Abstract
Multiple primary cancers (MPC) refer to the occurrence of two or more histologically distinct tumor types in a single individual, either simultaneously or sequentially. This report presents a rare and instructive case of a 71-year-old male who developed primary small intestinal diffuse large B-cell lymphoma (DLBCL) nine years after curative treatment for synchronous cardia and lung adenocarcinomas. The patient presented with dizziness and fatigue. Initial evaluation at a local hospital revealed moderate anemia (hemoglobin 66 g/L). Esophagogastroduodenoscopy and colonoscopy showed no remarkable findings. Subsequently, capsule endoscopy was performed. The capsule endoscopy demonstrated a segment of abnormal small bowel mucosa, showing marked edema, extensive erosions, and deep ulcers. The patient was then admitted to our center for further diagnosis and treatment. Chest computed tomography (CT) demonstrated postoperative changes from the prior lung and cardia cancers. Contrast-enhanced abdominal CT revealed focal ileal wall thickening and multiple small lymph nodes, suggestive of a neoplastic lesion. Further investigation was recommended. Single-balloon enteroscopy revealed a circumferential neoplastic growth, approximately 10 cm in length, with an ulcerative appearance and a dirty purulent coating in the ileum. Biopsy of the ileal lesion revealed a malignant lymphohematopoietic tumor with ulceration and necrosis, which on immunohistochemistry supported a diagnosis of aggressive B-cell lymphoma. Then the patient completed positron emission tomography - computed tomography (PET-CT) showing primary small-bowel lymphoma, Lugano staging 1E. To alleviate anemia and reduce the tumor burden, a partial resection of the ileum was performed. The segmental ileal resection confirmed DLBCL. Postoperatively, the patient was advised to be transferred to the oncology department for R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemoimmunotherapy. This case highlights the diagnostic challenge of evaluating new symptoms in cancer survivors and underscores the critical importance of considering metachronous hematologic malignancies, even with atypical presentations like isolated anemia, to avoid anchoring bias towards prior solid tumors.
Introduction
Over the past two decades, the frequency of patients presenting with two or more anatomically and temporally distinct primary malignancies—commonly termed multiple primary cancers (MPC)—has been steadily increasing worldwide, currently representing 2–17% of all newly diagnosed tumors (1, 2). The biological basis for MPC is multifactorial, involving a complex interplay among viral infections, cumulative environmental exposures, modifiable lifestyle factors, such as chewing betel nuts and smoking, chronic immune dysregulation, and the carcinogenic sequelae of prior oncological therapies (3–6). The literature on MPC often focuses on combinations of solid tumors, the subsequent development of a hematologic malignancy following dual solid primaries is exceptionally rare and presents a unique diagnostic dilemma. Patients with a history of cancer are at a perpetually elevated risk for new malignancies; however, when non-specific symptoms like anemia arise, clinical reasoning can be inadvertently constrained by “anchoring bias”. This cognitive bias leads to an over-reliance on the pre-existing medical narrative, often attributing new symptoms to the recurrence or late effects of known prior cancers, which can delay the diagnosis of a distinct, new disease entity. Lymphoma is a heterogeneous entity that includes Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL) (7). Diffuse large B-cell lymphoma (DLBCL) is the most common type of primary small intestinal lymphoma, with about 25% to 30% of patients presenting with localized disease at initial diagnosis (8). DLBCL represents one-third of all NHL cases, with incidence rates varying from 20% to 50% across different countries (9). The risk of developing DLBCL increases monotonically with age, and in all racial categories, the incidence rate is higher in males than in females. Individuals of white race have the highest incidence of DLBCL (10–12).
Herein, we present a case that exemplifies this diagnostic dilemma: a patient with a history of synchronous cardia and lung adenocarcinomas who, nine years later, presented with progressive dizziness and fatigue, ultimately diagnosed with primary small bowel DLBCL.
Case presentation
General information
A 71-year-old male patient was admitted to the local hospital due to progressive dizziness, fatigue, and a weight loss of 6 kg over the past two months. Initial evaluation revealed moderate anemia (hemoglobin 66 g/L). Esophagogastroduodenoscopy and colonoscopy showed no remarkable findings, prompting the performance of capsule endoscopy. The capsule transit lasted 14 hours and 44 minutes. From 7 hours and 24 minutes to 12 hours and 57 minutes, the capsule traversed a segment of small bowel characterized by marked mucosal edema, extensive erosions and deep ulcers (Supplementary Figure 1). The patient was admitted to our center for further diagnosis and treatment. The patient’s past oncological history was notable for two metachronous primary malignancies. In December 2016 the patient underwent curative-intent total gastrectomy for a poorly differentiated adenocarcinoma of the gastroesophageal junction (pT3N1M0). Histology showed invasion into the muscularis propria with focal extension to the serosa, lymphovascular invasion, and metastasis in 2 of 14 perigastric lymph nodes. At the same time, a wedge resection of the left upper lobe was performed for a 2-cm, moderately differentiated invasive adenocarcinoma classified as lung primary (pT1bN0M0). Regular chemotherapy was administered postoperatively.
