- 1Department of Medicine, Surgery and Dentistry “Scuola Medica Salernitana”, University of Salerno, Baronissi, Italy
- 2FIBROSYS srl, University of Salerno, Baronissi, Italy
- 3Center for Advanced Studies and Technology (CAST), “G. d’Annunzio” University of Chieti-Pescara, Chieti, Italy
- 4Department of Innovative Technologies in Medicine and Dentistry, “G. d’Annunzio” University of Chieti-Pescara, Chieti, Italy
- 5Department of Human, Philosophical and Educational Sciences (DISUFF), University of Salerno, Fisciano, Italy
Previous studies identified BAG3 as a stress-induced protein with pro-survival functions in various tumors. Based on this assumption, we analyzed the expression and secretion of BAG3 in 24 cancer cell lines representing ten types of cancer and compared these results with samples from primary tumors. BAG3 was ubiquitously expressed and secreted by all cell lines. Serum levels of BAG3 were significantly elevated in patients with liver, pancreatic, and ovarian cancers versus healthy controls. Immunohistochemical analysis confirmed widespread high BAG3 expression across multiple tumor types, often correlating with tumor grade. These data support BAG3 as a key regulator of tumor survival and a promising biomarker and therapeutic target.
Introduction
Bcl-2-associated athanogene 3 (BAG3) (1) is a multifunctional protein whose expression is induced by stressful stimuli, mainly through the activation of Heat Shock Factor (HSF) 1 (2), while is constitutive in human muscle cells, including cardiomyocytes (3–6), in brain and peripheral nervous system cells (6) and in some primary tumors (7–20). BAG3 interacts with the heat shock protein (Hsp)70 through its BAG domain, and with other partners through its WW domain, proline-rich (PXXP) repeat and IPV (Ile-Pro-Val) motifs, thereby regulating various intracellular pathways, including autophagy and mitophagy, apoptosis, mechanotransduction, excitation-contracting coupling, mitochondrial functions, cytoskeleton organization and motility, inflammasome modulation and structural stabilization of the sarcomere (6, 21–28). Stressful stimuli can induce the release of BAG3 through unconventional secretory pathways in certain cell types, such as cardiomyocytes and fibroblasts. BAG3 has indeed been found in the blood of patients with various cardiac or fibrotic diseases, including heart failure (29–32) and systemic sclerosis (33–35). Furthermore, BAG3 is detectable in the blood of patients affected by pancreatic adenocarcinoma (12, 15, 36).
The pro-survival activity of BAG3 (7, 8, 22–24) suggests that constitutive expression of this protein is a common characteristic of neoplastic cells, a hypothesis supported by analyses of multiple primary tumor types (7–20). In this study, BAG3 expression data integrated from numerous prior publications were reassessed, underscoring its widespread and robust association with tumor biology. Furthermore, the present work demonstrates that BAG3 is actively secreted by diverse human cancer cell lines, thereby extending previous knowledge that primarily focused on intracellular expression. Importantly, elevated serum levels of BAG3 were detected in patients with liver, pancreatic, and ovarian carcinomas compared to healthy controls, substantiating BAG3’s potential as a circulating biomarker reflective of tumor burden. These findings collectively reinforce the utility of BAG3 not only as a tissue-level marker but also as a secreted protein detectable in blood, opening new avenues for non-invasive cancer diagnostics and targeted therapeutic strategies.
Methods
Cell cultures
The human pancreatic cancer cell line HPAAPC (Cytion, Freiburg, Germany) was grown in a 1:1 mix of DMEM and Ham’s F12 medium with 5% FBS, 1% penicillin/streptomycin (P/S), 0.5 mM sodium pyruvate, 0.002 mg/mL insulin, 0.005 mg/mL transferrin, 40 ng/mL hydrocortisone, and 10 ng/mL mouse epidermal growth factor. The anaplastic thyroid carcinoma cell line 8505C (ECACC, Salisbury, UK) was cultured in EMEM with 10% FBS, 2 mM glutamine, and 1% non-essential amino acids (NEAA). Fibrosarcoma HT-1080 cells (ATCC) were grown in EMEM with 10% FBS. Liver cancer lines SK-Hep-1 and HepG2 (ATCC) were cultured in EMEM with 10% FBS and 1% P/S, while SNU-475, SNU-423, and SNU-387 (ATCC) were grown in RPMI-1640 with 10% FBS and 1% P/S. The gastric adenocarcinoma MKN-45 line (DSMZ, Germany) was cultured in RPMI-1640 with 20% FBS and 1% P/S. Head and neck cancer cell lines A-253, Detroit 562, SCC-9, and FaDu (ATCC) were cultured in different media: A-253 in McCoy’s 5A with 10% FBS and 1% P/S; Detroit 562 and FaDu in EMEM with 10% FBS and 1% P/S; SCC-9 in 1:1 DMEM and Ham’s F12 with 10% FBS, 1% P/S, 0.5 mM sodium pyruvate, and 400 ng/mL hydrocortisone. Melanoma lines A375, SK-Mel-24, SK-Mel-28, C8161, and UACC25 (ATCC) were cultured as follows: SK-Mel-24 and SK-Mel-28 in EMEM with 15% FBS and 1% P/S; C8161 in 1:1 DMEM and Ham’s F12 with 10% FBS; UACC25 in RPMI-1640 with 10% FBS and 1% P/S. Ovarian cancer lines PEA-1 and PEA-2 (ECACC) were grown in RPMI-1640 with 10% FBS, 2 mM glutamine, 2 mM sodium pyruvate, and 1% P/S. Breast cancer lines MCF-7 and MDA-MB-231 (ATCC) were cultured with MCF-7 in EMEM plus 10% FBS, 2 mM glutamine, 1% NEAA, and 1% P/S, and MDA-MB-231 in DMEM with 10% FBS and 1% P/S. All cell lines were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Isolation of cell culture supernatants
Tumor cells were plated in complete medium at a density of 1x106 cells/ml. The day after, were washed twice with 1X PBS and incubated for 16 hours in serum-free DMEM. The conditioned medium was collected and subjected to sequential centrifugation steps at 4°C to eliminate dead cells and cellular debris. The cleared supernatant was precipitated overnight at -20°C using cold acetone using a volume ratio ratio 1:3. After incubation, the samples were centrifuged again at 10, 000 x g for 30 minutes, and the supernatant was discarded. The protein pellet was then analyzed by Western Blot.
