- Department of Paediatrics, University of Otago, Christchurch, New Zealand
Tobacco exposure increases mortality and morbidity of the fetus, the child, the adolescent, and their children in turn. Nearly half the children in the world are exposed. Smoking is not merely personal choice or personal responsibility; those subtle phrases undermine those who have no choice in the matter. Tobacco control must take a multi-pronged attack. Smoking cessation by adults in childbearing years must take center stage of these efforts, because it is the only way to ensure a smoke-free environment for children. Smoke-free parents provide a role model for smoke-free young people, and erode the image of smoking as a desirable adult behavior to emulate. Pediatricians and pediatric pulmonologists have a key role to play here. This goal will reduce morbidity and mortality among adults and children. Legislation regarding taxation, environments, tobacco constituents, product placement and display, packaging, and media education are all key to this core goal. Smoke-free policy must be protected from attack based on trade agreements. Research is needed into more effective ways to attract and help people give up smoking, and into educating and re-deploying tobacco industry workers in emerging and developed countries.
“A custome lothsome to the eye, hatefull to the Nose, harmefull to the braine, dangerous to the Lungs” [sic]
Since 1604 when James I of England wrote the above description of tobacco smoking, people have recognized the common sense link between smoking and disease, conclusively documented by Doll and Bradford Hill (1) in the British doctors’ study. In 1967 the first effects of passive smoking on children were sought (2), but it was not until 1981 that Hirayama’s study of non-smoking women showed that passive smoking was a serious risk to health (3). The massive extent and prevalence of disease attributable to tobacco smoke exposure in children has slowly emerged and continues to be documented (Table 1). Cigarette smoke contains many carcinogens and cellular poisons that are especially high in sidestream smoke (4), but nicotine itself is a neural teratogen, a genotoxin and potentially a carcinogen in its own right (5–8).

Table 1. Increased fetal or child morbidity and mortality risks that have been associated with exposure to tobacco smoke (indicative, not exhaustive).
The tobacco industry maintained there was doubt about the links of tobacco smoking with lung cancer and other health concerns, and denied the addictiveness of cigarettes into the 1990s. However, once the internal memos of tobacco companies were forced into the public arena by landmark court cases, they were compelled, on the surface at least, to admit some of the harm of smoking (while continuing to this day to indicate there is doubt about key findings). They framed this damage in terms of personal choice and personal responsibility. People knew the risks and they chose the pleasure of smoking in spite of this; the industry seems almost to imply it has a responsibility to continue to supply this legal consumer demand.
Children Do Not have a Personal Choice
Addiction, passive smoking, and the risks to children totally negate the argument of personal choice and personal responsibility. A parent who is addicted to nicotine will find it extremely difficult not to expose their child to smoking, even if they smoke outside the home (9, 10). And that child has no choice about whether they are exposed. The fetus and the young child are the most vulnerable to, and the least able to avoid, the health consequences of exposure to smoking by their parents. Older children and teenagers are, in addition, vulnerable to the role modeling of smoking by their parents (11, 12), and to the attractive portrayal of cigarettes as a badge of adulthood. Every day nearly 100,000 young people worldwide start smoking (13). In turn, this group becomes the next generation of smoking parents.
Smoking exposure in the womb and in childhood increases risk factors for adult disease, aside from the risk of the child becoming a smoker themselves. These include (see Table 1) increased blood pressure and serum lipids (cardiovascular disease), decreased pulmonary function, and increased asthma severity (asthma and COPD), and genotoxic effects which may increase the risk of adult cancers.
So-called third-hand smoke has potential health effects on children. Third-hand smoke is when smoke is adsorbed onto clothing or fabric, and volatile substances then “off-gas” into the air again. This gas contains significant amounts of carcinogens and toxins (14). This means that smoking outside the house does not remove the risks to children and can only be recommended if it is a step toward quitting. Third-generation effects have been documented, in which tobacco-induced DNA alterations (methylation or mutations) occur in a smoker’s germ cells, and are passed on via the germline to their children and grandchildren (15, 16).
Pregnancy and childhood are thus periods when one person’s smoking intensely and intimately exposes other highly vulnerable people, and perpetuates a transgenerational cycle of smoking initiation. For these reasons elimination of smoking in pregnancy and in parents is an absolutely key issue for the immediate and future health of the population, and for reduction of uptake of tobacco smoking by the young.
A Matter of Global Injustice and Environmental Concern
Whereas once cigarette smoking was the luxury of the elite, it is now heavily overrepresented in lower socioeconomic, and disadvantaged minority groups, who have the least resources to be able to cope with the health effects, or advocate on their own behalf. Tobacco marketing has targeted these groups (17–19).
