- 1Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal
- 2Centro de Imunodeficiências Primárias, Lisboa, Portugal
- 3Clinica Universitária de Imunoalergologia, Hospital de Santa Maria, Centro Hospitalar Lisboa Norte, Lisboa, Portugal
The naïve CD4+ T-cell compartment is considered essential to guarantee immune competence throughout life. Its replenishment with naïve cells with broad diverse receptor repertoire, albeit with reduced self-reactivity, is ensured by the thymus. Nevertheless, cumulative data support a major requirement of post-thymic proliferation both for the establishment of the human peripheral naïve compartment during the accelerated somatic growth of childhood, as well as for its lifelong maintenance. Additionally, a dynamic equilibrium is operating at the cell level to fine-tune the T-cell receptor threshold to activation and survival cues, in order to counteract the continuous naïve cell loss by death or conversion into memory/effector cells. The main players in these processes are low-affinity self-peptide/MHC and cytokines, particularly IL-7. Moreover, although naïve CD4+ T-cells are usually seen as a homogeneous population regarding stage of maturation and cell differentiation, increasing evidence points to a variety of phenotypic and functional subsets with distinct homeostatic requirements. The paradigm of cells committed to a distinct lineage in the thymus are the naïve regulatory T-cells, but other functional subpopulations have been identified based on their time span after thymic egress, phenotypic markers, such as CD31, or cytokine production, namely IL-8. Understanding the regulation of these processes is of utmost importance to promote immune reconstitution in several clinical settings, namely transplantation, persistent infections, and aging. In this mini review, we provide an overview of the mechanisms underlying human naïve CD4+ T-cell homeostasis, combining clinical data, experimental studies, and modeling approaches.
Introduction and Aim
The thymus provides a unique microenvironment to support the differentiation of hematopoietic progenitors into T-cells with a diverse repertoire of T-cell receptors (TCR), albeit with low self-reactivity (1). After thymus egression, the expression of the chemokine receptor CCR7 and L-selectin (CD62L) enables the naïve T-cells to patrol the body, through a continuous recirculation between the blood and secondary lymphoid organs (SLO) (2). The naïve compartment represents, therefore, the lifelong reservoir of T-cells able to mount specific responses to new antigens and to replenish the pool of memory-effector T-cells (3, 4). In addition to thymic output, several rounds of post-thymic proliferation in SLO are considered necessary to fill the peripheral naïve T-cell compartment of a growing child (5–9). Moreover, its maintenance throughout life requires a dynamic equilibrium between thymic output and peripheral homeostatic proliferation and survival, in order to counteract the naïve cell loss through death or conversion into memory/effector cells (3, 4). The understanding of the fine-tuning of these processes is of utmost importance to ensure immune competence and to promote immune reconstitution in many clinical settings, namely transplantation, persistent infections, and aging.
We review here the homeostasis of human naïve T-cells, focusing on the CD4+ population given its pivotal role in immune response orchestration. Data from clinical settings, experimental studies, and modeling approaches were summarized to address the establishment of the naïve compartment, and the relative contribution of thymus and peripheral mechanisms to ensure the maintenance of naïve CD4+ T-cells throughout life. We further outline the cumulative evidence pointing to age-dependent maturation processes in the periphery that may alter the cell threshold to homeostatic cues (2, 10–14). Finally, the existence of phenotypic/functional naïve CD4+ T-cell subpopulations with distinct homeostasis is reviewed (13, 15–18), with a particular focus on thymic-derived regulatory T-cells (naïve-Tregs) that given their high self-reactivity are considered essential to prevent autoimmunity (19, 20). This suppressive lineage, defined by FOXP3 expression, has recently been shown to rely on unique homeostatic requirements (19, 21).
Establishment of the Naïve CD4+ T-Cell Compartment
The human thymus was shown to be active from very early in embryonic life, with reports of progenitor colonization of the thymic primordium by the eighth week of gestation and of mature T-cells featuring an already diverse TCR repertoire by the end of first trimester (22–24). Human T-cell development is a tightly controlled multistep process [reviewed in Ref. (1)], that is out of the scope of this review. αβ T-cell diversity depends on random TCR rearrangements and assembly of β- and α-chains, as well as on selection processes (1, 25). Life or death decisions are mainly dictated by the strength of TCR signaling upon interaction with peptide/MHC complexes, ultimately resulting in generation of CD4 or CD8 single-positive (SP) cells, based on MHC-II or MHC-I restriction, respectively (1, 25–27). Thymocytes expressing high-affinity TCR for self-peptide/MHC complexes are deleted by apoptosis, a negative selection process critical for self-tolerance, as attested by the multiorgan autoimmune disease associated with autoimmune regulator (AIRE) gene mutations that impair the expression of peripheral self-peptides by thymic epithelial cells (28, 29). Of note, self-peptide low-affinity interactions are later important for the peripheral homeostasis of the naïve compartment, by providing tonic signals that promote cell survival and low-level homeostatic proliferation (26).
The ultimate αβTCR diversity has been estimated to be as large as 1015 (26). It is worth emphasizing that the available tools to evaluate TCR diversity have significant limitations: spectratyping, which is based on length distribution of the most variable TCR region, poorly discriminates between loss of TCR specificities and biased clonal expansion (30, 31); and although next generation sequence (NGS) is a promising approach, the current algorithms still need improvements to fully account all variables inherent to the biology of TCR generation (25, 32, 33).
The thymus remains active until at least the sixth decade of life (34), as attested by de novo T-cell production in several clinical lymphopenic conditions, namely HIV/AIDS, hematopoietic stem cell transplantation (HSCT), and chemotherapy (35–38). Some authors claimed that thymic output peaks in first year of life and subsequently declines at annual rates of ~3% until 35–45 years of age, and ~1% thereafter, while others reported that the output of naïve T-cells only starts to decline in early adulthood (39, 40). This heterogeneity is, at least in part, related to differences in the methodological approaches used, namely histology versus measurement of thymic output by T-cell receptor excision circle (TREC) quantification (8, 39–43).
The DNA excised during β- and α-chain rearrangements results in several types of TRECs (44–47). sjTRECs, generated during the α-chain edition and containing signal joint (sj) sequence, have been broadly used to evaluate thymic activity (34). This PCR-based assay performed in circulating lymphocytes is also used in neonatal screening of major T-cell defects (48). TREC levels are influenced by peripheral events, namely cell proliferation and redistribution, or alterations in cell survival (41, 49). Therefore, total sjTRECs/microliter levels represent a better estimate of thymic output than sjTREC quantification within a given subpopulation, which is manifestly influenced by post-thymic proliferation (41, 49, 50). In line with this, the sj/βTREC ratio is considered a more accurate measurement of thymic activity, although the quantification of the TRECs generated during earliest TCRβ locus rearrangements is technically complex, precluding its generalized applicability (51). Since TRECs are not duplicated during mitosis, and are therefore diluted out with each cellular division (44, 52), the sj/βTREC ratio provides a good measurement of the proliferation occurring between the β and α gene rearrangement during T-cell development, a direct correlate of thymic output (41, 51, 53).
