 6) and Egger’s test (p=0.054), no statistically significant publication bias was detected. However, as the p-value was close to the conventional threshold of 0.05, a potential publication bias cannot be entirely ruled out.
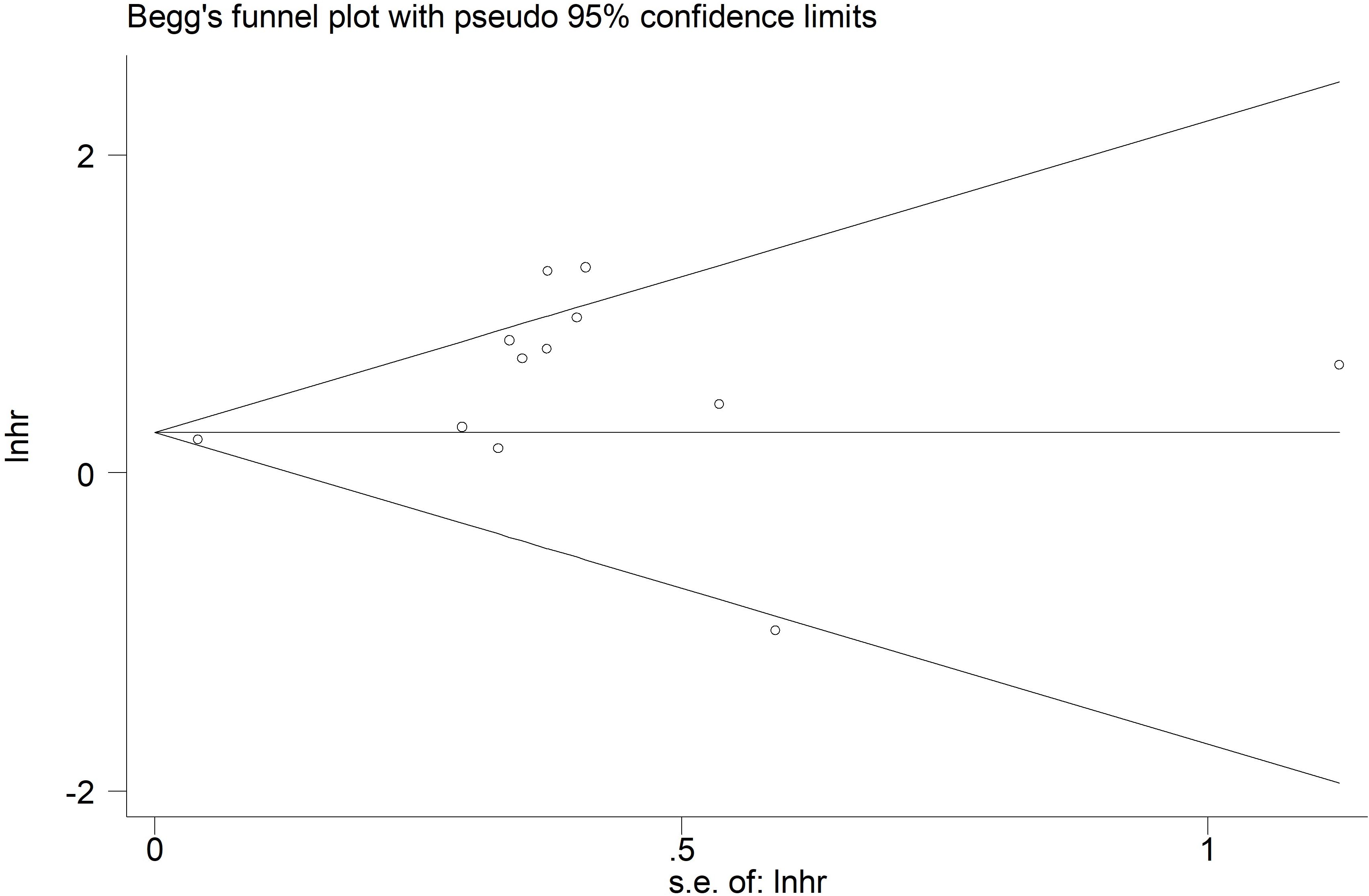
Figure 6. Begg’s funnel plots for the association of preoperative controlling nutritional status score with overall survival among surgical patients with esophageal cancer.