The inequity of exposure and health effects extends to the international and global scene. Tobacco companies, like many other industries, have exploited the emerging and developing countries of the world in China (the world’s largest tobacco-growing nation), India, Africa, and South America. Tobacco has been offered as a quick cash crop to peasant farmers who become locked into dependence on the industry supply of fertilizers, and often enter a debt trap, because tobacco impoverishes the soil (20). In 1999 it was estimated that over 200,000 hectares of forest or woodland were cleared every year in developing countries for tobacco plantations and to fuel the curing of tobacco (21, 22). And thousands of children are employed in unsafe and toxic conditions in tobacco manufacturing plants and in the extensive home-based manufacture of bidis (cheap cigarettes) in India, China, and other countries (13, 23, 24).
For these reasons tobacco use is a global cause of injustice and environmental concern, and is not just an issue of personal health (25). The WHO Framework Convention on Tobacco Control (2003) required signatory nations to meet key core principles and objectives from February 2005 (26). Hard-hitting legislation – including increased taxation, the banning of sales to minors, the banning of smoking in workplaces, restaurants, bars, casinos, the removal of vending machines and tobacco point-of-sale displays, requiring package health warnings and media campaigns – has been effective in reducing smoking rates in many countries to levels below 20%. In 2007, Frieden and Bloomberg estimated that reduction of smoking prevalence from 25 to 20% worldwide would prevent 73 million premature deaths in adults, 50 million deaths in children, and 50 million antenatal deaths by 2030 (27).
Legislation on the table for many countries includes plain-packaging (following Australia’s lead), banning smoking in cars carrying children, in parks and beaches, and regulation of tobacco constituents1. There is evidence that these approaches will further reduce smoking levels. Aotearoa/New Zealand has committed to being a smoke-free country (defined as a smoking prevalence of less than 5%) by 2025.
The Womb and the Home – Still Unprotected
And yet, as Tobacco Endgame conferences presage the demise of tobacco sales and smoking, the greatest area of harm to children remains the womb and the home, where legislation’s arm often falls short. Almost half of the world’s children who never smoked are exposed to tobacco smoke in the home (13). Many countries have legislated against physical punishment of children, and against neglect or leaving children younger than a certain age on their own at home. Can the home be protected from smoke by legislation, and how will it be regulated? We know that smoking outside the house does not protect children (9). We also know that measures directly addressing education of young people and teenagers are often not effective on their own in preventing smoking initiation (28). The only effective way to reduce smoking exposure in the home is for parents to give up smoking. Similarly the best way to change the image of tobacco from being seen by young people as a desirable badge of adult, independent status is for adult smokers in their childbearing years to give up smoking. We need continuing research efforts into the best way to counter media advertising, provide positive media messages, and make smoking cessation attractive as well as feasible to the majority of smokers.
From Corporate Social Responsibility to Challenging Public Health Legislation
The other major challenge that is being faced comes from a new tactic of the tobacco industry. Since the early 90s the industry has often tried to portray itself as a caring, community-responsible enterprise that has no wish to advertise to children or young people and supports educational activities. For example a tobacco company produced and distributed an education package for schools in Australia and New Zealand on responsible decision-making (“I’ve got the Power”), and another is a sponsor for the Keep New Zealand Beautiful trust. Through such initiatives (part of the public relations front termed “Corporate Social Responsibility” (29, 30)) the industry has tried to maintain this image, despite continuing to market a deadly product, and to use product placement in movies attractive to young people. Part of this has been an industry tactic to emphasize that smoking is an adult behavior, with the double effect of appearing to discourage youth smoking while doing the opposite, because youth seek to emulate adult behaviors (31).
“Acting in a manner that draws the clearest, sharpest possible line between who should and who should not have access to cigarettes will reinforce the right of adults to obtain and enjoy a legal product, and thus prevent marketing bans down the road that are driven by the youth access issue.” (Speech Presented at Philip Morris Invitational in 1995 (32)).
Now, as plain-packaging legislation has been enacted in Australia, and is planned in many other countries, including the UK and New Zealand, the tobacco industry has started to use tactics directly opposed to public health and the public good. Big Tobacco is pitting its massive financial might against governments through trade agreements, alleging threats to the industry’s profits and trading based on their intellectual property – including their logos and package design (33). This is clearly an area that bites deeply. Challenges have been brought to Australia based on the legislation being unconstitutional (this challenge has been overturned by the Australian High Court), being a technical barrier to trade (an action brought by several countries through the World Trade Organization, with legal support from tobacco companies) and being in breach of a longstanding trade agreement between Australia and Hong Kong (an industry shifted their office to Hong Kong in order to bring this dispute).
Australia has commendably not backed down on the legislation and has good reasons for defeating the challenges. But this will not be achieved without considerable legal expense (predicted to exceed AUD $10 million), and this has already resulted in a “chilling” effect – a delay in the planned introduction of plain packaging by the New Zealand government, and other governments (34). New Zealand and several other Pacific nations are at the same time negotiating a “new generation” trade agreement which is predicted to have a much greater reach into domestic policy. The Trans Pacific Partnership is backed by the US Foreign Trade Representative, and several tobacco companies have already intimated that they are prepared to use such an agreement to litigate to protect their investments (including intellectual property such as logos and packaging) if necessary (33–35). Tobacco companies and tobacco-growing states have major influence in US trade, regardless of the denouncement of tobacco by the US Surgeon General. It is essential that tobacco control strategies and the medical and legal professions work together to advocate against the loss of democratic powers and public health muscle that are potentially put on sale for the sake of trade.
