- 1Department of Medicine, Texas Tech University Health Sciences Center, El Paso, TX, USA
- 2Department of Pediatrics, Texas Tech University Health Sciences Center, El Paso, TX, USA
- 3Department of Pathology, Texas Tech University Health Sciences Center, El Paso, TX, USA
Background: Streptococcus bovis bacteremia has been associated with gastrointestinal diseases, especially colon cancer, neoplastic colon polyps, and other malignancies of the GI tract in adults. Sporadic cases of S. bovis disease have also been reported in neonates and young infants. Although uncommon, S. bovis infection can cause fulminant neonatal sepsis and meningitis.
Objectives: We report a series of pediatric patients with S. bovis bacteremia in a county hospital in a United States–Mexico border city in order to examine the demographic and clinical associations.
Methods: We characterized the demographic and clinical features in all pediatric patients with blood cultures positive for S. bovis at University Medical Center in El Paso, TX, USA between January 2000 and December 2010. Hospital records were systematically reviewed by using a standardized protocol.
Results: A total of seven episodes of S. bovis bacteremia were documented in seven pediatric patients (four female and three male). Mean age was 1.2 days (range 1–3 days), all were Hispanic, average birth weight (3.25 kg). Mode of delivery was spontaneous vaginal delivery (five) and Caesarian section (two). All of our patients developed early (<1 week) onset disease and presented with signs of respiratory distress. Five out of seven babies presented with abdominal distention and diarrhea. Six had clinical evidence of sepsis at presentation. Respiratory distress was the most common manifestation of sepsis (seven). Aspiration pneumonia was diagnosed in two of them. Most patients were treated with a combination of antibiotics (six), either ampicillin and gentamicin or ampicillin and cefotaxime, and one with ampicillin alone. None of the pediatric patients had endoscopy and none of them died.
Conclusion: Streptococcus bovis is considered as an uncommon pathogen in the newborn, but can be associated with substantial morbidity and mortality if not identified and treated early. Physicians should be alert to the less common presentation of neonatal bacteremia due to S. bovis.
Introduction
Streptococcus bovis is a non-enterococcal streptococcus in the Lancefield group D that may be found as a normal inhabitant of the human gastrointestinal tract in up to 16% of the population. The association between S. bovis bacteremia with endocarditis and gastrointestinal diseases, especially colon cancer, neoplastic colon polyps, and other gastrointestinal malignancies in adults has been well established in the literature (1–7). A meta-analysis reported that patients with positive S. bovis infection had higher concomitant rates of adenomas (43%) and colon carcinomas (18%) compared with the general asymptomatic population (8). Fernandez-Ruiz et al. reported that adenomatous polyps were found in 21/59 patients (36%) and was the most common finding among all abnormal colonic pathology results (9).
Several cases of S. bovis bacteremia have been reported in neonates and young infants (10–14). The clinical conditions that predispose to S. bovis infection in this population are unclear (15). The purpose of this study was to review the presentation of S. bovis bacteremia in pediatric patients. We report the demographic and clinical features of pediatric patients with S. bovis bacteremia in a United States–Mexico border city.
Materials and Methods
Between January 2000 and December 2010, all blood cultures positive for S. bovis submitted to the Bacteriologic Reference Laboratory of Texas Tech University Health Science Center, El Paso, TX, USA were identified.
After the approval of the protocol by the Institutional Review Board of Texas Tech University Health Science Center, the patient’s clinical records were systematically reviewed. Using a standardized protocol, we characterized their birth records, infant’s gestational age, birth weight, sex, and age at presentation. Maternal age, antenatal problems, and problems during labor were also identified from infant’s birth records.
The Texas Public Use Data File (PUDF) was used to estimate the total number of deliveries that occurred at the county hospital during the 11-year study period (January 2000 through December 2010). ICD-9-CM code V27 was used to identify deliveries. PUDF data covering the years 2004 through 2007 were readily available to the authors and were used to extrapolate the number of deliveries to the study period. The number of cases of S. bovis bacteremia was divided by the estimated number of deliveries at our county hospital.
Concomitant clinical variables were explored. Descriptive statistics of clinical characteristics were conducted by using the means, average, and percentages of data collected. Continuous variables were described using mean and SD, whereas categorical variables were described using frequencies and percentages.
