- 1Division of Vascular and Interventional Radiology, Department of Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States
- 2Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 3Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 4Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, GA, United States
- 5Department of Medicine, Hematology and Oncology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
Introduction: The treatment landscape of chronic myeloid leukemia (CML) has evolved with the introduction of second- and third-generation tyrosine kinase inhibitors (TKIs), oCering potential advantages over imatinib. We analyzed prescription trends and public awareness of TKIs to assess the adoption of newer agents.
Methods: Monthly US prescription data from the IQVIA National Prescription Audit (NPA) and Google Trends search volumes from March 1, 2017, to November 31, 2024, were analyzed for visual and quantitative trends and correlation patterns. Studied TKIs included imatinib (first generation), dasatinib, bosutinib, nilotinib (second generation), and ponatinib, asciminib (third generation).
Results: Second-generation TKIs increased by 14.4% (15,171 to 17,363 average monthly prescriptions) between 2017 and 2024, while the use of imatinib declined (-10.0%, from 18,704 to 16,835). Bosutinib (+144.1%) and dasatinib (+27.8%) usage increased during this period, while nilotinib prescriptions decreased (-26.0%). Third-generation TKIs saw substantial growth (696 to 2,123 average monthly prescriptions), led by ponatinib (+86.6%) and asciminib (+235.0% since 2022). Online search volumes strongly correlated with prescription trends, particularly for newer TKIs: asciminib (r = 0.85), bosutinib (r = 0.74), nilotinib (r = 0.74), ponatinib (r = 0.63), and dasatinib (r = 0.51). Imatinib showed little correlation (r = 0.21). Prescription patterns varied across disciplines, with Advanced Practice Providers (APPs) prescribing imatinib 9% less frequently than internists/primary care physicians (PCPs).
Discussion: These data highlight a shift toward newer TKIs in CML treatment, mirroring guideline recommendations and rising public awareness. Online search trends complement traditional prescription monitoring, oCering near real-time insights into evolving prescribing practices and drug adoption.
1 Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm characterized by overproduction of maturing myeloid precursor cells. The hallmark of CML is the Philadelphia (Ph) chromosome, generated by a specific chromosomal translocation between chromosomes 9 and 22 (t(9;22)(q34;q11)), resulting in the BCR::ABL1 fusion gene. This oncogene encodes the functional BCR-ABL1 protein, which leads to constitutive activation of tyrosine kinase and promotes growth of CML cells (1, 2). Due to the critical role of BCR::ABL1 in the pathogenesis of CML, tyrosine kinase inhibitors (TKIs) have become the cornerstone of modern CML therapy (3) with molecular response to TKI treatment serving as the key determinant of long-term prognosis in CML (4). Under TKI therapy, patients have demonstrated 8-year survival rates of 87% (5). Typically, patients remain on TKI treatment for extended periods often spanning several years and often achieving treatment-free remission. Treatment modifications occur primarily in patients who develop resistance to the therapy or experience intolerable side effects (3).
First-line therapy for chronic myeloid leukemia (CML) involves the use of first-generation TKI imatinib (Gleevec) or second-generation TKIs such as bosutinib (Bosulif), nilotinib (Tasigna), and dasatinib (Sprycel) (3). However, patients who do not respond and/or become refractory to one of these agents generally receive treatment with a newer third-generation TKI, including ponatinib (Iclusig) and asciminib (Scemblix). These drugs are of particular importance in the treatment of disease resistance, including patients with T315I mutation or for drug intolerance (6, 7), which, given the long course of treatment, occur in a fraction of patients (8–10).
Online search behavior has become a useful means of assessing public health trends, particularly prescription trends, in near real-time (11, 12). Several studies could indicate correlations between online search behavior and public health related topics such as infection outbreaks of influenza or COVID-19 (13, 14), hospital admissions, cardiovascular disease and prescription trends, thus offering an easily accessible means of mirroring prescription trends and in general interest in different drugs (15–18). With more than 90% of all online searches, Google represents the main online search engine. To this end, Google Trends has become the main resource for the analyses of public health-related online search patterns and trends (12). Given the evolving therapeutic landscape of TKI-based treatments for CML, we aimed to evaluate prescription trends and public awareness represented by online searches, as well as prescription behaviors across medical specialties. Additionally, we sought to explore how online searches might reflect actual prescriptions and potentially mirror prescription trends.
2 Materials and methods
2.1 Drug collection – prescription data
Prescription data were gathered from the IQVIA National Prescription Audit (NPA) database. The NPA provides a measure of overall US national prescription dispensing information from retail, mail-order, and long-term care pharmacies. These data include prescription data from approximately 90% of all outpatient prescription activity in the United States and are then projected to estimate all retail transactions. Dispensed prescriptions are recorded irrespective of the payer type, including both insured and self-pay cases. Further detailed information on the data collection process can be found elsewhere (16, 19, 20). In brief, IQVIA links the NPA to the American Medical Association’s Physician Masterfile and other professional organization records to confirm the primary specialty of prescribers.