It has also been suggested that some thymocytes may egress the thymus before switching from CD45RO to CD45RA, and only acquire the typical CD45RA+ naïve phenotype in the periphery (54), which has implications for correctly estimating thymic output and rate of recent thymic emigrant (RTE) incorporation in the naïve T-cell pool. Moreover, there are no clear markers to identify RTEs, since while thymocytes express high levels of CD31 molecule at thymus egress, CD31bright cells may persist in circulation (55–57), and not all RTEs express protein tyrosine kinase 7 (PTK7), the other suggested marker (16, 58).
Both thymic epithelial cell development defects, namely DiGeorge syndrome and FOXN1 deficiency, and defects of hematopoietic progenitors have severe clinical impact, which illustrates the non-redundant thymic contribution for the establishment of T-cell compartment (59, 60). Studies on primary immunodeficiency (61, 62), and on the immunological reconstitution achieved by the appropriate correction of these defects with HSCT (63), gene therapy (64–66), or thymus transplantation (67, 68), have been instrumental to better understand T-cell development. As an illustrative example of the knowledge that can be gathered from these clinical cases, we showed that the activity of thymus explants, evaluated by sj/βTREC ratio, drastically diminished 3 years post-thymic transplantation in a case of athymia due to FOXN1 deficiency (68). Nevertheless, this period was apparently sufficient to establish a sustained naïve T-cell compartment with a diverse TCR repertoire (68, 69).
Thymic Versus Peripheral Contribution for Naïve CD4+ T-Cell Maintenance
The thymus also contributes to the maintenance of naïve CD4+ T-cell compartment, as demonstrated by the marked contraction observed in individuals submitted to complete thymectomy in early infancy due to corrective cardiac surgery [reviewed in Ref. (70)]. However, the thymus ability to adjust its output to peripheral requirements is still controversial, despite reports of thymic rebound in lymphopenic clinical settings (37, 71–75).
The dynamics of naïve T-cell compartment is also constrained by pressure to memory-effector differentiation (3). It has been suggested that naïve CD4+ T-cells may adjust their threshold for TCR activation with the length of time in circulation since thymus export (2, 8, 76) and with the aging of the individual (14, 77, 78).
Of note, in spite of the continuous environmental antigenic stimulation and the age-associated reduction in thymic output, the size of the human naïve T-cell pool features only a slight decline throughout adulthood (34, 55, 79, 80). Moreover, while sjTREC levels within CD4+ T-cells decrease 50–100 times with age (34, 75), the absolute numbers of naïve CD4+ T-cells decline only by a factor of 2–3 (79, 80). Therefore, thymic output per se is insufficient to guarantee the size of the human naïve CD4+ T-cell compartment, and a major contribution of post-thymic cell proliferation is required, as supported by in silico models (5–9, 81, 82). In contrast, the naïve T-cell pool in mice is almost entirely maintained by thymic output (5), emphasizing the significant differences in T-cell development and homeostasis between the two species (2, 5, 83).
Naïve CD4+ T-cells feature low levels of proliferation while maintaining their naïve phenotype, as demonstrated by studies using in vivo incorporation of deuterated water or Bromodeoxyuridine (BrdU) (7, 84, 85). It remains unclear whether naïve cell turnover changes with age (8, 77, 86, 87), as well as with the time span since thymic egress (2, 8, 76). The main proliferative cues appear to be low-affinity peptide/MHC interactions and cytokines, mainly IL-7, a γ-chain (γC) cytokine produced by stromal cells in SLO (56, 88–96). Experimental studies support that the low-affinity peptides, presented by MHC-II in a non-immunogenic fashion, are mainly self-antigens possibly related to those displayed in the thymus (97–99). In this scenario, naïve CD4+ T-cell proliferation could be viewed as a peripheral selection process, in which the repertoire of CD4+ T-cells is restricted by low-affinity self-peptides. Conversely, cytokines are thought to induce homeostatic proliferative responses without the bias of TCR specificity, and, thus, being vital to preserve broad diversity (100).
IL-7 signaling [reviewed in Ref. (101)] is strictly modulated by the expression of the α-chain of its receptor (IL-7Rα, CD127). IL-7 itself (102, 103), other γC-cytokines (102), and TCR stimulation (88, 103, 104) down-modulate IL-7Rα expression, which is upregulated in the absence of its cognate cytokine (102, 103). In adults, this cytokine was shown to preferentially drive the proliferation of CD31+ naïve CD4+ T-cells, while sustaining the expression of CD31 in a PI3K-dependent manner (21, 57). Conversely, the IL-7-driven upregulation of the antiapoptotic molecule Bcl-2 was shown to occur irrespectively of CD31 expression and to be independent of the PI3K pathway (21, 57, 105). Interestingly, CD31 engagement inhibits TCR-mediated signal transduction via the immune-receptor tyrosine-based inhibitory motifs (ITIMs) present in its cytoplasmic domain, raising the hypothesis that CD31 expression hampers TCR triggering, and thus favors the cytokine-driven homeostatic proliferation of CD31+ cells (56, 94, 106). On the other hand, TCR activation and cell division result in loss of CD31 expression (107). Therefore, the CD31− naïve subset has been proposed to result and be maintained by TCR triggering with low-affinity antigens (13, 94). Accordingly, CD31− naïve CD4+ T-cells express higher levels of the antiapoptotic BFL1/A1 than their CD31+ counterparts, a marker specifically induced by TCR signaling (94, 108, 109). As a result, the CD31− subset proliferation is thought to cause TCR repertoire contraction, in contrast to the expected maintenance of diversity upon IL-7 driven proliferation of the CD31+ naïve CD4+ T-cell subset (94, 110).
Accordingly, the therapeutic use of human recombinant IL-7 was shown to induce preferential expansion of naïve CD4+ T-cells with a diverse TCR repertoire in several lymphopenia settings, namely HIV/AIDS (111, 112) and oncology (113, 114). The sjTREC content within CD31+ naïve CD4+ T-cells was reported to decrease following IL-7 administration in humans, supporting a significant degree of proliferation (114).
The maintenance of the naïve T-cell pool also depends on survival signals. During recirculation through SLO, naïve T-cells encounter IL-7, self-peptide/MHC complexes, and CCR7 ligands, all of which cooperate to produce homeostatic survival signals, namely upregulation of Bcl-2 (89, 115, 116). The relevance of cell survival pathways is further supported by the progressive loss of naïve CD4+ T-cells in association with defective Bcl-2 expression in patients with MST1 deficiency (117). An increase in the peripheral survival of naïve CD4+ T-cells in the elderly has been predicted by in silico models, in agreement with experimental data in mice (118).
Naïve CD4+ T-Cell Heterogeneity
Naïve CD4+ T-cells are usually seen as a homogeneous population regarding stage of maturation and cell differentiation, although their phenotypic and functional variety is increasingly recognized (13, 15, 16, 18, 19). Next, we overview the main factors contributing to this heterogeneity, as well as the principal subpopulations identified. The paradigm of a naïve CD4+ T-cell population committed to a distinct lineage in the thymus is the naïve-Treg subset (19, 119), reviewed in the next section.
