- 1Department of Pediatrics, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada
- 2Alberta Research Centre for Health Evidence, University of Alberta, Knowledge Translation Platform, Alberta SPOR SUPPORT Unit, Edmonton, AB, Canada
- 3Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 4Pediatric Pulmonary and Sleep Unit, Al-Sabah Hospital, Kuwait City, Kuwait
- 5Women and Children’s Health Research Institute, University of Alberta, Edmonton, AB, Canada
- 6Faculty of Medicine and Dentistry, School of Dentistry, University of Alberta, Edmonton, AB, Canada
Background: The use of long-term non-invasive ventilation (NIV) to treat sleep and breathing disorders in children has increased substantially in the last decade; however, less data exist about its use in infants. Given that infants have distinct sleep and breathing patterns when compared to older children, the outcomes of infants on long-term NIV may differ as well. The aim of this study is to systematically review the use and outcomes of long-term NIV in infants.
Methods: Ovid Medline, Ovid Embase, CINAHL (via EbscoHOST), PubMed, and Wiley Cochrane Library were systematically searched from January 1990 to July 2017. Studies on infants using long-term NIV outside of an acute care setting were included. Data were extracted on study design, population characteristics, and NIV outcomes.
Results: A total of 327 studies were full-text reviewed, with final inclusion of 60. Studies were distributed across airway (40%), neuromuscular (28%), central nervous system (10%), cardio-respiratory (2%), and multiple (20%) disease categories. Of the 18 airway studies reporting on NIV outcomes, 13 (72%) reported improvements in respiratory parameters. Of the 12 neuromuscular studies exclusively on spinal muscular atrophy type 1 (SMA1), six (50%) reported decreased hospitalizations and nine (75%) reported on mortality outcomes. Risk of bias was moderate to serious, and quality of the evidence was low to very low for all studies. Most studies had an observational design with no control group, limiting the potential for a meta-analysis.
Conclusion: The outcomes reported in studies differed by the disease category being studied. Studies on airway conditions showed improvements in respiratory parameters for infants using NIV. Studies on neuromuscular disorder, which were almost exclusively on SMA1, reported decreased hospitalizations and prolonged survival. Overall, it appears that NIV is an effective long-term therapy for infants. However, the high risk of bias and low quality of the available evidence limited strong conclusions.
Introduction
Rationale
Long-term non-invasive ventilation (NIV), defined as respiratory support delivered through an interface outside the airway, has become the treatment of choice for a number of chronic conditions resulting in respiratory insufficiency or sleep and breathing disorders in infants and children (1–3). These conditions include airway disorders, neuromuscular disorders (NMDs), and disorders of the central nervous system (CNS) (3–6). The shift toward NIV therapies may have been driven by improvements in NIV technology, a greater emphasis on home-based care, and a growing acceptance of NIV as a viable long-term respiratory support (1, 6, 7). With the increasing number of infants and children living at home using NIV, understanding the benefits and risks of NIV is becoming important not only for specialists involved in starting this therapy but also for pediatricians and primary care physicians providing care to these children within the community and policy makers responsible for decisions about provision of healthcare resources.
While there is a considerable body of work describing the use of long-term NIV, including continuous positive airway pressure (CPAP) and bi-level positive airway pressure (BPAP), in a broad range of pediatric populations, less is known about its use in infants (8–10). Without sufficient data to suggest otherwise, similar NIV treatment approaches are likely followed in both infants and older children, despite key physiological differences in sleep and breathing patterns in infancy. Both sleep and breathing processes are immature at birth and continue to develop through infancy, resulting in change in sleep patterns and breathing control that continue through early life (11). Sleep occupies a greater proportion of time in infants compared to older children (12), which makes infants more vulnerable to respiratory disorders that disrupt sleep. Immaturity of central respiratory centers in infants contributes to increased respiratory events and a greater variability in oxygen saturation, both of which may be important for the normal development of respiratory control (11, 13). Since sleep and breathing processes differ by age, especially in early life, the type of respiratory and sleep disorders treated with NIV, the response to NIV treatment, and the outcomes for NIV may also differ in infants as compared to older children.
Most data available on long-term NIV use in infants is limited to single-center observational studies with relatively small sample sizes (8). Aggregation of the available data for combined data analysis will improve our understanding of the risks and benefits of NIV therapy in the infant population.
Objective
The objective of this systematic review is to summarize the available evidence on the use of long-term NIV for infants and to estimate effect sizes for specific sub-populations and clinical outcomes compared to alternative respiratory care strategies.
Research Question
Does the use of NIV, compared to supportive care, or invasive ventilation, improve clinical outcomes for infants under the age of 2 years with chronic conditions resulting in respiratory insufficiency or sleep and breathing disorders?
Methods
Study Design
This review was conducted using systematic review methodology.
Participants
The inclusion criteria for this systematic review were as follows: (1) infants, defined by the Public Health Agency of Canada as ages 0–24 months inclusive (14); (2) NIV use, defined as breathing support delivered from outside the airway; and (3) long-term NIV use, defined as greater than three months outside of an acute care setting. For studies that examined a broader age range, the mean age of NIV initiation had to be less than 24 months in order to be included in this review, or data had to be presented separately for infants. We did not place any restrictions on study design or outcome eligibility.
Systematic Review Protocol
The protocol for this systematic review was developed according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (15). The full protocol has been registered in the PROSPERO database for international prospective reviews (16).
Search Strategy
This systematic review is an extension of a prior scoping review on long-term NIV in children (8). The scoping review search strategy, using Medical Subject Headings (MeSH) and free-text terms for “child” and “non-invasive ventilation,” was developed for MEDLINE (Ovid) and adapted for subsequent electronic databases with the full protocol published elsewhere (17) [see Table 1 for original MEDLINE (Ovid) search strategy]. Human studies published from 1990 onward were searched in MEDLINE (Ovid), Embase (Ovid), CINAHL (Ebsco), Cochrane Library (Wiley), and PubMed between November 17 and 28, 2014, with no restriction on study design. Gray literature, in the form of conference abstracts on respiratory and sleep medicine, was identified from 2012 to 2014. The literature search was re-run on April 29, 2016, and July 12, 2017, using the same search strategy in Ovid MEDLINE, Ovid Embase, CINAHL, and Wiley Cochrane Library to identify additional studies.

Table 1. Search strategy used in the Ovid Medline database for the scoping review to identify literature on the use of long-term non-invasive ventilation in children.
Data sources, Study Selection, and Data Extraction
The titles and abstracts of studies identified by the literature search were screened by two reviewers (JEM and MCC) to determine eligibility for full-text retrieval. English, French, Spanish, and Portuguese studies that were considered eligible were full-text reviewed for inclusion by two reviewers (JEM and MCC). The final included studies pertaining to children 0–18 years were then full-text screened by two reviewers (PKB and MMA) to identify studies relevant to infants for inclusion in this systematic review. Any disagreement at the screening, eligibility, and inclusion levels were discussed until a consensus was reached. The reference lists of studies meeting inclusion were also reviewed to identify any additional relevant literature.
Data were entered into a pre-established data collection form in Microsoft Excel (version 14.0.4760, Microsoft Corporation, 2010). These data included author’s name, year of publication, country of publication, study design, sample size, age of NIV initiation, NIV type, primary underlying disease conditions, co-morbidities, and primary and secondary outcome measures. One reviewer (PKB) extracted the data, and 20% of data extraction was verified by a second reviewer (MCC).
Risk of Bias
The Cochrane Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool (18) was used to assess the risk of bias in individual studies. The tool measured confounding, selection, measurement, missing data, and reporting bias. Bias was ranked as low, moderate, severe, critical, or no information. Risk of bias in individual studies was independently assessed by two reviewers (PKB and MMA), with disagreements resolved by discussion and consensus.
Quality Assessment
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool (19) was used to determine the quality of studies at an outcome level. Two reviewers (PKB and MMA) independently assessed the quality of studies, with disagreements being resolved through discussion and consensus. Meta-analysis was performed to calculate risk ratios for appropriate outcomes using Review Manager (version 5.3., Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Synthesis of Results
Studies were grouped by disease category (airway, NMD, CNS, cardio-respiratory or multiple disorders) after the data collection stage, to allow for adequate pathophysiological comparisons. Within each disease category, studies were grouped based on primary disease conditions. We included studies with infants who had multiple disease conditions under one disease heading if >75% of the infant cohort had the same disease condition; otherwise these studies were included in the multiple disorders category.