An Unethical Industry Can be Run by Decent People with a Blind Spot
The tobacco industry is unethical and immoral in that it continues to manufacture and market a lethal, addictive, and child-damaging product in full knowledge of these effects. The industry thus displays a disregard for human life, for the rights of children, for the status of the poor, and for the environment. Tobacco corporations have made duplicitous statements in public and in their public relations efforts that have been termed “Corporate Social Responsibility” as noted above. This is not to say that every worker in the industry is corrupt, but the industry and its corporate entities exhibit this unethical behavior and ethos. Many tobacco industry workers may have blind spots in regards to the medical, social, and environmental damage caused by their company. The controlling story within tobacco companies is not that of an unethical business producing a lethal and child-damaging product, but rather of a thriving business (despite attacks from fringe medical scientists) that benefits the community and country, treats its workers well and offers good job security, much like a food or pharmaceutical corporation (36). There has been little research into how tobacco industry employes may be best educated about the damage to the public. One interfaith group has bought industry shares in order to challenge and influence company policy, with some success (37).
A multi-pronged tobacco control strategy continues to be required (Figure 1) because of the many ways in which the industrial and legal might of the tobacco industry can be brought to bear to circumvent, penetrate, or challenge smoke-free measures and legislation. As child health professionals, our goal is that every child experiences their right to grow up in a smoke-free environment.
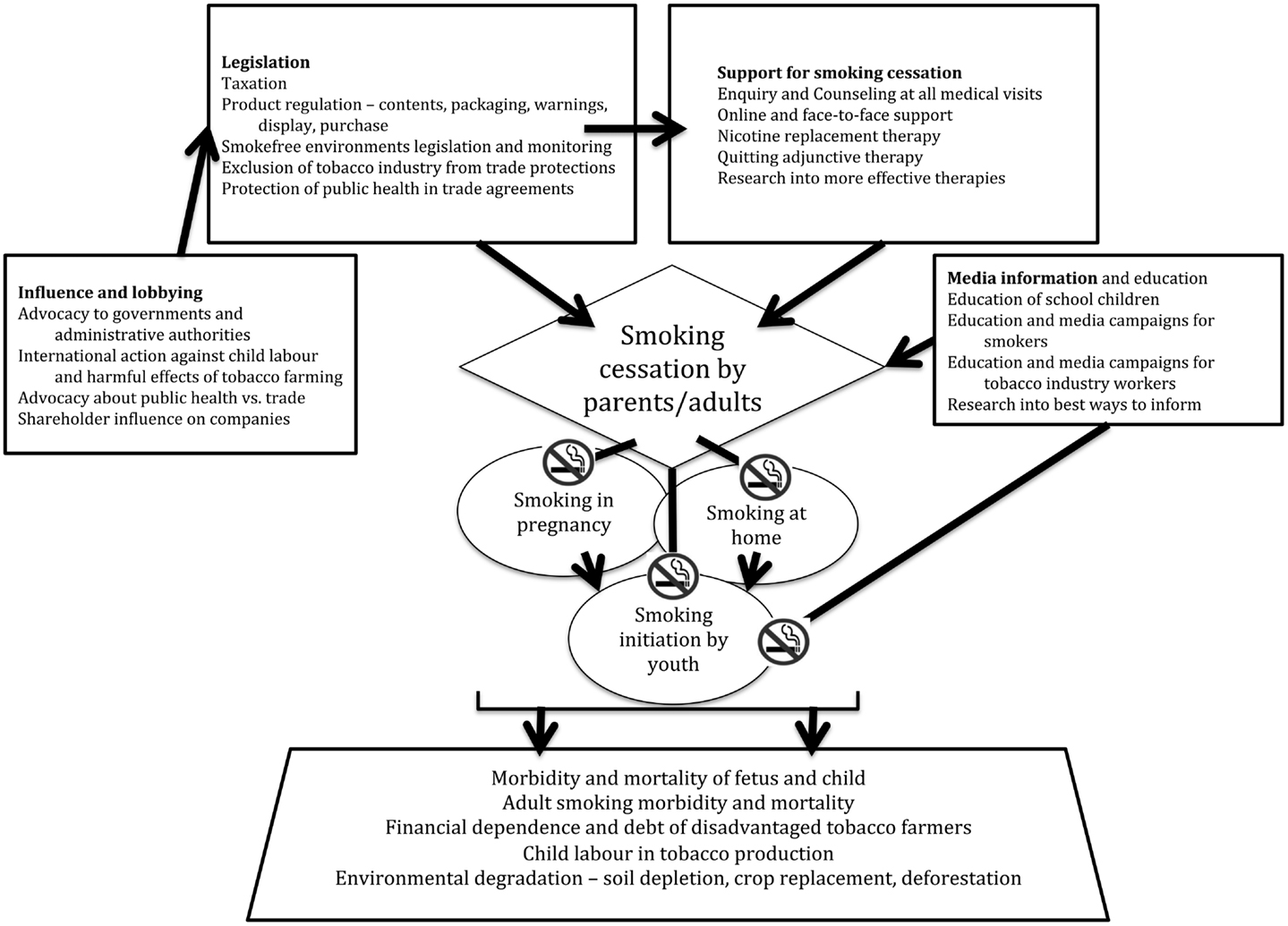
Figure 1. Multi-pronged approach to reducing tobacco-related mortality, morbidity, and smoking initiation among children, focused on reducing smoking prevalence in adults.