Discussion
According to our results, preoperative CONUT score may serve as a useful prognostic indicator in surgical esophageal cancer. Patients with an elevated CONUT score are exposed to significantly higher risk of worse clinical outcomes including the increased risk of postoperative complications and decreased long-term survival. However, owing to the limitations in included studies and this meta-analysis, more studies are still needed to further verify the above findings.
CONUT score is formulated according to the serum albumin level, cholesterol level, and total lymphocyte level. Cholesterol levels and lymphocyte counts in patients with esophageal cancer are closely related to their critical roles in the immune system and nutritional metabolism. Cholesterol is an essential component of cell membranes and participates in various physiological functions, such as cell signaling and hormone synthesis (35). Low cholesterol levels often indicate poor nutritional status, particularly in patients with cancer, where increased metabolic demands from the tumor and activation of inflammatory responses can reduce cholesterol synthesis (36). Moreover, low cholesterol levels are associated with impaired immune function, as cholesterol plays a crucial role in immune cell function (37). Studies have shown that patients with low cholesterol levels are more susceptible to infections, and tumor growth and metastasis may also be affected, leading to poorer prognosis (38). Lymphocytes are an important type of immune cell responsible for recognizing and eliminating abnormal cells, such as tumor cells, and foreign pathogens (39). Patients with cancer, particularly those with esophageal cancer, often face immune suppression, especially due to long-term disease progression and treatments like chemotherapy, which can result in reduced lymphocyte counts (40, 41). Low lymphocyte counts typically indicate a weakened immune system, unable to effectively identify and attack tumor cells, thereby affecting the anti-tumor immune response and increasing the risk of tumor recurrence or metastasis (40, 41).
In this context, the CONUT score may be viewed not merely as a simple nutritional index, but as a pragmatic surrogate that bridges systemic nutritional-immune status with underlying molecular and metabolic alterations in esophageal cancer. Its predictive value may therefore reflect, in part, the combined effects of nutritional deficiency, immune suppression, and tumor-metabolic rewiring. Future studies are warranted to validate its role in conjunction with more specific molecular biomarkers and to explore whether interventions to improve nutrition and immune function can favorably modulate the risk profiles indicated by CONUT.
Actually, there are two meta-analyses investigating the prognostic value of CONUT score in patients with esophageal cancer. Takagi et al. included five studies with only 952 patients and indicated that CONUT score was associated with OS (HR = 2.51, p<0.001), CSS (HR = 2.60, p<0.001), and DFS (HR = 2.08, p<0.001) among surgical patients with esophageal cancer (42). In another meta-analysis by Lv et al., 11 studies were included and similar associations between the pretreatment CONUT and survival were revealed (43). However, they did not focus on resected patients with esophageal cancer (43). In our meta-analysis, a total of 18 studies focusing on survival of patients with esophageal cancer were involved. Moreover, the prognostic role of CONUT score for short-term clinical outcomes presenting as postoperative complications was also systematically identified. In summary, our study provides stronger evidence for the application of CONUT score in the prognostic evaluation of patients with esophageal cancer who have undergone surgical treatment.
Although the pooled analysis demonstrated a significant association between elevated preoperative CONUT score and poorer OS, a moderate level of heterogeneity was observed (I² = 63.3%). This heterogeneity may be attributed to variations in patient characteristics and treatment strategies across the included studies. In particular, the proportion of patients receiving neoadjuvant therapy and the predominant pathological subtype differed among studies, both of which can substantially influence postoperative nutritional status and long-term outcomes. Subgroup analyses partly supported this explanation, as the association between CONUT score and OS remained significant in most subgroups, although it did not reach statistical significance in patients without neoadjuvant therapy. Moreover, differences in CONUT cutoff values, tumor stage distribution, and perioperative management practices could have contributed to the residual heterogeneity. These factors should be considered when interpreting the pooled results, and future studies with more standardized designs are warranted to further clarify these relationships.
Furthermore, the CONUT score provides a comprehensive reflection of a patient’s preoperative nutritional and immune status by simultaneously incorporating serum albumin, total cholesterol, and lymphocyte count. Compared with other commonly used indices such as the Prognostic Nutritional Index (PNI), Geriatric Nutritional Risk Index (GNRI), and neutrophil-to-lymphocyte ratio (NLR), CONUT has several clinical advantages. PNI and GNRI mainly evaluate protein reserves and body composition, while NLR emphasizes systemic inflammation. In contrast, CONUT integrates both nutritional and immunological dimensions, offering a more balanced assessment of the host’s metabolic and inflammatory state. Previous studies have suggested that CONUT exhibits superior prognostic performance for postoperative complications and long-term survival in gastrointestinal malignancies, including esophageal cancer, and can be easily obtained from routine laboratory data, making it a practical preoperative screening tool for risk stratification and perioperative optimization (23, 24, 44).