We gathered monthly prescription data for the US from March 1, 2017, to November 31, 2024. Total monthly dispensed prescriptions, prescriber specialty, and brand names of the drugs used among all patients were extracted. Our analyses considered total dispensed prescriptions (TRx), which encompass new and refill prescriptions. In this analysis, “prescriptions” hereafter refers to total dispensed prescriptions. For prescriber-related information, physician assistants and nurse practitioners were categorized as advanced practice providers (APP). General practitioners were referred to as primary care physicians (PCP)/internists, including family practice, general practice, general preventive medicine, geriatrics, internal medicine, internal medicine/pediatrics, osteopathic medicine, and pediatrics, as previously reported (20).
2.2 Data collection – online search data
We extracted monthly search data using the Google Trends for Health Application Programming Interface (16, 20, 21). Data were retrieved from March 1, 2017, to November 31, 2024. Online search volumes were measured as the number of searches per 10 million Google searches. Search data for following brand and generic names of TKIs were extracted: Imatinib (Gleevec), Bosutinib (Bosulif), Nilotinib (Tasigna), Dasatinib (Sprycel), Ponatinib (Iclusig) and Asciminib (Semblix). Hereafter reported TKI search data represent aggregated online search volumes for both brand and generic names to ensure representation. As such, the online search data analyzed in this study represent the combined online searches of generic and brand searches.
2.3 Statistical and graphical analysis
All analysis was done using the python programming language version 3.12. Libraries used for data aggregation and statistical analysis, including computation of Spearman’s correlation coefficient, were NumPy, Pandas and SciPy. Data visualization was carried out using Matplotlib and Seaborn.
3 Results
3.1 Prescription trends for individual first, second- and third-generation TKIs
During the study period between 03/2017 and 11/2024, imatinib/Gleevec, the first TKI approved by the FDA for the treatment of CML in 2001, showed the highest US prescription volumes with an average of 18,704 combined monthly prescriptions in 2017 (Figure 1a, Supplementary Tables 1, 2). Over time, US monthly prescriptions of imatinib decreased to an average of 16,835 in 2024 (Table 1). This represented a relative decrease of -10.0% between 2017 and 2024. While Gleevec alone accounted for 5,223 US monthly prescriptions in 2017 (27.9% of total imatinib prescriptions), Gleevec prescriptions decreased to 1,149 in 2024 (6.8% of total imatinib prescriptions).

Figure 1. Prescription trends for tyrosine kinase inhibitors (TKIs) used in CML treatment. Prescription data for individual first (A), second (B), and third (C) generation TKIs. The blurred-out curves show monthly prescription trends for each drug, and the focused curves show the 6-month moving average (not for generic dasatinib). CML indicates Chronic myeloid leukemia. Source: IQVIA National Prescription Audit (March 2017-November 2024).
Among newer, second-generation TKIs approved by the FDA from 2006 onward, dasatinib/Sprycel emerged as the most frequently dispensed TKI (Figure 1b, Supplementary Tables 1, 2). Between 2017 and 2024, US average monthly prescriptions increased from 8,354 to 10,673, showing a 27.8% growth (Table 1). Before its patent expiration in September 2024, Sprycel accounted for all second-generation TKI prescriptions (10,423 US prescriptions in August 2024). Following the availability of generic dasatinib, Sprycel prescriptions declined to 4,209 (-59.6%) by November 2024, while generic prescriptions increased to 7,188. Bosutinib/Bosulif had an average of 968 US monthly prescriptions 2017. However, when compared to other first- and second-generation TKIs, bosutinib showed the highest growth, increasing by 144.1% to 2,363 US monthly dispensed prescriptions in 2024. In contrast, nilotinib prescriptions declined from a monthly average of 5,849 in 2017 to 4,328 in 2024 (-26.0%).
For third-generation TKIs, Ponatinib showed continuously increasing US prescriptions from 2017 to 2024, from 696 average US monthly prescriptions (2017) to 1,299 (2024), representing an 86.6% increase over time (Figure 1c, Supplementary Table 1). For Asciminib, first US prescriptions were recorded in 11/2021 following the FDA approval for CML treatment in 10/2021 (22). In 2022, US average monthly prescriptions reached 246, which increased to 824 (+235.0%) for 2024.
Next, we analyzed prescription trends across TKI generations, as shown in Figure 1. Imatinib (Gleevec) was the only first-generation TKI, serving as the sole representative in the dataset. Second-generation TKIs saw a 14.4% increase, from 15,171 US monthly prescriptions in 2017 to 17,363 in 2024 (Supplementary Table 3). In contrast, third-generation TKIs had lower prescription volumes but experienced strong growth (+204.9%), rising from 696 US average monthly prescriptions in 2017 to 2,123 in 2024.
3.2 Prescription trends across specialties
In a next step, the drug choices across the top 3 prescribing specialties in the US, oncologists, APP and PCP/internists were analyzed (Figure 2). Across all TKI generations, oncologists were the top prescribing discipline accounting for 73.7% of all TKI prescriptions during the study period, followed by APP (17.5%) and PCPs/internists (8.1%) (Figure 2, Supplementary Table 4). Other medical disciplines showed a minimal role (< 1%).