At the same time we must never assume that the tobacco industry is going to roll over and surrender, or transmute into a charitable agency.
The Ongoing Fight with a Formidable Opponent
The tobacco companies have more economic and legal might than many countries. In countries like America and China they have entangled themselves in every stratum of politics and economics, from farmer benefits, to states, to congress and government trade agencies (36) (although their proportional contribution to these countries’ economies may be overstated (38)). The WHO estimates that the number of smokers worldwide will increase from 1.1 to 1.6 billion by 2025 (13). So what hope do we have? Are we condemned after each public health gain to expect ever-augmented strength from the industry?
The outcome cannot be predicted with certainty, but our stance as child health professionals and respiratory specialists is clear. We must expose and oppose the injustice to the young and the marginalized that we see on a massive and largely unappreciated scale. What we do has ramifications beyond just tobacco. The alcohol industry has been following the tobacco industry’s strategies closely, emphasizing personal responsibility and targeting the “problem drinker” rather than the industry taking responsibility for its own product and marketing to youth. Other industries that are operating at the cost of health interests may follow.
What counts in our favor is that we have a transparent agenda that is in the child’s and the community’s best interests, that is devoid of vested interests, that truly captures the moral high ground, and has the support of the international community, WHO and most of our governments (albeit that governments are always having to deal a large hand to trade interests). As with those who opposed the slave trade, we cannot underestimate the strength of the financial and corporate interests opposing change, but we have a responsibility to act and refuse to stand down on behalf of those who cannot advocate for themselves. This is a war that can be won, but it requires major commitment on our part, ongoing research into key aspects of tobacco control, and ongoing dialog with our health departments and governments.
To conclude, it is naturally of direct relevance to pediatric pulmonologists that tobacco smoke from conception onward has major effects on the development, functioning, and wellness of the entire respiratory system, and through the respiratory system on sudden infant death syndrome, meningococcal disease, and many other systemic diseases. It poses a long-term risk for adult chronic cardiorespiratory disease, and for smoking initiation, with its own incumbent risks. As well as this, we should be aware that growth and manufacture of tobacco products pose global risks to the health and wellbeing of children.
For the practicing pediatrician and pediatric pulmonologist it is vital that we consistently ask parents and older children about smoking. For parents and expectant parents who smoke it is helpful to ask about their willingness to quit, to advise them strongly to quit (in a brief sentence), and to provide them with verbal and/or written information about the risks to their children and even grandchildren of exposure. Brief motivational interviewing is an accepted method of doing this (39). Children and adolescents who are smokers are more difficult to advise and counsel effectively: helpfulness and clear advice to quit are important.
Parents who are smokers and are willing to quit should be reminded of the positive immediate aspects of quitting (improved child health, improved breath, taste, fitness etc.), encouraged to set a quit date, and provided with nicotine replacement. Referral to a smoking cessation provider should be offered, and follow-up arranged. For those who relapse they should be congratulated for trying, encouraged that relapse is part of the process of quitting, and assisted to learn from their attempt.
As expert leaders in our organizations and communities we should endeavor to speak up about the impact of tobacco exposure and marketing on our children, and the impact of the industry on the environment and children in the developing world. This may involve discussing the effects of tobacco smoke with colleagues such as obstetricians, input into smoke-free initiatives, letters to the media or to MPs, and lobbying of governments for protection of children through legislation. We should also be asking our governments about the effects of their trade relationships with the tobacco industry or tobacco exporting states on public health and on global injustice to children and families.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author thanks Professor KR Thankappan, and Sreedevi Padmajam, of Achutha Menon Centre for Health Science Studies, SCT Institute for Medical Sciences and Technology, Trivandrum, Kerala, India for information regarding the bidi industry in India.
Footnote
- ^Note that harm reduction strategies that involve substituting cigarettes with smokeless forms of tobacco (electronic cigarettes etc) rather than cessation are not ideal because of the toxicity of nicotine itself to the fetus, to the child, and to the germline. See paragraph 1.