Western blot
Intracellular proteins were obtained by using the TNT buffer (20mM HEPES (pH 7.5), 150mM NaCl, 0.1% Triton) containing a protease inhibitor cocktail (Sigma‐Aldrich), and subjected to 3 cycles of freezing/thawing. Lysates were then centrifuged for 20 min at 15, 000g, and the cleared supernatants were stored at -80°C. Protein concentration was determined by Bradford assay (Bio‐Rad), and 20 μg of total protein were separated on 10% SDS‐PAGE gels and electrophoretically transferred onto a nitrocellulose membrane. Nitrocellulose blots were blocked with 10% nonfat dry milk in TBST buffer (20mM Tris‐HCl at pH 7.4, 500mM NaCl, and 0.01% Tween), and incubated with primary antibodies in TBST containing 5% nonfat dry milk overnight at 4°C. An anti-BAG3 polyclonal antibody obtained by immunizing rabbits with the full- lenght recombinant BAG3 protein, anti-GAPDH monoclonal antibody (sc-32, 233, Santa Cruz Biotechnology), anti-Calregulin polyclonal antibody (sc-11398, Santa Cruz Biotechnology), anti-beta actin monoclonal antibody (sc-47778, Santa Cruz Biotechnology), anti-beta Tubulin monoclonal antibody (sc-166729, Santa Cruz Biotechnology), and anti-TRAP-1 monoclonal antibody (sc-73604, Santa Cruz Biotechnology), were used at a 1:5000 dilution. Immunoreactivity was detected using an ImageQuant ™ LAS 4000 (GE Healthcare).
Serum samples
Aliquots of serum samples were purchased at BIOIVT (West Sussex, United Kingdom) or at ReproCELL USA, Inc. (Maryland, USA) and stored at -80°C. The data on sera from healthy subjects analyzed in this study have been previously published (34).
BAG3 determination by the ELISA test
The BAG3 protein content in serum was measured using an enzyme-linked immunosorbent assay (ELISA). 96-well microplates (MediSorp™, cat. no. 467320, Thermo Scientific, Waltham, MA, USA) were coated with a proprietary monoclonal anti-BAG3 coating mAb and then blocked for non-specific binding sites. BAG3 standard protein or serum samples were then added to the wells. BAG3 content in sera samples was then determined using a second recombinant anti-BAG3 HRP-conjugated antibody as previously described (32).
Statistical analysis
Results were analyzed by GraphPad Prism software version 8.0.1 (Boston, MA, USA) and G*power software version 3.1.9.4. For variables non-normally distributed, p-values were assessed by a non-parametric Mann-Whitney U test to compare BAG3 serum level in individual patient populations with different carcinomas to healthy subjects. The statistical power analysis was conducted assuming these input parameters: statistical test: Mann-Whitney test; tail(s): one; parent distribution: min ARE; alpha error prob.: 0.05; effect size: calculated from the mean and standard deviation values obtained from the ELISA assay.
Results
BAG3 presence in the media from tumor cells and in patients’ serum
The results shown in Figure 1A demonstrate that the BAG3 protein was found in both cell extracts (IN) and culture supernatants (OUT) from a variety of human cancer cell lines, indicating active secretion into the extracellular environment. In particular, Western blot analyses revealed that pancreatic cancer cell lines displayed BAG3 signals in both cell lysates and supernatants, confirming previous findings that indicated BAG3 secretion by pancreatic ductal adenocarcinoma cells (37). Other tumor cell lines, including anaplastic thyroid carcinoma (8505C), fibrosarcoma (HT-1080), hepatocellular carcinoma (SK-Hep-1, SNU-475, SNU-423, SNU-387, HepG2), gastric adenocarcinoma (MKN-45), head and neck cancer (A-253, Detroit 562, SCC-9, FaDu), melanoma (A375, SK-Mel-24, SK-Mel-28, C8161, UACC257), ovarian cancer (PEA-1, PEA-2), small cell lung cancer (NCI-H69, NCI-H446), and breast cancer (MCF-7, MDA-MB-231), also displayed intracellular BAG3 expression and BAG3 release, although secretion levels varied among different lines.
