- 1Division of Pediatric Critical Care Medicine, Department of Pediatrics, Children’s Healthcare of Atlanta, Emory University School of Medicine, Atlanta, GA, USA
- 2Division of Infectious Disease, Department of Pediatrics, Emory University School of Medicine, Atlanta, GA, USA
- 3Division of Neonatal-Perinatal Medicine, Department of Pediatrics, Emory University School of Medicine, Atlanta, GA, USA
- 4Division of Pulmonology, Allergy & Immunology, Cystic Fibrosis, and Sleep, Department of Pediatrics, Emory University School of Medicine, Atlanta, GA, USA
Background: Oxidative stress is known to play a role in critical illness due to an imbalance in reactive oxygen species and reactive nitrogen species, and the body’s ability to detoxify pro-oxidants using small molecule anti-oxidants and anti-oxidant enzymes.
Objective: To compare the concentrations of plasma redox metabolites and redox potentials for the Cys/CySS and GSH/GSSG thiol/disulfide pairs in critically ill children with healthy control children.
Methods: We performed a prospective clinical observational study of children ages ≤18 years and weight ≥6 kg, who were hospitalized between January 2010 and April 2012 in a 30-bed multidisciplinary medical-surgical pediatric intensive care unit (PICU). We measured the plasma concentrations of Cys, CySS, GSH, and GSSG within the first 24 h of PICU arrival, and we calculated the redox potential for the Cys/CySS (Eh Cys/CySS) and GSH/GSSG (Eh GSH/GSSG) thiol/disulfide pairs in the plasma of 61 critically ill children and 16 healthy control children.
Results: Critically ill children have less Cys (p = 0.009), less CySS (p = 0.011), less Total Cys ([Cys] + 2[CySS], p = 0.01), more GSSG (p < 0.001), and more oxidized Eh GSH/GSSG (p < 0.001) compared to healthy children.
Conclusion: Our results demonstrate that in the presence of pediatric critical illness, the Total Cys/CySS thiol pool decreases while GSH is likely one component of the cellular redox system that reduces CySS back to Cys, thus maintaining Eh Cys/CySS. The Total Cys pool is more abundant than the Total GSH pool in the plasma of children. Further investigation is needed to elucidate the differences in redox potentials in subgroups of critically ill children, and to determine whether differences in redox metabolite concentrations and redox potentials correlate with severity of critical illness and clinical outcomes.
Introduction
Oxidative stress (OS) causes irreversible damage to DNA, lipids, and proteins (1–3), and is defined as a disruption of redox signaling and control (4–6). The thiols cysteine (Cys) and glutathione (GSH) are common sources of reducing equivalents for neutralizing oxidative stress. Critical Cys residues of many enzymes, receptors, ion channels, transporters, and transcription factors also sense oxidative stress and the oxidative state of these Cys residues influence protein function (4, 5, 7–9). While other small molecule anti-oxidants such as ascorbic acid, uric acid, tocopherols, and carotenoids are important in maintaining redox state of cells, the relative contribution of low molecular weight thiols to total antioxidant capacity is not clear. In addition, the most abundant thiols available for reducing equivalents in serum are albumin and other protein thiols (10, 11).
Cysteine undergoes rapid autoxidation and is toxic to mammalian cells at concentrations exceeding the normal, low micromolar concentrations found in plasma (12). The toxicity of Cys is controlled by incorporating cysteine into the tripeptide GSH (13). While Cys and CySS constitute the predominant low-molecular-weight thiol-disulfide pool in human plasma (14), GSH is more likely to remain reduced in an oxidative environment than Cys. GSH is also maintained in tissues at millimolar concentration with a relatively reduced redox state (15), indicating that the Cys/CySS and GSH/GSSG pool are not in equilibrium (14).