Treatment course
Initial investigations at our center identified anemia (hemoglobin 89 g/L) and a positive fecal occult blood test. Imaging studies were subsequently performed. Chest computed tomography (CT) revealed postoperative changes of left upper lobe lung cancer and cardia cancer, with scattered solid nodules present in the upper lobes of both lungs and the right middle lobe. Contrast-enhanced abdominal CT further indicated a suspicious ileal lesion, characterized by wall thickening, and associated small lymph nodes (Figure 1). Further investigation was recommended. Single-balloon enteroscopy with cannula support was advanced 100 cm proximal to the ileocecal valve, where a circumferential neoplastic growth, approximately 10 cm in length, with an ulcerative appearance and a dirty purulent coating in the ileum was identified (Figure 2). Biopsy of the ileal lesion revealed a malignant lymphohematopoietic tumor with ulceration and necrosis; immunohistochemistry supported the diagnosis of an aggressive B-cell lymphoma. Immunohistochemical staining revealed the following results: Tumor cells were positive for CD20 (3+), while negative for CD3 (-, reactive T cells), CKpan (-), CK7 (-), CK20 (-), CDX-2 (-), CD56 (-), CgA (-), Syn (-). Other markers showed INI-1 (2+), BRG1 (3+), P53 (2+), and Ki-67 (approximately 80%+). EBER in situ hybridization (-). Then the patient completed positron emission tomography - computed tomography (PET-CT) showing primary small-bowel lymphoma, Lugano staging 1E. Considering the patient’s long-term anemia, we have decided to perform a partial ileal resection for the patient. During the operation, an 11-cm-long tumor was observed in the ileum, and it was adhered to the right posterior wall of the bladder and the sigmoid colon. After careful and slow dissection, we performed ileum tumor resection, sigmoid colon repair, and bladder repair (Figure 3). Postoperative pathology showed: “Partial ileum” resection specimen: Malignant tumor of the lymphohematopoietic system, with extensive necrosis. In view of the immunohistochemical findings, DLBCL is favored (germinal center subtype; ulcerative mass; size 11x7x1.5 cm); tumor tissue invades the entire intestinal wall to the serosal fibro-fatty tissue; no tumor cells are seen at both ends of the specimen and the circumferential margin, and no tumor tissue metastasis is seen in the peritoneal lymph nodes (0/8). Notes: 1. Immunohistochemical staining shows: Tumor cells are positive for CD20 (3+), CD79a (3+), Pax-5 (3+), Bcl-6 (2+), Bcl-2 5% (+), c-myc approximately 90% (+), CD10 (2+), CD3 (2+), CD43 (1+), CD30 (-), CKpan (-), GranB (-), TIA-1 (-), CD4 (-), CD8 (2+), Mum-1 (-), CD56 (-), ALK1 (-), Ki-67 approximately 80% (+). 2. In situ hybridization: EBER (-). 3. B-cell gene rearrangement: Detection of monoclonal band for IGH: D region (+); Detection of monoclonal band for IGK: A and B regions (+). 4. T-cell gene rearrangement: Detection of monoclonal band for TCRB (-); Detection of monoclonal band for TCRD (-); Detection of monoclonal band for TCRG (-) (Figure 4). The postoperative patient has made a good recovery. It is recommended that he be transferred to the oncology department for R-CHOP regimen. To date, five cycles have been completed. The treatment plan is to complete a total of six cycles, after which a formal response assessment will be performed.
Figure 1
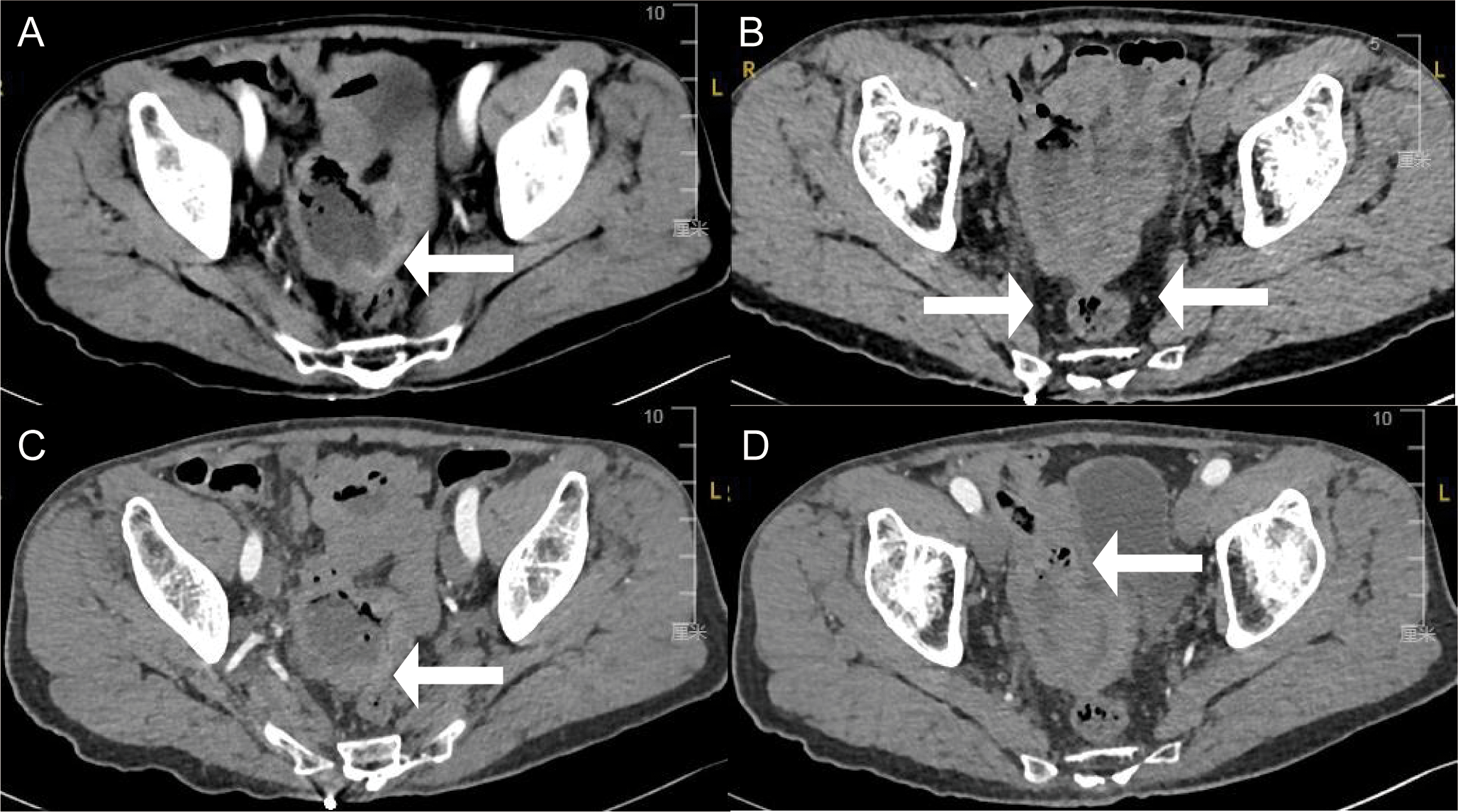
Enhance abdominal computed tomography cross-section images showing (A) thickening of part of the ileal wall (white arrows), (B) multiple small lymph nodes around the lesion (white arrows), (C) indistinct surrounding spaces with the sigmoid colon (white arrows), and (D) closely to the right posterior bladder wall (white arrows).