Results
A total of seven pediatric patients were diagnosed with S. bovis bacteremia during the study period. The estimated number of deliveries at the county hospital during the study period was 54,714 and 98% of the babies delivered at UMC were Hispanic, resulting in an unadjusted incidence of S. bovis bacteremia of 7/54,714 or 1.3 cases/10,000 births. The proportion of all bacteremias in neonates during that time period due to S. bovis was 7/756 (0.93%). If we remove blood cultures positive for Staphylococcus epidermidis, a probable contaminant, the clinically relevant proportion would be 7/433 (1.62%).
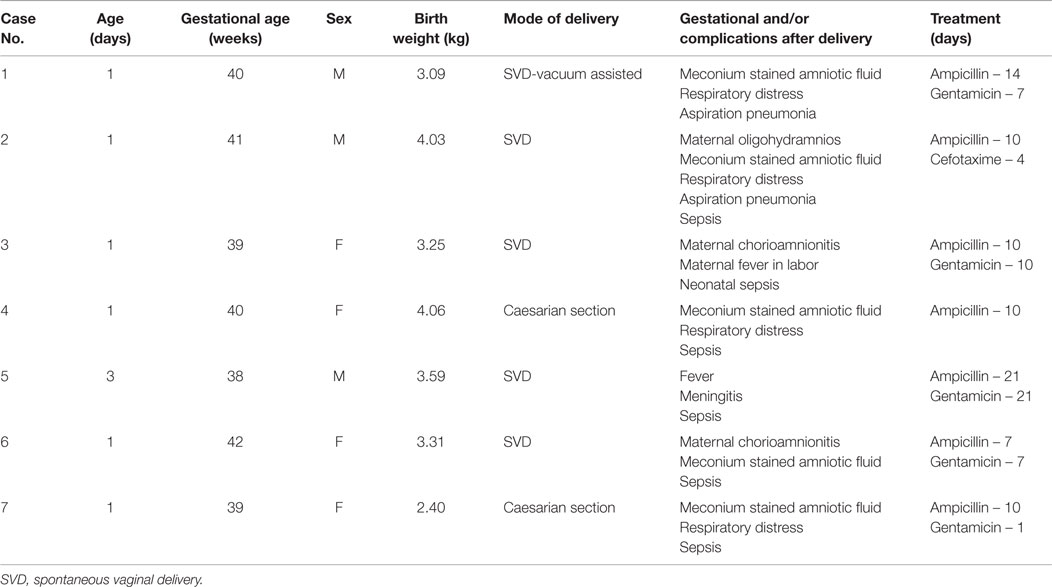
The neonates with S. bovis bacteremia included four (57%) female and three (42%) male, mean age of 1.2 days (range 1–3 days), and all were Hispanic. The clinical features of these babies with S. bovis bacteremia are summarized in Table 1. The mothers’ ages ranged from 17 to 33 years. One mother had a prior history of hydrops fetalis. All other obstetrical histories from previous pregnancies were normal. Maternal diagnosis during pregnancy, labor, and delivery included chorioamnionitis (two), oligohydramnios (two), and meconium stained fluid (five). Two had two of these complications. Review of labor and deliveries revealed the following: mode of delivery was spontaneous vaginal delivery (SVD) in five (71%) and caesarian section in two (28%). Six were uncomplicated and one SVD, which had be converted into vacuum assisted. Mean gestational age was 39 weeks (range 38–42); average birth weight was (3.25 kg).
Five out of seven babies presented with abdominal distention and diarrhea. Respiratory distress was present in all babies; all of them had evidence of meconium in the airway based on endotracheal intubation done at birth. Aspiration pneumonia secondary to meconium aspiration was diagnosed in two of them. Clinical evidence of sepsis was present in most of them 6 (75%). All patients except one were treated with combination antibiotics (six), either parenteral ampicillin and gentamicin or ampicillin and cefotaxime. One patient was treated with ampicillin alone. Duration of antibiotic therapy varied from 7 to 21 days (mean 12 days). Endoscopic evaluation was not performed in any of them since it was not clinically indicated. No other complications were noticed at the time of discharge. None of the patients died, but subsequent follow-up was not performed as it was beyond the scope of this study.
Discussion
In our study of S. bovis bacteremia in predominantly Hispanic neonatal patients in a county hospital setting, we found that S. bovis is an uncommon pathogen in the newborn that should be identified and treated early to avoid possible substantial morbidity and mortality.