Clinical experience in adults has demonstrated that redox balances of Cys and its disulfide cystine (CySS), and GSH and its glutathione disulfide (GSSG) reflect antioxidant function (16). However, the redox balance of plasma Cys/CySS and GSH/GSSG have not been well characterized in critically ill children. This study compared the concentrations of Cys, CySS, GSH, GSSG, and the redox potentials of the Cys/CySS (Eh Cys/CySS) and GSH/GSSG (Eh GSH/GSSG) couples in plasma of healthy and critically ill children. Once a difference between redox potentials between healthy control and critically ill children is established, correlation of these redox metabolites with diagnoses, severity of illness scores, and clinical outcomes may lead to the development of new biomarkers of pediatric critical illness.
Patients, Materials, and Methods
We performed a prospective clinical observational study in the pediatric intensive care unit (PICU) at Children’s Healthcare of Atlanta at Egleston between January 2010 and April 2012. The PICU is a 30-bed multidisciplinary medical-surgical ICU providing quaternary care for patients 0–21 years of age. This study was approved by the Institutional Review Boards of Emory University and Children’s Healthcare of Atlanta. Informed consent was obtained from each patient’s guardian.
Human Subjects
Pediatric intensive care unit (PICU) patients aged 0–18 years weighing ≥6 kg were eligible for enrollment. Blood draws were performed within 24 h of PICU admission. Healthy control children were recruited by advertisement for comparison with PICU patients, and controls were not compensated for their participation in this study. Exclusion criteria for controls included any medication use and a physician diagnosis of chronic disease. Control children were evaluated during an outpatient-only research visit that was rescheduled if upper respiratory infection or other illnesses were present.
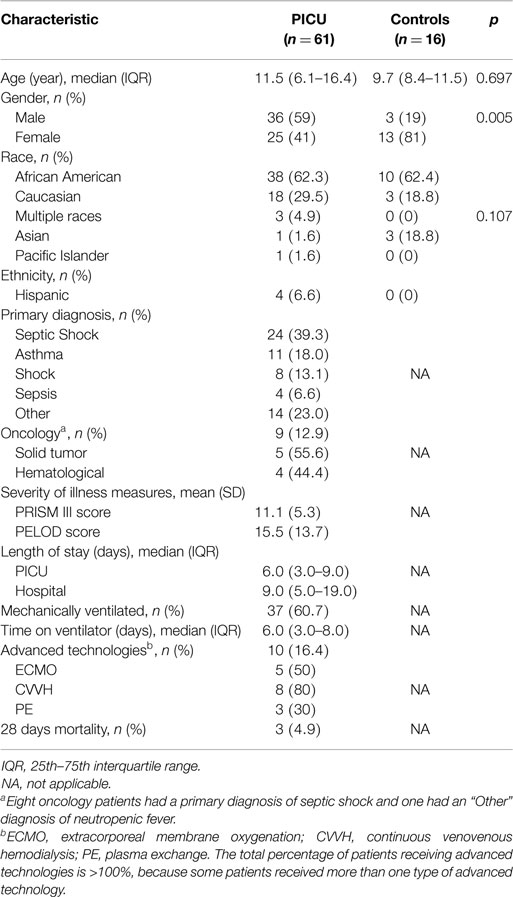
Demographic and clinical data such as age, gender, race, primary diagnosis, duration of mechanical ventilation, length of pediatric intensive care unit stay (PICU LOS), length of hospital stay (Hospital LOS), need for advanced technologies such as extracorporeal membranous oxygenation (ECMO), continuous veno-venous hemofiltration (CVVH), and plasma exchange (PE) were noted.
Determination of Severity of Illness
Septic shock was defined and classified according to the American College of Critical Care Medicine (ACCM) definitions of cardiovascular support (17). Severity of illness scores were calculated using the Pediatric Risk of Mortality score III (PRISM III) and pediatric logistic organ dysfunction (PELOD) score (18–20).