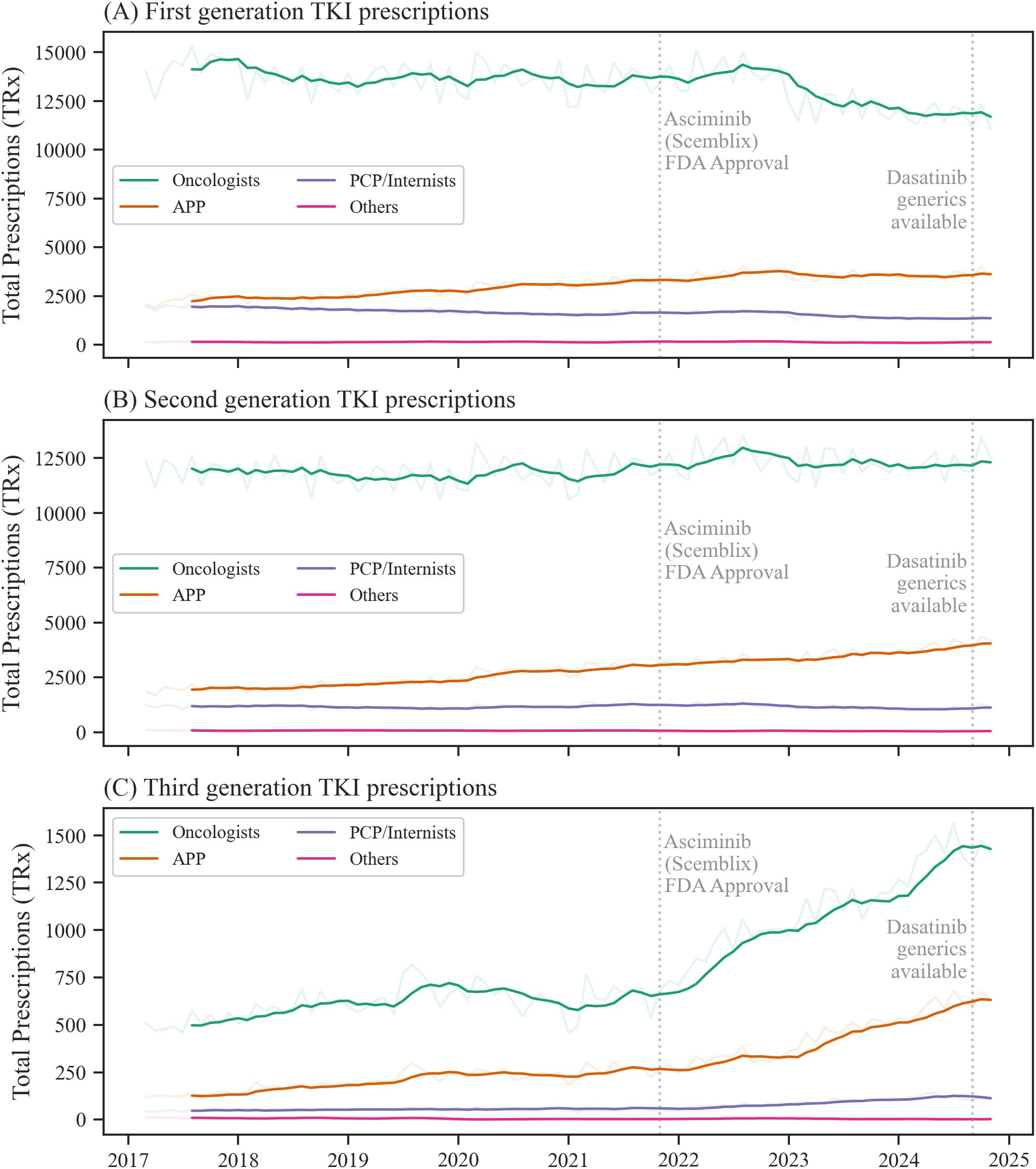
Figure 2. Prescription trends for tyrosine kinase inhibitor (TKI) generations across top prescribing specialties. Prescription data for first (A), second (B), and third (C) generation TKIs for each top prescribing discipline. The blurred-out curves show monthly prescription trends for each prescribing discipline, and the focused curves show the 6-month moving average. APP indicates advanced practice providers, PCP, primary care physicians. Source: IQVIA National Prescription Audit (March 2017-November 2024).
For imatinib/first-generation TKIs, oncologists’ average prescription rate in relation to total TKI prescriptions by specialty was 53.4% in 2017, which decreased to 46.3% in 2024 (Supplementary Figure 2a, Supplementary Table 4). Among APP, imatinib prescription rates decreased from 52.5% to 44.0% (Supplementary Figure 2b, Supplementary Table 4) during the same time period. For PCP/internists, the proportion of imatinib/first-generation TKI among all TKI was higher when compared to other specialties, accounting for 61.3% of TKIs in 2017 and decreased to 53.0% in 2024 (Supplementary Figure 2c, Supplementary Table 4).
Second-generation TKIs were used at a rate of 44.7% in 2017 by oncologists, compared to 48.3% in 2024. For APPs, second-generation TKIs represented 44.6% of all TKIs prescriptions in 2017 and reached 48.5% in 2024, while for PCPs/internists, their share increased from 37.2% to 42.4%.