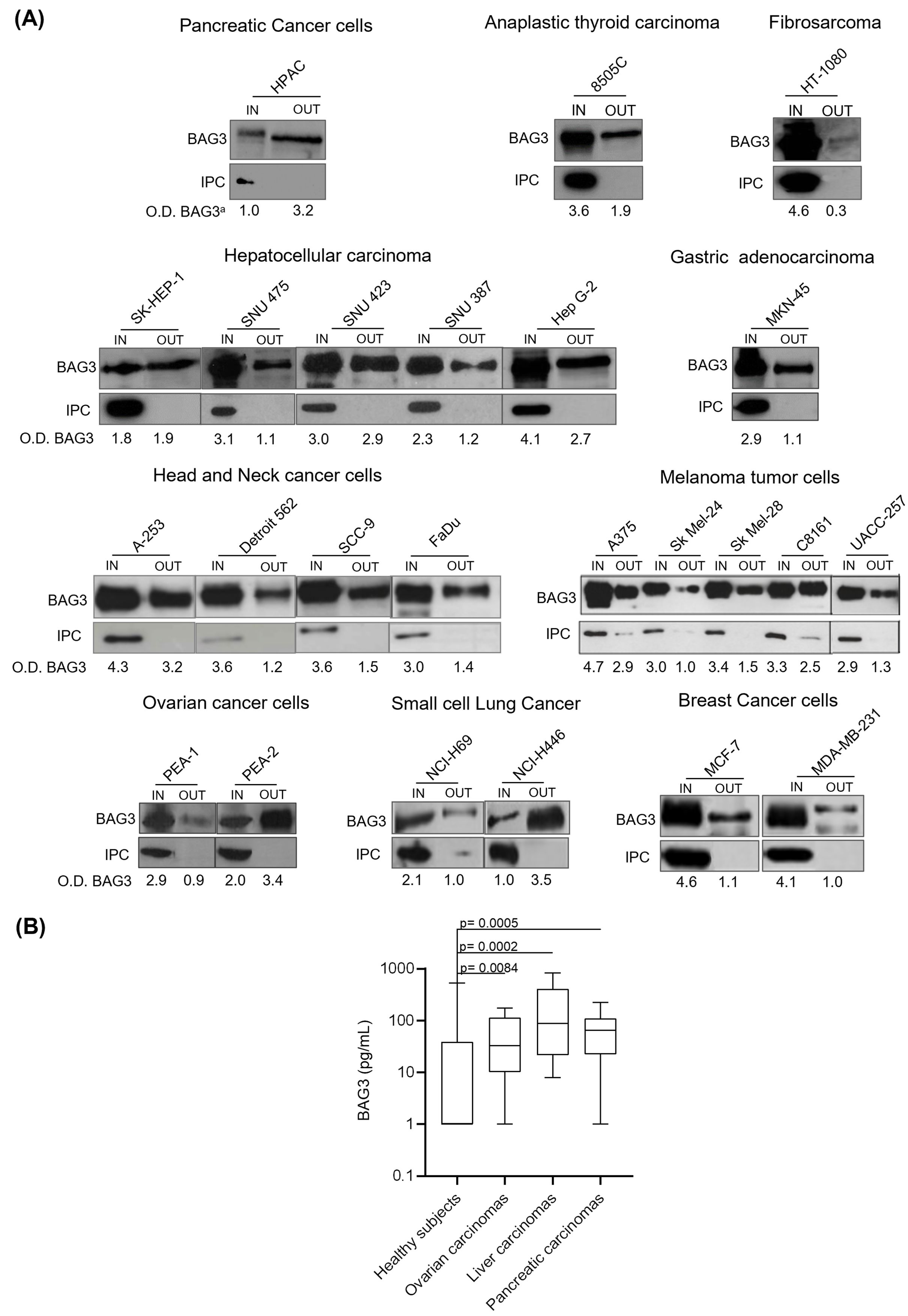
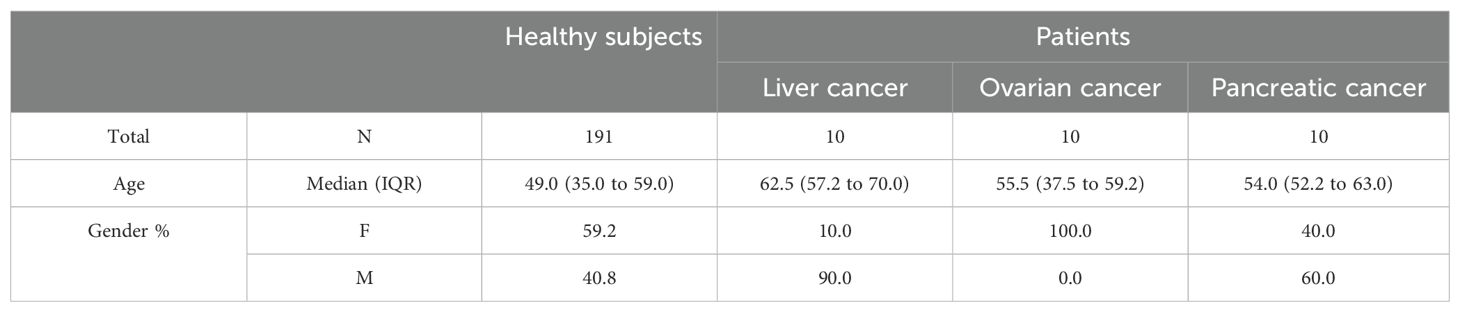
Figure 1. Detection of BAG3 in conditioned media from different cell types and in serum samples from patients with oncological diseases. (A) Human tumor cells were seeded at a density of 1x106 cells/mL. Twenty-four hours later, the cells were incubated for 16 hours in serum-free DMEM at 37°C in a 5% CO2 atmosphere. Total protein extracts from the cells (IN) and proteins from supernatants (OUT) were analyzed by Western blotting using a proprietary anti-BAG3 polyclonal antibody. Antibodies against GAPDH, β-actin, β-tubulin, calregulin, and TRAP1 were used as intracellular protein controls (IPC). O.D. BAG3a: optical density of BAG3 protein normalized to cell number. (B) Serum from patients with ovarian carcinoma (N=10), pancreatic carcinoma (N=10), and liver carcinoma (N=10) was tested for BAG3 protein levels through an ELISA assay. The graph shows the BAG3 values of patients’ sera compared to healthy subjects (N=191).