Sample Collection, Processing, and Redox State Calculations
After obtaining informed consent, plasma samples were immediately placed on ice, and transported to the Emory+Children’s Biomarker Core laboratory. Samples were placed in derivatization solution to stop oxidation and analyses of plasma were performed using high performance liquid chromatography as previously described (21–23). Briefly, blood was collected from controls by peripheral venipuncture or from PICU patients by accessing a central venous line, and 0.25 ml was immediately transferred to a microcentrifuge tube containing 0.25 ml of a preservation solution containing 100 mM serine-borate (pH 8.5) containing (per ml) 0.5 mg sodium heparin, 1 mg bathophenanthroline disulfonate sodium salt (BPDS), and 2 mg iodoacetic acid (21, 22). This procedure has been shown to minimize autoxidation and hemolysis (22). Following centrifugation to remove blood cells, aliquots (125 μl) were transferred to tubes containing 125 μl of 10% (w/v) perchloric acid containing 0.2M boric acid and 10 μM γ-l- glutamyl-l-glutamate as an internal standard. Samples were stored at −80°C prior to further processing to form N-dansyl derivatives and analysis by HPLC with fluorescence detection. Metabolites were identified by co-elution with standards, and quantification was obtained by integration relative to the internal standard (22). Total Cys and Total GSH were calculated using the equations: [Cys] + 2[CySS] and [GSH] + 2[GSSG], respectively. The redox states (Eh) of the thiol/disulfide pools were calculated with the Nernst equation using standard potentials for the GSH/GSSG (−264 mV) and Cys/CySS (−250 mV) pairs at pH 7.4, as previously described (16, 24).
Statistical Methods
All statistical analyses were performed using SAS 9.3 (Cary, NC, USA) and statistical significance was assessed at the 0.05 significance level unless otherwise noted. Since specific differences in metabolite and redox values are difficult to predict, statistical power was evaluated by utilization of an effect size. Samples of 61 cases and 16 controls were obtained giving 80% power to detect an effect size of 0.8 using a two-sample Wilcoxon-test. Prior to the analysis, the assumption of normality for the metabolite and redox values was assessed using the Shapiro-Wilk test for normality and by visual inspection of the histograms. As has been previously reported (16), evaluation of metabolite data demonstrated that none of the metabolites, with the exception of the redox potentials (Eh), were normally distributed, and thus, values were log transformed and adjusted for differences in age, gender, and race using generalized linear models. Back-transformed least-squares means and 95% confidence intervals are reported. Discrete variables are shown by frequency counts and percentages.
Results
Subjects and Clinical Characteristics
Demographic data are listed in Table 1. Primary diagnoses in the category labeled “Other” included: severe head trauma, post-operative from spinal fusion surgery, cerebral hypertension, tuberculosis, status epilepticus, brain abscess, post-bronchial foreign body, pertussis, traumatic brain injury, acute chest syndrome, parapneumonic effusion, neutropenic fever, and demyelinating central nervous system disease.
Redox Status of PICU Subjects Compared with Healthy Children
Redox data from the PICU subjects and healthy control children are shown in Figure 1. After adjusting for age, gender, and race, children admitted to the PICU had 2.5 times less Cys (2.29 vs. 0.91 μM; p = 0.009), 2.8 times less CySS (2.98 vs. 1.05 μM; p = 0.011), and 2.5 times less Total Cys (8.52 vs. 3.41 μM; p = 0.01) (Figure 1A). Children admitted to the PICU had no difference in GSH concentrations (0.29 vs. 0.22 μM; p = 0.573) or Total GSH (0.37 vs. 0.69 μM; p = 0.106), but PICU patients had 13 times more GSSG (0.01 vs. 0.13 μM; p < 0.001) than healthy control children (Figure 1B). Finally, children admitted to the PICU showed no difference in Eh Cys/CySS (−77 vs. −67 mV; p = 0.095) compared to healthy children; however, critically ill children had a ~40 mV more oxidized Eh GSH/GSSG (−113 vs. −71 mV; p < 0.001, Figure 1C) compared to healthy control children.