With ponatinib as the only approved third-generation TKI from 2017 to 2020 and asciminib expanding the class after October 29, 2021, third-generation TKIs accounted for 1.9% of total TKI prescriptions in 2017 among oncologists, increasing to 5.5% in 2024. Third-generation TKIs were more frequently prescribed by APPs, with their prescription rate increasing from 2.9% to 7.4% of all TKIs prescribed by APPs. The prescription rate among PCPs/internists also increased from 1.5% to 4.6% during this period.
3.3 Online search trends for TKIs
Online search trends for TKIs were subsequently analyzed. Search interest for imatinib decreased by 22.2% during the study period to an average of 13.2 per 10 million searches in 2024 (Figure 3, Supplementary Table 5). Dasatinib was the second most searched TKI after imatinib, with search volumes rising by 21.6% to 8.7 per 10 million searches. In contrast, nilotinib search interest declined by 32.9% to 3.5 per 10 million searches, mirroring its decreasing prescription trends. Online searches for bosutinib, a second-generation TKI, demonstrated a relative increase of 42.0% resulting in 2.0 per 10 million searches in 2024. Similarly, search volumes for ponatinib also surged by 46.7% to 2.3 per 10 million searches. Following its 2021 approval, asciminib saw a significant surge in search interest, reaching an average of 2.5 per 10 million searches in 2024.

Figure 3. Online searches for tyrosine kinase inhibitors (TKIs). Online search volumes as searches per 10 million searches for aggregated brand and generic names of individual TKIs. The blurred-out curves show monthly online searches for each TKI, and the focused curves show the 6-month moving average. Source: Google Trends (March 2017-November 2024).
Aggregated online search data by TKI generation revealed comparable volumes for first-generation (imatinib) and second-generation TKIs in 2017, with 16.9 and 13.8 per 10 million searches, respectively (Supplementary Table 6). Notably, searches for second-generation TKIs surpassed those for first-generation TKIs in 2020, reaching 14.2 per 10 million in 2024, compared to 13.2 for imatinib/Gleevec. Like third-generation TKI prescription trends, search interest increased significantly, rising from 1.5 per 10 million in 2017 to 4.8 in 2024 - a 212.6% increase, aligning with the 204.9% rise in prescriptions.
3.4 Correlation of prescriptions and Google Trends
Ultimately, we analyzed correlation patterns between TKI prescriptions and corresponding online searches. As depicted in Figure 4, the correlation matrix shows a size and color-coded representation of the correlation coefficients between quarterly prescription rates and quarterly online searches. For imatinib/first-generation TKIs no correlation (r= 0.21 (95%-CI: -0.15 – 0.52), p = 0.25) between dispensed prescriptions and online searches was observed. For the second-generation TKIs, dasatinib showed a moderate correlation (r = 0.51 (95%-CI: 0.20 – 0.73), p < 0.1). Bosutinib showed a strong positive correlation of 0.74 (95%-CI: 0.53 – 0.86, p < 0.1). Nilotinib, a drug for which prescriptions and search interest decreased over time, showed a correlation coefficient of 0.74 (95%-CI: 0.53 – 0.86, p < 0.1). Asciminib exhibited the strongest correlation between prescription rates and Google trends data, with a correlation of 0.85 (95%-CI: 0.71 – 0.92, p < 0.1). Ponatinib showed a moderate correlation of 0.63 (95%-CI: 0.36 – 0.80, p < 0.1).
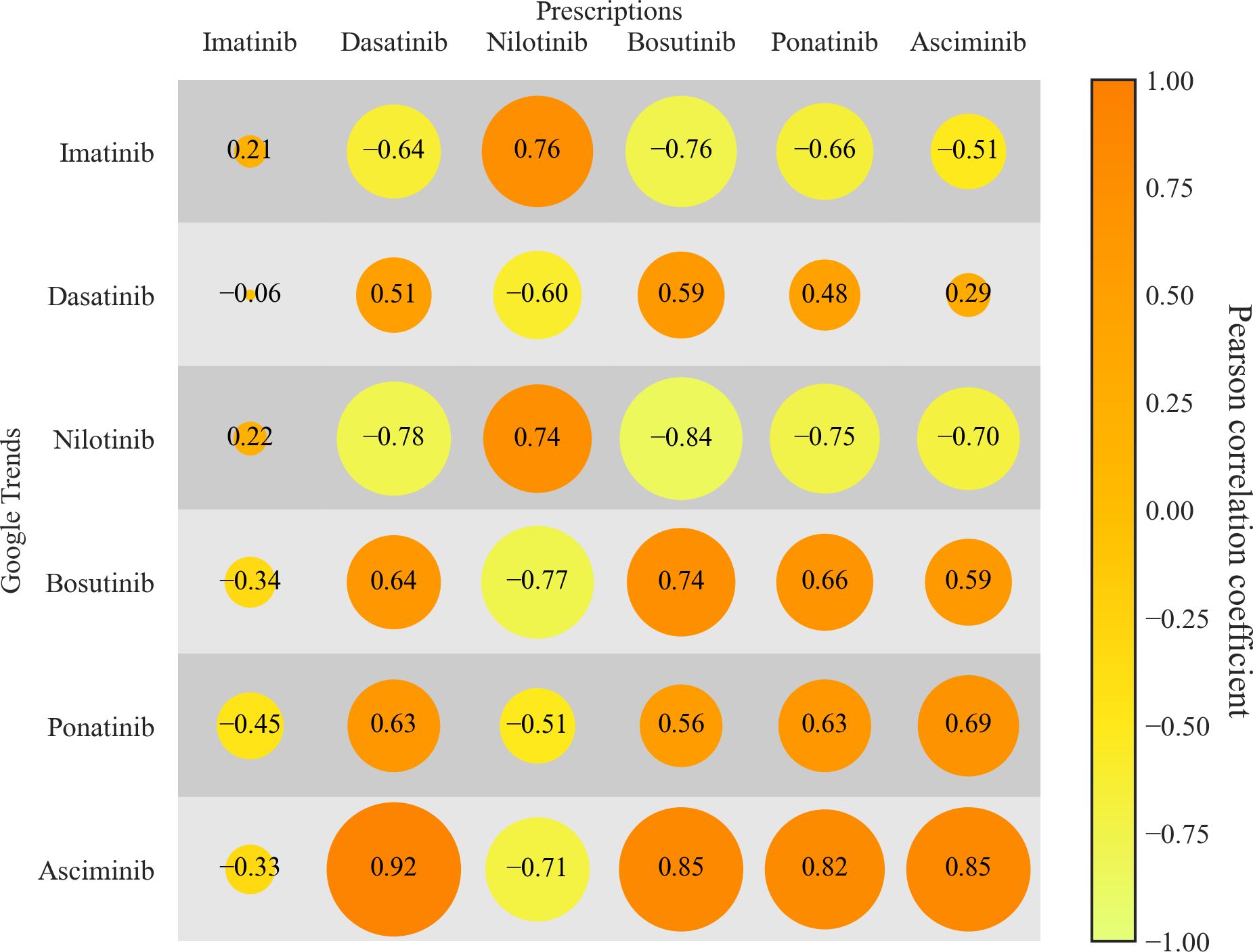