Primary and secondary outcomes were established after data collection, during synthesis of the data, based on the most common and clinically relevant outcomes reported in studies with the same disease condition. Primary outcomes were as follows: (1) objective changes in respiratory parameters, (2) discontinuation of NIV, (3) hospitalizations, and (4) mortality. Secondary outcomes were as follows: (1) improvements in underlying disease conditions, (2) improvements in growth parameters, (3) NIV facilitation of extubation, (4) predictors of NIV requirement, (5) NIV success/failure, (6) adherence to respiratory support, and (7) mask complications. Studies were included in the synthesis if they reported on at least one primary or secondary outcome. Continuous data were presented as a weighted mean (standard deviation) or median (interquartile range) where appropriate. Results were grouped and reported based on the primary underlying disease category being studied. Primary outcomes were reported in both tabular and narrative format, while secondary outcomes were only reported narratively.
Results
Study Selection and Characteristics
The search strategy, after removal of duplicates, identified 12,594 studies and additional records (Figure 1). After screening of the titles and abstracts, and with the addition of records from additional sources, 1046 studies met eligibility for review. After full-text review, 327 studies on children ages 0–18 years met the inclusion criteria for the scoping review. Full-text review of these 327 articles identified 64 studies meeting the infant inclusion criteria. Four conference proceedings met inclusion criteria but were excluded because of insufficient data reporting, leaving 60 articles reporting on a total of 977 infants for inclusion in this systematic review (Table 2) (3, 7, 9, 10, 20–75).
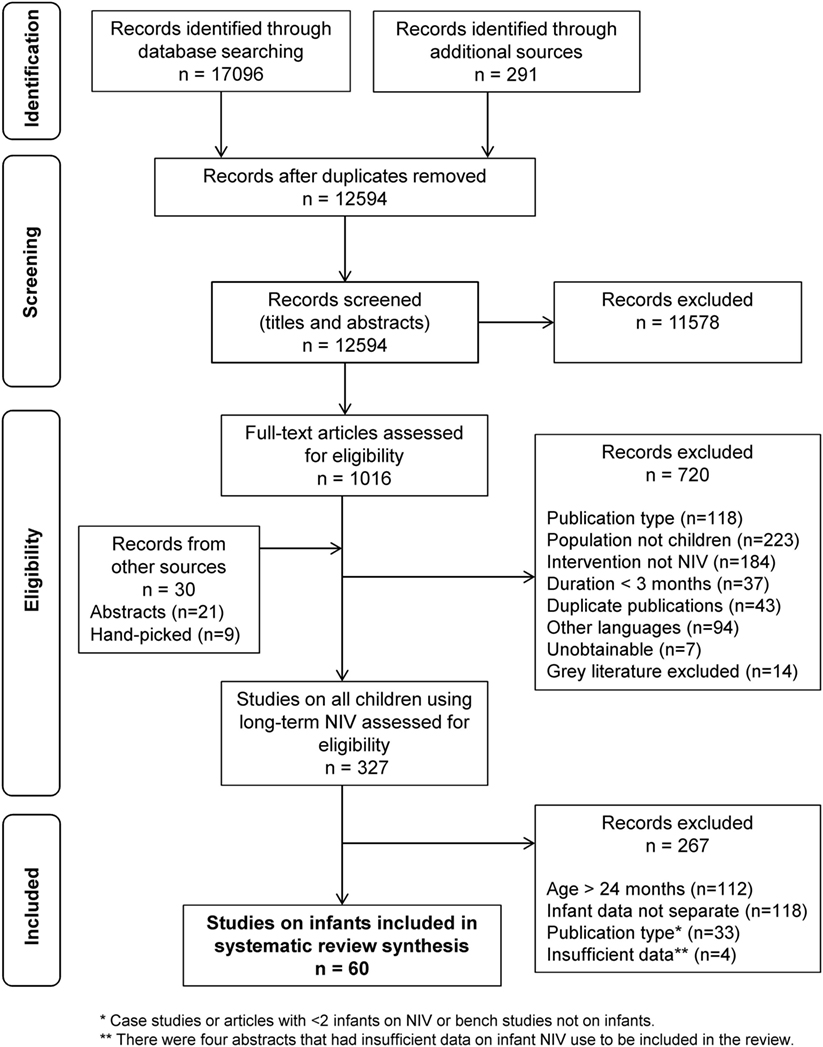
Figure 1. Flow diagram outlining the study selection process for the systematic review, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (15).
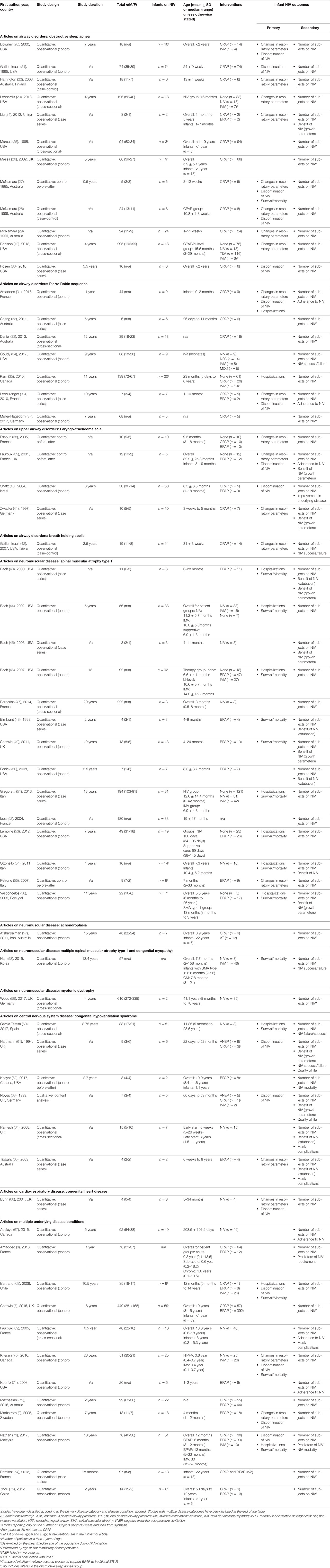
Table 2. Characteristics and outcomes of 60 studies included in the systematic review on infants using long-term NIV.
The majority of studies were retrospective (41/60, 68%), quantitative (59/60, 98%), and single-center studies (54/60, 90%). The most common study design was observational, which included cohort studies (31/60, 52%), case series (13/60, 25%), and cross-sectional studies (8/60, 13%). Forty-eight percent of studies were exclusively on the infant population. Based on primary underlying disease categories, the studies were distributed across airway disorders (24/60, 40%), NMD (17/60, 28%), CNS (6/60, 10%), cardio-respiratory diseases (1/60, 2%), and multiple disease categories (12/60, 20%; Table 2). Thirteen studies did not report NIV outcomes, only the number of infants using NIV, and were excluded from further analysis (7, 25, 26, 32, 33, 37, 47, 52, 57, 59, 72, 74, 75).
Obstructive Sleep Apnea
Obstructive sleep apnea (OSA) was the most common airway disorder studied in the infant population, with 12 studies (12/60, 20%) reporting on this condition (Table 2). Of these, 10 studies reported on infant NIV outcomes and were synthesized in the review (10, 20–24, 27–30). These studies included infants with multiple underlying conditions, the most common being a history of acute life-threatening events (ALTE), family history of sudden infant death syndrome (SIDS), and craniofacial malformations. Eight studies (8/10, 80%) reported on changes in respiratory parameters, with seven of these studies (7/10, 70%) showing improvements in central, obstructive, and/or mixed apneas from a diagnostic to titration polysomnography (Table 3) (10, 20, 22, 23, 27–29) Only one study (1/10, 10%) included diagnostic polysomnography results after long-term NIV use (weighted mean of 12 months), which showed an overall decrease in respiratory events, normalization of respiratory gases, and increased arousals during REM sleep (29). Five studies (5/10, 50%) reported discontinuation of NIV in infants because of improvements in respiratory parameters, with discontinuation rates ranging from 14 to 100% (weighted mean 70 ± 26%) (20, 21, 27, 29, 30). No studies reported on hospitalization outcomes (Table 4). One study (1/10, 10%) of five infants using NIV reported mortality outcomes, with all infants alive at the time of study publication (27).