References
1. Doll R, Hill AB. The mortality of doctors in relation to their smoking habits; a preliminary report. Br Med J (1954) 1:1451–5. doi:10.1136/bmj.1.4877.1451
2. Cameron P. The presence of pets and smoking as correlates of perceived disease. J Allergy (1967) 40:12–5. doi:10.1016/0021-8707(67)90054-8
3. Hirayama T. Non-smoking wives of heavy smokers have a higher risk of lung cancer: a study from Japan. Bull World Health Organ (1981) 282:183–5. doi:10.1136/bmj.282.6259.183
4. International Agency for Research on Cancer. Tobacco smoke. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (Tobacco Smoke and Involuntary Smoking). [Internet]. Vol. 83. Lyons: World Health Organization (2004) [cited 2013 Jul 1]; p. 51–94. Available from: http://monographs.iarc.fr/ENG/Monographs/vol83/mono83.pdf
5. Slotkin TA, Seidler FJ, Spindel ER. Prenatal nicotine exposure in rhesus monkeys compromises development of brainstem and cardiac monoamine pathways involved in perinatal adaptation and sudden infant death syndrome: amelioration by vitamin C. Neurotoxicol Teratol (2011) 33:431–4. doi:10.1016/j.ntt.2011.02.001
6. Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther (1998) 285:931–45.
7. Demirhan O, Demir C, Tunc E, Nandiklioglu N, Sutcu E, Sadikoglu N, et al. The genotoxic effect of nicotine on chromosomes of human fetal cells: the first report described as an important study. Inhal Toxicol (2011) 23:829–34. doi:10.3109/08958378.2011.617398
8. Tonini G, D’onofrio L, Dell’aquila E, Pezzuto A. New molecular insights in tobacco-induced lung cancer. Future Oncol (2013) 9:649–55. doi:10.2217/fon.13.32
9. Matt GE, Quintana PJ, Hovell MF, Bernert JT, Song S, Novianti N, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control (2004) 13:29–37. doi:10.1136/tc.2003.003889
10. Rumchev K, Jamrozik K, Stick S, Spickett J. How free of tobacco smoke are ‘smoke-free’ homes? Indoor Air (2008) 18:202–8. doi:10.1111/j.1600-0668.2008.00517.x
11. Scragg R, Laugesen M, Robinson E. Parental smoking and related behaviours influence adolescent tobacco smoking: results from the 2001 New Zealand national survey of 4th form students. N Z Med J (2003) 116:U707.
12. Gilman SE, Rende R, Boergers J, Abrams DB, Buka SL, Clark MA, et al. Parental smoking and adolescent smoking initiation: an intergenerational perspective on tobacco control. Pediatrics (2009) 123:e274–81. doi:10.1542/peds.2008-2251
13. Lando HA, Hipple BJ, Muramoto M, Klein JD, Prokhorov AV, Ossip DJ, et al. Tobacco is a global paediatric concern. Bull World Health Organ (2010) 88:2. doi:10.2471/BLT.09.069583
14. Matt GE, Quintana PJ, Destaillats H, Gundel LA, Sleiman M, Singer BC, et al. Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect (2011) 119:1218–26. doi:10.1289/ehp.1103500
15. Rehan VK, Liu J, Naeem E, Tian J, Sakurai R, Kwong K, et al. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med (2012) 10:129. doi:10.1186/1741-7015-10-129
16. Leslie FM. Multigenerational epigenetic effects of nicotine on lung function. BMC Med (2013) 11:27. doi:10.1186/1741-7015-11-27
17. Anderson SJ. Marketing of menthol cigarettes and consumer perceptions: a review of tobacco industry documents. Tob Control (2011) 20(Suppl 2):ii20–8. doi:10.1136/tc.2010.041939
18. Apollonio DE, Malone RE. Marketing to the marginalised: tobacco industry targeting of the homeless and mentally ill. Tob Control (2005) 14:409–15. doi:10.1136/tc.2005.011890
19. Siahpush M, Jones PR, Singh GK, Timsina LR, Martin J. The association of tobacco marketing with median income and racial/ethnic characteristics of neighbourhoods in Omaha, Nebraska. Tob Control (2010) 19:256–8. doi:10.1136/tc.2009.032185
20. Prasad VM. Case Study of Tobacco Cultivation and Alternate Crops in India. [Internet]. Geneva: WHO (2007) [cited 2013 Jul 1]; p. 1–15. Available from: http://www.who.int/tobacco/framework/cop/events/2007/india_case_study.pdf
21. Geist HJ. Global assessment of deforestation related to tobacco farming. Tob Control (1999) 8:18–28. doi:10.1136/tc.8.1.18
22. Chacha BK. Killing Trees to Cure Tobacco: Tobacco and Environmental Change in Kuria District, Kenya, 1969–1999. [Internet]. Rome: Food and Agriculture Organization of the United Nations (1999) [cited 2013 Jul 1]. Available from: http://www.fao.org/docrep/ARTICLE/WFC/XII/0900-B1.HTM
23. John S. History and culture of bidis in India: production, employment, marketing and regulations. In: Gupta PC, Asma S, editors. Bidi Smoking and Public Health [Internet]. Chapter 1.1. New Delhi: Ministry of Health and Family Welfare (2008) [cited 2013 Jul 1]; p. 1–9. Available from: http://www.who.int/tobacco/publications/prod_regulation/bidi_smoking_public_health.pdf
24. Hipple B, Lando H, Klein J, Winickoff J. Global teens and tobacco: a review of the globalization of the tobacco epidemic. Curr Probl Pediatr Adolesc Health Care (2011) 41:216–30. doi:10.1016/j.cppeds.2011.02.010
25. Syed S, Hammond R. Tobacco and the Rights of the Child. [Internet]. Geneva: WHO (2001) [cited 2013 Jul 1]; p. 1–62. Available from: http://whqlibdoc.who.int/hq/2001/WHO_NMH_TFI_01.3_Rev.1.pdf
26. WHO. WHO Report on the Global Tobacco Epidemic. [Internet]. Geneva: WHO (2013) [cited 2013 Jul 1]; p. 1–105. Available from: http://apps.who.int/iris/bitstream/10665/85380/1/9789241505871_eng.pdf
27. Frieden TR, Bloomberg MR. How to prevent 100 million deaths from tobacco. Lancet (2007) 369:1758–61. doi:10.1016/S0140-6736(07)60782-X
28. Hruba D, Zaloudikova I. What limits the effectiveness of school-based anti-smoking programmes? Cent Eur J Public Health (2012) 20:18–23.