At thymus egress, naïve CD4+ T-cells feature unique phenotypic and functional properties (2, 13, 16, 76, 120). Moreover, in agreement with studies using manipulated murine models, the cell-intrinsic properties in terms of turnover, survival, and threshold for TCR activation is also modulated by prolonged time span in circulation (2, 8, 10–12, 121). It is possible that naïve CD4+ T-cells acquire properties before and/or after leaving the thymus that will impact on their differentiation into distinct memory-effector subsets (122).
Work from our lab and others identified two subsets within the naïve CD4+ T-cell compartment with distinct proliferative histories defined by the expression of CD31, which are maintained throughout life by different homeostatic mechanisms (13, 56, 57, 94). As discussed above, the proliferation/survival of the CD31+ subset, which includes the RTEs, is mainly driven by IL-7, whereas CD31− cells proliferate in response to TCR stimulation by low-affinity self-peptide/MHC (13, 56, 57, 94). The CD31+ subset features a higher sjTREC content and telomere length than the CD31−, which has experienced more rounds of post-thymic proliferation (13, 56, 57, 94). The proportion of CD31+ cells within the naïve CD4+ compartment of human cord blood is up to 90–95% (56). Both absolute numbers and frequency of the CD31+ subset in peripheral blood decrease with aging, in parallel with the decline in their sjTREC content (13, 55, 56, 94, 123). In contrast, the counts of the CD31− subset remain relatively constant throughout adult life despite thymic involution, leading to a progressive increase in the relative proportion of CD31− within total naïve CD4+ T-cells with aging (13, 55, 94, 123).
In fact, age is the obvious determinant of naïve T-cell biology, impacting both on thymic activity and on SLO microenvironment where peripheral homeostatic mechanisms operate (43, 124). The functional properties of naïve CD4+ T-cells change along infancy, with a clear trend to differentiation into a Th2 profile of cytokine production in early life (125, 126). Additionally, thymic-generated functional populations that are hardly found beyond the first decade of life have been described with a yet unclear role in immunity, as illustrated by the IL-8-producing subset (15, 18).
Finally, the human naïve CD4+ T-cell compartment has been recently shown to include a small population of memory cells (<1%), as attested by rapid interferon-γ production upon TCR stimulation, which could be identified by the expression of Fas (CD95) and β-chain of the IL-2 receptor (CD122), in the absence of CD45RO (17, 18). These so-called memory-stem cells are generated during primary immune responses and are considered a reservoir for the memory pool, in line with their unique ability to self-renew through still unclear mechanisms (17).
The Uniqueness of the Naïve Regulatory CD4+ T-Cell Compartment
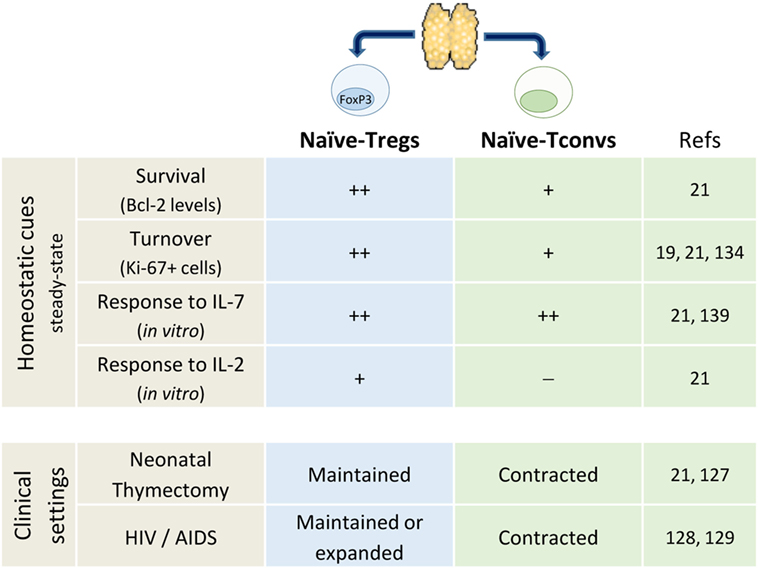
The subpopulation defined by FOXP3 expression is a hallmark of naïve CD4+ T-cell heterogeneity (19, 119). It is noteworthy that thymus removal early in infancy during corrective cardiac surgery was not associated with significant contraction of Foxp3+ naïve-Treg compartment (21, 127). Moreover, other clinical settings known to impact on thymic output and leading to conventional naïve CD4+ T-cell loss, namely HIV-1 infection, have been shown to feature preserved naïve-Tregs (128, 129). Altogether, as illustrated in Figure 1, these data support no thymic dependency and the existence of robust homeostatic peripheral mechanisms that ensure the maintenance of the naïve-Treg compartment, even in extreme clinical settings.
The preservation of naïve-Tregs in thymectomized and HIV-infected individuals was shown to be associated with increased turnover, as assessed by the cell cycling marker Ki-67 (21, 128). Moreover, in healthy subjects naïve-Tregs feature much higher rates of turnover than conventional naïve CD4+ T-cells, questioning the extent of niche sharing and competition for resources between these two naïve subsets (19, 21). Of note, matched blood and tonsil samples obtained from children submitted to routine tonsillectomy revealed higher frequency of cycling naïve-Tregs in the tonsils, with a larger fold-increase than in the conventional naïve CD4+ T-cell compartment (21).
T-cell receptor-affinity is thought to determine thymic Treg commitment, contributing to generate a repertoire skewed toward self-recognition (130–133). However, it has been shown that the high-affinity for self-peptides promotes their rapid differentiation into memory-Tregs upon TCR stimulation (19, 134–136), pointing to cytokines as important drivers of naïve-Treg homeostatic proliferation (21, 131).
Naïve-Tregs are known to express the α-chain of the IL-2 receptor (CD25), although at lower levels than memory-Tregs, and to similarly express reduced levels of IL-7Rα (19). Several studies have addressed the impact of IL-7 on other Treg subsets, both in human and mouse, with heterogeneous results (137–141). Our in vitro studies revealed that IL-7 induced PI3K-dependent proliferation, as well as Bcl-2 upregulation within naïve-Tregs, while preserving their naïve phenotype and suppressive capacity (21). Notably, their proliferation was significantly higher in response to IL-7 than IL-2 (21). Accordingly, an in vivo expansion of the naïve-Treg compartment was observed in patients submitted to IL-7 and to IL-2 therapy (112, 142–145).
Altogether, these data support thymic-independent maintenance of the naïve-Treg compartment (Figure 1), stressing the relevance of future research on the mechanisms counteracting the expected telomere loss and cell senescence.