Additionally, we measured BAG3 protein levels in serum samples from patients with various carcinomas (Figure 1B). The median BAG3 concentrations in these patients were higher than in healthy subjects. Specifically, median values were 88 pg/ml in liver carcinoma patients (p value vs healthy subjects = 0.0002; power (1-𝛽) = 0.86), 65 pg/ml in pancreatic carcinoma patients (p value vs healthy subjects = 0.0005; power (1-𝛽) = 0.65), and 33 pg/ml in ovarian carcinoma patients (p value vs healthy subjects = 0.0084; power (1-𝛽) = 0.35), while the median level in healthy subjects was under 15 pg/ml, which corresponds to the assay’s lower limit of detection. No significant association was observed between BAG3 levels and patients’ age or sex within the studied populations. These baseline characteristics data are summarized in Table 1.
High BAG3 expression across a spectrum of human cancers
BAG3 protein expression was previously analyzed in our lab in various human tumors, showing high positivity rates consistent with its role as an anti-apoptotic factor. As summarized in Figure 2 and in Supplementary Table 1, high levels of cytoplasmic BAG3 positivity were detected in various malignancies, often exceeding 90%. Specifically, endometrial tumors, PDAC (pancreatic ductal adenocarcinoma), and prostate carcinomas all showed positivity in 100% of the 515 cases analyzed. Equally high rates were observed in thyroid tumors (96% of 56 cases) and brain tumors (91% of 151 cases). While thyroid tumors showed consistently high BAG3 expression in all subtypes (papillary 96%, follicular 93%, and anaplastic 100%), expression in brain tumors varied by grade, with grade I glial tumors showing 77%, while grade II and III astrocytomas and glioblastoma multiforme all showed values above 89%. In addition, lung tumors (79% of 66 cases) showed 100% positivity in squamous cell carcinoma, adenocarcinoma, and large cell carcinoma, dropping to 61% in SCLC (Small Cell Lung Cancer). Head and neck squamous cell carcinoma (HNSCC) also showed high rates (86% overall, with oral cavity 80%, oropharynx 88%, and larynx 89%). The lowest overall positivity rate was found in melanomas (65% of 165 cases), where positivity was high in cutaneous melanomas (70%) and melanomas at other sites (67%), but significantly lower in ocular melanomas (23%). These results, derived from our previous publications (11, 13, 15, 17, 20, 38–42), underscore the widespread and elevated expression of the anti-apoptotic BAG3 protein in diverse cancers.