29. Friedman LC. Tobacco industry use of corporate social responsibility tactics as a sword and a shield on secondhand smoke issues. J Law Med Ethics (2009) 37:819–27. doi:10.1111/j.1748-720X.2009.00453.x
30. Thomson G. Trust Us We’re Socially Responsible. [Internet]. Auckland: ASH NZ (2005) [cited 2013 Jul 1]; p. 1–55. Available from: http://www.ash.org.nz/wp-content/uploads/2013/01/Research_commisoned_by_ASH/Trust_us_we_re_socially_responsible.pdf
31. Wakefield M, McLeod K, Perry CL. “Stay away from them until you’re old enough to make a decision”: tobacco company testimony about youth smoking initiation. Tob Control (2006) 15(Suppl 4):iv44–53. doi:10.1136/tc.2005.011536
32. Coombs J, Bond L, Van V, Daube M. “Below the line”: the tobacco industry and youth smoking. Australas Med J (2011) 4:655–73. doi:10.4066/AMJ.20111018
33. Kelsey J. New-generation free trade agreements threaten progressive tobacco and alcohol policies. Addiction (2012) 107:1719–21. doi:10.1111/j.1360-0443.2012.03874.x
34. Kelsey J. The trans-pacific partnership agreement: a gold-plated gift to the global tobacco industry? Am J Law Med (2013) 39:237–64.
35. Gleeson D, Friel S. Emerging threats to public health from regional trade agreements. Lancet (2013) 381:1507–9. doi:10.1016/S0140-6736(13)60312-8
36. Rosenblatt R. How Do Tobacco Executives Live With Themselves? [Internet]. New York Times (1994). Available from: http://www.nytimes.com/1994/03/20/magazine/how-do-tobacco-executives-live-with-themselves.html?pagewanted=print&src=pm
37. Crosby MH. Religious challenge by shareholder actions: changing the behaviour of tobacco companies and their allies. BMJ (2000) 321:375–7. doi:10.1136/bmj.321.7257.375
38. Warner KE. The economics of tobacco: myths and realities. Tob Control (2000) 9:78–89. doi:10.1136/tc.9.1.78
39. Lai DT, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev (2010):CD006936. doi:10.1002/14651858.CD006936.pub2
40. Zwink N, Jenetzky E, Brenner H. Parental risk factors and anorectal malformations: systematic review and meta-analysis. Orphanet J Rare Dis (2011) 6:25. doi:10.1186/1750-1172-6-25
41. Chang JS, Selvin S, Metayer C, Crouse V, Golembesky A, Buffler PA. Parental smoking and the risk of childhood leukemia. Am J Epidemiol (2006) 163:1091–100. doi:10.1093/aje/kwj143
42. Milne E, Greenop KR, Scott RJ, Bailey HD, Attia J, Dalla-Pozza L, et al. Parental prenatal smoking and risk of childhood acute lymphoblastic leukemia. Am J Epidemiol (2012) 175:43–53. doi:10.1093/aje/kwr275
43. Boffetta P, Tredaniel J, Greco A. Risk of childhood cancer and adult lung cancer after childhood exposure to passive smoke: a meta-analysis. Environ Health Perspect (2000) 108:73–82. doi:10.1289/ehp.0010873
44. Rudant J, Menegaux F, Leverger G, Baruchel A, Lambilliotte A, Bertrand Y, et al. Childhood hematopoietic malignancies and parental use of tobacco and alcohol: the ESCALE study (SFCE). Cancer Causes Control (2008) 19:1277–90. doi:10.1007/s10552-008-9199-5
45. Sorahan T, McKinney PA, Mann JR, Lancashire RJ, Stiller CA, Birch JM, et al. Childhood cancer and parental use of tobacco: findings from the inter-regional epidemiological study of childhood cancer (IRESCC). Br J Cancer (2001) 84:141–6. doi:10.1054/bjoc.2000.1556
46. Pang D, McNally R, Birch JM. Parental smoking and childhood cancer: results from the United Kingdom Childhood Cancer Study. Br J Cancer (2003) 88:373–81. doi:10.1038/sj.bjc.6600774
47. Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, et al. A review of human carcinogens – part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol (2009) 10:1033–4. doi:10.1016/S1470-2045(09)70326-2