Concluding Remarks
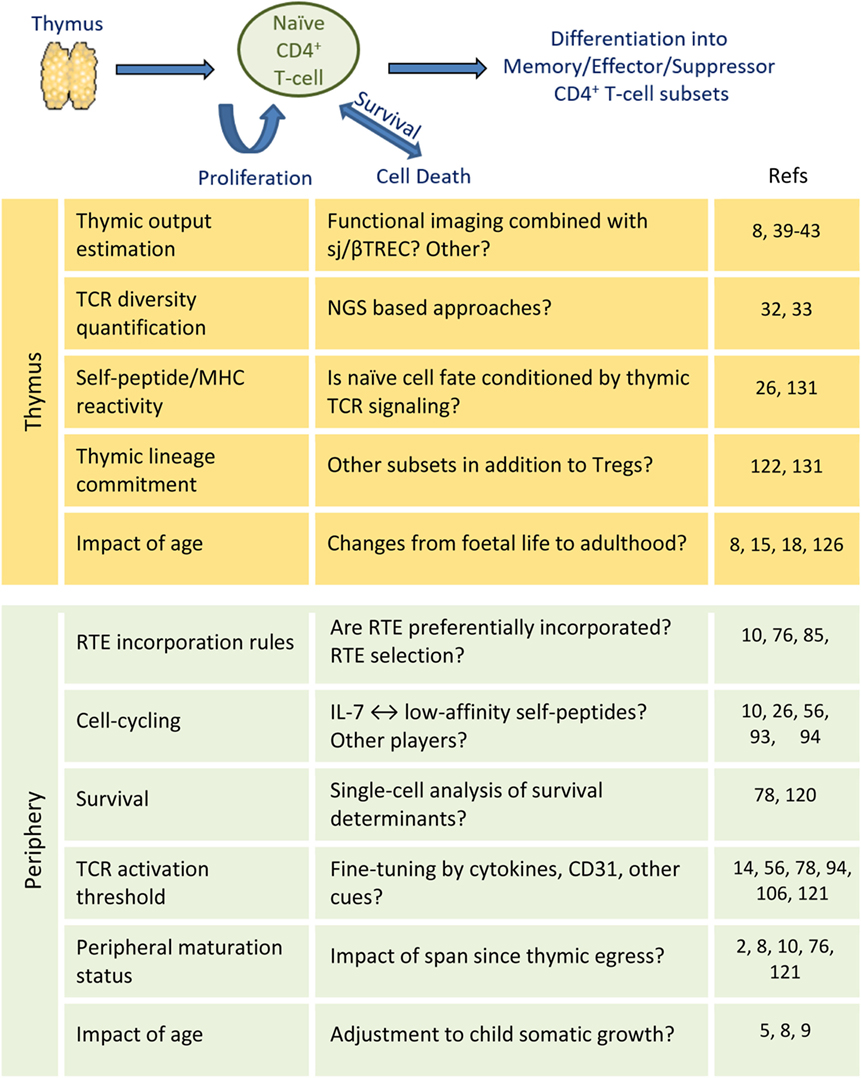
The accelerated somatic growth and overexposure to new antigens in infancy is expected to significantly impact on size and diversity of the naïve CD4+ T-cell compartment, although longitudinal data are limited (146). Pediatric studies are currently facilitated by the decrease in sample size allowed by recent NGS and flow-cytometry advances (147, 148). These studies will ultimately provide a comprehensive understanding of the establishment and maturation of the naïve CD4+ T-cell compartment of unique value for vaccination, persistent infections, and immune reconstitution clinical settings. Figure 2 outlines the main open questions discussed in this brief review, as well as the perspectives opened by recent methodological developments. Understanding the mechanisms underlying human naïve CD4+ T-cell homeostasis is ultimately critical to ensure immune competence throughout life.
Author Contributions
SS and AS prepared and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Helena Nunes-Cabaço, Iris Caramalho, Maria V. Soares, and Rui M. M. Victorino for the critical reading of the manuscript.
Funding
SS received a scholarship from Fundação para a Ciência e Tecnologia, Portugal.
References
1. Spits H. Development of [alpha][beta] T cells in the human thymus. Nat Rev Immunol (2002) 2(10):760–72. doi:10.1038/nri913
2. Fink PJ. The biology of recent thymic emigrants. Annu Rev Immunol (2013) 31:31–50. doi:10.1146/annurev-immunol-032712-100010
3. Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity (2008) 29(6):848–62. doi:10.1016/j.immuni.2008.11.002
4. Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol (2009) 9(12):823–32. doi:10.1038/nri2657
5. den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity (2012) 36(2):288–97. doi:10.1016/j.immuni.2012.02.006
6. Pekalski ML, Ferreira RC, Coulson RM, Cutler AJ, Guo H, Smyth DJ, et al. Postthymic expansion in human CD4 naive T cells defined by expression of functional high-affinity IL-2 receptors. J Immunol (2013) 190(6):2554–66. doi:10.4049/jimmunol.1202914
7. Westera L, van Hoeven V, Drylewicz J, Spierenburg G, van Velzen JF, de Boer RJ, et al. Lymphocyte maintenance during healthy aging requires no substantial alterations in cellular turnover. Aging Cell (2015) 14(2):219–27. doi:10.1111/acel.12311
8. Bains I, Thiebaut R, Yates AJ, Callard R. Quantifying thymic export: combining models of naive T cell proliferation and TCR excision circle dynamics gives an explicit measure of thymic output. J Immunol (2009) 183(7):4329–36. doi:10.4049/jimmunol.0900743
9. Hazenberg MD, Otto SA, van Rossum AM, Scherpbier HJ, de Groot R, Kuijpers TW, et al. Establishment of the CD4+ T-cell pool in healthy children and untreated children infected with HIV-1. Blood (2004) 104(12):3513–9. doi:10.1182/blood-2004-03-0805
10. Kim HK, Waickman AT, Castro E, Flomerfelt FA, Hawk NV, Kapoor V, et al. Distinct IL-7 signaling in recent thymic emigrants versus mature naive T cells controls T-cell homeostasis. Eur J Immunol (2016) 46(7):1669–80. doi:10.1002/eji.201546214
11. Soares MV, Borthwick NJ, Maini MK, Janossy G, Salmon M, Akbar AN. IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire. J Immunol (1998) 161(11):5909–17.