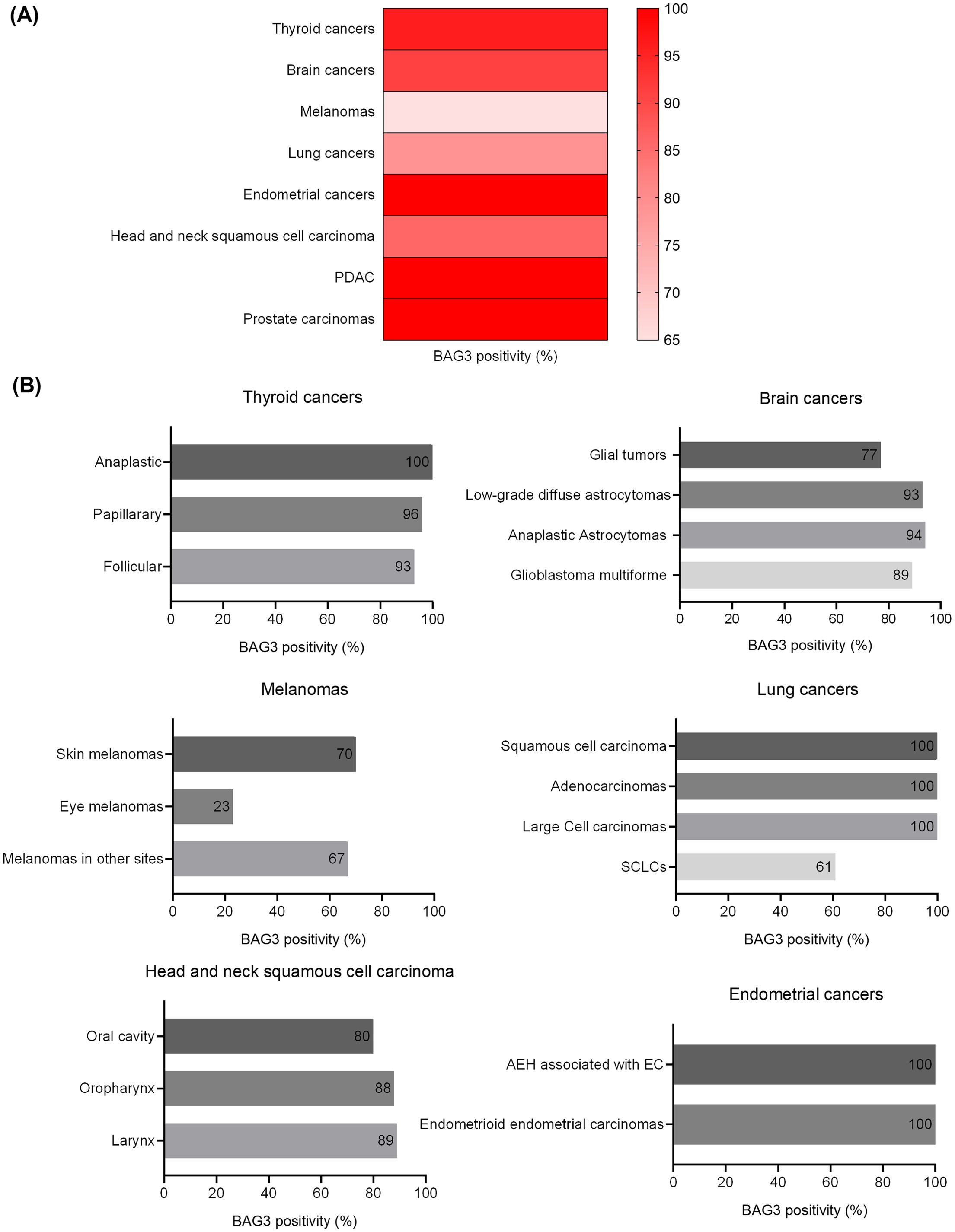
Figure 2. BAG3 positivity rates across human cancers and specific subtypes. The figure illustrates the percentage of BAG3-positive cases identified across a panel of human malignancies. The data show the overall positivity rate for each primary cancer type (A) and the specific rates for their respective histological subtypes (B).
Discussion
Our data reveal that BAG3 is both expressed and secreted by 24 tumor cell lines from diverse origins, highlighting the need for further investigation into its role in tumor growth in vitro. These findings confirm that BAG3’s function goes beyond its well-known intracellular activities to include secretion by cancer cells, which aligns with its involvement in regulating survival, proliferation, and intercellular signaling within the tumor microenvironment. Moreover, elevated serum BAG3 levels in carcinoma patients compared to healthy controls further support its potential as a biomarker for disease presence and activity.
This study reinforces and broadens existing evidence that the anti-apoptotic protein BAG3 exhibits high expression across a broad range of human malignancies, frequently exceeding 90% positivity. Specific tumor types such as endometrial, pancreatic ductal adenocarcinoma, and prostate cancers show near-universal BAG3 expression. Significant expression is also observed in certain thyroid, brain, lung, and head and neck cancer subtypes, accompanied by notable intertumoral heterogeneity, especially in melanomas and small cell lung cancers. These data substantiate BAG3’s function as a pivotal survival factor in tumor biology. Notably, aggressive tumor subtypes, including anaplastic thyroid carcinoma and glioblastoma—which are characterized by therapy resistance and poor prognosis—demonstrate elevated BAG3 positivity, suggesting BAG3-driven mechanisms contribute to their aggressive phenotype. Lower BAG3 levels in ocular melanomas and small cell lung cancer may indicate distinct cellular origins or regulatory processes. While BAG3 positivity correlates with tumor grade in some contexts, BAG3-negative tumors likely depend on alternative molecular alterations or signaling inputs from the microenvironment to maintain viability.
Nevertheless, the widespread secretion of BAG3 by tumors emphasizes its critical role in shaping the tumor microenvironment. Indeed, extracellular BAG3, through interaction with the IFITM2 receptor on macrophages and fibroblasts, fosters a pro-tumorigenic milieu that supports tumor growth, invasion, and immune evasion (37, 43, 44). Therapeutically, targeting extracellular BAG3 with monoclonal neutralizing antibodies in murine pancreatic adenocarcinoma models reduced fibrosis and macrophage infiltration, resulting in inhibited tumor progression (37, 45). Moreover, combining extracellular BAG3 blockade with immune checkpoint inhibitors (such as anti-SIRP-α or anti-PD-1 antibodies) (46, 47) yields synergistic enhancement of anti-tumor immune responses beyond immune checkpoint inhibition alone. These findings highlight BAG3’s potential as both a biomarker of tumor aggressiveness and a promising therapeutic target to disrupt malignant cell-microenvironment crosstalk.