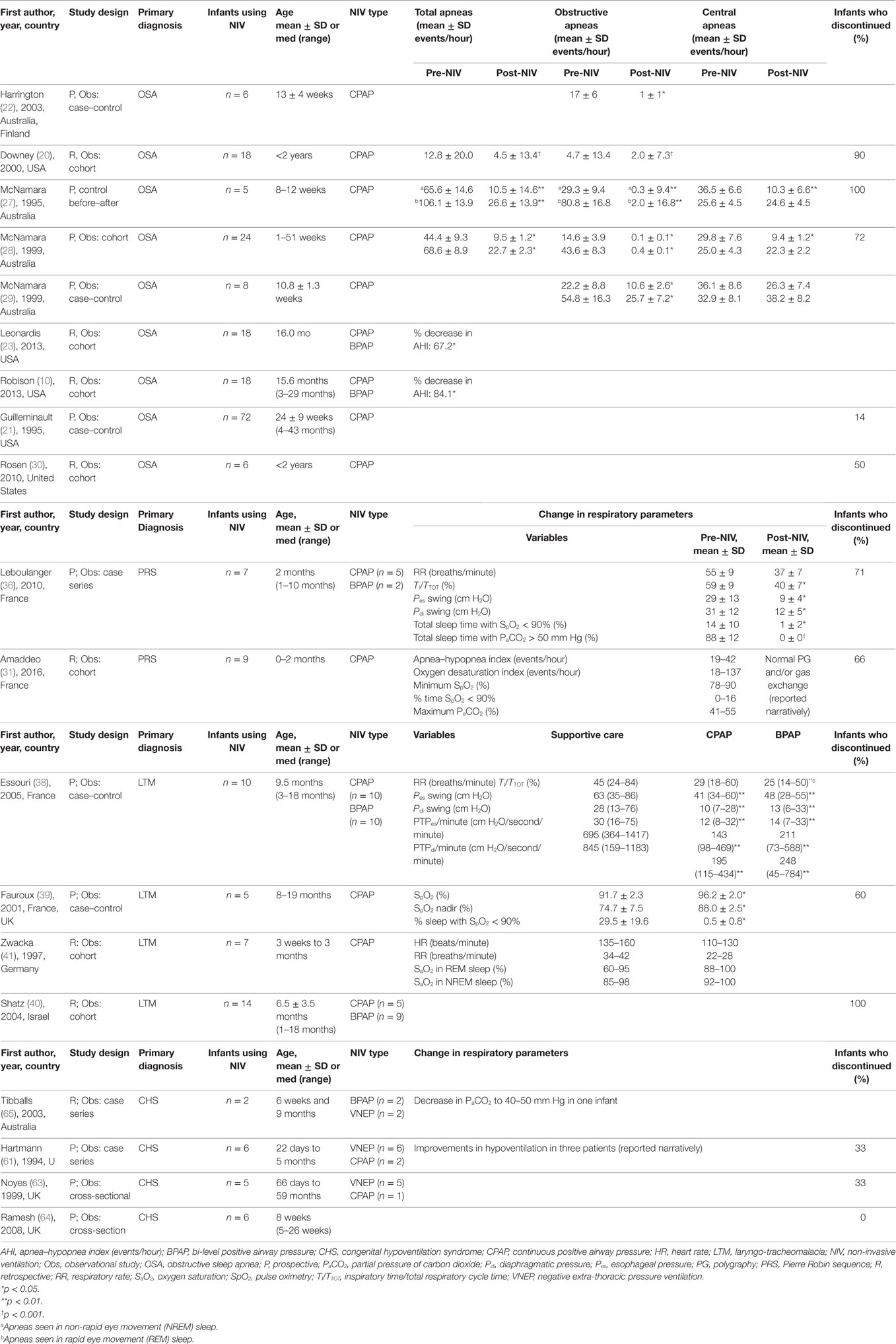
Table 3. Studies on infants using long-term NIV reporting change in respiratory parameters and discontinuation outcomes.
Pierre Robin Sequence
Seven studies (7/60, 12%) reported on infants with Pierre Robin sequence (PRS) using long-term NIV (Table 2). Four studies (4/7, 57%) reported on primary or secondary outcomes and were synthesized for this review (31, 34–36). A cohort study reported normalization of polygraphy parameters and gas exchange post-NIV initiation (Table 3) (31). A case series reported a decrease in respiratory rates, statistically significant improvements in respiratory effort, and normalization of respiratory gases after administration of NIV therapy in infants with PRS (36). Two studies on 16 infants with PRS reported discontinuation from NIV in 11 (69%) infants because of improvements in respiratory parameters (31, 36). Two studies comparing infants on NIV and invasive mechanical ventilation showed that the length of hospitalization were shorter for infants on NIV than for those receiving invasive mechanical ventilation via a tracheostomy (Table 4) (31, 35). No studies addressed survival outcomes in infants with PRS using long-term NIV. Adherence of infants to NIV was reported as excellent, showing more than 8 hours of NIV use per day in two studies (31, 36), with only a 1–2 week period required to adjust to the mask ventilation (31, 35). An additional cohort study demonstrated that infants with PRS using NIV were 10.43 times more likely to progress to a surgical airway compared to infants who required less advanced respiratory supports such as prone positioning and a nasopharyngeal airway (34).
Laryngo-Tracheomalacia
All four studies (4/60, 7%) on infants with laryngo-tracheomalacia (LTM) using long-term NIV reported on primary or secondary outcomes and were synthesized in the review (Table 2) (38–41). Three studies (3/4, 75%) reported on changes in respiratory parameters (Table 3) (38, 39, 41). A case–control study of 10 infants with LTM showed improvements in respiratory frequency and respiratory effort in infants using CPAP or BPAP compared to spontaneous breathing (38). Normalization of arterial oxygen saturations after NIV use was seen in two studies (39, 41). NIV discontinuation was reported in two studies, with a combined discontinuation due to improvement rate of 81% (13/16 infants) (39, 40). No studies examined hospitalization or mortality outcomes. Improvement in chest wall deformity after NIV use in three patients and normalization of weight in four patients was reported in one case–control study (39). The same study also reported an average NIV use per day of 10.2 hours/day in seven infants (50).
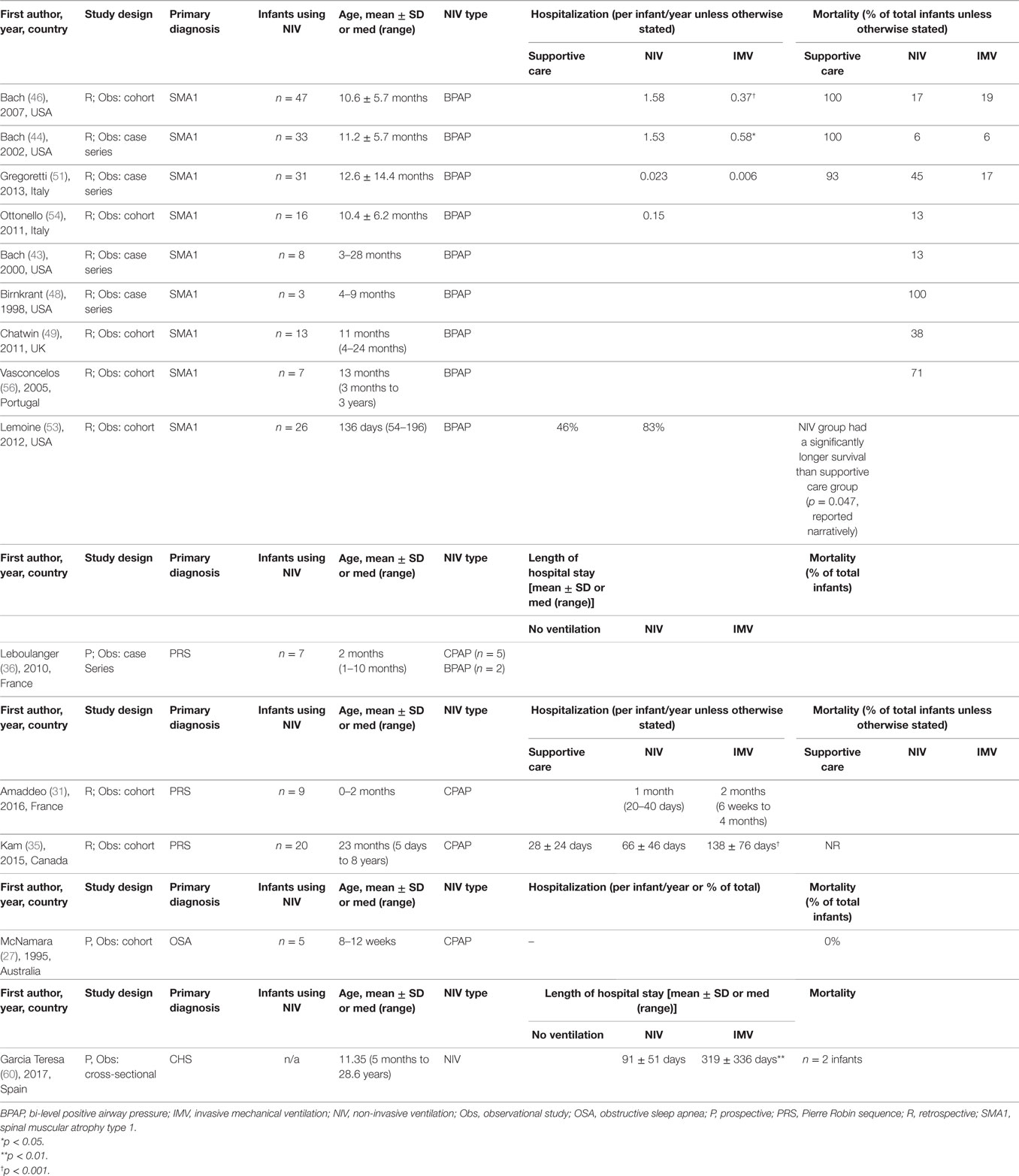
Spinal Muscular Atrophy Type 1
There were 14 studies (14/60, 23%) of infants with spinal muscular atrophy type 1 (SMA1) using long-term NIV (Table 2). Twelve of these studies reported on primary or secondary outcomes and were synthesized (43–46, 48–51, 53–56). Only one study (1/12, 8%) reported on changes in respiratory parameters and showed improvements in respiratory effort and normalization of respiratory gases in SMA1 patients using NIV therapy (Table 3) (55). Six studies (6/12, 50%) reported on hospitalization outcomes (Table 4) (43, 44, 46, 51, 53, 54). Of these, two studies reported that hospitalizations per patient per year were significantly higher in infants on NIV than infants with a tracheostomy until after three years of age (44, 46). Nine studies (9/12, 75%) reported on mortality outcomes (43, 44, 46, 48, 49, 51, 53, 54, 56); four of these studies compared infants on supportive care with those using NIV, showing prolonged survival in the NIV group (44, 46, 51, 53). Three studies (3/12, 25%) reported improvements in growth parameters, seen by resolution of chest wall deformity (pectus excavatum) after the initiation of NIV therapy (43, 45, 49). An additional three studies showed that NIV helped facilitate extubation in infants with SMA1 (43, 48, 50).