Figure 2
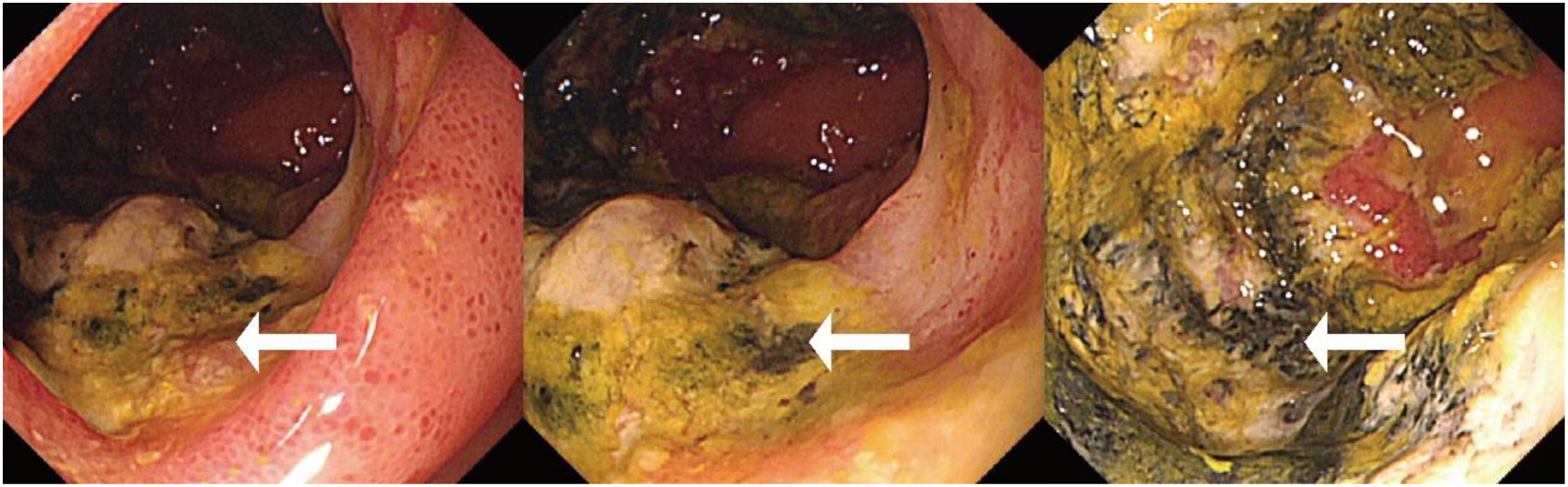
Single-balloon enteroscopy showing a circumferential neoplastic growth, approximately 10 cm in length, with an ulcerative appearance and a dirty purulent coating in the ileum (white arrows).