12. Dardalhon V, Jaleco S, Kinet S, Herpers B, Steinberg M, Ferrand C, et al. IL-7 differentially regulates cell cycle progression and HIV-1-based vector infection in neonatal and adult CD4+ T cells. Proc Natl Acad Sci U S A (2001) 98(16):9277–82. doi:10.1073/pnas.161272698
13. Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Mowes B, Radbruch A, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med (2002) 195(6):789–94. doi:10.1084/jem.20011756
14. Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, et al. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med (2012) 18(10):1518–24. doi:10.1038/nm.2963
15. Gibbons D, Fleming P, Virasami A, Michel ML, Sebire NJ, Costeloe K, et al. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat Med (2014) 20(10):1206–10. doi:10.1038/nm.3670
16. Haines CJ, Giffon TD, Lu LS, Lu X, Tessier-Lavigne M, Ross DT, et al. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med (2009) 206(2):275–85. doi:10.1084/jem.20080996
17. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med (2011) 17(10):1290–7. doi:10.1038/nm.2446
18. van den Broek T, Delemarre EM, Janssen WJ, Nievelstein RA, Broen JC, Tesselaar K, et al. Neonatal thymectomy reveals differentiation and plasticity within human naive T cells. J Clin Invest (2016) 126(3):1126–36. doi:10.1172/JCI84997
19. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity (2009) 30(6):899–911. doi:10.1016/j.immuni.2009.03.019
20. Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol (2010) 10(7):490–500. doi:10.1038/nri2785
21. Silva SL, Albuquerque AS, Serra-Caetano A, Foxall RB, Pires AR, Matoso P, et al. Human naive regulatory T-cells feature high steady-state turnover and are maintained by IL-7. Oncotarget (2016) 7(11):12163–75. doi:10.18632/oncotarget.7512
22. Haynes BF, Heinly CS. Early human T cell development: analysis of the human thymus at the time of initial entry of hematopoietic stem cells into the fetal thymic microenvironment. J Exp Med (1995) 181(4):1445–58. doi:10.1084/jem.181.4.1445
23. Farley AM, Morris LX, Vroegindeweij E, Depreter ML, Vaidya H, Stenhouse FH, et al. Dynamics of thymus organogenesis and colonization in early human development. Development (2013) 140(9):2015–26. doi:10.1242/dev.087320
24. Haddad R, Guimiot F, Six E, Jourquin F, Setterblad N, Kahn E, et al. Dynamics of thymus-colonizing cells during human development. Immunity (2006) 24(2):217–30. doi:10.1016/j.immuni.2006.01.008
25. Attaf M, Huseby E, Sewell AK. αβ T cell receptors as predictors of health and disease. Cell Mol Immunol (2015) 12(4):391–9. doi:10.1038/cmi.2014.134
26. Vrisekoop N, Monteiro JP, Mandl JN, Germain RN. Revisiting thymic positive selection and the mature T cell repertoire for antigen. Immunity (2014) 41(2):181–90. doi:10.1016/j.immuni.2014.07.007
27. Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol (2003) 21:139–76. doi:10.1146/annurev.immunol.21.120601.141107
28. Kisand K, Peterson P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy: known and novel aspects of the syndrome. Ann N Y Acad Sci (2011) 1246:77–91. doi:10.1111/j.1749-6632.2011.06308.x
29. Matsumoto M, Nishikawa Y, Nishijima H, Morimoto J, Matsumoto M, Mouri Y. Which model better fits the role of aire in the establishment of self-tolerance: the transcription model or the maturation model? Front Immunol (2013) 4:210. doi:10.3389/fimmu.2013.00210
30. Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science (1999) 286(5441):958–61. doi:10.1126/science.286.5441.958
31. Pannetier C, Even J, Kourilsky P. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today (1995) 16(4):176–81. doi:10.1016/0167-5699(95)80117-0
32. Goronzy JJ, Qi Q, Olshen RA, Weyand CM. High-throughput sequencing insights into T-cell receptor repertoire diversity in aging. Genome Med (2015) 7(1):117. doi:10.1186/s13073-015-0242-3
33. Qi Q, Zhang DW, Weyand CM, Goronzy JJ. Mechanisms shaping the naive T cell repertoire in the elderly – thymic involution or peripheral homeostatic proliferation? Exp Gerontol (2014) 54:71–4. doi:10.1016/j.exger.2014.01.005
34. Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature (1998) 396(6712):690–5. doi:10.1038/25374
35. Touloumi G, Pantazis N, Karafoulidou A, Mandalaki T, Goedert JJ, Kostrikis LG, et al. Changes in T cell receptor excision DNA circle (TREC) levels in HIV type 1-infected subjects pre- and post-highly active antiretroviral therapy. AIDS Res Hum Retroviruses (2004) 20(1):47–54. doi:10.1089/088922204322749495
36. Svaldi M, Lanthaler AJ, Dugas M, Lohse P, Pescosta N, Straka C, et al. T-cell receptor excision circles: a novel prognostic parameter for the outcome of transplantation in multiple myeloma patients. Br J Haematol (2003) 122(5):795–801. doi:10.1046/j.1365-2141.2003.04482.x
37. Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest (2005) 115(4):930–9. doi:10.1172/JCI22492
38. Gautier D, Beq S, Cortesao CS, Sousa AE, Cheynier R. Efficient thymopoiesis contributes to the maintenance of peripheral CD4 T cells during chronic human immunodeficiency virus type 2 infection. J Virol (2007) 81(22):12685–8. doi:10.1128/JVI.01131-07
39. Bertho JM, Demarquay C, Moulian N, Van Der Meeren A, Berrih-Aknin S, Gourmelon P. Phenotypic and immunohistological analyses of the human adult thymus: evidence for an active thymus during adult life. Cell Immunol (1997) 179(1):30–40. doi:10.1006/cimm.1997.1148
40. Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol (1985) 22(5):563–75. doi:10.1111/j.1365-3083.1985.tb01916.x
41. Dion ML, Sekaly RP, Cheynier R. Estimating thymic function through quantification of T-cell receptor excision circles. Methods Mol Biol (2007) 380:197–213. doi:10.1007/978-1-59745-395-0_12
42. Steffens CM, Al-Harthi L, Shott S, Yogev R, Landay A. Evaluation of thymopoiesis using T cell receptor excision circles (TRECs): differential correlation between adult and pediatric TRECs and naive phenotypes. Clin Immunol (2000) 97(2):95–101. doi:10.1006/clim.2000.4938
43. Chaudhry MS, Velardi E, Dudakov JA, van den Brink MR. Thymus: the next (re)generation. Immunol Rev (2016) 271(1):56–71. doi:10.1111/imr.12418
44. Livak F, Schatz DG. T-cell receptor alpha locus V(D)J recombination by-products are abundant in thymocytes and mature T cells. Mol Cell Biol (1996) 16(2):609–18. doi:10.1128/MCB.16.2.609
45. Verschuren MC, Wolvers-Tettero IL, Breit TM, Noordzij J, van Wering ER, van Dongen JJ. Preferential rearrangements of the T cell receptor-delta-deleting elements in human T cells. J Immunol (1997) 158(3):1208–16.
46. Kong F, Chen CH, Cooper MD. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity (1998) 8(1):97–104. doi:10.1016/S1074-7613(00)80462-8
47. Kong FK, Chen CL, Six A, Hockett RD, Cooper MD. T cell receptor gene deletion circles identify recent thymic emigrants in the peripheral T cell pool. Proc Natl Acad Sci U S A (1999) 96(4):1536–40. doi:10.1073/pnas.96.4.1536
48. van Zelm MC, van der Burg M, Langerak AW, van Dongen JJ. PID comes full circle: applications of V(D)J recombination excision circles in research, diagnostics and newborn screening of primary immunodeficiency disorders. Front Immunol (2011) 2:12. doi:10.3389/fimmu.2011.00012
49. Ribeiro RM, Perelson AS. Determining thymic output quantitatively: using models to interpret experimental T-cell receptor excision circle (TREC) data. Immunol Rev (2007) 216:21–34. doi:10.1111/j.1600-065X.2006.00493.x
50. Lorenzi AR, Patterson AM, Pratt A, Jefferson M, Chapman CE, Ponchel F, et al. Determination of thymic function directly from peripheral blood: a validated modification to an established method. J Immunol Methods (2008) 339(2):185–94. doi:10.1016/j.jim.2008.09.013
51. Dion ML, Poulin JF, Bordi R, Sylvestre M, Corsini R, Kettaf N, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity (2004) 21(6):757–68. doi:10.1016/j.immuni.2004.10.013
52. Takeshita S, Toda M, Yamagishi H. Excision products of the T cell receptor gene support a progressive rearrangement model of the alpha/delta locus. EMBO J (1989) 8(11):3261–70.