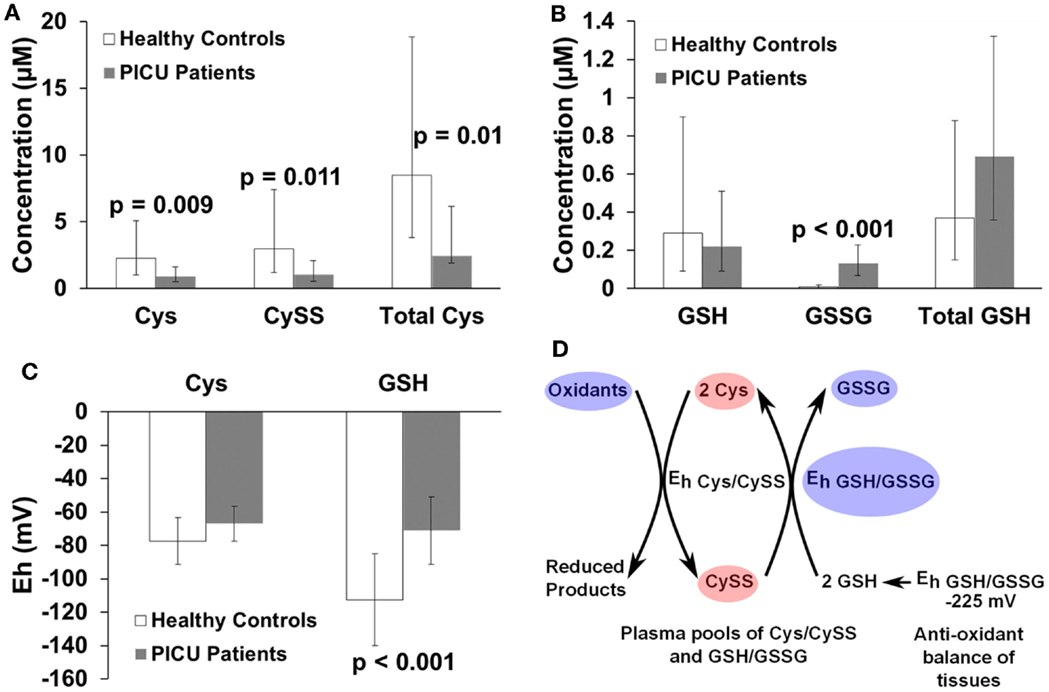
Figure 1. Bar graphs comparing concentrations of (A) Cys (μM), CySS (μM), and Total Cys (μM); (B) GSH (μM), GSSG (μM), and Total GSH (μM); (C) Eh Cys/CySS (mV) and Eh GSH/GSSG (mV) of healthy children controls with PICU patients. Individual bars indicate least-squares mean values and the whiskers represent 95% confidence intervals. Metabolite concentration data were log-transformed, and the back-transformed least-squares means are presented. The Eh are normally distributed, and a less negative number reflects a more oxidized redox value. All metabolite and Eh values were adjusted for age, gender, and race. p-Values are listed above the pairs being compared. (D) Represents a model summarizing the redox data in (A–C), adapted from Jones et al. (16). The Total Cys concentration is higher than that of Total GSH. Because Cys is more abundant and more readily oxidized than GSH (as indicated by a less negative redox potential), Cys is preferentially oxidized to CySS in plasma. GSH in the plasma serves as a reducing pool to convert CySS back to Cys, thus maintaining the Eh Cys/CySS in critically ill children nearly equal to that of healthy children. The redox balance of Cys is preserved by the supply of GSH from tissues. Red ovals represent a significant decrease in concentration of a metabolite. Blue ovals represent a significant increase in concentration of a metabolite or a more oxidized redox potential (Eh). Ovals are not representative of the magnitude of change and are not drawn to scale.