Several cases of S. bovis bacteremia have previously been reported in neonates and young infants (14–16). S. bovis is considered as an uncommon pathogen in the newborn. This uncommon infection appears to be associated with substantial morbidity and mortality (17). The pathogenesis or clinical conditions that predispose to S. bovis infection in this population is unclear. It has been suggested that early-onset (up to 1 week) of S. bovis infection might result from transmission of bacteria either intrapartum or during passage through a colonized birth canal and late-onset disease may occur as a consequence of postnatal acquisition of the organism (15).
Different microbiological classification of S. bovis subspecies may have various relationships to colorectal neoplasia in adults (8, 18, 19). Patients infected with S. gallolyticus subsp gallolyticus (biotype I) were noted to have an increased rate of colorectal neoplasia and infective endocarditis (8). In contrast to the S. bovis biotype I, S. bovis biotype II caused more biliary tract infections and acute cholecystitis than biotype I (18, 19). It would be interesting to known if biotype I was involved in this study but our laboratory did not perform genotype testing.
Since the 1970s, several large series in adults with S. bovis bacteremia and its association with colorectal neoplasia has been reported (1, 3, 16, 20). There have been five case series that included both adult and pediatric patients with S. bovis bacteremia (10–14). In these previous reported studies, there were 137 adults and only 16 pediatric cases. Other researchers also noted the association of S. bovis bacteremia with extra-colonic malignancies, including carcinoma of the esophagus, squamous cell carcinoma of the mouth, adenocarcinoma of the stomach, endometrial cancer, Kaposi sarcoma pancreatic cancer, and gastric lymphoma (11, 18–22). However, these complications have not been reported in children and were not present in our cohort (11, 21–25).
It seems that S. bovis bacteremia has different implications in adults and in children. The hypotheses of disease pathophysiology are probably different, as well as the variables of interest. In our series of predominantly Hispanic patients, seven infants were found to have S. bovis bacteremia. Contrary to what was described by Gerber et al. (15), all of our patients developed early-onset (<1 week) disease and presented with signs of respiratory distress, which included cyanosis, tachypnea, and apnea attacks. In their case series, most infants presented with gastrointestinal illness with late-onset of disease (>1 week). Five out of seven babies presented with abdominal distention and diarrhea in our series. In both series, it remains uncertain how these babies acquired the infection, but it is possible that early-onset S. bovis infection might result from transmission of bacteria either intrapartum or during passage through a colonized birth canal. It has been speculated that gut ischemia from anoxia can compromise the intestinal mucosa allowing translocation of previously commensal bacteria with subsequent systemic dissemination. However, in neonates, respiratory distress is a major sign of neonatal sepsis. Our entire cohort presented with respiratory distress soon after birth, which we could hypothesize to be a sign of an early infection with S. bovis, but there was also evidence of meconium in their airway which can also cause respiratory distress in this age group. Another important finding is the presence of meconium stained deliveries as we can speculate the presence of neonatal distress secondary to an early S. bovis infection. Late-onset disease may occur as a consequence of postnatal acquisition of the organism (15). It is possible that S. bovis may have colonized the meconium (with or without premature rupture of membrane). No other bacteria were reported to grow in blood culture (to support a mixed infection as a cause).
All except one of the babies in our study were treated with combination antibiotics. One was treated with ampicillin alone. All babies received antibiotics for 7–21 days. The response time was not clearly documented. So, we cannot determine how quickly the neonates responded to antibiotic therapy. The most common choice was ampicillin and gentamicin as is the standard of care in the neonatal intensive care unit to treat patients with respiratory distress and suspected sepsis. As reported in a prospective study by Corredoira et al., beta-lactam group of antibiotics are a reasonable first line treatment of S. bovis infection given that in their study there was no penicillin resistance among S. bovis isolates (26).
Our study has the limitation of being a retrospective study with limited patient size and no control group in a single medical center. This study is limited to infants who developed symptoms after birth and had a blood culture, although there could be an asymptomatic infection that could present later on in life or at another healthcare facility. However, this is one of the few clinical studies in pediatric patients in a predominantly Hispanic population in the United States. Our study was not designed to determine if there were any complications in our patients after their discharge. Specifically, no documented follow-up for meningitis was assessed in our study. At this point, there is no clear evidence of any of the adult complications of S. bovis infection for those patients with S. bovis bacteremia as newborns. Further studies need to be designed to determine if a neonatal infection will increase the risk of colon cancer as adults.