Figure 4. Correlations between TKI prescriptions and online searches. Correlation between TKI-specific prescription data and corresponding online search volumes, expressed as Pearson correlation coefficients (displayed as numbers within the bubbles). Data for prescriptions and online searches were aggregated by quarterly intervals. Orange bubbles represent positive correlations, whereas yellow/green bubbles represent negative correlations. Source: IQVIA National Prescription Audit (March 2017-November 2024).
4 Discussion
The present study provides a comprehensive analysis of US prescription trends and public awareness of TKIs commonly used for the treatment of CML between 2017 and 2024. Our study has four main findings. First, imatinib remained the most frequently prescribed TKI in the US among all other approved TKIs. Second-generation TKI usage has increased substantially, while third-generation TKIs, despite a rapid ongoing overall rise, proportionately remained at approximately 6% of all TKI prescriptions in 2024. Third, oncologists continued to be the main prescribers of TKIs used for CML treatment, while TKI prescriptions by APPs increased by 84% over time. Ultimately, public awareness as represented by online search volumes closely followed prescription trends for bosutinib, nilotinib, asciminib and ponatinib with highest correlations for asciminib.
To the best of our knowledge, this study is the first to comprehensively analyze US drug usage of TKIs, which are primarily approved for the treatment of CML. Consistent with previous reports highlighting the superior efficacy of second-generation TKIs in CML (23–25), imatinib may still be preferred due to its more favorable side effect profile (26). Additionally, as of September 2018 NCCN guidelines have recommended second-generation TKIs as first-line treatment for intermediate- or high-risk patients and younger patients (3, 27). This is in line with our findings, showing overall comparable usage patterns between imatinib/first-generation and second-generation TKIs. Nevertheless, given the higher increase in the proportion of second-generation TKI usage compared to imatinib/first-generation TKIs, this finding may be explained by the potentially higher efficacy of second-generation TKIs (23, 28, 29) as well as changes in guideline recommendations. The availability of generic imatinib helped reduce the cost of CML treatment (30), leading to a clear shift toward generic imatinib over Gleevec. Similarly, our data suggest a rapid transition from Sprycel prescriptions to generic dasatinib after September 2024 following its patent expiration. Thus, the introduction of second-generation generics might further facilitate the adoption of second-generation TKIs in first-line CML therapy. As of January 2024, the US patent for Tasigna has expired; however, no generic versions of nilotinib have been made available in the US at the time of writing. In August 2024, the European Medicines Agency (EMA) approved Nilotinib Accord as the first nilotinib generic (31), raising questions about potential US market entry.
Our analyses additionally revealed that non-physician professionals, such as APPs, including nurse practitioners and physician assistants, accounted for a large proportion of all US TKI prescriptions throughout the study period. Notably, there were no substantial differences in TKI drug (generation) usage between APPs and oncologists, the primary prescribing specialty. Previous studies have shown that APPs play an increasing role in prescribing, particularly in the management of oncology patients, where they are integral to patient care (32, 33). This has as well become evident in the management of patients chronic diseases (33), where APPs manage patient for counseling, drug prescriptions, treatment and follow-up visits (34). Our data further highlight the growing role of APPs in the management of CML patients.