The limited sample sizes for each tumor type, especially for ovarian carcinoma, where the statistical power was low, restrict the strength of our conclusions regarding serum BAG3 levels. Notably, ovarian carcinoma cells, particularly the chemoresistant line (PEA-2), display a distinct pattern of BAG3 secretion compared to other cancers. While our current study did not explicitly investigate the influence of metabolic changes on BAG3 secretion, the established reliance of ovarian cancer on oxidative metabolism suggests a potential metabolic link that warrants further exploration. Additionally, the cross-sectional nature of the serum analysis and the proximity of healthy subject BAG3 levels to the assay’s detection limit suggest the need for larger, longitudinal studies to validate BAG3’s utility as a reliable circulating biomarker in oncology.
Collectively, BAG3 expression and secretion are characteristic of many neoplasms, and understanding their precise functional roles in diverse tumor types can provide insights into tumor survival mechanisms and inform the development of innovative therapeutic strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
PM: Formal analysis, Investigation, Visualization, Writing – original draft. AC: Investigation, Visualization, Writing – original draft. JD: Investigation, Writing – original draft. AF: Formal analysis, Writing – review & editing. AR: Data curation, Writing – original draft. LM: Data curation, Writing – original draft. MT: Supervision, Writing – review & editing. VD: Supervision, Writing – original draft. GS: Investigation, Writing – original draft. MDM: Conceptualization, Data curation, Formal analysis, Writing – review & editing. AB: Conceptualization, Data curation, Formal analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. We used QuillBot to check the grammar.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1725674/full#supplementary-material
References
1. Takayama S, Xie Z, and Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. (1999) 274:781–6. doi: 10.1074/jbc.274.2.781
2. Franceschelli S, Rosati A, Lerose R, De Nicola S, Turco MC, and Pascale M. Bag3 gene expression is regulated by heat shock factor 1. J Cell Physiol. (2008) 215:575–7. doi: 10.1002/jcp.21397
3. Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, and Takayama S. BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol. (2006) 169:761–73. doi: 10.2353/ajpath.2006.060250
4. De Marco M, Turco MC, and Rosati A. BAG3 protein is induced during cardiomyoblast differentiation and modulates myogenin expression. Cell Cycle. (2011) 10:850–2. doi: 10.2353/ajpath.2006.060250
5. d’Avenia M, Citro R, De Marco M, Veronese A, Rosati A, Visone R, et al. A novel miR-371a-5p-mediated pathway, leading to BAG3 upregulation in cardiomyocytes in response to epinephrine, is lost in Takotsubo cardiomyopathy. Cell Death Dis. (2015) 6:e1948. doi: 10.1038/cddis.2015.280
6. Brenner CM, Choudhary M, McCormick MG, Cheung D, Landesberg GP, Wang JF, et al. BAG3: nature’s quintessential multi-functional protein functions as a ubiquitous intra-cellular glue. Cells. (2023) 12:937. doi: 10.3390/cells12060937
7. Romano MF, Festa M, Pagliuca G, Lerose R, Bisogni R, Chiaruzzi F, et al. BAG3 protein controls B-chronic lymphocytic leukaemia cell apoptosis. Cell Death Differ. (2003) 10:383–5. doi: 10.1038/sj.cdd.4401167
8. Romano MF, Festa M, Petrella A, Rosati A, Pascale M, Bisogno R, et al. BAG3 protein regulates cell survival in childhood acute lymphoblastic leukemia cells. Cancer Biol Ther. (2003) 2:508–10. doi: 10.4161/cbt.2.5.524
9. Bonelli P, Petrella A, Rosati A, Romano MF, Lerose R, Pagliuca MG, et al. BAG3 protein regulates stress-induced apoptosis in normal and neoplastic leukocytes. Leukemia. (2004) 18:358–60. doi: 10.1038/sj.leu.2403219
10. Ammirante M, De Laurenzi V, Graziano V, Turco MC, and Rosati A. BAG3 is required for IKKα nuclear translocation and emergence of castration resistant prostate cancer. Cell Death Dis. (2011) 2:e139. doi: 10.1038/cddis.2011.23
11. Festa M, Del Valle L, Khalili K, Franco R, Scognamiglio G, Graziano V, et al. BAG3 protein is overexpressed in human glioblastoma and is a potential target for therapy. Am J Pathol. (2011) 178:2504–12. doi: 10.1016/j.ajpath.2011.02.002