In conclusion, S. bovis bacteremia may be associated with gastrointestinal sepsis with respiratory distress as the most common symptom. In any patient with unexplained S. bovis bacteremia, clinicians should be aware of the possibility substantial morbidity and mortality and treat with antibiotics when appropriate.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Nancy Casner, CCRC, Zuber Mulla, Ph.D., and Georgina Grado for their assistance with data collection and manuscript preparation.
Abbreviations
S. bovis, Streptococcus bovis.
References
1. Klein RS, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. Streptococcus bovis septicemia and carcinoma of the colon. Ann Intern Med (1979) 91(4):560–2. doi: 10.7326/0003-4819-91-4-560
2. Beeching NJ, Christmas TI, Ellis-Pegler RB, Nicholson GI. Streptococcus bovis bacteraemia requires rigorous exclusion of colonic neoplasia and endocarditis. Q J Med (1985) 56(220):439–50.
3. Waisberg J, Matheus Cde O, Pimenta J. Infectious endocarditis from Streptococcus bovis associated with colonic carcinoma: case report and literature review. Arq Gastroenterol (2002) 39(3):177–80. doi:10.1590/S0004-28032002000300008
4. Wong SS, Woo PC, Ho PL, Wang TK. Continuous ambulatory peritoneal dialysis-related peritonitis caused by Streptococcus bovis. Eur J Clin Microbiol Infect Dis (2003) 22(7):424–6. doi:10.1007/s10096-003-0951-1
5. Mc CW, Mason JM III. Enterococcal endocarditis associated with carcinoma of the sigmoid; report of a case. J Med Assoc State Ala (1951) 21(6):162–6.
6. Ballet M, Gevigney G, Gare JP, Delahaye F, Etienne J, Delahaye JP. Infective endocarditis due to Streptococcus bovis. A report of 53 cases. Eur Heart J (1995) 16(12):1975–80.
7. Jean SS, Teng LJ, Hsueh PR, Ho SW, Luh KT. Bacteremic Streptococcus bovis infections at a university hospital, 1992-2001. J Formos Med Assoc (2004) 103(2):118–23.
8. Boleij A, van Gelder MM, Swinkels DW, Tjalsma H. Clinical importance of Streptococcus gallolyticus infection among colorectal cancer patients: systematic review and meta-analysis. Clin Infect Dis (2011) 53(9):870–8. doi:10.1093/cid/cir609
9. Fernández-Ruiz M, Villar-Silva J, Llenas-García J, Caurcel-Díaz L, Vila-Santos J, Sanz-Sanz F, et al. Streptococcus bovis bacteraemia revisited: clinical and microbiological correlates in a contemporary series of 59 patients. J Infect (2010) 61(4):307–13. doi:10.1016/j.jinf.2010.07.007
10. Murray HW, Roberts RB. Streptococcus bovis bacteremia and underlying gastrointestinal disease. Arch Intern Med (1978) 138(7):1097–9. doi:10.1001/archinte.1978.03630320037013
11. Reynolds JG, Silva E, McCormack WM. Association of Streptococcus bovis bacteremia with bowel disease. J Clin Microbiol (1983) 17(4):696–7.
12. Robson SC, Cannan C. Streptococcus bovis bacteremia and endocarditis at Groote Schuur Hospital. S Afr Med J (1989) 75(12):597.