Redox Status as a Function of Age, Gender, Primary Diagnosis, Severity of Illness Scores, Use of Advanced Technologies, and Clinical Outcomes in PICU Patients
Children in the PICU with organ dysfunction requiring advanced technologies (n = 10) such as ECMO, CVVH, and PE have less Cys [Median (IQR) 0.5 μM (0.3–0.57) vs. 1.02 μM (0.25–2.6); p = 0.027], less CySS [0.24 μM (0.1–0.54) vs. 0.79 μM (0.44–3.64); p = 0.027], and less Total Cys [1.02 μM (0.64–1.60) vs. 2.85 μM (1.26–11.15); p = 0.019] when compared with other children admitted to the PICU (n = 51). There was no difference in Eh Cys/CySS [Mean (SD) −64 mV (13) vs. −66 mV (7); p = 0.911] or any of the GSH redox parameters (p > 0.8) measured when comparing the patients receiving advanced technologies with the remaining PICU patients. We compared redox metabolite values, Eh Cys/CySS, and Eh GSH/GSSG across age and gender and found no significant differences for the PICU patients. There were no strong correlations (ρ ≥ 0.4) significant between primary diagnosis, severity of illness scores, such as PELOD and PRISM III score, or clinical outcomes such as, PICU LOS, Hospital LOS, or days of mechanical ventilation with redox metabolites, Eh Cys/CySS, or Eh GSH/GSSG.
Discussion
This study compared the plasma redox metabolite concentrations and redox potentials for the Cys/CySS and GSH/GSSG pairs between critically ill and healthy children. We found that in critically ill children, the total Cys/CySS thiol pool was ~2.5 times less than that in healthy children. Despite the reduction in Total Cys in critically ill children, the Eh Cys/CySS in critically ill children was maintained at healthy control levels. However, the Eh GSH/GSSG was ~40 mV more oxidized in critically ill vs. healthy children. These findings suggest that while Cys/CySS is the most abundant low-molecular-weight thiol-disulfide pool in plasma (23 times more abundant in plasma of healthy children than the GSH/GSSG pool), GSH is a better reducing agent than Cys as denoted by a more negative redox potential. Therefore, GSH reduces CySS back to Cys in critically ill children, thus maintaining Eh Cys/CySS as summarized in Figure 1D, based on a model derived by Jones et al. (16).
This is not the first study to measure cysteine and glutathione concentrations in children. Lyons and colleagues measured Cys metabolism and glutathione synthesis in 10 septic pediatric patients and compared with 10 post-operative controls (25). Results from the Lyons study showed that there was no difference in Cys or CySS levels when methionine intake was controlled; however, a 60% decrease in GSH synthesis rates was noted in septic patients vs. controls (25). Németh and Boda measured a xanthine oxidase activity index and a GSSG/GSH ratio in infants and children with septic shock and showed that these indices were significantly correlated with each other, with severity of illness scores (PRISM), and were increased in patients in a proinflammatory state (26). Our results extend this work by adding the Eh Cys/CySS and Eh GSH/GSSH to the literature of pediatric acute critical illness. Redox potential, calculated using the Nernst equation, accounts for the stoichiometry of two electron transfers as two GSH are involved in the oxidation to GSSG (6). As an example, the ratio of GSH/GSSG is also used to quantify reducing force for this thiol/disulfide pair; however, a GSH/GSSG ratio of 100 has very different reducing power for a GSH of 10 mM and a GSSG of 100 μM with an Eh GSH/GSSG of −264 mV vs. a GSH of 100 μM and a GSSG of 1 μM with an Eh GSH/GSSG of −204 mV (27, 28). While many assays exist for measuring individual pro-oxidant and anti-oxidant levels, the reason for quantifying the Eh Cys/CySS and Eh GSH/GSSG in serum is that it provides an overall measure of the pro-oxidant/anti-oxidant balance (6, 28).