Central Hypoventilation Syndrome
There were six studies (6/60, 10%) on NIV use for infants with central hypoventilation syndrome (CHS) that reported primary or secondary outcomes, and all six were summarized (Table 2) (60–65). The diagnosis of CHS was confirmed clinically in two studies (61, 65), via PHOX2B gene mutation analysis in three studies (60, 62, 64), and unreported in one study (63). NIV was used in conjunction with negative extra-thoracic pressure ventilation (VNEP) therapy in two studies: in one study, it was used as the primary therapy (65) and, in the second study, CPAP was used to relieve upper airway obstruction not resolved with VNEP (61). Improvements in respiratory parameters were reported in two studies: one showed the normalization of the partial pressure of carbon dioxide and resolution of pulmonary hypertension following the use of NIV (65) and the other study showed improvements in hypoventilation for 50% (3/6) of infants (Table 3) (61). One study with six infants reported NIV discontinuation in two infants (33%) because of improvements in respiratory parameters; the remaining four infants were using NIV only during sleep (61). One cohort study reported mortality outcomes and a higher hospitalization time for infants using invasive mechanical ventilation compared to NIV (Table 4) (60). Two studies showed parent-reported improvements in growth and development after NIV initiation using the results of a parent questionnaire (61, 63). An additional two studies reported pressure-related effects of mask use, which were predominantly skin breakdown and mid-face hypoplasia (64, 65). One cross-sectional study showed that it took less than a week for five of the six infants to adjust to NIV (61). A control before-after study of infants using two BPAP ventilators showed comparable sleep and respiratory parameters with both ventilators, with the exception of a greater decrease in the maximum transcutaneous carbon dioxide with the intelligent volume-assured pressured support compared to a traditional BPAP ventilator (62).
Synthesized Findings
After examining studies for all disease categories and respective outcomes, only three studies on infants with SMA1 reporting mortality outcomes were eligible for meta-analysis (44, 46, 51). The results of meta-analysis showed that there was a statistically significant decrease in the relative risk of mortality in the NIV group compared to the supportive care group (Figure 2).
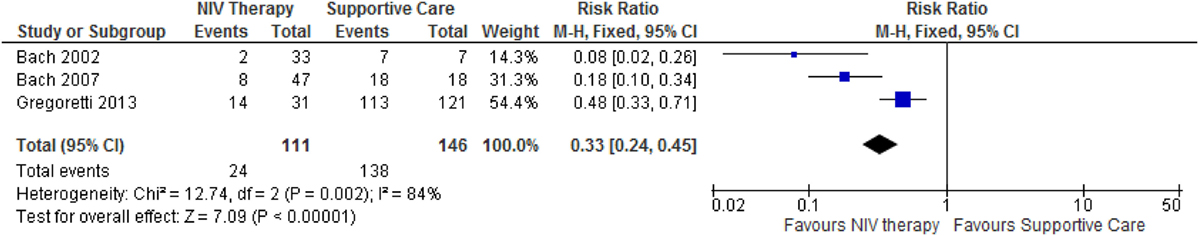
Figure 2. A meta-analysis on the effect of non-invasive ventilation (NIV) on the relative risk of mortality in infants with spinal muscular atrophy. The meta-analysis shows that the relative risk of mortality is significantly lower in infants using NIV compared to infants on supportive care. This decrease may be attributed to prolonged survival in infants using long-term NIV compared to supportive care.
Risk of Bias and Quality Assessment of Outcomes
Risk of bias ranged from moderate to severe in all studies synthesized in this review (Table 5). Study design was the main contributor to the low quality assessment of the studies. Almost all the included studies had an observational study design, which contributed to confounding bias in participant selection and selected reporting of results. Grading of the quality of the evidence for outcomes such as changes in respiratory parameters, discontinuation of NIV, hospitalizations, and mortality showed that the quality of evidence ranged from low to very low for all studies (Table 6).
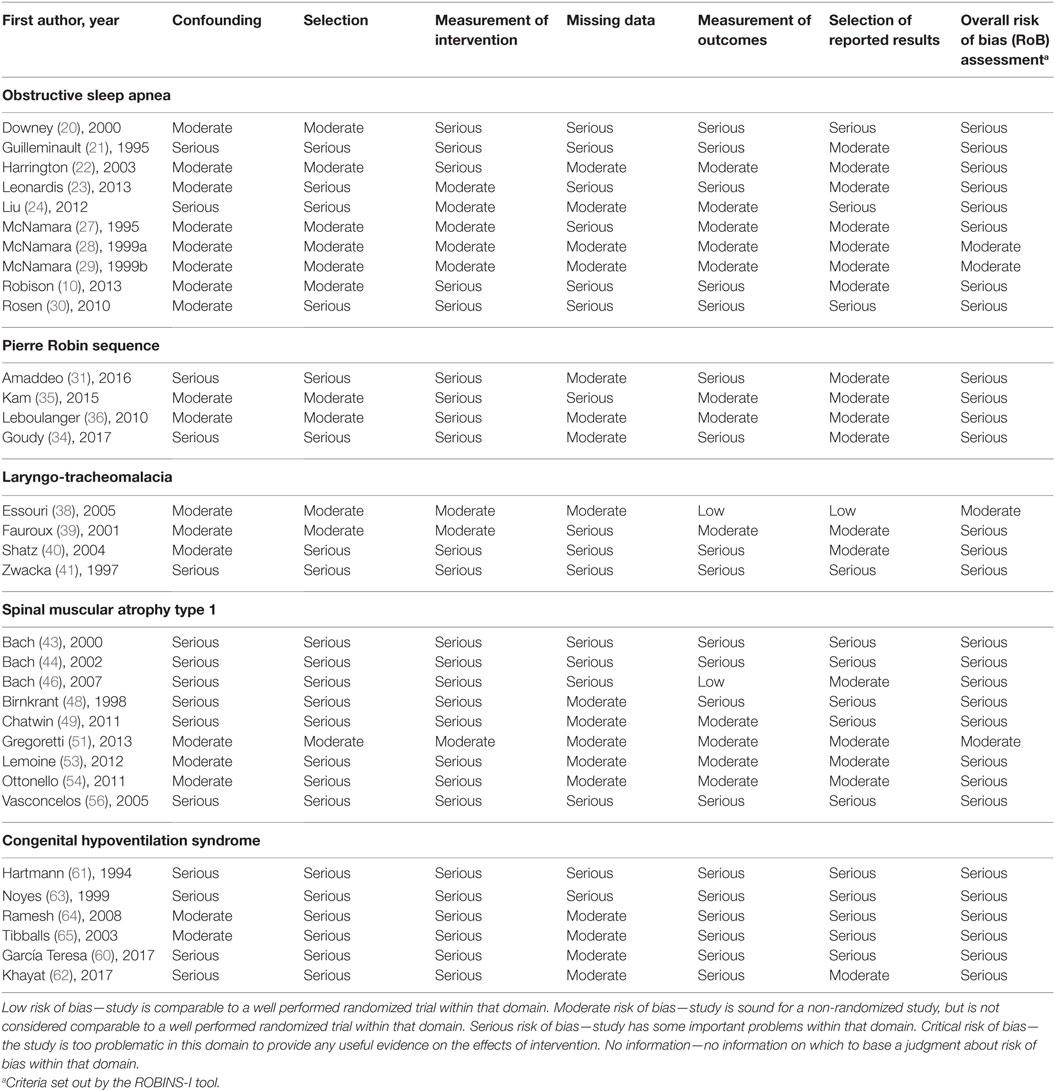
Table 5. Assessment of risk of bias in studies synthesized in the systematic review on long-term non-invasive ventilation in infants using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool (18).