Among APPs, third-generation TKIs were more frequently prescribed when compared to other disciplines, suggesting that APPs seemed to be involved in more complex treatment decisions for patients with relapsed or refractory CML. Overall, oncologists and APPs showed similar prescribing patterns, favoring second- and third-generation TKIs more often than internists, who tended to prescribe imatinib more frequently.
Our Google Trends search analysis revealed a strong correlation for asciminib, the most recently FDA-approved TKI for CML (approved October 29, 2021), consistent with prior studies linking increasing drug usage to higher online search interest (15, 16, 35). Notably, even drugs with declining prescriptions, such as nilotinib, showed strong correlations, suggesting Google Trends can also track downward trends for drugs in CML treatment. These findings highlight Google Trends as a potential near real-time tool to complement traditional prescription analyses. Google Trends can offer avenue for monitoring low-latency situational awareness: spikes in TKI can prompt targeted patient education/clinician outreach and pharmacy planning; regulators and health systems/manufacturers can anticipate short-term demand. Additionally, Google Trends data could further inform public health related research and may aid surveillance of guideline adherence.
Our study has limitations. First, the prescription data were not exclusive to CML patients, as these TKIs are also prescribed for conditions such as acute lymphoblastic leukemia (ALL) and gastrointestinal stromal tumors (GIST). Despite similar U.S. incidences for CML (~2.0/100,000) (36) and ALL (~1.9/100,000) (37), TKIs are used in nearly all CML cases, whereas in ALL they are largely confined to the Philadelphia chromosome–positive subset (38), ~20–30% of (adult) cases (39). GIST is less common (~0.7/100,000) (40), and TKIs are used primarily for unresectable/metastatic disease and as adjuvant therapy in high-risk resected tumors (41). As such, the presented data are expected to be driven mainly by CML, while smaller contributions from Ph-positive ALL and GIST cannot be entirely excluded.
Second, the prescription data from the National Prescription Audit is derived from a sample of outpatient pharmacies and extrapolated to estimate total prescriptions, which may introduce some margin of error. Online search interest may be driven by news coverage, regulatory announcements, guideline changes, and high-profile publications, creating spikes that are not directly tied to prescriptions activity. Such events can also precede or follow changes in prescribing with variable lags. Accordingly, the association between search activity and prescriptions should be interpreted as observational.
While we observed a clear increase in TKI prescriptions by APPs since 2017, we cannot determine whether this trend reflects a CML-specific shift or is part of a broader increase in non-physician prescribing. The physician assistant profession grew by 27.9% between 2019 and 2023 (42), suggesting that the increase in prescriptions may in part reflect general workforce expansion. Therefore, additional research into APP prescription behavior across other medical fields is warranted to better understand and determine whether the observed increase in TKI prescribing is specific to hematology or mirrors broader trends across all specialties. Prescription data analyzed in this study are representative of the US only and thus cannot be projected to other markets.
This study highlights the growing adoption of second- and third-generation TKIs in CML treatment, alongside the increasing role of non-physician professions such as advanced practice providers in prescribing decisions. Additionally, our findings reveal a strong link between TKI prescription trends and public awareness as represented by online searches, underscoring its potential as a real-time tool to for tracking the uptake and changes of approved TKI therapies used for CML treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
AS: Conceptualization, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. TJ: Writing – review & editing. AM: Writing – review & editing. FT: Writing – review & editing. JM: Writing – review & editing. GK: Writing – review & editing. AdS: Writing – review & editing. JB: Writing – review & editing. MC: Writing – review & editing. AS: Writing – review & editing. PC: Writing – review & editing. MB: Writing – review & editing. PB: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. OD: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. O.D. received support from National Institutes of Health grant T32 HL007227 and from the Dean’s Scholar in Clinical Research Program at the University of Texas Southwestern Medical Center. The other authors did not receive any financial assistance related to this work.
Acknowledgments
The statements, findings, conclusions, views and opinions contained and expressed in this article are based in part on data obtained under license from the following: IQVIA National Prescription Audit, all rights reserved. The statements, findings, conclusions, views and opinions contained and expressed herein are not necessarily those of IQVIA, Inc. or any of its affiliated or subsidiary entities.