Figure 3
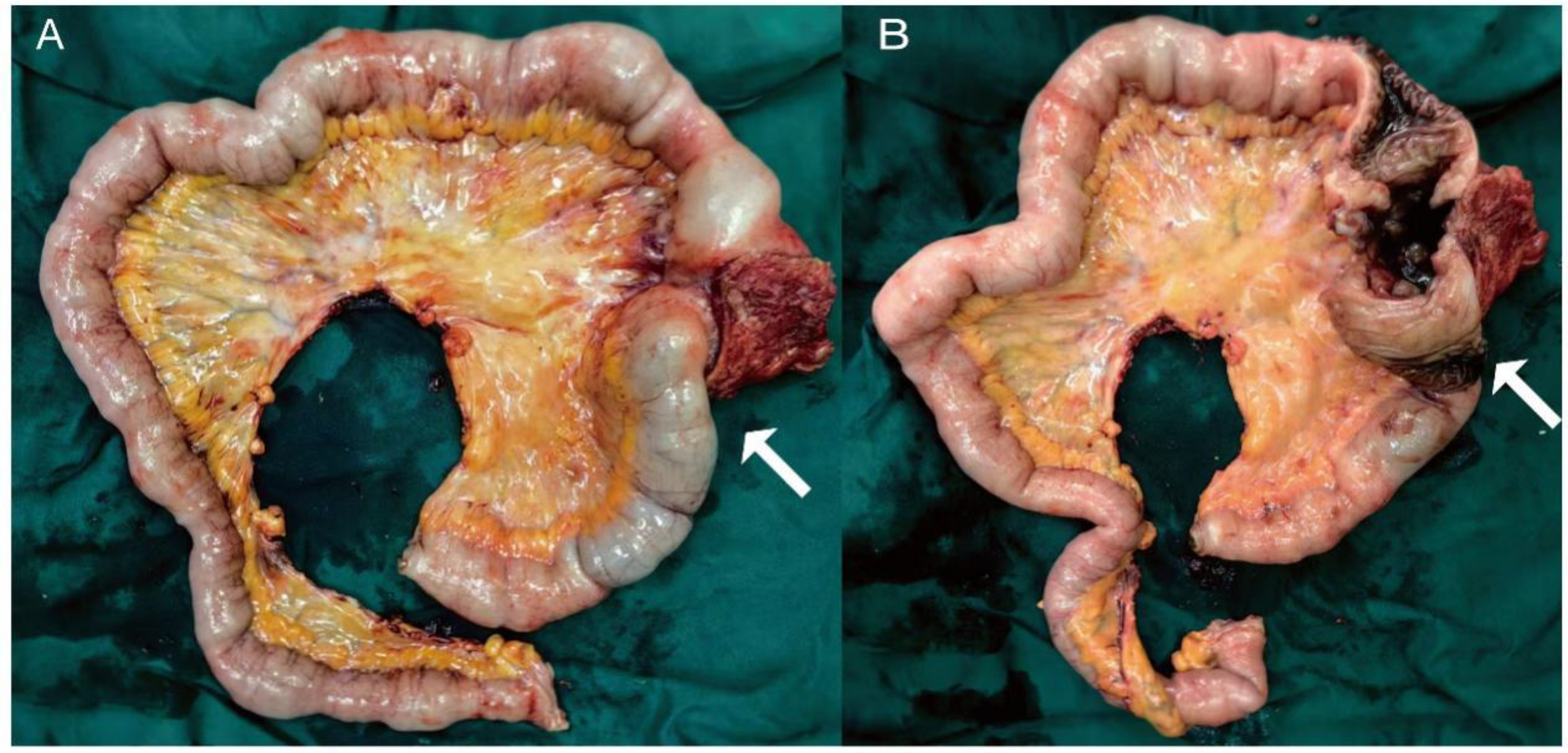
The gross specimen of the surgery (A) an 11-cm-long mass observed in the ileum (white arrows), (B) appearance of the mass after dissection.
Figure 4
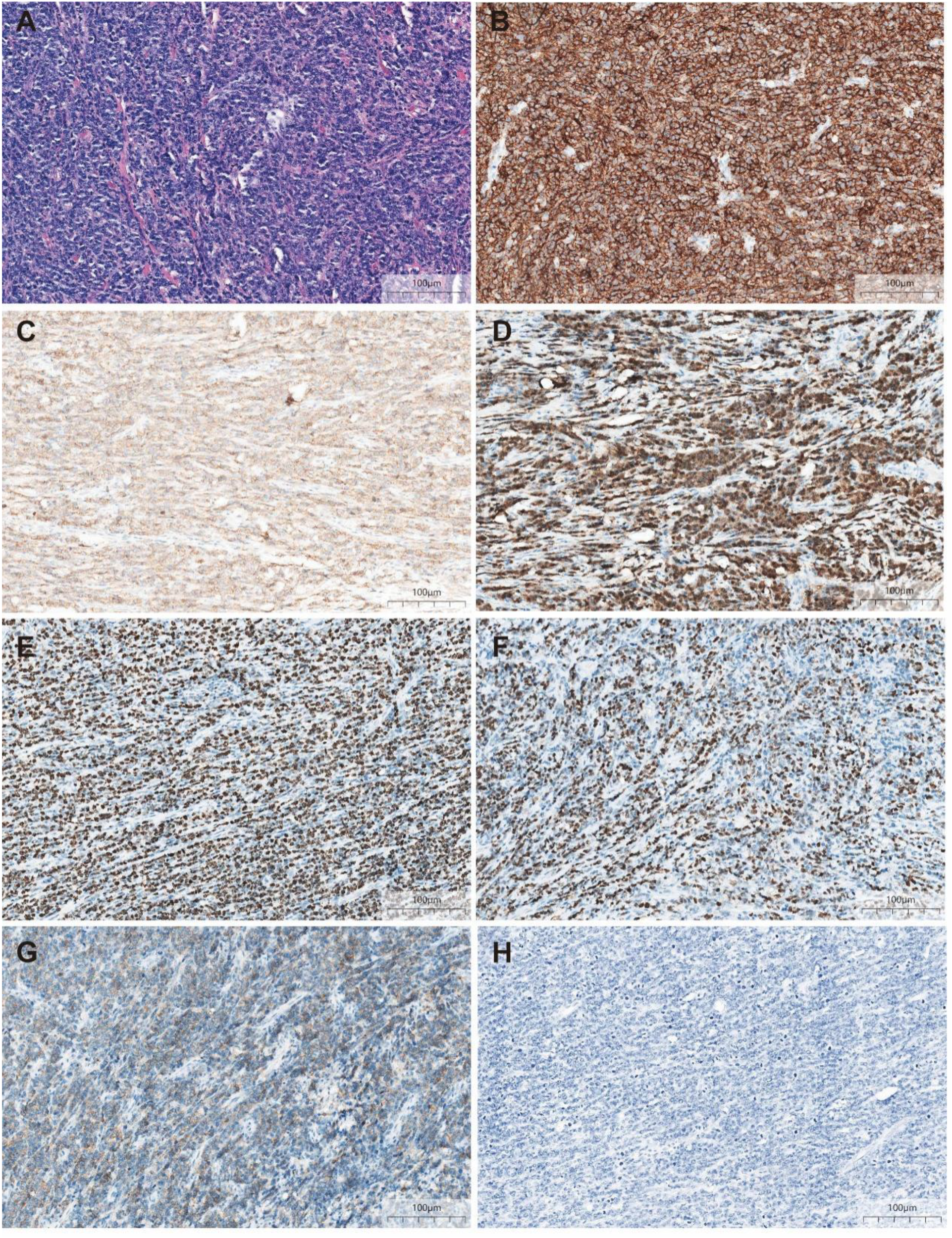
Postoperative pathology (A) ileum with a dense and diffuse large lymphocytic infiltrate (HE, ×200) (B) Atypical cells diffusely positive for marker CD20 (IHE CD20, ×200) (C) Atypical cells diffusely positive for marker CD79a (IHE CD79a, ×200) (D) Atypical cells diffusely positive for marker Pax-5 (IHE Pax-5, ×200) (E) Ki-67 hot spots at about 80% (+) (IHE Ki-67, ×200) (F) Atypical cells partly positive for marker BCL-6 (IHE BCL-6, ×200) (G) Atypical cells partly positive for marker CD10 (IHE CD10, ×200) (H) EBER in situ hybridization (-) (×200).