53. Almeida AR, Borghans JA, Freitas AA. T cell homeostasis: thymus regeneration and peripheral T cell restoration in mice with a reduced fraction of competent precursors. J Exp Med (2001) 194(5):591–9. doi:10.1084/jem.194.5.591
54. Bofill M, Akbar AN, Salmon M, Robinson M, Burford G, Janossy G. Immature CD45RA(low)RO(low) T cells in the human cord blood. I. Antecedents of CD45RA+ unprimed T cells. J Immunol (1994) 152(12):5613–23.
55. Kilpatrick RD, Rickabaugh T, Hultin LE, Hultin P, Hausner MA, Detels R, et al. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol (2008) 180(3):1499–507. doi:10.4049/jimmunol.180.3.1499
56. Kohler S, Thiel A. Life after the thymus: CD31+ and CD31− human naive CD4+ T-cell subsets. Blood (2009) 113(4):769–74. doi:10.1182/blood-2008-02-139154
57. Azevedo RI, Soares MV, Barata JT, Tendeiro R, Serra-Caetano A, Victorino RM, et al. IL-7 sustains CD31 expression in human naive CD4+ T cells and preferentially expands the CD31+ subset in a PI3K-dependent manner. Blood (2009) 113(13):2999–3007. doi:10.1182/blood-2008-07-166223
58. Lewis DB, Haines C, Ross D. Protein tyrosine kinase 7: a novel surface marker for human recent thymic emigrants with potential clinical utility. J Perinatol (2011) 31(Suppl 1):S72–81. doi:10.1038/jp.2010.187
59. Pignata C, D’Assante R, Sousa AE. Thymic stromal alterations and genetic disorders of immune system. Front Immunol (2015) 6:81. doi:10.3389/fimmu.2015.00081
60. Cirillo E, Giardino G, Gallo V, D’Assante R, Grasso F, Romano R, et al. Severe combined immunodeficiency – an update. Ann N Y Acad Sci (2015) 1356:90–106. doi:10.1111/nyas.12849
61. Ma CS, Wong N, Rao G, Nguyen A, Avery DT, Payne K, et al. Unique and shared signaling pathways cooperate to regulate the differentiation of human CD4+ T cells into distinct effector subsets. J Exp Med (2016) 213(8):1589–608. doi:10.1084/jem.20151467
62. Wiekmeijer AS, Pike-Overzet K, IJspeert H, Brugman MH, Wolvers-Tettero IL, Lankester AC, et al. Identification of checkpoints in human T-cell development using severe combined immunodeficiency stem cells. J Allergy Clin Immunol (2016) 137(2):517.e–26.e. doi:10.1016/j.jaci.2015.08.022
63. Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N Engl J Med (2014) 371(5):434–46. doi:10.1056/NEJMoa1401177
64. Qasim W, Gennery AR. Gene therapy for primary immunodeficiencies: current status and future prospects. Drugs (2014) 74(9):963–9. doi:10.1007/s40265-014-0223-7
65. Touzot F, Hacein-Bey-Abina S, Fischer A, Cavazzana M. Gene therapy for inherited immunodeficiency. Expert Opin Biol Ther (2014) 14(6):789–98. doi:10.1517/14712598.2014.895811
66. Touzot F, Moshous D, Creidy R, Neven B, Frange P, Cros G, et al. Faster T-cell development following gene therapy compared with haploidentical HSCT in the treatment of SCID-X1. Blood (2015) 125(23):3563–9. doi:10.1182/blood-2014-12-616003
67. Davies EG. Immunodeficiency in DiGeorge syndrome and options for treating cases with complete athymia. Front Immunol (2013) 4:322. doi:10.3389/fimmu.2013.00322
68. Albuquerque AS, Marques JG, Silva SL, Ligeiro D, Devlin BH, Dutrieux J, et al. Human FOXN1-deficiency is associated with alphabeta double-negative and FoxP3+ T-cell expansions that are distinctly modulated upon thymic transplantation. PLoS One (2012) 7(5):e37042. doi:10.1371/journal.pone.0037042
69. Markert ML, Marques JG, Neven B, Devlin BH, McCarthy EA, Chinn IK, et al. First use of thymus transplantation therapy for FOXN1 deficiency (nude/SCID): a report of 2 cases. Blood (2011) 117(2):688–96. doi:10.1182/blood-2010-06-292490
70. Appay V, Sauce D, Prelog M. The role of the thymus in immunosenescence: lessons from the study of thymectomized individuals. Aging (Albany NY) (2010) 2(2):78–81. doi:10.18632/aging.100122
71. Sandgaard KS, Lewis J, Adams S, Klein N, Callard R. Antiretroviral therapy increases thymic output in children with HIV. AIDS (2014) 28(2):209–14. doi:10.1097/QAD.0000000000000063
72. Roux E, Dumont-Girard F, Starobinski M, Siegrist CA, Helg C, Chapuis B, et al. Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood (2000) 96(6):2299–303.
73. Azevedo RI, Soares MV, Albuquerque AS, Tendeiro R, Soares RS, Martins M, et al. Long-term immune reconstitution of naive and memory t cell pools after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant (2013) 19(5):703–12. doi:10.1016/j.bbmt.2013.01.017
74. Hazenberg MD, Otto SA, de Pauw ES, Roelofs H, Fibbe WE, Hamann D, et al. T-cell receptor excision circle and T-cell dynamics after allogeneic stem cell transplantation are related to clinical events. Blood (2002) 99(9):3449–53. doi:10.1182/blood.V99.9.3449
75. Zhang L, Lewin SR, Markowitz M, Lin HH, Skulsky E, Karanicolas R, et al. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J Exp Med (1999) 190(5):725–32. doi:10.1084/jem.190.5.725
76. Bains I, Yates AJ, Callard RE. Heterogeneity in thymic emigrants: implications for thymectomy and immunosenescence. PLoS One (2013) 8(2):e49554. doi:10.1371/journal.pone.0049554
77. Appay V, Sauce D. Naive T cells: the crux of cellular immune aging? Exp Gerontol (2014) 54:90–3. doi:10.1016/j.exger.2014.01.003
78. Dowling MR, Hodgkin PD. Modelling naive T-cell homeostasis: consequences of heritable cellular lifespan during ageing. Immunol Cell Biol (2009) 87(6):445–56. doi:10.1038/icb.2009.11
79. Utsuyama M, Hirokawa K, Kurashima C, Fukayama M, Inamatsu T, Suzuki K, et al. Differential age-change in the numbers of CD4+CD45RA+ and CD4+CD29+ T cell subsets in human peripheral blood. Mech Ageing Dev (1992) 63(1):57–68. doi:10.1016/0047-6374(92)90016-7
80. Stulnig T, Maczek C, Bock G, Majdic O, Wick G. Reference intervals for human peripheral blood lymphocyte subpopulations from ‘healthy’ young and aged subjects. Int Arch Allergy Immunol (1995) 108(3):205–10. doi:10.1159/000237155
81. Dutilh BE, de Boer RJ. Decline in excision circles requires homeostatic renewal or homeostatic death of naive T cells. J Theor Biol (2003) 224(3):351–8. doi:10.1016/S0022-5193(03)00172-3
82. Murray JM, Kaufmann GR, Hodgkin PD, Lewin SR, Kelleher AD, Davenport MP, et al. Naive T cells are maintained by thymic output in early ages but by proliferation without phenotypic change after age twenty. Immunol Cell Biol (2003) 81(6):487–95. doi:10.1046/j.1440-1711.2003.01191.x