Conflict of interest
MB: Grants: NIH, FDA, AHA, Novo Nordisk, Bayer; Advisory Board: Novo Nordisk, Eli Lilly, Bayer, Novartis, Boehringer Ingelheim, Astra Zeneca, Genentech, Idorsia, Agepha, Vectura, New Amsterdam; Speaker: Novo Nordisk; Regulatory: Former voting member of EMDAC for the FDA.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Reviewer EA declared a shared affiliation with the author PC at the time of review.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1711453/full#supplementary-material
References
1. Rinaldi I and Winston K. Chronic myeloid leukemia, from pathophysiology to treatment-free remission: A narrative literature review. J Blood Med. (2023) 14:261–77. doi: 10.2147/JBM.S382090
2. Chereda B and Melo JV. Natural course and biology of CML. Ann Hematol. (2015) 94 Suppl 2:S107–21. doi: 10.1007/s00277-015-2325-z
3. Shah NP, Bhatia R, Altman JK, Amaya M, Begna KH, Berman E, et al. Chronic myeloid leukemia, version 2.2024, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2024) 22:43–69. doi: 10.6004/jnccn.2024.0007
4. Hochhaus A, Breccia M, Saglio G, Garcia-Gutierrez V, Rea D, Janssen J, et al. Expert opinion-management of chronic myeloid leukemia after resistance to second-generation tyrosine kinase inhibitors. Leukemia. (2020) 34:1495–502. doi: 10.1038/s41375-020-0842-9
5. Kantarjian H, O’Brien S, Jabbour E, Garcia-Manero G, Quintas-Cardama A, Shan J, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. (2012) 119:1981–7. doi: 10.1182/blood-2011-08-358135
6. Hughes TP and Shanmuganathan N. Management of TKI-resistant chronic phase CML. Hematol Am Soc Hematol Educ Program. (2022) 2022:129–37. doi: 10.1182/hematology.2022000328
7. Okabe S, Tauchi T, Tanaka Y, and Ohyashiki K. Efficacy of ponatinib against ABL tyrosine kinase inhibitor-resistant leukemia cells. Biochem Biophys Res Commun. (2013) 435:506–11. doi: 10.1016/j.bbrc.2013.05.022
8. Hughes TP, Mauro MJ, Cortes JE, Minami H, Rea D, DeAngelo DJ, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. (2019) 381:2315–26. doi: 10.1056/NEJMoa1902328
9. Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. (2018) 132:393–404. doi: 10.1182/blood-2016-09-739086
10. Alves R, Goncalves AC, Rutella S, Almeida AM, De Las Rivas J, Trougakos IP, et al. Resistance to tyrosine kinase inhibitors in chronic myeloid leukemia-from molecular mechanisms to clinical relevance. Cancers (Basel). (2021) 13:22. doi: 10.3390/cancers13194820
11. Arora VS, McKee M, and Stuckler D. Google Trends: Opportunities and limitations in health and health policy research. Health Policy. (2019) 123:338–41. doi: 10.1016/j.healthpol.2019.01.001
12. Mavragani A and Ochoa G. Google trends in infodemiology and infoveillance: methodology framework. JMIR Public Health Surveill. (2019) 5:e13439. doi: 10.2196/13439
13. Saegner T and Austys D. Forecasting and surveillance of COVID-19 spread using google trends: literature review. Int J Environ Res Public Health. (2022) 19:3–10. doi: 10.3390/ijerph191912394
14. Yang S, Santillana M, and Kou SC. Accurate estimation of influenza epidemics using Google search data via ARGO. Proc Natl Acad Sci U S A. (2015) 112:14473–8. doi: 10.1073/pnas.1515373112
15. Berning P, Schroer AE, Adhikari R, Razavi AC, Cornelis FH, Erinjeri JP, et al. Online searches for hepatocellular carcinoma drugs mirror prescription trends across specialties and changes in guideline recommendations. Front Oncol. (2024) 14:1324095. doi: 10.3389/fonc.2024.1324095
16. Dzaye O, Berning P, Razavi AC, Adhikari R, Jha K, Nasir K, et al. Online searches for SGLT-2 inhibitors and GLP-1 receptor agonists correlate with prescription rates in the United States: An infodemiological study. Front Cardiovasc Med. (2022) 9:936651. doi: 10.3389/fcvm.2022.936651
17. Senecal C, Gulati R, and Lerman A. Google trends insights into reduced acute coronary syndrome admissions during the COVID-19 pandemic: infodemiology study. JMIR Cardio. (2020) 4:e20426. doi: 10.2196/20426
18. Senecal C, Mahowald M, Lerman L, Lopes-Jimenez F, and Lerman A. Increasing utility of Google Trends in monitoring cardiovascular disease. Digit Health. (2021) 7:20552076211033420. doi: 10.1177/20552076211033420
19. Turner LW, Nartey D, Stafford RS, Singh S, and Alexander GC. Ambulatory treatment of type 2 diabetes in the U.S., 1997-2012. Diabetes Care. (2014) 37:985–92. doi: 10.2337/dc13-2097
20. Adhikari R, Jha K, Dardari Z, Heyward J, Blumenthal RS, Eckel RH, et al. National trends in use of sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists by cardiologists and other specialties, 2015 to 2020. J Am Heart Assoc. (2022) 11:e023811. doi: 10.1161/JAHA.121.023811
22. FDA approves asciminib for Philadelphia chromosome-positive chronic myeloid leukemia . Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-asciminib-philadelphia-chromosome-positive-chronic-myeloid-leukemia (Accessed October 30, 2025).