Literature search
Although previous reports on small intestinal DLBCL exist, cases involving MPC remain rare. We conducted a literature search using the terms “gastrointestinal diffuse large B-cell lymphoma” and “multiple primary cancers” or “simultaneous cancer” or “metachronous cancer” to retrieve relevant case reports in PubMed databases up to September 2025. We analyzed and summarized the clinical characteristics and outcomes of the three detected cases (13–15), with the summarized information presented in Supplementary Table 1.
Discussion
Small bowel tumors (SBTs) are a rare entity, accounting for 0.6% of all newly diagnosed cancer cases in the United States, and only 3% of all gastrointestinal tumors, despite the small intestine being the longest part of the gastrointestinal tract (16, 17). SBTs have approximately 40 different histological subtypes, the most common of which are adenocarcinoma (30–45%), neuroendocrine neoplasms (20–40%), lymphoma (10–20%), and sarcoma (10–15%) (18, 19). Primary small bowel lymphoma most commonly involves the ileum. The main histopathological types include DLBCL, mantle cell lymphoma (MCL), follicular lymphoma (FL), marginal zone lymphoma (MALT), and Burkitt lymphoma (BL) (20, 21).
DLBCL exhibits diversity in both the underlying etiology of the disease and clinical treatment outcomes. Known risk factors for DLBCL include severe immune deficiency and organ transplant recipients. Factors leading to chronic immune deficiency include a variety of autoimmune diseases (such as Sjogren’s syndrome, systemic lupus erythematosus, rheumatoid arthritis, Crohn’s Disease), viral infections (such as human immunodeficiency virus, hepatitis C virus, hepatitis B virus, Epstein-Barr virus), and obesity. A family history of NHL/DLBCL, personal history of cancer, and multiple genetic susceptibility loci are also established risk factors for DLBCL (22–24). In this case, the onset of DLBCL in the patient may be related to a history of concurrent presence of lung cancer and cardia cancer. This is likely due to a variety of biological factors, including cancer predisposition syndromes and immune impairments.
Primary intestinal DLBCL (PI-DLBCL) is a malignant tumor that originates from lymphocytes in the intestine. The diagnostic criteria for PI-DLBCL are based on Dawson’s criteria (25). PI-DLBCL predominantly affected males, accounting for 68.18% of cases, with a median age at onset of 57 years (26). The clinical manifestations of PI-DLBCL typically include abdominal pain, ascites, hepatomegaly, splenomegaly, bleeding, general fatigue, anorexia, and weight loss. Some patients presented with acute surgical complications, such as perforation, bowel obstruction, ileal intussusception, or severe occult gastrointestinal bleeding (27–29). Another study has also shown that the most common symptoms in patients with DLBCL are occult blood in stool (70.0%) and weight loss (60.0%) (30). The main manifestations of the patient reported in this case are moderate anemia caused by occult blood in the stool and weight loss.
Most PI-DLBCL patients are not easy to diagnose clinically. The common diagnostic methods for PI-DLBCL include ultrasound, CT, PET-CT, magnetic resonance imaging (MRI) and endoscopy, such as esophagogastroduodenoscopy, colonoscopy and capsule endoscopy (28). A study has shown that in cases of primary small intestinal lymphoma, patients with DLBCL have longer involved bowel segments and more frequent bowel wall thickening on abdominal CT compared with those with indolent B-cell lymphoma (BCL) (p = 0.003). Moreover, compared with patients with indolent BCL, those with DLBCL were more likely to have aneurysmal dilation (p = 0.020) (30). The abdominal CT of the patient reported in this case was consistent with the above results, showing that the wall of the ileum was partially thickened, the surrounding space was blurred. At present, one of the most recommended examination methods for lymphoma patients is PET-CT, which has irreplaceable advantages. In cases of PI-DLBCL, PET-CT can show the accumulation of 18F-fluorodeoxyglucose metabolism at the site of the lesion, accurately locating the intra- and extra-intestinal lesions of PI-DLBCL. The PET-CT scan of this patient showed that the lymphoma was confined to the ileum and no involvement of other organs or surrounding lymph nodes was observed. In addition to emergency surgery, endoscopy is also an essential tool for diagnosing intestinal lymphoma. Endoscopic examination can visually observe the entire affected intestine, determine the exact location of the lesion, and perform biopsies to achieve an accurate preoperative diagnosis of PI-DLBCL.