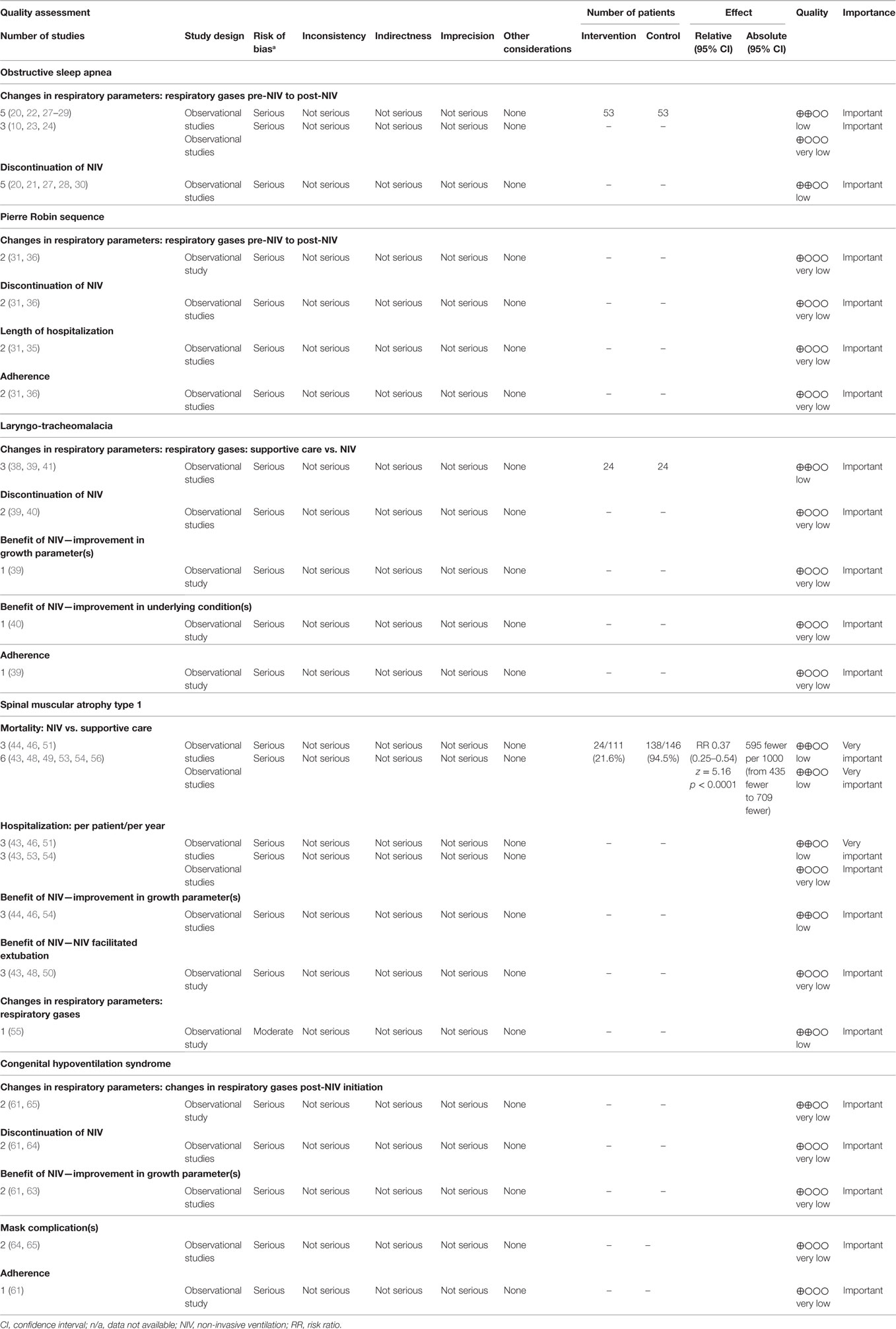
Table 6. Quality assessment of outcomes of infants using long-term non-invasive ventilation using the Grading of Recommendations Assessment, Development and Evaluation criteria (19).
Discussion
Summary of Main Findings
To our knowledge, this is the first systematic review on the use of long-term NIV in infants. We identified studies on a diverse range of airway conditions in which NIV therapy improved the results of polysomnographic and respiratory parameters. With data available for NMD and CNS disorders limited to SMA1 and CHS, extrapolation of NIV benefits to other NMD and CNS disorders in infants is challenging. Not all outcomes were studied in all disease categories; length of hospitalization was the focus in studies of PRS, while hospitalizations and mortality were the focus in studies of SMA, and respiratory events and NIV discontinuation in the remaining groups. The overall quality of evidence to support appropriate conclusions was low to very low for all studies included in this review.
There is a diverse range of airway disorders that may benefit from NIV therapy. Previous studies have identified many conditions that can predispose infants to upper airway obstruction, including craniofacial disorders, laryngeal disorders, and nasal obstruction (76). Similarly, in this review, we identified NIV use in a wide variety of diseases associated with compromised airway function, the most common being OSA, PRS, ALTE, infants at risk for SIDS, and LTM. The improvement in respiratory parameters reported in infants with airway disorders reflects an overall benefit from NIV therapy. In addition, the underlying airway conditions have potential for improvement, as seen with the infants discontinuing due to underlying improvements, so there may be less risk with NIV compared to invasive mechanical ventilation. Extrapolating these results to conditions with a similar pathophysiology, but for which there is no evidence for NIV use in the literature, may be reasonable given the diversity of disorders represented in the available evidence.
By contrast, extrapolation of outcomes for long-term NIV use in NMD and CNS disorders may be more challenging. The data relevant to long-term NIV use for NMD and CNS disorders are almost exclusively from two conditions: SMA1 and CHS. SMA1 is a progressively deteriorating disorder that is usually fatal during infancy. This contrasts with other NMD disorders presenting in infancy, such as congenital myopathy and congenital muscular dystrophy, which may have a better prognosis or steadier course (7, 58). The difference in prognoses of these conditions makes generalizing outcomes for NIV use in SMA1 to other NMD less appropriate. Similarly, CHS was the only CNS disorder for which data on long-term NIV use was available. NIV may be useful for other CNS disorders with accompanying respiratory compromise, such as congenital or acquired brain injury. Given the potentially unique physiology of CHS extrapolating the outcomes of NIV use for infants with CHS to other CNS conditions with different underlying respiratory pathophysiology may not be appropriate. Creation of national disease registries for infants and children using NIV will provide the opportunity to aggregate data on rare or minimally studied diseases and examine the use and outcomes of long-term NIV in these populations.
The outcomes that were reported in studies differed depending on the primary underlying disease category that was being examined. Studies of airway conditions predominantly reported on changes in respiratory parameters reported via polysomnography results and discontinuation of NIV. In addition, most studies reported short-term overnight polysomnography results; only one study had data on polysomnography results after long-term follow-up periods of NIV use in infants (29). Only one study on upper airway disorders reported on mortality outcomes (27) and none on hospitalization outcomes. Long-term outcomes, such as hospitalizations, intercurrent illness, growth and development, and quality of life warrant further study. Interestingly, studies on SMA1 predominantly reported on mortality and hospitalization outcomes, with only one study reporting on changes in respiratory parameters.
While the overall quality of the evidence available for the use of long-term NIV in infants is low to very low, there is a body of evidence that may help guide clinical practice. The reason for the low quality of the evidence included the study design and a high risk of bias due to the lack of blinding and randomization, and control for confounding variables. While these findings highlight the need for future studies of strong design and lower risk of bias, the available data still provide important information to inform treatment decisions for conditions where long-term NIV is being considered.
Limitations of the Included Studies
We identified a number of research gaps present in the studies included within this review. There was only one study that compared the efficacy of CPAP and BPAP ventilation in a cohort of infants (38). Similarly, while some studies reported mask complications (9, 21), only one compared the efficacy and practicality of different infant NIV masks (74). Only single studies were identified on the use of long-term NIV for infants with breath holding (42) and cardiac disease (66). Additionally, there were no studies on the clinical supports necessary for infants to be placed on NIV. It is important to know whether infants receive consultation and support from physicians, registered nurses, home care support, or a combination thereof, to determine whether a multidisciplinary NIV care plan is necessary for this population. The lack of comparison groups and/or homogeneity of outcomes reported precluded meta-analysis for most topics.