23. Brummendorf TH, Cortes JE, Milojkovic D, Gambacorti-Passerini C, Clark RE, le Coutre P, et al. Bosutinib versus imatinib for newly diagnosed chronic phase chronic myeloid leukemia: final results from the BFORE trial. Leukemia. (2022) 36:1825–33. doi: 10.1038/s41375-022-01589-y
24. Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boque C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J Clin Oncol. (2016) 34:2333–40. doi: 10.1200/JCO.2015.64.8899
25. Kantarjian HM, Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre P, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. (2021) 35:440–53. doi: 10.1038/s41375-020-01111-2
26. Vener C, Banzi R, Ambrogi F, Ferrero A, Saglio G, Pravettoni G, et al. First-line imatinib vs second- and third-generation TKIs for chronic-phase CML: a systematic review and meta-analysis. Blood Adv. (2020) 4:2723–35. doi: 10.1182/bloodadvances.2019001329
27. Radich JP, Deininger M, Abboud CN, Altman JK, Berman E, Bhatia R, et al. Chronic myeloid leukemia, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2018) 16:1108–35. doi: 10.6004/jnccn.2018.0071
28. Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. (2010) 362:2260–70. doi: 10.1056/NEJMoa1002315
29. Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. (2010) 362:2251–9. doi: 10.1056/NEJMoa0912614
30. Gorkin L and Kantarjian H. Targeted therapy: Generic imatinib - impact on frontline and salvage therapy for CML. Nat Rev Clin Oncol. (2016) 13:270–2. doi: 10.1038/nrclinonc.2016.59
31. Nilotinib accord: European medicines agency (2024). Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/nilotinib-accord (Accessed October 30, 2025).
32. Reynolds RB and McCoy K. The role of Advanced Practice Providers in interdisciplinary oncology care in the United States. Chin Clin Oncol. (2016) 5:44. doi: 10.21037/cco.2016.05.01
33. Ross AC, Polansky MN, Parker PA, and Palmer JL. Understanding the role of physician assistants in oncology. J Oncol Pract. (2010) 6:26–30. doi: 10.1200/JOP.091062
34. Bruinooge SS, Pickard TA, Vogel W, Hanley A, Schenkel C, Garrett-Mayer E, et al. Understanding the role of advanced practice providers in oncology in the United States. J Adv Pract Oncol. (2018) 9:585–98. doi: 10.1188/18.ONF.786-800
35. Berning P, Adhikari R, Schroer AE, Jelwan YA, Razavi AC, Blaha MJ, et al. Longitudinal analysis of obesity drug use and public awareness. JAMA Netw Open. (2025) 8:e2457232. doi: 10.1001/jamanetworkopen.2024.57232
36. Cancer stat facts: leukemia — Chronic myeloid leukemia (CML) . Available online at: https://seer.cancer.gov/statfacts/html/cmyl.html (Accessed October 4, 2025).
37. Cancer stat facts: leukemia — Acute lymphocytic leukemia (ALL) . Available online at: https://seer.cancer.gov/statfacts/html/alyl.html (Accessed October 4, 2025).
38. Hoelzer D, Bassan R, Boissel N, Roddie C, Ribera JM, Jerkeman M, et al. ESMO Clinical Practice Guideline interim update on the use of targeted therapy in acute lymphoblastic leukaemia. Ann Oncol. (2024) 35:15–28. doi: 10.1016/j.annonc.2023.09.3112
39. Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. (2007) 109:3189–97. doi: 10.1182/blood-2006-10-051912
40. Patel N and Benipal B. Incidence of gastrointestinal stromal tumors in the United States from 2001-2015: A United States cancer statistics analysis of 50 states. Cureus. (2019) 11:e4120. doi: 10.7759/cureus.4120
41. von Mehren M, Kane JM, Riedel RF, Sicklick JK, Pollack SM, Agulnik M, et al. NCCN guidelines(R) insights: gastrointestinal stromal tumors, version 2.2022. J Natl Compr Canc Netw. (2022) 20:1204–14. doi: 10.6004/jnccn.2022.0058
42. NCCPA 2023 statistical profile of board certified PAs 2024 [03.21.2025] . Available online at: https://www.nccpa.net/wp-content/uploads/2024/05/2023-Statistical-Profile-of-Board-Certified-PAs5_3_24.pdf (Accessed October 30, 2025).
Keywords: tyrosine kinase inhibitor usage, CML treatment, public awareness, infodemiology, Google Trends
Citation: Schroer AE, Javadi T, Mehta A, Torlak F, McFarland III JR, Kumar G, da Silva AL, Benjamin J, Choudhary MM, Sadeghi A, le Coutre P, Blaha MJ, Berning P and Dzaye O (2025) Longitudinal analysis of usage and public awareness of tyrosine kinase inhibitors for CML. Front. Oncol. 15:1711453. doi: 10.3389/fonc.2025.1711453
Received: 23 September 2025; Accepted: 24 October 2025;
Published: 17 November 2025.
Edited by:
Simona Bernardi, University of Brescia, ItalyReviewed by:
Elisabetta Abruzzese, University of Rome Tor Vergata, ItalyMario Tiribelli, University of Udine, Italy
Copyright © 2025 Schroer, Javadi, Mehta, Torlak, McFarland, Kumar, da Silva, Benjamin, Choudhary, Sadeghi, le Coutre, Blaha, Berning and Dzaye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Omar Dzaye, b21hci5kemF5ZUB1dHNvdXRod2VzdGVybi5lZHU=
†These authors share senior authorship
 Adrian E. Schroer
Adrian E. Schroer Tiffany Javadi4
Tiffany Javadi4 Jamaal Benjamin
Jamaal Benjamin Philipp le Coutre
Philipp le Coutre Philipp Berning
Philipp Berning Omar Dzaye
Omar Dzaye