A study has shown that approximately 15% of DLBCL patients have a history of MPC. Among the 123 DLBCL patients with MPC, 103, 16, and 4 had 1, 2, and 3 other primary malignant tumors respectively before receiving treatment for DLBCL. Gastric cancer was the most common, followed by colorectal cancer, lung cancer, prostate cancer, and breast cancer. Hematological malignancies were less common than solid cancers (31). This patient was found to have lung cancer and cardia cancer simultaneously before being diagnosed with DLBCL. The above study also showed that compared with patients without MPC, the overall survival (OS) and progression-free survival (PFS) of DLBCL patients with MPC are significantly shorter (P < 0.01 for both) (31).
The treatment for PI-DLBCL typically involves surgery, chemotherapy, and immunotherapy. The standard first-line chemotherapy regimen is R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) (32). However, in patients with NHL of the small intestine, chemotherapy can occasionally lead to perforation, which may be life-threatening. This increased risk is due to full-thickness damage caused by tumor or tissue necrosis following chemotherapy. A 37-year retrospective study of patients with gastrointestinal lymphoma found that 9% of patients experienced perforation, with 55% of perforations occurring after chemotherapy. The most common site of perforation was the small intestine (59%), followed by the large intestine (22%) and the stomach (16%) (33). Given the patient’s advanced age and the potential risks associated with chemotherapy, the initial step was to perform surgical resection of the diseased ileum. Complete resection of the affected area not only alleviates the patient’s symptoms but also reduces the risk of complications such as perforation, bleeding, obstruction, or intussusception. Moreover, early pathological diagnosis and clinical staging obtained from biopsies acquired through surgery are instructive for clinical diagnosis and treatment and do not increase the risk of patient mortality (34, 35). A study has shown that compared with surgery alone, in addition to surgically removing the primary site, systemic chemotherapy is associated with a significant increase in the survival rate of patients with non-metastatic primary small intestinal lymphoma. Therefore, the recommended treatment for PI-DLBCL is a combination of surgery and adjuvant therapy (including radiotherapy, chemotherapy, and targeted therapy), which can lead to better prognosis (36). After this patient’s surgery, we recommended that he visit the oncology department for chemoimmunotherapy.
The principal novelty of this case is twofold. First, to our knowledge, this represents a rare documented instance of a patient with synchronous dual solid tumors (cardia and lung adenocarcinoma) subsequently developing a primary small intestinal DLBCL presenting with unexplained anemia as the initial symptom. This sequence underscores the diverse spectrum of MPC and reinforces that cancer survivors require lifelong vigilance for new primary malignancies across all lineages, including hematologic ones. Second, and more importantly, this case serves as a compelling teaching tool regarding diagnostic reasoning and cognitive bias in oncology. The patient’s complex history created a high risk for “anchoring bias,” where clinicians might prematurely attribute his anemia to his past cancers or their treatment effects. The absence of typical DLBCL symptoms (e.g., pain, bleeding) further allowed this bias to persist. Our diagnostic odyssey - from unremarkable standard endoscopies to the pivotal findings on capsule endoscopy and enteroscopy - illustrates the necessity of persisting with a broad differential diagnosis and utilizing advanced diagnostic modalities when initial tests are inconclusive. We propose that such cases necessitate a deliberate, two-pronged diagnostic mindset: actively investigating for recurrence or complications of known diseases while simultaneously screening for new, unrelated primaries.
Clinical implications
Based on our experience, we propose the following key considerations for clinicians managing similar cases. First, in cancer survivors presenting with unexplained, non-specific symptoms such as anemia, a systematic workup must actively include the possibility of a new, metachronous malignancy (both solid and hematologic) alongside evaluation for recurrence or treatment sequelae. This deliberate mindset is the primary defense against anchoring bias. Second, when standard endoscopic evaluations (esophagogastroduodenoscopy and colonoscopy) are unrevealing in the context of persistent anemia, capsule endoscopy should be promptly considered as a non-invasive tool to screen the entire small bowel. Following a tissue diagnosis, PET-CT is indispensable for accurate staging of lymphomas, defining disease extent, and guiding treatment planning. Third, for localized primary small intestinal DLBCL, surgical resection prior to chemotherapy can serve a dual purpose. It provides definitive histopathological diagnosis, reduces tumor burden, and crucially, mitigates the risk of perforation or bleeding that can occur during chemotherapy-induced tumor lysis.
Limitations
While bone marrow biopsy is an important tool in the staging of lymphoma. However, in this case, the patient and family declined the procedure after considering the following factors: (1) the whole-body PET-CT scan showed no evidence of bone marrow involvement; and (2) peripheral blood tests revealed only anemia, without other cytopenias or abnormal cells indicative of bone marrow infiltration. We acknowledge that the lack of biopsy confirmation is a limitation in definitively establishing Lugano staging IE. Nevertheless, based on this comprehensive non-invasive assessment, the clinical team believes the likelihood of limited-stage disease is high.