Fitzpatrick and colleagues have measured the Eh Cys/CySS and Eh GSH/GSSH of mild-to-moderate and severe asthmatics (23). In comparison, PICU patients from the current study (mean Eh Cys/CySS −65 mV) have a significantly more oxidized Cys/CySS redox potential than mild-to-moderate asthmatics (mean Eh Cys/CySS −95 mV) and are similarly oxidized to severe asthmatics in the Fitzpatrick study (mean Eh Cys/CySS −55 mV). The subset of PICU patients admitted with a primary diagnosis of asthma (n = 11) tended to be the most oxidized subset of patients in the PICU with a mean Eh Cys/CySS −51 mV. For the GSH/GSSG redox state, severe asthmatics in the Fitzpatrick study (23) have a mean Eh GSH/GSSH of −90 mV similar to asthmatics admitted to the PICU in this study (Eh GSH/GSSG −93 mV). Due to the small numbers of asthmatics enrolled in our study, the Eh Cys/CySS and Eh GSH/GSSG results of patients admitted to the PICU with a primary diagnosis of asthma are not significantly different when compared with PICU patients with other primary diagnoses.
The current prospective study has several limitations. It is a small single-center study comparing critically ill children with diverse diagnoses. Because of the small number of patients with a variety of diagnoses, the study was not adequately powered to detect correlations between redox markers, clinical diagnoses, severity of illness scores, or outcomes such as length of PICU stay or duration of mechanical ventilation. We measured plasma redox markers, but measurements of local tissue redox metabolites, for example redox metabolites in the lung of asthmatics or in those with acute lung injury, may be a more relevant marker of OS in the microenvironment of the lung. We did not compare other biomarkers of oxidative stress or anti-oxidant potential with Eh Cys/CySS and Eh GSH/GSSG, such as levels of ascorbic acid, 4-hydroxynonenal, malondialdehyde, F2-isoprostanes, or enzymatic activity of common redox enzymes such as superoxide dismutase, glutathione peroxiredoxin, or thioredoxin. This lack of comparison is not unique to our study, and there are several studies comparing plasma biomarkers of oxidative stress in adults have not resulted in correlations among oxidative stress markers or clinical outcomes (29–33). In addition, a recently published study of lipopolysaccharide (LPS) induced sepsis in a porcine model did not show any correlation of many redox markers with each other or with clinical severity of illness (34). Despite these limitations, our data are in agreement with some of the findings of prior studies in septic children (25, 26), and confirm that significant differences exist in the Eh GSH/GSSG, rather than Eh Cys/CySS, of children admitted to a PICU when compared to healthy control children.
In summary, a decrease in the abundance of Total Cys, an increase in GSSG, and a more oxidized Eh GSH/GSSG may serve as potential markers of OS events in the plasma of critically ill children. Understanding changes that occur in redox metabolites, redox potentials, and redox signaling pathways in childhood diseases may lead to novel prognostic markers and therapeutic targets in pediatric critical illness. Future studies will work toward assessing the role of protein thiols, other markers of oxidative stress, and signal transduction pathways to elucidate the mechanism of oxidative stress in critically ill children. In addition to studying the biological mechanisms of how changes in Eh Cys/CySS and Eh GSH/GSSG correlate with other markers of OS and influence redox sensitive redox signaling pathways, we plan on evaluating whether the balance of Cys/CySS and GSH/GSSG can differentiate subpopulations of critically ill children with differing severity of critical illness.
Conflict of Interest Statement
The authors Jocelyn R. Grunwell, Scott E. Gillespie, Janine M. Ward, Theresa W. Gauthier, Lou Ann Brown, and Kiran B. Hebbar have indicated they have no financial relationships relevant to the article to disclose. Anne M. Fitzpatrick has received consulting fees from the following companies: Boehringer Ingelheim, Genentech Consulting, GlaxoSmithKline Scientific Advisory Board, MedImmune, Inc., Consulting, and Merck Scientific Advisory Board.
Acknowledgments
Funding Source: This study was supported by an internal award from the Children’s Healthcare of Atlanta Friends grant number 38234, by RO1 NR012021, and by award number UL1TR000454 from the National Center for Advancing the Translational Sciences. We also acknowledge the Emory+Children’s Statistical Core for assistance and the Emory+Children’s Biomarkers Core for analyte analyses.