There are some limitations in this meta-analysis. First, most studies are from China or Japan, which might cause some bias. Second, it is hard to conduct more subgroup analyses based on other parameters such as the comparison method of CONUT score and age. Further research should investigate the influence of these parameters on the prognostic role of CONUT score in esophageal cancer. Third, since all included studies were retrospective in nature, potential confounding factors could not be fully eliminated. Therefore, the findings should be interpreted with caution, and prospective multicenter studies are warranted to validate the prognostic value of CONUT in esophageal cancer. Fourth, the language restriction may have introduced a potential language bias.
Conclusion
Preoperative CONUT score might serve as a prognostic indicator in surgical esophageal cancer, and patients with an elevated CONUT score are more likely to experience worse long-term and short-term clinical outcomes. However, more studies are needed to further verify our conclusion.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YJ: Conceptualization, Investigation, Resources, Supervision, Visualization, Writing – original draft. LW: Data curation, Formal analysis, Software, Writing – review & editing. XM: Formal analysis, Resources, Software, Writing – original draft. EZ: Data curation, Investigation, Software, Validation, Writing – review & editing. LL: Data curation, Investigation, Methodology, Visualization, Writing – original draft. MY: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1694236/full#supplementary-material
References
1. Wang K and Yuan S. Current status and prospect of particle therapy for esophageal cancer. Precis Radiat Oncol. (2024) 8:92–8. doi: 10.1002/pro6.1232
2. Zhou X, Xue J, Chen L, Qin S, and Zhao Q. Exploration of individualized neoadjuvant therapy model for operable esophageal cancer: A Surveillance, Epidemiology, and End Results database analysis. Precis Radiat Oncol. (2024) 8:218–26. doi: 10.1002/pro6.1249
3. Siegel RL, Miller KD, Wagle NS, and Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
4. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
5. Huang FL and Yu SJ. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg. (2018) 41:210–5. doi: 10.1016/j.asjsur.2016.10.005
6. Uhlenhopp DJ, Then EO, Sunkara T, and Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. (2020) 13:1010–21. doi: 10.1007/s12328-020-01237-x
7. Wang Y, Hu X, Huang Y, Xu WY, Wu YM, Li PF, et al. Prognostic value of the C-reactive protein to albumin ratio in esophageal cancer: A systematic review and meta-analysis. Kaohsiung J Med Sci. (2020) 36:54–61. doi: 10.1002/kjm2.12129
8. Chen P, Chen M, Bu Y, Che G, Cheng C, and Wang Y. Prognostic role of lymph node regression in patients with esophageal cancer undergoing neoadjuvant therapy. Pathol Oncol Res. (2024) 30:1611844. doi: 10.3389/pore.2024.1611844
9. Meza-Valderrama D, Marco E, Dávalos-Yerovi V, Muns MD, Tejero-Sánchez M, Duarte E, et al. Sarcopenia, malnutrition, and cachexia: adapting definitions and terminology of nutritional disorders in older people with cancer. Nutrients. (2021) 13:761. doi: 10.3390/nu13030761
10. Arends J. Malnutrition in cancer patients: Causes, consequences and treatment options. Eur J Surg Oncol. (2024) 50:107074. doi: 10.1016/j.ejso.2023.107074
11. Takagi K, Domagala P, Polak WG, Buettner S, Wijnhoven BPL, and Ijzermans JNM. Prognostic significance of the controlling nutritional status (CONUT) score in patients undergoing gastrectomy for gastric cancer: a systematic review and meta-analysis. BMC Surg. (2019) 19:129. doi: 10.1186/s12893-019-0593-6
12. Takagi K, Buettner S, and Ijzermans JNM. Prognostic significance of the controlling nutritional status (CONUT) score in patients with colorectal cancer: A systematic review and meta-analysis. Int J Surg. (2020) 78:91–6. doi: 10.1016/j.ijsu.2020.04.046
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed). (2021) 372:n71. doi: 10.1136/bmj.n71
14. Peng J, Hao Y, Rao B, and Cao Y. Prognostic impact of the pre-treatment controlling nutritional status score in patients with non-small cell lung cancer: A meta-analysis. Med (Baltimore). (2021) 100:e26488. doi: 10.1097/MD.0000000000026488
15. Wang Y, Li J, Chang S, Dong Y, and Che G. Risk and influencing factors for subsequent primary lung cancer after treatment of breast cancer: A systematic review and two meta-analyses based on four million cases. J Thorac Oncol. (2021) 16:1893–908. doi: 10.1016/j.jtho.2021.07.001