Additional issues relevant to long-term NIV use in infants that are not addressed in the current literature include: limitations in availability of masks and headgear; limitations in the availability of BPAP machines that are sufficiently sensitive to detect flow rates; the impact of NIV use on craniofacial growth and the impact of craniofacial growth on NIV use; co-morbidities in infants using NIV; the impact of NIV on somatic growth and psychomotor development; and, most importantly, the impact of NIV use on quality of life for both infants and caregivers.
Limitations of the Review
Our review relied on the search methods and primary-level screening decisions of a scoping review on NIV in children with subsequent development of the research questions on NIV in infants. The methods to identify studies for the scoping review, however, were sufficiently inclusive to capture all relevant evidence on NIV in infants. We defined NIV for the scoping review on long-term NIV as breathing support outside the airway via an interface, consistent with the MeSH terminology for NIV and, therefore, included CPAP as well as BPAP. Some investigators, however, do not consider CPAP as a mode of NIV because it requires spontaneous breathing from the patient (1, 77). To address this concern, we reported the different ventilation types used by infants in the tables included in this review. Finally, we defined infants as ages 0–2 years based on the Public Health Agency of Canada definition (14). Some investigators may not agree with this definition, as the Centre for Disease Control defines infants as less than one year of age (78). Regardless of the definition used, it is still unclear whether there are differences in the outcomes of pediatric NIV with respect to age. Future work should consider whether infants represent a distinct group within children using long-term NIV.
Conclusion
This systematic review examines the use and outcomes of long-term NIV in infants across a range of respiratory and sleep disorders. Improvements in respiratory parameters and discontinuation from NIV due to improvement in underlying conditions have been shown for a broad range of upper airway disorders, such as OSA, PRS, and LTM, in infants. Long-term NIV use in infants with SMA1 decreased hospitalizations and prolonged survival compared to infants on supportive care. Infants with CHS may also show improvements in respiratory parameters after using NIV and potentially avoid tracheostomy. NIV appears to be a feasible method of providing long-term respiratory support for infants with a wide range of underlying conditions; however, several methodological weaknesses limit any strong categorical conclusions. The findings of this systematic review are relevant to a broad range of stakeholders and can be used to help guide clinicians on the use of long-term NIV in infants.
Author Contributions
PB conceptualized and designed the review, assessed articles for inclusion, extracted and analyzed data, interpreted the data, drafted the initial manuscript, and completed all subsequent revisions until submission. MC conceptualized and designed the review, assessed articles for inclusion, verified data extraction, and critically reviewed the manuscript. RF developed the search strategy, carried out the literature searches, and critically reviewed the manuscript. MA and BA assessed articles for inclusion, and critically reviewed the manuscript. AK provided guidance on study design and critically reviewed the manuscript. CF provided guidance on study design and review methodology and critically reviewed the manuscript. JM conceptualized and designed the review, assessed articles for inclusion, verified data extraction, interpreted the data, and critically reviewed the manuscript. All authors reviewed the manuscript and approved the final manuscript for submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MP and handling editor declared their shared affiliation.
Acknowledgments
The authors thank Dr. Meghan Sebastianski (Knowledge Translation Platform, Alberta SPOR SUPPORT Unit, University of Alberta) for her expertise and guidance on systematic review methodology.
Funding
The authors have no financial relationships relevant to this article to disclose. PB received salary support through a Pediatric Non-Invasive Ventilation studentship from the Department of Pediatrics, University of Alberta. The project was supported by a mini-grant from the Respiratory Health Strategic Clinical Network of Alberta Health Services.
Abbreviations
ALTE, acute life-threatening events; BPAP, bi-level positive airway pressure; CHS, central hypoventilation syndrome; CNS, central nervous system; CPAP, continuous positive airway pressure; GRADE, Grading of Recommendations Assessment, Development and Evaluation; LTM, laryngo-tracheomalacia; NIV, non-invasive ventilation; NMD, neuromuscular disorder; OSA, obstructive sleep apnea; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PRS, Pierre Robin sequence; ROBINS-I, Risk of Bias in Non-Randomized Studies of Interventions; SIDS, sudden infant death syndrome; SMA1, spinal muscular atrophy type 1.
References
1. Amin R, Sayal P, Syed F, Chaves A, Moraes TJ, MacLusky I. Pediatric long-term home mechanical ventilation: twenty years of follow-up from one Canadian center. Pediatr Pulmonol (2014) 49(8):816–24. doi:10.1002/ppul.22868
2. McDougall CM, Adderley RJ, Wensley DF, Seear MD. Long-term ventilation in children: longitudinal trends and outcomes. Arch Dis Child (2013) 98(9):660–5. doi:10.1136/archdischild-2012-303062
3. Amaddeo A, Moreau J, Frapin A, Khirani S, Felix O, Fernandez-Bolanos M, et al. Long term continuous positive airway pressure (CPAP) and noninvasive ventilation (NIV) in children: initiation criteria in real life. Pediatr Pulmonol (2016) 51(9):968–74. doi:10.1002/ppul.23416
4. Katz S, Selvadurai H, Keilty K, Mitchell M, MacLusky I. Outcome of non-invasive positive pressure ventilation in paediatric neuromuscular disease. Arch Dis Child (2004) 89(2):121–4. doi:10.1136/adc.2002.018655
5. Simonds AK, Ward S, Heather S, Bush A, Muntoni F. Outcome of paediatric domiciliary mask ventilation in neuromuscular and skeletal disease. Eur Respir J (2000) 16(3):476–81. doi:10.1034/j.1399-3003.2000.016003476.x
6. Jardine E, O’Toole M, Paton JY, Wallis C. Current status of long term ventilation of children in the United Kingdom: questionnaire survey. BMJ (1999) 318(7179):295–9. doi:10.1136/bmj.318.7179.295
7. Chatwin M, Tan HL, Bush A, Rosenthal M, Simonds AK. Long term non-invasive ventilation in children: impact on survival and transition to adult care. PLoS One (2015) 10(5):e0125839. doi:10.1371/journal.pone.0125839
8. Castro-Codesal ML, Dehaan K, Featherstone R, Bedi PK, Martinez Carrasco C, Katz SL, et al. Long-term non-invasive ventilation therapies in children: a scoping review. Sleep Med Rev (2018) 37:148–58. doi:10.1016/j.smrv.2017.02.005
9. Markstrom A, Sundell K, Stenberg N, Katz-Salamon M. Long-term non-invasive positive airway pressure ventilation in infants. Acta Paediatr (2008) 97(12):1658–62. doi:10.1111/j.1651-2227.2008.00990.x
10. Robison JG, Wilson C, Otteson TD, Chakravorty SS, Mehta DK. Analysis of outcomes in treatment of obstructive sleep apnea in infants. Laryngoscope (2013) 123(9):2306–14. doi:10.1002/lary.23685
11. MacLean JE, Fitzgerald DA, Waters KA. Developmental changes in sleep and breathing across infancy and childhood. Paediatr Respir Rev (2015) 16(4):276–84. doi:10.1016/j.prrv.2015.08.002
12. Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics (2003) 111(2):302–7. doi:10.1542/peds.111.2.302
13. Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care Med (2001) 164(1):16–30. doi:10.1164/ajrccm.164.1.2008171