Abbreviations
Cys, cysteine; CySS, cystine; CySSG, cysteine-glutathione disulfide (mixed disulfide); Eh, redox state calculated from Nernst equation; GSH, glutathione; GSSG, glutathione disulfide.
References
1. Chevion M, Berenshtein E, Stadtman ER. Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radic Res (2000) 33(Suppl):S99–108.
2. Onorato JM, Thorpe SR, Baynes JW. Immunohistochemical and ELISA assays for biomarkers of oxidative stress in aging and disease. Ann N Y Acad Sci (1998) 854:277–90. doi: 10.1111/j.1749-6632.1998.tb09909.x
3. Tahara S, Matsuo M, Kaneko T. Age-related changes in oxidative damage to lipids and DNA in rat skin. Mech Ageing Dev (2001) 122(4):415–26. doi:10.1016/S0047-6374(00)00257-8
4. Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med (2000) 28(3):463–99. doi:10.1016/S0891-5849(99)00242-7
5. Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal (1999) 11(1):1–14. doi:10.1016/S0898-6568(98)00037-0
6. Jones DP. Redefining oxidative stress. Antioxid Redox Signal (2006) 8(9–10):1865–79. doi:10.1089/ars.2006.8.1865
7. Paulsen CE, Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol (2010) 5(1):47–62. doi:10.1021/cb900258z
8. Paulsen CE, Carroll KS. Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery. Chem Rev (2013) 113(7):4633–79. doi:10.1021/cr300163e
9. Truong TH, Carroll KS. Redox regulation of epidermal growth factor receptor signaling through cysteine oxidation. Biochemistry (2012) 51(50):9954–65. doi:10.1021/bi301441e
10. Turell L, Radi R, Alvarez B. The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic Biol Med (2013) 65:244–53. doi:10.1016/j.freeradbiomed.2013.05.050
11. Anraku M, Chuang VT, Maruyama T, Otagiri M. Redox properties of serum albumin. Biochim Biophys Acta (2013) 1830(12):5465–72. doi:10.1016/j.bbagen.2013.04.036
12. Janaky R, Varga V, Hermann A, Saransaari P, Oja SS. Mechanisms of l-cysteine neurotoxicity. Neurochem Res (2000) 25(9–10):1397–405. doi:10.1023/A:1007616817499
13. Ookhtens M, Kaplowitz N. Role of the liver in interorgan homeostasis of glutathione and cyst(e)ine. Semin Liver Dis (1998) 18(4):313–29. doi:10.1055/s-2007-1007167
14. Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med (2000) 28(4):625–35. doi:10.1016/S0891-5849(99)00275-0
15. Kirlin WG, Cai J, Thompson SA, Diaz D, Kavanagh TJ, Jones DP. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med (1999) 27(11–12):1208–18. doi:10.1016/S0891-5849(99)00145-8
16. Jones DP, Mody VC Jr, Carlson JL, Lynn MJ, Sternberg P Jr. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med (2002) 33(9):1290–300. doi:10.1016/S0891-5849(02)01040-7
17. Hollenberg SM, Ahrens TS, Astiz ME, Chalfin DB, Dasta JF, Heard SO, et al. Practice parameters for hemodynamic support of sepsis in adult patients in sepsis: task force of the American college of critical care medicine, society of critical care medicine. Crit Care Med (1999) 27:695–7.
18. Available from: http://www.sfar.org/scores2/pelod2.html
19. Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet (2003) 362(9379):192–7. doi:10.1016/S0140-6736(03)13908-6
20. Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med (1996) 24(5):743–52. doi:10.1097/00003246-199605000-00004
21. Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol (2002) 348:93–112. doi:10.1016/S0076-6879(02)48630-2
22. Jones DP, Carlson JL, Samiec PS, Sternberg P Jr, Mody VC Jr, Reed RL, et al. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta (1998) 275(2):175–84. doi:10.1016/S0009-8981(98)00089-8
23. Fitzpatrick AM, Stephenson ST, Hadley GR, Burwell L, Penugonda M, Simon DM, et al. Thiol redox disturbances in children with severe asthma are associated with posttranslational modification of the transcription factor nuclear factor (erythroid-derived 2)-like 2. J Allergy Clin Immunol (2011) 127(6):1604–11. doi:10.1016/j.jaci.2011.03.031