48. George L, Granath F, Johansson AL, Anneren G, Cnattingius S. Environmental tobacco smoke and risk of spontaneous abortion. Epidemiology (2006) 17:500–5. doi:10.1097/01.ede.0000229984.53726.33
49. Courage CM. Environmental tobacco smoke. In: Tamburlini G, von Ehrenstein OB, editors. Children’s Health and Environment: A Review of Evidence [Internet]. Chapter 10. Copenhagen: World Health Organization Regional Office for Europe and European Environment Agency (2002) [cited 2013 Jul 1]; p. 142–151. Available from: http://www.euro.who.int/__data/assets/pdf_file/0007/98251/E75518.pdf
50. Stick S. The effects of in-utero tobacco-toxin exposure on the respiratory system in children. Curr Opin Allergy Clin Immunol (2006) 6:312–6. doi:10.1097/01.all.0000244789.10863.c4
51. Lammer EJ, Shaw GM, Iovannisci DM, Finnell RH. Maternal smoking, genetic variation of glutathione s-transferases, and risk for orofacial clefts. Epidemiology (2005) 16:698–701. doi:10.1097/01.ede.0000172136.26733.4b
52. Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci (2010) 116:364–74. doi:10.1093/toxsci/kfq103
53. Zhang K, Wang X. Maternal smoking and increased risk of sudden infant death syndrome: a meta-analysis. Leg Med (Tokyo) (2013) 15:115–21. doi:10.1016/j.legalmed.2012.10.007
54. Robertson J, Pattemore PK, Ford RP. The effect of maternal smoking on admission to hospital in infancy. N Z Med J (1993) 106:476–7.
55. Murray RL, Britton J, Leonardi-Bee J. Second hand smoke exposure and the risk of invasive meningococcal disease in children: systematic review and meta-analysis. BMC Public Health (2012) 12:1062. doi:10.1186/1471-2458-12-1062
56. Dezateux C, Stocks J, Dundas I, Fletcher ME. Impaired airway function and wheezing in infancy: the influence of maternal smoking and a genetic predisposition to asthma. Am J Respir Crit Care Med (1999) 159:403–10. doi:10.1164/ajrccm.159.2.9712029
57. Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics (2012) 129:735–44. doi:10.1542/peds.2011-2196
58. Alati R, Al Mamun A, O’Callaghan M, Najman JM, Williams GM. In utero and postnatal maternal smoking and asthma in adolescence. Epidemiology (2006) 17:138–44. doi:10.1097/01.ede.0000198148.02347.33
59. Hayatbakhsh MR, Sadasivam S, Mamun AA, Najman JM, Williams GM, O’Callaghan MJ. Maternal smoking during and after pregnancy and lung function in early adulthood: a prospective study. Thorax (2009) 64:810–4. doi:10.1136/thx
60. Cohen RT, Raby BA, Van Steen K, Fuhlbrigge AL, Celedon JC, Rosner BA, et al. In utero smoke exposure and impaired response to inhaled corticosteroids in children with asthma. J Allergy Clin Immunol (2010) 126:491–7. doi:10.1016/j.jaci.2010.06.016
61. Difranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics (2004) 113:1007–15.
62. Lalwani AK, Liu YH, Weitzman M. Secondhand smoke and sensorineural hearing loss in adolescents. Arch Otolaryngol Head Neck Surg (2011) 137:655–62. doi:10.1001/archoto.2011.109
63. Mattsson K, Kallen K, Longnecker MP, Rignell-Hydbom A, Rylander L. Maternal smoking during pregnancy and daughters’ risk of gestational diabetes and obesity. Diabetologia (2013) 56:1689–95. doi:10.1007/s00125-013-2936-7
64. Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) (2008) 32:201–10. doi:10.1038/sj.ijo.0803760
65. Mendez MA, Torrent M, Ferrer C, Ribas-Fito N, Sunyer J. Maternal smoking very early in pregnancy is related to child overweight at age 5-7 y. Am J Clin Nutr (2008) 87:1906–13.
66. Harris HR, Willett WC, Michels KB. Parental smoking during pregnancy and risk of overweight and obesity in the daughter. Int J Obes (Lond) (2013). doi:10.1038/ijo.2013.101. [Epub ahead of print].