83. Vicente R, Swainson L, Marty-Gres S, De Barros SC, Kinet S, Zimmermann VS, et al. Molecular and cellular basis of T cell lineage commitment. Semin Immunol (2010) 22(5):270–5. doi:10.1016/j.smim.2010.04.016
84. Macallan DC, Wallace D, Zhang Y, De Lara C, Worth AT, Ghattas H, et al. Rapid turnover of effector-memory CD4(+) T cells in healthy humans. J Exp Med (2004) 200(2):255–60. doi:10.1084/jem.20040341
85. Vrisekoop N, den Braber I, de Boer AB, Ruiter AF, Ackermans MT, van der Crabben SN, et al. Sparse production but preferential incorporation of recently produced naive T cells in the human peripheral pool. Proc Natl Acad Sci U S A (2008) 105(16):6115–20. doi:10.1073/pnas.0709713105
86. Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, et al. The influence of age on T cell generation and TCR diversity. J Immunol (2005) 174(11):7446–52. doi:10.4049/jimmunol.174.11.7446
87. Sauce D, Larsen M, Fastenackels S, Roux A, Gorochov G, Katlama C, et al. Lymphopenia-driven homeostatic regulation of naive T cells in elderly and thymectomized young adults. J Immunol (2012) 189(12):5541–8. doi:10.4049/jimmunol.1201235
88. Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol (2000) 1(5):426–32. doi:10.1038/80868
89. Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A (2001) 98(15):8732–7. doi:10.1073/pnas.161126098
90. Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science (1997) 276(5321):2057–62. doi:10.1126/science.276.5321.2057
91. Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J Exp Med (1997) 186(8):1223–32. doi:10.1084/jem.186.8.1223
92. Boursalian TE, Bottomly K. Survival of naive CD4 T cells: roles of restricting versus selecting MHC class II and cytokine milieu. J Immunol (1999) 162(7):3795–801.
93. van der Geest KS, Abdulahad WH, Teteloshvili N, Tete SM, Peters JH, Horst G, et al. Low-affinity TCR engagement drives IL-2-dependent post-thymic maintenance of naive CD4+ T cells in aged humans. Aging Cell (2015) 14(5):744–53. doi:10.1111/acel.12353
94. Kohler S, Wagner U, Pierer M, Kimmig S, Oppmann B, Mowes B, et al. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol (2005) 35(6):1987–94. doi:10.1002/eji.200526181
95. Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol (2005) 174(11):6571–6. doi:10.4049/jimmunol.174.11.6571
96. Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol (2011) 11(5):330–42. doi:10.1038/nri2970
97. Witherden D, van Oers N, Waltzinger C, Weiss A, Benoist C, Mathis D. Tetracycline-controllable selection of CD4(+) T cells: half-life and survival signals in the absence of major histocompatibility complex class II molecules. J Exp Med (2000) 191(2):355–64. doi:10.1084/jem.191.2.355
98. Viret C, Wong FS, Janeway CA Jr. Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity (1999) 10(5):559–68. doi:10.1016/S1074-7613(00)80055-2
99. Moses CT, Thorstenson KM, Jameson SC, Khoruts A. Competition for self ligands restrains homeostatic proliferation of naive CD4 T cells. Proc Natl Acad Sci U S A (2003) 100(3):1185–90. doi:10.1073/pnas.0334572100
100. Hassan J, Reen DJ. Human recent thymic emigrants – identification, expansion, and survival characteristics. J Immunol (2001) 167(4):1970–6. doi:10.4049/jimmunol.167.4.1970
101. Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol (2012) 24(3):209–17. doi:10.1016/j.smim.2012.04.010
102. Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity (2004) 21(2):289–302. doi:10.1016/j.immuni.2004.07.016
103. Alves NL, van Leeuwen EM, Derks IA, van Lier RA. Differential regulation of human IL-7 receptor alpha expression by IL-7 and TCR signaling. J Immunol (2008) 180(8):5201–10. doi:10.4049/jimmunol.180.8.5201
104. Swainson L, Verhoeyen E, Cosset FL, Taylor N. IL-7R alpha gene expression is inversely correlated with cell cycle progression in IL-7-stimulated T lymphocytes. J Immunol (2006) 176(11):6702–8. doi:10.4049/jimmunol.176.11.6702
105. Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med (2004) 200(5):659–69. doi:10.1084/jem.20040789
106. Newton-Nash DK, Newman PJ. A new role for platelet-endothelial cell adhesion molecule-1 (CD31): inhibition of TCR-mediated signal transduction. J Immunol (1999) 163(2):682–8.
107. Demeure CE, Byun DG, Yang LP, Vezzio N, Delespesse G. CD31 (PECAM-1) is a differentiation antigen lost during human CD4 T-cell maturation into Th1 or Th2 effector cells. Immunology (1996) 88(1):110–5. doi:10.1046/j.1365-2567.1996.d01-652.x
108. Gonzalez J, Orlofsky A, Prystowsky MB. A1 is a growth-permissive antiapoptotic factor mediating postactivation survival in T cells. Blood (2003) 101(7):2679–85. doi:10.1182/blood-2002-04-1229
109. Verschelde C, Walzer T, Galia P, Biemont MC, Quemeneur L, Revillard JP, et al. A1/Bfl-1 expression is restricted to TCR engagement in T lymphocytes. Cell Death Differ (2003) 10(9):1059–67. doi:10.1038/sj.cdd.4401265
110. Bousso P, Wahn V, Douagi I, Horneff G, Pannetier C, Le Deist F, et al. Diversity, functionality, and stability of the T cell repertoire derived in vivo from a single human T cell precursor. Proc Natl Acad Sci U S A (2000) 97(1):274–8. doi:10.1073/pnas.97.1.274
111. Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest (2009) 119(4):997–1007. doi:10.1172/JCI38052
112. Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood (2009) 113(25):6304–14. doi:10.1182/blood-2008-10-186601
113. Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother (2006) 29(3):313–9. doi:10.1097/01.cji.0000210386.55951.c2
114. Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med (2008) 205(7):1701–14. doi:10.1084/jem.20071681
115. Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol (2007) 8(11):1255–65. doi:10.1038/ni1513
116. Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, et al. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol (2001) 19:23–45. doi:10.1146/annurev.immunol.19.1.23
117. Nehme NT, Pachlopnik Schmid J, Debeurme F, Andre-Schmutz I, Lim A, Nitschke P, et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood (2012) 119(15):3458–68. doi:10.1182/blood-2011-09-378364
118. Tsukamoto H, Clise-Dwyer K, Huston GE, Duso DK, Buck AL, Johnson LL, et al. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci U S A (2009) 106(43):18333–8. doi:10.1073/pnas.0910139106