14. Infancy (Birth – Two Years of Age). Public Health Agency of Canada (2016). Available from http://www.phac-aspc.gc.ca/hp-ps/dca-dea/stages-etapes/childhood-enfance_0-2/index-eng.php
15. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (2009) 339:b2535. doi:10.1136/bmj.b2535
16. Bedi PK, Castro-Codesal ML, Featherstone R, AlBalawi MA, Alkhaledi B, Kozyrskyj A, et al. Long-term non-invasive ventilation in infants: a systematic review. PROSPERO 2016:CRD42016051302. (2016). Available from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016051302
17. Castro-Codesal ML, Featherstone R, Martinez Carrasco C, Katz SL, Chan EY, Bendiak GN, et al. Long-term non-invasive ventilation therapies in children: a scoping review protocol. BMJ Open (2015) 5(8):e008697. doi:10.1136/bmjopen-2015-008697
18. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (2016) 355:i4919. doi:10.1136/bmj.i4919
19. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (2008) 336(7650):924–6. doi:10.1136/bmj.39489.470347.AD
20. Downey R III, Perkin RM, MacQuarrie J. Nasal continuous positive airway pressure use in children with obstructive sleep apnea younger than 2 years of age. Chest (2000) 117(6):1608–12. doi:10.1378/chest.117.6.1608
21. Guilleminault C, Pelayo R, Clerk A, Leger D, Bocian RC. Home nasal continuous positive airway pressure in infants with sleep-disordered breathing. J Pediatr (1995) 127(6):905–12. doi:10.1016/S0022-3476(95)70026-9
22. Harrington C, Kirjavainen T, Teng A, Sullivan CE. nCPAP improves abnormal autonomic function in at-risk-for-SIDS infants with OSA. J Appl Physiol (2003) 95(4):1591–7. doi:10.1152/japplphysiol.00354.2002
23. Leonardis RL, Robison JG, Otteson TD. Evaluating the management of obstructive sleep apnea in neonates and infants. JAMA Otolaryngol Head Neck Surg (2013) 139(2):139–46. doi:10.1001/jamaoto.2013.1331
24. Liu D, Zhou J, Liang X, Huang Z, Tan Z, Zhong J. Remote monitoring of home-based noninvasive ventilation in children with obstructive sleep apnea-hypopnea syndrome. Sleep Breath (2012) 16(2):317–28. doi:10.1007/s11325-011-0516-y
25. Marcus CL, Ward SL, Mallory GB, Rosen CL, Beckerman RC, Weese-Mayer DE, et al. Use of nasal continuous positive airway pressure as treatment of childhood obstructive sleep apnea. J Pediatr (1995) 127(1):88–94. doi:10.1016/S0022-3476(95)70262-8
26. Massa F, Gonsalez S, Laverty A, Wallis C, Lane R. The use of nasal continuous positive airway pressure to treat obstructive sleep apnoea. Arch Dis Child (2002) 87(5):438–43. doi:10.1136/adc.87.5.438
27. McNamara F, Harris MA, Sullivan CE. Effects of nasal continuous positive airway pressure on apnoea index and sleep in infants. J Paediatr Child Health (1995) 31(2):88–94. doi:10.1111/j.1440-1754.1995.tb00753.x
28. McNamara F, Sullivan CE. Effects of nasal CPAP therapy on respiratory and spontaneous arousals in infants with OSA. J Appl Physiol (1999) 87(3):889–96. doi:10.1152/jappl.1999.87.3.889
29. McNamara F, Sullivan CE. Obstructive sleep apnea in infants and its management with nasal continuous positive airway pressure. Chest (1999) 116(1):10–6. doi:10.1378/chest.116.1.10
30. Rosen D. Some infants with Down syndrome spontaneously outgrow their obstructive sleep apnea. Clin Pediatr (Phila) (2010) 49(11):1068–71. doi:10.1177/0009922810378037
31. Amaddeo A, Abadie V, Chalouhi C, Kadlub N, Frapin A, Lapillonne A, et al. Continuous positive airway pressure for upper airway obstruction in infants with Pierre Robin sequence. Plast Reconstr Surg (2016) 137(2):609–12. doi:10.1097/01.prs.0000475799.07597.23
32. Cheng ATL, Corke M, Loughran-Fowlds A, Birman C, Hayward P, Waters KA. Distraction osteogenesis and glossopexy for Robin sequence with airway obstruction. ANZ J Surg (2011) 81(5):320–5. doi:10.1111/j.1445-2197.2010.05588.x
33. Daniel M, Bailey S, Walker K, Hensley R, Kol-Castro C, Badawi N, et al. Airway, feeding and growth in infants with Robin sequence and sleep apnoea. Int J Pediatr Otorhinolaryngol (2013) 77(4):499–503. doi:10.1016/j.ijporl.2012.12.019
34. Goudy S, Jiramongolchai P, Chinnadurai S. Logistic regression analysis of Pierre Robin sequence patients requiring surgical intervention. Laryngoscope (2017) 127(4):945–9. doi:10.1002/lary.26143
35. Kam K, McKay M, MacLean J, Witmans M, Spier S, Mitchell I. Surgical versus nonsurgical interventions to relieve upper airway obstruction in children with Pierre Robin sequence. Can Respir J (2015) 22(3):171–5. doi:10.1155/2015/798076
36. Leboulanger N, Picard A, Soupre V, Aubertin G, Denoyelle F, Galliani E, et al. Physiologic and clinical benefits of noninvasive ventilation in infants with Pierre Robin sequence. Pediatrics (2010) 126(5):e1056–63. doi:10.1542/peds.2010-0856
37. Muller-Hagedorn S, Buchenau W, Arand J, Bacher M, Poets CF. Treatment of infants with Syndromic Robin sequence with modified palatal plates: a minimally invasive treatment option. Head Face Med (2017) 13(1):4. doi:10.1186/s13005-017-0137-1
38. Essouri S, Nicot F, Clément A, Garabedian EN, Roger G, Lofaso F, et al. Noninvasive positive pressure ventilation in infants with upper airway obstruction: comparison of continuous and bilevel positive pressure. Intensive Care Med (2005) 31(4):574–80. doi:10.1007/s00134-005-2568-6
39. Fauroux B, Pigeot J, Polkey MI, Roger G, Boulé M, Clément A, et al. Chronic stridor caused by laryngomalacia in children: work of breathing and effects of noninvasive ventilatory assistance. Am J Respir Crit Care Med (2001) 164(10 Pt 1):1874–8. doi:10.1164/ajrccm.164.10.2012141
40. Shatz A, Goldberg S, Picard E, Kerem E. Pharyngeal wall collapse and multiple synchronous airway lesions. Ann Otol Rhinol Laryngol (2004) 113(6):483–7. doi:10.1177/000348940411300613
41. Zwacka G, Scholle S, Kemper G, Rieger B. Nasal CPAP therapy for infants with congenital stridor. Sleep Breath (1997) 2(4):85–97.
42. Guilleminault C, Huang Y-S, Chan A, Hagen CC. Cyanotic breath-holding spells in children respond to adenotonsillectomy for sleep-disordered breathing. J Sleep Res (2007) 16(4):406–13. doi:10.1111/j.1365-2869.2007.00605.x
43. Bach JR, Niranjan V, Weaver B. Spinal muscular atrophy type 1: a noninvasive respiratory management approach. Chest (2000) 117(4):1100–5. doi:10.1378/chest.117.4.1100
44. Bach JR, Baird JS, Plosky D, Navado J, Weaver B. Spinal muscular atrophy type 1: management and outcomes. Pediatr Pulmonol (2002) 34(1):16–22. doi:10.1002/ppul.10110
45. Bach JR, Bianchi C. Prevention of pectus excavatum for children with spinal muscular atrophy type 1. Am J Phys Med Rehabil (2003) 82(10):815–9. doi:10.1097/01.PHM.0000083669.22483.04
46. Bach JR, Saltstein K, Sinquee D, Weaver B, Komaroff E. Long-term survival in Werdnig-Hoffmann disease. Am J Phys Med Rehabil (2007) 86(5):339–345; quiz 346–338, 379. doi:10.1097/PHM.0b013e31804a8505
47. Barnérias C, Quijano S, Mayer M, Estournet B, Cuisset JM, Sukno S, et al. [Multicentric study of medical care and practices in spinal muscular atrophy type 1 over two 10-year periods]. Arch Pediatr (2014) 21(4):347–54. doi:10.1016/j.arcped.2014.01.017
48. Birnkrant DJ, Pope JF, Martin JE, Repucci AH, Eiben RM. Treatment of type I spinal muscular atrophy with noninvasive ventilation and gastrostomy feeding. Pediatr Neurol (1998) 18(5):407–10. doi:10.1016/S0887-8994(97)00227-0
49. Chatwin M, Bush A, Simonds AK. Outcome of goal-directed non-invasive ventilation and mechanical insufflation/exsufflation in spinal muscular atrophy type I. Arch Dis Child (2011) 96(5):426–32. doi:10.1136/adc.2009.177832
50. Ednick M, Wong B, Inge T, Knue M, Kalra M. Post-operative respiratory outcomes using a standard extubation protocol after elective gastrostomy tube placement in pediatric patients with spinal muscular atrophy type 1. J Pe1diatr Neurol (2008) 6(3):249–52. doi:10.1055/s-0035-1557467