13. Gold JS, Bayar S, Salem RR. Association of Streptococcus bovis bacteremia with colonic neoplasia and extracolonic malignancy. Arch Surg (2004) 139(7):760–5. doi:10.1001/archsurg.139.7.760
14. Lazarovitch T, Shango M, Levine M, Brusovansky R, Akins R, Hayakawa K, et al. The relationship between the new taxonomy of Streptococcus bovis and its clonality to colon cancer, endocarditis, and biliary disease. Infection (2013) 41(2):329–37. doi:10.1007/s15010-012-0314-x
15. Gerber JS, Glas M, Frank G, Shah SS. Streptococcus bovis infection in young infants. Pediatr Infect Dis J (2006) 25(11):1069–73. doi:10.1097/01.inf.0000240334.91713.48
16. Alazmi W, Bustamante M, O’Loughlin C, Gonzalez J, Raskin JB. The association of Streptococcus bovis bacteremia and gastrointestinal diseases: a retrospective analysis. Dig Dis Sci (2006) 51(4):732–6. doi:10.1007/s10620-006-3199-7
17. Beneteau A, Levy C, Foucaud P, Béchet S, Cohen R, Raymond J, et al. Childhood meningitis caused by Streptococcus bovis group: clinical and biologic data during a 12-year period in France. Pediatr Infect Dis J (2015) 34(2):136–9. doi:10.1097/INF.0000000000000513
18. Corredoira J, Alonso MP, García-Garrote F, García-Pais MJ, Coira A, Rabuñal R, et al. Streptococcus bovis group and biliary tract infections: an analysis of 51 cases. Clin Microbiol Infect (2014) 20(5):405–9. doi:10.1111/1469-0691.12333
19. Medina L, Mora L, Garcia V, Santos J. Acute cholecystitis and bacteraemia due to Streptococcus bovis biotype II. Enferm Infecc Microbiol Clin (2011) 29(1):70–1. doi:10.1016/j.eimc.2010.04.017
20. Sharara AI, Abou Hamdan T, Malli A, El-Halabi MM, Hashash JG, Ghaith OA, et al. Association of Streptococcus bovis endocarditis and advanced colorectal neoplasia: a case-control study. J Dig Dis (2013) 14(7):382–7. doi:10.1111/1751-2980.12059
21. Herrington P, Finkelman D, Balart L, Hines C Jr, Ferrante W. Streptococcus bovis septicemia and pancreatic adenocarcinoma. Ann Intern Med (1980) 92(3):441. doi:10.7326/0003-4819-92-3-441_1
22. Pigrau C, Lorente A, Pahissa A, Martinez-Vazquez JM. Streptococcus bovis bacteremia and digestive system neoplasms. Scand J Infect Dis (1988) 20(4):459–60. doi:10.3109/00365548809032490
23. Gelfand MS, Alford RH. Streptococcus bovis endocarditis and squamous-cell carcinoma of the mouth. N Engl J Med (1981) 305(5):284–5. doi:10.1056/NEJM198107303050516
24. Anaf V, Noel JC, Thys JP, Simon P, Buxant F. A first case of Streptococcus bovis bacteremia and peritonitis from endometrial cancer origin. Acta Chir Belg (2001) 101(1):38–9.
25. Glaser JB, Landesman SH. Streptococcus bovis bacteremia and acquired immunodeficiency syndrome. Ann Intern Med (1983) 99(6):878. doi:10.7326/0003-4819-99-6-878_1
26. Corredoira JC, Alonso MP, García JF, Casariego E, Coira A, Rodriguez A, et al. Clinical characteristics and significance of Streptococcus salivarius bacteremia and Streptococcus bovis bacteremia: a prospective 16-year study. Eur J Clin Microbiol Infect Dis (2005) 24(4):250–5. doi:10.1007/s10096-005-1314-x
Keywords: Streptococcus bovis, bacteremia, Hispanic, neonates
Citation: Alvarez A, Jia Y, Garcia CJ, Rosas-Blum ED, Boman D and Zuckerman MJ (2015) Streptococcus bovis bacteremia in neonates in a predominantly Hispanic population. Front. Pediatr. 3:92. doi: 10.3389/fped.2015.00092
Received: 23 June 2015; Accepted: 14 October 2015;
Published: 30 October 2015
Edited by:
Hans Van Rostenberghe, Universiti Sains Malaysia, MalaysiaReviewed by:
Corentin Babakissa, University of Sherbrooke, CanadaEytan Wine, University of Alberta, Canada
Copyright: © 2015 Alvarez, Jia, Garcia, Rosas-Blum, Boman and Zuckerman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc J. Zuckerman, bWFyYy56dWNrZXJtYW5AdHR1aHNjLmVkdQ==
 Alicia Alvarez
Alicia Alvarez Yi Jia
Yi Jia Cesar J. Garcia1
Cesar J. Garcia1 Eduardo D. Rosas-Blum
Eduardo D. Rosas-Blum Marc J. Zuckerman
Marc J. Zuckerman