67. Sorensen HT, Norgard B, Pedersen L, Larsen H, Johnsen SP. Maternal smoking and risk of hypertrophic infantile pyloric stenosis: 10 year population based cohort study. BMJ (2002) 325:1011–2. doi:10.1136/bmj.325.7371.1011
68. Al Mamun A, O’Callaghan FV, Alati R, O’Callaghan M, Najman JM, Williams GM, et al. Does maternal smoking during pregnancy predict the smoking patterns of young adult offspring? A birth cohort study. Tob Control (2006) 15:452–7. doi:10.1136/tc.2006.016790
69. Kwok MK, Schooling CM, Ho LM, Leung SS, Mak KH, McGhee SM, et al. Early life second-hand smoke exposure and serious infectious morbidity during the first 8 years: evidence from Hong Kong’s “Children of 1997” birth cohort. Tob Control (2008) 17:263–70. doi:10.1136/tc.2007.023887
70. Wilson KM, Pier JC, Wesgate SC, Cohen JM, Blumkin AK. Secondhand tobacco smoke exposure and severity of influenza in hospitalized children. J Pediatr (2013) 162:16–21. doi:10.1016/j.jpeds.2012.06.043
71. Tinuoye O, Pell JP, Mackay DF. Meta-analysis of the association between secondhand smoke exposure and physician-diagnosed childhood asthma. Nicotine Tob Res (2013). doi:10.1093/ntr/ntt033. [Epub ahead of print].
72. Simonetti GD, Schwertz R, Klett M, Hoffmann GF, Schaefer F, Wuhl E. Determinants of blood pressure in preschool children: the role of parental smoking. Circulation (2011) 123:292–8. doi:10.1161/CIRCULATIONAHA.110.958769
73. Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest (2002) 122:409–15. doi:10.1378/chest.122.2.409
74. Radic SD, Gvozdenovic BS, Pesic IM, Zivkovic ZM, Skodric-Trifunovic V. Exposure to tobacco smoke among asthmatic children: parents’ smoking habits and level of education. Int J Tuberc Lung Dis (2011) 15:276–80.
75. Stapleton M, Howard-Thompson A, George C, Hoover RM, Self TH. Smoking and asthma. J Am Board Fam Med (2011) 24:313–22. doi:10.3122/jabfm.2011.03.100180
76. McCarville M, Sohn MW, Oh E, Weiss K, Gupta R. Environmental tobacco smoke and asthma exacerbations and severity: the difference between measured and reported exposure. Arch Dis Child (2013) 98:510–4. doi:10.1136/archdischild-2012-303109
77. Bisgaard H, Jensen SM, Bonnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med (2012) 185:1183–9. doi:10.1164/rccm.201110-1922OC
78. Chen X, Abdulhamid I, Woodcroft K. Maternal smoking during pregnancy, polymorphic CYP1A1 and GSTM1, and lung-function measures in urban family children. Environ Res (2011) 111:1215–21. doi:10.1016/j.envres.2011.08.003
79. Kalliola S, Pelkonen AS, Malmberg LP, Sarna S, Hamalainen M, Mononen I, et al. Maternal smoking affects lung function and airway inflammation in young children with multiple-trigger wheeze. J Allergy Clin Immunol (2013) 131:730–5. doi:10.1016/j.jaci.2013.01.005
80. Pileggi C, Carbone V, Nobile CG, Pavia M. Blood pressure and related cardiovascular disease risk factors in 6-18 year-old students in Italy. J Paediatr Child Health (2005) 41:347–52. doi:10.1111/j.1440-1754.2005.00629.x
81. Le-Ha C, Beilin LJ, Burrows S, Huang RC, Oddy WH, Hands B, et al. Gender difference in the relationship between passive smoking exposure and HDL-cholesterol levels in late adolescence. J Clin Endocrinol Metab (2013) 98:2126–35. doi:10.1210/jc.2013-1016
82. Moskowitz WB, Mosteller M, Schieken RM, Bossano R, Hewitt JK, Bodurtha JN, et al. Lipoprotein and oxygen transport alterations in passive smoking preadolescent children. The MCV Twin Study. Circulation (1990) 81:586–92. doi:10.1161/01.CIR.81.2.586
Keywords: tobacco, passive smoking, children, environmental risk, ethics
Citation: Pattemore PK (2013) Tobacco or healthy children: the two cannot co-exist. Front. Pediatr. 1:20. doi: 10.3389/fped.2013.00020
Received: 12 July 2013; Accepted: 06 August 2013;
Published online: 23 August 2013.
Edited by:
Anne B. Chang, Queensland University of Technology, AustraliaReviewed by:
James Francis Chmiel, Western Reserve University, USAAnne B. Chang, Queensland University of Technology, Australia
Copyright: © 2013 Pattemore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philip Keith Pattemore, Department of Paediatrics, University of Otago, PO Box 4345, Christchurch Mail Centre, Christchurch 8140, New Zealand e-mail:cGhpbGlwLnBhdHRlbW9yZUBvdGFnby5hYy5ueg==