Conclusion
This case highlights that in patients with prior malignancies, non-specific symptoms like anemia should trigger a systematic workup that explicitly includes the possibility of a metachronous hematologic cancer. Although the exact pathogenesis is not fully understood, potential contributing factors may include genetic susceptibility, immune microenvironmental dysregulation, and shared carcinogenic exposures. Unexplained anemia should prompt thorough investigation, including endoscopy, radiology, pathology, and immunohistochemical characterization (CD20, CD79a, Pax-5, Ki-67, etc.) for accurate differentiation. Prompt diagnosis and appropriate treatment, as demonstrated in this case, are crucial for managing such complex presentations and improving patient outcomes.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the studies involving humans because The patient provided informed consent for the publication of their anonymized data. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BD: Data curation, Writing – original draft. CZ: Writing – original draft. YY: Conceptualization, Writing – review & editing. CD: Methodology, Writing – review & editing. NZ: Writing – review & editing. WY: Writing – review & editing. JZ: Writing – review & editing. JJ: Writing – review & editing. ZW: Writing – review & editing. LG: Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the Jinling Hospital’s Scientific Innovation Research Project (Grant No. 2024JCYJQN100, 2024JCYJQN114).
Acknowledgments
The authors extend their appreciation to the patient, who provided consent for publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2026.1706363/full#supplementary-material
References
1
Pan SY Huang CP Chen WC . Synchronous/metachronous multiple primary Malignancies: review of associated risk factors. Diagnostics (Basel). (2022) 12:1940. doi: 10.3390/diagnostics12081940
2
Vogt A Schmid S Heinimann K Frick H Herrmann C Cerny T et al . Multiple primary tumours: challenges and approaches, a review. ESMO Open. (2017) 2:e000172. doi: 10.1136/esmoopen-2017-000172
3
Wu Y Qu Z Wu Z Zhuang J Wang Y Wang Z et al . Multiple primary Malignancies and gut microbiome. BMC Cancer. (2025) 25:516. doi: 10.1186/s12885-025-13894-7
4
Schlenker B Schneede P . The role of human papilloma virus in penile cancer prevention and new therapeutic agents. Eur Urol Focus. (2019) 5:42–5. doi: 10.1016/j.euf.2018.09.010
5
Li CI Nishi N Mcdougall JA Semmens EO Sugiyama H Soda M et al . Relationship between radiation exposure and risk of second primary cancers among atomic bomb survivors. Cancer Res. (2010) 70:7187–98. doi: 10.1158/0008-5472.CAN-10-0276
6
Daly MB Pilarski R Yurgelun MB Berry MP Buys SS Dickson P et al . NCCN guidelines insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 1.2020. J Natl Compr Canc Netw. (2020) 18:380–91. doi: 10.6004/jnccn.2020.0017
7
Armitage JO Gascoyne RD Lunning MA Cavalli F . Non-hodgkin lymphoma. Lancet. (2017) 390:298–310. doi: 10.1016/S0140-6736(16)32407-2
8
Li S Young KH Medeiros LJ . Diffuse large B-cell lymphoma. Pathology. (2018) 50:74–87. doi: 10.1016/j.pathol.2017.09.006
9
Miranda-Filho A Piñeros M Znaor A Marcos-Gragera R Steliarova-Foucher E Bray F . Global patterns and trends in the incidence of non-Hodgkin lymphoma. Cancer Causes Control. (2019) 30:489–99. doi: 10.1007/s10552-019-01155-5
10
Wang SS . Epidemiology and etiology of diffuse large B-cell lymphoma. Semin Hematol. (2023) 60:255–66. doi: 10.1053/j.seminhematol.2023.11.004
11
Kanas G Ge W Quek RGW Keeven K Nersesyan K Arnason JE . Epidemiology of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) in the United States and Western Europe: population-level projections for 2020–2025. Leuk Lymphoma. (2022) 63:54–63. doi: 10.1080/10428194.2021.1975188
12
Cerhan JR Kricker A Paltiel O Flowers CR Wang SS Monnereau A et al . Medical history, lifestyle, family history, and occupational risk factors for diffuse large B-cell lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr. (2014) 2014:15–25. doi: 10.1093/jncimonographs/lgu010
13
Wang W Li P . Coexistence of colon adenocarcinoma, diffuse large B-cell lymphoma, and myelodysplastic syndrome: A case report. Med (Baltimore). (2019) 98:e16742. doi: 10.1097/MD.0000000000016742
14
Sugimoto K Uejima S Uchiyama Y Yasue R Nambu K Ishikawa J et al . Metachronous primary cancer of the tongue and Malignant lymphoma of the small intestine: A case report. Med (Baltimore). (2021) 100:e24806. doi: 10.1097/MD.0000000000024806
15
Ding W Luo H Wang Y . Synchronous multiple primary cancers involving rectal cancer and diffuse large B-cell lymphoma of the right colon: A case report. Cureus. (2025) 17:e83921. doi: 10.7759/cureus.83921
16
Vlachou E Koffas A Toumpanakis C Keuchel M . Updates in the diagnosis and management of small-bowel tumors. Best Pract Res Clin Gastroenterol. (2023) 101860:64–5. doi: 10.1016/j.bpg.2023.101860