12. Falco A, Rosati A, Festa M, Basile A, De Maro M, d’Avenia M, et al. BAG3 is a novel serum biomarker for pancreatic adenocarcinomas. Am J Gastroenterol. (2013) 108:1178–80. doi: 10.1038/ajg.2013.128
13. Franco R, Scognamiglio G, Salerno V, Sebastiani A, Cennamo G, Ascierto PA, et al. Expression of the anti-apoptotic protein BAG3 in human melanomas. J Invest Dermatol. (2012) 132:252–4. doi: 10.1038/jid.2011.257
14. Rosati A, Basile A, Falco A, d’Avenia M, Festa M, Graziano G, et al. Role of BAG3 protein in leukemia cell survival and response to therapy. Biochim Biophys Acta. (2012) 1826:365–9. doi: 10.1016/j.bbcan.2012.06.001
15. Rosati A, Bersani S, Tavano F, Dalla Pozza E, De Marco M, Palmieri M, et al. Expression of the antiapoptotic protein BAG3 is a feature of pancreatic adenocarcinoma and its overexpression is associated with poorer survival. Am J Pathol. (2012) 181:1524–9. doi: 10.1016/j.ajpath.2012.07.016
16. Guerriero L, Chong K, Franco R, Rosati A, Festa M, Graziano G, et al. BAG3 protein expression in melanoma metastatic lymph nodes correlates with patients’ survival. Cell Death Dis. (2014) 5:e1173. doi: 10.1038/cddis.2014.143
17. Esposito V, Baldi C, Zeppa P, Festa M, Guerriero L, d’Avenia M, et al. BAG3 protein is over-expressed in endometrioid endometrial adenocarcinomas. J Cell Physiol. (2017) 232:309–11. doi: 10.1002/jcp.25489
18. De Marco M, Turco MC, and Marzullo L. BAG3 in tumor resistance to therapy. Trends Cancer. (2020) 6:985–8. doi: 10.1016/j.trecan.2020.07.001
19. De Marco M, Falco A, Iaccarino R, Raffone A, Mollo A, Guida M, et al. An emerging role for BAG3 in gynaecological Malignancies. Br J Cancer. (2021) 125:789–97. doi: 10.1038/s41416-021-01446-2
20. De Luca P, Salzano FA, Camaioni A, Raffone A, Mollo A, Guida M, et al. BAG3 positivity as prognostic marker in head and neck squamous cell carcinoma. Cancers (Basel). (2025) 17:1843. doi: 10.3390/cancers17111843
21. Behl C. Breaking BAG: the co-chaperone BAG3 in health and disease. Trends Pharmacol Sci. (2016) 37:672–88. doi: 10.1016/j.tips.2016.04.007
22. De Marco M, Basile A, Iorio V, Feste M, Falco A, Ranieri B, et al. Role of BAG3 in cancer progression: A therapeutic opportunity. Semin Cell Dev Biol. (2018) 78:85–92. doi: 10.1016/j.semcdb.2017.08.049
23. Marzullo L, Turco MC, and De Marco M. The multiple activities of BAG3 protein: Mechanisms. Biochim Biophys Acta Gen Subj. (2020) 1864:129628. doi: 10.1016/j.bbagen.2020.129628
24. Kirk JA, Cheung JY, and Feldman AM. Therapeutic targeting of BAG3: considering its complexity in cancer and heart disease. J Clin Invest. (2021) 131:e149415. doi: 10.1172/JCI149415
25. Turco MC. BAG3 in the heart. JACC Basic Transl Sci. (2023) 8:840–2. doi: 10.1016/j.jacbts.2023.02.012
26. Martin TG, Myers VD, Dubey P, Dubey S, Perez E, Moravec CS, et al. Cardiomyocyte contractile impairment in heart failure results from reduced BAG3-mediated sarcomeric protein turnover. Nat Commun. (2021) 12:2942. doi: 10.1038/s41467-021-23272-z
27. Wang J, Tomar D, Martin TG, Dubey S, Dubey PK, Song J, et al. Bag3 regulates mitochondrial function and the inflammasome through canonical and noncanonical pathways in the heart. JACC Basic Transl Sci. (2023) 8:820–39. doi: 10.1016/j.jacbts.2022.12.013
28. Martin TG, Sherer LA, and Kirk JA. BAG3 localizes to mitochondria in cardiac fibroblasts and regulates mitophagy. Am J Physiol Heart Circ Physiol. (2024) 326:H1124–30. doi: 10.1152/ajpheart.00736.2023
29. De Marco M, Falco A, Basile A, Rosati A, Festa M, d’Avenia M, et al. Detection of soluble BAG3 and anti-BAG3 antibodies in patients with chronic heart failure. Cell Death Dis. (2013) 4:e495. doi: 10.1038/cddis.2013.8
30. De Marco M, D’Auria R, Rosati A, Vitulano G, Gigantino A, Citro R, et al. BAG3 protein in advanced-stage heart failure. JACC Heart Fail. (2014) 2:673–5. doi: 10.1016/j.jchf.2014.05.012
31. Gandhi PU, Gaggin HK, Belcher AM, Harisiades JE, Basile A, Falco A, et al. Analysis of BAG3 plasma concentrations in patients with acutely decompensated heart failure. Clin Chim Acta. (2015) 445:73–8. doi: 10.1016/j.cca.2015.02.048
32. De Marco M, Basile A, Rosati A, Marzullo L, Turco MC, Ciccarelli M, et al. BAG3 concentrations and heart failure events among individuals undergoing coronary and peripheral angiography. NPJ Cardiovasc Health. (2025) 2. doi: 10.1038/s44325-025-00092-4