16. Begg CB and Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
17. Egger M, Davey Smith G, Schneider M, and Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed). (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
18. Toyokawa T, Kubo N, Tamura T, Sakurai K, Amano R, Tanaka H, et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. (2016) 16:722. doi: 10.1186/s12885-016-2696-0
19. Yoshida N, Harada K, Baba Y, Kosumi K, Iwatsuki M, Kinoshita K, et al. Preoperative controlling nutritional status (CONUT) is useful to estimate the prognosis after esophagectomy for esophageal cancer. Langenbecks Arch Surg. (2017) 402:333–41. doi: 10.1007/s00423-017-1553-1
20. Hirahara N, Matsubara T, Hayashi H, Takai K, Nakada S, and Tajima Y. Prognostic importance of controlling nutritional status in patients undergoing curative thoracoscopic esophagectomy for esophageal cancer. Am J Ther. (2018) 25:E524–32. doi: 10.1097/MJT.0000000000000414
21. Xu J. Analysis of preoperativve nutritional data and prognostic model of esophageal squamous cell carcinoma. Doctor. (2018).
22. Hikage M, Taniyama Y, Sakurai T, Sato C, Takaya K, Okamoto H, et al. The influence of the perioperative nutritional status on the survival outcomes for esophageal cancer patients with neoadjuvant chemotherapy. Ann Surg Oncol. (2019) 26:4744–53. doi: 10.1245/s10434-019-07742-9
23. Sakai M, Sohda M, Saito H, Ubukata Y, Nakazawa N, Kuriyama K, et al. Comparative analysis of immunoinflammatory and nutritional measures in surgically resected esophageal cancer: A single-center retrospective study. In Vivo. (2020) 34:881–7. doi: 10.21873/invivo.11853
24. Yoon J, Nam J, Zainal Abidin MFB, Kim S, Lee E, Choi I, et al. Comparison of preoperative nutritional indexes for outcomes after primary esophageal surgery for esophageal squamous cell carcinoma. Nutrients. (2021) 13:4086–6. doi: 10.3390/nu13114086
25. Urabe M, Ueno M, Ogawa Y, Yago A, Shimoyama H, Honda A, et al. Comparative analysis of the prognostic utility of preoperative nutritional parameters in patients with resectable esophageal carcinoma. Gen Thorac Cardiovasc Surg. (2021) 69:326–35. doi: 10.1007/s11748-020-01555-4
26. Wang PY, Chen XK, Liu Q, Xu L, Zhang RX, Liu XB, et al. Application of four nutritional risk indexes in perioperative management for esophageal cancer patients. J Cancer Res Clin Oncol. (2021) 147:3099–111. doi: 10.1007/s00432-021-03585-8
27. Feng J, Wang L, Yang X, Chen Q, and Cheng X. The usefulness of pretreatment controlling nutritional status score for predicting recurrence in patients with esophageal squamous cell carcinoma undergoing neoadjuvant immunochemotherapy: A real-world study. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.1015365
28. He Y. Significance of CONUT Scores and PNI in Predicting Postoperative complications of esophageal Cancer. Master. (2022).
29. Horinouchi T, Yoshida N, Harada K, Eto K, Sawayama H, Iwatsuki M, et al. A retrospective study of preoperative malnutrition based on the Controlling Nutritional Status score as an associated marker for short-term outcomes after open and minimally invasive esophagectomy for esophageal cancer. Langenbecks Arch Surg. (2022) 407:3367–75. doi: 10.1007/s00423-022-02655-w
30. Fujiwara Y, Endo S, Higashida M, Kubota H, Yoshimatsu K, and Ueno T. The prognostic significance of preoperative nutritional/inflammatory markers and clinicopathological features in resectable esophagectomy patients: possibility of nutritional intervention. Esophagus. (2023) 20:234–45. doi: 10.1007/s10388-022-00961-2
31. Nonogaki I, Kanda M, Shimizu D, Inokawa Y, Hattori N, Hayashi M, et al. Controlling nutritional status score serves as a prognosticator in esophageal squamous cell carcinoma: optimal timing of evaluation of patients undergoing neoadjuvant treatment. World J Surg. (2023) 47:217–26. doi: 10.1007/s00268-022-06773-w
32. Yu H. Model construction of CT image for evaluation of postoperative nutritional indicators of esophageal squamous cell carcinoma. Master. (2023) 217–226. doi: 10.12677/ACM.2023.1361428
33. Fang P, Zhou J, Liang Z, Yang Y, Luan S, Xiao X, et al. The prognostic value of controlling nutritional status score on esophageal squamous cell carcinoma patients with neoadjuvant therapy followed by esophagectomy-a retrospective research. J Thorac Dis. (2024) 16:4460–4473. doi: 10.21037/jtd-24-187
34. Gao X and Yan L. Effect of preoperative AAPR and CONUT scores on prognosis of patients with esophageal cancer after radical surgery with adjuvant chemotherapy. Guide China Med. (2024) 22:1–4.