119. Fritzsching B, Oberle N, Pauly E, Geffers R, Buer J, Poschl J, et al. Naive regulatory T cells: a novel subpopulation defined by resistance toward CD95L-mediated cell death. Blood (2006) 108(10):3371–8. doi:10.1182/blood-2006-02-005660
120. Hogan T, Gossel G, Yates AJ, Seddon B. Temporal fate mapping reveals age-linked heterogeneity in naive T lymphocytes in mice. Proc Natl Acad Sci U S A (2015) 112(50):E6917–26. doi:10.1073/pnas.1517246112
121. Clise-Dwyer K, Huston GE, Buck AL, Duso DK, Swain SL. Environmental and intrinsic factors lead to antigen unresponsiveness in CD4(+) recent thymic emigrants from aged mice. J Immunol (2007) 178(3):1321–31. doi:10.4049/jimmunol.178.3.1321
122. Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature (2012) 484(7395):514–8. doi:10.1038/nature10957
123. Junge S, Kloeckener-Gruissem B, Zufferey R, Keisker A, Salgo B, Fauchere JC, et al. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. Eur J Immunol (2007) 37(11):3270–80. doi:10.1002/eji.200636976
124. Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol (2012) 33(6):306–14. doi:10.1016/j.it.2012.04.002
125. Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol (2009) 30(12):585–91. doi:10.1016/j.it.2009.09.002
126. Hebel K, Weinert S, Kuropka B, Knolle J, Kosak B, Jorch G, et al. CD4+ T cells from human neonates and infants are poised spontaneously to run a nonclassical IL-4 program. J Immunol (2014) 192(11):5160–70. doi:10.4049/jimmunol.1302539
127. Schadenberg AW, van den Broek T, Siemelink MA, Algra SO, de Jong PR, Jansen NJ, et al. Differential homeostatic dynamics of human regulatory T-cell subsets following neonatal thymectomy. J Allergy Clin Immunol (2014) 133(1):.e1–6. doi:10.1016/j.jaci.2013.08.030
128. Foxall RB, Albuquerque AS, Soares RS, Baptista AP, Cavaleiro R, Tendeiro R, et al. Memory and naive-like regulatory CD4+ T cells expand during HIV-2 infection in direct association with CD4+ T-cell depletion irrespectively of viremia. AIDS (2011) 25(16):1961–70. doi:10.1097/QAD.0b013e32834b3554
129. Simonetta F, Lecuroux C, Girault I, Goujard C, Sinet M, Lambotte O, et al. Early and long-lasting alteration of effector CD45RA(-)Foxp3(high) regulatory T-cell homeostasis during HIV infection. J Infect Dis (2012) 205(10):1510–9. doi:10.1093/infdis/jis235
130. Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity (2012) 37(3):475–86. doi:10.1016/j.immuni.2012.07.009
131. Caramalho I, Nunes-Cabaco H, Foxall RB, Sousa AE. Regulatory T-cell development in the human thymus. Front Immunol (2015) 6:395. doi:10.3389/fimmu.2015.00395
132. Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity (2004) 21(2):267–77. doi:10.1016/j.immuni.2004.07.009
133. Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol (2006) 7(4):401–10. doi:10.1038/ni1318
134. Booth NJ, McQuaid AJ, Sobande T, Kissane S, Agius E, Jackson SE, et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol (2010) 184(8):4317–26. doi:10.4049/jimmunol.0903781
135. Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest (2005) 115(7):1953–62. doi:10.1172/JCI23963
136. Seddiki N, Santner-Nanan B, Tangye SG, Alexander SI, Solomon M, Lee S, et al. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood (2006) 107(7):2830–8. doi:10.1182/blood-2005-06-2403
137. Heninger AK, Theil A, Wilhelm C, Petzold C, Huebel N, Kretschmer K, et al. IL-7 abrogates suppressive activity of human CD4+CD25+FOXP3+ regulatory T cells and allows expansion of alloreactive and autoreactive T cells. J Immunol (2012) 189(12):5649–58. doi:10.4049/jimmunol.1201286
138. Kim GY, Ligons DL, Hong C, Luckey MA, Keller HR, Tai X, et al. An in vivo IL-7 requirement for peripheral Foxp3+ regulatory T cell homeostasis. J Immunol (2012) 188(12):5859–66. doi:10.4049/jimmunol.1102328
139. Younas M, Hue S, Lacabaratz C, Guguin A, Wiedemann A, Surenaud M, et al. IL-7 modulates in vitro and in vivo human memory T regulatory cell functions through the CD39/ATP axis. J Immunol (2013) 191(6):3161–8. doi:10.4049/jimmunol.1203547
140. Simonetta F, Gestermann N, Martinet KZ, Boniotto M, Tissieres P, Seddon B, et al. Interleukin-7 influences FOXP3+CD4+ regulatory T cells peripheral homeostasis. PLoS One (2012) 7(5):e36596. doi:10.1371/journal.pone.0036596
141. Schmaler M, Broggi MA, Lagarde N, Stocklin BF, King CG, Finke D, et al. IL-7R signaling in regulatory T cells maintains peripheral and allograft tolerance in mice. Proc Natl Acad Sci U S A (2015) 112(43):13330–5. doi:10.1073/pnas.1510045112
142. Perales MA, Goldberg JD, Yuan J, Koehne G, Lechner L, Papadopoulos EB, et al. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood (2012) 120(24):4882–91. doi:10.1182/blood-2012-06-437236
143. Weiss L, Letimier FA, Carriere M, Maiella S, Donkova-Petrini V, Targat B, et al. In vivo expansion of naive and activated CD4+CD25+FOXP3+ regulatory T cell populations in interleukin-2-treated HIV patients. Proc Natl Acad Sci U S A (2010) 107(23):10632–7. doi:10.1073/pnas.1000027107
144. Bell CJ, Sun Y, Nowak UM, Clark J, Howlett S, Pekalski ML, et al. Sustained in vivo signaling by long-lived IL-2 induces prolonged increases of regulatory T cells. J Autoimmun (2015) 56:66–80. doi:10.1016/j.jaut.2014.10.002
145. Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med (2013) 5(179):179ra43. doi:10.1126/scitranslmed.3005265
146. Gollwitzer ES, Marsland BJ. Impact of early-life exposures on immune maturation and susceptibility to disease. Trends Immunol (2015) 36(11):684–96. doi:10.1016/j.it.2015.09.009
147. Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, et al. Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci U S A (2014) 111(36):13139–44. doi:10.1073/pnas.1409155111
Keywords: human T-cells, naïve CD4+ T-cells, naïve T-cell homeostasis, thymus, thymic activity, IL-7
Citation: Silva SL and Sousa AE (2016) Establishment and Maintenance of the Human Naïve CD4+ T-Cell Compartment. Front. Pediatr. 4:119. doi: 10.3389/fped.2016.00119
Received: 02 September 2016; Accepted: 17 October 2016;
Published: 31 October 2016
Edited by:
Claudio Pignata, University of Naples Federico II, ItalyReviewed by:
Christina E. Zielinski, Technische Universität München, GermanyLisa Renee Forbes, Baylor College of Medicine, USA
Copyright: © 2016 Silva and Sousa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana E. Sousa, YXNvdXNhQG1lZGljaW5hLnVsaXNib2EucHQ=
 Susana L. Silva
Susana L. Silva Ana E. Sousa
Ana E. Sousa