24. Clark WM. Oxidation-Reduction Potentials of Organic Systems. Baltimore, MD: Williams & Wilkins (1960).
25. Lyons J, Rauh-Pfeiffer A, Ming-Yu Y, Lu XM, Zurakowski D, Curley M, et al. Cysteine metabolism and whole blood glutathione synthesis in septic pediatric patients. Crit Care Med (2001) 29(4):870–7. doi:10.1097/00003246-200104000-00036
26. Nemeth I, Boda D. Xanthine oxidase activity and blood glutathione redox ratio in infants and children with septic shock syndrome. Intensive Care Med (2001) 27(1):216–21. doi:10.1007/s001340000791
27. Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med (2009) 47(10):1329–38. doi:10.1016/j.freeradbiomed.2009.08.021
28. Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med (2001) 30(11):1191–212. doi:10.1016/S0891-5849(01)00480-4
29. Watters JL, Satia JA, da Costa KA, Boysen G, Collins LB, Morrow JD, et al. Comparison of three oxidative stress biomarkers in a sample of healthy adults. Biomarkers (2009) 14(8):587–95. doi:10.3109/13547500903183954
30. Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, et al. Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem (2007) 53(9):1652–7. doi:10.1373/clinchem.2006.083923
31. Sakhi AK, Russnes KM, Thoresen M, Bastani NE, Karlsen A, Smeland S, et al. Pre-radiotherapy plasma carotenoids and markers of oxidative stress are associated with survival in head and neck squamous cell carcinoma patients: a prospective study. BMC Cancer (2009) 9:458. doi:10.1186/1471-2407-9-458
32. Bar-Or D, Bar-Or R, Rael LT, Brody EN. Oxidative stress in severe acute illness. Redox Biol (2015) 4C:340–5. doi:10.1016/j.redox.2015.01.006
33. Batra S, Kumar R, Kapoor AK, Ray G. Alterations in antioxidant status during neonatal sepsis. Ann Trop Paediatr (2000) 20(1):27–33. doi:10.1080/02724930092039
Keywords: oxidative stress, pediatric, critical illness, glutathione, cysteine, redox potential
Citation: Grunwell JR, Gillespie SE, Ward JM, Fitzpatrick AM, Brown LA, Gauthier TW and Hebbar KB (2015) Comparison of glutathione, cysteine, and their redox potentials in the plasma of critically ill and healthy children. Front. Pediatr. 3:46. doi: 10.3389/fped.2015.00046
Received: 15 March 2015; Accepted: 10 May 2015;
Published: 26 May 2015
Edited by:
Kanwaljeet J. S. Anand, University of Tennessee Health Science Center, USAReviewed by:
Felice Su, Stanford University, USAMichael Shoykhet, Washington University in St. Louis School of Medicine, USA
Copyright: © 2015 Grunwell, Gillespie, Ward, Fitzpatrick, Brown, Gauthier and Hebbar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jocelyn R. Grunwell, Division of Pediatric Critical Care Medicine, Department of Pediatrics, Children’s Healthcare of Atlanta at Egleston, Emory University School of Medicine, 1405 Clifton Rd. N.E., Atlanta, GA 30303, USA,amdydW53ZUBlbW9yeS5lZHU=
 Jocelyn R. Grunwell
Jocelyn R. Grunwell Scott E. Gillespie
Scott E. Gillespie Janine M. Ward
Janine M. Ward Anne M. Fitzpatrick4
Anne M. Fitzpatrick4 Lou Ann Brown
Lou Ann Brown Theresa W. Gauthier
Theresa W. Gauthier Kiran B. Hebbar
Kiran B. Hebbar