35. Kubo Y, Igaue S, Utsunomiya D, Kubo K, Kurita D, Ishiyama K, et al. Association between preoperative serum zinc level and prognosis in patients with advanced esophageal cancer in the neoadjuvant treatment era. Ann Gastroenterological Surg. (2024) 8:595–603. doi: 10.1002/ags3.12781
36. Espiau-Romera P, Gordo-Ortiz A, Ortiz-de-Solórzano I, and Sancho P. Metabolic features of tumor-derived extracellular vesicles: challenges and opportunities. Extracell Vesicles Circ Nucl Acids. (2024) 5:455–70. doi: 10.20517/evcna.2024.12
37. Fonseca P, Vardaki I, Occhionero A, and Panaretakis T. Metabolic and signaling functions of cancer cell-derived extracellular vesicles. Int Rev Cell Mol Biol. (2016) 326:175–99. doi: 10.1016/bs.ircmb.2016.04.004
38. Qin J, Tang Y, Zhu R, Feng X, Bie J, Shu Y, et al. IL2RB remodels the immune microenvironment and promotes the progression of esophageal squamous cell carcinoma. Ann Clin Lab Sci. (2025) 55:309–20.
39. Xi Y, Shen Y, Chen L, Tan L, Shen W, and Niu X. Exosome-mediated metabolic reprogramming: Implications in esophageal carcinoma progression and tumor microenvironment remodeling. Cytokine Growth Factor Rev. (2023) 73:78–92. doi: 10.1016/j.cytogfr.2023.08.010
40. Zeng D, Ye S, Yin W, Lian D, and Zhou S. Influence of IL-38 as a novel biomarker on the pathophysiological processes of esophageal cancer. Cancer Genet. (2025) 296-297:117–24. doi: 10.1016/j.cancergen.2025.06.010
41. Dings MPG, van der Zalm AP, Bootsma S, van Maanen TFJ, Waasdorp C, van den Ende T, et al. Estrogen-related receptor alpha drives mitochondrial biogenesis and resistance to neoadjuvant chemoradiation in esophageal cancer. Cell Rep Med. (2022) 3:100802. doi: 10.1016/j.xcrm.2022.100802
42. Takagi K, Buettner S, Ijzermans JNM, and Wijnhoven BPL. Systematic review on the controlling nutritional status (CONUT) score in patients undergoing esophagectomy for esophageal cancer. Anticancer Res. (2020) 40:5343–9. doi: 10.21873/anticanres.14541
43. Lv J, Chen P, Wu J, and Hu C. Prognostic value of pretreatment Controlling Nutritional Status score in esophageal cancer: a meta-analysis. Pathol Oncol Res. (2023) 29:1611221. doi: 10.3389/pore.2023.1611221
Keywords: controlling nutritional status score, esophageal cancer, surgery, long-term outcomes, short-term outcomes, meta-analysis
Citation: Ji Y, Wang L, Mei X, Zheng E, Lin L and Yang M (2025) Association of preoperative controlling nutritional status score with clinical outcomes among surgical patients with esophageal cancer: a meta-analysis. Front. Oncol. 15:1694236. doi: 10.3389/fonc.2025.1694236
Received: 28 August 2025; Accepted: 27 October 2025;
Published: 11 November 2025.
Edited by:
Hailin Tang, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Hengrui Liu, University of Cambridge, United KingdomJian Wan, Guangdong Women and Children Hospital, China
Copyright © 2025 Ji, Wang, Mei, Zheng, Lin and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mei Yang, eWFuZ21laXh3MjAyNUAxNjMuY29t
 Yanli Ji
Yanli Ji Mei Yang
Mei Yang