33. De Marco M, Basile A, Cammarota AL, Iannone C, Falco A, Marzullo L, et al. Response to antifibrotic therapy and decrease of circulating BAG3 protein levels in systemic sclerosis patients with reduced forced vital capacity. BioMed Pharmacother. (2024) 174:116578. doi: 10.1016/j.biopha.2024.116578
34. De Marco M, Armentaro G, Falco A, Iannone C, Falco A, Marzullo L, et al. Overexpression of BAG3 (Bcl2-associated athanogene 3) in serum and skin of patients with systemic sclerosis. Clin Exp Rheumatol. (2024) 42:1623–8. doi: 10.1016/j.biopha.2024.116578
35. Freedman P, De Marco M, Rosati A, Liberato M, Del Papa N, Turco MC, et al. Extracellular BAG3 is elevated in early diffuse systemic sclerosis. Mil Med Res. (2025) 12:37. doi: 10.1186/s40779-025-00628-w
36. Firpo MA, Boucher KM, Bleicher J, Khanderao GD, Rosati A, Poruk KE, et al. Multianalyte serum biomarker panel for early detection of pancreatic adenocarcinoma. JCO Clin Cancer Inform. (2023) 7:e2200160. doi: 10.1200/CCI.22.00160
37. Rosati A, Basile A, D’Auria R, d’Avenia M, De Marco, Falco A, et al. BAG3 promotes pancreatic ductal adenocarcinoma growth by activating stromal macrophages. Nat Commun. (2015) 6:8695. doi: 10.1038/ncomms9695
38. Chiappetta G, Ammirante M, Basile A, Rosati A, Festa M, Monaco M, et al. The antiapoptotic protein BAG3 is expressed in thyroid carcinomas and modulates apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. J Clin Endocrinol Metab. (2007) 92:1159–63. doi: 10.1210/jc.2006-1712
39. Staibano S, Mascolo M, Di Benedetto M, Vecchione ML, Ilardi G, Di Lorenzo G, et al. BAG3 protein delocalisation in prostate carcinoma. Tumour Biol. (2010) 31:461–9. doi: 10.1007/s13277-010-0055-3
40. Chiappetta G, Basile A, Barbieri A, Falco A, Rosati A, Festa M, et al. The anti-apoptotic BAG3 protein is expressed in lung carcinomas and regulates small cell lung carcinoma (SCLC) tumor growth. Oncotarget. (2014) 5:6846–53. doi: 10.18632/oncotarget.2261
41. Guerriero L, Palmieri G, De Marco M, Cossu A, Remondelli P, Capunzo M, et al. The anti-apoptotic BAG3 protein is involved in BRAF inhibitor resistance in melanoma cells. Oncotarget. (2017) 8:80393–404. doi: 10.18632/oncotarget.18902
42. De Marco M, Troisi J, Giugliano L, Rosati A, D’Antonio A, Iaccarino R, et al. BAG3 interacts with p53 in endometrial carcinoma. Cell Oncol (Dordr). (2020) 43:957–60. doi: 10.1007/s13402-020-00543-3
43. De Marco M, Del Papa N, Reppucci F, Iorio V, Basile A, Falco A, et al. BAG3 induces α-SMA expression in human fibroblasts and its over-expression correlates with poorer survival in fibrotic cancer patients. J Cell Biochem. (2022) 123:91–101. doi: 10.1002/jcb.30171
44. Iorio V, De Marco M, Basile A, Eletto D, Capunzo M, Remondelli P, et al. CAF-derived IL6 and GM-CSF cooperate to induce M2-like TAMs-letter. Clin Cancer Res. (2019) 25:892–3. doi: 10.1158/1078-0432.CCR-18-2455
45. Rosati A, Marzullo L, De Marco M, De Laurenzi V, D’Amico MF, Turco MC, et al. Toxicity in combined therapies for tumours treatments: a lesson from BAG3 in the TME? Front Immunol. (2023) 14:1241543. doi: 10.3389/fimmu.2023.1241543
46. De Marco M, Gauttier V, Pengam S, Mary C, Ranieri B, Basile A, et al. Concerted BAG3 and SIRPα blockade impairs pancreatic tumor growth. Cell Death Discov. (2022) 8:94. doi: 10.1038/s41420-022-00817-9
Keywords: BAG3, tumor, cancer cell lines, human serum, biomarker
Citation: Manzo P, Cammarota AL, Dimitrov J, Falco A, Rosati A, Marzullo L, Turco MC, De Laurenzi V, Sala G, De Marco M and Basile A (2025) BAG3 in human tumors. Front. Oncol. 15:1725674. doi: 10.3389/fonc.2025.1725674
Received: 15 October 2025; Accepted: 31 October 2025;
Published: 24 November 2025.
Edited by:
Giovanni Li Volti, University of Catania, ItalyReviewed by:
Sebastiano Giallongo, Kore University of Enna, ItalyFranca Esposito, University of Naples Federico II, Italy
Copyright © 2025 Manzo, Cammarota, Dimitrov, Falco, Rosati, Marzullo, Turco, De Laurenzi, Sala, De Marco and Basile. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Margot De Marco, bWRlbWFyY29AdW5pc2EuaXQ=
 Paola Manzo
Paola Manzo Anna Lisa Cammarota
Anna Lisa Cammarota Jelena Dimitrov
Jelena Dimitrov Antonia Falco
Antonia Falco Alessandra Rosati
Alessandra Rosati Liberato Marzullo
Liberato Marzullo Maria Caterina Turco
Maria Caterina Turco Vincenzo De Laurenzi
Vincenzo De Laurenzi Gianluca Sala
Gianluca Sala Margot De Marco
Margot De Marco Anna Basile
Anna Basile