17
Jasti R Carucci LR . Small bowel neoplasms: A pictorial review. Radiographics. (2020) 40:1020–38. doi: 10.1148/rg.2020200011
18
Chung CS Tai CM Huang TY Chang CW Chen KC Tseng CM et al . Small bowel tumors: A digestive endoscopy society of Taiwan (DEST) multicenter enteroscopy-based epidemiologic study. J Formos Med Assoc. (2018) 117:705–10. doi: 10.1016/j.jfma.2017.09.003
19
Rondonotti E Koulaouzidis A Georgiou J Pennazio M . Small bowel tumours: update in diagnosis and management. Curr Opin Gastroenterol. (2018) 34:159–64. doi: 10.1097/MOG.0000000000000428
20
Shirwaikar Thomas A Schwartz M Quigley E . Gastrointestinal lymphoma: the new mimic. BMJ Open Gastroenterol. (2019) 6:e000320. doi: 10.1136/bmjgast-2019-000320
21
Olszewska-Szopa M Wróbel T . Gastrointestinal non-Hodgkin lymphomas. Adv Clin Exp Med. (2019) 28:1119–24. doi: 10.17219/acem/94068
22
Huguet M Navarro JT Moltó J Ribera JM Tapia G . Diffuse large B-cell lymphoma in the HIV setting. Cancers (Basel). (2023) 15:3191. doi: 10.3390/cancers15123191
23
Olén O Smedby KE Erichsen R Pedersen L Halfvarson J Hallqvist-Everhov Å et al . Increasing risk of lymphoma over time in crohn’s disease but not in ulcerative colitis: A scandinavian cohort study. Clin Gastroenterol Hepatol. (2023) 21:3132–42. doi: 10.1016/j.cgh.2023.04.001
24
Berndt SI Vijai J Benavente Y Camp NJ Nieters A Wang Z et al . Distinct germline genetic susceptibility profiles identified for common non-Hodgkin lymphoma subtypes. Leukemia. (2022) 36:2835–44. doi: 10.1038/s41375-022-01711-0
25
Senyondo G Bella S Saleem A Mehdi SA Sidhu J . Ileo-colonic fistula due to diffuse large B cell lymphoma: unusual presentation of a rare disease. Cureus. (2021) 13:e12956. doi: 10.7759/cureus.12956
26
Chen X Wang J Liu Y Lin S Shen J Yin Y et al . Primary intestinal diffuse large B-cell lymphoma: novel insights and clinical perception. Front Oncol. (2024) 14:1404298. doi: 10.3389/fonc.2024.1404298
27
Simões AB Baruffi GD Pauletti MGT Valentini Junior DF . Adult ileocolic intussusception due to primary diffuse large B-cell lymphoma. Rev Esp Enferm Dig. (2025) 117:276–8. doi: 10.17235/reed.2023.9809/2023
28
Dias E Medas R Marques M Andrade P Cardoso H Macedo G . Clinicopathological characteristics and prognostic factors of small bowel lymphomas: a retrospective single-center study. Porto BioMed J. (2023) 8:e217. doi: 10.1097/j.pbj.0000000000000217
29
Pawlak KM Martínez-Alcalá A Kröner PT Fry LC Mönkemüller K . Small-bowel B-cell lymphoma presenting as autoimmune hemolytic anemia and severe obscure gastrointestinal bleeding. Endoscopy. (2023) 55:E854–e5. doi: 10.1055/a-2098-0883
30
Li L Ma H Niu M Chen C Yu C Zhang H et al . Characterization of primary small intestinal lymphoma: a retrospective study based on double balloon endoscopy. BMC Gastroenterol. (2024) 24:116. doi: 10.1186/s12876-024-03193-z
31
Tanba K Chinen Y Uchiyama H Uoshima N Shimura K Fuchida S et al . Prognostic impact of a past or synchronous second cancer in diffuse large B cell lymphoma. Blood Cancer J. (2018) 8:1. doi: 10.1038/s41408-017-0043-6
32
Barraclough A Hawkes E Sehn LH Smith SM . Diffuse large B-cell lymphoma. Hematol Oncol. (2024) 42:e3202. doi: 10.1002/hon.3202
33
Matsumoto M Kasahara K Nagakawa Y Katsumata K Tsuchida A . Multiple intestinal perforations caused by diffuse large B-cell lymphoma-A case report. Gan To Kagaku Ryoho. (2022) 49:1377–9.
34
Liu X Lv T Zhang X Li J . Extensive small intestinal diffuse large B cell lymphoma. Rev Esp Enferm Dig. (2023) 115:267. doi: 10.17235/reed.2022.9100/2022
35
Wang M Ma S Shi W Zhang Y Luo S Hu Y . Surgery shows survival benefit in patients with primary intestinal diffuse large B-cell lymphoma: A population-based study. Cancer Med. (2021) 10:3474–85. doi: 10.1002/cam4.3882
36
Lu PW Fields AC Yoo J Irani J Goldberg JE Bleday R et al . Surgical management of small bowel lymphoma. J Gastrointest Surg. (2021) 25:757–65. doi: 10.1007/s11605-020-04730-3
Summary
Keywords
anemia, cardia adenocarcinoma, ileum diffuse large B-cell lymphoma, lung adenocarcinoma, multiple primary cancers
Citation
Da B, Zhao C, Yao Y, Dai C, Zhu N, Yan W, Zhang J, Jiang J, Wang Z and Guo L (2026) Unmasking small-bowel DLBCL in an elderly patient with simultaneous multiple primary cancers through anemia: a case report and literature review. Front. Oncol. 16:1706363. doi: 10.3389/fonc.2026.1706363
Received
16 September 2025
Revised
30 January 2026
Accepted
05 February 2026
Published
19 February 2026
Volume
16 - 2026
Edited by
Jeffrey J. Pu, Tufts University, United States
Reviewed by
John L. Vaughn, New York University, United States
Fouad Nahhat, Mayo Clinic Arizona, United States
Updates
Copyright
© 2026 Da, Zhao, Yao, Dai, Zhu, Yan, Zhang, Jiang, Wang and Guo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiming Wang, wzmdoc@163.com; Lei Guo, guolei900809@126.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.