51. Gregoretti C, Ottonello G, Chiarini Testa MB, Mastella C, Ravà L, Bignamini E, et al. Survival of patients with spinal muscular atrophy type 1. Pediatrics (2013) 131(5):e1509–14. doi:10.1542/peds.2012-2278
52. Ioos C, Leclair-Richard D, Mrad S, Barois A, Estournet-Mathiaud B. Respiratory capacity course in patients with infantile spinal muscular atrophy. Chest (2004) 126(3):831–7. doi:10.1378/chest.126.3.831
53. Lemoine TJ, Swoboda KJ, Bratton SL, Holubkov R, Mundorff M, Srivastava R. Spinal muscular atrophy type 1: are proactive respiratory interventions associated with longer survival? Pediatr Crit Care Med (2012) 13(3):e161–5. doi:10.1097/PCC.0b013e3182388ad1
54. Ottonello G, Mastella C, Franceschi A, Bosticco D, Wolfler A, Pedemonte M, et al. Spinal muscular atrophy type 1: avoidance of hospitalization by respiratory muscle support. Am J Phys Med Rehabil (2011) 90(11):895–900. doi:10.1097/PHM.0b013e318232883a
55. Petrone A, Pavone M, Testa MBC, Petreschi F, Bertini E, Cutrera R. Noninvasive ventilation in children with spinal muscular atrophy types 1 and 2. Am J Phys Med Rehabil (2007) 86(3):216–21. doi:10.1097/PHM.0b013e31802ef774
56. Vasconcelos M, Fineza I, Felix M, Estevao MH. Spinal muscular atrophy – noninvasive ventilatory support in pediatrics. Rev Port Pneumol (2005) 11(5):443–55. doi:10.1016/S0873-2159(15)30520-1
57. Afsharpaiman S, Sillence DO, Sheikhvatan M, Ault JE, Waters K. Respiratory events and obstructive sleep apnea in children with achondroplasia: investigation and treatment outcomes. Sleep Breath (2011) 15(4):755–61. doi:10.1007/s11325-010-0432-6
58. Han YJ, Park JD, Lee B, Choi YH, Suh DI, Lim BC, et al. Home mechanical ventilation in childhood-onset hereditary neuromuscular diseases: 13 years’ experience at a single center in Korea. PLoS One (2015) 10(3):e0122346. doi:10.1371/journal.pone.0122346
59. Wood L, Cordts I, Atalaia A, Marini-Bettolo C, Maddison P, Phillips M, et al. The UK myotonic dystrophy patient registry: facilitating and accelerating clinical research. J Neurol (2017) 264(5):979–88. doi:10.1007/s00415-017-8483-2
60. García Teresa MA, Porto Abal R, Rodríguez Torres S, García Urabayen D, García Martínez S, Trang H, et al. [Spanish patients with central hypoventilation syndrome included in the European Registry. The 2015 data]. An Pediatr (2017) 86(5):255–63. doi:10.1016/j.anpedi.2016.05.008
61. Hartmann H, Jawad MH, Noyes J, Samuels MP, Southall DP. Negative extrathoracic pressure ventilation in central hypoventilation syndrome. Arch Dis Child (1994) 70(5):418–23. doi:10.1136/adc.70.5.418
62. Khayat A, Medin D, Syed F, Moraes TJ, Bin-Hasan S, Narang I, et al. Intelligent volume-assured pressured support (iVAPS) for the treatment of congenital central hypoventilation syndrome. Sleep Breath (2017) 21(2):513–9. doi:10.1007/s11325-017-1478-5
63. Noyes J, Hartmann H, Samuels M, Southall D. The experiences and views of parents who care for ventilator-dependent children. J Clin Nurs (1999) 8(4):440–50. doi:10.1046/j.1365-2702.1999.00258.x
64. Ramesh P, Boit P, Samuels M. Mask ventilation in the early management of congenital central hypoventilation syndrome. Arch Dis Child Fetal Neonatal Ed (2008) 93(6):F400–3. doi:10.1136/adc.2008.139931
65. Tibballs J, Henning RD. Noninvasive ventilatory strategies in the management of a newborn infant and three children with congenital central, hypoventilation syndrome. Pediatr Pulmonol (2003) 36(6):544–8. doi:10.1002/ppul.10392
66. Bunn HJ, Roberts P, Thomson AH. Noninvasive ventilation for the management of pulmonary hypertension associated with congenital heart disease in children. Pediatr Cardiol (2004) 25(4):357–9. doi:10.1007/s00246-003-0501-8
67. Adeleye A, Ho A, Nettel-Aguirre A, Buchhalter J, Kirk V. Noninvasive positive airway pressure treatment in children less than 12 months of age. Can Respir J (2016) 2016:7654631. doi:10.1155/2016/7654631
68. Bertrand P, Fehlmann E, Lizama M, Holmgren N, Silva M, Sanchez I. [Home ventilatory assistance in Chilean children: 12 years’ experience]. Arch Bronconeumol (2006) 42(4):165–70. doi:10.1157/13086621
69. Fauroux B, Lavis JF, Nicot F, Picard A, Boelle PY, Clément A, et al. Facial side effects during noninvasive positive pressure ventilation in children. Intensive Care Med (2005) 31(7):965–9. doi:10.1007/s00134-005-2669-2
70. Kherani T, Sayal A, Al-Saleh S, Sayal P, Amin R. A comparison of invasive and noninvasive ventilation in children less than 1 year of age: a long-term follow-up study. Pediatr Pulmonol (2016) 51(2):189–95. doi:10.1002/ppul.23229
71. Koontz KL, Slifer KJ, Cataldo MD, Marcus CL. Improving pediatric compliance with positive airway pressure therapy: the impact of behavioral intervention. Sleep (2003) 26(8):1010–5. doi:10.1093/sleep/26.8.1010
72. Machaalani R, Evans CA, Waters KA. Objective adherence to positive airway pressure therapy in an Australian paediatric cohort. Sleep Breath (2016) 20(4):1327–36. doi:10.1007/s11325-016-1400-6
73. Nathan AM, Loo HY, de Bruyne JA, Eg KP, Kee SY, Thavagnanam S, et al. Thirteen years of invasive and noninvasive home ventilation for children in a developing country: a retrospective study. Pediatr Pulmonol (2017) 52(4):500–7. doi:10.1002/ppul.23569
74. Ramirez A, Delord V, Khirani S, Leroux K, Cassier S, Kadlub N, et al. Interfaces for long-term noninvasive positive pressure ventilation in children. Intensive Care Med (2012) 38(4):655–62. doi:10.1007/s00134-012-2516-1
75. Zhou J, Liu DB, Zhong JW, Huang ZY, Qiu SY, Zhou YP, et al. Feasibility of a remote monitoring system for home-based non-invasive positive pressure ventilation of children and infants (Provisional abstract). Int J Pediatr Otorhinolaryngol (2012) 76(12):1737–40. doi:10.1016/j.ijporl.2012.08.012
76. Katz ES, Mitchell RB, D’Ambrosio CM. Obstructive sleep apnea in infants. Am J Respir Crit Care Med (2012) 185(8):805–16. doi:10.1164/rccm.201108-1455CI
77. Amaddeo A, Caldarelli V, Fernandez-Bolanos M, Moreau J, Ramirez A, Khirani S, et al. Polygraphic respiratory events during sleep in children treated with home continuous positive airway pressure: description and clinical consequences. Sleep Med (2015) 16(1):107–12. doi:10.1016/j.sleep.2014.07.030
78. Centres for Disease Control and Prevention. Child Development: Infants (0-1 Year of Age). (2017). Available from: https://www.cdc.gov/ncbddd/childdevelopment/positiveparenting/infants.html
Keywords: continuous positive airway pressure, bi-level positive airway pressure, obstructive sleep apnea, Pierre Robin sequence, laryngo-tracheomalacia, spinal muscular atrophy type 1, central hypoventilation syndrome
Citation: Bedi PK, Castro-Codesal ML, Featherstone R, AlBalawi MM, Alkhaledi B, Kozyrskyj AL, Flores-Mir C and MacLean JE (2018) Long-term Non-Invasive Ventilation in Infants: A Systematic Review and Meta-Analysis. Front. Pediatr. 6:13. doi: 10.3389/fped.2018.00013
Received: 20 November 2017; Accepted: 12 January 2018;
Published: 12 February 2018
Edited by:
Renato Cutrera, Bambino Gesù Ospedale Pediatrico (IRCCS), ItalyReviewed by:
Martino Pavone, Bambino Gesù Ospedale Pediatrico (IRCCS), ItalyJean-Paul Praud, Université de Sherbrooke, Canada
Copyright: © 2018 Bedi, Castro-Codesal, Featherstone, AlBalawi, Alkhaledi, Kozyrskyj, Flores-Mir and MacLean. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joanna E. MacLean, am9hbm5hLm1hY2xlYW5AdWFsYmVydGEuY2E=
 Prabhjot K. Bedi
Prabhjot K. Bedi Maria Luisa Castro-Codesal
Maria Luisa Castro-Codesal Robin Featherstone2
Robin Featherstone2 Mohammed M. AlBalawi
Mohammed M. AlBalawi Bashar Alkhaledi
Bashar Alkhaledi Anita L. Kozyrskyj
Anita L. Kozyrskyj